Abstract
Because the use of live attenuated mutants of Shigella spp. represents a promising approach to protection against bacillary dysentery (M. E. Etherridge, A. T. M. Shamsul Hoque, and D. A. Sack, Lab. Anim. Sci. 46:61–66, 1996), it becomes essential to rationalize this approach in animal models in order to optimize attenuation of virulence in the vaccine candidates, as well as their route and mode of administration, and to define the correlates of protection. In this study, we have compared three strains of Shigella flexneri 5—the wild-type M90T, an aroC mutant, and a double purE aroC mutant—for their pathogenicity, immunogenicity, and protective capacity. Protection against keratoconjunctivitis, induced by wild-type M90T, was used as the protection read out in guinea pigs that were inoculated either intranasally or intragastrically. Following intranasal immunization, the aroC mutant elicited weak nasal tissue destruction compared to M90T and achieved protection correlated with high levels of local anti-lipopolysaccharide immunoglobulin A (IgA), whereas the purE aroC double mutant, which also elicited weak tissue destruction, was not protective and elicited a low IgA response. Conversely, following intragastric immunization, only the M90T purE aroC double mutant elicited protection compared to both the aroC mutant and the wild-type strain. This mutant caused mild inflammatory destruction, particularly at the level of Peyer's patches, but it persisted much longer within the tissues. This could represent an essential parameter of the protective response that, in this case, did not clearly correlate with high anti-lipopolysaccharide IgA titers.
Shigella flexneri is the major etiological agent of endemic bacillary dysentery, a severe form of diarrhea responsible for approximately 1 million fatalities annually (20). The disease is characterized by bacterial invasion of colonic epithelial cells, leading to an intense inflammatory response with severe destruction of the colonic mucosa (22). The invasion process requires the concerted action of specific virulence factors encoded by a plasmid (41, 45) and chromosomally encoded metabolic functions necessary for the bacteria to survive within the host (3, 34). In ligated intestinal loops of rabbits, early entry of S. flexneri occurs via M cells of the follicle-associated epithelium of Peyer's patches (PPs) (51, 44). Similarly, after intragastric (i.g.) inoculation of macaques, S. flexneri uses lymphoid structures associated with the colonic and rectal mucosae as its primary site of entry (43). Once the lymphoid structures have been reached, bacteria encounter resident macrophages that are rapidly killed by apoptosis, due to the ability of IpaB, a 62-kDa bacterial invasin, to activate caspase 1 (14, 55, 56). The death of the macrophages may facilitate bacterial survival in these tissues, but it causes a substantial release of interleukin-1β (IL-1β), a cytokine that elicits early inflammation (35) from the dying macrophages (57). Immigration of polymorphonuclear cells (PMNs), recruited to the site of infection, disrupts intercellular junctions between epithelial cells and thereby facilitates bacterial access to the basolateral side of the epithelium where bacteria can efficiently invade (29, 36). This invasion eventually results in extensive inflammation and tissue destruction of the villous epithelium adjacent to lymphoid follicles (35, 43).
Our current understanding of Shigella infection mechanisms is at a stage in which identification and characterization of the major factors of the invasion process must be integrated into a scheme that can be established only by using in vivo models of infection. This integration is essential for design and improvement of live attenuated vaccine candidates. However, in vivo studies are complicated by the relatively innate resistance of most animals to oral infection by S. flexneri (24), which impairs the coordinated analysis of invasion, inflammation, and immunity. In studies aimed at exploring protection against shigellosis, Shigella vaccines are often evaluated using methods of inoculation that differ from the normal route of infection (11). A murine model based on intranasal (i.n.) infection has been developed and used in various protection experiments (25). Virulent shigellae invade murine bronchial and alveolar epithelia, where they elicit both acute suppurative infiltrates and epithelial necrosis (26, 49). Mice immunized with wild-type S. flexneri strains or attenuated vaccine strains exhibit partial protective immunity to subsequent i.n. challenges with a lethal Shigella inoculum (37). The ability of Shigella spp. to invade the corneal epithelium of guinea pigs, rabbits, and mice, spread to contiguous cells, and cause keratoconjunctivitis (i.e., the Sereny test [46]) has also become a major model system. This model has been used to test potential vaccine candidates for attenuation (12, 13, 47) and to evaluate vaccine efficacy (11, 15, 30, 32). Nevertheless, the oral route has also been used to administer Shigella strains and vaccine candidates to various animals (18, 24, 31, 54) to stimulate local protective immunity that reflects the natural route of infection. Despite this variety of approaches, the response of the immune system to Shigella infection and its consequences on both bacterial survival and tissue alteration and repair are still not known in detail.
The purpose of this study is to analyze the relationships that exist between invasiveness, inflammation, and immunogenicity in order to define guidelines that would allow the construction of Shigella vaccine candidates. With this aim we have evaluated the impact of both the route of infection and the intensity of the innate immune response on the development of adaptive immune responses, using the guinea pig model. Infection and vaccination were performed using two routes of administration: the i.n. route to study the immune potential and pathogenicity of shigellae in the respiratory tract and the i.g. route to explore the effect of Shigella invasion under conditions nearest to those that occur during natural infection. To manipulate the degree of innate immune responses, strains of Shigella with increasing degrees of attenuation were used: the wild-type strain M90T and two strains with mutations in metabolic functions necessary for bacterial growth in tissues, M90T aroC, which is auxotrophic for p-aminobenzoic acid (PABA), and M90T purE aroC, which is auxotrophic for PABA and adenine. These three strains were analyzed for invasion ability, induction of inflammation, and immunogenicity in guinea pigs. Our strategy involved the i.n. or i.g. immunization of one set of guinea pigs to evaluate the immunogenicity and the protective efficacy of M90T, M90T aroC, and M90T purE aroC by using the Sereny test and the i.n. or i.g. infection of another set of guinea pigs to correlate the immune response to the actual degree of pathogenicity and reactogenicity of the strains. These parameters were evaluated by monitoring (i) the ability of mutant strains to colonize different tissues, (ii) the persistence of bacteria within these tissues, and (iii) the histopathological and immunohistochemical analysis of infected tissues. Our results show that the ability to induce a protective immune response depends on the immunization route, which indicates that both persistence of bacteria in infected tissues and low reactogenicity are the major factors conferring protective immunity.
MATERIALS AND METHODS
Bacterial strains and media.
Vaccine strains used in this study are listed in Table 1. The wild-type S. flexneri 5 strain M90T and Escherichia coli 395.1 were routinely grown in Trypticase soy broth (Becton Dickinson and Co., Cockeysville, Md.) or Trypticase soy broth agar (TCSA) (Difco Laboratories, Detroit, Mich.). M90T aroC::Tn10 and M90T ΔpurE aroC::Tn10 mutants were also cultured in brain heart infusion (Bacto BHI; Difco). TCSA containing 100 mg of Congo red dye per liter was used to select invasive clones of Shigella spp. (28). When necessary, tetracycline was added at a concentration of 10 μg/ml.
TABLE 1.
Vaccine strains used and relevant characteristics
| Strain | Relevant characteristics | Growth requirement | Reference |
|---|---|---|---|
| M90T | Shigella flexneri 5 wild-type strain harboring 220-kb invasion plasmid | Nicotinic acid | 41 |
| M90T aroC::Tn10 (M90T aroC) | M90T derivative | Nicotinic acid, Phe, Trp, Tyr, p-aminobenzoate, 2,3-dihydroxybenzoate | 3 |
| M90T ΔpurE aroC::Tn10 (M90T purE aroC) | M90T derivative | Nicotinic acid, Phe, Trp, Tyr, p-aminobenzoate, 2,3-dihydroxybenzoate, adenine | 3 |
| E. coli 395.1 | E. coli K-12 | 42 |
Animals.
Female Hartley guinea pigs weighing approximately 400 g were used for infection and immunization experiments. The animals were housed in individual cages.
Immunizations.
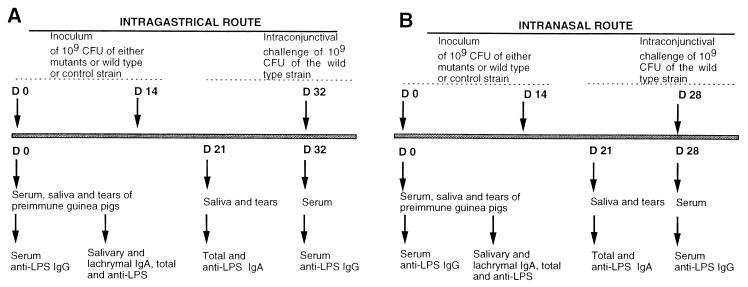
Guinea pigs were anesthesized with 20 mg of ketamine per liter (Imalgène 1000; Rhone Mérieux, Lyon, France) and immunized i.n. or i.g. with 109 microorganisms harvested from overnight-growth plates. A total of 10 animals were immunized for each strain (M90T, M90T aroC::Tn10, M90T ΔpurE aroC::Tn10, and E. coli 395.1). The immunization schemes are shown in Fig. 1A and B.
