Abstract
Background
The prognostic role of BNP (B‐type natriuretic peptide) in patients with cardiac sarcoidosis without evident heart failure is unknown.
Methods and Results
This is a post hoc analysis of ILLUMINATE‐CS (Illustration of the Management and Prognosis of Japanese Patients With Cardiac Sarcoidosis), a multicenter, retrospective, and observational study that evaluated the clinical characteristics and prognosis of cardiac sarcoidosis. We analyzed patients with cardiac sarcoidosis without evident heart failure at the time of diagnosis. The association between baseline BNP levels and prognosis was investigated. The primary end point was the combined end point of all‐cause death, heart failure hospitalization, and fatal ventricular arrhythmia. In total, 238 patients (61.0±11.1 years, 37% men) were analyzed, and 61 primary end points were observed during a median follow‐up period of 3.0 (interquartile range, 1.7–5.8) years. Patients with high BNP (BNP above the median value of BNP) were older and had a lower renal function and left ventricular ejection fraction than those with low BNP values. Kaplan–Meier curve analysis indicated that high BNP levels were significantly associated with a high incidence of primary end points (log‐rank P=0.004), and this association was retained even in multivariable Cox regression (hazard ratio, 2.06 [95% CI, 1.19–3.55]; P=0.010). Log‐transformed BNP as a continuous variable was associated with the primary end point (hazard ratio, 2.12 [95% CI, 1.31–3.43]; P=0.002).
Conclusions
High baseline BNP level was an independent predictor of future adverse events in patients with cardiac sarcoidosis without heart failure at the time of diagnosis.
Registration
URL: https://www.umin.ac.jp/english/; Unique Identifier: UMIN‐CTR: UMIN000034974.
Keywords: B‐type natriuretic peptide, biomarker, cardiac sarcoidosis, prognosis, heart failure, steroid
Subject Categories: Cardiomyopathy, Arrhythmias, Biomarkers, Heart Failure
Nonstandard Abbreviations and Acronyms
- CS
cardiac sarcoidosis
- LASSO
least absolute shrinkage and selection operator
Clinical Perspective.
What Is New?
In patients with cardiac sarcoidosis without heart failure, high BNP (B‐type natriuretic peptide) at the time of diagnosis was significantly associated with the primary outcome (the combination of all‐cause death, heart failure hospitalization, and fatal ventricular arrhythmia).
High BNP levels (≥101.3 pg/mL), steroid therapy after diagnosis, and the manifestations of ventricular tachycardia/ventricular fibrillation were independent prognostic factors of the primary outcome.
Furthermore, BNP as a continuous variable was independently associated with the primary outcome.
What Are the Clinical Implications?
In patients with cardiac sarcoidosis, clinicians should assess BNP level at the time of diagnosis even in those without a history or signs of heart failure.
When the BNP level is elevated at the time of diagnosis, close examination for heart failure and careful follow‐up are required.
Sarcoidosis is a multisystemic inflammatory disease of unknown cause. 1 Sarcoid granulomas resulting from inflammation can occur in multiple organs, including the heart, and it has been well demonstrated that the prognosis of patients with sarcoidosis relies significantly on the involvement of the heart. The involvement of the heart, known as cardiac sarcoidosis (CS), causes heart failure, ventricular arrhythmias, and even sudden cardiac death. 2 Therefore, the risk stratification of patients with CS is important. However, few studies have evaluated the prognostic factors in patients with CS, and few prognostic biomarkers have been established in this population.
BNP (B‐type natriuretic peptide) is a cardiac hormone that is stimulated mainly by ventricular load, and BNP level reflects the degree of ventricular burden. 3 BNP can be easily measured and is useful for screening and diagnosing heart failure, and its value in terms of diagnosis and prognosis in patients with heart failure has been well established. 4 , 5 , 6
BNP has been reported to be useful for identifying the involvement of cardiac lesions in patients with sarcoidosis. 7 , 8 However, the prognostic value of BNP in patients with CS, especially those with no history or symptoms of heart failure, has not been clarified, although BNP has been clearly shown to be a predictor of adverse cardiac events in patients without heart failure in the general population. 9 , 10 Therefore, we hypothesized that increased BNP level at the time of diagnosis is associated with poor prognosis even in patients with CS without heart failure.
METHODS
The data underlying this study are available on reasonable request to the corresponding author.
