Abstract
Background
Stiffness of the proximal aorta may play a critical role in adverse left ventricular (LV)–vascular interactions and associated LV diastolic dysfunction. In a community‐based sample, we sought to determine the association between proximal aortic stiffness measured by cardiovascular magnetic resonance (CMR) and several clinical measures of LV diastolic mechanics.
Methods and Results
Framingham Heart Study Offspring adults (n=1502 participants, mean 67±9 years, 54% women) with available 1.5T CMR and transthoracic echocardiographic measures were included. Measures included proximal descending aortic strain and aortic arch pulse wave velocity by CMR (2002–2006) and diastolic function (mitral Doppler E and A wave velocity, E wave area, and LV tissue Doppler e' velocity) by echocardiography (2005–2008). Multivariable linear regression analysis was used to relate CMR aortic stiffness measures to measures of echocardiographic LV diastolic function. All continuous variables were standardized. In multivariable‐adjusted regression analyses, aortic strain was inversely associated with E wave deceleration time (estimated β=−0.10±0.032, P=0.001), whereas aortic arch pulse wave velocity was inversely associated with E/A ratio (estimated β=−0.094±0.027, P=0.0006), E wave area (estimated β=−0.070±0.027, P=0.010), and e' (estimated β=−0.061±0.027, P=0.022), all indicating associations of higher aortic stiffness by CMR with less favorable LV diastolic function. Compared with men, women had a larger inverse relationship between pulse wave velocity and E/A ratio (interaction β=−0.085±0.031, P=0.0064). There was no significant effect modification by age or a U‐shaped (quadratic) relation between aortic stiffness and LV diastolic function measures.
Conclusions
Higher proximal aortic stiffness is associated with less favorable LV diastolic function. Future studies may clarify temporal relations of aortic stiffness with varying patterns and progression of LV diastolic dysfunction.
Keywords: aortic strain, cardiac magnetic resonance (CMR), diastolic function, proximal aortic stiffness
Subject Categories: Magnetic Resonance Imaging (MRI)
Nonstandard Abbreviations and Acronyms
- FHS
Framingham Heart Study
- LVEDV
left ventricular end‐diastolic volume
- PWV
pulse wave velocity
- TTE
transthoracic echocardiography
Clinical Perspective.
What Is New?
In community‐dwelling individuals, proximal aortic stiffness as measured by cardiac magnetic resonance imaging is associated with left ventricular diastolic dysfunction.
What Are the Clinical Implications?
Stiffness of the aorta and left ventricle, hallmarks of diastolic dysfunction, are important causes of morbidity and mortality, but their subclinical relations are not well understood.
The results inform pathophysiologic understanding of the relation of proximal aortic stiffness with left ventricular diastolic function in individuals without prevalent cardiovascular disease.
Aortic stiffness is an important marker of vascular aging and is associated with left ventricular (LV) systolic dysfunction 1 , 2 and incident cardiovascular disease (CVD). 3 , 4 , 5 , 6 , 7 , 8 Global aortic stiffness, well characterized by carotid femoral pulse wave velocity (PWV), largely captures stiffness of the descending thoraco‐abdominal aorta. Stiffness of the proximal aorta is critical in dynamic coupling of the LV to the aorta. Located immediately downstream of the LV, the proximal aorta receives flow directly from the LV and buffers pressure swings in the distal aorta and throughout the arterial tree. In addition, the proximal aorta, which contains more elastic tissue, is embryologically distinct from the distal aorta, and the assessment of this region is not fully captured by analysis of global aortic stiffness using carotid femoral PWV. 7
Growing evidence indicates that aortic stiffness imposes afterload to the LV and contributes to adverse LV remodeling. 1 , 2 , 9 , 10 Prior studies demonstrated that higher aortic stiffness is associated with alterations in LV mass, volumes, and systolic function. 2 , 9 , 10 , 11 , 12 However, our understanding of the relation of aortic stiffness with diastolic function is limited. The dynamic interaction between the aorta and LV influence mutual dysfunction, 7 , 13 and stiffness of the LV with diastolic dysfunction may represent early precursors of heart failure. 14 This totality of evidence underscores the importance of further elucidating the significance of proximal aortic stiffness on pathologic vascular–ventricular dynamics. Aortic strain is a noninvasive imaging measure of local aortic stiffness properties and vascular aging, with demonstrated associations with age, sex, and CVD in large groups of both asymptomatic healthy adults as well as individuals with CVD. 15 , 16 , 17 Thus, we sought to investigate the associations of cardiovascular magnetic resonance (CMR) measures of proximal aortic stiffness with echocardiographic measures of diastolic dysfunction in a well‐phenotyped community cohort to further understand the functional interrelations between the aorta and LV. We hypothesized that CMR evidence of higher aortic stiffness would be associated with clinical echocardiographic measures of diastolic dysfunction.
