Abstract
Background
The patterns of depressive symptom change during the first month after discharge, as well as their prognostic implications, and predictors of persistent or new‐onset depressive symptoms are not well characterized.
Methods and Results
We included patients hospitalized for heart failure undergoing Patient Health Questionnaire‐2 before discharge and at 1 month after discharge in a multicenter prospective cohort. We characterized 4 patterns of change in depressive symptoms—persistent, new‐onset, remitted depressive symptoms, and no depressive symptom—and examined the associations between the 4 patterns and 1‐year clinical outcomes. We analyzed the factors associated with persistent or new‐onset depressive symptoms. A total of 4130 patients were included. Among 1175 (28.5%) symptomatic patients and 2955 (71.5%) symptom‐free patients before discharge, 817 (69.5%) had remission, and 366 (12.2%) had new‐onset depressive symptoms, respectively. Compared with no depressive symptom, persistent depressive symptoms were associated with an increased risk of cardiovascular death (hazard ratio [HR], 2.10 [95% CI, 1.59–2.79]) and heart failure rehospitalization (HR, 1.56 [95% CI, 1.30–1.87]); new‐onset depressive symptoms were associated with an increased risk of cardiovascular death (HR, 1.78 [95%CI, 1.32–2.40]) and heart failure rehospitalization (HR, 1.54 [95% CI, 1.29–1.83]). Remitted depressive symptoms were associated with a slightly increased risk of cardiovascular death but had no significant association with heart failure rehospitalization. Patients who were female or had poor socioeconomic status, stroke history, renal dysfunction, or poor health status had a higher risk of persistent or new‐onset depressive symptoms.
Conclusions
Sex, socioeconomic status, clinical characteristics, and health status help identify patients with high risks of depressive symptoms at 1 month after discharge. Dynamic capture of depressive symptom change during this period informs long‐term risk stratifications and targets patients who require psychological interventions and social support to improve clinical outcomes.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier (NCT02878811).
Keywords: death, depressive symptom, heart failure, postdischarge period, rehospitalization
Subject Categories: Mental Health, Heart Failure
Nonstandard Abbreviations and Acronyms
- China PEACE5p‐HF Study
China Patient‐Centered Evaluative Assessment of Cardiac Events Prospective Heart Failure Study
- KCCQ‐12
Kansas City Cardiomyopathy Questionnaire‐12
- PHQ‐2
Patient Health Questionnaire‐2
- RASI
renin‐angiotensin system inhibitors
Clinical Perspective.
What Is New?
By leveraging a contemporary multicenter prospective cohort of patients hospitalized for heart failure, our study first examined the associations between change in depressive symptoms during the first month after discharge and 1‐year risk of death or rehospitalization.
Patients with persistent, new‐onset, or remitted depressive symptoms at 1 month after discharge had 110%, 80%, and 30% higher risk of 1‐year cardiovascular death compared with those without depressive symptom, respectively.
Patients who were female or had poor socioeconomic status, stroke history, renal dysfunction, or poor health status had a higher risk of persistent or new‐onset depressive symptoms at 1 month after discharge.
What Are the Clinical Implications?
Persistent or new‐onset depressive symptoms during the first month of discharge are associated with worse clinical outcomes among patients hospitalized for heart failure, highlighting the importance of repeated assessment of depression to target high‐risk patients for psychological intervention and social support to improve their outcomes.
Heart failure (HF) is a leading cause of hospitalization associated with substantial mortality, morbidity, and health care expenditures. 1 , 2 Depression is a frequent and debilitating comorbidity affecting 10% to 30% of patients with acute HF, 3 , 4 and the number of those with depressive symptoms is even larger. Comorbid depressive symptoms compromised patients' adherence to therapies 5 and healthy lifestyles 6 and were associated with impaired quality of life, 7 recurrent rehospitalization, 8 increased mortality, 4 and augmented costs. 9
Depressive symptoms are characterized by relapsing and remitting courses, 10 and repeated measurements of symptoms after discharge may better reflect future risks of adverse events than a single measurement. Johansson et al reported that among patients with HF enrolled in a randomized controlled trial, 26.2% of patients had persistent or new‐onset depressive symptoms at 18 months after hospital discharge that were associated with a higher risk of death or rehospitalization. 11 However, the long time interval may have concealed any remission or recurrence. Although the preliminary results from a small study suggested patients with acute HF experienced significant change in depressive symptoms during the first month of discharge, 12 the patterns of depressive symptom change in this vulnerable period, 13 , 14 as well as their prognostic significance and associated patient characteristics, remain unknown. Multiple stressors during this period, including changes in diet, lack of physical activity, or confrontation with family or social stresses, 15 can hypothetically arouse fluctuations of ongoing depressive symptoms or even provoke new depressive episodes.
Accordingly, we used data from a multicenter prospective cohort of real‐world patients hospitalized for HF and aimed to characterize the changes in depression symptoms from before discharge to 1 month after discharge, to examine the associations between the patterns of depressive symptom change and 1‐year clinical outcomes and to identify the predictors of persistent depressive symptoms or new‐onset depressive symptoms. Addressing these issues could provide important insights for targeting psychological intervention and social support to improve clinical outcomes.
METHODS
Data Availability
The data underlying this article currently cannot be shared publicly.
Study Design and Population
China PEACE 5p‐HF Study (China Patient‐Centered Evaluative Assessment of Cardiac Events Prospective Heart Failure Study) is a nationwide prospective cohort of patients with acute HF from 52 hospitals located in 20 provinces between August 2016 and May 2018. The design has been detailed elsewhere. 16 Briefly, we invited consecutive patients who were aged 18 years or older and hospitalized for newly diagnosed or acute decompensated HF to participate in the study within 48 hours of their admission. Patients who signed informed consent were enrolled and followed up at 1, 6, and 12 months after discharge. The diagnosis criteria of HF were based on the Chinese guidelines of HF, 17 which are consistent with those of the American College of Cardiology/American Heart Association 18 and European Society of Cardiology. 19 Patients who did not complete the Patient Health Questionnaire‐2 (PHQ‐2), a simple and user‐friendly screening test for depressive symptoms, either before discharge or at 1 month after discharge were excluded from the current analysis. The study was approved by ethics committees of Fuwai Hospital and local sites. The study was registered on www.clinicaltrials.gov (NCT02878811).
We divided the patients into 4 groups according to their baseline and 1‐month PHQ‐2 score: no depressive symptom (no depressive symptom before discharge and 1 month), remitted depressive symptoms (depressive symptoms before discharge and remitted at 1 month), new‐onset depressive symptoms (no depressive symptom before discharge and developed at 1 month), and persistent depressive symptoms (depressive symptoms before discharge and 1 month).
