Abstract
Objectives
The emergence of SARS-CoV-2 variants raised questions about the extent to which vaccines designed in 2020 have remained effective. We aimed to assess whether vaccine status was associated with the severity of Omicron SARS-CoV-2 infection in hospitalized patients.
Methods
We conducted an international, multi-centric, retrospective study in 14 centres (Bulgaria, Croatia, France, and Turkey). We collected data on patients hospitalized for ≥24 hours between 1 December 2021 and 3 March 2022 with PCR-confirmed infection at a time of exclusive Omicron circulation and hospitalization related or not related to the infection. Patients who had received prophylaxis by monoclonal antibodies were excluded. Patients were considered fully vaccinated if they had received at least two injections of either mRNA and/or ChAdOx1-S or one injection of Ad26.CoV2-S vaccines.
Results
Among 1215 patients (median age, 73.0 years; interquartile range, 57.0–84.0; 51.3% men), 746 (61.4%) were fully vaccinated. In multivariate analysis, being vaccinated was associated with lower 28-day mortality (Odds Ratio [95% Confidence Interval] (OR [95CI]) = 0.50 [0.32–0.77]), intensive care unit admission (OR [95CI] = 0.40 [0.26–0.62]), and oxygen requirement (OR [95CI] = 0.34 [0.25–0.46]), independent of age and comorbidities. When co-analysing these patients with Omicron infection with 948 patients with Delta infection from a study we recently conducted, Omicron infection was associated with lower 28-day mortality (OR [95CI] = 0.53 [0.37–0.76]), intensive care unit admission (OR [95CI] = 0.19 [0.12–0.28]), and oxygen requirements (OR [95CI] = 0.50 [0.38–0.67]), independent of age, comorbidities, and vaccination status.
Discussion
Originally designed vaccines have remained effective on the severity of Omicron SARS-CoV-2 infection. Omicron is associated with a lower risk of severe forms, independent of vaccination and patient characteristics.
Keywords: Breakthrough infection, Omicron, SARS-CoV-2, Severity, Vaccine
Introduction
First identified in November 2021, the Omicron (B.1.1.529) variant of concern (VOC) of SARS-CoV-2 replaced the Delta (B.1.617.2) VOC globally and became virtually the only circulating variant in most countries in December 2021 and January 2022. Between December 2021 and April 2022, it was responsible for periods of highest incidence of SARS-CoV-2 infection in Europe, North America, China, and Japan since the beginning of the pandemic [1]. The extent of Omicron VOC waves is considered to be related to increased infectivity and immune escape from neutralizing antibodies resulting from previous SARS-CoV-2 infection, vaccination, or both [2,3].
In most European countries, the unprecedented numbers of SARS-CoV-2 infections during Omicron waves were associated with a decreased case-fatality rate compared with the Delta VOC [[4], [5], [6]]. Whether related to an intrinsically lower risk of severe COVID-19 or the high vaccine coverage, this Omicron VOC calls into question the impact of vaccination at both the individual and the populational level. Meanwhile, the picture provided by the aforementioned studies is incomplete because most milder cases will not be admitted to the hospital or even diagnosed.
We aimed to determine the extent to which vaccination was still associated with protection against severe forms of Omicron infection by assessing severity among people hospitalized with SARS-CoV-2 infection when considering their vaccine status and other risk factors. We also explored the influence of VOC by coupling our data with previously published data on the Delta variant and using the same methodology.
Methods
We performed a retrospective, multicentre study across 14 hospital centres from four countries, namely Bulgaria (Sofia), Croatia (Olsijek and Zagreb), France (Annecy, Paris/Béclère, Caen, Grenoble, Le Mans, Limoges, Nantes and Poitiers) and Turkey (Ankara and Karaman). Hospitalized patients with a PCR-confirmed SARS-CoV-2 infection from 1 December 2021 to 3 March 2022 (i.e. when the Omicron [B.1.1.529] variant [subvariant BA.1] was virtually the only circulating SARS-CoV-2 variant in these countries) were included chronologically regardless of the indication for admission, the objective being to include at least the first 50–100 patients per centre. Patients were considered infected by the Omicron VOC either through mutation screening or because it was the only variant circulating at that time in the area according to the virologist from each centre.
A patient was considered fully vaccinated if he/she had received at least either one injection of the Ad26.CoV2-S vaccine (Janssen) or two injections of the ChAdOx1-S vaccine (AstraZeneca) and/or RNA vaccines (tozinameran [Pfizer/BioNTech] and elasomeran [Moderna]), with the last injection administered at least 14 days before the date of PCR. Vaccinated and unvaccinated patients were included concurrently according to the order of hospitalization in the participating centre.
Data were anonymously collected with a form similar to the one used in a recent study on SARS-CoV-2 Delta infection [7], providing similar variables (supplementary material and Table 1, Table 2 ), with the exception of antiviral treatments, which were not available at the time data were collected for the Delta study. The ethics committee of the French-speaking Society of Infectious Diseases (IRB00011642) gave its approval for the study (N°2022-0101), which was declared to the French National Commission for Informatics and Liberties (CNIL MR004: n°2224742).
Table 1.