Study Design
This study was performed as a secondary analysis of the ILLUMINATE‐CS (Illustration of the Management and Prognosis of Japanese Patients With Cardiac Sarcoidosis), which was a multicenter retrospective registry to investigate the clinical characteristics and outcomes of patients with CS. 11 Patients with CS, first diagnosed between 2001 and 2017 at 33 hospitals, were enrolled in the registry. Patients who refused enrollment after being notified that they were included in the registry were excluded. The diagnosis of CS was based on either the Japanese Circulation Society criteria proposed in 2016 or the Heart Rhythm Society 2014 consensus statement. 12 , 13
This study was conducted in accordance with the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. The study protocol was approved by the ethics committee of each participating hospital, and informed consent was waived because of the retrospective nature of the study.
Data Collection of Clinical Variables
Clinical information, such as demographic data, medical histories, medications, blood samples including BNP, and findings on cardiovascular imaging, were obtained during the initial diagnostic process for CS. Presenting manifestations (documented during the diagnosis of CS) were determined, and heart failure was diagnosed on the basis of the Framingham criteria. 14 Further, a previous diagnosis of heart failure was defined as a history of heart failure before the diagnosis of CS. Patients who had no heart failure at the time of diagnosis of CS or no history of heart failure before the diagnosis of CS were regarded as those “without heart failure.”
Outcomes and Follow‐Up
The primary outcome of the current study was the combined outcome of all‐cause death, heart failure hospitalization, and fatal ventricular arrhythmia event. All the outcomes were retrospectively obtained from the medical records by a board‐certified cardiologist. Fatal ventricular arrhythmia event was regarded as a composite of sudden cardiac death and either documented ventricular fibrillation (VF), sustained ventricular tachycardia (VT) lasting for >30 seconds, or appropriate implantable cardioverter defibrillator therapy. The causes of death, including sudden cardiac death and hospitalization for heart failure, were in accordance with the definitions recently proposed by the Heart Failure Collaboratory and Academic Research Consortium. 15
Statistical Analysis
Categorical variables were reported as numbers and percentages. Normally distributed continuous variables were presented as the mean±SD, while those that were nonnormally distributed were shown as the median and interquartile range. Student's t test or the Mann–Whitney U test for continuous variables and chi‐square or Fisher's exact tests for categorical variables were used for comparison between the groups, as appropriate. When needed, the variables were transformed for further analysis.
Kaplan–Meier curves with log‐rank analysis were used to compare the cumulative incidence of the primary end point between the groups. Cox regression analysis was used to assess for potential variables associated with the primary outcome. Accordingly, the proportional hazards assumption of Cox regression was checked by analysis of the scaled Schoenfeld residuals. Further, we used a backward stepwise approach using the P value criterion (P<0.05) to build the optimal model because well‐established prognostic factors for patients with CS without heart failure have not been elucidated. In addition, we performed least absolute shrinkage and selection operator (LASSO)–Cox regression as a sensitivity analysis. For variable selection, we first performed multiple imputation to take into account missing data on the derivation cohort and created 20 imputed data sets without missing data using a chained‐equations procedure. Further, we conducted a LASSO logistic regression separately for each of the aforementioned data sets. 16 We used the covariates that were selected in at least half of the imputed data sets (ie, ≥10 data sets). 17 For the LASSO regression, the shrinkage parameter (lambda) was chosen as 1 standard error of the minimum mean squared prediction error according to the 10‐fold cross validation. All variables collected as baseline characteristics were evaluated in LASSO logistic regression as potential prognostic factors, and the model identified those rendering the most parsimonious risk prediction with the highest prognostic prediction performance. Finally, multivariable Cox regression analysis including variables selected by LASSO regression was performed.
All the statistical analyses were performed using the R software version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria; ISBN 3‐900 051‐07‐0, URL http://www.r‐project.org). Statistical significance was set at P<0.05.
RESULTS
Among the 512 patients who were registered with ILLUMINATE‐CS, 145 had heart failure and 139 had missing data on BNP or the history of heart failure. Among the 238 patients who were analyzed (aged 61.0±11.1 years), 36.6% were male patients.
The patients were divided into 2 groups according to the median BNP (101.3 pg/mL). Table 1 shows the baseline characteristics of the patients. Patients with high BNP levels were older and had a lower estimated glomerular filtration rate and a lower left ventricular ejection fraction (LVEF).
Table 1.