Data Collection and Definition
Demographics (age, sex), socioeconomic status (education level, marital status, employment status, medical debt), smoking, and self‐reported health status were collected by standardized questionnaire through face‐to‐face interviews during index hospitalization. Education level was categorized as less than high school or high school or above. Employed was defined as undertaking nonmanual or manual work before the index hospitalization. Unemployed was defined as previously employed (including retirement, layoff, unable to work because of permanent illness or disability, unwilling to work, and the need to take care of family) or never employed. 20 Medical debt was defined as having borrowed money from others to pay for medical expenses during the past 12 months. Patients' health status at admission was measured by Kansas City Cardiomyopathy Questionnaire‐12 (KCCQ‐12). The total score ranged from 0 to 100, and a lower score was regarded as worse health status. 21 Cognitive function was assessed by Mini‐Cog test before discharge, with scores ranging from 0 to 5 (scores ≤2 indicating cognitive impairment). 22 Cardiovascular risk factors, New York Heart Association class, comorbidities, systolic blood pressure, and heart rate were obtained through central medical record abstraction. Left ventricular ejection fraction (LVEF) was measured before discharge. LVEF phenotypes were categorized as HF with reduced EF (HFrEF, LVEF<40%), HF with mildly reduced EF (40% ≤LVEF<50%) and HF with preserved EF (LVEF≥50%). NT‐proBNP N‐terminal pro‐brain‐type natriuretic peptide and creatinine were analyzed at central laboratory with samples collected within 48 hours of admission. We calculated the estimated glomerular filtration rate (eGFR) with an equation developed by adaptation of the Modification of Diet in Renal Disease equation based on data from Chinese patients with chronic kidney disease. 23 Self‐reported use of medications was recorded at each follow‐up. Major adverse cardiovascular events (MACE) were collected and adjudicated centrally based on medical records by trained clinicians, comprising hospitalization for angina, nonfatal myocardial infarction, cardiac arrest with successful resuscitation, HF rehospitalization, and stroke.
Assessment of Depressive Symptoms
Depressive symptoms were assessed before discharge by PHQ‐2, a short version of PHQ‐9. PHQ‐2 has comparable reliability in identifying patients at an increased risk of adverse outcomes, and higher feasibility than PHQ‐9. 24 PHQ‐2 evaluates the symptoms of depressed mood and anhedonia via 2 items, including “little interest or pleasure in doing things” and “feeling down, depressed, or hopeless.” For each symptom, a score of 0, 1, 2, or 3 is assigned to 4 response options ranging from “not at all” to “nearly every day” to estimate the frequency. The total score ranges from 0 to 6, and exceeding 3 is considered as having depression symptoms, with a sensitivity of 83% and a specificity of 92% for depression in patients from primary care settings. 25
Outcomes
The outcomes of the study included cardiovascular death, all‐cause death, HF rehospitalization, and all‐cause rehospitalization during 1 year of discharge and were collected and adjudicated centrally based on medical records by trained clinicians. Survival status and cause of death were further confirmed according to the national database of death cause.
Statistical Analysis
Continuous variables were described by mean with SD or median with interquartile range, and categorical variables were described by frequency with percentage. Baseline characteristics were compared using Kruskal–Wallis test for continuous variables and the chi‐square test for categorical variables, grouped by patterns of depressive symptom change. We plotted outcomes of 4 patterns by Kaplan–Meier curves and compared them by the log‐rank test.
Cox proportional hazards models with shared gamma frailty approach were used to assess the associations of depressive symptom change and the outcomes, counting hospitals as random effects, and adjusting for age (<65, ≥65 years), sex (male, female), smoking status (current smoker, nonsmoker), education level (less than high school, high school or above), New York Heart Association class at admission (II, III, IV), prior HF (new‐onset HF, decompensated chronic HF), hypertension, diabetes, prior myocardial infarction, stroke, anemia, systolic blood pressure at admission (<120, 120–140, ≥140 mm Hg), LVEF phenotype (HFrEF, HF with mildly reduced EF, HF with preserved EF), NT‐proBNP (stratified into trichotomies), and eGFR (<45, ≥45 mL/min per 1.73 m2), self‐reported use of medications at 1 month, including renin‐angiotensin system inhibitors, β‐blocker, and spironolactone and MACE within 1 month after discharge. Proportional hazard assumptions were not violated according to Schoenfeld residual analyses. For cardiovascular death or rehospitalization, noncardiovascular death or all‐cause death was censored, respectively. We also added interaction terms of age (<65, ≥65 years), sex (male, female), and LVEF phenotype (HFrEF, HF with mildly reduced EF, HF with preserved EF) to identify potential modification effects. We also examined the association of 4 patterns of change in depressed mood or anhedonia identified from 2 PHQ‐2 items with 1‐year clinical outcomes separately. For each item, we considered score exceeding 0 as having corresponding symptoms and generated 4 patterns of change in corresponding symptoms: persistent (having symptoms before discharge and 1 month), new‐onset (no symptom before discharge and developed at 1 month), remitted (having symptoms before discharge and remitted at 1 month) symptoms, and no symptom (no symptom before discharge and 1 month). To confirm the robustness of the results from the Cox proportional hazards models, we also fitted generalized linear mixed models counting hospitals as random effects adjusting for the same variables as the Cox models.
A generalized linear mixed model counting hospitals as random effects was used to determine the factors associated with persistent or new‐onset depressive symptoms at 1 month after discharge, with no depressive symptom or remitted depressive symptoms as reference. Candidate variables in the multivariate analysis included age (<65, ≥65 years), sex (male, female), marital status (in marriage, not in marriage), education level (under high school, high school or above), employment status (employed, unemployed), medical debt, prior HF (new‐onset HF, decompensated chronic HF), hypertension, diabetes, prior myocardial infarction, stroke, anemia, LVEF phenotype (HFrEF, HF with mildly reduced EF/HF with preserved EF), eGFR (<45, ≥45 mL/min per 1.73 m2), NT‐proBNP (stratified into dichotomies), 5‐unit decrease of KCCQ score at admission, and 1‐unit decrease in Mini‐Cog score before discharge and MACE within 1 month after discharge. For consistency analyses, we repeated the generalized linear mixed model using a multinomial distribution with patterns of depressive symptoms change as a 4‐level dependent variable, with no depressive symptom as reference. We also incorporated persistent, new‐onset, or remitted depressive symptoms as an event of interest and determined the factors associated with this event.
To evaluate the effect of missing PHQ‐2 score on the results, we conducted a sensitivity analysis using a propensity method. 26 Propensity scores were generated using logistic regression to estimate the probability of missing PHQ‐2 score before discharge or at 1 month after discharge, incorporating demographic and clinical characteristics and health status as predictors. We used the inverse of the propensity score as a means of weighting the observed proportion of 4 patterns of depressive symptom change 27 and reperformed the similar Cox regression models and generalized linear mixed models as the main analyses. To minimize the effect of false negative results from PHQ‐2 measurement, we altered the cutoff score of PHQ‐2 from 3 to 2, which was reported to have a higher sensitivity, 28 and assessed the associations of depressive symptom change and the outcomes in a similar approach.
Rates of missing value ranged from 0.1% (creatinine) to 4.4% (LVEF). Missing values of continuous variables were imputed with the Markov chain Monte‐Carlo method, taking the average of 10× imputation value as the final value. A 2‐sided P value of <0.05 was considered statistically significant, and all statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
We enrolled 4907 patients in the China PEACE 5p‐HF Study. We excluded 335 patients who did not complete the PHQ‐2 before discharge and another 442 who did not complete the PHQ‐2 at 1 month after discharge. Finally, 4130 patients were included in the current analysis. Compared with the study cohort, patients who did not complete the PHQ‐2 were less likely to have low education level, be marrried, be unemployed, have medical debt, and be current smokers; they were more likely to have anemia, higher NT‐proBNP level, lower eGFR level, lower systolic blood pressure level, higher heart rate level, HFrEF, lower KCCQ‐12 score, lower Mini‐Cog score; less likely to receive renin‐angiotensin system inhibitors, β‐blocker, or spironolactone at discharge; and more likely to have MACE within 1 month after discharge (Table S1).