FIG. 1.
Immunization schedules (A) via the i.g. route and (B) via the i.n. route. Upper arrows of each schedule indicate the days of immunization and the day of challenge with the wild-type strain. The first row of lower arrows indicates the days of bleeding and the days when saliva and tears were collected while the second row indicates when IgG and IgA analyses were performed. Inoculation and challenge doses are also indicated.
(i)i.g. immunization.
Approximately 0.5 ml of bacterial cell suspension was delivered into the stomach of a guinea pig through a 1-mm-diameter polyethylene feeding tube inserted into the esophagus, immediately after administration by the same route of about 0.5 ml of 1.4% sodium bicarbonate in order to buffer gastric acidity. Animals were immunized twice (day 0 and day 14). At days 0 and 32, sera were collected from a foot vein. At days 0 and 21, samples of saliva were recovered as follows: saliva was collected with a wick tampon (Polyfiltronics Group Inc., Rockland, Mass.) left in the mouth for 3 min for full absorption. The wicks were then soaked in ice, weighed, dipped in a 0.5-ml solution of phosphate-buffered saline (PBS) containing 5% milk and protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, and 0.5 μg of leupeptin per ml, all products of Sigma Chemical Co., St. Louis, Mo.) (4). Subsequently, the samples were vortexed and centrifuged for 2 min at 12,700 × g. Finally, the liquid phase was collected and stored at −80°C.
(ii) i.n. immunization.
Before immunization, animals were treated as described above; then a suspension of 109 bacteria in 0.1 ml was applied dropwise to the nares (0.05 ml for each nare). At days 0 and 28, sera were collected from a foot vein, whereas at days 0 and 21, samples of saliva were removed as specified above.
(iii) Intranconjunctival challenge.
At days 32 and 28 for i.g. and i.n. administration, respectively, guinea pigs were challenged with 0.01 ml of an M90T suspension containing 109 bacteria. The suspension was deposited into the conjunctival sac of one eye using a dropper, and lids were slightly massaged to ensure that the inoculum was distributed over the entire eye. Following challenge with M90T, the animals were inspected daily for 7 days for the development of keratoconjunctivitis. The degree of keratoconjunctivitis was rated on the basis of time of development, severity, and (when possible) rate of clearance of the symptoms, using the following scheme: 0, no disease; 1, mild conjunctivitis; 2, keratoconjunctivitis with no purulence; and 3, fully developed keratoconjunctivitis with purulence (11). The percentage of protection was defined as follows: full protection, percentage of eyes with rating of 0; partial protection, percentage of eyes with rating of 1.
Infections.
Nine guinea pigs were infected i.n. or i.g. using each strain of bacteria (M90T, M90T aroC::Tn10, and M90T ΔpurE aroC::Tn10). One untreated animal was sacrificed, and its relevant tissues were used as a control.
(i) i.g. infection.
Animals were infected i.g. with a suspension of 109 bacteria following the procedure described for i.g. immunization. At days 1, 3, and 7, three animals were sacrificed for each bacterial strain. Samples of liver and PPs were removed and prepared for both histopathological analysis and counts of viable bacteria. Briefly, relevant intestinal segments were dissected longitudinally, opened on their mesenteric side, and extensively washed with PBS. Four to seven PPs for each animal were punched off the tissue by using a 4-mm-diameter skin biopsy punch (Stiefel, Nanterre, France). Most of the overlying mucus was peeled off by direct contact with absorbing paper, followed by several washings with PBS. The liver was removed, weighed, and cut, and the sections were kept for further investigation. For histopathological analysis, PPs and liver samples were fixed in 4% formalin, whereas for bacterial counts, saline buffer was added to samples, and they were immediately stored in ice until further manipulation.
(ii) i.n. infection.
At days 1, 3, and 7, three animals were sacrificed for each bacterial strain. Nasal mucosa or alternatively the nasal parts of the head in addition to the parotid glands and lungs were removed for both histopathological analysis and bacterial counts. Briefly, the nasal section was cut off along the line under the eyes, and the tip of the nose was removed after stripping the skin from the head. For bacterial counts, nasal mucosa was removed from the nasal cavities. For histopathological analysis the nasal section was stored in a solution of 4% formalin and 5% trichloroacetic acid. Parotid glands were removed by excision of the skin in the posterolateral section of each ear, and the lungs were removed from the thoracic cavity. For histopathological analysis, parotid glands and lungs were fixed in 4% formalin, whereas for bacterial counts saline buffer was added, and the samples were immediately stored in ice.
Bacterial counts in tissue samples.
Tissue samples in ice-cold 0.9% NaCl (10 ml for lungs, 5 ml for nasal mucosa, parotids, and lungs, and 1 ml for each PP) were ground with an Ultraturrax apparatus (Janke and Kunkel, GmbH and Co., Staufen, Germany). Serial dilutions of the resulting solutions were plated on TCSA with or without tetracycline added and were incubated overnight at 37°C. Bacterial counts were normalized to the dilution factor and to the weight of the sample.
Histopathological analysis.
After treatment with formalin, samples were dehydrated, embedded in paraffin, and sectioned in 5-μm-thick slices at various levels. Cuts were stained with hematoxylin-eosin or immunostained for observation. Damage to the architecture of the intestinal villi was evaluated by the index of intestinal atrophy, which involved measurement of the length of a villus divided by its width (L/W ratio). About 80 villi were analyzed, their length and width were recorded, and the L/W ratio was calculated. For the guinea pig control, the mean L/W ratio was 5.59.
Immunocytochemistry was performed as follows: the sections were deparaffinated and rehydrated, and endogenous peroxidases were blocked by 0.3% hydrogen peroxide in methanol. Bacterial lipopolysaccharide (LPS) was labeled using a biotinylated primary mouse monoclonal antibody (immunoglobulin G3 [IgG3], kappa chain) directed against the S. flexneri serotype 5 somatic antigen at a concentration of 5 μg/ml (37, 44). The preparations were incubated overnight at 4°C. The reaction was amplified by using a Vectastain ABC kit (Vector Laboratories, Inc., Burlingame, Calif.). Staining was obtained by incubation with the substrate chromogen (DAB) solution (3-3′ diaminobenzidine tetrahydrochloride-H2O2 [Vector Laboratories, Inc.]). Counterstaining was obtained with Harris hematoxylin (Merck & Co., Rahway, N.J.). Final mounting was done on glycerol gel (Dako Corp., Carpinteria, Calif.).
Serum and saliva analysis.
An enzyme-linked immunosorbent assay (ELISA) was used to quantitate serum and salivary IgG and IgA against LPS.
A solution containing 10 μl of S. flexneri 5 purified LPS per ml of coating buffer (20 mM Na2CO3 [pH 9.6]) was used to coat 96-well microtiter plates (Immunoplates F96, Maxi Sorp; Nunc, Roskilde, Denmark) (100 μl per well) overnight at room temperature. The plates were washed with PBS–0.05% Tween 20, covered with a filler solution (1% bovine serum albumin in washing buffer), and incubated at room temperature for 1 h. The solution was aspirated, and the plates were washed with washing buffer. Guinea pig sera or saliva, serially diluted in filler solution, was added to the wells and incubated 1 h at 37°C. After this time and following washes with washing buffer, the plates were treated separately for the dosage of IgA or IgG.
For anti-LPS IgA, the plates were incubated with a sheep primary antibody specific for guinea pig IgA (Bethyl Lab. Inc., Montgomery, Tex.), for 1 h at 37°C at a concentration of 1:1,000. After washing, a horseradish peroxidase (HRP)-conjugated donkey secondary-antibody anti-sheep IgG (heavy and light chains) (Bethyl Lab. Inc.) was added at a dilution of 1:5,000 and left 1 h at 37°C.
Total IgA was evaluated as follows. First, a rabbit anti-guinea pig IgA (α chain) (Bethyl Lab. Inc.) was used at a concentration of 1:2,000 to coat the wells; then different dilutions of saliva were added and IgA was quantitated with a sheep primary antibody anti-guinea pig IgA (1:1,000 concentration). The HRP donkey anti-sheep IgG (heavy and light chains) (1:5,000) (Bethyl Lab. Inc.) was used again in the revelation step.
