Abstract
Background
We hypothesized that stroke outcome is related to multiple baseline hydration‐related factors including volume contracted state (VCS) and diuretic use.
Methods and Results
We analyzed a prospective cohort of subjects with ischemic stroke <24 hours of onset enrolled in acute treatment trials within VISTA (Virtual International Stroke Trials Archive). A VCS was defined based on blood urea nitrogen‐to‐creatinine ratio. The primary end point was modified Rankin Scale score at 90 days. Primary analysis used generalized ordinal logistic regression over the mRS range, adjusted for Totaled Health Risks in Vascular Events score, onset‐to‐enrollment time, and thrombolytic use. Of 5971 eligible patients with stroke, 42% were taking diuretics at the time of hospitalization, and 44% were in a VCS. Patients in a VCS were older, had more vascular risk factors, were more likely taking diuretics, and had more severe strokes. Diuretic use was associated with both reduced chance of achieving a good functional outcome (odds ratio [OR], 0.57 [95% CI, 0.52–0.63]) and increased mortality at 90 days (OR, 2.30 [95% CI, 2.04–2.61]). VCS was associated with greater mortality 90 days after stroke (OR, 1.53 [95% CI, 1.33–1.76]). There was no evidence of effect modification among the 3 exposures of VCS, diuretic use, or hypokalemia in relation to outcome.
Conclusions
A VCS at the time of hospitalization was associated with more severe stroke and odds of death but not associated with worse functional outcome when accounting for relevant characteristics. Diuretic use and low serum potassium at the time of stroke onset were associated with worse outcome and may be worthy of further investigation.
Keywords: acute, diuretic, hydration, stroke
Subject Categories: Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- ENOS
Efficacy of Nitric Oxide
- mRS
modified Rankin Scale
- THRIVE
Totaled Health Risks in Vascular Events
- VCS
volume contracted state
- VISTA
Virtual International Stroke Trials Archive
Clinical Perspective.
What Is New?
Hydration status at the time of stroke may impact clinical outcome, and dehydration is associated with increased chance of death within 90 days from stroke.
Diuretic medications may contribute to a dehydrated state and reduce the odds of good functional recovery in patients with acute stroke.
What Are the Clinical Implications?
These data support additional exploration of the benefits of hydration algorithms at the time of hospitalization for acute stroke in case this is a modifiable factor to improve recovery.
The pathophysiology of acute ischemic stroke depends upon the complex interactions among cerebral and systemic hemodynamic parameters. Previous studies have suggested that the use of diuretics and other antihypertensive medications may reduce the risk of, reduce the severity of, and improve the outcome after stroke. 1 , 2 , 3 However, these medications have the potential to increase the likelihood of dehydration, or more accurately, a volume contracted state (VCS) at the time of stroke, which may worsen functional outcome. 4 A common side effect of these medications is also change in electrolytes, most notably depletion of serum potassium, which may also be associated with risk of stroke and stroke‐related death. 5 , 6 , 7 Whether diuretics, hydration status, and potassium levels have an effect in the acute setting has not been determined.
In the acute setting, patients with ischemic stroke are commonly administered intravenous fluid under the premise that dehydration can lead to reduced cerebral perfusion and ultimately worse clinical outcome. 4 , 8 , 9 The 2018 American Heart Association/American Stroke Association guidelines for early management of patients with acute ischemic stroke merely state that, “Hypotension and hypovolemia should be corrected to maintain systemic perfusion levels necessary to support organ function….” with an admission that, “There are no data to guide volume and duration of parenteral fluid delivery.”10 Although the class of recommendation for this guideline is 1 (strong), the evidence for this merely comes from consensus of expert opinion based on clinical experience. Surprisingly, data on the hydration practices and efficacy of this practice are sparse. 11 , 12 A few studies have recently begun to look at the effect of a VCS in patients with acute stroke on poststroke outcomes. 13 , 14 , 15 , 16 A single‐center study found that those in a VCS had worse clinical outcome at 90 days than those who were euvolemic. 16 A recent substudy of the ENOS (Efficacy of Nitric Oxide) trial involving data from 310 participants found an unfavorable shift in the modified Rankin Scale (mRS) and increased death at day 90 in patients with increased urea; however, other markers of dehydration did not yield consistent findings. 17
The VISTA (Virtual International Stroke Trials Archive) is a combined resource that includes data contributed by principal investigators of numerous international acute stroke trials. 18 It has been anonymized with the goal of providing a large and centrally collated data set for investigators wishing to perform exploratory analyses. All included patients had baseline assessment within 24 hours of stroke onset and confirmed stroke diagnosis using cerebral imaging. We used this cohort to investigate the relationship between diuretic use, the consequences of diuretic use at the time of stroke, including hypokalemia and volume contraction, and association with stroke outcome.
We aimed to investigate the potential consequences of diuretic use and the associations among these factors with outcome after acute ischemic stroke in an international multicenter database. We hypothesized that patients on diuretics and in a VCS would have worse functional outcomes at 90 days than those who were euvolemic upon arrival to the hospital.
METHODS
Data for this study are hosted by the Virtual Trials Archives (http://www.virtualtrialsarchives.org). Because the data collected for this study contain human subjects’ information, reasonable requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to vista.coordinator@glasgow.ac.uk.