The average age of the study cohort was 65.1±13.5 years, and 38.0% were female. PHQ‐2 was performed after 7 (6–10) days of admission. There were 1175 (28.5%) patients with depressive symptoms before discharge, 817 (69.5%) of whom had remission at 1 month after discharge. Among 2955 (71.5%) symptom‐free patients before discharge, 366 (12.4%) developed new‐onset depressive symptoms at 1 month after discharge. The patterns of depressive symptom change were generally similar across subgroups of age, sex, and LVEF phenotype (Figure 1). Compared with the patients without depressive symptom, those with persistent or new‐onset depressive symptoms were more likely to be female and unemployed and have low education level, medical debt, poor New York Heart Association cardiac function, history of diabetes or stroke, higher NT‐proBNP level, lower eGFR, and lower KCCQ‐12 score; were less likely to have HFrEF and to receive evidence‐based medications both at discharge and at 1 month postdischarge; and were more likely to have MACE within 1 month after discharge. The characteristics of patients with remitted depressive symptoms mostly resembled those of patients with persistent or new‐onset depressive symptoms, except that the prevalence of HFrEF was similar to that in patients without depressive symptom (Table 1).
Figure 1. Patterns of depressive symptom change stratified by age, sex, and LVEF phenotype.
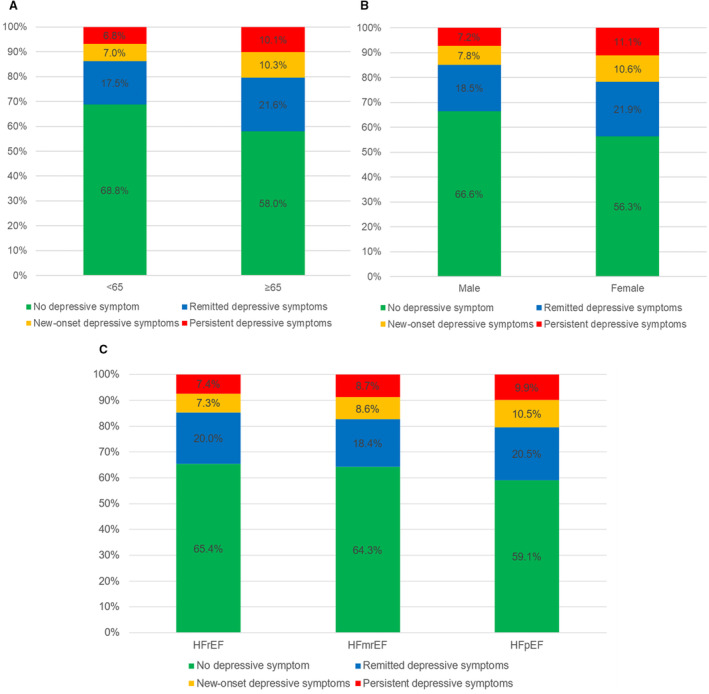
(A) Age (<65, ≥65), (B) sex (male, female), (C) LVEF phenotype (HFrEF, HFmrEF, HFpEF). No depressive symptom: PHQ‐2 <2 before discharge and <2 at 1 month; remitted depressive symptoms: PHQ‐2 ≥3 before discharge and <2 at 1 month; new‐onset depressive symptoms: PHQ‐2 <2 before discharge and ≥3 at 1 month; persistent depressive symptoms: PHQ‐2 ≥3 before discharge and ≥3 at 1 month. HFmrEF indicates heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; and LVEF, left ventricular ejection fraction.
Table 1.
Baseline Characteristics by Patterns of Depressive Symptom Change at 1 Month After Discharge
| Variables | Total (N=4130) | No depressive symptom (N=2589) | Remitted depressive symptoms (N=817) | New‐onset depressive symptoms (N=366) | Persistent depressive symptoms (N=358) | P value |
|---|---|---|---|---|---|---|
| Age, y, mean±SD | 65.1±13.5 | 63.5±13.7 | 67.2±13.1 | 67.7±12.3 | 69.0±12.0 | <0.001 |
| Female sex, n (%) | 1569 (38.0) | 884 (34.1) | 344 (42.1) | 167 (45.6) | 174 (48.6) | <0.001 |
| Socioeconomics, n (%) | ||||||
| Education level: under high school | 2964 (71.8) | 1803 (69.6) | 608 (74.4) | 283 (77.3) | 270 (75.4) | 0.001 |
| In marriage | 3328 (80.6) | 2161 (83.5) | 622 (76.1) | 279 (76.2) | 266 (74.3) | <0.001 |
| Unemployed | 3400 (82.3) | 2010 (77.6) | 718 (87.9) | 336 (91.8) | 336 (93.9) | <0.001 |
| Medical debt | 418 (10.1) | 193 (7.5) | 111 (13.6) | 48 (13.1) | 66 (18.4) | <0.001 |
| Cardiovascular risk factors, n (%) | ||||||
| Current smoker | 1055 (25.5) | 749 (28.9) | 173 (21.2) | 72 (19.7) | 61 (17.0) | <0.001 |
| Hypertension | 2421 (58.6) | 1499 (57.9) | 472 (57.8) | 219 (59.8) | 231 (64.5) | 0.104 |
| Diabetes | 1305 (31.6) | 787 (30.4) | 261 (31.9) | 118 (32.2) | 139 (38.8) | 0.015 |
| Decompensated chronic HF, n (%) | 2914 (70.6) | 1730 (66.8) | 622 (76.1) | 268 (73.2) | 294 (82.1) | <0.001 |
| New York Heart Association class, n (%) | <0.001 | |||||
| II | 605 (14.7) | 426 (16.5) | 96 (11.7) | 45 (12.3) | 38 (10.6) | |
| III | 1864 (45.1) | 1199 (46.3) | 346 (42.4) | 185 (50.5) | 134 (37.4) | |
| IV | 1661 (40.2) | 964 (37.2) | 375 (45.9) | 136 (37.2) | 186 (52.0) | |
| Comorbidities, n (%) | ||||||
| Myocardial infarction | 867 (21.0) | 529 (20.4) | 179 (21.9) | 64 (17.5) | 95 (26.5) | 0.017 |
| Stroke | 842 (20.4) | 462 (17.8) | 186 (22.8) | 85 (23.2) | 109 (30.4) | <0.001 |
| Anemia | 919 (22.3) | 501 (19.4) | 214 (26.2) | 92 (25.1) | 112 (31.3) | <0.001 |
| Laboratory test | ||||||
| N‐terminal pro‐B‐type natriuretic peptide, pg/mL, median (interquartile range) | 1370 (574, 2996) | 1204 (517, 2624) | 1659 (709, 3301) | 1527 (649, 3559) | 2184 (729, 4829) | <0.001 |
| Estimated glomerular filtration rate, mean±SD | 73.5±25.2 | 75.6±24.7 | 71.2±24.9 | 72.0±26.6 | 65.4±25.4 | <0.001 |
| Clinical characteristics | ||||||
| Systolic blood pressure, mm Hg, mean±SD | 134±24 | 134±24 | 134±25 | 133±23 | 135±27 | 0.797 |
| Heart rate, bpm, mean±SD | 89±23 | 89±22 | 90±23 | 90±24 | 88±22 | 0.845 |
| Left ventricular EF phenotype, n (%) | 0.001 | |||||
| HF with reduced EF | 1537 (37.2) | 1005 (38.8) | 307 (37.6) | 112 (30.6) | 113 (31.6) | |
| HF with mildly reduced EF | 1007 (24.4) | 647 (25.0) | 185 (22.6) | 87 (23.8) | 88 (24.6) | |
| HF with preserved EF | 1586 (38.4) | 937 (36.2) | 325 (39.8) | 167 (45.6) | 157 (43.9) | |
| Mini‐Cog score, mean±SD | 3.4±1.7 | 3.5±1.6 | 3.1±1.7 | 3.1±1.7 | 2.9±1.8 | <0.001 |
