Abstract
Durable implantable left ventricular assist devices (LVADs) have been shown to improve survival and quality of life for patients with stage D heart failure. Even though LVADs remain underused overall, the number of patients with heart failure supported with LVADs is steadily increasing. Therefore, general cardiologists will increasingly encounter these patients. In this review, we provide an overview of the field of durable LVADs. We discuss which patients should be referred for consideration of advanced heart failure therapies. We summarize the basic principles of LVAD care, including medical and surgical considerations. We also discuss the common complications associated with LVAD therapy, including bleeding, infections, thrombotic issues, and neurologic events. Our goal is to provide a primer for the general cardiologist in the recognition of patients who could benefit from LVADs and in the principles of managing patients with LVAD. Our hope is to “demystify” LVADs for the general cardiologist.
Keywords: chronic systolic heart failure, end stage heart failure, left ventricular assist device, mechanical circulatory support, stage D heart failure
Subject Categories: Cardiomyopathy, Heart Failure
Heart failure (HF) is a complex clinical syndrome resulting from any structural or functional impairment of ventricular filling or ejection of blood 1 that affects >6 million Americans. 2 Stage D/advanced HF is defined as the presence of refractory symptoms despite optimal medical, surgical, and device therapy. 3 It is estimated that ≈ 580 000 individuals have stage D HF in the US. When only guideline directed medical therapy is used, these patients have a dismal prognosis, with 1‐year survival ranging from 6% to 25%. 4 , 5 , 6 Given its poor prognosis, recognition of the transition to stage D HF is critical, as only a few treatment options are available to prolong and improve quality of life, including palliative inotropes, heart transplantation (HT), and left ventricular assist device (LVAD). Although ≈ 62 000 patients are eligible for these advanced therapies yearly, roughly only 10 000 receive an LVAD or an HT, leaving a large percentage of eligible patients deprived of these life‐saving therapies. 7 Despite efforts to increase the donor pool, HT remains limited by the scarcity of donor hearts. However, LVAD therapy does not share the same constraints; its underuse is multifactorial and includes general unfamiliarity with the technology and under‐recognition of patients with advanced HF who are eligible.
Nonetheless, with the growing number of patients who are being implanted with durable (dischargeable) LVADs, general cardiologists will increasingly encounter patients with these devices. The goal of this paper is to provide general cardiologists with a broad overview of implanted durable LVADs.
LVAD AS THERAPY FOR ADVANCED HF
The first generation of implantable durable LVADs were based on pulsatile flow. Subsequent technological advances led to the advent of LVADs that use continuous flow (CF) instead of pulsatile flow, which allowed for miniaturization, improved durability of the devices, and improved long‐term survival. CF‐LVADs have become the standard of care for medically refractory Stage D HF as either bridge to transplant, destination therapy (long term), or bridge to decision. 8 , 9 , 10 Between June 2006 and December 2018, nearly 25 000 adult patients received a Food and Drug Administration‐approved durable mechanical circulatory support device, with dramatic improvements in survival after LVAD implantation over time (Figure 1; Table S1). In the landmark REMATCH (Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure) trial, 1 year survival with the first generation pulsatile Heartmate XVE LVAD was 52% compared with 25% in the medical therapy group (P=0.002). 4 More recently, the MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3) trial demonstrated that survival with the centrifugal‐flow fully magnetically levitated pump HeartMate 3 (HM3 [Abbott Laboratories]) LVAD has reached 79% at 2 years. 11
Figure 1. Trends in the rate of 1‐year survival across clinical study groups over time.
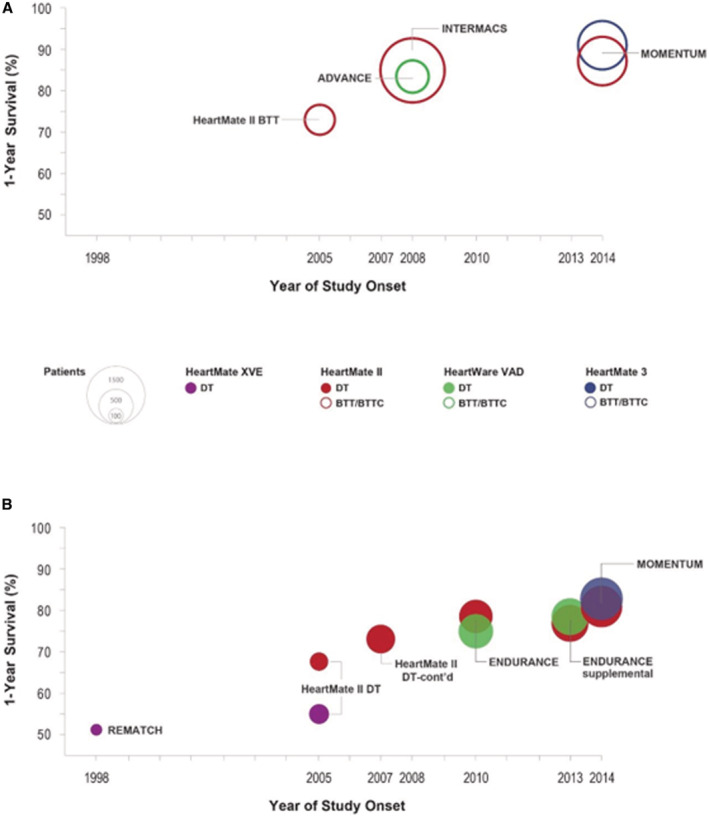
A and B, Display the 1‐year survival rates for patients enrolled in large studies of left ventricular assist devices. A, Patients implanted as bridge‐to‐transplant or bridge‐to‐transplant‐candidacy (both represented as open circles). B, Patients implanted as destination therapy (solid circles). Data are shown according to the respective start dates of each study. Each circle is color coded for each type of implanted device with purple for HeartMate XVE, maroon for HeartMate II, green for HeartWare, and blue for HeartMate 3. The study is labeled on the figure and data are listed in Table S1. The area of each circle represents the sample size in each group (reference sizes are shown in the key). BTC indicates bridge to candidacy; BTT, bridge to transplant; BTTC, bridge to transplant candidacy; DT, destination therapy; and VAD, ventricular assist device.
These benefits in survival have not only been shown in clinical trials but have also been observed in real world data. Current generation CF‐LVADs have a 30‐day mortality of only 5% and a 1‐year survival of 84%. Slightly more than half of the patients are alive at 4 years after implant, with 29% of patients supported on mechanical circulatory support and 33% having undergone HT. In comparison, healthier ambulatory patients with advanced HF treated with medical therapy had a 53% 2‐year survival without LVAD placement or HT. 12
HT waitlist mortality has also decreased over the past decade, from 14.6 deaths per 100 waitlist‐years in 2005 to 9.7 deaths per 100 waitlist‐years in 2015, 13 likely because of improvements in guideline directed medical therapy, changes in organ allocation systems, but also because of the use of CF‐LVADs as a bridge to transplant and to the aforementioned improvements in survival on these devices. Indeed, pretransplant mortality among LVAD supported patients dropped from 43.2 per 100 waitlist‐years in 2006 to 8.0 per 100 waitlist‐years in 2016. 13 It should be noted that following the latest revision to the organ allocation system in 2018, the number of patients who are being bridged to HT with a durable LVAD has dropped precipitously, as the new system prioritizes patients on temporary mechanical circulatory support. However, even with this decline, >2000 LVADs are still being implanted annually. Therefore, it is important for all cardiologists to have a basic understanding of the principles of managing patients with these devices.
Importantly, durable LVADs have not only been shown to improve survival, but they also improve quality of life for patients with HF. Patients with end‐stage HF treated with a durable LVAD have a significant reduction in New York Heart Association functional class, by 2 to 3 classes, as well as significant improvement in quality of life. These results were validated in each of the Food and Drug Administration‐approved implantable LVADs. 4 , 11 , 14
WHO TO REFER FOR LVAD THERAPY
Despite the demonstrated benefits of LVAD therapy in patients with advanced HF, a substantial number of these patients are either not referred for consideration of this therapy or are referred too late. 15 This has been attributed to the difficulty in recognition of when patients with HF have transitioned from stage C to stage D HF, as the inexorable progression is often slow and indolent, without a widely available biomarker to aid the clinician in making the diagnosis.
Multiple classification systems have been developed to assess the functional capacity and prognosis of patients with HF. The original and most used classification, is the New York Heart Association class, which characterizes patients based on their exercise capacity and symptom burden. This system was limited by its subjectivity, its inability to guide HF therapy, and the fact that patients could both improve and decline in status. Given these shortcomings, the American College of Cardiology/American Heart Association released a staging system in 2001 that placed emphasis on antecedent risk factors and progressive stages of disease similar to the approach commonly used in cancer staging. 16 Most recently, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) classification was developed to provide a more granular classification of the severity of HF in patients with advanced New York Heart Association class III or IV symptoms. 17 In patients defined as advanced by any of these classification systems, cardiopulmonary exercise testing can be performed for further risk stratification. In cardiopulmonary exercise testing, analysis of gas exchange is performed at rest, during exercise, and during recovery which allows measurements of oxygen uptake (VO2), carbon dioxide output (VCO2), ventilation, and the slope of ventilation/VCO2. 18
As per the International Society for Heart and Lung Transplantation guidelines, 19 patients not on beta blockade with a peak VO2 of <14 mL/kg per minute 20 or those on beta blockade with a peak VO2 of <12 mL/kg per minute 21 should be referred for evaluation for advanced therapies. Additionally, in select populations, metrics including predicted VO2 ≤50% and ventilation/VCO2 slope of >35 should also merit evaluation for HT. Unfortunately, this test is not widely available, and most physicians and health care providers are not familiar with it.
