Abstract
Background
Nitrates and nitrites are used as food additives in processed meats. They are also commonly ingested from water and several foods. Several short‐term clinical studies suggested beneficial effects of dietary nitrates on blood pressure, while deleterious effects on oxidative damage have been suggested in some experimental studies. However, there is a lack of evidence from longitudinal epidemiological studies linking foods and water‐originated and additives‐originated nitrites and nitrates, separately, to hypertension and cardiovascular diseases risk. We aimed to study these associations in a large population‐based cohort.
Methods and Results
Overall, 106 288 adults from the French NutriNet‐Santé cohort (2009–2022) were included. Associations between nitrites and nitrates intakes and hypertension and cardiovascular disease risks were assessed using multi‐adjusted Cox proportional hazard models. During follow‐up, 3810 incident cases of hypertension and 2075 cases of cardiovascular diseases were ascertained. Participants with higher intakes of additives‐originated nitrites (sodium nitrite in particular [European code e250]) had a higher hypertension risk compared with nonconsumers (hazard ratio, 1.19 [95% CI, 1.08–1.32], P=0.001, and 1.19 [95% CI, 1.08–1.32], P=0.002), respectively. No association was detected between foods and water‐originated nitrites, or nitrates with hypertension risk (all P values >0.3). We found no association between nitrites or nitrates and risks of cardiovascular diseases (all P values >0.2).
Conclusions
These results do not support a protective role of nitrites or nitrates in cardiovascular health. Instead, they suggest a positive association between nitrites from food additives and hypertension risk, which needs confirmation in other large‐scale studies. These findings provide new evidence in the context of current discussions about updating regulations on the use of nitrites as food additives.
Keywords: cardiovascular diseases, food additives, hypertension, nitrates, nitrites, processed meats, prospective cohort
Subject Categories: High Blood Pressure
We are commonly exposed to dietary nitrites and nitrates, through natural presence in vegetables, as well as water and soil contamination. Nitrites and nitrates are also widely used as food additives because of their preservative properties and the pink coloration they provide to some processed meats. Experimental and clinical studies have suggested potential benefits of dietary nitrites and nitrates on cardiometabolic health, including lowering blood pressure and improving endothelial function 1 : There is now a general consensus that dietary nitrate/nitrite ingestion is a source of endogenous nitric oxide (NO) through the activity of NO2‐ reductases. 1 Short‐term interventional human studies 2 , 3 (2 hours to 42 days duration) have mostly suggested that inorganic nitrate supplementation, in the form of beetroot juice, rocket salad beverage, or spinach beverage, may lower blood pressure through bioconversion to nitrite by oral bacteria, followed by a reduction to nitrous acid, which spontaneously decomposes to NO and other bioactive nitrogen oxides. Yet, other interventional studies showed no such effect on blood pressure and arterial stiffness. 4 In addition, evidence from observational studies is incomplete, showing mixed results, and no distinction between foods and water‐originated and additives‐originated nitrates. 5 , 6 On the other hand, a possible role of nitrites and nitrates in inducing oxidative damage has been postulated in cellular models, 7 and a few studies suggested that excessive nitrate intakes stimulate angiotensin II type 1 receptor, 8 , 9 leading to increased production of oxygen radicals by both endothelial cells and smooth muscle cells. 10 In a context where several public health authorities are considering banning nitrites/nitrates as additives because of carcinogenic properties of their by‐product nitrosamines, 11 we investigated using data from the French cohort study NutriNet‐Santé, whether dietary exposures to nitrites/nitrates were associated with risks of cardiovascular disease and hypertension, distinguishing between foods and water‐originated and food additives‐originated nitrites/nitrates.
Methods
The French NutriNet‐Santé cohort was launched in 2009, aiming to investigate the links between diet and health. It is conducted according to the Declaration of Helsinki guidelines and was approved by the Institutional Review Board of the French Institute for Health and Medical Research (IRB Inserm n°0000388FWA00005831) and the “Commission Nationale de l'Informatique et des Libertés” (CNIL n°908 450/n°909 216). The study is registered at clinicaltrials.gov as NCT03335644. Electronic informed consent is obtained from each participant. Data described in the article, code book, and analytic code will be made available upon request pending application and approval. Researchers from public institutions can submit a collaboration request including information on the institution and a brief description of the project to collaboration@etude-nutrinet-sante.fr. All requests will be reviewed by the steering committee of the NutriNet‐Santé study. If the collaboration request is accepted, a data access agreement will be necessary and appropriate authorizations from the relevant administrative authorities may be needed. In accordance with existing regulations, no personal identification data will be accessible.