| Kansas City Cardiomyopathy Questionnaire‐12 score, mean±SD | 44.5±22.7 | 49.7±22.1 | 36.3±21.2 | 40.3±21.1 | 29.6±19.4 | <0.001 |
| Medications at discharge, n (%) | ||||||
| RASI | 2187 (53.0) | 1449 (56.0) | 398 (48.7) | 169 (46.2) | 171 (47.8) | <0.001 |
| ACEI | 1319 (31.9) | 880 (34.0) | 247 (30.2) | 91 (24.9) | 101 (28.2) | 0.001 |
| ARB | 880 (21.3) | 576 (22.2) | 153 (18.7) | 79 (21.6) | 72 (20.1) | 0.177 |
| β‐receptor blocker | 2478 (60.0) | 1652 (63.8) | 455 (55.7) | 190 (51.9) | 181 (50.6) | <0.001 |
| Aldosterone antagonists | 2642 (64.0) | 1698 (65.6) | 516 (63.2) | 216 (59.0) | 212 (59.2) | 0.014 |
| Medications at 1 mo, n (%) | ||||||
| RASI | 1550 (37.5) | 132 (36.1) | 1035 (40.0) | 125 (34.9) | 258 (31.6) | <0.001 |
| ACEI | 916 (22.2) | 66 (18.0) | 620 (23.9) | 72 (20.1) | 158 (19.3) | 0.005 |
| ARB | 641 (15.5) | 67 (18.3) | 419 (16.2) | 53 (14.8) | 102 (12.5) | 0.030 |
| β‐receptor blocker | 2305 (55.8) | 185 (50.5) | 1509 (58.3) | 179 (50.0) | 432 (52.9) | <0.001 |
| Aldosterone antagonists | 2063 (50.0) | 178 (48.6) | 1238 (47.8) | 190 (53.1) | 457 (55.9) | <0.001 |
| Major adverse cardiovascular events within 1 mo after discharge, n (%) | 321 (7.8) | 176 (6.8) | 70 (8.6) | 37 (10.1) | 38 (10.6) | 0.012 |
No depressive symptom: PHQ‐2 <2 before discharge and <2 at 1 month; remitted depressive symptoms: PHQ‐2 ≥3 before discharge and <2 at 1 month; new‐onset depressive symptoms: PHQ‐2 <2 before discharge and ≥3 at 1 month; persistent depressive symptoms: PHQ‐2 ≥3 before discharge and ≥3 at 1 month. ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blocker; EF, ejection fraction; HF, heart failure; PHQ‐2, Patient Health Questionnaire‐2; and RASI, renin‐angiotensin system inhibitor.
Association Between Clinical Outcomes and 4 Patterns of Depressive Symptom Change
During 1‐year follow‐up, 522 (12.6%) patients died, and 456 (11.0%) died of cardiovascular causes; 2024 (49.0%) patients experienced rehospitalization, and 1364 (33.0%) had HF rehospitalization. Cumulative incidences of the outcomes differed significantly among the 4 patterns of depressive symptom change (all P<0.001). Cumulative incidences of death demonstrated a downward gradient from patients with persistent, new‐onset, or remitted depressive symptoms compared with those with no depressive symptom; patients with persistent or new‐onset depressive symptoms had similar incidences of HF rehospitalization or all‐cause rehospitalization and substantially higher incidences than those with remitted depressive symptoms or no depressive symptom (Figure 2).
Figure 2. Outcomes of patients stratified by patterns of depressive symptom change.
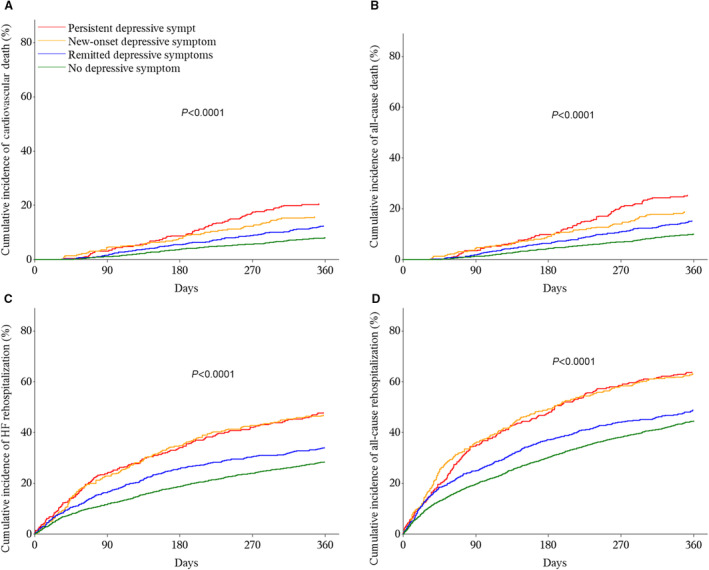
(A) Cardiovascular death, (B) all‐cause death, (C) heart failure rehospitalization, (D) all‐cause rehospitalization. No depressive symptom: PHQ‐2 <2 before discharge and <2 at 1 month; remitted depressive symptoms: PHQ‐2 ≥3 before discharge and <2 at 1 month; new‐onset depressive symptoms: PHQ‐2 <2 before discharge and ≥3 at 1 month; persistent depressive symptoms: PHQ‐2 ≥3 before discharge and ≥3 at 1 month. HF indicates heart failure; and PHQ‐2, Patient Health Questionnaire‐2.
After adjusting potential confounders, compared with no depressive symptom, persistent depressive symptoms had an increased risk of cardiovascular death (hazard ratio [HR], 2.10 [95% CI, 1.59–2.79], P<0.001), all‐cause death (HR, 1.91 [95% CI, 1.46–2.48], P<0.001), HF rehospitalization (HR, 1.56 [95% CI, 1.30–1.87], P<0.001), and all‐cause rehospitalization (HR, 1.36 [95% CI, 1.17–1.58], P<0.001). Similarly, new‐onset depressive symptoms had an increased risk of cardiovascular death (HR, 1.78 [95% CI, 1.32–2.40], P<0.001), all‐cause death (HR, 1.60 [95% CI, 1.20–2.12], P=0.001), HF rehospitalization (HR, 1.54 [95% CI, 1.29–1.83], P<0.001), and all‐cause rehospitalization (HR, 1.46 [95% CI, 1.26–1.69], P<0.001). Although remitted depressive symptoms had a slightly increased risk of cardiovascular death (HR, 1.30 [95% CI, 1.02–1.66], P=0.032), no significant association was observed with all‐cause death (HR, 1.23 [95% CI, 0.98–1.54], P=0.076), HF rehospitalization (HR, 1.14 [95% CI, 0.98–1.31], P=0.083), or all‐cause rehospitalization (HR, 1.03 [95% CI, 0.92–1.16], P=0.588) (Figure 3). After fitting generalized linear mixed models, the results were similar with the main analyses (Figure S1). There was no significant heterogeneity across subgroups of age, sex, and LVEF phenotype (Tables S2 through S4). After separating 2 symptoms identified from PHQ‐2 to assess the association with outcomes, new‐onset or persistent depressed mood was associated with higher risks of death or hospitalization. Similar findings were observed for new‐onset or persistent anhedonia, although the effect size seemed larger (Table S5).
Figure 3. Associations between 4 patterns of depressive symptom change at 1 month after discharge and clinical outcomes.