METHODS
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Study Sample
The FHS (Framingham Heart Study) Offspring cohort 18 was recruited from 1971 to 1974 and includes individuals who are children of the FHS Original cohort and the spouses of those children. Every 4 to 6 years, participants undergo comprehensive evaluations including a physician‐administered medical history and physical examination and routine bloodwork. This investigation included adult members of the FHS Offspring cohort who underwent CMR (2002–2006) (n=1787) and attended examination cycle 8 (2005–2008), during which transthoracic echocardiography (TTE) was conducted (n=3021). CMR was offered to participants who lived in a state contiguous with Massachusetts using random sampling from strata of decade age, sex, and quintile of Framingham Risk Score. 19 There were 1692 individuals who underwent CMR and participated in exam 8. After excluding 158 individuals with inadequate CMR stiffness measures and 32 with inadequate TTE diastolic function measures, 1502 individuals were available for this investigation. The Boston University Medical Center Institutional Review Board approved the study protocol. All participants provided written informed consent.
Covariates
Covariates were measured as part of the FHS Offspring cohort study during examination cycle 8 (2005–2008). Diabetes was defined by a fasting glucose of ≥126 mg/dL and/or treatment of diabetes with medication. Current smoking was defined as reported smoking ≥1 cigarette per day during the year preceding their FHS examination. Serological blood testing was performed in the fasting state using standard enzymatic methods for blood glucose, serum total cholesterol, and high‐density lipoprotein cholesterol. Height and weight were measured in inches and pounds, respectively. The examination also included a physician‐performed medical history with patient‐reported medication history and a physical exam with noninvasive brachial systolic and diastolic blood pressure measurements by sphygmomanometry. Prevalent CVD was adjudicated by a 3‐physician panel that reviewed all pertinent medical records.
CMR Imaging Acquisition
CMR was performed using a 1.5T system (Gyroscan ACS NT, release 9, or Achieva, release 1; Philips Healthcare, Best, the Netherlands), with a 5‐element commercial cardiac array receiver coil, as previously described. 20 Localizing scans were performed to determine the position and orientation of the heart. ECG‐gated, balanced steady‐state free precession sequences were used to acquire contiguous short‐axis images through the heart (temporal resolution, 39 ms; repetition time, 3.2 ms = R‐R interval; echo time, 9 ms; flip angle, 30°; field of view, 400 mm; matrix size, 208×256; slice thickness, 10 mm; gap, 0). An ECG‐gated, transverse‐phase contrast slice was localized orthogonal to the proximal ascending and descending thoracic aorta at the level of the pulmonary artery bifurcation during an end‐expiration breath hold (temporal resolution, 30–40 ms; repetition time, 15 ms; echo time, 6.5 ms; field of view, 300 mm; matrix size, 256×256; slice thickness, 6 mm).
LV Structure and Function Analysis
CMR measures of LV structure and systolic function were determined by a semiquantitative analysis tool (EasyVision version 5.1, Philips Medical Systems). An observer blinded to clinical data manually traced epicardial and endocardial borders at end diastole and end systole. 21 LV end‐diastolic volume (LVEDV) and LV end‐systolic volume were computed by method of summation of discs, and their difference was calculated as stroke volume. LV ejection fraction was determined by the ratio of stroke volume to LVEDV. LV mass was computed as the summation of myocardial volume in all short‐axis slices multiplied by myocardial density. LVEDV, LV end‐systolic volume, and LV mass were indexed to body surface area to account for body size in these measures (LV end‐diastolic volume index, LV end‐systolic volume index, and LV mass index).
Proximal Descending Aortic Strain and Aortic Arch PWV Analysis
Similarly, 2 blinded observers analyzed all CMR PWV and aortic strain data using Image J (version 1.5 22 ) and EasyVision (version 5.1, Philips Medical Systems), respectively. Aortic strain and PWV were calculated as previously described. 2 , 23 Maximum and minimum cross‐sectional areas of the proximal descending aorta throughout the cardiac cycle were measured using a semiautomated contouring method (Figure 1A). Percent aortic strain, change in cross‐sectional area over the cardiac cycle, was calculated as , where ΔA(%) is change in cross‐sectional area of the aorta, Amax is the largest aortic cross‐sectional area, Amin is the smallest aortic cross‐sectional area. PWV of the aortic arch was calculated as the distance between phase contrast acquisitions in the ascending and proximal descending aorta divided by the transit time as has been previously described (Figure 1B). 2 , 23 Transit time was measured after peak flow normalization as an average time difference using the least squares estimate between all data points on the upslope of the ascending and descending aortic flow curves during systole. 2 , 23 Higher aortic stiffness was indicated by lower aortic strain and higher aortic arch PWV.
Figure 1. Cardiac magnetic resonance imaging stiffness measures.