Helpful hints of progression towards stage D HF include a gradual decrease in exercise tolerance (which often goes unnoticed by the patient because of the slow progression), requirement for down titration of previously tolerated doses of neurohormonal antagonist medications, persistence of symptoms in spite of adequate goal directed medical therapy +/− cardiac resynchronization therapy, recurrent decompensation or hospitalizations, requirement for escalating doses of diuretics, severe left ventricular (LV) dilation (LV >7 cm), young age with no evidence of improvement on guideline directed medical therapy, or early signs of end‐organ damage (worsening kidney function or liver function) (Table). Patients with any of these characteristics should be referred to an HF specialist for comanagement of their HF as well as for consideration of advanced therapies including HT and LVAD placement.
Table 1.
Triggers for Patient With Heart Failure Referral to a Specialist/Program*
|
ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor‐neprilysin inhibitor; BNP, B‐type natriuretic peptide; BUN, blood urea nitrogen; EF, ejection fraction; HF, heart failure; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and NYHA, New York Heart Association.
Adapted from 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment. 91
Beyond assessment of volume status, we strongly encourage physicians to evaluate their patients with HF for early symptoms and signs of disease progression during each clinical encounter. They should refer patients with confirmed or suspected HF progression to an HF specialist and need not worry that a referral may be “inappropriate” or “too early”.
For many of these patients, HF specialists will initiate an evaluation into candidacy for advanced HF therapies. This is usually a multidisciplinary assessment that includes a comprehensive and detailed medical, surgical, and psychosocial evaluation. Often there are medical or psychosocial barriers to HT or LVADs that are uncovered or identified during this evaluation. Early referral allows time to devise interventions that eliminate or mitigate these obstacles, so that the patients' eligibility for these lifesaving therapies is not compromised.
BASIC PRINCIPLES OF LVAD CARE
Each LVAD consists of an inflow cannula, implantable pump, and an outflow graft, which are not visible to the patient, and external parts including the driveline exiting the abdominal wall, the controller, and an energy source (Figure 2). The controller is a smartphone‐sized “computer” that gives the patient and provider basic diagnostics and can trigger alarms in certain clinical situations or pump issues. The energy source is connected to the controller and comes from either a set of batteries that provide up to 17 hours of uninterrupted power, or from an alternating current power source.
Figure 2. Components of contemporary left ventricular assist devices.
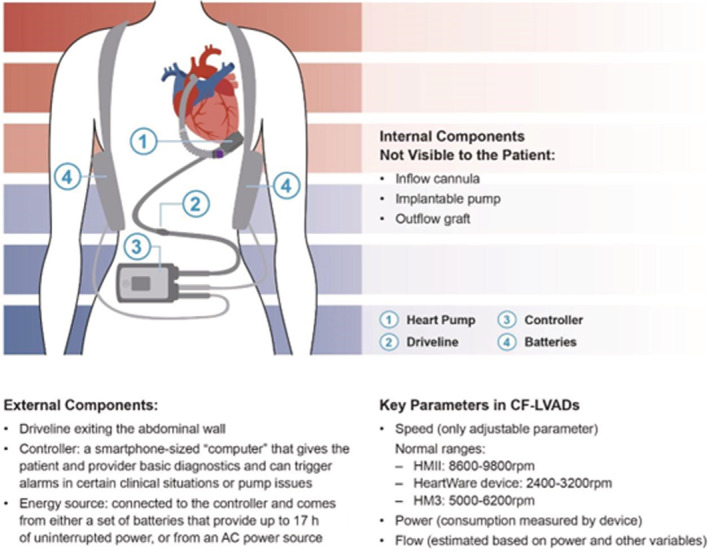
This figure summarizes key internal and external components of contemporary left ventricular assist devices. (1) Blood flows from the left ventricle to the inflow cannula (not shown but inserted into the left ventricular apex) through the pump and into the outflow graft to the aorta; (2) the driveline extends from the pump and out through the abdominal wall to the (3) external controller; (4) the main energy source for the pump is external batteries or an AC power source (not shown). AC indicates alternating Current; CV‐LVADs, continuous flow left ventricular assist device; HMII, HeartMate II; HM3, HeartMate 3; and rpm, rotations per minute.
There are 3 important parameters to know in CF‐LVADs: speed, power, and flow. The speed of the LVAD is the only one that can be manually adjusted. It is selected by the LVAD clinician and is device specific, with normal ranges of 8600‐9800RPM for the axial flow HeartMate II (HMII) LVAD, 2400‐3200RPM for the HeartWare device, and 5000‐6200RPM for the HM3 pump, which is the only Food and Drug Administration‐approved LVAD that is currently being manufactured. Speed is originally set in the operating room at the time of device implantation and is specifically adjusted with the goal of optimizing cardiac output and LV unloading without impairing right ventricular function. Ideally, the optimal speed will ensure minimal mitral regurgitation, an intermittently opening aortic valve (every 3 to 4 beats), and a midline interventricular septum. 22 Echocardiograms are performed on a periodic basis in both the inpatient and outpatient settings to re‐assess the optimal speed for the patient over time. Additionally, right heart catheterizations may be used to optimize a patient's LVAD speed based on hemodynamic data and have been associated with higher rates of speed changes, medication adjustments, and trends towards decreased adverse events. 23 The power of the device is directly measured, and the flow is calculated based on the power and LVAD speed, and hematocrit in certain devices (HeartWare, HM3). Some devices (HeartWare) also provide waveforms, which reflect the instantaneous flow through the pump and may be helpful in assessing a patient's volume status, afterload, right ventricular function, and heart rhythm. 24
ALARMS
Alarms appear on a patient's controller to allow for the early recognition of potential adverse events or device malfunction. The causes of these alarms and their evaluations vary by pump type. While this section is not an exhaustive guide, it will focus on the most clinically relevant alarms.
HeartWare
Alarms on this device are broken into 3 categories: high priority, medium priority, and low priority. These can be distinguished based on the alarm color and tone.
There are 3 high priority alarms, all of which require immediate attention: ventricular assist device (VAD) stoppage, controller failure, and critical battery. VAD stoppage may occur because of multiple reasons and is most commonly related to an issue with the driveline or the controller. Based on the cause of the LVAD stoppage, the patient may be instructed to reconnect their driveline or to change their controller. In the setting of a controller failure or critical battery, the patient would be advised to change their controller or replace their battery.
Medium priority alarms include high watts, low flow alarms, and controller faults. Potential etiologies of a high watt alarm include pump thrombosis, high flow, or an LVAD electrical fault. Basic evaluation for this alarm should include blood work to assess International Normalized Ratio (INR) and markers of hemolysis as well as an echocardiogram. Low flows may be attributable to suction events, increased afterload (hypertension), impaired VAD filling (tamponade, hypovolemia, right heart failure (RHF), ventricular arrhythmias, or inflow/outflow graft obstruction/kinking). Physical examination, ECG, echocardiogram, as well as a potential contrast enhanced high‐resolution cardiac computed tomography may aid in diagnosing the cause of the low flow alarms. 25
HMII AND HM3
Alarms in patients with these devices include messages and active symbols on their controller screen. Alarms are broken down into 2 categories: hazard and advisory notices. Hazard alarms require immediate clinical attention and include pump off, low flow, driveline disconnect, double power disconnect, and critical battery. 26 The management and work up of these alarms are similar to the HeartWare device as described above.
It is important that all patients (and their caregivers) receive extensive training on alarms, how to best address them, and when to contact the LVAD team. In general, non‐LVAD trained cardiologists are unlikely to be familiar with LVAD alarms. Therefore, when faced with an LVAD alarm, we recommend that they contact the LVAD team. All programs should have an on‐call person who will be able to assist in troubleshooting, resolving, and or planning the next steps required to best help the patient.
IMPLANTATION PROCEDURE
The most common surgical approach to LVAD implantation is through a median sternotomy, using cardiopulmonary bypass without a cross clamp of the aorta. Computed tomography of the chest is often used as a “roadmap” for reoperative and complex anatomic planning. Recently, less invasive approaches including left thoracotomy access with partial sternotomy or limited right anterior thoracotomy have gained interest, as they may make chest re‐entry easier and safer in patients with previous sternotomies and those who are being implanted as bridge to transplantation. Early, nonrandomized data suggest that these minimally invasive techniques are safe, 27 , 28 cost effective, 29 and may improve length of stay. 29 A clinical study evaluating the effects of access techniques other than full median sternotomy and their impact on postimplant length of stay is currently underway. 30
VALVULAR DISEASE
Aortic insufficiency of mild or greater significance requires concomitant correction during LVAD placement, 31 otherwise it would create a “closed‐circulatory loop” between the pump, aortic valve, left ventricle and pump again 32 (Figure 3). Options to correct include: oversewing the aortic valve with Park's stitch or modified Park's stitch, closure of the ventriculo‐aortic junction with a surgical patch, or valve replacement. Patients who have a previously placed mechanical valve should undergo replacement with a bioprosthetic valve 33 because of higher rates of thromboembolic events in mechanical valves secondary to blood stasis around the valve and intermittent valve opening. 34 It is important to note that patients who have undergone oversewing of the aortic valve or patch closure are completely dependent on their LVAD for cardiac output and are at risk for sudden death in the setting of LVAD dysfunction.
