Abstract
Background
The EROSION (Effective Anti‐Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography‐Based Management in Plaque Erosion) study demonstrated that antithrombotic therapy without stenting was safe and feasible in selected patients with acute coronary syndrome caused by plaque erosion. However, the factors related to the prognosis of these patients are not clear. This study aimed to explore the predictors of an adverse prognosis of a nonstent strategy in a larger sample size.
Methods and Results
A total of 252 (55 patients were from the EROSION study) patients with acute coronary syndrome with plaque erosion who met the inclusion criteria of the EROSION study and completed clinical follow‐up were enrolled. Patients were divided into 2 groups according to the occurrence of major adverse cardiovascular events (MACE), which were defined as the composite of cardiac death, recurrent myocardial infarction, ischemia‐driven target lesion revascularization, rehospitalization because of unstable or progressive angina, major bleeding, and stroke. Among 232 patients with acute coronary syndrome included in the final analysis, 50 patients (21.6%) developed MACE at a median follow‐up of 2.9 years. Compared with patients without MACE, patients with MACE were older and had a higher degree of percentage of area stenosis (72.2%±9.4% versus 64.2%±15.7%, P<0.001) and thrombus burden (24.4%±10.4% versus 20.4%±10.9%, P=0.010) at baseline. Multivariate Cox regression analysis confirmed that age, percentage of area stenosis, and thrombus burden were predictors of MACE. The best cutoff values of predictors were age ≥60 years, percentage of area stenosis ≥63.5%, and thrombus burden ≥18.5%, respectively, and when they were all present, the rate of MACE rose to 57.7%.
Conclusions
The nonstent treatment strategy of patients with acute coronary syndrome caused by plaque erosion was heterogeneous, and patients aged ≥60 years, percentage of area stenosis ≥63.5%, and thrombus burden ≥18.5% may predict a worse clinical outcome.
Keywords: acute coronary syndrome, nonstent strategy, optical coherence tomography, plaque erosion, prognosis
Subject Categories: Clinical Studies
Clinical Perspective.
What Is New?
The long‐term clinical prognosis of patients was studied in a larger sample size of the population eligible for the EROSION (Effective Anti‐Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography‐Based Management in Plaque Erosion) study.
The predictors of major adverse cardiovascular events caused by a nonstent strategy were explored.
What Are the Clinical Implications?
This study provided new inclusion conditions that reduced the risk of adverse events for patients enrolled in the EROSION study with a nonstent strategy.
Patients aged ≥60 years, with area stenosis percentage ≥63.5% and with thrombus burden ≥18.5% had a significantly increased rate of major adverse cardiovascular events, and the cumulative incidence of major adverse cardiovascular events increased by 4.2 times when the 3 predictors were combined; therefore, a nonstent treatment might not be suitable for these patients.
Nonstandard Abbreviations and Acronyms
- AS%
percentage of area stenosis
- EROSION
Effective Anti‐Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography‐Based Management in Plaque Erosion
- MACE
major adverse cardiovascular events
- TB
thrombus burden
Acute coronary syndrome (ACS) mainly includes 3 underlying pathological types: plaque rupture, plaque erosion, and calcified nodules, of which plaque erosion accounts for about one‐third. 1 , 2 Current clinical guidelines recommended that stent implantation should be priorly selected for patients with ACS. 3 However, stent‐related complications, such as in‐stent thrombosis, restenosis, and neoatherosclerosis remain major problems. 4 , 5 The EROSION (Effective Anti‐Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography‐Based Management in Plaque Erosion) study, with a 1‐year and 4‐year follow‐up, suggested that antithrombotic therapy without stenting was safe and feasible in selected patients with ACS caused by plaque erosion. 6 , 7 , 8 However, at the 4‐year follow‐up, 23.1% of patients still experienced adverse cardiovascular events. 8 Therefore, we aimed to explore the predictors of the adverse clinical prognosis of this treatment strategy in a larger sample size by using optical coherence tomography (OCT).
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
This study was a single‐center observational study and was a retrospective analysis of patients from the EROSION study (n=55) and subsequent patients with plaque erosion who met the inclusion criteria of the EROSION study and received no stent implantation (n=197). Among 252 patients, 20 patients were excluded for the following reasons: (1) dual antiplatelet therapy discontinued because of aortic ulcer at 1‐month follow‐up (n=1), (2) suboptimal image quality (n=4), (3) previous stent implantation or coronary artery bypass graft (n=3); (4) imaging after predilatation (n=2); (5) incomplete demographic and clinical data (n=6), and (6) patient lost follow‐up (n=4). The final analysis included 232 patients (Figure 1). Patients were divided into 2 groups according to the occurrence or absence of major adverse cardiovascular events (MACE).
Figure 1. Study flowchart.
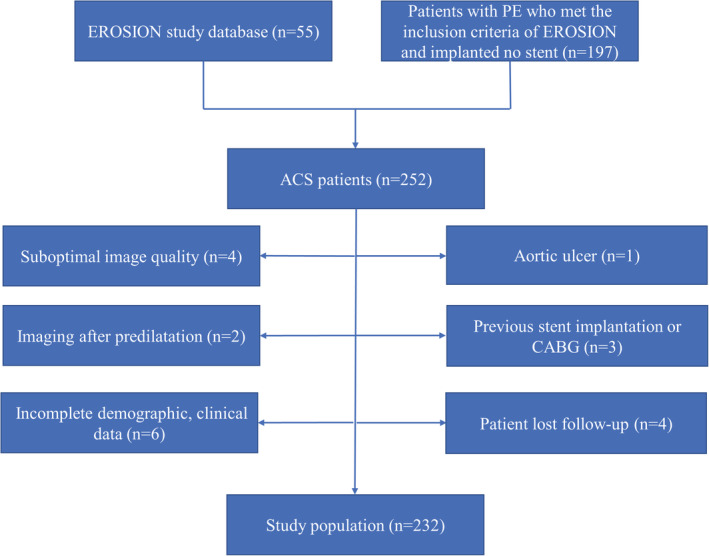
ACS indicates acute coronary syndrome; CABG, coronary artery bypass grafting; EROSION, Effective Anti‐Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography‐Based Management in Plaque Erosion; and PE, plaque erosion.