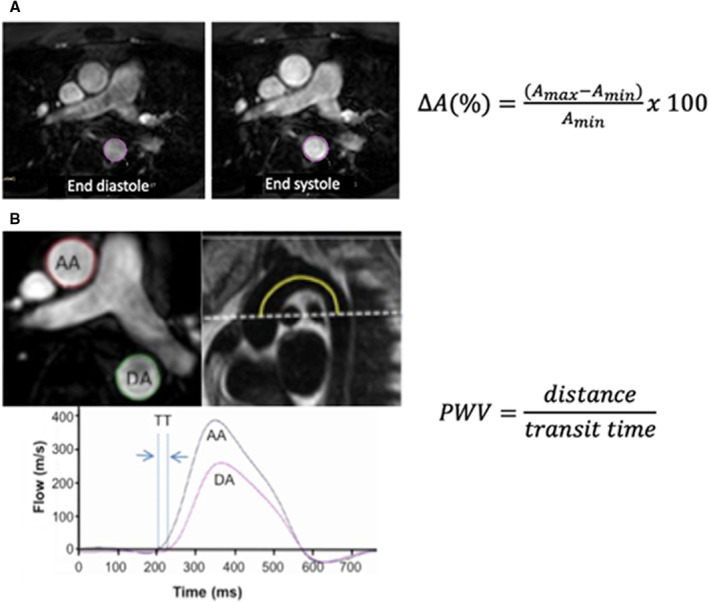
A, Aortic strain measured as percent change of cross‐sectional area of the proximal descending aorta at the end diastole and end systole, ΔA(%) is change in cross‐sectional area, Amax is largest aortic cross‐sectional area, Amin is smallest aortic cross‐sectional area. B, PWV of the aortic arch was calculated as the distance between phase contrast acquisitions at the AA and DA divided by the TT. TT was measured after peak flow normalization as an average time difference using the least squares estimate between all data points on the upslope of the AA and DA flow curves during systole. AA indicates ascending aorta; DA, descending aorta; PWV, pulse wave velocity; and TT, transit time.
Interreader reproducibility in a random sample of 25 cases by 2 individuals was excellent for both strain and PWV (intraclass correlation coefficients 0.95 and 0.97, respectively). Bland–Altman plots demonstrating good limits of agreement are presented in Figure S1.
Transthoracic Echocardiography
TTE was performed at mean 26.3±13 months from CMR with a Philips HP Sonos 5500 ultrasound machine (Philips Healthcare). Participants were imaged in the left lateral decubitus position. Pulsed wave Doppler inflow across the mitral valve in diastole was measured with the sample volume placed at the level of the mitral leaflet tips to track the leading edge of the E and A waves. Mitral pulse wave Doppler measures included peak E wave and A wave velocities, peak E/A velocity ratio, E wave area, and E wave deceleration time (Figure 2). Tissue Doppler velocities were acquired with the sample volume placed at the center of the lateral mitral annulus to measure the early diastolic velocity of the mitral valve annulus e'. Previous studies have demonstrated strong interobserver correlation of the E′, E, and A waves with interobserver correlation coefficients demonstrated as >0.97. 9 , 24
Figure 2. Measures of diastolic function.
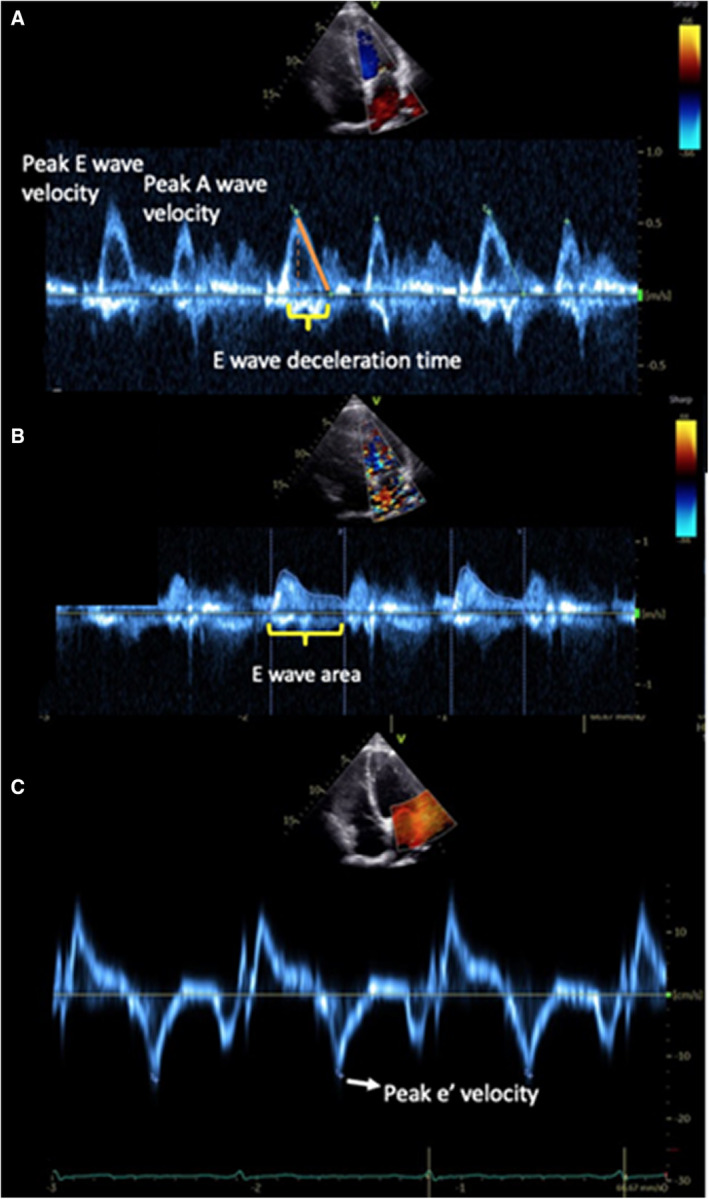
A, Transmitral peak E and A wave velocity measurements. B, E wave area. C, Tissue Doppler lateral e' velocity measurement.