Baseline Characteristics of the Study
| Low BNP | High BNP | P value | |
|---|---|---|---|
| n=119 | n=119 | ||
| BNP, pg/mL | 40.3 (23.3–63.6) | 201.8 (144.5–454.5) | – |
| Age, y | 57.7±10.9 | 64.4±10.3 | <0.001 |
| Male sex, n (%) | 48 (40.3) | 39 (32.8) | 0.282 |
| LVEF, % | 56 (48–64) | 46 (38–59) | <0.001 |
| Medical history | |||
| Atrial fibrillation, n (%) | 4 (3.4) | 12 (10.2) | 0.067 |
| Diabetes, n (%) | 30 (25.2) | 25 (21.2) | 0.539 |
| Hypertension, n (%) | 32 (26.9) | 47 (39.5) | 0.054 |
| Pacemaker implantation, n (%) | 27 (22.9) | 33 (28.2) | 0.372 |
| ICD/CRT‐D implantation, n (%) | 9 (7.6) | 14 (12.2) | 0.277 |
| Gallium‐67 accumulation, n (%) | 23 (33.3) | 32 (45.1) | 0.170 |
| PET accumulation, n (%) | 74 (93.7) | 71 (97.3) | 0.445 |
| LGE on CMR, n (%) | 69 (87.3) | 66 (95.7) | 0.088 |
| Manifestation at diagnosis | |||
| Atrioventricular block, n (%) | 47 (39.5) | 62 (52.1) | 0.068 |
| Basal thinning of the IVS, n (%) | 7 (5.9) | 3 (2.5) | 0.333 |
| Wall motion abnormality, n (%) | 54 (45.4) | 60 (50.4) | 0.517 |
| VT/VF, n (%) | 21 (17.6) | 24 (20.2) | 0.741 |
| eGFR, mL/min per 1.73 m2 | 94.4 (82.2–102.4) | 84.2 (67.8–95.7) | <0.001 |
| Creatinine, mg/dL | 0.74 (0.63–0.91) | 0.80 (0.68–0.99) | 0.030 |
| ACEi/ARB at baseline, n (%) | 43 (36.1) | 61 (51.7) | 0.019 |
| Beta blocker at baseline, n (%) | 31 (26.1) | 45 (38.5) | 0.051 |
| Steroid use after diagnosis, n (%) | 105 (88.2) | 109 (91.6) | 0.519 |
Continuous variables are presented as mean ± SD or median (interquartile range). ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, B‐type natriuretic peptide; CMR, cardiac magnetic resonance; CRT‐D, cardiac resynchronization therapy defibrillator; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; IVS, interventricular septum; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; PET, positron emission tomography; VF, ventricular fibrillation; and VT, ventricular tachycardia.
During a median follow‐up period of 3.0 (interquartile range, 1.7–5.8) years, 61 primary outcomes were observed, including 18 hospitalizations for heart failure, 42 documented fatal ventricular arrhythmia events, and 20 all‐cause deaths. The Kaplan–Meier curves showed that patients with high BNP levels were significantly associated with a high incidence of primary outcomes (log‐rank P=0.004) (Figure 1). In the univariate Cox regression analysis, variables with a P value of <0.1 were age and history of atrial fibrillation and VT/VF, no use of steroids after diagnosis, LVEF, estimated glomerular filtration rate, log‐transformed BNP (log BNP) levels, and the manifestations of VT/VF (Table 2). After the backward stepwise approach, steroid therapy after diagnosis, log BNP level, and a history of VT/VF were the final optimal models (steroid use: hazard ratio, 0.49 [95% CI, 0.25–0.98]; P=0.044; log BNP: hazard ratio, 2.12 [95% CI, 1.31–3.43]; P=0.002; and a history of VT/VF: hazard ratio, 2.94 [95% CI, 1.65–5.25]; P<0.001) (Table 2). Similarly, patients with high BNP levels were independently associated with a higher incidence of the primary outcome than those with low BNP levels after the backward stepwise approach (hazard ratio, 2.06 [95% CI, 1.19–3.55]; P=0.010).
Figure 1. Kaplan–Meier curves for the primary outcomes according to the BNP levels.
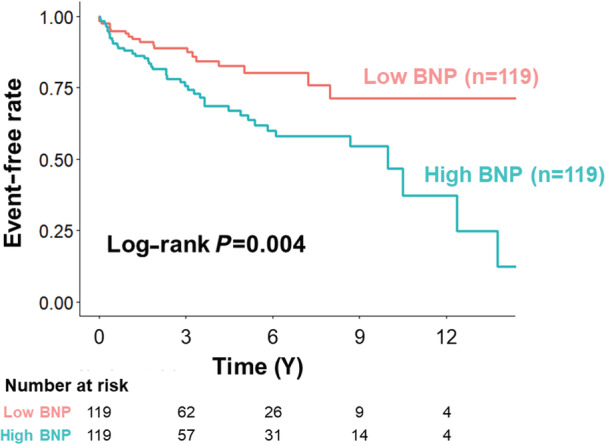
Patients were divided into 2 groups according to the median value of BNP (101.3 pg/mL) at the time of diagnosis of cardiac sarcoidosis. BNP indicates B‐type natriuretic peptide.
Table 2.