We conducted a cohort study of patients who were prospectively enrolled in clinical trials within the VISTA. 18 Patients were included if they had an ischemic stroke diagnosis within 24 hours of onset or time last known to be normal. There was no limitation to National Institutes of Health Stroke Scale (NIHSS) score for inclusion in this archive. Patients were excluded if their stroke was caused by a primary intracranial hemorrhage or if they were undergoing dialysis before the stroke admission. To be included in this analysis, subjects were additionally required to have the following data available: mRS score at 90 days, NIHSS score at baseline and at 90 days, time from stroke onset, whether or not they received intravenous thrombolysis (tissue plasminogen activator), baseline medications (eg, diuretics), and medical history including hypertension, atrial fibrillation, and diabetes. In all of the VISTA trials included, mRS score was expected to be measured in person around 90 days as one of the key outcomes. Because the VISTA is a conglomerate of trials, each trial had its own allowances for the time window around 90 days, the use of telephone mRS score for patients unable to be evaluated in person, and information carried forward from prior visits if needed.
A VCS was defined a priori as a blood urea nitrogen (BUN)‐to‐creatinine ratio of >20 (urea:creatinine >100) as has previously been defined, and is referred to here as VCS‐20. 17 We additionally conducted similar analysis using a post hoc definition of BUN‐to‐creatinine ratio >30 (urea:creatinine >150), referred to as VCS‐30. All baseline laboratory values were measured before randomization in each of the individual trials, typically upon arrival to the hospital at the time of stroke. Estimated glomerular filtration rate was calculated based on age, sex, race, and initial serum creatinine, and then chronic kidney disease stage was determined. All included patients were from sites with institutional review board approval for study enrollment. Written informed consent was not required for this retrospective study using VISTA database which provided deidentified data from multiple trials. All participants gave written informed consent prior to participating in their specific trial.
Statistical Analysis
Baseline characteristics of subjects with and without VCS were compared using the Student t test for continuous variables, χ2 test for categorical variables, and Wilcoxon rank sum test for ordinal variables. The primary end point was functional outcome at 90 days, as measured by the mRS. The mRS ranges from 0 (completely normal) to 5 (severely disabled and dependent), and 6 is assigned if the patient died. Generalized ordinal logistic regression was performed over the full range of mRS scores, comparing these outcomes between patients with a VCS and euvolemic in univariate analysis. For the multivariable analyses, we calculated the Totaled Health Risks in Vascular Events (THRIVE) score, which includes age, baseline NIHSS score, hypertension, diabetes, and atrial fibrillation to minimize the number of covariates used in the models. 19 We prespecified adjustment for the THRIVE score, onset‐to‐enrollment time, and intravenous tissue plasminogen activator use. Stratified analyses and tests for interaction were performed based on the use of diuretics before stroke and thrombolysis for the event. Secondary analyses of the primary end point were a comparison of the proportion of patients with a bad outcome, defined as mRS >2 at 90 days, with similar adjustments and stratifications as above. We also assessed change in the NIHSS score from baseline to day 90 and mortality by day 90 as secondary end points. All analyses were performed using Stata/SE 12.1 (StataCorp, College Station, TX).
RESULTS
The VISTA database yielded 7444 patients who met the eligibility criteria for this analysis, with 3051 out of 7444 (41%) of this subset prescribed diuretics at the time of hospital presentation. Of this group, 5971 (80%) had sufficient laboratory testing to measure a baseline VCS. Forty‐four percent (2626/5971) demonstrated VCS‐20. Patients in VCS‐20 were older, more often women, and had higher NIHSS scores (Table 1). They were also more likely to have hypertension, diabetes, atrial fibrillation, and congestive heart failure. THRIVE score was higher in the VCS‐20 group, indicating a higher risk for worse outcome. Patients in the VCS‐20 group were more likely to be taking diuretics. There was no difference in stroke onset‐to‐enrollment time and use of intravenous thrombolysis between both groups. Of the 6833 patients with available serum potassium data, 624 (9.1%) had low serum potassium levels (<3.5 mmol/L), whereas 344 (5.0%) had high serum potassium (≥5.0 mmol/L). Using the VCS‐30 definition yielded similar outcomes (Table S1).
Table 1.
Baseline Characteristics of Patients Prescribed Diuretics and Characteristics of Those With VCS‐20: VCS Defined as Blood Urea Nitrogen/Creatinine Ratio >20