The diagnosis of ACS included ST‐segment–elevation myocardial infarction, non–ST‐segment–elevation myocardial infarction, and unstable angina pectoris, as previously described. 9 , 10 ST‐segment–elevation myocardial infarction was defined as continuous chest pain for >30 minutes, arrival at the hospital within 12 hours from symptom onset, ST‐segment elevation >0.1 mV in at least 2 contiguous leads or new left bundle‐branch block on the 12‐lead ECG, and elevated cardiac markers (troponin I or creatine kinase‐myocardial band). 9 Non–ST‐segment–elevation myocardial infarction was defined as ischemic symptoms in the absence of ST‐segment elevation on the ECG with elevated cardiac markers. 10 Unstable angina pectoris was defined as having newly developed/accelerating chest symptoms on exertion or rest angina within 2 weeks without biomarker release. 10 The culprit lesion was identified based on angiographic findings, ECG changes, and/or left ventricular wall motion abnormalities. In patients with multiple stenoses, the plaque with the most severe stenosis or with evidence of acute thrombus on angiography or OCT was considered to be the culprit.
This study was approved by the ethics committee of the Second Affiliated Hospital of Harbin Medical University and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all enrolled patients.
Catheterization Procedures
Patients were routinely treated with aspirin (300 mg), ticagrelor (180 mg), and unfractionated heparin (100 IU/kg) before catheter intervention. Coronary angiography was performed via radial or femoral approach using a 6F or 7F sheath after intracoronary administration of 100 to 200 μg nitroglycerin. The procedural strategy, including the use of glycoprotein IIb/IIIa inhibitor (tirofiban, bolus of 25 mg/kg administered over 3 minutes followed by continuous intravenous infusion of 0.15 mg/kg per minute) or manual aspiration thrombectomy (Export V aspiration catheter; Medtronic Cardio Vascular, Santa Rosa, CA) were at the discretion of the interventional cardiologist. The duration of glycoprotein IIb/IIIa inhibitor infusion was 12 to 24 hours. The culprit lesion was assessed using OCT after antegrade coronary flow was restored. When plaque erosion was diagnosed by OCT, the residual diameter stenosis was <70% on angiogram, thrombolysis in myocardial infarction flow grade was 3, and the patient was stable without symptoms, no stent was implanted. All patients were treated with dual antiplatelet therapy after discharge as recommended by clinical guidelines.
Coronary Angiography Analysis
Quantitative coronary angiography analysis was performed using Cardiovascular Angiography Analysis System (CASS 5.10.1; Pie Medical Imaging BV, Maastricht, the Netherlands). Coronary flow was assessed with the thrombolysis in myocardial infarction flow grade classification at baseline. Reference vessel diameter, minimal lumen diameter, diameter stenosis, and lesion length were measured.
OCT Image Acquisition and Analysis
OCT imaging was performed using a commercially available frequency‐domain OCT system (ILUMIEN OPTIS or OPTIS Integrated System; Abbott Vascular, Santa Clara, CA) as previously reported. 11 All OCT images were submitted to the Intravascular Imaging and Physiology Core Laboratory of the Second Affiliated Hospital of Harbin Medical University and analyzed by 2 independent investigators who were blinded to patients' information, using an offline review workstation. In the case of discordance, a consensus reading was achieved from a third senior investigator. Quantitative and qualitative OCT analyses of the culprit and nonculprit lesions were performed according to previously established criteria and consensus. 12 , 13 The reference was the site with the largest lumen area either proximal or distal to the stenosis, and plaque length was determined as the distance between the distal and proximal reference. Minimal lumen area was the smallest lumen area within the length of the plaque, and percentage of area stenosis (AS%) was calculated as ([Mean reference lumen area—minimal lumen area]/Mean reference lumen area) × 100.
Plaque erosion was identified by the presence of attached thrombus overlying an intact and visualized plaque, luminal surface irregularity at the culprit lesion in the absence of thrombus, or attenuation of underlying plaque by thrombus without superficial lipid or calcification immediately proximal or distal to the site of thrombus. 1 Plaques underlying erosions were divided into fibrous or lipid plaques. Fibrous plaques were defined by the presence of high backscattering and homogeneous signal‐rich regions; lipid plaques were identified as signal‐poor regions with diffuse borders. Lipid arc was analyzed at 1‐mm intervals, and lipid length was obtained on the longitudinal view. Lipid index was calculated by multiplication of lipid length and the mean value of lipid arc. Fibrous cap thickness was calculated by the average of 3 different measurements performed at the thinnest part of fibrous cap covering a lipid core. Thin‐cap fibroatheroma was defined as a plaque with a lipid arc larger than 90° and with the thinnest part of the fibrous cap measuring <65 μm. Macrophage accumulations were defined as the presence of signal‐rich, distinct, or confluent punctate regions that exceeded the intensity of background speckle noise. Microchannels were identified as signal‐poor vesicular or tubular structure delineated in at least 3 contiguous frames. Calcifications were identified as an area with low backscattering signal and a sharp border inside a plaque. Cholesterol crystals were identified as thin and linear regions of high signal intensity with high backscattering within a plaque. Thrombus was defined as an irregular mass attached to the luminal surface or floating into the lumen with a diameter >250 μm. The type of thrombus was categorized as either red or white thrombus. A red thrombus was highly backscattering with high attenuation, whereas a white thrombus was homogeneous with low attenuation. When the thrombus contained both red and white elements, it was defined as mixed. The quantitative method for thrombus analysis has been previously described. 6 , 14 In brief, the lumen area and flow area were measured in each frame as mentioned above, and the thrombus area was calculated as lumen area minus flow area. The thrombus length was measured as the longitudinal distance between the most distal and the most proximal frame that showed intraluminal thrombus. Thrombus volume was calculated as the mean thrombus area multiplied by the thrombus length. Thrombus burden (TB) was defined as the mean thrombus area divided by the mean lumen area.