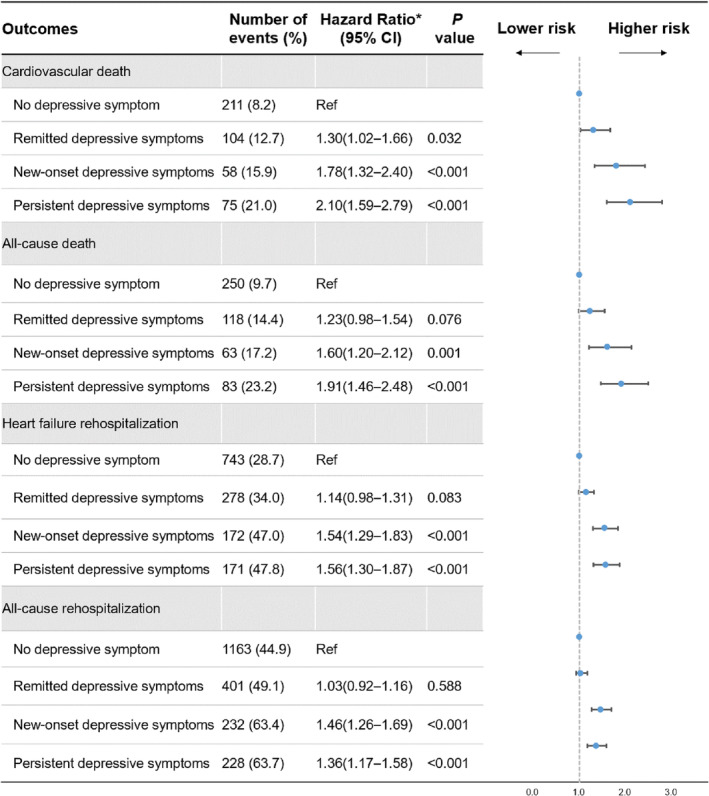
No depressive symptom: PHQ‐2 <2 before discharge and <2 at 1 month; remitted depressive symptoms: PHQ‐2 ≥3 before discharge and <2 at 1 month; new‐onset depressive symptoms: PHQ‐2 <2 before discharge and ≥3 at 1 month; persistent depressive symptoms: PHQ‐2 ≥3 before discharge and ≥3 at 1 month. *Counting hospitals as random effects and adjusting for age (<65, ≥65 years), sex (male, female), smoking status (current smoker, nonsmoker), education level (less than high school, high school or above), prior HF, New York Heart Association class (II, III, IV), hypertension, diabetes, prior myocardial infarction, stroke, anemia, systolic blood pressure (<120, 120–140, ≥140 mm Hg), LVEF phenotype (HFrEF, HFmrEF, HFpEF), N‐terminal pro‐B‐type natriuretic peptide (stratified into trichotomies), eGFR (<45, ≥45 mL/min per 1.73 m2), self‐report use of medications at 1 month after discharge (including renin‐angiotensin system inhibitors, β‐blocker, and spironolactone), and MACE within 1 month after discharge. eGFR indicates estimated glomerular filtration rate; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; KCCQ‐12; LVEF, left ventricular ejection fraction; OR, odds ratio; MACE, major adverse cardiovascular events; and PHQ‐2, Patient Health Questionnaire‐2.
Factors Associated With Change in Depressive Symptoms
Unemployed status (odds ratio [OR], 1.68 [95% CI, 1.21–2.34]), medical debt (OR, 1.78 [95% CI, 1.38–2.31]), eGFR<45 mL/min per 1.73 m2 (OR, 1.30 [95% CI, 1.01–1.68]), a 5‐unit decrease of KCCQ‐12 score at admission (OR, 1.10 [95% CI, 1.08–1.12]), and a 1‐unit decrease in Mini‐Cog score before discharge (OR, 1.08 [95% CI, 1.02–1.14]) were associated with a higher risk of persistent or new‐onset depressive symptoms (Table 2). Female sex (OR, 1.20 [95% CI, 1.00–1.45]) and stroke history (OR, 1.22 [95% CI, 0.99–1.50]) were marginally associated with a higher risk. When treating persistent, new‐onset, or remitted depressive symptoms as a 4‐level dependent variable, most factors associated with persistent or new‐onset depressive symptoms remained consistent with those identified in the main analyses (Table S6). When treating persistent, new‐onset, or remitted depressive symptoms as an entity, all the aforementioned factors were consistently relevant, except that eGFR<45 mL/min per 1.73 m2 was not significant, and there were additional significant factors of prior HF and high NT‐proBNP level (Table S7).
Table 2.
Patient Characteristics Associated With Persistent or New‐Onset Depressive Symptoms
| Factors (reference or unit of change) | Multivariate analysis | |
|---|---|---|
| Odds ratio (95% CI) | P value | |
| Age ≥65 y (<65 y) | 1.03 (0.84–1.26) | 0.793 |
| Female sex (male sex) | 1.20 (1.00–1.45) | 0.056 |
| In marriage (not in marriage) | 0.97 (0.79–1.20) | 0.793 |
| Less than high school (high school or above) | 1.02 (0.83–1.27) | 0.825 |
| Unemployed (employed) | 1.68 (1.21–2.34) | 0.002 |
| Medical debt (no medical debt) | 1.78 (1.38–2.31) | <0.001 |
| Decompensated chronic HF (new‐onset HF) | 1.18 (0.96–1.45) | 0.118 |
| Hypertension (no hypertension) | 0.99 (0.82–1.19) | 0.875 |
| Diabetes (no diabetes) | 1.11 (0.92–1.34) | 0.284 |
| Prior MI (no MI) | 0.94 (0.76–1.17) | 0.591 |
| Stroke (no stroke) | 1.22 (0.99–1.50) | 0.064 |
| Anemia (no anemia) | 1.12 (0.90–1.38) | 0.308 |
| HF with mildly reduced EF/HF with preserved EF (HF with reduced EF) | 1.15 (0.94–1.41) | 0.183 |
| Estimated glomerular filtration rate <45 (≥45 mL/min per 1.73 m2) | 1.30 (1.01–1.68) | 0.041 |
| High N‐terminal pro‐B‐type natriuretic peptide level (low level) | 1.11 (0.92–1.34) | 0.280 |
| Kansas City Cardiomyopathy Questionnaire‐12 score (per 5‐unit decrease) | 1.10 (1.08–1.12) | <0.001 |
| Mini‐Cog score (per 1‐unit decrease) | 1.08 (1.02–1.14) | 0.008 |
| MACE within 1 mo after discharge (no MACE) | 1.32 (0.98–1.77) | 0.063 |
New‐onset depressive symptoms: PHQ‐2 <2 before discharge and ≥3 at 1 month; persistent depressive symptoms: PHQ‐2 ≥3 before discharge and ≥3 at 1 month. EF indicates ejection fraction; HF, heart failure; MACE, major adverse cardiovascular events; MI, myocardial infarction; and PHQ‐2, Patient Health Questionnaire‐2.
Sensitivity Analyses
After accounting for missing PHQ‐2 scores by adjusting them with the inverse of propensity score weighting, the proportion of 4 patterns of depressive symptom change did not differ considerably with the main analyses (Figure S2). Association of change in depressive symptoms with the outcomes, as well as predictors of persistent or new‐onset depressive symptoms, were also similar (Table S8, Figure S3). Applying a lower PHQ‐2 cutoff score of 2 did not affect the association of change in depressive symptoms with the outcomes, except that remitted depressive symptoms were associated with all‐cause death (HR, 1.29 [95% CI, 1.02–1.64], P=0.035) (Figure S4).