| Variable | Diuretic, n=3051 | No diuretic, n=4393 | P value | VCS, VCS‐20, n=2626 | No VCS, VCS‐20, n=3345 | P value |
|---|---|---|---|---|---|---|
| Age, y | 72±11 | 67±13 | <0.001 | 72±11 | 67±13 | <0.001 |
| Sex, women | 1525 (50%) | 1874 (43%) | <0.001 | 1499 (57%) | 1220 (36%) | <0.001 |
| Race | ||||||
| Black | 114 (4%) | 91 (2%) | <0.001* | 34 (1%) | 156 (5%) | <0.001* |
| Asian | 156 (5%) | 306 (7%) | 157 (6%) | 285 (9%) | ||
| White | 2478 (81%) | 3218 (73%) | 2296 (87%) | 2730 (82%) | ||
| Other, or not specified | 303 (10%) | 778 (18%) | 139 (5%) | 174 (5%) | ||
| Continent and country | ||||||
| Europe/Australia/South Africa/Israel | 1763 (58%) | 2804 (64%) | … | 1638 (62%) | 2212 (66%) | … |
| North America | 1118 (37%) | 1234 (38%) | 810 (31%) | 824 (25%) | ||
| South America | 37 (1%) | 64 (1%) | 50 (2%) | 46 (1%) | ||
| East Asia | 128 (4%) | 277 (6%) | 128 (5%) | 263 (8%) | ||
| Hypertension | 2573 (84%) | 2580 (59%) | <0.001 | 1912 (73%) | 2342 (70%) | 0.018 |
| Diabetes | 850 (28%) | 760 (17%) | <0.001 | 640 (24%) | 660 (20%) | <0.001 |
| Atrial fibrillation | 1079 (35%) | 825 (19%) | <0.001 | 762 (29%) | 742 (22%) | <0.001 |
| Heart failure | 563 (19%) | 114 (3%) | <0.001 | 275 (10%) | 230 (7%) | <0.001 |
| Previous myocardial infarction | 495 (16%) | 421 (10%) | <0.001 | 313 (12%) | 416 (12%) | 0.545 |
| Previous stroke | 555 (19%) | 669 (16%) | 0.002 | 480 (18%) | 644 (19%) | 0.337 |
| Previous TIA | 235 (8%) | 326 (8%) | 0.583 | 207 (8%) | 287 (9%) | 0.342 |
| THRIVE score | 4 (3–5) | 3 (2–4) | <0.001 | 4 (3–5) | 3 (2–5) | <0.001 |
| Chronic kidney disease stage | ||||||
| 0 | 502 (17%) | 1195 (29%) | <0.001 | 717 (27%) | 719 (21%) | 0.093 |
| 1 | 1318 (46%) | 2117 (51%) | 1234 (47%) | 1696 (51%) | ||
| 2 | 698 (24%) | 628 (15%) | 470 (18%) | 660 (20%) | ||
| 3 | 303 (11%) | 165 (4%) | 183 (7%) | 219 (7%) | ||
| 4 | 59 (2%) | 27 (1%) | 21 (1%) | 47 (1%) | ||
| 5 | 5 (0.2%) | 1 (0.02%) | 1 (0.04%) | 4 (0.1%) | ||
| Diuretics | ||||||
| Potassium sparing only | 63 (2%) | … | 261 (10%) | 259 (8%) | 0.003 | |
| Non–potassium sparing only | 2441 (80%) | 1160 (44%) | 1290 (39%) | <0.001 | ||
| Both | 547 (18%) | 229 (9%) | 234 (7%) | 0.013 | ||
| Neither | 1431 (54%) | 2017 (60%) | <0.001 | |||
| Any diuretic | 3051 (100%) | 4393 (100%) | 1192 (45%) | 1315 (39%) | <0.001 | |
| Baseline laboratory results | ||||||
| Blood urea nitrogen, mg/dL | 20±9 | 18±7 | <0.001 | 23±8 | 15±6 | <0.001 |
| Creatinine, mg/dL | 1.03±0.37 | 0.92±0.27 | <0.001 | 0.91±0.27 | 1.01±0.33 | <0.001 |
| Sodium, mmol/L | 139±4 | 140±3 | <0.001 | 140±4 | 140±4 | 0.785 |
| Potassium, mmol/L | 4.1±0.5 | 4.1±0.5 | 0.011 | 4.1±0.5 | 4.1±0.5 | 0.024 |
| Glucose, mg/dL | 144±59 | 132±54 | <0.001 | 142±58 | 133±52 | <0.001 |
| Hemoglobin, g/dL | 13.5±1.9 | 13.8±1.7 | <0.001 | 13.5±1.7 | 14.0±1.8 | <0.001 |
| Baseline blood pressure, mm Hg | ||||||
| Systolic | 154±25 | 151±23 | <0.001 | 152±24 | 153±24 | 0.436 |
| Diastolic | 82±14 | 81±16 | 0.014 | 80±15 | 83±15 | <0.001 |
| Onset to enrollment, h | 4.4±2.5 | 4.8±2.8 | <0.001 | 4.0±1.1 | 4.0±1.2 | 0.155 |
| Baseline NIHSS score | 14 (9–18) | 12 (9–16) | <0.001 | 13 (9–17) | 12 (8–16) | <0.001 |
| IV thrombolysis, rtPA | 1035 (34%) | 1323 (30%) | 0.001 | 990 (38%) | 1205 (36%) | 0.212 |
| Calculated osmolality, mmol/L | 294 ± 8 | 293 ± 8 | <0.001 | 296 ± 8 | 292 ± 8 | <0.001 |
NIHSS indicates National Institutes of Health Stroke Scale; rtPA, tissue plasminogen activator; THRIVE, Totaled Health Risks in Vascular Events; TIA, transient ischemic attack; and VCS, volume contracted state. *Race comparison groups are White vs non‐White.
Race comparison groups are White vs non‐White.
In the final cohort of patients with acute stroke, 3051 out of 5971 (51%) were prescribed diuretic medications. Diuretic use was associated with greater odds of achieving a worse outcome after 90 days following stroke in both unadjusted and adjusted models (odds ratio [OR], 1.31 [95% CI, 1.20–1.42]; P<0.001). There were also significantly increased odds of death for patients who were prescribed diuretics at the time of stroke as compared with those who were not, even after adjustment (adjusted OR, 1.64 [95% CI, 1.44–1.88]; P<0.001) (Table 2). The adverse relationship was driven predominantly by the 2988 out of 3051 (98%) patients prescribed non–potassium‐sparing diuretics at the time of hospitalization (Table 2). Change in the NIHSS score from baseline to day 90 was not different when comparing patients who were taking and those who were not taking diuretic medications. Patients prescribed diuretics were more likely to be in a VCS as compared with those who were not (P<0.001). There was no evidence of effect modification (statistical treatment interaction) between VCS and diuretic use in either VCS‐20 or VCS‐30 groups (P interaction=0.997 and P interaction=0.536, respectively).
Table 2.