Clinical Follow‐Up
Patients were followed after discharge by hospital visit or phone call. MACE were defined as composites of cardiac death, recurrent myocardial infarction, ischemia‐driven target lesion revascularization, rehospitalization caused by unstable or progressive angina, major bleeding, and stroke. Cardiac death was defined as death in the presence of acute coronary syndrome, significant cardiac arrhythmia, refractory congestive heart failure, or death attributed to cardiovascular cause at postmortem. Recurrent myocardial infarction was defined as typical chest pain accompanied by a rise of >2 times the upper reference limit of troponins, development of new Q waves on the ECG, or both. Ischemia‐driven target lesion revascularization was defined as either percutaneous or surgical revascularization at the culprit lesion site identified at index procedure for angina or angina‐equivalent symptoms. Unstable or progressive angina were evaluated according to Braunwald Unstable Angina Classification. Major bleeding was defined as any fatal bleed, intracranial bleed, intrapericardial bleed with cardiac tamponade, or hypovolemic shock/severe hypotension caused by bleeding requiring pressor or surgery; a fall in hemoglobin of ≥5 g/dL; or a need for transfusion of ≥4 units of red cell concentrates. Other major bleeding was defined as significantly disabling bleeding, a fall in hemoglobin of ≥3 g/dL but <5 g/dL, or a need for transfusion of at least 2 units of red cell concentrates. Stroke was defined as a new acute episode of neurologic dysfunction thought to be vascular in origin, with signs or symptoms lasting >24 hours, preferably supported by an imaging procedure such as computed tomography or magnetic resonance imaging. The MACE were adjudicated by 3 experienced cardiologists who reviewed original source documents and were unaware of baseline OCT data.
Statistical Analysis
Statistical analysis was performed with SPSS version 21.0 (IBM, Armonk, NY). Categorical data were expressed as absolute frequencies and percentages and compared using the χ2 test or Fisher exact test, as appropriate. Data distribution was assessed by using the Kolmogorov‐Smirnov test. Continuous variables were shown as mean±SD for normally distributed data or as median (25th–75th percentiles) for nonnormally distributed data and compared using the t test or Mann‐Whitney U test. The survival analysis was estimated by the Kaplan‐Meier method and were compared with the log‐rank test. The predictors of patients with a poor prognosis were determined by multivariable Cox analysis with stepwise selection. Variables exhibiting a P<0.1 in the univariate analysis were tested in the multivariable analysis. Receiver operating characteristics with area under the curve were used to determine the best cutoff value for continuous predictors. A 2‐sided P value <0.05 was considered statistically significant.
Results
Among 232 patients with ACS included in the study, 50 patients (21.6%) developed MACE at a median follow‐up of 2.9 years (interquartile range, 2.0–3.9 years), including 6 patients (2.6%) with cardiac deaths, 3 patients (1.3%) with nonfatal reinfarction, 29 patients (12.5%) with target lesion revascularization, 36 patients (15.5%) with rehospitalization attributable to angina pectoris, 2 patients (0.9%) with severe bleeding, and 5 patients (2.2%) with stroke (Table 1). Clinical, angiographic, and OCT features were compared in patients with or without MACE.
Table 1.
Clinical Prognosis of Included 232 Patients
| MACE | No. of patients (%) |
|---|---|
| Any events | 50/232 (21.6) |
| Cardiac death | 6 (2.6) |
| Recurrent myocardial infarction | 3 (1.3) |
| Ischemia‐driven TLR | 29 (12.5) |
| Rehospitalization because of unstable or progressive angina | 36 (15.5) |
| Major bleeding | 2 (0.9) |
| Stroke | 5 (2.2) |
MACE indicates major adverse cardiovascular events; and TLR, target lesion revascularization.
Clinical and Angiography Characteristics
The clinical and coronary angiography characteristics are detailed in Table 2. Compared with patients without MACE, patients with MACE were older (55.7±10.6 years versus 51.0±10.2 years, P=0.004). The total ischemic time (419.9±289.0 minutes versus 335.7±238.7 minutes, P=0.059) and the prevalence of diabetes (18.0% versus 8.8%, P=0.063) were numerically greater in patients with MACE. As for the usage of dual antiplatelet therapy, most patients used ticagrelor (72.0% in the MACE group and 65.9% in the non‐MACE group, P=0.418), and some used clopidogrel (28.0% in the MACE group and 34.1% in the non‐MACE group, P=0.418). No significant differences were found in other clinical characteristics and coronary angiography data between the 2 groups.
Table 2.
Clinical and Angiography Characteristics
| Variables | MACE, n=50 | No MACE, n=182 | P value |
|---|---|---|---|
| Age, y | 55.7±10.6 | 51.0±10.2 | 0.004 |
| Men | 38 (76.0) | 149 (81.9) | 0.353 |
| Current smoking | 32 (64.0) | 136 (74.7) | 0.133 |
| Dyslipidemia | 20 (40.0) | 92 (50.5) | 0.186 |
| Hypertension | 15 (30.0) | 45 (24.7) | 0.451 |
| Diabetes | 9 (18.0) | 16 (8.8) | 0.063 |
| CKD | 2 (4.0) | 3 (1.6) | 0.310 |
| Clinical presentation | |||
| STEMI | 40 (80.0) | 157 (86.3) | 0.273 |
| NSTE‐ACS | 10 (20.0) | 25 (13.7) | |
| Total ischemic time, min | 419.9±289.0 | 335.7±238.7 | 0.059 |
| Laboratory data | |||
| TG, mg/dL | 144.5±76.0 | 142.5±100.8 | 0.898 |
| TC, mg/dL | 173.5±40.5 | 173.2±39.4 | 0.956 |
| LDL‐C, mg/dL | 105.4±31.8 | 105.8±32.7 | 0.930 |
| HDL‐C, mg/dL | 50.8±16.1 | 50.6±13.6 | 0.945 |
| Hs‐CRP, mg/L | 5.5±5.0 | 6.5±5.0 | 0.823 |
| HbA1c, % | 5.9±1.1 | 6.0±1.1 | 0.331 |
| CK‐MB, μg/L | 123.0 (58.3–200.1) | 149.1 (35.5–285.8) | 0.240 |
| cTnI, μg/L | 42.0 (11.6–79.8) | 45.2 (8.6–123.1) | 0.379 |
| NT‐proBNP, pg/mL | 396.0 (145.8–1548.5) | 617.5 (166.5–1216.8) | 0.845 |
| Medications | |||
| Aspirin | 50 (100) | 182 (100) | … |
| Clopidogrel | 14 (28.0) | 62 (34.1) | 0.418 |
| Ticagrelor | 36 (72.0) | 120 (65.9) | 0.418 |
| Statins | 50 (100) | 182 (100) | … |
| ACEI/ARB | 31 (62.0) | 108 (59.3) | 0.734 |
| β‐Blocker | 38 (76.0) | 128 (70.3) | 0.431 |
| Lesion location | |||
| LAD | 37 (74.0) | 130 (71.4) | 0.945 |
| LCX | 4 (8.0) | 14 (7.7) | |
| RCA | 9 (18.0) | 38 (20.9) | |
| RVD, mm | 3.1±0.6 | 3.1±0.6 | 0.467 |
| MLD, mm | 1.3±0.4 | 1.4±0.5 | 0.163 |
| DS, % | 56.2±10.6 | 54.5±10.5 | 0.323 |
| Lesion length, mm | 15.9±5.1 | 16.9±5.9 | 0.249 |
| Manual thrombectomy | 40 (80.0) | 127 (69.8) | 0.154 |
| GPI | 24 (48.0) | 99 (54.7) | 0.401 |
| Initial TIMI flow grade | |||
| 0–1 | 37 (74.0) | 138 (75.8) | 0.724 |
| 2–3 | 13 (26.0) | 44 (24.2) | |
Values are mean±SD, n (%), or median (25th–75th percentiles).