Cox Proportional Hazard Analysis for the Primary Outcome
| Unadjusted model | Adjusted Model* | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Log‐transformed BNP | 2.19 | 1.38–3.47 | <0.001 | 2.12 | 1.31–3.43 | 0.002 |
| Age, y | 1.03 | 1.01–1.05 | 0.016 | |||
| Male sex | 1.33 | 0.79–2.22 | 0.280 | |||
| LVEF, per 10% | 0.81 | 0.68–0.96 | 0.015 | |||
| Medical history | ||||||
| Atrial fibrillation | 2.01 | 0.91–4.44 | 0.084 | |||
| Diabetes | 0.61 | 0.32–1.18 | 0.145 | |||
| Hypertension | 1.21 | 0.71–2.05 | 0.487 | |||
| Pacemaker implantation | 0.87 | 0.49–1.55 | 0.633 | |||
| ICD/CRT‐D implantation | 1.61 | 0.76–3.41 | 0.211 | |||
| Gallium‐67 accumulation | 1.40 | 0.74–2.65 | 0.306 | |||
| PET accumulation | 0.46 | 0.14–1.51 | 0.200 | |||
| LGE on CMR | 1.62 | 0.49–5.40 | 0.433 | |||
| Manifestation at diagnosis | ||||||
| Atrioventricular block | 0.76 | 0.46–1.27 | 0.291 | |||
| Basal thinning of the IVS | 1.38 | 0.82–2.31 | 0.221 | |||
| Wall motion abnormality | 1.15 | 0.69–1.91 | 0.585 | |||
| VT/VF | 3.28 | 1.91–5.63 | <0.001 | 2.94 | 1.65–5.25 | <0.001 |
| eGFR, per 10 mL/min per 1.73 m2 | 0.84 | 0.76–0.94 | 0.002 | |||
| Creatinine, mg/dL | 1.38 | 1.07–1.70 | 0.011 | |||
| ACEi/ARB at baseline | 1.52 | 0.92–2.52 | 0.101 | |||
| Beta blocker at baseline | 1.35 | 0.80–2.29 | 0.267 | |||
| Steroid use after diagnosis | 0.43 | 0.26–0.84 | 0.012 | 0.49 | 0.25–0.98 | 0.044 |
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, B‐type natriuretic peptide; CMR, cardiac magnetic resonance; CRT‐D, cardiac resynchronization therapy defibrillator; eGFR, estimated glomerular filtration rate; HR, hazard ratio; ICD, implantable cardioverter‐defibrillator; IVS, interventricular septum; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; PET, positron emission tomography; VF, ventricular fibrillation; and VT, ventricular tachycardia.
Adjusted model was determined using backward stepwise approach.
The sensitivity analysis performed by LASSO–Cox regression analysis showed that log BNP (20/20 data sets), history of VT/VF (20/20 data sets), estimated glomerular filtration rate (20/20 data sets), and steroid therapy after diagnosis (16/20 data sets) were selected in more than half of the 20 imputed data sets. Accordingly, they were included in the multivariable Cox regression model, which confirmed that log BNP was independently associated with the primary outcome (Table 3).
Table 3.
Multivariable Cox Regression for Primary End Point With Variables Selected With LASSO Regression
| Multivariable Cox regression | |||
|---|---|---|---|
| HR | 95% CI | P value | |
| Log‐transformed BNP | 1.81 | 1.11–2.96 | 0.018 |
| eGFR, per 10 mL/min per 1.73 m2 | 0.88 | 0.78–0.99 | 0.038 |
| VT/VF as a manifestation at diagnosis | 2.56 | 1.40–4.69 | 0.002 |
| Steroid use after diagnosis | 0.65 | 0.31–1.37 | 0.254 |
BNP indicates B‐type natriuretic peptide; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LASSO, least absolute shrinkage and selection operator; VF, ventricular fibrillation; and VT, ventricular tachycardia.
A total of 22 patients were diagnosed histologically from myocardial tissue, and 150 were diagnosed histologically from myocardial tissue or extracardiac tissue. The histological evidence did not influence the prognostic impact of BNP for the primary end point (Figure 2).
Figure 2. Cox proportional hazard analysis of log‐transformed BNP for the primary end point according to histological diagnosis.
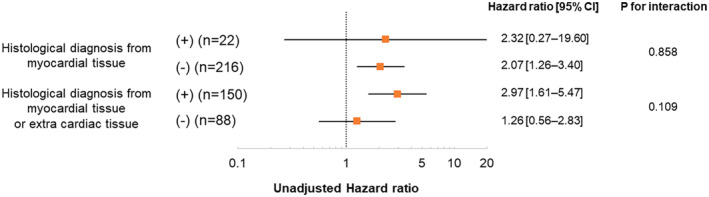
There was no significant interaction between the prognostic impact of log‐transformed BNP and histological evidence. BNP indicates B‐type natriuretic peptide.