Comparison of Functional Outcomes and Mortality for Patients With and Without Diuretic Use Including Detail by Class of Diuretic and Potassium‐Sparing Versus Non–Potassium‐Sparing Classes
| Variable | Any diuretic, n=3051 | No diuretic, n=4393 | OR (95% CI) | aOR (95% CI) |
|---|---|---|---|---|
| Change in NIHSS score, baseline–90 d | 7 (5–10) | 7 (5–10) | … | … |
| mRS >2, bad outcome | 33.0% | 46.4% | 1.76 (1.60–1.94) | 1.17 (1.05–1.31) |
| mRS score | 4 (2–5) | 3 (1–4) | 1.93 (1.78–2.10) | 1.31 (1.20–1.42) |
| Mortality | 24.5% | 12.2% | 2.33 (2.06–2.64) | 1.64 (1.44–1.88) |
| Potassium‐sparing diuretic, n=610 | Not taking a potassium‐sparing diuretic, n=6834 | OR (95% CI) | aOR (95% CI) | |
|---|---|---|---|---|
| Change in NIHSS score, baseline–90 d | 7 (5–10) | 7 (5–10) | … | … |
| mRS >2, bad outcome | 41.1% | 40.9% | 0.99 (0.84–1.18) | 0.79 (0.65–0.95) |
| mRS score | 3 (1–5) | 3 (1–4) | 1.09 (0.94–1.27) | 0.90 (0.77–1.05) |
| Mortality | 21.4% | 16.9% | 1.34 (1.09–1.65) | 1.17 (0.94–1.45) |
| Non–potassium‐sparing diuretic, n=2988 | Not taking a non–potassium‐sparing diuretic, n=4456 | OR (95% CI) | aOR (95% CI) | |
|---|---|---|---|---|
| Change in NIHSS score, baseline–90 d | 7 (5–10) | 7 (5–10) | … | … |
| mRS >2, bad outcome | 33.0% | 46.3% | 1.75 (1.59–1.93) | 1.17 (1.05–1.31) |
| mRS score | 4 (2–5) | 3 (1–4) | 1.92 (1.76–2.09) | 1.30 (1.19–1.42) |
| Mortality | 24.5% | 12.4% | 2.30 (2.04–2.61) | 1.64 (1.43–1.87) |
aOR adjusted for Totaled Health Risks in Vascular Events score, onset‐to‐treatment time, and thrombolytic use. Tests for interaction for dichotomous mRS outcome: VCS‐20*diuretic P=0.994, VCS‐30*diuretic P=0.536; for mRS shift VCS‐20 P=0.940, VCS‐30*diuretic P=0.649. aOR indicates adjusted odds ratio; mRS, modifed Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; and OR, odds ratio.
In this cohort, 2626 out of 5971 (44%) presented to the hospital in a VCS as defined by (BUN/creatinine ratio>20). VCS‐20 also appeared to be associated with an adverse shift in mRS scores, worse outcome, and higher mortality compared with those without VCS‐20 in crude analyses (Table 3). After adjustment for THRIVE scores, onset‐to‐treatment time, and the use of thrombolysis, the effects on mRS scores were no longer evident. Change in NIHSS from baseline to day 90 was not different between groups when comparing patients with and without VCS‐20. VCS‐20 was associated with 1.18 adjusted odds of death at 90 days (adjusted OR, 1.01–1.31; P=0.032). There was no evidence of effect modification on functional outcome (mRS score) between VCS‐20 and diuretic use (P interaction=0.940). The distribution of predicted 90‐day mRS scores after adjustment in relation to initial volume status is shown in Figure 1 (VCS‐20) and Figure 2 (VCS‐30). In a sensitivity analysis, we adjusted our model for the individual components of THRIVE rather than the summary score, which yielded similar results. Analyses using the post hoc VCS‐30 definition yielded similar magnitudes of the associations for the mRS score, NIHSS score, and mortality, although the latter was no longer significant (Table 3).
Table 3.
Comparison of 90‐Day Functional Outcomes and Mortality for Patients With and Without VCS Using 2 Thresholds of BUN/Cr Ratio (VCS‐20 BUN/Cr Ratio >20) and (VCS‐30 BUN/Cr Ratio>30)
| VCS‐20, n=2626 | No VCS‐20, n=3345 | OR (95% CI) | aOR (95% CI) | |
|---|---|---|---|---|
| Change in NIHSS score, baseline–90 d | 7 (5–10) | 7 (5–10) | … | … |
| mRS >2, bad outcome | 37.6% | 46.6% | 1.45 (1.30–1.61) | 1.09 (0.97–1.23) |
| mRS score | 3 (1–5) | 3 (1–4) | 1.43 (1.30–1.57) | 1.09 (1.00–1.20) |
| Mortality | 19.8% | 13.8% | 1.53 (1.33–1.76) | 1.18 (1.01–1.31) |
| VCS‐30, n=462 | No VCS‐30, n=5509 | OR (95% CI) | aOR (95% CI) | |
|---|---|---|---|---|
| Change in NIHSS score, baseline–90 d | 7 (5–11) | 7 (5–10) | … | … |
| mRS >2, bad outcome | 31.5% | 43.5% | 1.67 (1.36–2.06) | 1.19 (0.95–1.50) |
| mRS score | 4 (2–5) | 3 (1–4) | 1.64 (1.38–1.95) | 1.17 (0.98–1.39) |
| Mortality | 23.7% | 15.8% | 1.65 (1.31–2.07) | 1.19 (0.93–1.52) |
aOR adjusted for Totaled Health Risks in Vascular Events score, onset‐to‐treatment time, and thrombolytic use. aOR indicates adjusted odds ratio; BUN/Cr, blood urea nitrogen/creatinine; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; and VCS, volume contracted state.