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CKD, chronic kidney disease; CK‐MB, creatine kinase myocardial band; cTnI, cardiac troponin I; DS, diameter stenosis; GPI, glycoprotein IIb/IIIa inhibitor; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; Hs‐CRP, high‐sensitivity C‐reactive protein; LAD, left anterior descending artery; LCX, left circumflex artery; LDL‐C, low‐density lipoprotein cholesterol; MACE, major adverse cardiovascular events; MLD, minimal lumen diameter; NSTE‐ACS, non–ST‐segment–elevation acute coronary syndrome; NT‐pro BNP, N‐terminal pro‐B type natriuretic peptide; RCA, right coronary artery; RVD, reference vessel diameter; STEMI, ST‐segment–elevation myocardial infarction; TC, total cholesterol; TG, triglyceride; and TIMI, thrombolysis in myocardial infarction.
OCT Findings
The OCT findings are listed in Table 3. Patients with MACE had a lower prevalence of fibrous plaque (6.0% versus 22.0%, P=0.010) and had larger residual TB (24.4±10.4% versus 20.4±10.9%, P=0.010) than those without MACE. Moreover, the minimal lumen area was smaller (2.3±0.9 mm2 versus 2.9±2.0 mm2, P=0.001), and the AS% was significantly higher in patients with MACE (72.2±9.4% versus 64.2%±15.7%, P<0.001).
Table 3.
Optical Coherence Tomography Findings
| Variables | MACE, n=50 | No MACE, n=182 | P value |
|---|---|---|---|
| Underlying plaque | 0.010* | ||
| Fibrous plaque | 3 (6.0) | 40 (22.0) | |
| Lipid plaque | 47 (94.0) | 142 (78.0) | |
| FCT, μm | 100.6±45.7 | 106.0±49.0 | 0.775 |
| Mean lipid arc, degree | 205.5±42.7 | 198.5±47.3 | 0.211 |
| Lipid length, mm | 12.3±5.5 | 13.2±6.4 | 0.233 |
| Lipid index, mm, degree | 2571.2±1267.4 | 2694.8±1523.7 | 0.147 |
| Maximum lipid arc, degree | 292.3±73.0 | 283.8±71.9 | 0.712 |
| TCFA | 15 (30.0) | 32 (17.6) | 0.053* |
| Macrophage | 43 (86.0) | 146 (80.2) | 0.352 |
| Microchannel | 20 (40.0) | 77 (42.3) | 0.770 |
| Cholesterol crystals | 8 (16.0) | 44 (24.2) | 0.219 |
| Calcification | 13 (26.0) | 48 (26.4) | 0.958 |
| Thrombus type | 0.699 | ||
| Red | 7 (14.0) | 34 (19.2) | |
| White | 29 (58.0) | 97(54.8) | |
| Mixed | 14 (28.0) | 46 (26.0) | |
| Thrombus length, mm | 7.1±5.5 | 7.2±4.9 | 0.855 |
| Thrombus area, mm2 | 1.2±1.0 | 1.0±0.9 | 0.587 |
| Thrombus volume, mm3 | 5.6 (2.5–10.2) | 4.6 (1.8–10.8) | 0.637 |
| Thrombus burden | 24.4±10.4 | 20.4±10.9 | 0.010* |
| Thrombus score | 54.5 (35.0–85.5) | 60.0 (33.0–96.0) | 0.747 |
| MLA, mm2 | 2.3±0.9 | 2.9±2.0 | 0.001* |
| AS% | 72.2±9.4 | 64.2±15.7 | <0.001* |
Values are expressed as n (%), mean±SD, or median (25th–75th percentiles).
AS% indicates percentage of area stenosis; FCT, fibrous cap thickness; MACE, major adverse cardiovascular events; MLA, minimal lumen area; and TCFA, thin‐cap fibroatheroma.
P value<0.05 considered statistically significant.
Predictors for MACE
To investigate the independent predictors of MACE, clinical, angiographic, and OCT data of plaque features were included in univariate and multivariate Cox regression analyses. Univariate Cox regression analysis showed that age, diabetes, total ischemia time, fibrous plaque, thin‐cap fibroatheroma, TB, minimal lumen area, and AS% were related to MACE (Table S1). Multivariate Cox regression analysis confirmed that age (hazard ratio [HR], 1.035 [95% CI, 1.005–1.065]; P=0.021), AS% (HR, 1.043 [95% CI, 1.015–1.071]; P=0.003), and TB (HR, 1.026 [95% CI, 1.001–1.053]; P=0.044) were independent predictors of MACE (Table 4).
Table 4.