DISCUSSION
The novel findings of the present study were that the BNP level at the time of diagnosis was associated with subsequent worse clinical outcomes and that steroid therapy was significantly associated with a lower incidence of adverse events in patients with CS without heart failure.
Although BNP is widely known as one of the most powerful prognostic markers in patients with heart failure, 6 , 18 , 19 , 20 , 21 its prognostic value in patients with CS has not been well elucidated. A retrospective study of 49 patients with CS demonstrated that BNP level was a predictor of new‐onset heart failure after adjustment for LVEF. 22 Although this finding suggests the usefulness of BNP for predicting prognosis and is in line with our study results, the reliability of the results of previous studies is limited by the small number of events (only 7 cases of new‐onset heart failure were observed). Another study investigating 83 patients with CS before being treated with steroid therapy revealed that a high BNP level was associated with a composite of adverse events (advanced atrioventricular block, VT/VF, heart failure hospitalization, and all‐cause death) on univariate analysis, but the association was not retained after adjustment for age, estimated glomerular filtration rate, and LVEF. 23 However, as this study observed only 19 adverse events that occurred during follow‐up, these previous studies might be underpowered to evaluate the prognostic value of BNP. Although there are several other studies that have mentioned the association between BNP and prognosis in CS, 24 , 25 , 26 none have been able to examine whether BNP is associated with adverse events in patients with CS independent of other covariates associated with the BNP level and prognosis of CS, mostly because of the limited sample size and events. ILLUMINATE‐CS is one of the largest cohorts of CS, and this is the first study to clearly show that BNP is an independent prognostic factor in patients with CS, even after excluding those with heart failure at the time of diagnosis and the population in which the prognostic value of BNP has been established.
Although the prognostic value of BNP in patients with CS is unclear, previous studies have reported that BNP or NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) are significantly associated with mortality or cardiovascular diseases in a population without heart failure. 9 , 10 The pathophysiological background of this association is yet to be clarified; however, there are some possible hypotheses. First, increased natriuretic peptides may reflect subclinical cardiac impairment, including diastolic dysfunction. BNP is secreted in response to increased left ventricular end‐diastolic pressure, and several studies have described its usefulness for screening for early diastolic dysfunction. 27 , 28 Indeed, ventricular diastolic dysfunction was reported to be common in patients with sarcoidosis, even without cardiac diseases. 29 Second, BNP may be an indicator of silent myocardial ischemia. Myocardial ischemia, even without left ventricular dysfunction, was reported to augment the cardiac BNP gene and subsequently elevate the plasma BNP level. 30 A previous study involving patients with asymptomatic type 2 diabetes without clinical cardiac dysfunction showed that increased BNP could predict a positive exercise tolerance test. 31 Third, BNP may be an indicator of CS activity. A meta‐analysis showed that immunosuppressant therapy might improve atrioventricular nodal conduction. 32 In addition, improvement of CS activity by immunosuppressant therapy was associated with lower arrhythmia burden. 33 , 34 Thus, BNP, which is associated with poor outcomes, including fatal ventricular arrhythmia events in the current analysis, might be related to the CS activity. It is possible that BNP is a useful tool for guiding immune‐modulating therapies. However, as this hypothesis is not supported by sufficient evidence, further studies are needed. Finally, in our study, patients in the high‐BNP group were older than those in the low‐BNP group. Since the elderly patients are not as symptomatic as the young patients, even if they have heart failure, 35 heart failure may be overlooked in some elderly patients with increased BNP. Therefore, the clinical implications of our study are that in patients with CS, (1) the BNP level should be evaluated at the time of diagnosis even in those without heart failure;and (2) if the BNP level is high at the time of diagnosis, a close examination for heart failure and careful follow‐up are needed.
In our study, treatment with steroids was independently associated with a lower incidence of primary end points in patients with CS without heart failure. This finding is particularly interesting because no report has shown that steroid use improves clinical outcomes in patients with CS. Although there has been no consensus on the timing of steroid therapy for patients with CS, several studies have suggested that early steroid initiation leads to favorable clinical outcomes in patients with CS. In a retrospective study of 75 patients treated with steroids for CS, patients with an initial LVEF of ≥50% had a better survival rate than those with an initial LVEF of <50%. 36 Another study of patients with CS reported that steroid therapy was associated with improved left ventricular function and remodeling in patients with an initial LVEF of ≥30%, but not in those with progression of left ventricular impairment (LVEF <30%). 37 Considering these findings, the positive effect of steroids might be mitigated in the late stage of CS, and early initiation of steroids might be more effective. However, further research is required to clarify this hypothesis.