Figure 1. Comparison of 90‐day functional outcome by mRS score for patients with and without VCS‐20.
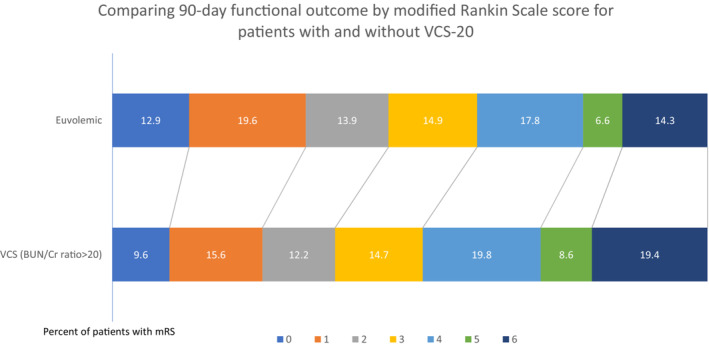
BUN/Cr indicates blood urea nitrogen/creatinine; mRS, modified Rankin Scale; and VCS, volume contracted state.
Figure 2. Comparison of 90‐day functional outcome by mRS score for patients with and without VCS‐30.
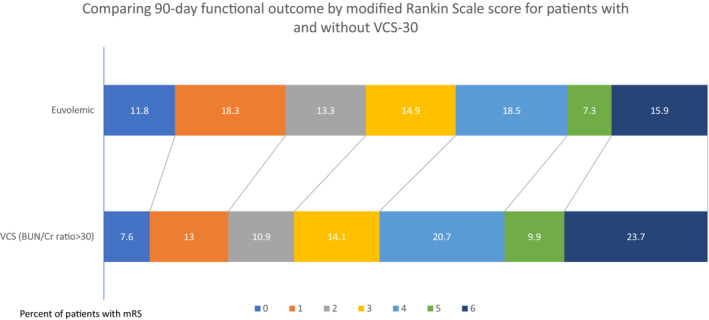
BUN/Cr indicates blood urea nitrogen/creatinine; mRS, modified Rankin Scale; and VCS, volume contracted state.
In this group, 969 out of 5971 (16%) demonstrated an abnormality in serum potassium at the time of hospital presentation for stroke. Low potassium at baseline was not associated with change in NIHSS scores, a shift in mRS scores, or mortality. Elevated potassium appeared to be associated with an adverse shift in mRS scores and higher mortality compared with euvolemia in crude analyses but not after adjustment (Table 4). There was no evidence of effect modification on functional outcome (mRS score) between VCS‐20 and low potassium level (P interaction=0.492).
Table 4.
Comparison of 90‐Day Outcomes for Patients Based on Serum Potassium Levels
| Serum potassium <3.5, n=624 | Serum potassium ≥3.5, n=6209 | OR (95% CI) | aOR (95% CI) | |
|---|---|---|---|---|
| Change in NIHSS score, baseline–90 d | 7 (5–10) | 7 (5–10) | … | … |
| mRS >2, bad outcome | 36.0% | 42.0% | 1.29 (1.08–1.53) | 1.26 (1.04–1.53) |
| mRS score | 3 (1–5) | 3 (1–4) | 1.13 (0.98–1.31) | 1.06 (0.91–1.23) |
| Mortality | 17.1% | 16.9% | 1.01 (0.81–1.27) | 0.96 (0.76–1.22) |
| Serum potassium ≥5.0, n=344 | Serum potassium <5.0, n=6489 | OR (95% CI) | aOR (95% CI) | |
|---|---|---|---|---|
| Change in NIHSS score, baseline–90 d | 7 (5–10) | 7 (5–10) | … | … |
| mRS >2, bad outcome | 36.4% | 41.7% | 1.25 (1.00–1.58) | 1.03 (0.80–1.34) |
| mRS score | 3 (2–5) | 3 (1–4) | 1.35 (1.11–1.64) | 1.12 (0.92–1.36) |
| Mortality | 22.7% | 16.6% | 1.48 (1.13–1.93) | 1.24 (0.93–1.65) |
aOR adjusted for Totaled Health Risks in Vascular Events score, onset‐to‐treatment time, and thrombolytic use. Tests for interaction for dichotomous mRS outcome: VCS‐20*low potassium P=0.492, VCS‐30*low potassium P=0.327; for mRS shift VCS‐20*low potassium P=0.413, VCS‐30*low potassium P=0.297. aOR indicates adjusted odds ratio; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; and OR, odds ratio.
DISCUSSION
We found that patients with a VCS had increased adjusted odds of death after 90 days as compared with patients who did not have a VCS. Furthermore, more patients who were prescribed diuretics were in a VCS, and diuretic use appeared to be associated with worse functional outcome and higher odds of death after adjustment for age, stroke severity, and comorbid conditions known to worsen stroke outcome. Taken together, these findings suggest additional physiologically relevant and potentially modifiable variables to predict and influence stroke outcome. There is a paucity of data about the relationship between a VCS and stroke outcome, and this is the first attempt to analyze the relationship to diuretic use and the relationship to functional outcome in a large group of prospectively recruited acute stroke patients with acute stroke from a group of international stroke study sites.