Characteristics of patients with SARS-CoV-2 (Omicron variant) infection according to vaccine status: demographics, underlying diseases, and presentation
| Vaccinated (N = 746) | Unvaccinated (N = 469) | p | |
|---|---|---|---|
| Age (y), n (%) | 0.300 | ||
| <35 y | 67 (9.0) | 54 (11.5) | |
| 35–65 y | 198 (26.5) | 128 (27.3) | |
| >65 y | 481 (64.5) | 287 (61.2) | |
| Age (y) | 74.0 [57.3–85.0] | 71.0 [55.0–83.0] | 0.041 |
| Sex: men, n (%) | 406 (54.4) | 217 (46.3) | 0.007 |
| BMI (kg/m2) | 25.9 [22.2–29.4] (n = 391) | 26.1 [22.6–29.2] (n = 232) | 0.670 |
| Overweight (BMI > 25), n (%) | 221 (56.5) (n = 391) | 134 (57.8) (n = 232) | 0.828 |
| Pre-existing comorbidities, n (%) | |||
| Cardiac failure | 121 (23.6) (n = 512) | 65 (18.3) (n = 355) | 0.073 |
| Respiratory disease | 103 (13.8) | 47 (10.0) | 0.062 |
| Oxygen at home | 15 (2.9) (n = 514) | 3 (0.8) (n = 354) | 0.063 |
| Kidney failure (GFR<30) | 88 (12.0) (n = 731) | 31 (6.8) (n = 459) | 0.004 |
| Diabetes | 184 (24.7) | 106 (22.7) | 0.452 |
| Hypertension | 261 (50.8) (n = 514) | 183 (51.5) (n = 355) | 0.877 |
| Solid cancer for <3 mo | 22 (3.0) (n = 745) | 9 (1.9) | 0.351 |
| Haematologic malignancy | 42 (5.6) | 10 (2.1) (n = 468) | 0.003 |
| Immunodepressiona | 77 (15.0) (n = 514) | 33 (9.3) (n = 355) | 0.013 |
| At least one comorbidity among the above | 523 (70.1) | 330 (70.4) | 0.949 |
| Number of comorbidities | 1.0 [0.0-2.0] | 1.0 [0.0-2.0] | 0.201 |
| Tobacco, active or discontinued, <3 y, n (%) | 44 (11.9) (n = 370) | 38 (15.4) (n = 247) | 0.258 |
| Previous SARS-CoV-2 infection, n (%) | 12 (1.9) (n = 633) | 10 (2.4) (n = 415) | 0.661 |
| Time from a previous infection (d) | 319.0 [43.0-344.0] (n = 9) | 215 [97.3-389.5] (n = 10) | 0.497 |
| Time from last vaccine dose (d) | 113.0 [64.0-178.2] | — | — |
| Admission for COVID-19 | 359 (48.7) (n = 737) | 317 (69.5) (n = 456) | <0.001 |
| Ct value (lowest value if many) | 21.4 [18.2-27.0] (n = 423) | 22.1 [19.0-27.3] (n = 273) | 0.125 |
| CRP (highest value) (mg/L) | 58.0 [21.5-117.5] (n = 575) | 66.0 [26.0-126.3] (n = 380) | 0.110 |
| The proportion of lungs involved in CT scans, n (%) | n = 179 | n = 168 | 0.003 |
| 0–10% | 90 (50.3) | 51 (30.4) | |
| 11–25% | 40 (22.3) | 55 (32.7) | |
| 26–50% | 32 (17.9) | 41 (24.4) | |
| 51–75% | 12 (6.7) | 18 (10.7) | |
| >75% | 5 (2.8) | 3 (1.8) |
Quantitative variables are presented as median (first quartile to third quartile). When data were missing or calculation is performed on a subgroup, the number of patients out of whom the statistic was calculated is mentioned alongside. BMI, body mass index; GFR, glomerular filtration rate; Ct, Cycle threshold; CT, computed tomography; CRP, C-reactive protein.
Immunosuppression is defined by the presence of one of the following: solid organ transplantation, haematopoietic stem cell transplantation, long-term steroid use, biotherapy, other immunosuppressive therapy, other cause of immunodepression.
Table 2.
Characteristics of patients with SARS-CoV-2 (Omicron variant) infection according to vaccine status: management and outcomes during hospital stay
| Vaccinated (N = 746) | Unvaccinated (N = 469) | p | |
|---|---|---|---|
| Requirement for oxygen, n (%) | 385 (51.6) | 304 (64.8) | <0.001 |
| Maximal oxygen flow (L/min) | 4.0 [2.0-10.0] | 7.0 [3.0-15.0] | <0.001 |
| Duration of oxygen therapy (d) | 5.0 [2.0-10.0] | 7.0 [4.0-11.8] | <0.001 |
| High-flow oxygen, n (%) | 32 (4.4) (n = 729) | 56 (12.0) (n = 468) | <0.001 |
| Steroid therapy | |||
| Prescribed n (%) | 297 (40.0) (n = 743) | 275 (58.8) (n = 468) | <0.001 |
| Median Dose (IQR) | 7.5 [6.0–11.1] (n = 290) | 8.0 [6.0–12.0] (n = 273) | 0.009 |
| Duration (d) | 5.0 [3.0-10.0] (n = 286) | 8.0 [4.0-10.0] (n = 272) | <0.001 |
| Tocilizumab, n (%) | 20 (2.7) (n = 745) | 29 (6.2) | 0.004 |
| Monoclonal Ab, n (%)a | 30 (4.0) (n = 745) | 19 (4.1) (n = 467) | 1.000 |
| Antiviral use, n (%)b | 27 (5.2) (n = 516) | 25 (7.0) (n = 355) | 0.336 |
| ICU admission, n (%) | 60 (8.0) | 72 (15.4) | <0.001 |
| ICU duration (d) | 7.0 [3.0-15.0] | 9.0 [5.0-15.0] | 0.095 |
| Invasive mechanical ventilation, n (%) | 17 (2.3) (n = 740) | 22 (4.7) (n = 464) | 0.030 |
| Invasive mechanical ventilation duration (d) | 5.0 [2.5-12.5] | 8.0 [7.0-20.0] | 0.037 |
| Death at day 28, n (%) | 71 (9.5) | 62 (13.2) | 0.044 |
| Death at day 28 and/or ICU admission, n (%) | 113 (15.2) | 116 (24.7) | <0.001 |
| Length of stay (d) | 7.0 [3.0-14.0] (n = 571) | 8.0 [4.0-13.0] (n = 377) | 0.472 |
Quantitative variables are presented as median (first quartile to third quartile). When data were missing, the number of patients out of whom the percentage was calculated is mentioned alongside the percentage. Ab, antibody; ICU, intensive care unit; IQR, interquartile range.
Among the 30 vaccinated patients who received monoclonal antibodies, 55.6% and 44.4% received tixagevimab/cilgavimab and sotrovimab, respectively. Among the 19 unvaccinated patients, 85.7% and 14.3% received tixagevimab/cilgavimab and sotrovimab, respectively.
Among the 27 vaccinated patients who received antiviral, 78.6% received remdesivir and 21.4% favipiravir. Among the 25 unvaccinated patients who received an antiviral, 57.7% received remdesivir and 42.3% received favipiravir.