Statistical Analysis
Descriptive statistics are displayed as mean±SD or percentages. Primary dependent variables were continuous E/A ratio and E/e' ratio, whereas continuous mitral Doppler E wave deceleration time and tissue Doppler e' were secondary variables. Predictor variables were continuous aortic strain and continuous aortic arch PWV. Variables were loge‐transformed, and distributions were visually assessed to evaluate the extent of skewness. Multivariable linear regression analysis was used to relate CMR aortic stiffness measures to TTE measures of LV diastolic function. Regression results for continuous dependent variables are presented as estimated β±SE, representing the increment of the dependent variable in SD units per SD unit increment in the predictor variable. Age‐ and sex‐adjusted as well as multivariable‐adjusted analyses were performed, adjusting for age, sex, height, weight, systolic blood pressure, diastolic blood pressure, heart rate, ratio of total:high‐density lipoprotein cholesterol, use of antihypertensive medication, use of lipid‐lowering medication, presence of diabetes, and current smoking. Furthermore, we evaluated the effect of time between CMR and TTE on the relations between predictors and outcomes by (1) including time between CMR and TTE in additional multivariable models and (2) evaluating for significant interactions between CMR aortic stiffness variables with time difference quantified both by tertiles and as an ordinal variable on the diastolic function outcomes. Separately, we also evaluated for effect modification by age and sex in the relations of aortic stiffness and LV diastolic function.
Models that included quadratic terms for independent variables were examined to evaluate for U‐shaped relationships of aortic stiffness with diastolic function. In addition, in secondary analyses, we performed additional models adjusting for LV end‐diastolic volume index, LV mass index, and LV ejection fraction to evaluate whether LV structural and functional changes could underlie associations of aortic stiffness and LV diastolic function. A P value <0.05 was considered statistically significant. The statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Study Sample
Characteristics of the 1502 study participants are listed in Table 1. Participants were middle to older aged with a similar proportion of men and women. Individuals had a low to moderate CVD risk profile, with approximately half the cohort reporting taking medications for hypertension or dyslipidemia and a relatively small proportion with prevalent diabetes and active smoking. Imaging results were consistent with a relatively healthy middle‐aged to older cohort, with mean LV ejection fraction and E/A and E/e' ratios consistent with normal to mildly reduced systolic and diastolic function (Table 1). Aortic stiffness measures were consistent with modestly increased stiffness, consistent with the age of cohort members.
Table 1.
Characteristics of Framingham Heart Study Offspring Cohort Sample (N=1502)
| Clinical characteristics | |
|---|---|
| Age, y | 67±9 |
| Women | 811 (54) |
| Height, meters | 1.68±0.1 |
| Weight, kg | 78.5±16.8 |
| Systolic blood pressure, mm Hg | 128±17 |
| Diastolic blood pressure, mm Hg | 74±10 |
| Heart rate, beats/min | 62±10 |
| Total:HDL cholesterol ratio | 3.5±1.0 |
| Use of antihypertensive medications | 758 (51) |
| Use of lipid‐lowering medications | 710 (47) |
| Diabetes | 190 (13) |
| Smoking | 118 (8) |
| Prevalent cardiovascular disease | 69 (5) |
| CMR measures | |
| Aortic arch pulse wave velocity, m/s | 9±5 |
| Aortic strain, % | 17±9 |
| LV end‐diastolic volume index, mL/m2 | 66±12 |
| LV end‐systolic volume index, mL/m2 | 22±8 |
| LV mass index, g/m2 | 55±12 |
| LV ejection fraction, % | 67±7 |
| Echocardiography measures | |
| E wave deceleration time, ms | 219±33 |
| E' wave area, cm2 | 0.9±0.3 |
| Transmitral E/A ratio | 0.9±0.3 |
| Mitral annular e' (lateral), cm/s | 10±2 |
| E/e' | 7±2 |
Data are reported as number (percentage) for categorical variables and mean (SD) for continuous variables. Prevalent cardiovascular disease as adjudicated by the Framingham Heart Study includes history of stroke, transient ischemic attack, and coronary heart disease. Coronary heart disease is defined as myocardial infarction (diagnostic ECG, cardiac biomarkers, and clinical presentation), coronary insufficiency (unstable angina), or stable angina. CMR indicates cardiac magnetic resonance; HDL, high‐density lipoprotein; and LV, left ventricular.