For anti-LPS IgG, an HRP-conjugated sheep primary antibody against guinea pig IgG (heavy and light chains) (Bethyl Lab. Inc.) was used at a dilution of 1:10,000.
After these treatments ELISA plates were washed again and developed with O-phenylenediamine (Sigma Chemical Co.) (0.3 mg/ml in citrate buffer [pH 5.6]) and H2O2 (0.03%). The reaction was blocked with H2SO4 (4 N), and the absorbance was read at 490 nm in an ELISA reader (model MR 4000; Dynatech Laboratories, Inc., Alexandria, Va.). The titers were defined as the reciprocal of the last dilution having an optical density at 490 nm of 0.1 or more.
Statistical analysis.
The attack rates for keratoconjunctivitis in vaccinated guinea pigs and controls following conjunctival sac challenge were compared by Fisher's exact test. An unpaired Student's t test was used to compare the mean value of CFU of bacteria recovered from infected tissues and the significance of the differences between the two immunization routes. The Mann-Whitney U test was performed to compare the mean titers of antibody elicited by the vaccine strains. The values were considered statistically significant for P < 0.05, whereas values of 0.05 < P < 0.09 were considered to be of borderline significance.
RESULTS
Immunizations.
Guinea pigs were immunized with S. flexneri 5 wild-type strain M90T, the auxotrophic mutants M90T aroC and M90T purE aroC, and E. coli strain 395.1, following the protocols described in Materials and Methods.
M90T aroC is an attenuated mutant in which auxotrophy for aromatic amino acids results in a negative Sereny test (3). In the plaque assay, which is an in vitro test that measures the ability of virulent shigellae to form areas of necrosis on a confluent HeLa cell monolayer, M90T aroC induces a cytopathic effect that is far weaker than that of the wild-type strain. Its intracellular generation time is longer than that of M90T (119 min versus 75 min) (3). M90T purE aroC, which harbors the adenine auxotrophy mutation (purE) in addition to the aroC mutation, is severely attenuated. It was found to be negative in the plaque assay and dramatically retarded in intracellular multiplication (intracellular generation time, 302 min), and it did not elicit a positive Sereny test (3). Although the ΔpurE mutant was only weakly attenuated (3), the combination of this mutation with either the aroC or aroD deletion further impaired the virulence of these strains (3). E. coli 395.1 was used as a negative control in all immunization trials. Animals were immunized following the schedule shown in Fig. 1. The Sereny test was used to evaluate the protective efficacy and immunogenicity of the vaccine candidates.
Normally, from 96 h postchallenge onwards, the severity of symptoms decreased up to complete clearance. Therefore, only data relative to this period are shown. Results are summarized in Table 2.
TABLE 2.
Immune protection of guinea pigs immunized i.g. or i.n. against the intraconjunctival challenge with the wild-type S. flexneri 5 strain M90T
| Immunization strain | No. of animals inoculated | No. of animals showing the following ratingsa at hours p.i.
|
Protectionb %
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 48 h
|
72 h
|
96 h
|
|||||||||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | Full | Partial | ||
| i.g. routec | |||||||||||||||
| M90T | 10 | 1 | 4 | 5 | 0 | 1 | 4 | 5 | 0 | 1 | 4 | 5 | 0 | 10 | 40 |
| M90T aroC::Tn10 | 10 | 0 | 3 | 7 | 0 | 0 | 2 | 8 | 0 | 0 | 1 | 9 | 0 | 0 | 20 |
| M90T ΔpurE aroC::Tn10 | 10 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 100 | 0 |
| E. coli 395.1 | 10 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 9 | 1 | 0 | 0 |
| i.n. routed | |||||||||||||||
| M90T | 10 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 100 | 0 |
| M90T aroC::Tn10 | 10 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 100 | 0 |
| M90T ΔpurE aroC::Tn10 | 10 | 10 | 0 | 0 | 0 | 4 | 2 | 2 | 2 | 4 | 3 | 3 | 0 | 40 | 20 |
| E. coli 395.1 | 10 | 0 | 0 | 8 | 2 | 0 | 0 | 1 | 9 | 0 | 0 | 1 | 9 | 0 | 0 |
Reaction ratings: 0, normal; 1, palpebral edema with conjunctival hyperemia; 2, conjunctival hyperemia, plus slight exudate; 3, full-blown purulent keratoconjunctivitis. Vaccine significance efficacy was evaluated by Fisher's exact test.
Protection was evaluated at 72 h postchallenge and was defined as follows: full, percentage of eyes with rating of 0; partial, percentage of eyes with rating of 1.
i.g. route vaccine efficacy significance: M90T, full protection: P = 0.0909; partial protection: P = 0.0010; aroC, partial protection: P = 0.0152; M90T ΔpurE aroC::Tn10, full protection: P < 0.0001.
i.n. route vaccine efficacy significance: M90T, full protection: P = 0.0003; aroC, full protection: P = 0.0003; M90T ΔpurE aroC::Tn10, full protection: P = 0.0606.
i.n. immunization with M90T or M90T aroC provided significant protection against a challenge with M90T (full protection, 100%), whereas only low levels of protection were obtained in animals vaccinated with M90T purE aroC (full protection, 40%). There was no protection, either partial or full, against M90T in animals immunized i.g. or i.n. with E. coli 395.1.
In contrast, among animals immunized i.g., only those treated with the M90T purE aroC mutant exhibited full protection against challenge with the virulent homologous strain (full protection, 100%). Surprisingly, wild-type M90T conferred only moderate protection (full protection, 10%; partial protection, 40%), whereas the aroC mutant elicited no significant protection (partial protection, 20%). There was no protection, either partial or full, against M90T in animals immunized i.g. or i.n. with E. coli 395.1.
Antibody response against M90T somatic antigen.
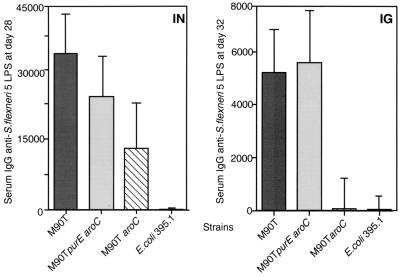
Immunogenicity of the vaccine candidates was measured by determining the levels of serum IgG and saliva IgA directed against S. flexneri 5 LPS. All guinea pigs showed null antibody titers against S. flexneri before vaccination (data not shown). Approximately 2 weeks after the second immunization (at days 32 and 28 for i.g. and i.n. immunization, respectively), animals were bled and the serum IgG response against LPS was evaluated by ELISA. At day 21 postinfection (p.i.), saliva was collected from animals, and anti-LPS IgA levels were measured. Results shown in Fig. 2 and Table 3 demonstrate significant differences in the levels of antibodies elicited by the two different immunization routes in that higher levels of both IgG and IgA were observed in animals vaccinated i.n. than in those vaccinated i.g. (P = 0.0034 for IgG i.n. versus IgG i.g., and P < 0.0001 for IgA i.n. versus IgA i.g.). At day 21, tears were also collected, and anti-LPS IgA levels in the tears were measured. The results obtained were comparable to those observed in saliva (data not shown).
FIG. 2.
Serum IgG titers against S. flexneri 5 LPS from animals immunized i.n. (IN) and i.g. (IG) at days 28 and 32 p.i., respectively. Data represent the geometric mean titers for each group of guinea pigs immunized i.n. or i.g. with M90T, M90T aroC, M90T purE aroC, and E. coli 395.1 Standard deviations (SDs) are shown. Preimmune serum IgG titers against S. flexneri 5 LPS were <50. The titers were defined as the reciprocal of the last dilution having an optical density at 490 nm of 0.1 or more.
TABLE 3.