Statistical analysis
Qualitative variables are expressed as counts (percentage) and frequency distributions compared with the chi-square test or Fisher's exact test when appropriate. Continuous variables were expressed as median (first quartile; third quartile), and differences were tested with the independent t-test for normally distributed variables or otherwise with the Mann-Whitney U test. Factors associated with severe forms of infection defined by three outcomes (requirement for oxygen, intensive care unit [ICU] admission, and death at day 28) were assessed. Univariate and multivariate logistic regressions based on general linear models were performed with a stepwise variable selection according to the Akaike information criterion [8] to select the potential risk factors for severe outcome (Table 3 ) for the multivariate model (Table 4 and supplementary material). Even though some data were missing at random, in many cases, this was because they did not exist. Patients could have been too severe to receive a computed tomography (CT) scan before mechanical ventilation or only mildly ill and went back home without any oxygen requirement or CT scan. To manage missing data not at random, multivariate analysis was initially performed only on the dataset with complete cases, after which the model was applied to the full dataset [9]. Results are presented on the basis of the full dataset. In addition, a multivariate analysis was similarly performed on the dataset of vaccinated patients only to independently assess the weight of comorbidities among vaccinated patients, taking into account the influence of the time elapsed since the most recent injection. Interactions were systematically searched and mentioned if found. Finally, this cohort of Omicron-infected patients was merged with a cohort of Delta-infected patients recently published [7], and an analysis was performed taking into account the VOC. All statistical analyses were performed with R version 4.1.2.
Table 3.
Association of patient characteristics with negative outcomes (bivariate logistic regression)
| Factors | Death at day 28 |
ICU |
Oxygen required |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age (y) | 1.05 [1.04–1.07] | <0.001 | 1.00 [0.99–1.00] | 0.306 | 1.03 [1.02–1.04] | <0.001 |
| Sex: male | 1.14 [0.79–1.64] | 0.485 | 1.48 [1.03–2.14] | 0.038 | 1.38 [1.10–1.73] | 0.006 |
| Diabetes mellitus | 1.55 [1.04–2.28] | 0.028 | 1.64 [1.10–2.40] | 0.014 | 1.31 [1.00–1.72] | 0.048 |
| Hypertension | 1.47 [0.97–2.27] | 0.073 | 1.21 [0.80–1.83] | 0.365 | 1.87 [1.43–2.45] | <0.001 |
| Tobacco | 1.44 [0.66–2.87] | 0.323 | 1.53 [0.67–3.14] | 0.278 | 1.16 [0.72–1.85] | 0.546 |
| BMI (kg/m2) | 0.94 [0.89–0.99] | 0.037 | 1.05 [1.00–1.09] | 0.029 | 1.07 [1.04–1.10] | <0.001 |
| Overweight (BMI > 25) | 0.54 [0.30–0.97] | 0.040 | 1.44 [0.83–2.59] | 0.207 | 1.71 [1.24–2.36] | 0.001 |
| Kidney failure (GFR < 30) | 2.86 [1.77–4.53] | <0.001 | 2.11 [1.26–3.42] | 0.003 | 1.81 [1.21–2.75] | 0.005 |
| Respiratory disease | 1.67 [1.01–2.66] | 0.036 | 1.40 [0.83–2.27] | 0.189 | 3.06 [2.06–4.64] | <0.001 |
| Oxygen at home | 0.97 [0.15–3.48] | 0.968 | 1.48 [0.34–4.59] | 0.539 | 15.04 [3.07–271.60] | 0.009 |
| Cardiac failure | 1.71 [1.06–2.69] | 0.024 | 1.39 [0.86–2.19] | 0.167 | 1.58 [1.14–2.22] | 0.007 |
| Solid cancer for <3 mo | 5.54 [2.56–11.57] | <0.001 | 0.88 [0.21–2.51] | 0.829 | 1.06 [0.52–2.23] | 0.874 |
| Haematologic malignancy | 2.29 [1.09–4.42] | 0.019 | 0.49 [0.12–1.36] | 0.236 | 1.34 [0.76–2.43] | 0.314 |
| Immunosuppression | 1.64 [0.92–2.80] | 0.082 | 1.28 [0.70–2.22] | 0.397 | 1.23 [0.82–1.85] | 0.318 |
| At least one comorbidity | 1.52 [1.00–2.36] | 0.055 | 1.93 [1.25–3.11] | 0.004 | 1.65 [1.29–2.11] | <0.001 |
| Number of comorbidities | 1.26 [1.11–1.42] | <0.001 | 1.20 [1.06–1.36] | 0.003 | 1.20 [1.10–1.30] | <0.001 |
| Previous SARS-CoV-2 infection | 0.40 [0.05–2.98] | 0.368 | 0.42 [0.06–3.17] | 0.402 | 0.81 [0.35–1.90] | 0.630 |
| Time from the last infection (d)a | 1.000 | 0.997 | 0.739 | |||
| Vaccination | 0.69 [0.48–0.99] | 0.045 | 0.48 [0.33–0.69] | <0.001 | 0.58 [0.46–0.73] | <0.001 |
| Time from last vaccine injection (d)a | 0.289 | 0.117 | 0.104 | |||
BMI, body mass index; GFR, glomerular filtration rate; ICU, intensive care unit.
For the time from the last infection and from the last vaccine injection, the scarcity of data made the calculation of CIs irrelevant.
Table 4.
Factors associated with negative outcomes, with a multivariate logistic regression (best model according to Akaike Information Criteria (AIC))
| Death on day 28 |
ICU |
Need for oxygen |
||||
|---|---|---|---|---|---|---|
| Best model | p | Best model | p | Best model | p | |
| Age | 1.06 [1.04-1.08] | <0.001 | 1.03 [1.02-1.03] | <0.001 | ||
| Sex: male | 1.25 [0.82-1.90] | 0.304 | 1.37 [1.02-1.83] | 0.036 | ||
| Diabetes | 1.51 [0.95-2.36] | 0.076 | ||||
| Kidney failure | 2.52 [1.44-4.31] | 0.001 | 2.30 [1.29-3.97] | 0.004 | ||
| Previous SARS-CoV-2 infection | 0.29 [0.02-1.59] | 0.247 | 0.36 [0.02-1.81] | 0.325 | ||
| Vaccination | 0.50 [0.32-0.77] | 0.002 | 0.40 [0.26-0.62] | <0.001 | 0.34 [0.25-0.46] | <0.001 |
| Pulmonary diseases | 1.51 [0.86-2.56] | 0.138 | 1.68 [0.96-2.85] | 0.060 | 1.91 [1.19-3.12] | 0.009 |
| O2 at home | 8.41 [1.58-156.23] | 0.044 | ||||
| Solid cancer for <3 mo | 7.99 [2.97-20.80] | <0.001 | ||||
| Haematological cancer | 3.84 [1.67-8.29] | 0.001 | ||||
| Immunosuppressed | 1.58 [1.03-2.46] | 0.038 | ||||
Models were explored with a full model including the variable ‘gender’, ‘Age’, ‘Diabetes’, ‘Hypertension (HTA)’, ‘KidneyFailure’, ‘O2_home’, ‘CardiacFailure’, ‘ImmunoSup’, ‘previousSARS-CoV-2 infection’, ‘Vaccination’, ‘PulmDis’, ‘SolidCancer3M’, ‘HematoK’, and ‘OneComorb’. ‘Tobacco’, ‘BMI’, and ‘overweight’ were not included owing to more than 50% of missing data. No interactions were found. ICU, intensive care unit.
