Abstract
Superparamagnetic iron oxide (SPIO)-labeling of cells has been applied for magnetic resonance imaging (MRI) cell tracking for over 30 years, having resulted in a dozen or so clinical trials. SPIO nanoparticles are biodegradable and can be broken down into elemental iron, and hence the tolerance of cells to magnetic labeling has been overall high. Over the years, however, single reports have accumulated demonstrating that the proliferation, migration, adhesion and differentiation of magnetically labeled cells may differ from unlabeled cells, with inhibition of chondrocytic differentiation of labeled human mesenchymal stem cells (hMSCs) as a notable example. This historical perspective provides an overview of some of the drawbacks that can be encountered with magnetic labeling. Now that magnetic particle imaging (MPI) cell tracking is emerging as a new in vivo cellular imaging modality, there has been a renaissance in the formulation of SPIO nanoparticles this time optimized for MPI. Lessons learned from the occasional past pitfalls encountered with SPIO-labeling of cells for MRI may expedite possible future clinical translation of (combined) MRI/MPI cell tracking.
Keywords: Magnetic resonance imaging, magnetic particle imaging, superparamagnetic iron oxide nanoparticles, immune cells, stem cells, cell tracking
Graphical Abstract
In some cases, magnetic intracellular labeling of cells for in vivo cell tracking with magnetic resonance imaging (MRI) or magnetic particle imaging (MPI) may affect cell viability, proliferation, migration, adhesion, or differentiation. A historical perspective of such reported differences in behavior between labeled and unlabelled cells is provided, with an urgent call for developing minimal standard requirements and protocols for clinical studies that aim to use magnetically labeled cells.
1. Introduction
During the War on Cancer in the 1970s, a better understanding of the overall interactions between our immune system and cancer was obtained. Realizing the potential to exploit our natural defense system, an explosion of clinical immune cell therapies occurred since the mid 1980s, with as primary effector cells lymphokine-activated killer cells,[1] tumor-infiltrating lyphocytes (TILs),[2] antigen-presentic dendritic cells,[3] chimeric antigen receptor (CAR) T-cells[4] and CAR natural killer cells.[5] Other than taking lymph node (LN) or tumor biopsies, nothing would be known about the selectivity of tumor homing and off-target site biodistribution if it wouldn’t be for in vivo cell tracking.[6] The same holds true for stem cell therapy and intended targets of tissue repair in regenerative medicine. During the late 1990’s/early 2000’s, the stem cell field rapidly expanded with the isolation of human embryonic stem cells,[7] adult human stem cells,[8] and more recently induced pluripotent stem cells.[9]
Immune cells[10] and bone marrow stem cells[11] were initially labeled with the radiotracer 111In-oxine to determine their whole body biodistribution in patients using γ-camera imaging. For the first time, it was possible to visualize cells everywhere without need of tissue biposies. However, radiotracers can exhibit significant cytotoxicity, lack an infinite half-life, and nuclear imaging techniques do not provide anatomical information and high spatial soft tissue contrast. This has led to the development of alternative non-invasive in vivo cell tracking approaches, with magnetic resonance imaging (MRI) historically being one of the primary clinical imaging modalities,[12] followed by magnetic particle imaging (MPI) as a more recent cell tracking technique albeit only pre-clinical at the present time.[13].
In order to be detected by MRI and MPI, cells need to be magnetically labeled with superparamagnetic iron oxide (SPIO) nanoparticles, acting as a (negative) contrast or tracer agent, respectively. On MRI, labeled cells are detected indirectly through the loss of proton signal, and on MPI directly through the generation of a magnetically induced signal (Figure 1). All SPIO particles will generate hypointense contrast on MRI, but not all particles can generate an MPI signal. Given that many new SPIO formulations are now being developed that are optimized for MPI,[14] we revisit here some of the past unwanted side effects of cellular magnetic labeling encountered with preparing cells for MRI.
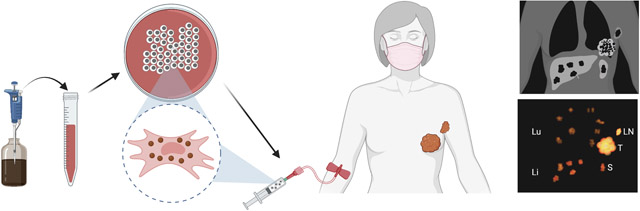
Figure 1:

Schematic outline of in vivo MPI/MRI cell tracking, as exemplified by monitoring of injected SPIO-labeled T cells in a breast cancer patient. (A) Cells are first magnetically labeled with SPIO nanoparticles through simple co-incubation in culture medium during normal expansion. After collecting and washing, cells are injected intravenously, and the patient is subjected to imaging follow-up. (B) Anticipated imaging findings are specific homing to the primary tumor (T) and a regional lymph node (LN) tumor metastasis, along with non-specific uptake in the lung (Lu), liver (Li), and spleen (S). While MRI cell tracking has entered the clinic, the use of MPI so far has only been pre-clinical.