Figure 3. Ineffective blood flow in patients with a left ventricular assist device and aortic insufficiency.
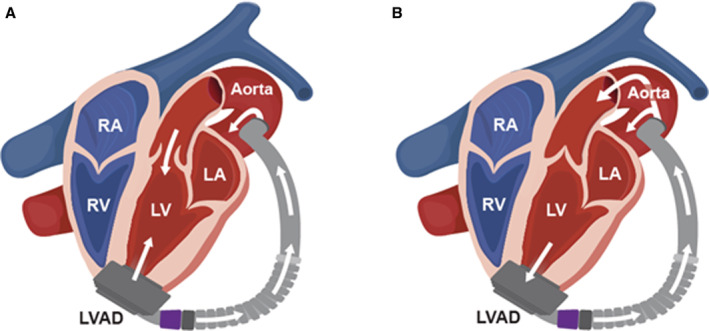
A, Displays the ineffective (circular) flow of blood in patients with a left ventricular assist device and aortic insufficiency. Blood is flowing primarily from the left ventricular apex to the pump, to the aorta, back through the incompetent aortic valve, and back to the left ventricular apex and pump without flowing to the periphery. B, Displays the normal flow of blood in patients with a left ventricular assist device and no aortic insufficiency. LA indicates left atrium; LV, left ventricle; LVAD, left ventricular assist device; RA, right atrium; and RV, right ventricle.
Mitral valve stenosis of moderate or greater severity should be corrected with a bioprosthetic valve. 31 Hemodynamically significant tricuspid regurgitation (≥moderate) has been shown to result in a longer requirement for inotropic support, higher rate of right ventricular assist device use, longer hospital stays, 35 and decreased survival. 36 Unfortunately, most studies that have examined the benefit of concomitant tricuspid valve repair have failed to demonstrate a survival benefit. 36 , 37 , 38 However, despite this, the guidelines recommend that ≥moderate tricuspid regurgitation should be repaired at the time of LVAD implantation (Class IIa, Level of Evidence: C). 31
ANTICOAGULATION AND ANTIPLATELET THERAPY
Because of the interaction of blood along the surface of the LVAD, with a resultant development of thrombus, all patients require anticoagulation with coumadin and aspirin. Once perioperative bleeding and coagulopathy subside, anticoagulation therapy with warfarin is usually initiated, with an INR goal of 2.0 to 3.0. International Society for Heart and Lung Transplantation guidelines recommend intravenous unfractionated heparin bridging within 48 hours after LVAD implantation. The recommended dose of aspirin is 325mg for patients with an HMII or HeartWare device, and 81 mg for an HM3 device.
However, it is important to remember that the anticoagulation regimen of an individual patient may be adjusted based on their personal history and their risk of bleeding versus clotting. This may result in some patients being on reduced or no anticoagulation.
BLOOD PRESSURE
Current automated blood pressure (BP) cuff technologies rely on peripheral pulsatility to measure BP. As patients with LVADs typically have a narrow arterial pulse pressure, their palpable pulse may be absent or intermittent, which may result in the inability of automated cuffs to register a BP. 39 In this situation, a Doppler device is used to assess BP by evaluating the pressure at which the Doppler sound returns during cuff deflation. This pressure has been shown to correspond to the mean arterial pressure (MAP). In contrast, in patients who do not have an LVAD or in those who have an LVAD but have a palpable pulse, the pressure at which the Doppler sound returns during cuff deflation corresponds to the systolic BP (Figure 4).
Figure 4. Method to obtain blood pressure in patients with left ventricular assist devices.
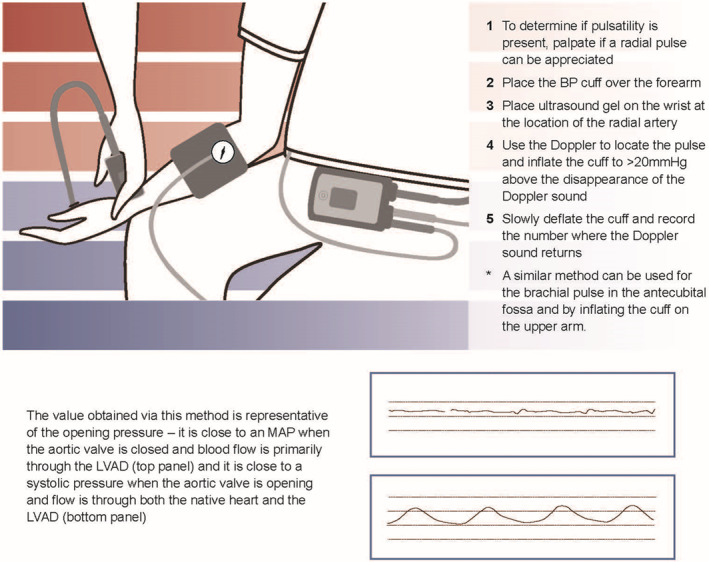
BP indicates blood pressure; LVAD, left ventricular assist device; and MAP, mean arterial pressure.
It is recommended that the MAP in patients with durable LVADs be maintained in the range between 75 and 85 mm Hg. Data from clinical trials have demonstrated that hypertension (MAP >90) is associated with an increase in both stroke 40 , 41 and pump thrombosis 40 , 42 in some (but not all) devices. Additionally, extremes of both low BP (MAP ≤75) and hypertension (MAP >100) have been associated with increased mortality in both the HMII and HeartWare devices. 43
ARRHYTHMIAS
Both atrial and ventricular arrhythmias are common in patients receiving LVADs secondary to pre‐existing abnormal myocardial substrate, reverse remodeling, and alterations in the electrical conduction system post‐LVAD placement. Pre‐existing atrial arrhythmias are present in up to 54% 44 of patients undergoing LVAD placement, with new onset atrial fibrillation developing in up to 32% 45 of patients.
A history of ventricular arrhythmia (VA) is present in 60% of patients undergoing LVAD placement with 37% developing new onset VA following LVAD placement. 46 Potential mechanisms for the VA include: underlying cardiomyopathy, scar around the LVAD inflow cannula, suction events (because of LV underfilling or high pump speed), or changes in the QT interval secondary to LV unloading. 47 The treatment of VA in patients with LVAD is based on the underlying mechanism. In patients with underlying cardiomyopathy or scar, first line therapies consist of antiarrhythmic drugs, followed by options including ventricular tachycardia ablation or stellate ganglion block in refractory cases.
CARDIOPULMONARY RESUSCITATION
The determination of whether a patient supported by an LVAD requires cardiopulmonary resuscitation is difficult as many of these patients do not have a palpable pulse. The first step in this evaluation is to auscultate the chest to see if there is an appreciable VAD hum, followed by an assessment of end organ perfusion. In patients without a VAD hum, or with signs of impaired perfusion, both the driveline and controller should be interrogated to ensure they are appropriately connected and that flows are maintained. In patients with persistent low‐flows as well as evidence of end organ dysfunction, such as significant hypotension (MAP <50), or a end‐tidal carbon dioxide tension (PetCO2) value of <20 mm Hg, routine advanced cardiovascular life support and basic life support protocols should be followed. 48 , 49 The risk of dislodging an LVAD or displacing a cannula/pump during chest compressions is only a theoretical one, and current guidelines still recommend CPR in the patients who need it. 48
Many patients with LVADs can tolerate ventricular arrhythmias for prolonged periods of time secondary to the development of a Fontan‐like circulation which occurs because of an extreme reduction in pulmonary vascular resistance. In patients with hemodynamic instability, cardioversion and defibrillation are not contraindicated and may be performed without disconnection of the LVAD. 49 For patients with refractory arrhythmias despite defibrillation, Veno‐Arterial Extracorporeal Membrane Oxygenation (VA‐Ecmo) can be considered.
CAREGIVER RESPONSIBILITIES AND BURDEN
Patients with LVADs usually require caregivers to support them during the recovery process and to provide long‐term assistance in case of device malfunction and overall patient care. Caregiver involvement varies significantly and depends on a patient's preoperative functional status and level of independence. These caregivers actively participate in preoperative and postoperative education and are crucial components of the overall therapeutic success for this population. At many centers, patients without caregivers may not be considered for LVAD therapy as lack of social support has been associated with worse outcomes. 50
ROLE OF VAD COORDINATORS
Like many other aspects of treating patients with cardiovascular diseases, patients with LVAD require a multidisciplinary approach, with collaboration between multiple services. A unique role belongs to the VAD coordinators who ensure close communication between patients and all involved parties, have significant expertise in device management, and provide continuity of care. Their specific roles and assignments may vary among programs, but any successful LVAD center depends on a strong and cohesive group of coordinators.
PALLIATION AND END OF LIFE
The use of palliative care specialists can help reduce suffering, improve symptoms, and set realistic expectations that concur with the patient and their beliefs. Patients with HF often face major treatment decisions over time and should be provided with an adequate support structure to help evaluate the benefits and burdens of each treatment option.
LVAD therapy is one of the advanced HF therapy modalities and its presence (or lack of) should not affect decisions about quality and quantity of life.