DISCUSSION
By leveraging a contemporary multicenter prospective cohort of patients hospitalized for HF, our study first demonstrated significant change in depressive symptoms during the first month of discharge, which was associated with 1‐year risk of death or rehospitalization. One‐third of patients with depressive symptoms remained depressed, and 1 in 10 symptom‐free patients developed new‐onset depressive symptoms. We revealed a stepwise gradient in the overall risks of adverse prognosis from persistent, new‐onset, and remitted symptoms to no depressive symptom. Compared with patients without depressive symptom, those with persistent, new‐onset, and remitted depressive symptoms had 110%, 80%, and 30% higher risks of cardiovascular death, respectively, regardless of demographics, socioeconomics, clinical profiles, health status, and therapies. Persistent or new‐onset depressive symptoms also had a higher risk of rehospitalization. Women and patients with poor socioeconomics, stroke history, lower eGFR, or poor health status had a higher risk of persistent or new‐onset depressive symptoms. Our study highlighted the importance of repeated PHQ‐2 measurements, particularly in patients with acute HF with an increased risk of depressive symptoms, and targeted psychological intervention and social support.
Based on the largest cohort with HF to capture the depressive symptom changes across 2 successive assessments, we provided real‐world evidence on the fluctuant nature of depressive symptoms during the early postdischarge period. Although over two‐ thirds of patients with depressive symptoms had remission along with HF stabilization over the postdischarge period, a significant proportion experienced persistent or even new‐onset depressive symptoms, regardless of age, sex, and LVEF phenotype. Previous small studies also reported that 12% of patients negative for depression at discharge screened by the Geriatric Depression Scale had a positive screen at 4 weeks, 12 and about 60% of patients with mild depression measured by Hamilton Depression Rating Scale went into remission at 6 weeks. 29 Similar patterns were also observed in the studies that had longer assessment intervals with more sophisticated assessment tools. 7 , 11 , 30
Our study was distinguished by revealing the distinctive clinical outcomes along with the different depressive symptom changes at 1 month after discharge. Although studies reported that positive depression screening before discharge was associated with worse outcomes, 31 , 32 our study indicated that a single measurement was insufficient to orientate the fluctuant depressive symptoms and may ignore some patients with substantially elevated risks of death and rehospitalization. Compared with remitted symptoms, persistent or new‐onset depressive symptoms markedly deviated from the prognostic trajectory and had a more deleterious impact on death and rehospitalization. The ongoing depressive symptoms compromised patients' subjective physical function, 33 , 34 health‐related quality of life, 35 and medication adherence 36 and therefore induced adverse events. 37 Data from a randomized trial showed that compared with patients without depressive symptom, those with new‐onset depressive symptoms at 18 months after discharge had an increased risk of 3‐year mortality, 11 whereas persistent depressive symptoms had a slightly higher risk. Undetected fluctuations of depressive symptoms during the long‐term interval and highly selective patients in this study may account for the difference in the conclusions. 11 Previous studies in patients with chronic HF also found that worsening depressive symptoms over the 1‐year period was associated with an increased risk of all‐cause death. 38 , 39 Our study highlighted that clinicians could easily perform PHQ‐2 screening before discharge as well as at 1 month after discharge to identify the patients with high risks of depressive symptoms. Those patients may benefit from cognitive behavior therapies 40 or collaborative care interventions including nurse‐led assessment and monitoring, as well as structured psychosocial care from social workers. 41
Our study informed clinical care by identifying sex, socioeconomics, clinical profiles, and health status that predispose patients to persistent or new‐onset depressive symptoms. Sex disparity prevails in depression, 42 and a previous study also demonstrated that women were more vulnerable to depressive symptoms than men among patients with HF. 43 As important social determinants of health in patients with HF, 44 unemployed status and medical debt have been identified to predict incident depressive symptoms, echoing the findings from previous studies in patients with HF 45 , 46 or general populations. 47 , 48 Impaired renal function has been found to correlate with the development of depression, 49 and depression could also in turn accelerate the progression of chronic kidney disease and lead to poor outcomes in patients with chronic kidney disease. 50 Stroke history was associated with distress and impaired functional status from poststroke disabilities, 51 which may disturb the emotion regulation process after discharge. Lower KCCQ‐12 score indicated a worse functioning status and quality of life, 52 which were strongly associated with lower positive emotions in patients. 53 , 54 Havranek et al found a graded relationship between lower KCCQ‐12 score and an increased risk of depression in patients with acute HF. 45 The correlation between cognitive impairment and depression has been reported, 55 , 56 and a meta‐analysis demonstrated that about 32% of patients with mild cognitive impairment had depression. 57 Deteriorating memory function in the early stage of cognitive decline may induce depressive symptoms. 58
Our findings should be interpreted in the light of the following limitations. First, we did not collect the information of other psychiatric diseases or prescriptions of antidepressants or assess the social support or stressful events that could possibly influence the courses of the depressive symptoms. 59 Second, we could not preclude false‐negative results from PHQ‐2. However, we performed sensitivity analyses by applying a lower threshold and obtained consistent results. Third, patients who did not complete PHQ‐2 at 1 month after discharge had lower KCCQ‐12 scores and Mini‐Cog scores than those who completed it, suggesting their vulnerability to depressive symptoms. The proportion of persistent or new‐onset depressive symptoms, therefore, may be underestimated. We also performed several sensitivity analyses by accounting for the missing value of PHQ‐2, which obtained similar results. Fourth, our observational design could not rule out unmeasured confounding factors, although we collected and adjusted extensive clinical and nonclinical factors.
CONCLUSIONS
In conclusion, the significant change in depressive symptoms during the first month of HF hospitalization discharge and its association with clinical outcomes highlight the importance of repeated PHQ‐2 screens before discharge and 1 month later. Particular attention should be paid to women and patients with poor socioeconomic status, stroke history, renal dysfunction, or poor health status. Timely identification of patients with persistent or new‐onset symptoms at 1 month after discharge can target psychological interventions and social support, which may improve patient outcomes.
Sources of Funding
This work was supported by the China Academy of Chinese Medical Sciences Innovation Fund for Medical Science (2021‐I2M‐1‐009) and the National Key Technology R&D Program (2015BAI12B02) from the Ministry of Science and Technology of China.
Disclosures
Dr Li reported receiving research grants, through Fuwai Hospital, from the Chinese government and Chinese Academy of Medical Sciences for work to improve the management of hypertension and blood lipids and to improve patient outcomes of cardiovascular disease and COVID‐19; receiving research agreements, through the National Center for Cardiovascular Diseases and Fuwai Hospital, from Amgen for a multicenter clinical trial assessing the efficacy and safety of omecamtiv mecarbil and for registration of dyslipidemic patients; receiving a research agreement, through Fuwai Hospital, from Sanofi for a multicenter clinical trial on the effects of sotagliflozin; receiving a research agreement, through Fuwai Hospital, with the University of Oxford for a multicenter clinical trial of empagliflozin; receiving a research agreement, through the National Center for Cardiovascular Diseases, from AstraZeneca for clinical research methods training outside the submitted work; and receiving a research agreement, through the National Center for Cardiovascular Diseases, from Lilly for physician training outside the submitted work. No other disclosures were reported.
Supporting information
Table S1–S8
Figure S1–S4
Acknowledgments
We appreciate the multiple contributions made by project teams in the realms of study operation and data collection. We also thank Prof. Harlan M. Krumholz from Yale University, Prof. Frederick A. Masoudi from University of Colorado, and Prof. John A. Spertus from University of Missouri for their advice on the design of China PEACE 5p‐HF Study. We are grateful for the support provided by the Chinese government.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027438
For Sources of Funding and Disclosures, see page 12.