Salivary IgA titers from animals immunized either i.g. or i.n. and challenged intraconjunctivally
| Strain | Titers (±SD) after type immunization
|
|||
|---|---|---|---|---|
| i.g.
|
i.n.
|
|||
| Total salivary IgA | Anti-LPS IgA/total IgA | Total salivary IgA | Anti-LPS IgA/total IgA | |
| M90T | 11,440 ± 4,894 | 5.41 | 3,001 ± 827 | 87.51 |
| M90T aroC::Tn10 | 16,256 ± 1,316 | 0 | 3,199 ± 1,216 | 68.32 |
| M90TΔpurE aroC::Tn10 | 28,217 ± 3,831 | 1.71 | 7,265 ± 1,126 | 30.28 |
| E. coli 395.1 | 2,363 ± 1,033 | 0 | 2,474 ± 897 | 0 |
As shown in Fig. 2, in i.n. trials, M90T purE aroC conferred only low protection compared to M90T, but the IgG response induced in animals immunized with this strain was comparable to that induced by M90T (P = 0.2475 for M90T purE aroC versus M90T). Despite the high total salivary IgA titer found with M90T purE aroC, the percentage of specific IgA against LPS was lower for M90T purE aroC than for M90T (P = 0.0286 for M90T purE aroC versus M90T) and aroC (P = 0.0190 for M90T purE aroC versus M90T aroC). Results are summarized in Table 3.
In i.g. immunizations, only M90T and M90T purE aroC induced significant levels of serum anti-LPS IgG. Also, in this case there was no significant difference (P = 0.3930 for M90T purE aroC versus M90T) between serum anti-LPS IgG titers measured in animals immunized with M90T purE aroC, which induced full protection, and M90T, which induced only partial protection. Salivary anti-LPS IgA was markedly low; only IgA responses elicited by M90T and M90T purE aroC appeared significant and reached a detectable level of anti-LPS specificity (5.41 and 1.71 for M90T and M90T purE aroC, respectively). It must be stressed that animals immunized with M90T were 10% fully and 40% partially protected, whereas those treated with M90T purE aroC were 100% protected. Therefore, no significant correlation was found between high salivary anti-LPS IgA titers and protection. All results are shown in Fig. 2 and in Table 3.
Guinea pigs immunized either i.n. or i.g. with the E. coli 395.1 control strain showed no significant antibody titers against LPS.
Infections.
The results obtained in immunization trials indicated that the vaccine strains demonstrated different capabilities for inducing protective immune responses depending on the immunization route. This finding suggested that the bacterial strains behaved differently in different host tissues. We reasoned that the probability of inducing an adaptive immune response relied on various factors including the ability of bacteria to colonize the host, to persist within an infected tissue, and to induce, to various degrees, an inflammatory response. To address these major points, M90T, M90T aroC, and M90T purE aroC were used to infect another pool of guinea pigs, either i.n. or i.g., and the ability of these strains to persist, disseminate, and induce an inflammatory response was then evaluated. Our aim was to compare the behavior of these strains in the tissues they infected in an attempt to detect an association between a specific bacterial trait in the host and the capacity to induce a protective immune response.
Two sets of guinea pigs were infected and sacrificed at different times p.i. Three animals for each strain in both infection protocols were sacrificed on days 1, 3, and 7, respectively. To measure the extent of bacterial dissemination in the host, several organs and tissues were processed. Briefly, nasal mucosa, parotids, and lungs were processed for animals infected i.n., and PPs and liver were processed for those challenged i.g. The number of bacteria was evaluated, and immunohistopathological analysis was performed. This analysis allowed us to monitor three essential parameters: bacterial colonization, dissemination, and persistence.
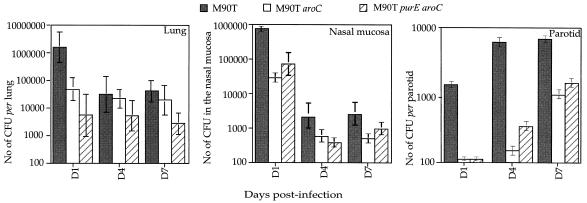
In the intranasally infected animals, all bacterial strains tested reached the lungs, where they persisted for at least 7 days and disseminated into the parotids, as shown in Fig. 3. Although the number of bacteria decreased from day 3, approximately 4 × 104 bacteria were isolated on day 7 from the lungs of animals infected with M90T. In the nasal mucosa the number of bacteria from animals infected with M90T dramatically decreased during the 7 days of observation, whereas in parotid glands the number of bacteria from all infected animals increased during the same period. M90T colonized the tissues more efficiently than the other strains, and bacterial counts from animals infected with M90T purE aroC were the lowest.
FIG. 3.
Shigella survival and dissemination in lungs, nasal mucosa, and parotids in guinea pigs infected i.n. with M90T, M90T aroC, and M90T purE aroC at days 1, 4 and 7 p.i. Data shown represent the mean number of CFU calculated per lung, within the nasal mucosa and per parotid glands. SDs are shown.
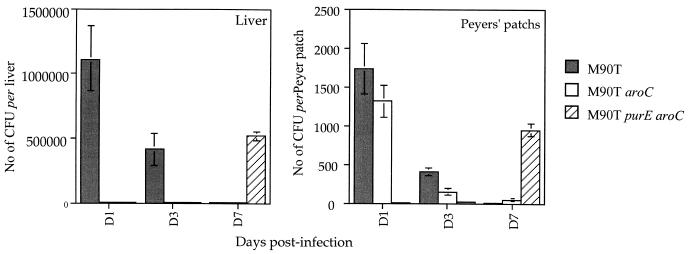
In animals infected i.g., both M90T and M90T purE aroC reached the liver whereas M90T aroC failed to do so, as shown in Fig. 4. The main difference was that M90T was found in the liver at days 1 and 3 and disappeared at day 7, whereas M90T purE aroC was found in the liver only on day 7. The same kinetics were observed for the PPs, where M90T and M90T aroC were recovered at days 1 and 3 and cleared at day 7. In contrast, M90T purE aroC accumulated in the PPs and was observed only on day 7. M90T aroC behaved in a manner similar to M90T, although they gave lower bacterial counts.
FIG. 4.
Shigella survival and dissemination in PPs and liver in guinea pigs infected i.g. with M90T, M90T aroC, and M90T purE aroC at days 1, 4 and 7 p.i. Data shown represent the mean number of CFU calculated per liver and per PP. SDs are shown.
Briefly, in the i.n. model, all three bacterial strains disseminated in the tissues that were investigated, where they persisted for 7 days, differing only in their ability to colonize the infected organs. In the i.g. model, the results of dissemination, colonization, and persistence differed for each strain. M90T colonized better than the other strains, but it was cleared at day 7, as was M90T aroC. In contrast to M90T, M90T purE aroC was barely present at days 1 and 3, but it persisted and multiplied at day 7. All three strains were isolated from PPs, but only M90T and M90T purE aroC disseminated into the liver.
Histopathology and immunohistochemistry performed on intestinal tissues of animals infected intragastrically.
Histopathological analysis was performed on tissue sections removed from the intestines of animals sacrificed at days 1, 3, and 7. The sections usually included PPs and the surrounding area containing villi.
To perform a quantitative evaluation of the damage induced by the presence of bacteria within the intestine, we measured the L/W ratio of the villi. Changes in this ratio are provoked by intestinal atrophy subsequent to Shigella infection. About 80 villi per strain per day p.i. were examined, their lengths and widths were recorded, and the ratio was calculated. Data obtained and statistical analysis are shown in Table 4.
TABLE 4.
L/W ratio of animals infected i.g.
| Strain | L/W ratio at day p.i
|
Control | |
|---|---|---|---|
| 1a | 7b | ||
| M90T | 1.99 ± 0.680 | 3.58 ± 1.03 | |
| M90T aroC | 3.37 ± 0.919 | 4.62 ± 0.830 | |
| M90T purE aroC | 4.50 ± 0.761 | 5.06 ± 0.567 | 5.59 ± 1.02 |
P < 0.0001 (M90T purE aroC versus M90T); P = 0.0737 (M90T aroC versus M90T) (Student's t test).
P = 0.0476 (M90T purE aroC versus M90T); P = 0.1950 (M90T aroC versus M90T) (Student's t test).
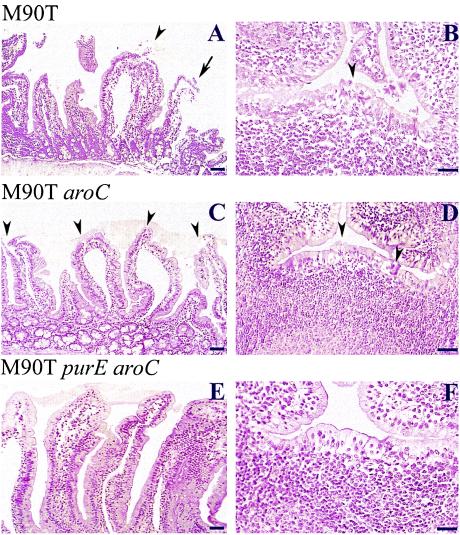
The typical villus from animals infected after 1 day with M90T is shown in Fig. 5A. At day 1 p.i., the villus architecture was severely altered with intestinal atrophy (L/W ratio = 1.99 ± 0.68), depletion of goblet cells, focal abscesses (arrowheads) and areas of detachment as well as destruction of the epithelial lining reflecting extensive epithelial necrosis (arrow). The lamina propria was characterized by the presence of a massive inflammatory infiltrate (mostly PMNs) causing edema and hemorrhagic foci. PPs were also altered (Fig. 5B), their follicle-associated epithelium being infiltrated by the inflammatory reaction that occurred in the dome area. In flares (arrowhead), the epithelium was even disrupted, and ulcers leaking inflammatory cells could be observed.