RECOVERY
Registries have demonstrated a recovery rate, defined as an increase in ejection fraction to allow for pump removal, in 1% to 2% 51 of patients implanted with LVADs, which was felt to be secondary to reverse remodeling attributable to LVAD unloading and/or recovery of underlying cardiac dysfunction. In the Remission from Stage D Heart Failure (RESTAGE‐HF) trial, protocolized adjustment of LVAD speeds and aggressive goal directed medical therapy optimization was found to be successful in achieving recovery in a select group of patients with nonischemic cardiomyopathy. 52
COMPLICATIONS OF LVADS
Bleeding
Bleeding is one of the most common adverse events in the early (<90 days) postoperative period following LVAD implantation and is a frequent complication in the late period (≥90 days), with nearly one third of patients experiencing a major bleeding episode by 1 year. 53 While the predominance of early bleeding is related to complications of the surgical intervention itself, late bleeding is most commonly secondary to gastrointestinal bleeding (GIB) which accounts for nearly 60% of all LVAD associated bleeding. 53 Although bleeding has not been shown to affect mortality, it does have a significant effect on morbidity and is one of the most common causes of re‐admissions. 54 , 55 Additionally, the requirement of frequent blood transfusions may increase a patient's sensitization to antibodies, making future heart transplantation increasingly difficult.
Because of varying technologies and device hemocompatibility, the rates of GIB differ based on device type, ranging from 12% to 25% in the first year after device implant. 56 Risk factors associated with an increased risk of GIB include post LVAD implant infection, prior GIB, elevated INR, lower platelet count, elevated right atrial pressure, low body mass index, female sex, and destination therapy indication for implantation. 57 , 58
Multiple hypotheses have been proposed to explain the pathophysiology of GIB in patients with LVAD including:
Requirement of both antiplatelet and anticoagulation therapies (aspirin and coumadin)
An acquired Von Willebrand syndrome that is attributable to CF physiology
The development of angiodysplasias during CF‐LVAD support
Angiogenesis related signaling cascade
However, it is more likely that each of these mechanisms are part of a larger, multihit theory. 59
Arteriovenous malformations and angiodysplasias in the upper gastrointestinal tract and small bowel are the most common source of gastrointestinal bleed and represent up to 60% of bleeds in LVADs. 60 Diagnostic strategies at the time of an acute GIB usually consist of endoscopic evaluation, but they may need to be escalated in some cases to video capsule study, tagged red blood cell scans or angiography. Therapeutic strategies are either procedural or pharmacologic. Procedurally based strategies during an acute LVAD GIB include cauterization, arterial embolization, and, in extreme cases, surgical intervention. Pharmacologic options during an acute event include cessation of antiplatelet therapy and/or anticoagulation as well as possible reversal with agents such as vitamin K, fresh frozen plasma, or Prothrombin complex concentrate.
Multiple pharmacologic therapies have been examined for their benefits as both primary and secondary prevention agents of GIB. Omega 3 fatty acids have been shown to significantly decrease the rates of gastrointestinal bleeds, number of blood product transfusions, days in the hospital, and gastrointestinal procedures in patients with LVAD which is thought to be mediated through its anti‐inflammatory properties. 61 Octreotide, a somatostatin analogue, has been extensively studied as a secondary prevention agent, and has been shown to decrease the frequency of GIB 62 secondary to increased splanchnic vasoconstriction, enhancement of platelet aggregation, and inhibition of gastrointestinal angiogenesis. 63 Patients on digoxin were also found to have fewer GIB from angiodysplasias attributed to suppression of neoangiogenesis. 64
In patients with recurrent gastrointestinal bleeds who have failed other measures, strategies such as holding or decreasing antiplatelet therapy dosing or pursuing lower INR goals are often entertained as possible options. However, these strategies should be considered with caution based on device type. While decreasing antiplatelet dosing may decrease GIB, it has also been shown to increase the rate of both ischemic and hemorrhagic strokes in patients with HeartWare devices. 41 Evaluation of the cessation of antiplatelet therapy in the HM3 device is currently underway, 65 and small studies have reported no difference in stroke rates in these devices at lower INR goals (1.5–1.9). 66
Infection
Although LVADs are internal, the driveline exits the abdominal wall, and this external portion is a large source of infection. Major infection is the most common adverse event among patients with LVAD in the STS INTERMACS Database. Forty percent of patients supported by an axial flow device, 43% with a CF‐Hybrid levitation device, and 33% with a CF‐Full magnetic levitation device experience a major infection during the first year of follow‐up after LVAD implantation. 56 The incidence of infection is highest in the early postimplant period, but the risk of infection persists during the entire duration of device support. 67 , 68 Infections in patients supported by an LVAD may be characterized as VAD‐specific, VAD‐related, or non‐VAD infections. 69 Most VAD‐specific infections are localized to the driveline, but these infections may also affect the LVAD pump, cannula, or pocket. VAD‐related infections include endocarditis, bloodstream infections, and mediastinitis from sternal wound or pocket infection. Common non‐VAD infections include pneumonia, urinary tract infection, cholecystitis, and clostridium difficile infection.
Risk factors for infections in patients with LVAD include obesity, diabetes, poor nutritional status, female sex, previous cardiac surgery, INTERMACS Profile 1 status, and longer hospitalization before LVAD implantation. 70 , 71 , 72 , 73 Staphylococcus aureus is the most common organism implicated in driveline infections, followed by enteric Gram‐negative bacteria, Pseudomonas aeruginosa, and coagulase negative staphylococci. 70 Poorly controlled driveline infections can lead to pump pocket infection, sepsis, and death. During long‐term follow‐up in the INTERMACS Database, infection was the third most common cause of death in patients with LVAD. 42 Infections in patients with LVAD have also been associated with a higher risk of both pump thrombosis and stroke, with hemorrhagic strokes more common than ischemic strokes. 68 Activation of the inflammatory system in the setting of infection may lead to stimulation of the coagulation system, mycotic aneurysms, increased vascular fragility, and hemorrhagic conversion of septic emboli.
Optimal driveline management is also important for the prevention of infections. Practices vary among institutions but typically include dressings that are changed under sterile conditions every 1 to 3 days and anchoring devices can help stabilize the driveline and reduce trauma at the exit site. Some institutions also recommend restriction of showering throughout the lifespan of the LVAD.
Driveline infections involving cellulitis and drainage may be treated with oral antibiotics. 70 Treatments for continued infection with increasing drainage and tunneling along the driveline include intravenous antibiotics and surgical debridement to expose the tunnel and scrub the driveline with disinfectants. Replacement of the LVAD with rerouting of the driveline may be considered but is associated with the risk of surgery and extension of infection to other areas. 74 Pump pocket infections are treated with parenteral antibiotics, surgical debridement, and continuous irrigation or vacuum‐assisted drainage. In some cases, complete pump exchange, pump explant, and HT may be considered.
Despite preventive measures, the burden of infectious complications in patients with LVAD is considerable given that the LVAD is a foreign object, and all commonly used durable LVADs rely on a driveline exiting the body to connect to a power source. Fully implantable LVADs are currently under development, and it is hoped that they will be associated with a lower incidence of infections in patients with LVAD in the future.
THROMBOEMBOLIC EVENTS
Pump Thrombosis
LVAD thrombotic events are decreasing over time because of innovations in LVAD pump design. In the ENDURANCE trial comparing the HeartWare to HMII in patients ineligible for transplant, LVAD exchange because of pump thrombosis seemed to be less frequent with the HeartWare than the HMII, occurring in 19 patients (6.4%) versus 16 patients (10.7%) (P=0.12). 55 In the MOMENTUM 3 trial comparing the HM3 to the HMII in patients with advanced HF, the incidence of suspected or confirmed LVAD thrombosis at 2 years was significantly lower with the HM3 than the HMII device, occurring in 7 patients (1.4%) versus 70 patients (13.9%), (P<0.001). 11 These improvements are attributable to the fact that, when compared with the HeartWare and HMII devices, the HM3 has wider gaps for blood flow through the LVAD, less friction during movement attributable to the absence of bearings, and more intrinsic pulsatility, which leads to less shear stress and stasis of blood. 75
Treatment for suspected LVAD thrombosis may include increased antithrombotic and antiplatelet therapies. If these are not sufficient, then other potential therapies include early LVAD exchange, LVAD explantation (in case of significant myocardial recovery), or consideration of urgent HT (in eligible candidates). While thrombolytics were previously used in patients with the HeartWare device, their use has fallen out of favor because of limited success and high rates of bleeding complications including hemorrhagic cerebrovascular accident. For centrifugal LVADs, device replacement can be performed through a less invasive anterior thoracotomy for HeartWare devices and a subcostal approach for HMII devices. 76
Neurologic Events
Neurologic events remain the most feared morbidity associated with LVADs because of their deleterious impact on functional capacity and quality of life. Ischemic and hemorrhagic strokes occur after LVAD implantation with an incidence ranging from 5% to 30%, varying by device type. 9 , 11 , 55 , 77 , 78 , 79 , 80 , 81 Stroke adversely affects survival, 81 , 82 and hemorrhagic stroke is associated with a higher mortality than ischemic stroke. Furthermore, the neurologic sequelae could also compromise a patient's candidacy for HT.