Results
We included 1215 hospitalized patients; of these, 746 (61.4%) were fully vaccinated and 469 (38.6%) were not vaccinated at all. The main characteristics of both the groups are described in Table 1. Patient included were from Bulgaria (Sofia, n = 43), Croatia (Olsijek, n = 22; Zagreb, n = 132), France (Annecy, n = 368; Paris/Béclère, n = 169; Caen, n = 131; Grenoble, n = 70; Le Mans, n = 49; Limoges, n = 34; Nantes, n = 40; Poitiers, n = 54), and Turkey (Ankara, n = 77; Karaman, n = 34).
Among vaccinated patients with complete information on their vaccine history (n = 674), most were vaccinated with Pfizer vaccines only (n = 431, 63.9%) or a mix of the two mRNA vaccines (n = 91, 13.5%), whereas a few had received a mix of mRNA and adenovirus vaccines (53, 7.9%) or only Moderna vaccines (n = 52, 7.7%), AstraZeneca vaccines (n = 23, 3.4%) or Janssen vaccines (n = 15, 2.2%). More than half of the vaccinated patients had received more than two doses (373 patients received 3 doses and 22 patients received 4 doses). The median time from the last vaccine dose was 113.0 days (interquartile range, 64.0–178.2).
Characteristics of the SARS-CoV-2 infection according to vaccine status
In bivariate analysis, vaccinated patients were slightly older, more frequently male, and more frequently presented with certain comorbidities (respiratory disease, haematologic malignancy, and immunodepression); however, they did not differ with unvaccinated patients in terms of median number of comorbidities.
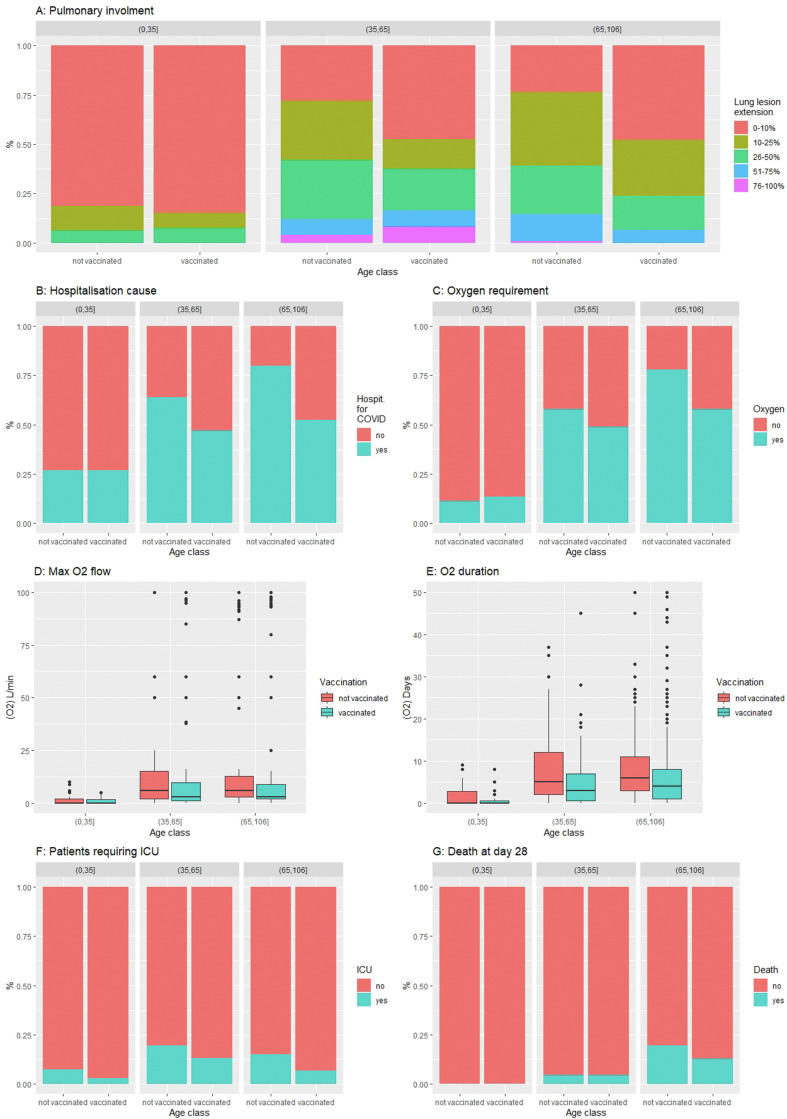
Notwithstanding this, unvaccinated patients had more severe SARS-CoV-2 infections (Table 1, Table 2): they were more frequently admitted because of COVID-19 instead of being admitted for another cause with an incident diagnosis of an asymptomatic SARS-CoV-2 infection (69.5 vs. 48.7%), their lung involvement was more extended on CT scan (69.6 vs. 49.7% had more than 10% of lung involved); they more frequently required oxygen supplementation (64.8 vs. 51.6%) at a higher flow (7 [3.0; 15.0] vs. 4 [2.0; 10.0] L/min) and for a longer duration (7.0 [4.0; 11.8] vs. 5.0 [2.0; 10.0] days), and they more frequently required high-flow oxygen therapy (12.0 vs. 4.4%) and immunomodulatory therapy, such as steroids (58.8 vs. 40.0%) or tocilizumab (6.2 vs. 2.7%). The protective effect of vaccination predominated at an older age (Fig. 1 ).
Fig. 1.
SARS-CoV-2 (Omicron variant) infection severity criteria according to vaccine status in the three age classes. (a) Extension of lung lesions on computed tomography scanner; (b) hospitalization cause; (c) proportion of patients requiring oxygen; (d) maximal oxygen flow; (e) duration of oxygen therapy; (f) proportion of patients requiring intensive care unit (ICU); (g) proportion of death at day 28.