FIG. 5.
Hematoxylin-eosin staining of tissue sections corresponding to villi (A, C, and E) and PPs (B, D, and F) from guinea pigs infected with M90T (A and B), M90T aroC (C and D) and M90T purE aroC (E and F) at day 1 p.i. In panels A and C, arrowheads point to focal abscesses. In panels B and D, arrowheads define areas of epithelium destruction where PMNs are streaming into the lumen. In panel A, the arrow points to an area of epithelium necrosis. Bars, 50 μm (A, C and E) and 20 μm (B, D and F).
M90T aroC also caused significant intestinal atrophy at day 1 with an L/W ratio of 3.37 ± 0.919. Figure 5C shows massive release of mucus and shortening of villi, with indentation of the epithelium reflecting the presence of focal subepithelial inflammatory infiltrates as well as ulcerated zones (arrowheads). The lamina propria appeared infiltrated as well as dilated, following development of the inflammatory infiltrates. Similarly, PPs (Fig. 5D) appeared altered with infiltrates and, in some areas, rupture of the follicle-associated epithelium (arrowheads). The severity of these lesions, however, was in general less dramatic than that observed with wild-type M90T, reflecting attenuation in response to the aroC mutation. M90T purE aroC, in general, caused very limited alteration of the intestinal mucosa. Intestinal atrophy was barely observed at day 1, with an L/W ratio of 4.5 ± 0.76. As shown in Fig. 5E, villi appeared elongated, with their width being slightly increased due to lamina propria edema in response to a clear but limited inflammatory infiltrate. In PPs (Fig. 5F), although the follicular-associated epithelium appeared somewhat infiltrated by inflammatory cells, neither abscesses nor ulcerations were observed.
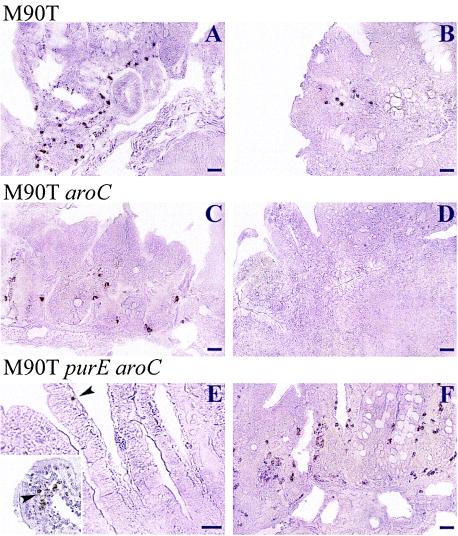
We then attempted to correlate the persistence of bacteria or bacterial material (i.e., through LPS staining) in infected intestinal tissues with the quality of immune protection obtained by the wild-type strain M90T and the M90T mutants aroC and purE aroC. Immunostaining for LPS was performed on sections from tissues obtained at day 1, 3, or 7 p.i. Figure 6 shows typical examples from villi and PPs. Whereas a significant amount of bacterial material was observed in severely altered tissues with M90T at day 1 (Fig. 6A), in agreement with the strong invasive capacity of this wild-type strain, only a limited amount of bacterial material could be detected after 7 days (Fig. 6B). With regard to M90T aroC, at day 1 (Fig. 6C) a significant amount of bacterial material was observed to be associated with the tissues. However, at day 7, all detectable bacterial material had been cleared (Fig. 6D). Although limited amounts of bacterial material were observed at day 1 p.i. with M90T purE aroC (Fig. 6E), mainly associated with the tip of the villi, it is striking that at day 7 p.i., large amounts of bacterial material could be detected, essentially in the deep portion of PPs and in the vicinity of crypts (Fig. 6F). These results are in agreement with the bacterial counts shown in Fig. 4, indicating that the double mutant has the capacity to survive and eventually to grow to significant numbers within 7 days. These data also suggest that the immunostaining corresponds not only to bacterial material but also to live bacteria.
FIG. 6.
Immunoperoxidase labeling of serotype 5 somatic antigen by anti-LPS monoclonal immunoglobulin G. (A, C, and E) Sections of PPs (A and C) and villi (E) from animals infected i.g. at day 1 p.i; (B, D, and F) sections of PPs of animals at day 7 p.i. (A and B) Sections from guinea pigs infected with M90T; (C and D) sections from guinea pigs infected with M90T aroC; (E and F) sections from guinea pigs infected with M90T purE aroC. Bars, 50 μm (A, B, C, D, and F) and 20 μm (E). The inset in panel E shows the tip of a villus in which LPS material can be observed. Arrowheads point to bacteria or bacterial components present within villi.
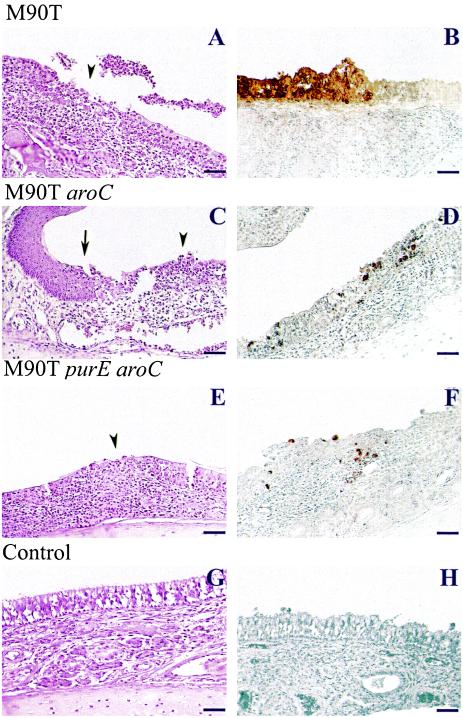
Histopathology and immunohistochemistry performed on upper respiratory tissues of animals infected intranasally.
Histopathological analysis was performed on tissue sections from the nasal mucosa of animals sacrificed at days 1, 3, and 7. As shown in Fig. 7A, after 1 day of infection, the wild-type strain M90T was able to cause massive inflammation (mostly PMNs) of the chorion with ulceration of the associated simple epithelium (arrowhead), reflecting necrosis of the epithelial lining. A massive efflux of PMNs was observed through the ulcerations, with formation of purulent plaques in the nasal cavity. In similar conditions, the M90T aroC mutant also caused epithelial alteration with ulceration and efflux of PMNs (arrowhead), although the inflammatory infiltrate in the chorion appeared weaker (Fig. 7C). It is interesting, as shown in Fig. 7, that the inflammation and destruction ceased at the transition between the simple epithelium concerned in the process and the stratified squamous epithelium, which was preserved (arrow), suggesting that bacterial invasion occurred only in simple epithelia. As shown in Fig. 7E, the M90T purE aroC double mutant also caused epithelial lesions, although of much weaker intensity, in both the chorion and epithelial lining. The arrowhead in this panel points to a typical zone of inflammatory infiltrate of the epithelial lining without significant rupture of this lining.
FIG. 7.
Infection of guinea pig nasal mucosa with M90T (A and B), M90T aroC (C and D) and M90T purE aroC (E and F) at day 1 p.i; (G and H) nasal mucosa from an uninfected animal. (A, C, E, and G) Hematoxylin-eosin stained tissue sections; (B, D, F, and H) immunoperoxidase labeling of serotype 5 somatic antigen by anti-LPS monoclonal IgG. Arrowheads (A, C, and E) point to areas of necrosis of the associated simple epithelium. Arrow (C) indicates the transition between the stratified squamous epithelium and the simple epithelium where areas of necrosis are observable. Bar, 20 μm.
As shown in Fig. 7B, immunolabeling of LPS confirmed massive bacterial invasion of the simple epithelium by M90T in areas corresponding to massive inflammatory destruction. The M90T aroC mutant, which caused weaker lesions, was accordingly present in smaller amounts than M90T, bacterial LPS being associated with the epithelial lining and, in very low amounts, with the chorion (Fig. 7D). Weak staining of the double mutant M90T purE aroC was observed compared to M90T (Fig. 7F).