Risk factors for stroke vary by device type and include age, 83 presence of infections, 84 antiplatelet and anticoagulant therapies, 41 and elevated BP. 41 , 85 Many ischemic strokes are thought to be thromboembolic in origin, though underlying intracranial or extracranial atherosclerotic disease may also contribute. Hemorrhagic strokes are thought to be related to any of the following: (1) elevated BP in the setting of anticoagulation, (2) high‐velocity intracranial flow, or (3) hemorrhagic conversion from thromboembolic strokes. 41
There are no standardized protocols for the management of neurologic events in patients with LVADs. A head computed tomography should be obtained as soon as a stroke is suspected to determine whether the cerebrovascular accident is ischemic or hemorrhagic in nature. In case of a hemorrhagic event, prompt neurosurgical consultation should be considered, whereas in case of an ischemic event, decisions would need to be made about the antiplatelet and anticoagulant therapies.
While innovations in LVAD pump design have reduced the rates of LVAD thrombotic and thromboembolic events, additional data are necessary to understand best medical practices for prevention. The ENDURANCE Supplemental Trial demonstrated that stringent control of BP in patients supported by the HeartWare LVAD, with frequent monitoring to maintain an MAP target of <85 mm Hg, was associated with a lower risk of stroke compared with the cohort of the ENDURANCE trial without strict BP parameters. 14 Whether antiplatelet agents are still required to reduce the risk of thrombotic and thromboembolic events in the era of fully magnetically levitated centrifugal‐flow LVADs is unclear.
Right Heart Failure
RHF is a common cause of morbidity and mortality in patients following LVAD placement. Although the exact incidence is not known attributable to changing diagnostic criteria, it is thought to occur in up to 53% of patients. 86 Multiple different models have been proposed to best predict patients who would develop RHF; however, the ability to identify these patients pre‐LVAD implantation remains a challenge. 87 , 88
According to the latest INTERMACS definition, RHF can be divided into 3 phases: early acute, early post implant, or late stages. Early acute RHF is defined as the requirement for implantation of a temporary or durable right VAD at the time of LVAD implantation. Early post‐implant RHF is defined as the need to implant a temporary/durable right VAD within 30 days of LVAD implant, failure to wean from inotropic, vasopressor, or inhaled NO support within 14 days of LVAD placement, or the need to initiate these therapies within 30 days of device implant and for a duration of at least 14 days. Approximately 5% of patients receiving an LVAD require right VAD therapy and an additional 10% to 15% require prolonged inotropes. 89 Late RHF is defined as the need for a right VAD >30 days after LVAD implantation, or hospitalizations occurring >30 days postdevice implant with associated clinical exam findings or hemodynamic parameters consistent with RHF, and which require IV diuresis or inotropic support for at least 72 hours.
Late RHF has been associated with higher incidences of rehospitalizations, gastrointestinal bleed, stroke, infection, and worsening quality of life. 88 Therapies for this condition are limited and include escalating diuretics, inotropes, and transplant in eligible patients. 88 , 90
Role of the General Cardiologist
General cardiologists have a pivotal role in the long‐term management of patients with LVAD. Routine BP measurements on every office visit are recommended to decrease the risk of stroke and intracranial bleeding. While adjustments of BP medications should be made in conjunction with the primary LVAD team, preferred agents are afterload reducing medications such as angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, angiotensin receptor‐neprilysin inhibitors, and hydralazine. The role of beta blockers in patients with LVAD is highly debated and their use should be based on individual patient characteristics. Patients who may benefit from their use include those undergoing evaluation for recovery/LVAD explantation as well as those with atrial or ventricular arrhythmias. However, caution should be used in patients with right ventricular failure as beta blockers could lead to acute decompensation.
Since the LVAD does not provide support to the right ventricle, many patients suffer varying degrees of right ventricular failure and require close follow‐up for volume status as well as sodium and volume restrictions. It is highly recommended to assess the volume status on every office visit and to discuss possible adjustments of the diuretic dose with the primary LVAD team. In some cases, additional testing such as echocardiography or right heart catheterization may be useful to help guide therapy.
Patients who have implantable cardioverter defibrillator should undergo device interrogation periodically or in the case of new or concerning symptoms. The presence of ventricular arrhythmias, their frequency, and their duration are important to recognize and address.
Finally, awareness of the unique physiology of patients with LVADs and the complications associated with these devices as outlined in this paper are essential for general cardiologists, since patients frequently approach them first with their initial symptoms. Early recognition of these complications allows treatments to be instituted which improve the clinical outcomes of this patient population.
CONCLUSIONS
Although the number of LVADs implanted per year continues to increase, many patients who could benefit from these devices are not offered this therapy because they are not referred to an advanced HF program in a timely manner, despite the proven beneficial impact on both survival and quality of life. This gap in therapy is secondary to unfamiliarity with device technology and difficulty in recognizing patients who progress to stage D HF.
As duration of support continues to increase, these devices will become increasingly common, so general cardiologists will be encountering more of these patients in their practice.
We hope that this primer will allow general cardiologists to become more comfortable with LVADs and more familiar with the principles of managing patients supported with an LVAD.
Sources of Funding
None.
Disclosures
Dr Chaudhry receives speaking honoraria from Medtronic and consultation fees from Edwards Lifesciences. Dr Devore receives research funding through his institution from the American Heart Association, Amgen, Biofourmis, Bodyport, Cytokinetics, American Regent, Inc, the National Heart, Lung, and Blood Institute, and Novartis. He receives consultation fees/honoraria from Abiomed, Amgen, AstraZeneca, Cardionomic, InnaMed, LivaNova, Natera, Novartis, Procyrion, Story Health, Vifor, and Zoll. Dr Vidual receives research funding from Abbott and the National Institutes of Health. Dr Nassif receives consultation fees from Vifor. Dr Birati receives research funding from American Regent and Medtronic. He receives speaking honoraria from AstraZeneca, Novo Nordisk, and CTS. Dr Gong receives research funding from the American Society of Transplantation Research Network/Natera. He receives speaking honoraria from AstraZeneca. Dr Alturi receives speaking honoraria from Abbott, Edwards Lifesciences, and Medtronic. Dr Pham receives consulting fees from Abbott, Medtronic, and Abiomed. Dr Sun receives consulting fees from Abbott. Dr Bansal receives research funding from Abbott and Livanova. He receives consultation fees from Abbott and LivaNova. He is an advisory board member for Abbott, Abiomed, LivaNova, and VADovation. Dr Najjar receives research funding from Abbott and Merck. He receives consulting fees from Abbott. Dr Mudy has no disclosures to report.
Supporting information
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027251
For Sources of Funding and Disclosures, see page 12.
REFERENCES
- 1. Writing Committee M, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 2. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics‐2020 Update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 3. Fang JC, Ewald GA, Allen LA, Butler J, Westlake Canary CA, Colvin‐Adams M, Dickinson MG, Levy P, Stough WG, Sweitzer NK, et al. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail. 2015;21:519–534. doi: 10.1016/j.cardfail.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 4. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, et al. Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175 [DOI] [PubMed] [Google Scholar]
- 5. Rogers JG, Butler J, Lansman SL, Gass A, Portner PM, Pasque MK, Pierson RN 3rd, Investigators IN. Chronic mechanical circulatory support for inotrope‐dependent heart failure patients who are not transplant candidates: results of the INTrEPID Trial. J Am Coll Cardiol. 2007;50:741–747. doi: 10.1016/j.jacc.2007.03.063 [DOI] [PubMed] [Google Scholar]
- 6. Hershberger RE, Nauman D, Walker TL, Dutton D, Burgess D. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail. 2003;9:180–187. doi: 10.1054/jcaf.2003.24 [DOI] [PubMed] [Google Scholar]