Dietary Data Collection
Online questionnaires (physical activity, socioeconomic status, anthropometry, and health) were regularly administered to participants, including a biannual series of 3 nonconsecutive validated web‐based 24‐hour dietary records (≥2 mandatory), with an estimation of portion sizes, merged with a detailed food composition database covering >3500 generic food/beverage items, with additional information on consumed commercial brands. These 24‐hour records were validated against biomarkers and an interview with a trained dietitian. Dietary underreporting was identified on the basis of the method proposed by Black 12 , using the basal metabolic rate and Goldberg cut‐off, and underenergy reporters were excluded.
Exposure to Nitrites and Nitrates
We estimated exposure to nitrites/nitrates from (1) foods and water (nonadditive sources), (ie, naturally occurring in food products and water contamination), and (2) food additives (as detailed elsewhere 13 ), that is, exposure to potassium nitrite (European code e249), sodium nitrite (e250), sodium nitrate (e251), and potassium nitrate (e252). Of note, potassium nitrite and sodium nitrate were not explored in the present study because they were consumed by <1% of the population. We also determined the exposure to total nitrites/nitrates, reflecting the exposure from both foods and water, and food additives. Foods and water–originated nitrites and nitrates were determined by food category using the European Food Safety Authority's concentration levels for natural sources and contamination from agricultural practices. The French official regional sanitary control of tap water (the governmental regional database SISE‐Eaux) was used to estimate exposure via water consumption, by region of residence, and year of 24‐hour dietary record. As regards all food additives in the NutriNet‐Santé cohort, we used a double qualitative/quantitative approach: the presence of food additives was determined using 3 databases: (1) OQALI, a national database hosted by the French food safety authority (Agence Nationale de Sécurité Sanitaire et Alimentaire Nationale) and National Research Institute for Agriculture, Food and the Environment to characterize the quality of the food supply; (2) Open Food Facts, an open collaborative database of food products marketed worldwide; and (3) Mintel Global New Products Database, an online database of innovative food products in the world. Quantitative assessment of food additives was performed, using in that order (1) ad‐hoc laboratory assays, (2) doses of generic foods as reported by European Food Safety Authority, and (3) from the Codex General Standard for Food Additives in case the first 2 options were not available.
Case Ascertainment
Participants were invited to report major health events and medications via an annual health questionnaire, a biannual dedicated health check questionnaire, or voluntarily at any time via a dedicated interface on the study website. Participants were asked to provide medical records (such as diagnostic support reports, hospital admissions, and/or anatomic pathology reports) to support their declaration of health problems. A panel of medical experts validated major health events after reviewing participants' medical records and obtaining additional information from participants' physicians or medical facilities. In case participants did not respond for >1 year on the study website, the expert committee contacted the participants' relatives or physicians. In addition to this process, which was the primary source of case identification, participants' cohort data were linked, subject to their consent, to the National Health Insurance Medical Administration Database (SNIIRAM, State Council Decree No. 2013–175). Finally, we used data from the French National Cause‐Specific Death Register (CépiDC) to identify possible missing cardiovascular events in deceased participants. The International Classification of Diseases, Clinical Modification (ICD‐CM, Tenth Revision) code was used to classify cardiovascular disease cases. In this study, all patients diagnosed between the date of enrollment and October 5, 2021 had coronary heart disease such as myocardial infarction (code I21), acute coronary syndrome (I21.4), angioplasty (Z95.8), and angina pectoris (I20.0), along with cerebrovascular accidents such as strokes (I64) and transient ischemic attacks (G45.8 and G45.9). Incident hypertension cases were identified through the annual health questionnaires in participants who reported a new diagnosis of hypertension and use of relevant blood pressure medication. In the full cohort, case ascertainment was based for 73% on a combined self‐reported diagnosis of hypertension and adequate vasoactive treatment, for 7% on self‐reported diagnosis of hypertension only, and for 20% on adequate vasoactive treatment report without having reported another pathology requiring such treatment.