These data are consistent with the frequency of a VCS seen in single‐site observational studies. 4 , 20 , 21 A VCS is more common in older patients with more comorbidities and more severe strokes. After adjusting for these variables, we found that patients in a VCS did not have worse outcomes at 90 days compared with those who were euvolemic on arrival to the hospital but appeared to have increased mortality. Prior studies have reported that VCS negatively affects outcome after acute ischemic stroke but may have had limited power to account for confounding by other clinical factors. 13 , 14 , 15 , 16 Our study improves upon the limitations of these studies by expanding the sample size to include subjects from a large database of stroke clinical trials with systematic assessments of outcomes, enhancing the reliability and precision of the results. Similarly, investigators from the ENOS trial found no evidence of a consistent relationship between markers of VCS and outcome. Together, these results suggest that these biochemical indicators of dehydration do not adequately reflect tissue perfusion, or that any effect of volume status on perfusion is small compared with other factors such as arterial recanalization.
We also observed a relationship between prescribed diuretic use before stroke admission and poor stroke outcome. This result seems to be mainly driven by the majority subgroup of patients on non–potassium‐sparing diuretics. This effect was independent from volume status. A prior report from a single‐center cohort suggested that diuretics, especially thiazide diuretics, may improve early outcome after stroke. 22 Our observation that diuretic use by itself is associated with worse outcome may be potentially attributed to the underlying comorbid conditions or provider concern that administration of intravenous fluids could contribute to decompensated heart failure, resulting in a more prolonged VCS. We do not have data specific to the frequency or rate of treatment of the VCS to validate this hypothesis, and this could be an interesting focus of future study. Prior studies have shown a strong association between diuretic use and dehydration in patients with stroke. 9 , 23 Moreover, given our finding that fewer good outcomes resulted from low serum potassium, it is conceivable that a potassium‐lowering medication would negatively affect outcome through this indirect route. In a similar vein, potassium‐sparing diuretics might indirectly affect outcome by preserving serum potassium levels.
Hypokalemia is another potentially modifiable parameter in patients presenting to the hospital with acute stroke, with a previously proposed mechanism involving adrenaline‐induced hypokalemia. 24 Previous studies have suggested that hypokalemia may lead to worse clinical outcome after stroke. 25 , 26 Our hypothesis that patients with acute ischemic stroke with low serum potassium at presentation will have worse clinical outcome at 90 days yielded mixed results, with no shift across the mRS but fewer good outcomes when dichotomized. This finding may be spurious because of multiple testing but should be evaluated in an independent data set. Although VCS, diuretic use, and low potassium are likely to be overlapping conditions, there did not appear to be any interactions among their relationships with clinical outcomes.
These findings are clinically important, though there were some notable limitations in our study. First, this was a retrospective analysis of prospective cohorts; therefore, we were limited to the data available in the VISTA database (ie, stroke trial data), each with specific eligibility criteria. This introduces the possibility of selection bias as a significant limitation. Notably, few patients likely received thrombectomy because it was not the standard of care when these trials were completed. Additionally, although patients were prescribed diuretics, we do not have any information about adherence with these medications. Next, the data for potentially relevant variables, such as urine‐specific gravity, urine sodium, serum bicarbonate, measured osmolality, and ejection fraction, are not routinely collected in stroke trials and therefore were not included in the analysis. Using a single measure at baseline likely does not fully reflect the dynamic situation of a patient with acute ischemic stroke. We additionally acknowledge that this cohort enrolled patients with more severe stroke and thus threatens the generalizability of these data to those with mild stroke. There are likely numerous unmeasured residual confounders, and both hypokalemia and VCS‐20 may simply reflect a marker of a sicker cohort of patients with stroke.
To untangle these phenomena, we used this commonly collected biomarker. Given that most stroke trials routinely collected BUN or urea and creatinine, we used BUN/creatinine ratio as a proxy for volume status and found that BUN/creatinine ratio >20, as has been used previously, defines about half of the subjects as VCS. 17 This finding suggests that either VCS is extremely common, or the definition is too imprecise, likely including other causes of elevated BUN/creatinine ratio such as gastrointestinal bleeding or steroid use. This is a significant limitation to interpreting these results and underscores the issue with not having a gold standard, objective definition for a VCS. Other studies have defined aVCS as BUN/creatinine ratio >15, given that this ratio is frequently thought to indicate azotemia and dehydration. 14 , 15 , 16 Had we used this definition in this study, 78% of subjects would have been defined as VCS. We also evaluated a more stringent ratio of 30, present in about 8% of patients, with largely similar results.
Despite these limitations, this is an important study to assist in understanding the complex relationship between hydration status and clinical outcome after stroke. For the majority of patients with stroke, avoiding metabolic complications in the early stroke period is the primary approach to improving patient outcomes. This cohort of patients takes an important next step in exploring possible mechanisms and begins developing clinical protocols to modify relevant variables. It is the first analysis of a cohort of international patients with prospective and hyperacute data. It is a large sample size, with a standardized and monitored data collection methodology. Investigators were blinded to the hypothesis of this study, and therefore hydration practices were likely consistent with real‐world practices, which are useful to clinicians who are treating similar patients around the world. Although these results cannot directly support a change in clinical practice related to rehydration after stroke, it provides critical foundational information to better understanding these associations.
CONCLUSIONS
A VCS is associated with increased odds of death in this cohort of patients with acute ischemic stroke. Patients with acute ischemic stroke taking diuretics and possibly those with low serum potassium appear to be associated with worse outcome. Future studies should investigate practices for patients with stroke related to these clinically important variables.