Association of Diastolic Dysfunction With Proximal Aortic Stiffness
Age‐ and sex‐adjusted as well as multivariable‐adjusted associations of aortic stiffness with LV diastolic measures are presented in Table 2. In multivariable models, lower aortic strain was associated with greater E wave deceleration time, indicating that greater stiffness is associated with prolonged early diastolic filling. Aortic arch PWV was associated with both lower transmitral E/A ratio and E wave area, consistent with mild LV diastolic dysfunction and decreased early diastolic contribution to LV filling, respectively. Higher PWV was also associated with lower lateral mitral annulus tissue Doppler e' velocity, consistent with LV diastolic dysfunction. Aortic arch PWV was not associated with E/e' ratio in age‐ and sex‐adjusted or multivariable‐adjusted analyses. Secondary analyses additionally adjusting for measures of LV structure and function including LV end‐diastolic volume index, LV mass index, and LV ejection fraction demonstrated similar inverse associations between mean aortic strain with E wave deceleration time (β=−0.11±0.03, P=0.0006) and PWV with E/A, E wave area, and e' (β=−0.093±0.029 [P=0.0014], β=−0.064±0.028 [P=0.021], and β=−0.052±0.027 [P=0.048], respectively). There was no significant change in any results with an additional adjustment of time between CMR and TTE as a covariate (Table S1). There was no significant interaction of aortic stiffness measures with time between CMR and TTE either by tertiles or as a continuous variable (all P>0.05). We did not observe quadratic associations of aortic stiffness terms with diastolic function measures (all P≥0.10).
Table 2.
Associations of Continuous Left Ventricular Diastolic Dysfunction With Aortic Stiffness
| E/A | E wave area | e' | E/e' | E wave deceleration time | |
|---|---|---|---|---|---|
| β±SE | β±SE | β±SE | β±SE | β±SE | |
| Age and sex adjusted | |||||
| Pulse wave velocity |
−0.099 (0.028) P=0.0005 |
−0.10 (0.027) P=0.0002 |
−0.070 (0.027) P=0.008 |
−0.032 (0.028) P=0.260 |
0.031 (0.030) P=0.299 |
| Aortic strain |
0.022 (0.029) P=0.444 |
−0.0048 (0.030) P=0.874 |
0.009 (0.029) P=0.753 |
−0.031 (0.030) P=0.300 |
−0.088 (0.032) P=0.0054 |
| Multivariable adjusted | |||||
| Pulse wave velocity |
−0.094 (0.027) P=0.0006 |
−0.070 (0.027) P=0.010 |
−0.061 (0.027) P=0.022 |
−0.014 (0.028) P=0.604 |
0.052 (0.030) P=0.087 |
| Aortic strain |
−0.023 (0.028) P=0.406 |
−0.029 (0.029) P=0.314 |
−0.005 (0.029) P=0.875 |
−0.040 (0.029) P=0.177 |
−0.10 (0.032) P=0.001 |
Data are presented as β (SE) per SD unit increment in the predictor variable. Aortic stiffness variables (proximal descending aortic strain, aortic arch pulse wave velocity) were loge transformed to achieve normal distribution. The multivariable‐adjusted model included age, sex, height, weight, systolic blood pressure, diastolic blood pressure, heart rate, total/high‐density lipoprotein cholesterol, usage of antihypertensive medication, usage of lipid‐lowering medication, diabetes status, and smoking status. All continuous variables were standardized.
A sex interaction was noted with PWV and E/A ratio (estimated β for women versus men=−0.085±0.031, P=0.0064). This more negative relationship between higher PWV with lower E/A and in women is consistent with a larger effect of the association between aortic stiffness and LV diastolic dysfunction in women.