REFERENCES
- 1. Bragazzi NL, Zhong W, Shu J, Abu Much A, Lotan D, Grupper A, Younis A, Dai H. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. 2021;28:1682–1690. doi: 10.1093/eurjpc/zwaa147 [DOI] [PubMed] [Google Scholar]
- 2. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats A. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2022. doi: 10.1093/cvr/cvac013 [DOI] [PubMed] [Google Scholar]
- 3. Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta‐analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055 [DOI] [PubMed] [Google Scholar]
- 4. Sokoreli I, de Vries JJG, Pauws SC, Steyerberg EW. Depression and anxiety as predictors of mortality among heart failure patients: systematic review and meta‐analysis. Heart Fail Rev. 2016;21:49–63. doi: 10.1007/s10741-015-9517-4 [DOI] [PubMed] [Google Scholar]
- 5. Wu JR, Lennie TA, Dekker RL, Biddle MJ, Moser DK. Medication adherence, depressive symptoms, and cardiac event‐free survival in patients with heart failure. J Card Fail. 2013;19:317–324. doi: 10.1016/j.cardfail.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conraads VM, Deaton C, Piotrowicz E, Santaularia N, Tierney S, Piepoli MF, Pieske B, Schmid JP, Dickstein K, Ponikowski PP, et al. Adherence of heart failure patients to exercise: barriers and possible solutions: a position statement of the study group on exercise training in heart failure of the heart failure Association of the European Society of Cardiology. Eur J Heart Fail. 2012;14:451–458. doi: 10.1093/eurjhf/hfs048 [DOI] [PubMed] [Google Scholar]
- 7. Dekker RL, Lennie TA, Albert NM, Rayens MK, Chung ML, Wu JR, Song EK, Moser DK. Depressive symptom trajectory predicts 1‐year health‐related quality of life in patients with heart failure. J Card Fail. 2011;17:755–763. doi: 10.1016/j.cardfail.2011.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel N, Chakraborty S, Bandyopadhyay D, Amgai B, Hajra A, Atti V, Das A, Ghosh RK, Deedwania PC, Aronow WS, et al. Association between depression and readmission of heart failure: a national representative database study. Prog Cardiovasc Dis. 2020;63:585–590. doi: 10.1016/j.pcad.2020.03.014 [DOI] [PubMed] [Google Scholar]
- 9. Husaini BA, Taira D, Norris K, Adhish SV, Moonis M, Levine R. Depression effects on hospital cost of heart failure patients in California: an analysis by ethnicity and gender. Indian J Community Med. 2018;43:49–52. doi: 10.4103/ijcm.IJCM_151_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Judd LL, Akiskal HS. Delineating the longitudinal structure of depressive illness: beyond clinical subtypes and duration thresholds. Pharmacopsychiatry. 2000;33:3–7. doi: 10.1055/s-2000-7967 [DOI] [PubMed] [Google Scholar]
- 11. Johansson P, Lesman‐Leegte I, Lundgren J, Hillege HL, Hoes A, Sanderman R, van Veldhuisen DJ, Jaarsma T. Time‐course of depressive symptoms in patients with heart failure. J Psychosom Res. 2013;74:238–243. doi: 10.1016/j.jpsychores.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 12. Fulop G, Strain JJ, Stettin G. Congestive heart failure and depression in older adults: clinical course and health services use 6 months after hospitalization. Psychosomatics. 2003;44:367–373. doi: 10.1176/appi.psy.44.5.367 [DOI] [PubMed] [Google Scholar]
- 13. Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol. 2015;12:220–229. doi: 10.1038/nrcardio.2015.14 [DOI] [PubMed] [Google Scholar]
- 14. Vader JM, LaRue SJ, Stevens SR, Mentz RJ, DeVore AD, Lala A, Groarke JD, AbouEzzeddine OF, Dunlay SM, Grodin JL, et al. Timing and causes of readmission after acute heart failure hospitalization‐insights from the heart failure network trials. J Card Fail. 2016;22:875–883. doi: 10.1016/j.cardfail.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Metra M, Gheorghiade M, Bonow RO, Dei CL. Postdischarge assessment after a heart failure hospitalization: the next step forward. Circulation. 2010;122:1782–1785. doi: 10.1161/CIRCULATIONAHA.110.982207 [DOI] [PubMed] [Google Scholar]
- 16. Huang X, Yu Y, Li X, Masoudi FA, Spertus JA, Yan X, Krumholz HM, Jiang L, Li J. The China patient‐centred evaluative assessment of cardiac events (PEACE) prospective heart failure study design. BMJ Open. 2019;9:e025144. doi: 10.1136/bmjopen-2018-025144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. CHFAoCMDA HFGoCSoCoCMA and Cardiology EBoCJo . Chinese guidelines for the diagnosis and treatment of heart failure 2018. Zhonghua Xin Xue Guan Bing Za Zhi. 2018;46:760–789. [DOI] [PubMed] [Google Scholar]
- 18. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 19. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 20. Ge Y, Zhang L, Gao Y, Wang B, Zheng X. Socio‐economic status and 1 year mortality among patients hospitalized for heart failure in China. ESC Heart Fail. 2022;9:1027–1037. doi: 10.1002/ehf2.13762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spertus JA, Jones PG. Development and validation of a short version of the Kansas City cardiomyopathy questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469–476. doi: 10.1161/CIRCOUTCOMES.115.001958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borson S, Scanlan JM, Chen P, Ganguli M. The mini‐cog as a screen for dementia: validation in a population‐based sample. J Am Geriatr Soc. 2003;51:1451–1454. doi: 10.1046/j.1532-5415.2003.51465.x [DOI] [PubMed] [Google Scholar]
- 23. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368 [DOI] [PubMed] [Google Scholar]
- 24. Piepenburg SM, Faller H, Gelbrich G, Störk S, Warrings B, Ertl G, Angermann CE. Comparative potential of the 2‐item versus the 9‐item Patient Health Questionnaire to predict death or rehospitalization in heart failure. Circ Heart Fail. 2015;8:464–472. doi: 10.1161/CIRCHEARTFAILURE.114.001488 [DOI] [PubMed] [Google Scholar]
- 25. Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire‐2: validity of a two‐item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- 26. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langkamp DL, Lehman A, Lemeshow S. Techniques for handling missing data in secondary analyses of large surveys. Acad Pediatr. 2010;10:205–210. doi: 10.1016/j.acap.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arroll B, Goodyear‐Smith F, Crengle S, Gunn J, Kerse N, Fishman T, Falloon K, Hatcher S. Validation of PHQ‐2 and PHQ‐9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8:348–353. doi: 10.1370/afm.1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koenig HG. Depression in hospitalized older patients with congestive heart failure. Gen Hosp Psychiatry. 1998;20:29–43. doi: 10.1016/S0163-8343(98)80001-7 [DOI] [PubMed] [Google Scholar]
- 30. Johansson P, Lesman‐Leegte I, Svensson E, Voors A, van Veldhuisen DJ, Jaarsma T. Depressive symptoms and inflammation in patients hospitalized for heart failure. Am Heart J. 2011;161:1053–1059. doi: 10.1016/j.ahj.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 31. Piepenburg SM, Faller H, Störk S, Ertl G, Angermann CE. Symptom patterns and clinical outcomes in women versus men with systolic heart failure and depression. Clin Res Cardiol. 2019;108:244–253. doi: 10.1007/s00392-018-1348-6 [DOI] [PubMed] [Google Scholar]