The present study has several limitations. First, some patients with CS were diagnosed on the basis of the guidelines of the Japanese Circulation Society. 12 Thus, patients diagnosed by a combination of clinical findings of other organ sarcoidosis or multiple cardiac presentations, such as imaging findings, were included. These results should be taken with caution, as histological proof was not performed in all the patients. Second, there are no established protocols regarding how to initiate, taper, or maintain steroid treatment. Third, because of the retrospective nature of this study, there was potential for sampling bias and incomplete data. For instance, we did not obtain data for active myocarditis examinations. Fourth, we analyzed BNP levels measured at the time of diagnosis and did not have data on protocolized BNP measurement; therefore, we could not analyze the association between serial changes in BNP levels and prognosis. Fifth, we did not obtain information for pulmonary arterial hypertension, although BNP was reported to be a prognostic marker in such populations. 38 Finally, we could not exclude patients with heart failure at the time of diagnosis, although almost all study patients (97.9%) underwent echocardiography. Further prospective studies with larger sample sizes and longer follow‐up periods are necessary to determine the association between BNP levels and prognosis.
CONCLUSIONS
Among patients with CS without heart failure at the time of diagnosis, higher BNP levels were associated with poorer long‐term clinical outcomes. The results of this study clearly suggest that BNP is a biomarker recommended to be measured in patients with CS in terms of risk stratification.
Sources of Funding
The ILLUMINATE‐CS study was partially supported by Novartis Pharma Research Grants and JSPS KAKENHI (grant nos. 21H03309 and 22K16147).
Disclosures
Dr Matsue received an honorarium from Otsuka Pharmaceutical Co. and Novartis Japan. Dr Okumura received honoraria from Ono Yakuhin, Otsuka, Novartis, and Astrazeneca; and research grants from Ono Yakuhin, Amgen Astellas, Pfizer, Alnylam, and Alexion (not in connection with the submitted work). The remaining authors have no disclosures to report.
For Sources of Funding and Disclosures, see page 7.
Contributor Information
Daichi Maeda, Email: daichimaeda0424@yahoo.co.jp.
Yuya Matsue, Email: yuya8950@gmail.com.
References
- 1. Drent M, Crouser ED, Grunewald J. Challenges of sarcoidosis and its management. N Engl J Med. 2021;385:1018–1032. doi: 10.1056/NEJMra2101555 [DOI] [PubMed] [Google Scholar]
- 2. Kouranos V, Sharma R. Cardiac sarcoidosis: state‐of‐the‐art review. Heart. 2021;107:1591–1599. doi: 10.1136/heartjnl-2019-316442 [DOI] [PubMed] [Google Scholar]
- 3. Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87:1402–1412. doi: 10.1172/JCI115146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts E, Ludman AJ, Dworzynski K, Al‐Mohammad A, Cowie MR, McMurray JJ, Mant J, Failure NGDGfAH . The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta‐analysis in the acute care setting. BMJ. 2015;350:h910. doi: 10.1136/bmj.h910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nishii M, Inomata T, Takehana H, Naruke T, Yanagisawa T, Moriguchi M, Takeda S, Izumi T. Prognostic utility of B‐type natriuretic peptide assessment in stable low‐risk outpatients with nonischemic cardiomyopathy after decompensated heart failure. J Am Coll Cardiol. 2008;51:2329–2335. doi: 10.1016/j.jacc.2007.11.085 [DOI] [PubMed] [Google Scholar]
- 6. Oremus M, Don‐Wauchope A, McKelvie R, Santaguida PL, Hill S, Balion C, Booth R, Brown JA, Ali U, Bustamam A, et al. BNP and NT‐proBNP as prognostic markers in persons with chronic stable heart failure. Heart Fail Rev. 2014;19:471–505. doi: 10.1007/s10741-014-9439-6 [DOI] [PubMed] [Google Scholar]
- 7. Yasutake H, Seino Y, Kashiwagi M, Honma H, Matsuzaki T, Takano T. Detection of cardiac sarcoidosis using cardiac markers and myocardial integrated backscatter. Int J Cardiol. 2005;102:259–268. doi: 10.1016/j.ijcard.2004.05.028 [DOI] [PubMed] [Google Scholar]