Statistical Analysis
Categories of exposure to nitrites/nitrates were defined using sex‐specific tertiles for foods and water‐originated nitrites and nitrates, and using a nonconsumer category along with low and high consumer categories (separated by sex‐specific median) for food additives. Cox proportional hazard models adjusted for age (as time‐scale), sex, daily energy intake (kcal/d), intakes of alcohol, sodium, sugar, saturated fatty acids, fiber, heme iron (g/d), body mass index (kg/m2), physical activity levels (high, moderate, low calculated according to the international physical activity questionnaire recommendations), smoking status (never smokers, former smokers, current light smokers, current heavy smokers), pack‐years, number of dietary records, family history of cardiovascular outcomes, educational level (primary, secondary, undergraduate, postgraduate) were performed. These adjustment factors were chosen since (1) some are well‐established risk or protective factors for cardiovascular outcomes and can be associated with a dietary pattern high in nitrates or nitrites intakes (smoking status and pack‐years, body mass index, alcohol, physical activity level, daily energy intake, educational level, sugar) or (2) others are concomitant components in foods containing high contents of nitrites and nitrates (fiber, saturated fatty acids, sodium, heme iron). All models were mutually adjusted for nitrate/nitrite intakes other than the specific one studied: for instance, when evaluating additives‐originated nitrites, we adjusted for foods and water‐originated nitrites and for total nitrates. Multiple Imputation by Chained Equations method by fully conditional specifications (20 imputed data sets) was performed to handle missing data for the following covariates: physical activity level (13.9%), level of education (6.2%), body mass index (0.8%), and smoking status (0.2% of missing data). The proportional hazard assumption was tested using the Schoenfeld residual method implemented in the survival R package. Participants contributed person‐time until date of diagnosis, last completed questionnaire, death, or October 5, 2021, whichever occurred first. The analyses were conducted using R software 3.5.2. P values <0.05 were considered statistically significant.
Results and Discussion
During follow‐up (median=7.2 years), 2075 of cardiovascular diseases (including 1004 cerebrovascular and 1079 coronary heart diseases) were diagnosed, and 3810 incident cases of hypertension were identified among 106 288 participants (79.3% women, media age at baseline, 41.2 years). The proportional hazard assumption was met. Dietary intakes of nitrites and nitrates are described in Figure 1. Food sources of nitrites and nitrates from additives were exclusively processed meats or meals and snacks containing processed meats (Figure 1). Exposure to nitrites from food additives was associated with higher hypertension risk (hazard ratio [HR]higher versus nonexposed, 1.19 [95% CI, 1.08–1.32], P=0.001; HR, 1.19 [1.08–1.32], P=0.002 for sodium nitrite specifically). There was no evidence for associations between foods and water‐originated nitrites or nitrates and hypertension risk (all P values>0.3) or between dietary nitrites or nitrates (whatever their source) with risks of cardiovascular (all P values >0.2), cerebrovascular (all P values >0.2), or coronary heart diseases (all P values >0.6) (details in Figure 2).
Figure 1.
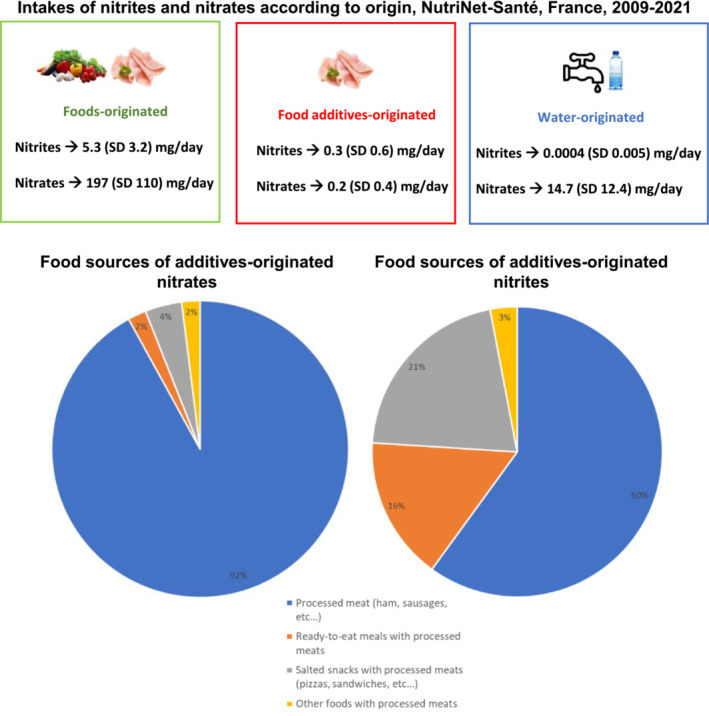
Intakes and Food Sources of Nitrites and Nitrates, NutriNet‐Santé Cohort, France, 2009 to 2021 (n=106 288).