- 7. Morris AA, Khazanie P, Drazner MH, Albert NM, Breathett K, Cooper LB, Eisen HJ, O'Gara P, Russell SD, American Heart Association Heart F , et al. Guidance for timely and appropriate referral of patients with advanced heart failure: a Scientific Statement from the American Heart Association. Circulation. 2021;144:e238–e250. doi: 10.1161/CIR.0000000000001016 [DOI] [PubMed] [Google Scholar]
- 8. Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Baldwin JT, Young JB. Fifth INTERMACS annual report: risk factor analysis from more than 6000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 9. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM 3rd, Long JW, et al. Advanced heart failure treated with continuous‐flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938 [DOI] [PubMed] [Google Scholar]
- 10. Griffith BP, Kormos RL, Borovetz HS, Litwak K, Antaki JF, Poirier VL, Butler KC. HeartMate II left ventricular assist system: from concept to first clinical use. Ann Thorac Surg. 2001;71:S116–S120; discussion S114–S116. doi: 10.1016/s0003-4975(00)02639-4 [DOI] [PubMed] [Google Scholar]
- 11. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, et al. A fully magnetically levitated left ventricular assist device ‐ final report. N Engl J Med. 2019;380:1618–1627. doi: 10.1056/NEJMoa1900486 [DOI] [PubMed] [Google Scholar]
- 12. Ambardekar AV, Kittleson MM, Palardy M, Mountis MM, Forde‐McLean RC, DeVore AD, Pamboukian SV, Thibodeau JT, Teuteberg JJ, Cadaret L, et al. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry. J Heart Lung Transplant. 2019;38:408–417. doi: 10.1016/j.healun.2018.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colvin M, Smith JM, Hadley N, Skeans MA, Carrico R, Uccellini K, Lehman R, Robinson A, Israni AK, Snyder JJ, et al. OPTN/SRTR 2016 Annual Data Report: Heart. Am J Transplant. 2018;18(Suppl 1):291–362. doi: 10.1111/ajt.14561 [DOI] [PubMed] [Google Scholar]
- 14. Milano CA, Rogers JG, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Mokadam NA, Mahr C, Miller JS, Markham DW, et al. HVAD: the ENDURANCE supplemental trial. JACC Heart Fail. 2018;6:792–802. doi: 10.1016/j.jchf.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 15. Herr JJ, Ravichandran A, Sheikh FH, Lala A, Chien CV, Hsiao S, Srivastava A, Pedrotty D, Nowaczyk J, Tompkins S, et al. Practices of referring patients to advanced heart failure centers. J Card Fail. 2021;27:1251–1259. doi: 10.1016/j.cardfail.2021.05.024 [DOI] [PubMed] [Google Scholar]
- 16. Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568 [DOI] [PubMed] [Google Scholar]
- 17. Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, Naftel DC, Ulisney K, Desvigne‐Nickens P, Kirklin JK. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009;28:535–541. doi: 10.1016/j.healun.2009.02.015 [DOI] [PubMed] [Google Scholar]
- 18. Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, et al. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69 [DOI] [PubMed] [Google Scholar]
- 19. Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger‐Isakov L, Kirklin JK, Kirk R, Kushwaha SS, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10‐year update. J Heart Lung Transplant. 2016;35:1–23. doi: 10.1016/j.healun.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 20. Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778 [DOI] [PubMed] [Google Scholar]
- 21. Peterson LR, Schechtman KB, Ewald GA, Geltman EM, de las Fuentes L, Meyer T, Krekeler P, Moore ML, Rogers JG. Timing of cardiac transplantation in patients with heart failure receiving beta‐adrenergic blockers. J Heart Lung Transplant. 2003;22:1141–1148. doi: 10.1016/s1053-2498(02)01225-1 [DOI] [PubMed] [Google Scholar]
- 22. Stainback RF, Estep JD, Agler DA, Birks EJ, Bremer M, Hung J, Kirkpatrick JN, Rogers JG, Shah NR, American Society of E . Echocardiography in the management of patients with left ventricular assist devices: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015;28:853–909. doi: 10.1016/j.echo.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 23. Uriel N, Burkhoff D, Rich JD, Drakos SG, Teuteberg JJ, Imamura T, Rodgers D, Raikhelkar J, Vorovich EE, Selzman CH, et al. Impact of hemodynamic ramp test‐guided HVAD speed and medication adjustments on clinical outcomes. Circ Heart Fail. 2019;12:e006067. doi: 10.1161/CIRCHEARTFAILURE.119.006067 [DOI] [PubMed] [Google Scholar]
- 24. Rich JD, Burkhoff D. HVAD flow waveform morphologies: theoretical foundation and implications for clinical practice. ASAIO J. 2017;63:526–535. doi: 10.1097/MAT.0000000000000557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. http://www.medtronic.me/content/dam/medtronic‐com/products/cardiac‐rhythm/ventricular‐assist‐device/documents/hvad‐controller‐alarm‐guide.pdf. Accessed September 27th.
- 26. Trinquero P, Pirotte A, Gallagher LP, Iwaki KM, Beach C, Wilcox JE. Left ventricular assist device management in the emergency department. West J Emerg Med. 2018;19:834–841. doi: 10.5811/westjem.2018.5.37023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McGee E Jr, Danter M, Strueber M, Mahr C, Mokadam NA, Wieselthaler G, Klein L, Lee S, Boeve T, Maltais S, et al. Evaluation of a lateral thoracotomy implant approach for a centrifugal‐flow left ventricular assist device: the LATERAL clinical trial. J Heart Lung Transplant. 2019;38:344–351. doi: 10.1016/j.healun.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 28. Hironaka CE, Deng B, Kawabori M, Critsinelis AC, Zhan Y, Chen FY, Vest A, DeNofrio D, Kiernan MS, Couper GS. Left thoracotomy vs full sternotomy for centrifugal durable LVAD implantation: 1‐year outcome comparison post‐LVAD and post‐heart transplantation. J Artif Organs. 2021;24:312–319. doi: 10.1007/s10047-021-01250-y [DOI] [PubMed] [Google Scholar]
- 29. Mahr C, McGee E Jr, Cheung A, Mokadam NA, Strueber M, Slaughter MS, Danter MR, Levy WC, Cheng RK, Beckman JA, et al. Cost‐effectiveness of thoracotomy approach for the implantation of a centrifugal left ventricular assist device. ASAIO J. 2020;66:855–861. doi: 10.1097/MAT.0000000000001209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Implantation of the HeartMate3 in Subjects with Heart Failure Using Surgical SWIFT HM3 PMS (SWIFT). Available at: https://clinicaltrials.gov/ct2/show/NCT04548128. Accessed November 15, 2022.
- 31. Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–187. doi: 10.1016/j.healun.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 32. Noly PE, Pagani FD, Noiseux N, Stulak JM, Khalpey Z, Carrier M, Maltais S. Continuous‐flow left ventricular assist devices and valvular heart disease: a comprehensive review. Can J Cardiol. 2020;36:244–260. doi: 10.1016/j.cjca.2019.11.022 [DOI] [PubMed] [Google Scholar]
- 33. Truby LK, Garan AR, Givens RC, Wayda B, Takeda K, Yuzefpolskaya M, Colombo PC, Naka Y, Takayama H, Topkara VK. Aortic insufficiency during contemporary left ventricular assist device support: analysis of the INTERMACS Registry. JACC Heart Fail. 2018;6:951–960. doi: 10.1016/j.jchf.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tulimat T, Osman B, Beresian J, Sfeir P, Borgi J. Management of a mechanical aortic valve during left ventricular assist device implantation in a previously replaced aortic root. Int J Artif Organs. 2022;45:152–154. doi: 10.1177/0391398821990667 [DOI] [PubMed] [Google Scholar]
- 35. Piacentino V III, Williams ML, Depp T, Garcia‐Huerta K, Blue L, Lodge AJ, Mackensen GB, Swaminathan M, Rogers JG, Milano CA. Impact of tricuspid valve regurgitation in patients treated with implantable left ventricular assist devices. Ann Thorac Surg. 2011;91:1342–1346; discussion 1346–1347. doi: 10.1016/j.athoracsur.2011.01.053 [DOI] [PubMed] [Google Scholar]
- 36. Zhigalov K, Szczechowicz M, Mashhour A, Kadyraliev BK, Mkalaluh S, Easo J, Ennker J, Eichstaedt HC, Weymann A. Left ventricular assist device implantation with concomitant tricuspid valve repair: is there really a benefit? J Thorac Dis. 2019;11:S902–S912. doi: 10.21037/jtd.2018.11.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Song HK, Gelow JM, Mudd J, Chien C, Tibayan FA, Hollifield K, Naftel D, Kirklin J. Limited utility of tricuspid valve repair at the time of left ventricular assist device implantation. Ann Thorac Surg. 2016;101:2168–2174. doi: 10.1016/j.athoracsur.2016.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robertson JO, Grau‐Sepulveda MV, Okada S, O'Brien SM, Matthew Brennan J, Shah AS, Itoh A, Damiano RJ, Prasad S, Silvestry SC. Concomitant tricuspid valve surgery during implantation of continuous‐flow left ventricular assist devices: a Society of Thoracic Surgeons database analysis. J Heart Lung Transplant. 2014;33:609–617. doi: 10.1016/j.healun.2014.01.861 [DOI] [PubMed] [Google Scholar]
- 39. Cowger JA, Estep JD, Rinde‐Hoffman DA, Givertz MM, Anderson AS, Jacoby D, Chen L, Brieke A, Mahr C, Hall S, et al. Variability in blood pressure assessment in patients supported with the HeartMate 3TM. ASAIO J. 2022;68:374–383. doi: 10.1097/MAT.0000000000001497 [DOI] [PubMed] [Google Scholar]