Appendix
VISTA Acute Steering Committee Members
Kennedy R. Lees (Chair): School of Cardiovascular & Metabolic Health, University of Glasgow, UK; Andrei Alexandrov: University of Tennessee Health Science Center, USA; Philip M. Bath: Institute of Neuroscience, University of Nottingham, UK; Erich Bluhmki: Boehringer Ingelheim, Biberach, Germany; Natan Bornstein: Professor of Neurology at the Tel‐Aviv University, Sackler Faculty of Medicine, Israel; Christopher Chen: Department of Pharmacology, National University of Singapore, Singapore; Stephen M. Davis: Department of Neurology, Royal Melbourne Hospital, University of Melbourne, Australia; Hans‐Christoph Diener: Department of Neurology, University Duisburg‐Essen, Hufelandstrasse, Essen, Germany; Geoffrey Donnan: Neurology, University of Melbourne, Australia; Marc Fisher: Department of Neurology, University of Massachusetts Medical School, USA; Myron Ginsberg: Department of Neurology, University of Miami Miller School of Medicine, Miami, USA; Barbara Gregson: Department of Neurosurgery, Newcastle University, Newcastle General Hospital, UK; James Grotta: Department of Neurology, University of Texas, Health Science Centre at Houston, USA; Werner Hacke: Department of Neurology, University of Heidelberg, Germany; Michael G. Hennerici: Department of Neurology, University of Heidelberg, Germany; Marc Hommel: Joseph Fourier University, Grenoble, France; Markku Kaste: Department of Neurology, Helsinki University Central Hospital, University of Helsinki, Finland; Patrick Lyden: Keck School of Medicine of University of Southern California, USA; John Marler: Food and Drug Administration, USA; Keith Muir: Institute of Neuroscience and Psychology, University of Glasgow, UK; Christine Roffe: Keele University, UK; Ralph Sacco: Miller School of Medicine, University of Miami, USA; Ashfaq Shuaib: Director, Stroke Program, University of Alberta, Canada; Philip Teal: Professor of Stroke Neurology, University of British Columbia, Vancouver; Narayanaswamy Venketasubramanian: Raffles Neuroscience Centre, Raffles Hospital, Singapore; Nils G. Wahlgren: Karolinska Hospital, Stockholm, Sweden; Steven Warach: Dell Medical School, University of Texas at Austin, USA; Christian Weimar: Department of Neurology, University Hospital Essen, University of Duisburg‐Essen, Essen, Germany.
Sources of Funding
Drs Renner and Kasner were supported by National Institutes of Health grants U10NS086474 and U24NS107224. Dr Bath is Stroke Association Professor of Stroke Medicine and Emeritus National Institute for Health and Care Research Senior Investigator. Dr Bahouth is supported by the American Heart Association (18CDA34110126).
Disclosures
Dr Kasner receives or has received grant support from WL Gore, Bristol‐Myers Squibb, Medtronic, Genentech, Remedy; consulting fees from Bristol‐Myers Squibb, AstraZeneca; and royalties from UpToDate; all unrelated to the current study.
Supporting information
Table S1
Acknowledgments
Statistical analysis was completed by Dr Kasner.
*A complete list of the VISTA Acute Steering Committee members can be found in the Appendix at the end of the article.
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Mona N. Bahouth, Email: mbahout1@jhmi.edu.
the VISTA Acute Steering Committee:
Kennedy R. Lees, Andrei Alexandrov, Philip M. Bath, Erich Bluhmki, Natan Bornstein, Christopher Chen, Stephen M. Davis, Hans‐Christoph Diener, Geoffrey Donnan, Marc Fisher, Myron Ginsberg, Barbara Gregson, James Grotta, Werner Hacke, Michael G. Hennerici, Marc Hommel, Markku Kaste, Patrick Lyden, John Marler, Keith Muir, Christine Roffe, Ralph Sacco, Ashfaq Shuaib, Philip Teal, Narayanaswamy Venketasubramanian, Nils G. Wahlgren, Steven Warach, and Christian Weimar
References
- 1. Psaty BM, Smith NL, Siscovick DS, Koepsell TD, Weiss NS, Heckbert SR, Lemaitre RN, Wagner EH, Furberg CD. Health outcomes associated with antihypertensive therapies used as first‐line agents. A systematic review and meta‐analysis. JAMA. 1997;277:739–745. doi: 10.1001/jama.1997.03540330061036 [DOI] [PubMed] [Google Scholar]
- 2. Tziomalos K, Giampatzis V, Bouziana SD, Spanou M, Papadopoulou M, Kazantzidou P, Kostaki S, Kouparanis A, Savopoulos C, Hatzitolios AI. Effects of different classes of antihypertensive agents on the outcome of acute ischemic stroke. J Clin Hypertens (Greenwich). 2015;17:275–280. doi: 10.1111/jch.12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shih HM, Lin WC, Wang CH, Lin LC. Hypertensive patients using thiazide diuretics as primary stroke prevention make better functional outcome after ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:2414–2418. doi: 10.1016/j.jstrokecerebrovasdis.2014.05.021 [DOI] [PubMed] [Google Scholar]
- 4. Schrock JW, Glasenapp M, Drogell K. Elevated blood urea nitrogen/creatinine ratio is associated with poor outcome in patients with ischemic stroke. Clin Neurol Neurosurg. 2012;114:881–884. doi: 10.1016/j.clineuro.2012.01.031 [DOI] [PubMed] [Google Scholar]