Multivariate Cox Regression Analysis
| Variables | P value | HR | 95% CI |
|---|---|---|---|
| Age, y | 0.021* | 1.035 | 1.005–1.065 |
| 30–40 y | 0.041 | 0.037 | 0.002–0.868 |
| 40–50 y | 0.616 | 0.837 | 0.418–1.678 |
| 50–60 y | 0.069 | 2.727 | 0.925–8.042 |
| 60–70 y | 0.028 | 3.519 | 1.144–10.823 |
| 70–80 y | 0.025 | 3.233 | 1.159–9.021 |
| Diabetes | 0.305 | 1.557 | 0.668–3.631 |
| Total ischemic time, min | 0.646 | 1.000 | 0.999–1.001 |
| Fibrous plaque | 0.289 | 0.329 | 0.042–2.569 |
| TCFA | 0.945 | 1.025 | 0.506–2.079 |
| Thrombus burden | 0.044* | 1.026 | 1.001–1.053 |
| AS% | 0.003* | 1.043 | 1.015–1.071 |
AS% indicates percentage of area stenosis; HR, hazard ratio; and TCFA, thin‐cap fibroatheroma.
P value<0.05 considered statistically significant.
Receiver operating curve analysis was performed to identify the best cutoff values to predict MACE, and the best cutoff values were 60 years for age (area under the curve, 0.609; P=0.019), 63.5% for AS% (area under the curve, 0.650; P=0.003), and 18.5% (area under the curve, 0.620; P=0.010) for TB.
MACE Cumulative Rate Analysis
The best cutoff values of age ≥60 years, AS% ≥63.5%, and TB≥18.5% were used for further MACE cumulative rate Kaplan‐Meier estimates analysis. Patients aged ≥60 years, AS% ≥63.5%, or TB ≥18.5% had a significantly increased risk of MACE than those without, respectively (Figure 2). When all 3 predictors were present, the rate of MACE increased to 57.7% (Figure 3).
Figure 2. Kaplan‐Meier survival curves analysis.
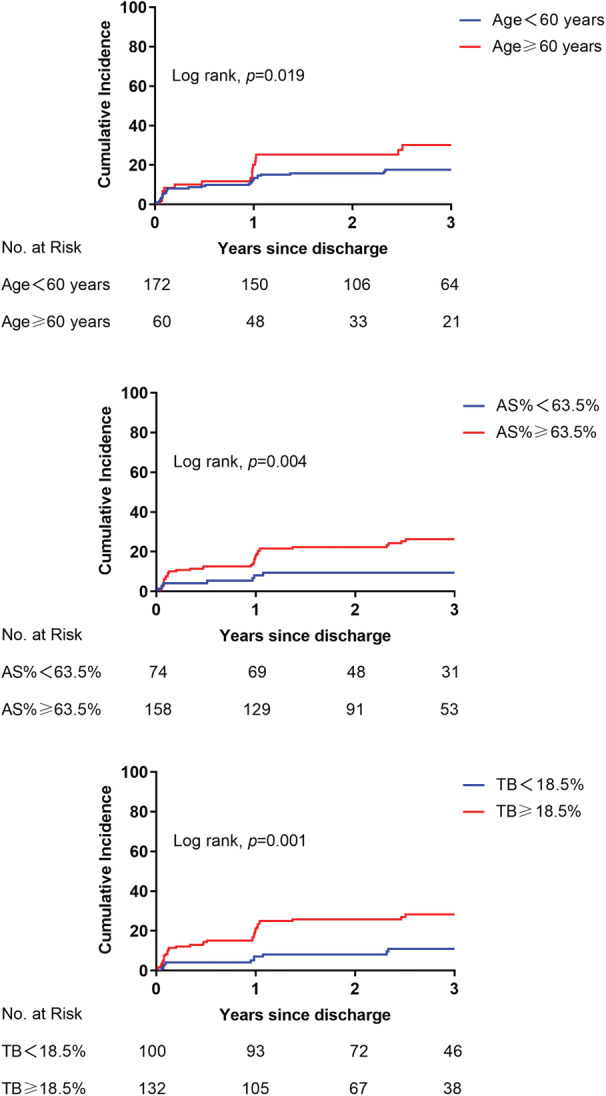
The Kaplan‐Meier analysis of MACE cumulative rate in patients aged ≥60 years, AS% ≥63.5%, or TB≥18.5%. AS% indicates percentage of area stenosis; MACE, major adverse cardiovascular events; and TB, thrombus burden.
Figure 3. Rate of MACE.
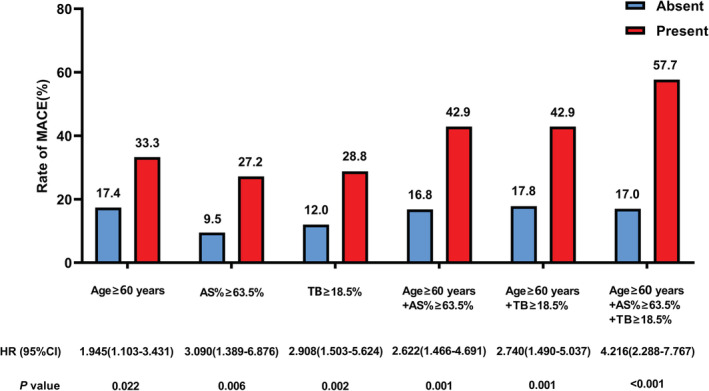
Specific OCT parameters alone or in combination and rate of MACE are shown. The best cutoff values of age ≥60 years, AS% ≥63.5%, and TB≥18.5% from the ROC curve analysis were used for further MACE cumulative rate Kaplan‐Meier estimate analyses.
AS% indicates percentage of area stenosis; HR, hazard ratio; MACE, major adverse cardiac events; OCT, optical coherence tomography; ROC, receiver operating characteristic; and TB, thrombus burden.
Discussion
The present study further investigated the prognosis of patients who met the inclusion criteria of the EROSION study and identified predictors of patients with MACE at a median follow‐up of 2.9 years. The main findings were as follows. The total incidence of MACE in patients with ACS with plaque erosion administrated with nonstent strategy was 21.6%. Patients with MACE were older and had a higher degree of lumen stenosis and larger TB. Age ≥60 years, AS% ≥63.5%, and TB ≥18.5% were predictors of MACE. Moreover, when all predictors were present, the cumulative rate of MACE increased to 57.7%.