Figure 2. Associations of Dietary Exposure to Nitrites and Nitrates With Cardiovascular and Hypertension Risks, NutriNet‐Santé Cohort, France, 2009 to 2021 (n=106 288).
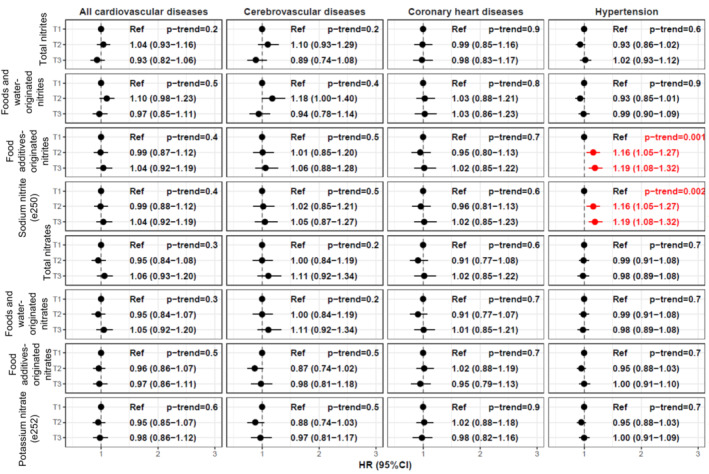
For total nitrite and nitrate intakes and from natural sources, sex‐specific tertiles of consumption were defined. Cutoffs were: 4.09 mg/d and 5.59 mg/d in women, and 5.21 mg/d and 7.52 mg/d in men for total nitrites; 152.3 mg/d and 233.4 mg/d in women, and 162.2 mg/d and 251 mg/d in men for total nitrates; 3.88 mg/d and 5.37 mg/d in women, and 4.94 mg/d and 7.10 mg/d in men for nitrites from natural sources; 152.2 mg/d and 233.2 mg/d in women, and 161.9 mg/d and 250.7 mg/d in men for nitrates from natural sources. For nitrites and nitrates from food additives, 3 categories of consumption were defined: nonconsumers, low consumers, and high consumers (separated by sex‐specific median among consumers). Cutoffs were: 0.19 mg/d in women and 0.25 mg/d in men for nitrites from food additives; 0.36 mg/d in women and 0.46 mg/d in men for nitrates from food additives; 2.23 mg/d in women and 2.23 mg/d in men for potassium nitrite (e249); 0.19 mg/d in women and 0.25 mg/d in men for sodium nitrite (e250); 0.04 mg/d in women and 0.05 mg/d in men for sodium nitrate (e251); and 0.36 mg/d in women and 0.46 mg/d in men for potassium nitrate (e252). HR indicates hazard ratio; and Ref, reference category.
While a beneficial role of dietary nitrate in the cardiovascular function has been suggested in some interventional studies in healthy volunteers and patients 2 , 3 and some observational studies, 6 our results do not support any evidence for cardiovascular benefits of nitrites or nitrates. On the contrary, we observed for the first time, associations between additives‐originated nitrites and a higher hypertension risk. The associations might be explained by the suggested role of nitrites in promoting oxidative damage in different organs. 7 , 8 In addition, N‐nitroso compounds (essentially formed during meat processing) have been shown to increase the risk of insulin resistance and coronary heart disease. 14 , 15 Limitations of this study encompass potential classification and residual confounding bias, because of its observational design. In addition, participants of the NutriNet‐Santé study were younger than the French general population, 16 which could explain the lack of association with cardiovascular disease, linked to reduced statistical power for this outcome. Strengths were the large sample size, prospective design, comprehensive assessment of exposure to nitrites/nitrates distinguishing different sources, and extensive adjustment for potential confounders, including sodium intake and heme iron (major components of processed meats).