- 8. Handa T, Nagai S, Ueda S, Chin K, Ito Y, Watanabe K, Tanizawa K, Tamaya M, Mishima M, Izumi T. Significance of plasma NT‐proBNP levels as a biomarker in the assessment of cardiac involvement and pulmonary hypertension in patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27:27–35. [PubMed] [Google Scholar]
- 9. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994 [DOI] [PubMed] [Google Scholar]
- 10. Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N‐terminal pro‐brain natriuretic peptide, C‐reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609 [DOI] [PubMed] [Google Scholar]
- 11. Nabeta T, Kitai T, Naruse Y, Taniguchi T, Yoshioka K, Tanaka H, Okumura T, Sato S, Baba Y, Kida K, et al. Risk stratification of patients with cardiac sarcoidosis: the ILLUMINATE‐CS registry. Eur Heart J. 2022;43:3450–3459. doi: 10.1093/eurheartj/ehac323 [DOI] [PubMed] [Google Scholar]
- 12. Terasaki F, Azuma A, Anzai T, Ishizaka N, Ishida Y, Isobe M, Inomata T, Ishibashi‐Ueda H, Eishi Y, Kitakaze M, et al. JCS 2016 guideline on diagnosis and treatment of cardiac sarcoidosis‐ digest version. Circ J. 2019;83:2329–2388. doi: 10.1253/circj.CJ-19-0508 [DOI] [PubMed] [Google Scholar]
- 13. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 14. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601 [DOI] [PubMed] [Google Scholar]
- 15. Abraham WT, Psotka MA, Fiuzat M, Filippatos G, Lindenfeld J, Mehran R, Ambardekar AV, Carson PE, Jacob R, Januzzi JL Jr, et al. Standardized definitions for evaluation of heart failure therapies: scientific expert panel from the heart Failure Collaboratory and academic research consortium. Eur J Heart Fail. 2020;22:2175–2186. doi: 10.1002/ejhf.2018 [DOI] [PubMed] [Google Scholar]
- 16. Goldstein BA, Navar AM, Carter RE. Moving beyond regression techniques in cardiovascular risk prediction: applying machine learning to address analytic challenges. Eur Heart J. 2017;38:1805–1814. doi: 10.1093/eurheartj/ehw302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Austin PC, Lee DS, Ko DT, White IR. Effect of variable selection strategy on the performance of prognostic models when using multiple imputation. Circ Cardiovasc Qual Outcomes. 2019;12:e005927. doi: 10.1161/CIRCOUTCOMES.119.005927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R. B‐type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105:2392–2397. doi: 10.1161/01.CIR.0000016642.15031.34 [DOI] [PubMed] [Google Scholar]
- 19. Koglin J, Pehlivanli S, Schwaiblmair M, Vogeser M, Cremer P, vonScheidt W. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol. 2001;38:1934–1941. doi: 10.1016/S0735-1097(01)01672-2 [DOI] [PubMed] [Google Scholar]
- 20. Tsutamoto T, Wada A, Maeda K, Hisanaga T, Mabuchi N, Hayashi M, Ohnishi M, Sawaki M, Fujii M, Horie H, et al. Plasma brain natriuretic peptide level as a biochemical marker of morbidity and mortality in patients with asymptomatic or minimally symptomatic left ventricular dysfunction. Comparison with plasma angiotensin II and endothelin‐1. Eur Heart J. 1999;20:1799–1807. doi: 10.1053/euhj.1999.1746 [DOI] [PubMed] [Google Scholar]
- 21. Balion C, Santaguida PL, Hill S, Worster A, McQueen M, Oremus M, McKelvie R, Booker L, Fagbemi J, Reichert S, et al. Testing for BNP and NT‐proBNP in the diagnosis and prognosis of heart failure. Evid Rep Technol Assess (Full Rep). 2006;142:1‐147. [PMC free article] [PubMed] [Google Scholar]
- 22. Kiko T, Yoshihisa A, Kanno Y, Yokokawa T, Abe S, Miyata‐Tatsumi M, Misaka T, Oikawa M, Kobayashi A, Ishida T, et al. A multiple biomarker approach in patients with cardiac sarcoidosis. Int Heart J. 2018;59:996–1001. doi: 10.1536/ihj.17-695 [DOI] [PubMed] [Google Scholar]
- 23. Kobayashi Y, Sato T, Nagai T, Hirata K, Tsuneta S, Kato Y, Komoriyama H, Kamiya K, Konishi T, Omote K, et al. Association of high serum soluble interleukin 2 receptor levels with risk of adverse events in cardiac sarcoidosis. ESC Heart Fail. 2021;8:5282–5292. doi: 10.1002/ehf2.13614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chiba T, Nakano M, Hasebe Y, Kimura Y, Fukasawa K, Miki K, Morosawa S, Takanami K, Ota H, Fukuda K, et al. Prognosis and risk stratification in cardiac sarcoidosis patients with preserved left ventricular ejection fraction. J Cardiol. 2020;75:34–41. doi: 10.1016/j.jjcc.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 25. Nagano N, Nagai T, Sugano Y, Morita Y, Asaumi Y, Aiba T, Kanzaki H, Kusano K, Noguchi T, Yasuda S, et al. Association between basal thinning of interventricular septum and adverse long‐term clinical outcomes in patients with cardiac sarcoidosis. Circ J. 2015;79:1601–1608. doi: 10.1253/circj.CJ-14-1217 [DOI] [PubMed] [Google Scholar]