- 32. Rollman BL, Herbeck Belnap B, Mazumdar S, Houck PR, He F, Alvarez RJ, Schulberg HC, Reynolds CF III, McNamara DM. A positive 2‐item patient health questionnaire depression screen among hospitalized heart failure patients is associated with elevated 12‐month mortality. J Card Fail. 2012;18:238–245. doi: 10.1016/j.cardfail.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shen BJ, Eisenberg SA, Maeda U, Farrell KA, Schwarz ER, Penedo FJ, Bauerlein EJ, Mallon S. Depression and anxiety predict decline in physical health functioning in patients with heart failure. Ann Behav Med. 2011;41:373–382. doi: 10.1007/s12160-010-9251-z [DOI] [PubMed] [Google Scholar]
- 34. Lossnitzer N, Feisst M, Wild B, Katus HA, Schultz JH, Frankenstein L, Stock C. Cross‐lagged analyses of the bidirectional relationship between depression and markers of chronic heart failure. Depress Anxiety. 2020;37:898–907. doi: 10.1002/da.23041 [DOI] [PubMed] [Google Scholar]
- 35. Scherer M, Düngen HD, Inkrot S, Tahirović E, Lashki DJ, Apostolović S, Edelmann F, Wachter R, Loncar G, Haverkamp W, et al. Determinants of change in quality of life in the cardiac insufficiency bisoprolol study in elderly (CIBIS‐ELD). Eur J Intern Med. 2013;24:333–338. doi: 10.1016/j.ejim.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 36. Shen BJ, Maeda U. Psychosocial predictors of self‐reported medical adherence in patients with heart failure over 6 months: an examination of the influences of depression, self‐efficacy, social support, and their changes. Ann Behav Med. 2018;52:613–619. doi: 10.1093/abm/kay003 [DOI] [PubMed] [Google Scholar]
- 37. Zuluaga MC, Guallar‐Castillón P, Rodríguez‐Pascual C, Conde‐Herrera M, Conthe P, Rodríguez‐Artalejo F. Mechanisms of the association between depressive symptoms and long‐term mortality in heart failure. Am Heart J. 2010;159:231–237. doi: 10.1016/j.ahj.2009.11.011 [DOI] [PubMed] [Google Scholar]
- 38. Chandra A, Alcala MAD, Claggett B, Desai AS, Fang JC, Heitner JF, Liu J, Pitt B, Solomon SD, Pfeffer MA, et al. Associations between depressive symptoms and HFpEF‐related outcomes. JACC Heart Fail. 2020;8:1009–1020. doi: 10.1016/j.jchf.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 39. Sherwood A, Blumenthal JA, Hinderliter AL, Koch GG, Adams KF Jr, Dupree CS, Bensimhon DR, Johnson KS, Trivedi R, Bowers M, et al. Worsening depressive symptoms are associated with adverse clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2011;57:418–423. doi: 10.1016/j.jacc.2010.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jeyanantham K, Kotecha D, Thanki D, Dekker R, Lane DA. Effects of cognitive behavioural therapy for depression in heart failure patients: a systematic review and meta‐analysis. Heart Fail Rev. 2017;22:731–741. doi: 10.1007/s10741-017-9640-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bekelman DB, Allen LA, McBryde CF, Hattler B, Fairclough DL, Havranek EP, Turvey C, Meek PM. Effect of a collaborative care intervention vs usual care on health status of patients with chronic heart failure: the CASA randomized clinical trial. JAMA Intern Med. 2018;178:511–519. doi: 10.1001/jamainternmed.2017.8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arias‐de la Torre J, Vilagut G, Ronaldson A, Serrano‐Blanco A, Martín V, Peters M, Valderas JM, Dregan A, Alonso J. Prevalence and variability of current depressive disorder in 27 European countries: a population‐based study. Lancet Public Health. 2021;6:e729–e738. doi: 10.1016/S2468-2667(21)00047-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dewan P, Rørth R, Jhund PS, Shen L, Raparelli V, Petrie MC, Abraham WT, Desai AS, Dickstein K, Køber L, et al. Differential impact of heart failure with reduced ejection fraction on men and women. J Am Coll Cardiol. 2019;73:29–40. doi: 10.1016/j.jacc.2018.09.081 [DOI] [PubMed] [Google Scholar]
- 44. White‐Williams C, Rossi LP, Bittner VA, Driscoll A, Durant RW, Granger BB, Graven LJ, Kitko L, Newlin K, Shirey M. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the American Heart Association. Circulation. 2020;141:e841–e863. [DOI] [PubMed] [Google Scholar]
- 45. Havranek EP, Spertus JA, Masoudi FA, Jones PG, Rumsfeld JS. Predictors of the onset of depressive symptoms in patients with heart failure. J Am Coll Cardiol. 2004;44:2333–2338. doi: 10.1016/j.jacc.2004.09.034 [DOI] [PubMed] [Google Scholar]
- 46. Aggelopoulou Z, Fotos NV, Chatziefstratiou AA, Giakoumidakis K, Elefsiniotis I, Brokalaki H. The level of anxiety, depression and quality of life among patients with heart failure in Greece. Appl Nurs Res. 2017;34:52–56. doi: 10.1016/j.apnr.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 47. Anthony JC, Petronis KR. Suspected risk factors for depression among adults 18–44 years old. Epidemiology. 1991;2:123–132. doi: 10.1097/00001648-199103000-00006 [DOI] [PubMed] [Google Scholar]
- 48. Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151:979–986. doi: 10.1176/ajp.151.7.979 [DOI] [PubMed] [Google Scholar]
- 49. Lin S, Luan X, He W, Ruan Y, Yuan C, Fan A, Chen X, He J. Post‐stroke depression and estimated glomerular filtration rate: a prospective stroke cohort. Neuropsychiatr Dis Treat. 2020;16:201–208. doi: 10.2147/NDT.S225905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hedayati SS, Minhajuddin AT, Afshar M, Toto RD, Trivedi MH, Rush AJ. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303:1946–1953. doi: 10.1001/jama.2010.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 52. Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City cardiomyopathy questionnaire in clinical trials and clinical care: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;76:2379–2390. doi: 10.1016/j.jacc.2020.09.542 [DOI] [PubMed] [Google Scholar]
- 53. Seale GS, Berges IM, Ottenbacher KJ, Ostir GV. Change in positive emotion and recovery of functional status following stroke. Rehabil Psychol. 2010;55:33–39. doi: 10.1037/a0018744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Heo S, Lennie TA, Okoli C, Moser DK. Quality of life in patients with heart failure: ask the patients. Heart Lung. 2009;38:100–108. doi: 10.1016/j.hrtlng.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bennett S, Thomas AJ. Depression and dementia: cause, consequence or coincidence? Maturitas. 2014;79:184–190. doi: 10.1016/j.maturitas.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 56. Rubin R. Exploring the relationship between depression and dementia. JAMA. 2018;320:961–962. doi: 10.1001/jama.2018.11154 [DOI] [PubMed] [Google Scholar]
- 57. Ismail Z, Elbayoumi H, Fischer CE, Hogan DB, Millikin CP, Schweizer T, Mortby ME, Smith EE, Patten SB, Fiest KM. Prevalence of depression in patients with mild cognitive impairment: a systematic review and meta‐analysis. JAMA Psychiatry. 2017;74:58–67. doi: 10.1001/jamapsychiatry.2016.3162 [DOI] [PubMed] [Google Scholar]
- 58. Jajodia A, Borders A. Memory predicts changes in depressive symptoms in older adults: a bidirectional longitudinal analysis. J Gerontol B Psychol Sci Soc Sci. 2011;66:571–581. doi: 10.1093/geronb/gbr035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brummett BH, Babyak MA, Barefoot JC, Bosworth HB, Clapp‐Channing NE, Siegler IC, Williams RB Jr, Mark DB. Social support and hostility as predictors of depressive symptoms in cardiac patients one month after hospitalization: a prospective study. Psychosom Med. 1998;60:707–713. doi: 10.1097/00006842-199811000-00008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S8
Figure S1–S4
Data Availability Statement
The data underlying this article currently cannot be shared publicly.