Factors associated with severity
Three outcomes were considered: the need to receive oxygen, the need to be admitted to an ICU, and death at day 28. In bivariate analysis, older age, male sex, and most comorbidities (as well as having at least one comorbidity or a higher number of comorbidities) were significantly associated with one or several negative outcomes. Conversely, vaccination was protective against these three outcomes (Table 3).
In multivariate analysis, the protective effect of vaccination persisted with regard to the need for oxygen, need for ICU admission, and risk of death, and it attenuated and practically discarded the negative impact of many comorbidities (Table 4). Older age, solid or haematological cancer, and kidney failure remained independent risk factors for a more severe outcome, notably death. Pulmonary diseases tended to negatively impact the outcome; however, only regarding the need for oxygen was the impact significant.
Influence of the VOC (Delta vs. Omicron)
Patients infected by the Omicron VOC presented less severe infection, either vaccinated or unvaccinated, compared with patients infected by the Delta VOC.
In bivariate analysis (Table 5, Table 6 ), whether vaccinated or not, Omicron-infected patients were significantly (or at least presented a trend) less hospitalized for COVID-19 (significantly among those vaccinated); had lower median C-reactive protein (CRP) (significantly among those vaccinated); had less extended lung lesion on CT scan (significantly among those unvaccinated); less frequently required oxygen supplementation, high-flow oxygen therapy, steroid therapy, or tocilizumab (significantly among both vaccinated and unvaccinated); less frequently required to be admitted in ICU (both vaccinated and unvaccinated); and had lower 28-day mortality (among those vaccinated).
Table 5.
Comparison of the Omicron infection with the Delta infection according to vaccination status: demographics, underlying diseases, and presentation
| Vaccinated (N = 1220) |
Unvaccinated (N = 950) |
|||||
|---|---|---|---|---|---|---|
| Omicron (n = 746) | Delta (n = 474) | p | Omicron (n = 469) | Delta (n = 481) | p | |
| Age (y), n (%) | 0.031 | <0.001 | ||||
| <35 y | 67 (9.0) | 27 (5.7) | 54 (11.6) | 97 (20.2) | ||
| 35–65 y | 198 (26.6) | 111 (23.4) | 128 (27.4) | 225 (46.8) | ||
| >65 y | 481 (64.5) | 336 (70.9) | 287 (61.2) | 159 (33.1) | ||
| Age (y) | 74.0 [57.3 85.0] | 75.0 [63.3-84.0] | 0.012 | 71.0 [55.0-83.0] | 55.0 [38.0-73.0] | <0.001 |
| Sex: men, n (%) | 406 (54.4) | 261 (55.1) | 0.873 | 217 (46.3) | 223 (46.4) | 1.000 |
| BMI (kg/m2) | 25.9 [22.2-29.4] | 25.8 [22.3-31.1] | 0.036 | 26.1 [22.6-29.2] | 27.2 [24.2-31.2] | <0.001 |
| Overweight (BMI > 25), n (%) | 221 (56.5) | 192 (56.6) | 1.000 | 134 (57.8) | 222 (69.4) | 0.006 |
| Pre-existing comorbidities, n (%) | ||||||
| Cardiac failure | 121 (23.6) | 50 (13.7) | <0.001 | 65 (18.3) | 29 (8.0) | <0.001 |
| Respiratory disease | 103 (13.8) | 91 (20.7) | 0.002 | 47 (10.0) | 54 (12.6) | 0.256 |
| Oxygen at home | 15 (2.9) | 14 (4.0) | 0.515 | 3 (0.8) | 5 (1.4) | 0.501 |
| Kidney failure (GFR < 30) | 88 (12.0) | 51 (15.2) | 0.182 | 31 (6.8) | 10 (3.2) | 0.041 |
| Diabetes | 184 (24.7) | 127 (28.7) | 0.141 | 106 (22.6) | 76 (17.8) | 0.086 |
| Hypertension | 261 (50.8) | 244 (55.1) | 0.206 | 183 (51.5) | 123 (28.7) | <0.001 |
| Solid cancer for <3 mo | 22 (3.0) | 17 (4.8) | 0.170 | 9 (1.9) | 8 (2.2) | 0.945 |
| Haematologic malignancy | 42 (5.6) | 25 (7.1) | 0.428 | 10 (2.1) | 6 (1.7) | 0.869 |
| Immunodepression | 77 (15.0) | 74 (20.3) | 0.048 | 33 (9.3) | 21 (5.8) | 0.110 |
| At least one comorbidity among those above | 523 (70.1) | 369 (90.4) | <0.001 | 330 (70.4) | 306 (86.9) | <0.001 |
| Number of comorbidities | 1.0 [0.0-2.0] | 2.0 [1.0-3.0] | <0.001 | 1.0 [0.0-2.0] | 1.0 [0.0-2.0] | 0.010 |
| Tobacco, active or discontinued < 3 y, n (%) | 44 (11.9) | 36 (10.3) | 0.580 | 38 (15.4) | 49 (14.3) | 0.800 |
| Previous SARS-CoV-2 infection, n (%) | 12 (1.9) | 19 (5.4) | 0.005 | 10 (2.4) | 12 (3.4) | 0.533 |
| Time from a previous infection (d) | 319.0 [43.0-344.0] | 226.0 [176.5-299.0] | 0.688 | 215.0 [97.3-389.5] | 69 [43.3-244.3] | 0.113 |
| Time from last vaccine dose (d)a | 113.0 [64.0-178.2] | 125.0 [82.0-166.5] | 0.716 | – | – | – |
| Admission for COVID-19 | 359 (48.7) | 281 (59.3) | <0.001 | 317 (69.5) | 361 (75.1) | 0.069 |
| Ct value (lowest value if many) | 21.4 [18.2-27.0] | 26.0 [18.0-34.0] | <0.001 | 22.1 [19.0-27.3] | 26.0 [21.0-33.0] | <0.001 |
| CRP (highest value) (mg/L) | 58.0 [21.5-117.5] | 87.5 [31.3-152.8] | <0.001 | 66.0 [26.0-126.3] | 86.0 [34.0-145.0] | 0.073 |
| The proportion of lungs involved on CT scan n (%) | 0.134 | <0.001 | ||||
| 0–10% | 90 (50.3) | 67 (36.8) | 51 (30.4) | 31 (13.5) | ||
| 11–25% | 40 (22.3) | 50 (27.5) | 55 (32.7) | 57 (24.9) | ||
| 26–50% | 32 (17.9) | 39 (21.4) | 41 (24.4) | 75 (32.8) | ||
| 51–75% | 12 (6.7) | 18 (9.9) | 18 (10.7) | 49 (21.4) | ||
| >75% | 5 (2.8) | 8 (4.4) | 3 (1.8) | 17 (7.4) | ||
BMI, body mass index; GFR, glomerular filtration rate; Ct, Cycle threshold; CT, computed tomography; CRP, C-reactive protein.