- 26. Nordenswan HK, Lehtonen J, Ekstrom K, Raisanen‐Sokolowski A, Mayranpaa MI, Vihinen T, Miettinen H, Kaikkonen K, Haataja P, Kerola T, et al. Manifestations and outcome of cardiac sarcoidosis and idiopathic Giant cell myocarditis by 25‐year Nationwide cohorts. J Am Heart Assoc. 2021;10:e019415. doi: 10.1161/JAHA.120.019415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lubien E, DeMaria A, Krishnaswamy P, Clopton P, Koon J, Kazanegra R, Gardetto N, Wanner E, Maisel AS. Utility of B‐natriuretic peptide in detecting diastolic dysfunction: comparison with doppler velocity recordings. Circulation. 2002;105:595–601. doi: 10.1161/hc0502.103010 [DOI] [PubMed] [Google Scholar]
- 28. Romano S, Di Mauro M, Fratini S, Guarracini L, Guarracini F, Poccia G, Penco M. Early diagnosis of left ventricular diastolic dysfunction in diabetic patients: a possible role for natriuretic peptides. Cardiovasc Diabetol. 2010;9:89. doi: 10.1186/1475-2840-9-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ozyilmaz E, Akilli R, Berk I, Deniz A, Ozturk OG, Baydar O, Saygideger Y, Seydaoglu G, Erken E. The frequency of diastolic dysfunction in patients with sarcoidosis and it's relationship with HLA DRB1* alleles. Sarcoidosis Vasc Diffuse Lung Dis. 2019;36:285–293. doi: 10.36141/svdld.v36i4.8606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goetze JP, Christoffersen C, Perko M, Arendrup H, Rehfeld JF, Kastrup J, Nielsen LB. Increased cardiac BNP expression associated with myocardial ischemia. FASEB J. 2003;17:1105–1107. doi: 10.1096/fj.02-0796fje [DOI] [PubMed] [Google Scholar]
- 31. Rana BS, Davies JI, Band MM, Pringle SD, Morris A, Struthers AD. B‐type natriuretic peptide can detect silent myocardial ischaemia in asymptomatic type 2 diabetes. Heart. 2006;92:916–920. doi: 10.1136/hrt.2005.071423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fazelpour S, Sadek MM, Nery PB, Beanlands RS, Tzemos N, Toma M, Birnie DH. Corticosteroid and immunosuppressant therapy for cardiac sarcoidosis: a systematic review. J Am Heart Assoc. 2021;10:e021183. doi: 10.1161/JAHA.121.021183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crouser ED, Ruden E, Julian MW, Raman SV. Resolution of abnormal cardiac MRI T2 signal following immune suppression for cardiac sarcoidosis. J Investig Med. 2016;64:1148–1150. doi: 10.1136/jim-2016-000144 [DOI] [PubMed] [Google Scholar]
- 34. Slivnick JA, Betz J, Kalbfleisch S, Crouser ED, Kahwash R. High‐dose intravenous glucocorticoids are effective in the acute management of ventricular arrhythmias in cardiac sarcoidosis: a case series. Heart Rhythm Case Rep. 2020;6:706–710. doi: 10.1016/j.hrcr.2020.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lam C, Smeltzer SC. Patterns of symptom recognition, interpretation, and response in heart failure patients: an integrative review. J Cardiovasc Nurs. 2013;28:348–359. doi: 10.1097/JCN.0b013e3182531cf7 [DOI] [PubMed] [Google Scholar]
- 36. Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T, Izumi T, Sekiguchi M, Central Japan Heart Study G . Prognostic determinants of long‐term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–1010. doi: 10.1016/S0002-9149(01)01978-6 [DOI] [PubMed] [Google Scholar]
- 37. Chiu CZ, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M, Kitakaze M, Tomoike H, Miyatake K. Prevention of left ventricular remodeling by long‐term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–146. doi: 10.1016/j.amjcard.2004.08.083 [DOI] [PubMed] [Google Scholar]
- 38. Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Kakishita M, Fukushima K, Okano Y, Nakanishi N, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.CIR.102.8.865 [DOI] [PubMed] [Google Scholar]