- 5. Green D, Ropper A, Kronmal R, Psaty B, Burke G. Serum potassium level and dietary potassium intake as risk factors for stroke. Neurology. 2002;59:314–320. doi: 10.1212/WNL.59.3.314 [DOI] [PubMed] [Google Scholar]
- 6. Khaw K, Barrett‐Connor E. Dietary potassium and stroke associated mortality: a 12‐year prospective population study. N Engl J Med. 1987;316:235–240. doi: 10.1056/NEJM198701293160502 [DOI] [PubMed] [Google Scholar]
- 7. Gao F, Wang CT, Chen C, Guo X, Yang LH, Ma XC, Han JF. Effect of hypokalemia on functional outcome at 3 months post‐stroke among first‐ever acute ischemic stroke patients. Med Sci Monit. 2017;10:2825–2832. doi: 10.12659/MSM.902464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Owens WB. Blood pressure control in acute cerebrovascular disease. J Clin Hypertens (Greenwich). 2011;13:205–211. doi: 10.1111/j.1751-7176.2010.00394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rowat A, Graham C, Dennis M. Dehydration in hospital‐admitted stroke patients. Stroke. 2012;43:857–859. doi: 10.1161/STROKEAHA.111.640821 [DOI] [PubMed] [Google Scholar]
- 10. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 11. Visvanathan A, Dennis M, Whiteley W. Parenteral fluid regimens for improving functional outcome in people with acute stroke. Cochrane Database Syst Rev. 2015;2015:CD011138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bahouth MN, Gottesmam RF, Szanton SL. Primary ‘dehydration’ and acute stroke: a systematic research review. J Neurol. 2018;265:2167–2181. doi: 10.1007/s00415-018-8799-6 [DOI] [PubMed] [Google Scholar]
- 13. Rowat A, Graham C, Dennis M. Dehydration in hospital admitted stroke patients: detection, frequency and association. Stroke. 2012;43:857–859. doi: 10.1161/STROKEAHA.111.640821 [DOI] [PubMed] [Google Scholar]
- 14. Lin LC, Yang JT, Weng HH, Hsiao CT, Lai SL, Fann WC. Predictors of early clinical deterioration after acute ischemic stroke. Am J Emerg Med. 2011;29:577–581. doi: 10.1016/j.ajem.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 15. Lin CJ, Yang JT, Huang YC, Tsai YN, Lee MH, Lee M, Hsiao CT, Hsaio KY, Lin LC. Favorable outcome of blood urea nitrogen/creatinine‐based hydration therapy 3 months after acute ischemic stroke. Am J Emerg Med. 2016;34:2414–2418. doi: 10.1016/j.ajem.2016.09.033 [DOI] [PubMed] [Google Scholar]
- 16. Bahouth MN, Gaddis A, Hillis AE, Gottesman RF. Pilot study of volume contracted state and hospital outcome after stroke. Neurol Clin Pract. 2018;8:21–26. doi: 10.1212/CPJ.0000000000000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Billington CK, Appleton JP, Berge E, Sprigg N, Glover M, Bath PMW. Impact of hydration status on haemodynamics, effects of acute blood pressure‐lowering treatment, and prognosis after stroke. Br J Clin Pharmacol. 2018;84:2914–2922. doi: 10.1111/bcp.13761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ali M, Bath PMW, Curram J, Davis SM, Diener HC, Donnan GA, Fisher M, Gregson BA, Grotta J, Hacke W, et al. The virtual international stroke trials archive. Stroke. 2007;38:1905–1910. doi: 10.1161/STROKEAHA.106.473579 [DOI] [PubMed] [Google Scholar]
- 19. Flint AC, Cullen SP, Faigeles BS, Rao VA. Predicting long‐term outcome after endovascular stroke treatment: the totaled health risks in vascular events score. Am J Neuroradiol. 2010;7:1192–1196. doi: 10.3174/ajnr.A2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodriguez GJ, Cordina SM, Vazquez G, Suri MF, Kirmani JF, Ezzeddine MA, Qureshi AI. The hydration influence on the risk of stroke (THIRST) study. Neurocrit Care. 2009;10:187–194. doi: 10.1007/s12028-008-9169-5 [DOI] [PubMed] [Google Scholar]
- 21. Miller JB, Lee A, Siszanski JP, Tustian M, Corcoran JL, Moore S, Rodriguez L, Lewandowski CA. Challenge of intravascular volume assessment in acute ischemic stroke. Am J Emerg Med. 2018;36:1018–1021. doi: 10.1016/j.ajem.2018.01.071 [DOI] [PubMed] [Google Scholar]
- 22. Wright JM, Musini VM. First‐line drugs for hypertension. Cochrane Database Syst Rev. 2018;4:CD001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Churchill M, Grimm S, Reding M. Risks of diuretic usage following stroke. Neurorehabil Neural Repair. 2004;18:161–165. doi: 10.1177/0888439004268163 [DOI] [PubMed] [Google Scholar]
- 24. Gariballa SE, Robinson TG, Fotherby MD. Hypokalemia and potassium excretion in stroke patients. J Am Geriatr Soc. 1997;45:1454–1458. doi: 10.1111/j.1532-5415.1997.tb03195.x [DOI] [PubMed] [Google Scholar]
- 25. Liu CH, Lin SC, Lin JR, Yang JT, Chang YJ, Chang CH, Chang TY, Huang KL, Ryu SJ, Lee TH. Dehydration is an independent predictor of discharge outcome and admission cost in acute ischaemic stroke. Eur J Neurol. 2014;21:1184–1191. doi: 10.1111/ene.12452 [DOI] [PubMed] [Google Scholar]
- 26. Lin LC, Lee JD, Hung YC, Chang CH, Yang JT. BUN/creatinine ratio based hydration for preventing stroke‐in‐evolution after acute ischemic stroke. Am J Emerg Med. 2014;32:709–712. doi: 10.1016/j.ajem.2014.03.045 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