- 40. Najjar SS, Slaughter MS, Pagani FD, Starling RC, McGee EC, Eckman P, Tatooles AJ, Moazami N, Kormos RL, Hathaway DR, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33:23–34. doi: 10.1016/j.healun.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 41. Teuteberg JJ, Slaughter MS, Rogers JG, McGee EC, Pagani FD, Gordon R, Rame E, Acker M, Kormos RL, Salerno C, et al. The HVAD left ventricular assist device: risk factors for neurological events and risk mitigation strategies. JACC Heart Fail. 2015;3:818–828. doi: 10.1016/j.jchf.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 42. Maltais S, Kilic A, Nathan S, Keebler M, Emani S, Ransom J, Katz JN, Sheridan B, Brieke A, Egnaczyk G, et al. PREVENtion of HeartMate II Pump thrombosis through clinical management: the PREVENT multi‐center study. J Heart Lung Transplant. 2017;36:1–12. doi: 10.1016/j.healun.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 43. Cowger JA, Shah P, Pagani FD, Grafton G, Stulak J, Chamogeorgakis T, Lanfear D, Nemeh H, Pinney S. Outcomes based on blood pressure in patients on continuous flow left ventricular assist device support: an Interagency Registry for Mechanically Assisted Circulatory Support analysis. J Heart Lung Transplant. 2020;39:441–453. doi: 10.1016/j.healun.2019.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deshmukh A, Kim G, Burke M, Anyanwu E, Jeevanandam V, Uriel N, Tung R, Ozcan C. Atrial arrhythmias and electroanatomical remodeling in patients with left ventricular assist devices. J Am Heart Assoc. 2017;6. doi: 10.1161/JAHA.116.005340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hickey KT, Garan H, Mancini DM, Colombo PC, Naka Y, Sciacca RR, Abrams MP, Solove M, Zeoli N, Flannery M, et al. Atrial fibrillation in patients with left ventricular assist devices: incidence, predictors, and clinical outcomes. JACC Clin Electrophysiol. 2016;2:793–798. doi: 10.1016/j.jacep.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 46. Gordon JS, Maynes EJ, Choi JH, Wood CT, Weber MP, Morris RJ, Massey HT, Tchantchaleishvili V. Ventricular arrhythmias following continuous‐flow left ventricular assist device implantation: a systematic review. Artif Organs. 2020;44:E313–E325. doi: 10.1111/aor.13665 [DOI] [PubMed] [Google Scholar]
- 47. Pettit SJ, Petrie MC, Connelly DT, Japp AG, Payne JR, Haj‐Yahia S, Gardner RS. Use of implantable cardioverter defibrillators in patients with left ventricular assist devices. Eur J Heart Fail. 2012;14:696–702. doi: 10.1093/eurjhf/hfs062 [DOI] [PubMed] [Google Scholar]
- 48. Peberdy MA, Gluck JA, Ornato JP, Bermudez CA, Griffin RE, Kasirajan V, Kerber RE, Lewis EF, Link MS, Miller C, et al. Cardiopulmonary resuscitation in adults and children with mechanical circulatory support: a scientific statement from the American Heart Association. Circulation. 2017;135:e1115–e1134. doi: 10.1161/CIR.0000000000000504 [DOI] [PubMed] [Google Scholar]
- 49. Givertz MM, DeFilippis EM, Colvin M, Darling CE, Elliott T, Hamad E, Hiestand BC, Martindale JL, Pinney SP, Shah KB, et al. HFSA/SAEM/ISHLT clinical expert consensus document on the emergency management of patients with ventricular assist devices. J Heart Lung Transplant. 2019;38:677–698. doi: 10.1016/j.healun.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 50. Bruce CR, Minard CG, Wilhelms LA, Abraham M, Amione‐Guerra J, Pham L, Grogan SD, Trachtenberg B, Smith ML, Bruckner BA, et al. Caregivers of patients with left ventricular assist devices: possible impacts on patients' mortality and interagency registry for mechanically assisted circulatory support‐defined morbidity events. Circ Cardiovasc Qual Outcomes. 2017;10. doi: 10.1161/CIRCOUTCOMES.116.002879 [DOI] [PubMed] [Google Scholar]
- 51. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–1086. doi: 10.1016/j.healun.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 52. Birks EJ, Drakos SG, Patel SR, Lowes BD, Selzman CH, Starling RC, Trivedi J, Slaughter MS, Alturi P, Goldstein D, et al. Prospective multicenter study of myocardial recovery using left ventricular assist devices (RESTAGE‐HF [Remission from Stage D Heart Failure]): medium‐term and primary end point results. Circulation. 2020;142:2016–2028. doi: 10.1161/CIRCULATIONAHA.120.046415 [DOI] [PubMed] [Google Scholar]
- 53. Molina EJ, Shah P, Kiernan MS, Cornwell WK 3rd, Copeland H, Takeda K, Fernandez FG, Badhwar V, Habib RH, Jacobs JP, et al. The society of thoracic surgeons intermacs 2020 annual report. Ann Thorac Surg. 2021;111:778–792. doi: 10.1016/j.athoracsur.2020.12.038 [DOI] [PubMed] [Google Scholar]
- 54. Mehra MR, Goldstein DJ, Uriel N, Cleveland JC Jr, Yuzefpolskaya M, Salerno C, Walsh MN, Milano CA, Patel CB, Ewald GA, et al. Two‐year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018;378:1386–1395. doi: 10.1056/NEJMoa1800866 [DOI] [PubMed] [Google Scholar]
- 55. Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376:451–460. doi: 10.1056/NEJMoa1602954 [DOI] [PubMed] [Google Scholar]
- 56. Teuteberg JJ, Cleveland JC Jr, Cowger J, Higgins RS, Goldstein DJ, Keebler M, Kirklin JK, Myers SL, Salerno CT, Stehlik J, et al. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: the changing landscape of devices and indications. Ann Thorac Surg. 2020;109:649–660. doi: 10.1016/j.athoracsur.2019.12.005 [DOI] [PubMed] [Google Scholar]
- 57. Molina TL, Krisl JC, Donahue KR, Varnado S. Gastrointestinal bleeding in left ventricular assist device: octreotide and other treatment modalities. ASAIO J. 2018;64:433–439. doi: 10.1097/MAT.0000000000000758 [DOI] [PubMed] [Google Scholar]
- 58. Yavar Z, Cowger JA, Moainie SL, Salerno CT, Ravichandran AK. Bleeding complication rates are higher in females after continuous‐flow left ventricular assist device implantation. ASAIO J. 2018;64:748–753. doi: 10.1097/MAT.0000000000000734 [DOI] [PubMed] [Google Scholar]
- 59. Imamura T, Kinugawa K, Uriel N. Therapeutic strategy for gastrointestinal bleeding in patients with left ventricular assist device. Circ J. 2018;82:2931–2938. doi: 10.1253/circj.CJ-18-0883 [DOI] [PubMed] [Google Scholar]
- 60. Kataria R, Jorde UP. Gastrointestinal bleeding during continuous‐flow left ventricular assist device support: state of the field. Cardiol Rev. 2019;27:8–13. doi: 10.1097/CRD.0000000000000212 [DOI] [PubMed] [Google Scholar]
- 61. Imamura T, Nguyen A, Rodgers D, Kim G, Raikhelkar J, Sarswat N, Kalantari S, Smith B, Chung B, Narang N, et al. Omega‐3 therapy is associated with reduced gastrointestinal bleeding in patients with continuous‐flow left ventricular assist device. Circ Heart Fail. 2018;11:e005082. doi: 10.1161/CIRCHEARTFAILURE.118.005082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Juricek C, Imamura T, Nguyen A, Chung B, Rodgers D, Sarswat N, Kim G, Raikhelkar J, Ota T, Song T, et al. Long‐acting octreotide reduces the recurrence of gastrointestinal bleeding in patients with a continuous‐flow left ventricular assist device. J Card Fail. 2018;24:249–254. doi: 10.1016/j.cardfail.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shah KB, Gunda S, Emani S, Kanwar MK, Uriel N, Colombo PC, Uber PA, Sears ML, Chuang J, Farrar DJ, et al. Multicenter evaluation of octreotide as secondary prophylaxis in patients with left ventricular assist devices and gastrointestinal bleeding. Circ Heart Fail. 2017;10. doi: 10.1161/CIRCHEARTFAILURE.117.004500 [DOI] [PubMed] [Google Scholar]
- 64. Vukelic S, Vlismas PP, Patel SR, Xue X, Shitole SG, Saeed O, Sims DB, Chinnadurai T, Shin JJ, Forest SJ, et al. Digoxin is associated with a decreased incidence of angiodysplasia‐related gastrointestinal bleeding in patients with continuous‐flow left ventricular assist devices. Circ Heart Fail. 2018;11:e004899. doi: 10.1161/CIRCHEARTFAILURE.118.004899 [DOI] [PubMed] [Google Scholar]
- 65. Mehra MR, Crandall DL, Gustafsson F, Jorde UP, Katz JN, Netuka I, Uriel N, Connors JM, Sood P, Heatley G, et al. Aspirin and left ventricular assist devices: rationale and design for the international randomized, placebo‐controlled, non‐inferiority ARIES HM3 trial. Eur J Heart Fail. 2021;23:1226–1237. doi: 10.1002/ejhf.2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Netuka I, Ivak P, Tucanova Z, Gregor S, Szarszoi O, Sood P, Crandall D, Rimsans J, Connors JM, Mehra MR. Evaluation of low‐intensity anti‐coagulation with a fully magnetically levitated centrifugal‐flow circulatory pump‐the MAGENTUM 1 study. J Heart Lung Transplant. 2018;37:579–586. doi: 10.1016/j.healun.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 67. Vidula H, Kutyifa V, Johnson BA, Strawderman RL, Harrington D, Polonsky B, Papernov A, Alexis JD. Readmission patterns during long‐term follow‐up after left ventricular assist device implantation. Am J Cardiol. 2018;122:1021–1027. doi: 10.1016/j.amjcard.2018.05.037 [DOI] [PubMed] [Google Scholar]
- 68. Shah P, Birk SE, Cooper LB, Psotka MA, Kirklin JK, Barnett SD, Katugaha SB, Phillips S, Looby MM, Pagani FD, et al. Stroke and death risk in ventricular assist device patients varies by ISHLT infection category: an INTERMACS analysis. J Heart Lung Transplant. 2019;38:721–730. doi: 10.1016/j.healun.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 69. Hannan MM, Husain S, Mattner F, Danziger‐Isakov L, Drew RJ, Corey GR, Schueler S, Holman WL, Lawler LP, Gordon SM, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant. 2011;30:375–384. doi: 10.1016/j.healun.2011.01.717 [DOI] [PubMed] [Google Scholar]