Rate of MACE in ACS Patients Caused by Plaque Erosion with Nonstent Strategy
In current practice, the main treatment strategy of patients with ACS is still to implant a stent, irrespective of underlying pathological types. The EROSION study has found that the nonstent strategy of conservative treatment with antithrombotic drugs is an alternative treatment for some selected patients with ACS caused by plaque erosion. The incidence of MACE in the EROSION study was 3.6% at 1‐month follow‐up, 7.5% at 1‐year follow‐up, and 23.1% at 4‐year follow‐up. 6 , 7 Previous studies have reported that the incidence of MACE in patients with ACS after stent treatment is 3% to 6% at 1 month, 15 , 16 6% to 9% at 1 year, 15 , 17 11% to 12% at 2 years, 18 , 19 and 13% at 3 years, 20 , 21 which is lower compared with that in the EROSION study, but this is because none of these studies included rehospitalization because of unstable or progressive angina as MACE. The proportion of these patients is 15.5% in our study. Therefore, after excluding MACE in this subset of patients, the incidence of MACE was similar between stent and nonstent treatment strategies, suggesting that the nonstent treatment strategy is safe and feasible for selected patients with ACS with plaque erosion. However, after 2.9 years of clinical follow‐up in this study, it is worth noting that about a fifth of enrolled patients still developed MACE, which is not ideal enough. These findings also indicated that the heterogeneous nature of this treatment strategy and other factors may affect the prognosis of this treatment, although the majority of patients were free from MACE.
Age and Prognosis of a Nonstent Strategy
In the EROSION study, at 1‐year follow‐up, patients with residual thrombus were older than those without 7 (P=0.067), and at 4‐year follow‐up, patients with target lesion revascularization tended to be older 8 (P=0.097). In our study, patients with MACE were significantly older than those without, and age was an independent predictor of MACE. We considered that this result may come from the following reasons. First, we further expanded the sample size of the EROSION study to explore the rate of MACE in a wider population with a nonstent strategy. Moreover, compared with young patients, older patients shared both a high ischemic and bleeding risk, and the effect of antithrombotic treatment in older patients is relatively poor, but the risk of adverse drug reactions and bleeding is higher, thus increasing the risk of MACE. 22 , 23 In addition, previous studies have found that the older patients experienced more MACE than the younger patients after stopping dual antiplatelet therapy and using a single antiplatelet drug, and age is an independent predictor of MACE events after cessation of dual antiplatelet therapy. 24
Effects of Lumen Area Stenosis and TB on Prognosis of a Nonstent Strategy
In our study, patients with MACE had more severe lumen stenosis and greater TB, and AS% and TB were independent predictors of adverse cardiovascular events. In general, more severe stenosis indicated greater plaque burden. In the previous the Providing Regional Observations to Study Predictors of Events in the Coronary Tree study, Stone et al reported that nonculprit lesions associated with recurrent events were more likely to have a plaque burden of 70% or greater. 25 Moreover, Nicholls et al also found that a direct relationship was observed between the baseline burden of coronary atherosclerosis and future adverse cardiovascular events. 26 When the diameter stenosis is >70%, the degree of area stenosis is >90%. 27 In this study and the EROSION study, patients with diameter stenosis <70% on coronary angiography were selected, indicating that some patients may have a >70% plaque burden. 6 Our study observed that AS% ≥63.5% was a predictor of patients with a worse prognosis, which meant that the area stenosis degree standard defined by OCT (AS% <63.5%) may be better than the diameter standard of <70% on angiography to have a better clinical prognosis.
Previous studies have found that high TB was an important predictor of adverse cardiovascular outcome. 28 In our study, we also observed that patients with adverse events had a higher TB, and with the high resolution of OCT, we identified that patients with TB ≥18.5% may have a worse clinical prognosis. At the 4‐year follow‐up of the EROSION study, He et al found patients with target lesion revascularization had greater TB at 1‐month OCT follow‐up, and concluded that the reduction of thrombus volume at 1 month was a predictor of the prognosis of a nonstent strategy. 8 Our study extended previous conclusions, and found that greater baseline TB was also associated with adverse cardiovascular events. Because of the high cost of OCT, many patients did not have a 1‐month OCT follow‐up. Therefore, it is more feasible to evaluate the future MACE risk of patients according to the baseline data, such as TB, area stenosis, age, diameter stenosis, thrombolysis in myocardial infarction, and symptoms, which will provide ideas for further optimization of treatment measures.
Clinical Implications
In the EROSION study and our study, although most patients with the nonstent strategy were free from adverse cardiovascular events, >20% of patients still had a poor prognosis during clinical follow‐up, indicating the heterogeneity of this treatment strategy. Therefore, we investigated the predictors of MACE and found patients aged ≥60 years, AS% ≥63.5%, and TB ≥18.5% had a significantly increased rate of MACE than patients without (Figure 4), and the MACE rate increased about 4.2 times when the 3 predictors were combined. In future clinical practice, patients without the 3 predictors may benefit more from a nonstent strategy, which gives us a hint about future exploration to further optimize inclusion criteria of this therapeutic strategy. In addition to the importance of routine clinical follow‐up, OCT can provide additional information, such as the decrease in thrombus volume over time under optimal medical treatment, which can provide ideas for further optimization of treatments. It may be more reasonable to find patients with poor response to antithrombotic drugs and administer stronger antithrombotic drugs and stent treatment if necessary.
Figure 4. The presence of age ≥60 years, AS% ≥63.5%, and TB≥18.5% may mean a worse clinical outcome in patients administered with nonstent strategy.