For the time from the last infection and from the last vaccine injection.
Table 6.
Comparison of the Omicron infection with the Delta infection according to vaccine status: management and outcomes during hospital stay
| Vaccinated (N = 1220) |
Unvaccinated (N = 950) |
|||||
|---|---|---|---|---|---|---|
| Omicron (n = 746) | Delta (n = 474) | p | Omicron (n = 469) | Delta (n = 481) | p | |
| Requirement for oxygen, n (%) | 385 (51.6) | 229 (57.5) | 0.064 | 304 (64.8) | 270 (73.0) | 0.014 |
| Maximal oxygen therapy (L/min) | 3.0 [1.0-9.0] | 3.0 [0.0-8.8] | 0.140 | 6.0 [2.0-15.0] | 6.0 [2.0-50.0] | 0.153 |
| Duration of oxygen therapy (d) | 3.0 [0.5-7.3] | 3.0 [0.0-8.0] | 0.369 | 6.0 [2.0-10.0] | 6.0 [2.0-12.0] | 0.629 |
| High-flow oxygen, n (%) | 32 (4.4) | 49 (13.6) | <0.001 | 56 (12.0) | 105 (29.4) | <0.001 |
| Steroid therapy | ||||||
| Prescribed, n (%) | 297 (40.0) | 174 (47.2) | 0.027 | 275 (58.8) | 250 (68.5) | 0.005 |
| Median dose (IQR) | 7.5 [6.0–10.7] | 6.0 [6.0–7.5] | <0.001 | 8.0 [6.0–12.0] | 6.0 [6.0–9.0] | <0.001 |
| Duration (d) | 5.0 [3.0-10.0] | 9.0 [5.0-10.0] | <0.001 | 8.0 [4.0-10.0] | 10.0 [7.0-10.0] | <0.001 |
| Tocilizumab, n (%) | 20 (2.7) | 21 (6.3) | 0.007 | 29 (6.2) | 59 (18.3) | <0.001 |
| Monoclonal Ab, n (%) | 30 (4.0) | 27 (7.3) | 0.030 | 19 (4.1) | 7 (1.9) | 0.122 |
| ICU admission, n (%) | 60 (8.0) | 60 (16.2) | <0.001 | 72 (15.4) | 133 (36.0) | <0.001 |
| Invasive mechanical ventilation, n (%) | 17 (2.3) | 25 (6.9) | <0.001 | 22 (4.7) | 59 (16.5) | <0.001 |
| Death at day 28, n (%) | 71 (9.5) | 74 (16.7) | <0.001 | 62 (13.2) | 53 (12.2) | 0.733 |
| Death at day 28 and/or ICU admission, n (%) | 113 (15.1) | 116 (30.7) | <0.001 | 116 (24.7) | 166 (44.6) | <0.001 |
| Length of stay | 7.0 [3.0-14.0] | 9.0 [4.0-16.0] | 0.003 | 8.0 [4.0-13.0] | 7.0 [3.0-15.0] | 0.866 |
ICU, intensive care unit; IQR, interquartile range; Ab, Antibody.
In multivariate analysis (Table 7 ), Omicron infection, compared with Delta, was associated with less need for oxygen therapy, less ICU admission, and fewer deaths, whereas vaccination and a history of SARS-CoV-2 infection were also protective. Only male sex, older age, kidney failure, immunosuppression, and solid cancer were still risk factors for mortality.
Table 7.
Multivariate analysis on the merged cohort (Delta + Omicron)
| Death on day 28 |
ICU |
Need for oxygen |
||||
|---|---|---|---|---|---|---|
| Best model | p | Best model | p | Best model | p | |
| Variant: Omicron | 0.53 [0.37-0.76] | 0.001 | 0.19 [0.12-0.28] | <0.001 | 0.50 [0.38-0.67] | <0.001 |
| Sex: male | 1.66 [1.15-2.41] | 0.007 | 1.37 [0.96-1.95] | 0.084 | 1.65 [1.25-2.18] | <0.001 |
| Age | 1.07 [1.05-1.08] | <0.001 | 1.04 [1.03-1.04] | <0.001 | ||
| Kidney failure | 2.32 [1.46-3.67] | <0.001 | 0.41 [0.15-0.93] | 0.050 | ||
| HTA | 1.28 [0.87-1.90] | 0.211 | ||||
| Immunosuppressed | 2.21 [1.30-3.66] | 0.003 | 0.79 [0.31-1.83] | 0.593 | 1.56 [1.05-2.33] | 0.028 |
| Previous SARS-CoV-2 infection | 0.14 [0.01-0.67] | 0.053 | 0.10 [0.01-0.50] | 0.028 | 0.08 [0.02-0.26] | <0.001 |
| Vaccination | 0.45 [0.30-0.66] | <0.001 | 0.26 [0.17-0.39] | <0.001 | 0.20 [0.14-0.28] | <0.001 |
| Solid cancer for <3 mo | 5.61 [2.53-12.57] | <0.001 | 0.41 [0.09-1.31] | 0.174 | ||
| Overweight | 1.72 [1.10-2.77] | 0.021 | 1.63 [1.17-2.27] | 0.004 | ||
| At least one comorbidity | 1.68 [0.84-3.45] | 0.146 | 1.61 [0.97-2.67] | 0.064 | ||
| O2 treatment at home | 2.86 [1.05-9.28] | 0.053 | ||||
| Interaction vaccination × immunosuppresseda | 2.95 [1.03-8.87] | 0.047 | ||||
| Interaction vaccination × previous COVID-19b | 5.60 [1.21-28.49] | 0.030 | ||||
In the model for ICU, a significant and positive interaction was found between being vaccinated and being immunosuppressed, which means that the protective effect of vaccination on the risk of ICU admission decreases among immunosuppressed patients.