- 70. Kirklin JK, Pagani FD, Goldstein DJ, John R, Rogers JG, Atluri P, Arabia FA, Cheung A, Holman W, Hoopes C, et al. American Association for Thoracic Surgery/International Society for Heart and Lung Transplantation guidelines on selected topics in mechanical circulatory support. J Thorac Cardiovasc Surg. 2020;159:865–896. doi: 10.1016/j.jtcvs.2019.12.021 [DOI] [PubMed] [Google Scholar]
- 71. Pavlovic NV, Randell T, Madeira T, Hsu S, Zinoviev R, Abshire M. Risk of left ventricular assist device driveline infection: a systematic literature review. Heart Lung. 2019;48:90–104. doi: 10.1016/j.hrtlng.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 72. Forest SJ, Xie R, Kirklin JK, Cowger J, Xia Y, Dipchand AI, Sivathasan C, Merry C, Lund LH, Kormos R, et al. Impact of body mass index on adverse events after implantation of left ventricular assist devices: an IMACS registry analysis. J Heart Lung Transplant. 2018;37:1207–1217. doi: 10.1016/j.healun.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Patel CB, Blue L, Cagliostro B, Bailey SH, Entwistle JW, John R, Thohan V, Cleveland JC Jr, Goldstein DJ, Uriel N, et al. Left ventricular assist systems and infection‐related outcomes: a comprehensive analysis of the MOMENTUM 3 trial. J Heart Lung Transplant. 2020;39:774–781. doi: 10.1016/j.healun.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 74. Bauer TM, Choi JH, Luc JGY, Weber MP, Moncho Escriva E, Patel S, Maynes EJ, Boyle AJ, Samuels LE, Entwistle JW, et al. Device exchange versus nonexchange modalities in left ventricular assist device‐specific infections: a systematic review and meta‐analysis. Artif Organs. 2019;43:448–457. doi: 10.1111/aor.13378 [DOI] [PubMed] [Google Scholar]
- 75. Bourque K, Cotter C, Dague C, Harjes D, Dur O, Duhamel J, Spink K, Walsh K, Burke E. Design rationale and preclinical evaluation of the HeartMate 3 left ventricular assist system for hemocompatibility. ASAIO J. 2016;62:375–383. doi: 10.1097/MAT.0000000000000388 [DOI] [PubMed] [Google Scholar]
- 76. Barac YD, Wojnarski CM, Junpaparp P, Jawitz OK, Billard H, Daneshmand MA, Agrawal R, Devore A, Patel CB, Schroder JN, et al. Early outcomes with durable left ventricular assist device replacement using the HeartMate 3. J Thorac Cardiovasc Surg. 2020;160:132–139.e1. doi: 10.1016/j.jtcvs.2019.09.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Miller LW, Deng M, Naka Y, et al. Impact of center volume on outcomes of left ventricular assist device implantation as destination therapy: analysis of the Thoratec HeartMate Registry, 1998 to 2005. Circ Heart Fail. 2009;2:3–10. doi: 10.1161/CIRCHEARTFAILURE.108.796128 [DOI] [PubMed] [Google Scholar]
- 78. Park SJ, Milano CA, Tatooles AJ, Rogers JG, Adamson RM, Steidley DE, Ewald GA, Sundareswaran KS, Farrar DJ, Slaughter MS, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail. 2012;5:241–248. doi: 10.1161/CIRCHEARTFAILURE.111.963991 [DOI] [PubMed] [Google Scholar]
- 79. Slaughter MS, Pagani FD, McGee EC, Birks EJ, Cotts WG, Gregoric I, Howard Frazier O, Icenogle T, Najjar SS, Boyce SW, et al. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2013;32:675–683. doi: 10.1016/j.healun.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 80. Acharya D, Loyaga‐Rendon R, Morgan CJ, Sands KA, Pamboukian SV, Rajapreyar I, Holman WL, Kirklin JK, Tallaj JA. INTERMACS analysis of stroke during support with continuous‐flow left ventricular assist devices: risk factors and outcomes. JACC Heart Fail. 2017;5:703–711. doi: 10.1016/j.jchf.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Colombo PC, Mehra MR, Goldstein DJ, Estep JD, Salerno C, Jorde UP, Cowger JA, Cleveland JC Jr, Uriel N, Sayer G, et al. Comprehensive analysis of stroke in the long‐term cohort of the MOMENTUM 3 study. Circulation. 2019;139:155–168. doi: 10.1161/CIRCULATIONAHA.118.037231 [DOI] [PubMed] [Google Scholar]
- 82. Parikh NS, Cool J, Karas MG, Boehme AK, Kamel H. Stroke risk and mortality in patients with ventricular assist devices. Stroke. 2016;47:2702–2706. doi: 10.1161/STROKEAHA.116.014049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Coffin ST, Haglund NA, Davis ME, Xu M, Dunlay SM, Cowger JA, Shah P, Aaronson KD, Pagani FD, Stulak JM, et al. Adverse neurologic events in patients bridged with long‐term mechanical circulatory support: a device‐specific comparative analysis. J Heart Lung Transplant. 2015;34:1578–1585. doi: 10.1016/j.healun.2015.08.017 [DOI] [PubMed] [Google Scholar]
- 84. Kato TS, Schulze PC, Yang J, Chan E, Shahzad K, Takayama H, Uriel N, Jorde U, Farr M, Naka Y, et al. Pre‐operative and post‐operative risk factors associated with neurologic complications in patients with advanced heart failure supported by a left ventricular assist device. J Heart Lung Transplant. 2012;31:1–8. doi: 10.1016/j.healun.2011.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nassif ME, Tibrewala A, Raymer DS, Andruska A, Novak E, Vader JM, Itoh A, Silvestry SC, Ewald GA, LaRue SJ. Systolic blood pressure on discharge after left ventricular assist device insertion is associated with subsequent stroke. J Heart Lung Transplant. 2015;34:503–508. doi: 10.1016/j.healun.2014.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Raina A, Seetha Rammohan HR, Gertz ZM, Rame JE, Woo YJ, Kirkpatrick JN. Postoperative right ventricular failure after left ventricular assist device placement is predicted by preoperative echocardiographic structural, hemodynamic, and functional parameters. J Card Fail. 2013;19:16–24. doi: 10.1016/j.cardfail.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 87. Frankfurter C, Molinero M, Vishram‐Nielsen JKK, Foroutan F, Mak S, Rao V, Billia F, Orchanian‐Cheff A, Alba AC. Predicting the risk of right ventricular failure in patients undergoing left ventricular assist device implantation: a systematic review. Circ Heart Fail. 2020;13:e006994. doi: 10.1161/CIRCHEARTFAILURE.120.006994 [DOI] [PubMed] [Google Scholar]
- 88. Rame JE, Pagani FD, Kiernan MS, Oliveira GH, Birati EY, Atluri P, Gaffey A, Grandin EW, Myers SL, Collum C, et al. Evolution of late right heart failure with left ventricular assist devices and association with outcomes. J Am Coll Cardiol. 2021;78:2294–2308. doi: 10.1016/j.jacc.2021.09.1362 [DOI] [PubMed] [Google Scholar]
- 89. Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant. 2015;34:1123–1130. doi: 10.1016/j.healun.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 90. Rich JD, Gosev I, Patel CB, Joseph S, Katz JN, Eckman PM, Lee S, Sundareswaran K, Kilic A, Bethea B, et al. The incidence, risk factors, and outcomes associated with late right‐sided heart failure in patients supported with an axial‐flow left ventricular assist device. J Heart Lung Transplant. 2017;36:50–58. doi: 10.1016/j.healun.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 91. Yancy CW, Januzzi JL Jr, Allen LA, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Jessup M, Lindenfeld J, Maddox TM, et al. 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;71:201–230. doi: 10.1016/j.jacc.2017.11.025 [DOI] [PubMed] [Google Scholar]
- 92. Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, et al. Use of a continuous‐flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758 [DOI] [PubMed] [Google Scholar]
- 93. Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, Conte JV, Bogaev RC, MacGillivray TE, Naka Y, et al. Extended mechanical circulatory support with a continuous‐flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–321. doi: 10.1016/j.jacc.2009.03.055 [DOI] [PubMed] [Google Scholar]
- 94. John R, Naka Y, Smedira NG, Starling R, Jorde U, Eckman P, Farrar DJ, Pagani FD. Continuous flow left ventricular assist device outcomes in commercial use compared with the prior clinical trial. Ann Thorac Surg. 2011;92:1406–1413; discussion 1413. doi: 10.1016/j.athoracsur.2011.05.080 [DOI] [PubMed] [Google Scholar]
- 95. Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, Jessup ML, Gregoric ID, Loyalka P, Frazier OH, et al. Use of an intrapericardial, continuous‐flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125:3191–3200. doi: 10.1161/CIRCULATIONAHA.111.058412 [DOI] [PubMed] [Google Scholar]
- 96. Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC Jr, Colombo PC, Walsh MN, Milano CA, Patel CB, Jorde UP, et al. A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med. 2017;376:440–450. doi: 10.1056/NEJMoa1610426 [DOI] [PubMed] [Google Scholar]
- 97. Goldstein DJ, Naka Y, Horstmanshof D, Ravichandran AK, Schroder J, Ransom J, Itoh A, Uriel N, Cleveland JC Jr, Raval NY, et al. Association of clinical outcomes with left ventricular assist device use by bridge to transplant or destination therapy intent: the multicenter study of maglev technology in patients undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) randomized clinical trial. JAMA Cardiol. 2020;5:411–419. doi: 10.1001/jamacardio.2019.5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