DISCUSSION
In this cross‐sectional study of community‐dwelling adults, we related local and regional (strain, PWV) stiffness of the proximal aorta to clinical echocardiographic measures of LV diastolic dysfunction and observed an association of higher proximal aortic stiffness with unfavorable measures of LV diastolic mechanics. Lower aortic strain, indicative of a less elastic, stiffer aorta, was associated with greater E wave deceleration time, indicating prolonged diastolic filling of the LV, as seen with diastolic dysfunction. Elevated PWV of the aortic arch was associated with lower E/A ratio, lower E wave area, and e' velocity, consistent with both reduced early, passive filling of the LV during diastole and reduced diastolic LV annular velocity. These associations persisted with additional statistical adjustment for LV structure and function, including LV mass, volume, and ejection fraction. Although aortic strain and PWV demonstrated heterogeneity of associations with specific LV diastolic function measures, the overall totality of data support a connection between stiffness of the aorta and that of the LV. Moreover, the relation between aortic stiffness and LV diastolic dysfunction was more pronounced in women. These findings contribute to a growing understanding of vascular stiffness in an older population as a potential mechanism of cardiovascular dysfunction and disease pathogenesis and highlight augmented pathologic vascular–ventricular relations in older women that may underlie their predominance of heart failure with preserved ejection fraction.
Higher passive LV stiffness and abnormal LV relaxation impair LV filling result in diastolic dysfunction, 14 evidenced by a prolonged mitral Doppler E wave deceleration time. Corollaries of prolonged filling are relatively greater atrial contribution to LV filling, demonstrated by lower peak E amplitude, E/A ratio, and area under the E wave curve. Greater LV stiffness results in reduced velocity of excursion of the LV base in early diastole, measured by lower peak e' velocity. Multiple mechanisms have been suggested to explain the contribution of proximal aortic stiffness to hemodynamic derangements leading to TTE manifestations of LV diastolic dysfunction, heart failure, and CVD. 7 Greater aortic stiffness increases afterload to the LV, leading to adaptive and adverse ventricular remodeling 1 , 2 , 10 , 11 , 12 and eventually diastolic and systolic dysfunction. 2 , 7 , 9 , 25 In addition, the late systolic loading that is seen with proximal aortic stiffening and loss of the normal cushioning function of the aorta negatively affects LV relaxation and the coupling of systole and diastole. 7 , 13 , 26 Furthermore, excess pulsatility from aortic stiffness may compromise coronary perfusion, resulting in both systolic and diastolic dysfunction. 7 , 24
Prior population‐based studies, including those in the FHS, have largely focused on relations of global aortic stiffness with LV structure and systolic function, such as LV mass, LV volume, and mid‐ventricular systolic strain. 2 , 9 , 10 Evaluation of the relationship between aortic stiffness, particularly of the proximal aorta, and broad measures of LV diastolic mechanics has previously been lacking. In the present work, we demonstrate the association between proximal aortic stiffness and TTE measures of LV diastolic dysfunction, demonstrating the importance of the proximal aortic segment in LV diastolic pathology that occurs independently from LV structure and systolic function. Although this study assessed local and regional aortic stiffness using CMR, our results are less reflective of the imaging modality per se but, rather, indicative of underlying mechanisms. Indeed, both echocardiography and CMR have been used to evaluate aortic strain 27 and have been demonstrated to have good correlation. 28
Our study also demonstrates a steeper association of higher PWV with lower E/A ratio in women. Low E/A ratio is a TTE marker of diastolic dysfunction, and its association with higher PWV suggests that higher proximal aortic stiffness is associated with a relative greater contribution of active atrial contribution to LV filling in diastole. 29 Thus, this sex interaction of higher aortic stiffness with lower E/A ratio suggests an interplay between large vessel stiffness and LV diastolic dysfunction that is more marked in women than in men. Heart failure with preserved ejection fraction is predominant in older women. Prior smaller studies have investigated the relations of vascular and LV function in middle‐aged 30 , 31 to elderly 10 adults, including those without known CVD and with heart failure with preserved ejection fraction. 32 These studies, which assessed central pressure pulsatility and arterial stiffness by applanation tonometry or invasive hemodynamics, have demonstrated predominant associations of central aortic stiffness with LV diastolic dysfunction more in women than in men. Our results in a larger group of community‐dwelling adults with mild LV diastolic function are consistent with the bulk of evidence suggesting a differential influence of sex in the relations between arterial stiffness and LV structure and diastolic function in older individuals. Although we did not observe an association of aortic stiffness with E/e', we did observe similar declines in both mitral E wave and tissue Doppler e' velocities.
Strengths and Limitations
In this large cohort of older adults, we described the association of CMR measures of proximal aortic stiffness with TTE measures of LV diastolic mechanics, which has not been well studied previously. The current study expands on prior work by evaluating local mechanical properties of the aorta (strain) additionally to aortic arch stiffness (PWV) alongside measures of LV diastolic filling that are routinely evaluated in clinical studies. Similar to prior publications, 15 , 16 , 17 we used aortic strain as a measure of aortic stiffness as contemporaneous blood pressures for calculation of aortic distensibility were unavailable. Strengths of this study include the large cohort of community‐dwelling individuals and use of consistently acquired and well‐validated evaluations of clinical risk factors as well as CMR and TTE measurements.