In the model for O2 need, a significant and positive interaction was found between being vaccinated and having a previous SARS-CoV-2 infection, which means that the effect of vaccination was decreased among patients having previously had SARS-CoV-2 infection. In addition, a trend towards the interaction between being vaccinated and being immunosuppressed was found, similar to the ICU model, but was not significant (p 0.08), which suggests an effect comparable to the ICU model. ICU, intensive care unit; HTA, Hypertension.
Interactions suggest that the benefit of vaccination is lesser on the protective effect on ICU need among immunosuppressed patients and among patients with a history of SARS-CoV-2 infection.
Discussion
Despite the fact that Omicron emerged as a new VOC at the end of 2021, our results show that vaccines designed in early 2020 still protect from severe forms. This protection persists even in older patients and patients with comorbidities and prevails regardless of whether the VOC is a Delta or Omicron. However, greater age, kidney failure, and solid or haematological cancer are still associated with mortality.
The large cohort of 2167 patients obtained by merging our two cohorts not only highlights the fact that the VOC is a risk factor in itself, independent from the known risk factors and vaccine status, with a greater risk of severe form associated with Delta rather than Omicron, but also highlights the impact of a history of SARS-CoV-2 infection. Indeed, the whole cohort provided sufficient power to show a significant influence of a past SARS-CoV-2 infection regarding mortality, need for ICU, or need for oxygen. For the Delta-only cohort, patients with a previous SARS-CoV-2 infection less frequently needed oxygen or ICU but only with a non-significant trend towards lower mortality [7]. A non-significant but likewise trend (for the three outcomes) was observed in the current study on Omicron. The fact that a prior infection is selected by the model suggests its importance; however, the lack of significance also suggests either a lack of power or a lesser importance compared with vaccination in protecting the patient. Consequently, immunity resulting from a prior infection may not be regarded as necessarily better or sufficient compared with vaccine-induced immunity. Another explanation could be that the antigenic distance between Omicron and the previous variants may decrease the protection provided by the Omicron infection as compared with a Delta infection.
Our results regarding the positive impact of vaccination on the SARS-CoV-2 infection are in line both with previous studies on previous variants (particularly Delta [7,10,11]) and Omicron [10,12]. Some of these studies evidenced a marked decrease in vaccine efficacy towards SARS-CoV-2 infection but conserved efficacy regarding severe infection and death. Most of the studies included patients even if they were not hospitalized; the fact that we observed this protection even among patients hospitalized for COVID-19 (compared with those with an incidental SARS-CoV-2 infection with no clinical COVID-19) is a strong argument confirming the benefits of the vaccine.
Our observation regarding the lower risk of severe forms of Omicron infection compared with Delta is in accordance with the current literature. Indeed, previous studies concluded that Omicron was associated with less severe infections [4,13]. However, these studies were carried out at the population level, where a large number of asymptomatic or mild cases could have gone undetected, thereby diluting the power to detect severe cases. Moreover, Delta and Omicron waves were not contemporaneous and have spread in potentially very different populations in terms of vaccine coverage, history of SARS-CoV-2 infection, etc. Such differences in the populations could generate differences misattributed to the virus. However, with a multivariate analysis applied to two cohorts with the same design, our study was able to decouple the effect of Omicron from vaccination and consequently confirmed that it produces less severe forms than Delta. Nonetheless, the lesser proportion of severe forms with Omicron compared with Delta is not as marked among older people compared with the general population [6]. It could be related to the difference of age between the Delta and the Omicron cohort but only partially because the multivariate analysis showed an effect independent between age and VOC. Consequently, severe forms do exist with Omicron and are not negligible.
Our study presents some limitations, in particular missing data, mostly because of its retrospective design; for that reason, some items were not taken into consideration in the analysis, such as vaccine type. Moreover, we included patients from four countries, and our results may have been different if more countries (either European or not) had been represented. In addition, we did not perform anti-Spike serology at admission; anti-Spike antibody level has been shown to be associated with prognosis [14,15], and it might have facilitated understanding of the severity of SARS-CoV-2 infection in some vaccinated subjects, in particular among immunosuppressed subjects who could present a lesser response to vaccines. In addition, the availability of antiviral treatment was not comparable between the Delta and the Omicron wave, which could have had an impact on mortality.
The main strength of our study is the large number of highly phenotyped patients, making our conclusions probably valid for most populations. In particular, vaccine status was individually checked, ensuring accurate classification of patients. In addition, the inclusion period we chose corresponds to a phase of homogeneous circulation of a unique variant, ensuring more precision to our conclusions. Lastly, the fact that the data collection was aligned with the Delta study performed in 2021 enabled us to conduct the comparison between the two variants with high validity.
Our study has several implications. Despite the immune escape by Omicron, vaccination with the currently available vaccines still retains efficacy against severe infections, even among older patients or those with comorbidities, which is an important rationale for vaccine campaigns to be remembered. It also questions the necessity of booster doses, as in haemodialysis patients for whom vaccine-induced protection is proportional to the number of doses [16,17].
Omicron is less associated with severe COVID-19 but is more contagious than Delta [2,3]; this explains why a significant number of deaths were still observed during the BA-1 and BA-2 waves [18], the low frequency of severe form being counterbalanced by the massive number of infections. This observation undermines the statements describing Omicron as a step of SARS-CoV-2 towards a more ‘banal’ coronavirus [[19], [20], [21]], such as 229E, NL63, OC43, and HKU1, and is an argument to maintain high vaccine coverage. Moreover, bivalent mRNA vaccines have received market authorization from the European Medicines Agency ([22] for Wuhan SARS-CoV-2 and Omicron BA.1 SARS-CoV-2), and from the U.S. Food and Drug Administration ([23] Wuhan SARS-CoV-2 and Omicron BA.4/5); our results suggest that patients with risk factors for severe COVID-19 should not wait for such vaccines before receiving a boosting injection (such an injection being recommended 3–6 months after the previous one), as currently available vaccines still retain efficacy.
During the second quarter of 2022, new Omicron subvariants, BA.4 and BA.5, progressively became predominant in Europe. Studies showed a decrease in the neutralization activities of antibodies elicited by vaccination [24] and of therapeutic monoclonal antibodies [25] towards them. New studies are needed to determine the extent to which vaccination status, the number of vaccine doses, and the timing of the last injection bring protection against severe infections in addition to the impact of the divalent vaccines.