DISCUSSION
To elicit the signaling cascade that ultimately induces the protective immune response, Shigella vaccine candidates probably need to retain sufficient invasiveness to deliver the appropriate signals but also to avoid causing symptomatic lesions by being sufficiently stealthy. However, finding a fine balance between invasiveness, inflammation, and immunogenicity is a particularly difficult objective to achieve (40), since Shigella invasiveness is mainly associated with the ability of the bacteria to induce a strong inflammatory reaction at the site of infection. In this work, we have attempted to further define rational bases for live vaccine design by means of virulence attenuation. In particular, we have analyzed in vivo the correlations existing between major parameters such as the degree of invasiveness, intensity of the inflammatory response, immunogenicity, and protective capacity of a S. flexneri 5 wild-type strain and of two of its attenuated mutants, auxotrophic for PABA (aroC) and for PABA and adenine (aroC purE), in guinea pigs infected by either the i.g. or the i.n. route. Immunogenicity was evaluated by assessing the ability of these strains to protect guinea pigs vaccinated either i.n. or i.g. against Shigella intraconjunctival challenge. In this way, we attempted to address several questions. (i) How does the route of inoculation influence the quality of the immune response with regard to protection against a virulent challenge? (ii) What differences can be observed among the wild-type strain and its mutants with regard to interaction with nasal and intestinal tissues (i.e., their ability to colonize, persist, disseminate within the host, and elicit an inflammatory reaction)? (iii) Is there a link between the degree of invasiveness, the inflammatory potential of strains, and their capacity to induce protective immunogenicity?
The first two questions focus on the differences between the two immunization routes. Our results indicate that the immune responses elicited by vaccine candidates are strongly influenced by the immunization routes, with the i.n. route being more efficient than the i.g. route (i.n. versus i.g.: P = 0.0063). Protection generated by i.n. immunization is accompanied by high specific anti-LPS IgA titers in saliva and tears (not shown). Accordingly, M90T purE aroC, which elicited the highest titer of total salivary IgA, was less protective than M90T and M90T aroC in that it elicited the lowest levels of specific salivary anti-LPS IgA. This issue is consistent with previous studies that reported, in the i.n. model of guinea pig vaccination, that high titers of anti-LPS IgA (31, 32) were associated with protection (33). Moreover, in the guinea pig keratoconjunctivitis model, high levels of antigen-specific antigen-secreting cells occur in superficial ventral cervical lymph nodes and correlate with the vaccine protective efficacy (12). In the mouse pulmonary model of shigellosis, high levels of IgA-ASC and high mucosal anti-LPS IgA titers were detected following two i.n. immunizations with wild-type S. flexneri 2a (49). Specifically, a monoclonal IgA directed against a serotype-specific epitope of S. flexneri LPS was demonstrated to confer protective immunity (37). However, this rationale does not completely account for the results obtained in i.g. immunizations. In this case, M90T and M90T purE aroC were the only strains conferring protective immunity, with the significant difference that M90T was only partially protective, whereas M90T purE aroC was fully protective. It is intriguing that only animals immunized with these two strains exhibited observable levels of anti-LPS IgA in tears (not shown) and saliva. Nevertheless, these titers were significantly lower than those measured in i.n. immunizations (P < 0.0001). The question of whether a low level of anti-LPS IgA alone supports the protection generated by the i.g. delivered M90T purE aroC and M90T remains unsolved.
Consistent with other studies (53) that focused on the differences between the immune responses following either i.g. or i.n. antigen administration, higher titers of serum anti-LPS IgG were found in guinea pigs vaccinated i.n. than in those immunized i.g. However, it has been shown that in the murine pulmonary model of shigellosis there is no correlation between high anti-LPS IgG and protection (25). In our study, under the i.g. regimen, only M90T and M90T purE aroC stimulated significant levels of anti-LPS IgG, perhaps suggesting that these antibodies influence protection. In humans, a significant correlation between anti-LPS IgG and resistance to shigellosis was observed (5, 6), and Shigella-LPS conjugates (composed of the O-specific polysaccharides conjugated to a carrier protein) are protective and induce high levels of anti-LPS IgG (7). Briefly, in the model of i.n. vaccinated guinea pigs, protective immunity appears to be correlated essentially with high levels of anti-LPS IgA, whereas in animals vaccinated i.g., protection seems to be a multifactorial process in which both anti-LPS IgA and IgG may contribute to the response. These findings also suggest a possible role for cell-mediated immune mechanisms in protective immunity following i.g. vaccination. In fact, several studies implicate cell-mediated immunity as an essential defense mechanism against shigellosis on the basis of results obtained in animal models (49) and in human natural infections (16, 17). A high production of both IL-10 and gamma interferon (IFN-γ) from peripheral blood mononuclear cells of volunteers experimentally infected with Shigella spp. was reported (39) to be accompanied by poor proliferative response to all Shigella antigens accounted for by the synergic effects of IL-10, IFN-γ, and transforming growth factor β in decreasing IL-2 and IL-15 production. We hypothesize that this pattern of cytokine induction and repression might be reversed in i.g. immunization with the M90T purE aroC mutant, which, being sufficiently stealthy, is able to persist longer in infected tissues. Therefore, T-cell proliferation might occur, thus further potentiating the immune response. Consistent with this hypothesis, a stronger CD45 response was observed at day 7 p.i. in T-cell areas of PPs of animals infected with M90T purE aroC than in those infected with M90T (data not shown).
In recent years, a certain number of Shigella attenuated strains have been constructed and evaluated as vaccine candidates (8, 23). These strains could be roughly classified into two groups: (i) mutants in which genes governing one or two main steps of the metabolism were inactivated (e.g., thyA, aroD, and aroA aroD) (1, 24, 50) and (ii) mutants harboring the icsA deletion in addition to one of the above-mentioned gene inactivations (2, 10, 21, 32, 34, 43, 54). The rationale underlying these constructions is that strains belonging to the first group are impaired in intracellular tissue replication, whereas the mutants in icsA are impaired also in intercellular dissemination. In the colonic and rectal mucosa of experimentally infected animals (43), inactivation of icsA restricts S. flexneri invasion to the epithelial cells overlying the lymphoid structures, strongly reducing bacterial colonization. The mutants used in this study are expected to disseminate into the infected tissues because they do not carry the icsA deletion. Their attenuation is based exclusively on a metabolic defect that reduces their intracellular multiplication, as previously shown in the tissue culture invasion assay (3). Following i.g. administration, M90T purE aroC was not recovered from PPs at day 1 p.i., but the number of bacteria progressively increased during the 7-day infection period. M90T bacterial counts showed opposite kinetics. Tissue lesions and inflammation were seldom seen with M90T purE aroC. By contrast, extensive alteration of the epithelial lining was seen in animals infected with M90T and M90T aroC, consistent with previous results obtained with M90T-infected ligated ileal loops in rabbits (44). At day 1 p.i., M90T was observed to be associated with the areas of intense destruction. Consequently, bacteria were rarely observed within the infected tissues after this point in time. At day 1 p.i., M90T purE aroC was located within the tips of villi and, at day 7 p.i., to a greater extent near the crypts. This latter observation indicates that this mutant is able to move within the epithelial layer and to reach areas far from the sites of entry. It was reported that in PPs, the number of dendritic cells (DCs) increases during infections at the sites of bacterial entry (19, 48). It could be hypothesized that M90T purE aroC may enter DCs in PPs and, through these cells, may reach the deeper layers of the intestinal epithelium. In fact, DCs were recently proposed as a vector for viable salmonellae or for Salmonella material during the course of salmonellosis (27). This tight interaction between salmonellae and DCs results in cytokine production and an increase in Salmonella dissemination. In support of this idea, M90T and M90T purE aroC were also isolated from liver tissue at days 1 and 3 and day 7, respectively, thus indicating that bacteria pass into the portal blood stream. Accordingly, S. flexneri 2a was also found to enter the blood stream in a rabbit model of shigellosis (9).