The study results should be contextualized in the setting of several potential limitations. First, as an observational, cross‐sectional study, no direct causal inferences can be made between aortic stiffness and LV diastolic dysfunction. Although we conducted comprehensive multivariable‐adjusted analyses, residual confounding cannot be fully excluded. Second, the demographics of the study sample may also limit its generalizability, as the participants studied are largely middle‐aged to older individuals of European descent with low to moderate risk for CVD. In addition, patients with advanced LV diastolic dysfunction were not well represented in the cohort. Third, imaging studies were performed at contemporaneous exams, although CMR studies of aortic stiffness preceded TTE assessment of LV diastolic function. It is possible that relations between aortic stiffness and LV diastolic function were attenuated as measures were not assessed in a single cross‐sectional evaluation. Although there was a time difference between CMR and TTE, several analytic models did not demonstrate a significant impact of this difference in the associations between aortic stiffness and LV diastolic dysfunction. Indeed, this time difference would rather bias our results toward the null, reinforcing our observations. Because cardiovascular remodeling has a prolonged time course, it is possible that only modest changes in these measures occurred during this time period or the relationship between these relative changes in aortic stiffness and diastolic function variables was preserved despite any progression in the measures. Fourth, the primary dependent variables of interest E/A and E/e' were prespecified, but additional testing of associations may have influenced statistical significance of the results through multiple testing, and thus future verification of our results through replication is warranted. Overall, our collective study results encourage future study of the temporal relations of vascular–ventricular dysfunction to determine the potential clinical implications of surveilling and modulating aortic stiffness on LV diastolic mechanics and outcomes.
CONCLUSIONS
Proximal aortic stiffness is associated with clinical measures of LV diastolic dysfunction. Our findings further contribute to an understanding of vascular–ventricular dynamics that may ultimately contribute to clinical CVD. Future studies may clarify temporal relations of aortic stiffness with patterns and progression of LV diastolic dysfunction and their impact on prognosis.
Sources of Funding
This study was supported by contracts from the National Institutes of Health (NIH; N01‐HC‐25195, HHSN268201500001I, and 75N92019D00031) to the Framingham Heart Study/Boston University School of Medicine and research grants R01HL70279, R01HL080124, R01HL107385, and R01HL142983 from the National Heart, Lung and Blood Institute. Dr Vasan is supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine. Dr Benjamin is supported by NIH R01HL092577 and 2U54HL120163 and American Heart Association AHA_18SFRN34110082. Dr Tsao is partially supported by NIH R03HL145195 and R01HL155717.
Disclosures
Dr O'Donnell is employed by Novartis Institutes for Biomedical Research (Cambridge, MA). Dr Mitchell is the owner of Cardiovascular Engineering, Inc. (Norwood, MA), a company that develops and manufactures devices to measure vascular stiffness, and is a consultant to and receives honoraria from Novartis, Merck, Bayer, Servier, Philips Healthcare, and deCODE genetics. Dr Tsao has been a consultant to Abiomed. All other authors report no disclosures.
Supporting information
Table S1
Figure S1
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027230
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1. Weber T, O'Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008;21:1194–1202. doi: 10.1038/ajh.2008.277 [DOI] [PubMed] [Google Scholar]
- 2. Ohyama Y, Ambale‐Venkatesh B, Noda C, Chugh AR, Teixido‐Tura G, Kim JY, Donekal S, Yoneyama K, Gjesdal O, Redheuil A, et al. Association of aortic stiffness with left ventricular remodeling and reduced left ventricular function measured by magnetic resonance imaging: the multi‐ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2016;9:9. doi: 10.1161/CIRCIMAGING.115.004426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mattace‐Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235 [DOI] [PubMed] [Google Scholar]
- 4. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation. 2010;121:505–501. doi: 10.1161/CIRCULATIONAHA.109.886655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sutton‐Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well‐functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628 [DOI] [PubMed] [Google Scholar]
- 6. Willum‐Hansen T, Staessen JA, Torp‐Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342 [DOI] [PubMed] [Google Scholar]
- 7. Chirinos JA, Segers P, Hughes T, Townsend R. Large‐artery stiffness in health and disease: state‐of‐the‐art review. J Am Coll Cardiol. 2019;74:1237–1263. doi: 10.1016/j.jacc.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236 [DOI] [PubMed] [Google Scholar]