Author contributions
GB and OE conceptualized the study, developed the methodology, and performed the software analysis. SA, NT, JFF, JM, ML, PV, CJ, and RC performed the validation. GB and OE performed the formal analysis. GB, SA, NT, JM, JFF, SB, ML, PV, RC, MD, CJ, and OE performed the investigation. All authors curated the data. GB and OE wrote the original draft of the manuscript. SA, ML, NT, PV, JFF, JM, SB, CJ reviewed and edited the manuscript. GB and OE visualized the data. GB and OE supervised the study. GB and OE performed the administration.
Transparency declaration
The authors declare that they have no conflicts of interest. No external funding was received.
Acknowledgements
The authors are indebted to Sami Kinikli and Metin Ozsoy for data capture and Jeffrey Arsham for English editing.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.12.020.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.2022. European Centre for Disease Prevention and Control. Country overview report: week 32 2022.https://covid19-country-overviews.ecdc.europa.eu/key_epidemiological_indicators.html Visited on 2022-08-24. [Google Scholar]
- 2.Syed A.M., Ciling A., Taha T.Y., Chen I.P., Khalid M.M., Sreekumar B., et al. Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2200592119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui Z., Liu P., Wang N., Wang L., Fan K., Zhu Q., et al. Structural and functional characterizations of infectivity and immune evasion of SARS-CoV-2 Omicron. Cell. 2022;185:860–871.e13. doi: 10.1016/j.cell.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyberg T., Ferguson N.M., Nash S.G., Webster H.H., Flaxman S., Andrews N., et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson A.G., Amin A.B., Ali A.R., Hoots B., Cadwell B.L., Arora S., et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132–138. doi: 10.15585/mmwr.mm7104e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auvigne V., Vaux S., Strat Y.L., Schaeffer J., Fournier L., Tamandjou C., et al. Severe hospital events following symptomatic infection with Sars-CoV-2 Omicron and Delta variants in France, December 2021-January 2022: a retrospective, population-based, matched cohort study. EClinicalMedicine. 2022;48 doi: 10.1016/j.eclinm.2022.101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epaulard O., Abgrall S., Lefebvre M., Faucher J.F., Michon J., Frentiu E., et al. Impact of vaccination on the symptoms of hospitalised patients with SARS-CoV-2 Delta variant (B.1.617.1) infection. Clin Microbiol Infect. 2022;28:1629–1635. doi: 10.1016/j.cmi.2022.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akaike H. In: International symposium on information theory. Petrov B.N., Csaki F., editors. Budapest: Academiai Kiado; 1973. Information theory and an extension of the maximum likelihood principle. [Google Scholar]
- 9.Jakobsen J.C., Gluud C., Wetterslev J., Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials–a practical guide with flowcharts. BMC Med Res Methodol. 2017;17:162. doi: 10.1186/s12874-017-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katikireddi S.V., Cerqueira-Silva T., Vasileiou E., Robertson C., Amele S., Pan J., et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil. Lancet. 2022;399:25–35. doi: 10.1016/S0140-6736(21)02754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harder T., Külper-Schiek W., Reda S., Treskova-Schwarzbach M., Koch J., Vygen-Bonnet S., et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.41.2100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brosh-Nissimov T., Hussein K., Wiener-Well Y., Orenbuch-Harroch E., Elbaz M., Lipman-Arens S., et al. Hospitalized patients with severe COVID-19 during the Omicron wave in Israel—benefits of a fourth vaccine dose. Clin Infect Dis. 2022 Jun 20 doi: 10.1093/cid/ciac501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn F., Bonander C., Moghaddassi M., Rasmussen M., Malmqvist U., Inghammar M., et al. Risk of severe COVID-19 from the Delta and Omicron variants in relation to vaccination status, sex, age and comorbidities—surveillance results from southern Sweden, July 2021 to January 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.9.2200121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plūme J., Galvanovskis A., Šmite S., Romanchikova N., Zayakin P., Linē A. Early and strong antibody responses to SARS-CoV-2 predict disease severity in COVID-19 patients. J Transl Med. 2022;20:176. doi: 10.1186/s12967-022-03382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanghavi D.K., Bhakta S., Wadei H.M., Bosch W., Cowart J.B., Carter R.E., et al. Low antispike antibody levels correlate with poor outcomes in COVID-19 breakthrough hospitalizations. J Intern Med. 2022;292:127–135. doi: 10.1111/joim.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng C.C., Platen L., Christa C., Tellenbach M., Kappler V., Bester R., et al. Improved SARS-CoV-2 neutralization of Delta and Omicron BA.1 variants of concern after fourth vaccination in hemodialysis patients. Vaccines. 2022;10:1328. doi: 10.3390/vaccines10081328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espi M., Charmetant X., Barba T., Mathieu C., Pelletier C., Koppe L., et al. A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int. 2022;101:390–402. doi: 10.1016/j.kint.2021.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santé Publique France. Coronavirus: chiffres cles et evolution de la covid-19 en France et dans le monde. n.d. https://www.santepubliquefrance.fr/dossiers/coronavirus-covid-19/coronavirus-chiffres-cles-et-evolution-de-la-covid-19-en-france-et-dans-le-monde Visited on 2022-12-14.
- 19.Balloux F., Tan C., Swadling L., Richard D., Jenner C., Maini M., et al. The past, current and future epidemiological dynamic of SARS-CoV-2. Oxf Open Immunol. 2022;3 doi: 10.1093/oxfimm/iqac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callaway E. Beyond Omicron: what’s next for COVID’s viral evolution. Nature. 2021;600:204–207. doi: 10.1038/d41586-021-03619-8. [DOI] [PubMed] [Google Scholar]
- 21.Gudina E.K., Ali S., Froeschl G. Omicron: a blessing in disguise? Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.875022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Medicines Agency. First adapted COVID-19 booster vaccines recommended for approval in the EU 2022. https://www.ema.europa.eu/en/news/first-adapted-covid-19-booster-vaccines-recommended-approval-eu . Visited on 2022-09-03
- 23.U.S. Food and Drug Administration. COVID-19 bivalent vaccine boosters 2022. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-bivalent-vaccine-boosters . Visited on 2022-09-03
- 24.Wang Q., Guo Y., Iketani S., Nair M.S., Li Z., Mohri H., et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608:603–608. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takashita E., Yamayoshi S., Simon V., van Bakel H., Sordillo E.M., Pekosz A., et al. Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med. 2022;387:468–470. doi: 10.1056/NEJMc2207519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.