Although these findings need confirmation in other large‐scale prospective and experimental studies in order to draw firm conclusions, they provide new evidence in the context of current discussions regarding the need for a reduction in the use of nitrite additives in processed meats by the food industry, as stated by the latest report of the French Agency for Food, Environmental and Occupational Health and Safety. Our results are in line with dietary guidelines recommending that the population limit their consumption of processed meats as well as foods containing controversial additives, among which is sodium nitrite.
Sources of Funding
The NutriNet‐Santé study was supported by the following public institutions: Ministère de la Santé, Santé publique France, Institut National de la Santé et de la Recherche Médicale (INSERM), Institut National de la Recherche Agronomique (INRAE), Conservatoire National des Arts et Métiers (CNAM), and University Sorbonne Paris Nord. EC was supported by a Doctoral Funding from University Sorbonne Paris Nord ‐ Galilée Doctoral School. This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No 864219), the French National Cancer Institute (INCa_14059), the French Ministry of Health (arrêté 29.11.19), the Initiative d'Excellence Université de Paris (ANR‐18‐IDEX‐0001), and the Bettencourt‐Schueller Foundation. This project was awarded the French network for Nutrition And Cancer Research (NACRe) Partnership Label. This work only reflects the authors' view and the funders are not responsible for any use that may be made of the information it contains. Researchers were independent from funders. Funders had no role in the study design, the collection, analysis, and interpretation of data, the writing of the report, and the decision to submit the article for publication.
Disclosures
FP received funding from the French Pork Institute (IFIP), for another experimental project on processed meats and colorectal cancer, aiming to evaluate technical solutions (eg, agricultural practices, formulation) to mitigate the well‐established deleterious impact of processed meat on colorectal cancer risk. It is not related at all to the present project/manuscript nor to cardiovascular outcomes. IFIP had no role in designing the present study nor funding it. The remaining authors have no disclosures to report.
Acknowledgments
We thank Nathalie Druesne‐Pecollo, PhD (operational coordinator); Younes Esseddik, Thi Hong Van Duong, Régis Gatibelza, Jagatjit Mohinder, Rizvane Mougamadou, and Aladi Timera (computer scientists); Fabien Szabo de Edelenyi, PhD, Julien Allegre, Nathalie Arnault, Laurent Bourhis, Nicolas Dechamp (data‐manager/statisticians); Cédric Agaësse, Alexandre De Sa, Rebecca Lutchia (dietitians); Paola Yvroud, MD (health event validator), and Maria Gomes (Nutrinaute support) for their technical contribution to the NutriNet‐Santé study. We thank all the volunteers of the NutriNet‐Santé cohort.
For Sources of Funding and Disclosures, see page 5 and 6.