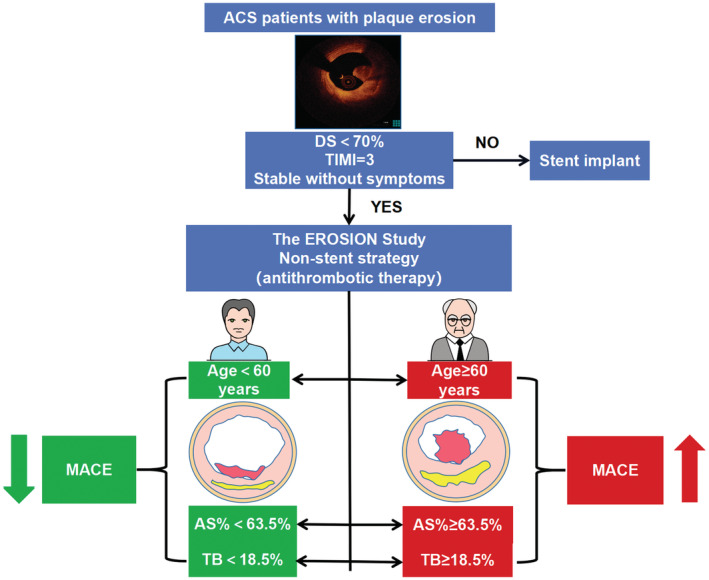
ACS indicates acute coronary syndrome; AS%, percentage of area stenosis; DS, diameter stenosis; EROSION, Effective Anti‐Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography‐Based Management in Plaque Erosion; MACE, major adverse cardiac events; TB, thrombus burden; and TIMI, thrombolysis in myocardial infarction.
Study Limitations
There are several limitations that should be acknowledged. First, this study was a single‐ center prospective observational study; therefore, selection bias was inevitable. Second, this study was a nonrandomized study and did not include a control group of patients treated with stents. Third, the use of thrombectomy was decided by the operators, which might have affected lesion morphologies, although care was taken to avoid excessive mechanical trauma. Fourth, investigators and patients were unblinded in our study. Fifth, the overlying thrombus might reduce the accuracy of OCT to assess underlying plaque characteristics. Sixth, the OCT definition of plaque erosion is a diagnosis of exclusion, requiring the absence of fibrous cap rupture. Seventh, the use of glycoprotein IIb/IIIa inhibitor in nearly 50% of patients does not translate to the practice in the United States and could have contributed to increased bleeding. Finally, in light of the rather conspicuously low retention of patients at year 4, the hazard ratios reported in the Kaplan‐Meier curves need to be compared with those at risk at the beginning of the trial.
Conclusions
The present study found that 21.6% of patients with ACS with plaque erosion receiving a nonstent strategy had MACE at about 3 years, indicating the heterogeneity effects of this treatment, and patients aged ≥60 years, AS% ≥63.5%, and TB≥18.5% may have a worse clinical outcome.
Source of Funding
This work was supported by the National Natural Science Foundation of China (number 82072091 to J.D.), Natural Science Foundation of Heilongjiang Province (YQ2020H017 to J.D. and TD2020H001 to B.Y.), and Hei Long Jiang Postdoctoral Foundation (grant number LBH‐Q21117 to J.D. and grant number LBH‐Z21186 to C.F.).
Disclosures
None.
Supporting information
Table S1
Acknowledgments
The authors sincerely thank all colleagues and patients who participated in this study.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026414
For Sources of Funding and Disclosures, see page 10.
See Editorial by Maino and Jaffer.
Contributor Information
Jiannan Dai, Email: daijiannandr@163.com.
Bo Yu, Email: yubodr@163.com.
References
- 1. Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–1758. doi: 10.1016/j.jacc.2013.05.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: The pathologists' view. Eur Heart J. 2013;34:719–728. doi: 10.1093/eurheartj/ehs411 [DOI] [PubMed] [Google Scholar]
- 3. Niccoli G, Montone RA, Di Vito L, Gramegna M, Refaat H, Scalone G, Leone AM, Trani C, Burzotta F, Porto I, et al. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J. 2015;36:1377–1384. doi: 10.1093/eurheartj/ehv029 [DOI] [PubMed] [Google Scholar]
- 4. Palmerini T, Biondi‐Zoccai G, Della Riva D, Mariani A, Genereux P, Branzi A, Stone GW. Stent thrombosis with drug‐eluting stents: Is the paradigm shifting? J Am Coll Cardiol. 2013;62:1915–1921. doi: 10.1016/j.jacc.2013.08.725 [DOI] [PubMed] [Google Scholar]
- 5. Taniwaki M, Windecker S, Zaugg S, Stefanini GG, Baumgartner S, Zanchin T, Wenaweser P, Meier B, Juni P, Raber L. The association between in‐stent neoatherosclerosis and native coronary artery disease progression: A long‐term angiographic and optical coherence tomography cohort study. Eur Heart J. 2015;36:2167–2176. doi: 10.1093/eurheartj/ehv227 [DOI] [PubMed] [Google Scholar]
- 6. Jia H, Dai J, Hou J, Xing L, Ma L, Liu H, Xu M, Yao Y, Hu S, Yamamoto E, et al. Effective anti‐thrombotic therapy without stenting: Intravascular optical coherence tomography‐based management in plaque erosion (the EROSION study). Eur Heart J. 2017;38:792–800. doi: 10.1093/eurheartj/ehw381 [DOI] [PubMed] [Google Scholar]
- 7. Xing L, Yamamoto E, Sugiyama T, Jia H, Ma L, Hu S, Wang C, Zhu Y, Li L, Xu M, et al. EROSION study (effective anti‐thrombotic therapy without stenting: Intravascular optical coherence tomography‐based Management in Plaque Erosion): A 1‐year follow‐up report. Circ Cardiovasc Interv. 2017;10:e005860. doi: 10.1161/CIRCINTERVENTIONS.117.005860 [DOI] [PubMed] [Google Scholar]
- 8. He L, Qin Y, Xu Y, Hu S, Wang Y, Zeng M, Feng X, Liu Q, Syed I, Demuyakor A, et al. Predictors of non‐stenting strategy for acute coronary syndrome caused by plaque erosion: 4‐year outcomes of the EROSION study. EuroIntervention. 2021;17:497–505. doi: 10.4244/EIJ-D-20-00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6 [DOI] [PubMed] [Google Scholar]
- 10. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the Management of Patients with non‐ST‐elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;130:e344–e426. doi: 10.1161/CIR.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 11. Fang C, Yin Y, Jiang S, Zhang S, Wang J, Wang Y, Li L, Wang Y, Guo J, Yu H, et al. Increased vulnerability and distinct layered phenotype at culprit and nonculprit lesions in STEMI versus NSTEMI. JACC Cardiovasc Imaging. 2022;15:672–681. doi: 10.1016/j.jcmg.2021.07.022 [DOI] [PubMed] [Google Scholar]