In i.n. infections, a great number of bacteria were isolated from infected tissues, and all strains persisted longer than 1 week and disseminated within the nose-associated organs, including the parotid glands. Broad damage characterized by abscesses showing PMN infiltrations was observed within the ciliated epithelium in the nasal tract, which appears to be the primary site of bacterial entry. Intensity of the lesions was inversely correlated with the attenuation of the strain. The numbers of both bacteria cultured from and bacteria detectable within these infected tissues were consistent with the alterations produced. Therefore, at day 7 p.i. higher numbers of both M90T and M90T aroC were found in all of the tissues examined when compared to M90T purE aroC. As also reported for the mouse model of shigellosis, the guinea pig nasal-infection model is mainly characterized by the development of an intense inflammatory reaction in the lungs with a leukocytic exudate that accumulates in bronchi (data not shown). In a study by Van de Verg et al., challenging of mice within injected S. flexneri 2a (49) led to a prolonged increase in the proportion of monocytes or macrophages in the lungs. Pulmonary IFN-γ levels were elevated, but only at earlier time points of infection, and these levels then rapidly decreased. Therefore, this early IFN-γ response could enhance the microbicidal activity of infiltrating macrophages at this time (49). Moreover, in humans, the frequency of IFN-γ mRNA-expressing cells is significantly reduced during the acute stage of shigellosis compared to that observed during the convalescent stage (38, 52). In guinea pigs infected with all strains used in this study, the number of bacteria recovered from lungs also decreased after 3 days p.i. However, after 7 days of infection, this number did not change. This finding may indicate that, as in humans and mice, the production of IFN-γ, responsible for the first clearance of bacteria, might be reduced in the acute stage of infection, thus allowing maintenance of shigellae within the lungs.
On the basis of the results obtained following the two routes of infections, it can be concluded that, in our model, bacterial persistence within the infected tissues is a prerequisite for establishment of protective immunity. This might partially explain the success of i.n. immunization compared to the i.g. regimen. After i.g. administration, M90T purE aroC, although more attenuated, was able to persist longer in the tissues and was the only strain able to confer protective immunity. On the other hand, after i.n. administration, all strains persisted for extended periods within the tissues analyzed but to different extents. In these tissues, M90T purE aroC was more easily cleared, thus resulting in less protective immunity compared to that induced by M90T aroC and M90T.
This study highlights the need to investigate further the mechanisms that mediate the transition from the innate immune response to the adaptive immune response and, consequently, to develop better experimental animal models that more closely mimic the naturally occurring disease.
ACKNOWLEDGMENTS
We thank Michel Huerre and Nicole Wuscher for histopathological procedures, Daniele Remotti and Mariateresa Taffuri for advice in microscopical analysis, Armelle Phalipon for providing us with the anti-S. flexneri 5 LPS antibody and for helpful discussions, and Dana Philpott for careful reading of the manuscript and suggestions.
This work was supported in part by grants from the World Health Organization (V27/181/79) and from the European Union (QLK2-1999-00938).
REFERENCES
- 1.Ahmed Z U, Mahfuzur R S, Sack D A. Protection of adult rabbits and monkeys against shigellosis by oral immunization with a thymine-requiring and temperature-sensitive mutant of Shigella flexneri Y. Vaccine. 1990;8:153–158. doi: 10.1016/0264-410x(90)90139-d. [DOI] [PubMed] [Google Scholar]
- 2.Bernardini M L, Mounier J, d'Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of icsA, a plasmid locus of Shigella flexneri that governs intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cersini A, Salvia A M, Bernardini M L. Intracellular multiplication and virulence of Shigella flexneri auxotrophic mutants. Infect Immun. 1998;66:549–557. doi: 10.1128/iai.66.2.549-557.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark B D, Bedrosian I, Schindler R, Cominelli F, Cannon J G, Shaw A R, Dinarello C A. Detection of interleukin 1 alpha and 1 beta in rabbit tissues during endotoxemia using sensitive radioimmunoassays. J Appl Physiol. 1991;71:2412–2418. doi: 10.1152/jappl.1991.71.6.2412. [DOI] [PubMed] [Google Scholar]
- 5.Cohen D, Green M S, Block C, Rouach T, Ofek L. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J Infect Dis. 1988;157:1068–1071. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- 6.Cohen D, Green M S, Block C, Slepon R, Ofek L. A prospective study on the association between serum antibodies to lipopolysaccharide and attack rate of shigellosis. J Clin Microbiol. 1991;29:386–389. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen D, Ashkenazi S, Green M, Lerman Y, Slepon R, Robin G, Orr N, Taylor D N, Sadoff J C, Chu C, Shiloach J, Schneerson R, Robbins J B. Safety and immunogenicity of investigational Shigella conjugate vaccines in Israeli volunteers. Infect Immun. 1996;64:4074–4077. doi: 10.1128/iai.64.10.4074-4077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coster T S, Hoge C W, Van De Verg L L, Hartman A B, Oaks E V, Venkatesan M M, Cohen D, Robin G, Fontaine-Thompson A, Sansonetti P J, Hale T L. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun. 1999;67:3437–3443. doi: 10.1128/iai.67.7.3437-3443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etherridge M E, Shamsul Hoque A T M, Sack D A. Pathologic study of a rabbit model for shigellosis. Lab Anim Sci. 1996;46:61–66. [PubMed] [Google Scholar]
- 10.Fontaine A, Arondel J, Sansonetti P J. Construction and evaluation of live attenuated vaccine strains of Shigella flexneri and Shigella dysenteriae. Res Microbiol. 1990;141:907–912. doi: 10.1016/0923-2508(90)90129-e. [DOI] [PubMed] [Google Scholar]
- 11.Hartman A B, Powell C, Schultz C L, Oaks E V, Eckels K H. Small animal model to measure efficacy and immunogenicity of Shigella vaccine strains. Infect Immun. 1991;59:4075–4083. doi: 10.1128/iai.59.11.4075-4083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartman A B, Van De Verg L L, Collins H H, Tang J, D B, Bendiuk N O, Taylor D N, Powell C J. Local immune response and protection in the guinea pig keratoconjunctivitis model following immunization with Shigella vaccines. Infect Immun. 1994;62:412–420. doi: 10.1128/iai.62.2.412-420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartman A B, Venkatesan M M. Construction of a stable attenuated Shigella sonnei ΔvirG vaccine strain, WRSS1, and protective efficacy and immunogenicity in the guinea pig keratoconjunctivitis model. Infect Immun. 1998;66:4572–4576. doi: 10.1128/iai.66.9.4572-4576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. The interleukin 1β-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect Immun. 1997;65:5165–5170. doi: 10.1128/iai.65.12.5165-5170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huan P T, Taylor R, Lindberg A A, Verma N K. Immunogenicity of the Shigella flexneri serotype Y (SFL 124) vaccine strain expressing cloned glucosyl transferase gene of converting bacteriophage SfX. Microbiol Immunol. 1995;39:467–472. doi: 10.1111/j.1348-0421.1995.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 16.Islam D, Bardhan P K, Lindberg A A, Christensson B. Shigella infection induces cellular activation of T and B cells and distinct species-related changes in peripheral blood lymphocyte subsets during the course of the disease. Infect Immun. 1995;63:2941. doi: 10.1128/iai.63.8.2941-2949.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Islam D, Wretlind B, Lindberg A A, Christensson B. Changes in the peripheral blood T-cell receptor VB repertoire in vivo and in vitro during shigellosis. Infect Immun. 1996;64:1391. doi: 10.1128/iai.64.4.1391-1399.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamell A, Stocker B A D, Katakura S, Reinholt F P, Lindberg A A. Live oral auxotrophic Shigella flexneri SFL124 vaccine with a deleted aroD gene: characterization and monkey protection studies. Vaccine. 1992;10:389–394. doi: 10.1016/0264-410x(92)90069-v. [DOI] [PubMed] [Google Scholar]
- 19.Kelsall B L, Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer's patch. J Exp Med. 1996;183:237–247. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotloff K L, Winickoff J P, Ivanoff B, Clemens J D, Swerdlow D L, Sansonetti P J, Adak G K, Levine M M. Global burden of Shigella infections: implications for vaccine development and implementation. Bull W H O. 1999;77:651–656. [PMC free article] [PubMed] [Google Scholar]