References
- 1. Lundberg JO, Carlström M, Weitzberg E. Metabolic effects of dietary nitrate in health and disease. Cell Metab. 2018;28:9–22. doi: 10.1016/j.cmet.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 2. Bahadoran Z, Mirmiran P, Kabir A, Azizi F, Ghasemi A. The nitrate‐independent blood pressure‐lowering effect of beetroot juice: A systematic review and meta‐analysis. Adv Nutr. 2017;8:830–838. doi: 10.3945/an.117.016717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackson JK, Patterson AJ, MacDonald‐Wicks LK, Oldmeadow C, McEvoy MA. The role of inorganic nitrate and nitrite in cardiovascular disease risk factors: A systematic review and meta‐analysis of human evidence. Nutrition Reviews. 2018;76:348–371. doi: 10.1093/nutrit/nuy005 [DOI] [PubMed] [Google Scholar]
- 4. Bondonno CP, Liu AH, Croft KD, Ward NC, Yang X, Considine MJ, Puddey IB, Woodman RJ, Hodgson JM. Short‐term effects of nitrate‐rich green leafy vegetables on blood pressure and arterial stiffness in individuals with high‐normal blood pressure. Free Radical Biology and Medicine. 2014;77:353–362. doi: 10.1016/j.freeradbiomed.2014.09.021 [DOI] [PubMed] [Google Scholar]
- 5. Bondonno CP, Dalgaard F, Blekkenhorst LC, Murray K, Lewis JR, Croft KD, Kyrø C, Torp‐Pedersen C, Gislason G, Tjønneland A, et al. Vegetable nitrate intake, blood pressure and incident cardiovascular disease: Danish diet, cancer, and health study. Eur J Epidemiol. 2021;36:813–825. doi: 10.1007/s10654-021-00747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackson JK, Zong G, MacDonald‐Wicks LK, Patterson AJ, Willett WC, Rimm EB, Manson JE, McEvoy MA. Dietary nitrate consumption and risk of CHD in women from the Nurses' health study. British Journal of Nutrition. 2019;121:831–838. doi: 10.1017/S0007114519000096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. May JM, Qu Z‐C, Li X. Nitrite generates an oxidant stress and increases nitric oxide in EA.hy926 endothelial cells. Free Radic Res. 2004;38:581–589. doi: 10.1080/10715760410001688366 [DOI] [PubMed] [Google Scholar]
- 8. Kurz S, Hink U, Nickenig G, Borthayre AB, Harrison DG, Münzel T. Evidence for a causal role of the renin‐angiotensin system in nitrate tolerance. Circulation. 1999;99:3181–3187. doi: 10.1161/01.CIR.99.24.3181 [DOI] [PubMed] [Google Scholar]
- 9. Hirai N, Kawano H, Yasue H, Shimomura H, Miyamoto S, Soejima H, Kajiwara I, Sakamoto T, Yoshimura M, Nakamura H, et al. Attenuation of nitrate tolerance and oxidative stress by an angiotensin II receptor blocker in patients with coronary spastic angina. Circulation. 2003;108:1446–1450. doi: 10.1161/01.CIR.0000089092.61590.A8 [DOI] [PubMed] [Google Scholar]
- 10. Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circulation Research. 1994;74:1141–1148. doi: 10.1161/01.RES.74.6.1141 [DOI] [PubMed] [Google Scholar]
- 11. International Agency for Research on Cancer (IARC) . IARC Monographs on the Evaluation of Carcinogenesis Risks to Humans some N‐Nitroso Compounds. Lyon: International Agency for Research on Cancer; 1998:17. [PMC free article] [PubMed] [Google Scholar]
- 12. Black A. Critical evaluation of energy intake using the Goldberg cut‐off for energy intake: Basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes. 2000;24:1119–1130. doi: 10.1038/sj.ijo.0801376 [DOI] [PubMed] [Google Scholar]
- 13. Chazelas E, Druesne‐Pecollo N, Esseddik Y, Szabo de Edelenyi F, Agaësse C, De Sa A, Lutchia R, Rebouillat P, Srour B, Debras C, et al. Exposure to food additive mixtures in 106,000 French adults from the NutriNet‐Santé cohort. Sci rep. 2021;11:19680. doi: 10.1038/s41598-021-98496-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Etemadi A, Sinha R, Ward MH, Graubard BI, Inoue‐Choi M, Dawsey SM, Abnet CC. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH‐AARP diet and health study: Population based cohort study. BMJ. 2017;357:j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tong M, Neusner A, Longato L, Lawton M, Wands JR, de la Monte SM. Nitrosamine exposure causes insulin resistance diseases: Relevance to type 2 diabetes mellitus, non‐alcoholic steatohepatitis, and Alzheimer's disease. J Alzheimers Dis. 2009;17:827–844. [PMC free article] [PubMed] [Google Scholar]
- 16. Andreeva VA, Salanave B, Castetbon K, Deschamps V, Vernay M, Kesse‐Guyot E, Hercberg S. Comparison of the sociodemographic characteristics of the large NutriNet‐Sante e‐cohort with French census data: the issue of volunteer bias revisited. JEpidemiolCommunity Health. 2015;69:893–898. doi: 10.1136/jech-2014-205263 [DOI] [PubMed] [Google Scholar]