- 9. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Cheng S, Aragam J, Levy D, Benjamin EJ, Vasan RS, et al. Relations of central hemodynamics and aortic stiffness with left ventricular structure and function: the Framingham heart study. J Am Heart Assoc. 2016;5:e002693. doi: 10.1161/JAHA.115.002693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bell V, Sigurdsson S, Westenberg JJ, Gotal JD, Torjesen AA, Aspelund T, Launer LJ, Harris TB, Gudnason V, de Roos A, et al. Relations between aortic stiffness and left ventricular structure and function in older participants in the age, gene/environment susceptibility–Reykjavik study. Circ Cardiovasc Imaging. 2015;8:e003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouthier JD, De Luca N, Safar ME, Simon AC. Cardiac hypertrophy and arterial distensibility in essential hypertension. Am Heart J. 1985;109:1345–1352. doi: 10.1016/0002-8703(85)90364-3 [DOI] [PubMed] [Google Scholar]
- 12. Rabkin SW, Chan SH. Correlation of pulse wave velocity with left ventricular mass in patients with hypertension once blood pressure has been normalized. Heart Int. 2012;7:e5. doi: 10.4081/hi.2012.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borlaug BA, Kass DA. Ventricular‐vascular interaction in heart failure. Cardiol Clin. 2011;29:447–459. doi: 10.1016/j.ccl.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 14. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566 [DOI] [PubMed] [Google Scholar]
- 15. Redheuil A, Yu WC, Wu CO, Mousseaux E, de Cesare A, Yan R, Kachenoura N, Bluemke D, Lima JA. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010;55:319–326. doi: 10.1161/HYPERTENSIONAHA.109.141275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scarabello M, Codari M, Secchi F, Cannaò PM, Alì M, Di Leo G, Sardanelli F. Strain of ascending aorta on cardiac magnetic resonance in 1027 patients: relation with age, gender, and cardiovascular disease. Eur J Radiol. 2018;99:34–39. doi: 10.1016/j.ejrad.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 17. Cavalcante JL, Lima JA, Redheuil A, Al‐Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. doi: 10.1016/j.jacc.2010.12.017 [DOI] [PubMed] [Google Scholar]
- 18. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813 [DOI] [PubMed] [Google Scholar]
- 19. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 20. Tsao CW, Gona PN, Salton CJ, Chuang ML, Levy D, Manning WJ, O'Donnell CJ. Left ventricular structure and risk of cardiovascular events: a Framingham heart study cardiac magnetic resonance study. J Am Heart Assoc. 2015;4:e002188. doi: 10.1161/JAHA.115.002188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salton CJ, Chuang ML, O'Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, Edelman RR, Levy D, Manning WJ. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham heart study offspring cohort. J Am Coll Cardiol. 2002;39:1055–1060. doi: 10.1016/s0735-1097(02)01712-6 [DOI] [PubMed] [Google Scholar]
- 22. Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Noda C, Ambale Venkatesh B, Ohyama Y, Liu CY, Chamera E, Redheuil A, Teixido‐Tura G, Chugh AR, Wu CO, Hundley GW, et al. Reproducibility of functional aortic analysis using magnetic resonance imaging: the MESA. Euro Heart J Cardiovasc Imaging. 2016;17:909–917. doi: 10.1093/ehjci/jev215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nwabuo CC, Duncan M, Xanthakis V, Peterson LR, Mitchell GF, McManus D, Cheng S, Vasan RS. Association of circulating ceramides with cardiac structure and function in the community: the Framingham heart study. J Am Heart Assoc. 2019;8:e013050. doi: 10.1161/JAHA.119.013050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weber T, Chirinos JA. Pulsatile arterial haemodynamics in heart failure. Eur Heart J. 2018;39:3847–3854. doi: 10.1093/eurheartj/ehy346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gillebert TC, Leite‐Moreira AF, De Hert SG. Load dependent diastolic dysfunction in heart failure. Heart Fail Rev. 2000;5:345–355. doi: 10.1023/a:1026563313952 [DOI] [PubMed] [Google Scholar]
- 27. Lehmann ED. Noninvasive measurements of aortic stiffness: methodological considerations. Pathol Biol (Paris). 1999;47:716–730. [PubMed] [Google Scholar]
- 28. Rong LQ, Palumbo MC, Rahouma M, Lopes AJ, Devereux RB, Kim J, Girardi LN, Gaudino M, Weinsaft JW. Descending aortic strain quantification by intra‐operative transesophageal echocardiography: multimodality validation via cardiovascular magnetic resonance. Echocardiography. 2020;37:1820–1827. doi: 10.1111/echo.14851 [DOI] [PubMed] [Google Scholar]
- 29. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 30. Shim CY, Park S, Choi D, Yang WI, Cho IJ, Choi EY, Chung N, Ha JW. Sex differences in central hemodynamics and their relationship to left ventricular diastolic function. J Am Coll Cardiol. 2011;57:1226–1233. doi: 10.1016/j.jacc.2010.09.067 [DOI] [PubMed] [Google Scholar]
- 31. Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. doi: 10.1161/HYPERTENSIONAHA.112.191148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lau ES, Panah LG, Zern EK, Liu EE, Farrell R, Schoenike MW, Namasivayam M, Churchill TW, Curreri L, Malhotra R, et al. Arterial stiffness and vascular load in HFpEF: differences among women and men. J Card Fail. 2022;28:202–211. doi: 10.1016/j.cardfail.2021.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