- 21.Kotloff K L, Noriega F R, Samandari T, Sztein M B, Losonsky G A, Nataro J P, Picking W D, Barry E M, Levine M M. Shigella flexneri 2a strain CVD 1207, with specific deletions in virG, sen, set, and guaBA, is highly attenuated in humans. Infect Immun. 2000;68:1034–1039. doi: 10.1128/iai.68.3.1034-1039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine M M. Bacillary dysentery: mechanisms and treatment. Med Clin N Am. 1982;66:623. doi: 10.1016/s0025-7125(16)31411-0. [DOI] [PubMed] [Google Scholar]
- 23.Levine M M, Galen J, Barry E, Noriega F, Tacket C, Sztein M, Chatfield S, Daugan G, Losonsky G, Kotloff K. Attenuated Salmonella typhi and Shigella as live oral vaccines and as live vectors. Behring Inst Mitt. 1997;98:120–123. [PubMed] [Google Scholar]
- 24.Lindberg A A, Kamell A, Stocker B A D, Katakura S, Sweiha H, Reinholt F. Development of an auxotrophic oral live Shigella flexneri vaccine. Vaccine. 1988;6:146–150. doi: 10.1016/s0264-410x(88)80018-5. [DOI] [PubMed] [Google Scholar]
- 25.Mallett C P, Van De Verg L L, Collins H H, Hale T L. Evaluation of Shigella vaccine safety and efficacy in an intranasal challenged mouse model. Vaccine. 1993;11:190–196. doi: 10.1016/0264-410x(93)90016-q. [DOI] [PubMed] [Google Scholar]
- 26.Mallett C P, Hale T L, Kaminski R W, Larsen T, Orr N, Cohen D, Lowell G H. Intranasal or intragastric immunization with proteosome-Shigella lipopolysaccharide vaccines protects against lethal pneumonia in a murine model of Shigella infection. Infect Immun. 1995;63:2382–2386. doi: 10.1128/iai.63.6.2382-2386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marriott I, Hammond T G, Thomas E K, Kenneth L B. Salmonella efficiently enter and survive within cultured CD11c+ dendritic cells initiating cytokine expression. Eur J Immunol. 1999;29:1107–1115. doi: 10.1002/(SICI)1521-4141(199904)29:04<1107::AID-IMMU1107>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Maurelli A T, Blackmon B, Curtiss R., III Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984;43:195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti P J. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992;60:237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noriega F R, Wang J Y, Losonsky G, Maneval D R, Hone D M, Levine M M. Construction and characterization of attenuated aroA and icsA Shigella flexneri strain CVD 1203, a prototype live oral vaccine. Infect Immun. 1994;62:5168–5172. doi: 10.1128/iai.62.11.5168-5172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noriega F R, Losonsky G, Wang J Y, Formal S B, Levine M M. Further characterization of ΔaroA ΔvirG Shigella flexneri 2A strain CVD 1203 as a mucosal Shigella vaccine and as a live-vector vaccine for delivering antigens of enterotoxigenic Escherichia coli. Infect Immun. 1996;64:23–27. doi: 10.1128/iai.64.1.23-27.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noriega F R, Losonsky G, Lauderbaugh C, Liao F M, Wang J Y, Levine M M. Engineered ΔguaB-A ΔvirG Shigella flexneri 2a strain CVD 1205: construction, safety, immunogenicity, and potential efficacy as a mucosal vaccine. Infect Immun. 1996;64:3055–3061. doi: 10.1128/iai.64.8.3055-3061.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noriega F R, Liao F M, Maneval D R, Ren S, Formal S B, Levine M M. Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun. 1999;67:782–788. doi: 10.1128/iai.67.2.782-788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okada N, Sasakawa C, Tobe T, Talukder K A, Komatsu K, Yoshikawa M. Construction of a physical map of the chromosome of Shigella flexneri 2A and the direct assignment of nine virulence-associated loci identified by Tn5 insertions. Mol Microbiol. 1991;5:2171–2180. doi: 10.1111/j.1365-2958.1991.tb02147.x. [DOI] [PubMed] [Google Scholar]
- 35.Perdomo J J, Cavaillon J M, Huerre M, Ohayon H, Gounon P, Sansonetti P J. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J Exp Med. 1994;180:1307–1319. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perdomo J J, Gaunon P, Sansonetti P J. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J Clin Invest. 1994;93:633–643. doi: 10.1172/JCI117015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phalipon A, Kaufmann M, Michetti P, Cavaillon J M, Huerre M, Sansonetti P J, Kraehembuhl J P. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J Exp Med. 1995;182:769–778. doi: 10.1084/jem.182.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raqib R, Gustafsson A, Andersson J, Bakhiet M. A systemic downregulation of gamma interferon production is associated with acute shigellosis. Infect Immun. 1997;65:5338–5341. doi: 10.1128/iai.65.12.5338-5341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samandari T, Kotloff K L, Losonsky G A, Picking W D, Sansonetti P J, Levine M M, Sztein M B. Production of IFN-γ and IL-10 to Shigella invasins by mononuclear cells from volunteers orally inoculated with a Shiga toxin-deleted Shigella dysenteriae type 1 strain. J Immunol. 2000;164:2221–2232. doi: 10.4049/jimmunol.164.4.2221. [DOI] [PubMed] [Google Scholar]
- 40.Sansonetti P J. Pathogenesis of shigellosis: from molecular and cellular biology of epithelial cell invasion to tissue inflammation and vaccine development. Jpn J Med Sci Biol. 1998;51(Suppl. 1):S69–S80. doi: 10.7883/yoken1952.51.supplement1_s69. [DOI] [PubMed] [Google Scholar]
- 41.Sansonetti P J, Kopecko D J, Formal S B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982;35:852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sansonetti P J, Hale T L, Dammin G J, Kapfer C, Collins H H, Jr, Formal S B. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect Immun. 1983;39:1392–1402. doi: 10.1128/iai.39.3.1392-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sansonetti P J, Arondel J, Fontaine A, d'Hauteville H, Bernardini M L. ompB (osmo-regulation) and icsA (cell to cell spread) mutants of Shigella flexneri: vaccine candidates and probes to study the pathogenesis of shigellosis. Vaccine. 1991;9:416–422. doi: 10.1016/0264-410x(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 44.Sansonetti P J, Arondel J, Cantey J R, Prevost M C, Huerre M. Infection of rabbit Peyer's patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect Immun. 1996;64:2752–2764. doi: 10.1128/iai.64.7.2752-2764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasakawa C, Kamata K, Sakai T, Makino S, Yamada M, Okada N, Yoshikawa M. Virulence-associated genetic regions comprising 31 kilobases of the 230-kilobase plasmid in Shigella flexneri 2a. J Bacteriol. 1988;170:2480–2484. doi: 10.1128/jb.170.6.2480-2484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sereny B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol Acad Sci Hung. 1957;4:367–376. [PubMed] [Google Scholar]
- 47.Sereny B. A new method for the measurement of protective potency of dysentery vaccines. Acta Microbiol Acad Sci Hung. 1962;9:55–60. [PubMed] [Google Scholar]
- 48.Sousa C R, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain R N, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van de Verg L L, Mallett C P, Collins H H, Larsen T, Hammack C, Hale T L. Antibody and cytokine responses in a mouse pulmonary model of Shigella flexneri serotype 2a infection. Infect Immun. 1995;63:1947–1954. doi: 10.1128/iai.63.5.1947-1954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verma N K, Lindberg A A. Construction of aromatic dependent Shigella flexneri 2a live vaccine candidate strains: deletion mutations in the aroA and the aroD genes. Vaccine. 1991;9:6–9. doi: 10.1016/0264-410x(91)90308-s. [DOI] [PubMed] [Google Scholar]
- 51.Wassef J S, Keren D F, Mailloux J L. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect Immun. 1989;57:858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Way S S, Borczuk A C, Dominitz R, Goldberg M B. An essential role for gamma interferon in innate resistance to Shigella flexneri infection. Infect Immun. 1998;66:1342–1348. doi: 10.1128/iai.66.4.1342-1348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu H-Y, Nikolova E B, Beagley K W, Eldrige J H, Russell M W. Development of antibody-secreting cells and antigen-specific T cells in cervical lymph nodes after intranasal immunization. Infect Immun. 1997;65:227–235. doi: 10.1128/iai.65.1.227-235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshikawa M, Sasakawa C, Okada N, Takasaka M, Nakayama M, Yoshikawa Y, Kohno A, Danbara H, Nariuchi H, Shimada H, Toriumi M. Construction and evaluation of a virG thyA double mutant of Shigella flexneri 2a as candidate live-attenuated oral vaccine. Vaccine. 1995;13:1436–1440. doi: 10.1016/0264-410x(95)00071-8. [DOI] [PubMed] [Google Scholar]
- 55.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 56.Zychlinsky A, Kenny B, Menard R, Prevost M C, Holland I B, Sansonetti P J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994;11:619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 57.Zychlinsky A, Fitting C, Cavaillon J M, Sansonetti P J. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J Clin Invest. 1994;94:1328–1332. doi: 10.1172/JCI117452. [DOI] [PMC free article] [PubMed] [Google Scholar]