- 12. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the international working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–1072. doi: 10.1016/j.jacc.2011.09.079 [DOI] [PubMed] [Google Scholar]
- 13. Raber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, Radu MD, Joner M, Yu B, Jia H, et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39:3281–3300. doi: 10.1093/eurheartj/ehy285 [DOI] [PubMed] [Google Scholar]
- 14. Amabile N, Hammas S, Fradi S, Souteyrand G, Veugeois A, Belle L, Motreff P, Caussin C. Intra‐coronary thrombus evolution during acute coronary syndrome: Regression assessment by serial optical coherence tomography analyses. Eur Heart J Cardiovasc Imaging. 2015;16:433–440. doi: 10.1093/ehjci/jeu228 [DOI] [PubMed] [Google Scholar]
- 15. Kedhi E, Joesoef KS, McFadden E, Wassing J, van Mieghem C, Goedhart D, Smits PC. Second‐generation everolimus‐eluting and paclitaxel‐eluting stents in real‐life practice (COMPARE): A randomised trial. The Lancet. 2010;375:201–209. doi: 10.1016/S0140-6736(09)62127-9 [DOI] [PubMed] [Google Scholar]
- 16. Windecker S, Serruys PW, Wandel S, Buszman P, Trznadel S, Linke A, Lenk K, Ischinger T, Klauss V, Eberli F, et al. Biolimus‐eluting stent with biodegradable polymer versus sirolimus‐eluting stent with durable polymer for coronary revascularisation (LEADERS): A randomised non‐inferiority trial. The Lancet. 2008;372:1163–1173. doi: 10.1016/S0140-6736(08)61244-1 [DOI] [PubMed] [Google Scholar]
- 17. Serruys PW, Silber S, Garg S, van Geuns R, Richardt G, Buszman P, Kelbaek H, van Boven A, Hofma S, Linke A, et al. Comparison of zotarolimus‐eluting and everolimus‐eluting coronary stents. N Engl J Med. 2010;363:136–146. doi: 10.1056/NEJMoa1004130 [DOI] [PubMed] [Google Scholar]
- 18. De Luca G, Damen S, Camaro C, Benit E, Verdoia M, Rasoul S, Liew H, Polad J, Ahmad W, Zambahari R, et al. Final results of the randomised evaluation of short‐term dual antiplatelet therapy in patients with acute coronary syndrome treated with a new‐generation stent (REDUCE trial). EuroIntervention. 2019;15:e990–e998. doi: 10.4244/EIJ-D-19-00539 [DOI] [PubMed] [Google Scholar]
- 19. Tröbs M, Achenbach S, Nef H, Gori T, Naber C, Neumann T, Richardt G, Schmermund A, Wöhrle J, Zahn R, et al. Everolimus eluting bioresorbable vascular scaffolds in patients with acute coronary syndromes: two‐year results from the German‐Austrian ABSORB registry. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions. 2021;98:E564–E570. doi: 10.1002/ccd.29831 [DOI] [PubMed] [Google Scholar]
- 20. Kereiakes D, Ellis S, Metzger C, Caputo R, Rizik D, Teirstein P, Litt M, Kini A, Kabour A, Marx S, et al. 3‐year clinical outcomes with Everolimus‐eluting Bioresorbable coronary scaffolds: The ABSORB III trial. J Am Coll Cardiol. 2017;70:2852–2862. doi: 10.1016/j.jacc.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 21. Picard F, Avram R, Marquis‐Gravel G, Tadros VX, Ly HQ, Dorval JF, Doucet S, Gosselin G, Asgar AW, Ibrahim R, et al. Clinical outcomes of bioresorbable vascular scaffold to treat all‐comer patients. Are patients with acute coronary syndrome better candidates for bioresorbable vascular scaffold? Cardiovasc revascula. 2019;20:228–234. doi: 10.1016/j.carrev.2018.06.022 [DOI] [PubMed] [Google Scholar]
- 22. Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn HP, Buttner HJ, Neumann FJ. Impact of cytochrome P450 2C19 loss‐of‐function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol. 2010;55:2427–2434. doi: 10.1016/j.jacc.2010.02.031 [DOI] [PubMed] [Google Scholar]
- 23. Kamran H, Jneid H, Kayani WT, Virani SS, Levine GN, Nambi V, Khalid U. Oral antiplatelet therapy after acute coronary syndrome: A review. JAMA. 2021;325:1545–1555. doi: 10.1001/jama.2021.0716 [DOI] [PubMed] [Google Scholar]
- 24. Mazlan‐Kepli W, Dawson J, Berry C, Walters M. Cessation of dual antiplatelet therapy and cardiovascular events following acute coronary syndrome. Heart. 2019;105:67–74. doi: 10.1136/heartjnl-2018-313148 [DOI] [PubMed] [Google Scholar]
- 25. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, et al. A prospective natural‐history study. N Engl J Med. 2011;364(3):226–235. doi: 10.1056/NEJMoa1002358 [DOI] [PubMed] [Google Scholar]
- 26. Nicholls SJ, Hsu A, Wolski K, Hu B, Bayturan O, Lavoie A, Uno K, Tuzcu EM, Nissen SE. Intravascular ultrasound‐derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol. 2010;55:2399–2407. doi: 10.1016/j.jacc.2010.02.026 [DOI] [PubMed] [Google Scholar]
- 27. Huebner T, Schuepbach WM, Seeck A, Sanz E, Meier B, Voss A, Pilgram R. Cardiogoniometric parameters for detection of coronary artery disease at rest as a function of stenosis localization and distribution. Med Biol Eng Comput. 2010;48:435–446. doi: 10.1007/s11517-010-0594-1 [DOI] [PubMed] [Google Scholar]
- 28. Jolly SS, Cairns JA, Lavi S, Cantor WJ, Bernat I, Cheema AN, Moreno R, Kedev S, Stankovic G, Rao SV, et al. Thrombus aspiration in patients with high thrombus burden in the TOTAL trial. J Am Coll Cardiol. 2018;72:1589–1596. doi: 10.1016/j.jacc.2018.07.047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