2. Magnetic imaging
2.1. MRI cell tracking
Inspired by the immune cell therapy nuclear imaging studies mentioned above, it is not suprising that initial efforts towards magnetic labeling and MRI cell tracking was directed towards white blood cells,[15] eventually resulting in a first clinical trial using dendritic cells (DCs).[16] Since whole body imaging with MRI is very difficult, and other sources of hypointensities exist, MRI cell tracking is best suited for monitoring local tissue engraftment, cellular homing in nearby organs and, first and foremost, real-time imaging of cell delivery.[17]
2.2. MPI cell tracking
Although MPI uses a magnetic field to detect the same SPIO particles used with MRI, it is a fundamentally different imaging modality.[18] Instead of proton spin relaxation, it detects changes in the superparamagnetic magnetization as a function of the magnetic field strength, that is moved around in space. Only when the drive field is near-zero, an MPI signal can be generated, with the signal intensity directly correlating to the amount of SPIO. Unlike MRI, this should allow a straightforward quantification of the local amount of magnetically labeled cells using fiducials with known cell concentrations.[19]
Indeed, using SPIO-labeled mesenchymal stem cells (MSCs) and neural stem cells (NSCs), the first MPI cell tracking study demonstrated that such linear cell quantification is possible both in cell phantoms and in tissues having different cell densities.[20] Other studies also reported on labeling and tracking of MSCs, showing in vivo accumulation in the brain, liver, and lung.[21] As for white blood cells, interest so far has been to visualize cytotoxic T cells[22] and DCs[23], again as related to immune cell therapy.
3. Methods for cellular magnetic labeling
Magnetic labeling of cells is in general simple and straightfoward, using simple co-incubation of cells and SPIO in culture medium, usually for 24–48 hrs and at an iron concentration of ~25 μg Fe/ml. SPIO particles can be prepared with an inherent cationic surface charge (positive zeta potential), complexed with cationic transfection agents[24], or combined with electroporation[25] or sonoporation[26] for general, non-specific labeling of non-phagocytic cells (Figure 2A). Alternatively, they may be conjugated to antibodies[15a, 15e, 27] for specific labeling of cells carrying the appropriate receptor. Entry mechanisms usually involve macropinocytosis with the SPIO particles ending up in endosomes, which eventually fuse with lysosomes (Figure 2B).[15b]. The endosomal-lysosomal system is a series of organelles in the endocytic pathway where SPIOs are internalized, recycled and modulated.[28] In such a low pH environment, SPIOs are biodegraded over time,[29] with the elemental iron eventually becoming part of the normal body iron pool. Some cells, such as macrophages, can fully biodegrade the nanoparticles within a short time,[30] whereas other (non-phagocytic) cells including hMSCs only partially decompose SPIO particles over time.[31] For hMSCs, it has even been reported that biodegraded, newly available iron species can be re-crystallized, mirroring the neosynthesis of nanoparticles.[32] On transmission electron microscopy these particles resemble magnetoferritin,[33] suggesting a possible role for endogenous apoferritin in this process.
Figure 2:

Methods for intracellular magnetic cell labeling. (A) Cells can endocytose SPIO nanoparticles by conjugating them to antibodies (AB) that induce receptor-mediated endocytosis, by synthesizing them with a cationic coating (CC), by complexing them with cationic transfection agents (TA), or by membrane permeabilization using magnetoelectroporation (MEP) or magnetosonoporation (MSP). (B) Upon invagination of the cell membrane and subsequent SPIO internalization into newly formed endosomes (E), the SPIO-containing endosomes rapidly fuse with lysosomes (L) to form endosomal-lysosomal complexes (EL). The SPIO particles are eventually degraded within the EL system to elemental iron (Fe), and incorporated into the normal body iron blood pool through natural iron-sequestering proteins.
4. Cellular impact of magnetic labeling
4.1. Cell viability and phenotype
Upon SPIO biodegradation in the acidic compartment of the lysosomes, the release of elemental Fe may lead to increased cell death due to depletion of innate cellular anti-oxidants and/or the generation of reactive oxygen species (ROS). This loss of cell viability can be partially reversed by adding the anti-oxidant quercetin to cell cultures.[34] Indeed, a non-significant transient increase in ROS production in SPIO-labeled hMSCs[35] and endothelial progenitor cells[36] has been reported, and it was shown that for SPIO-incubation times of 4 or 24 hours addition of the iron-chelator desferrioxamine reduced the ROS levels in half.[37] Since Fe2+ is responsible for the oxidative stress induced by the Fenton reaction, the ROS levels will depend on the core composition of the nanoparticles: maghemite (γFe2O3) has only Fe3+ species, whereas magnetite (Fe2O3.FeO or Fe3O4 in short) contains both Fe2+ and Fe3+ species. Toxicity will also depend on the level of cellular glutathione (GSH), a major antioxidant compound that is oxidized to glutathione disulfide (GSSG) in the presence of ROS.[38] As for potential changes in phenotype, the level of Oct-4 mRNA increased in SPIO-labeled hMSCs at day 1, with a change in the cellular phenotype from CD45−/CD44+/CD29+ to CD45low/CD44+/CD29+ cells at day 7 post-labeling, which closely resembles the phenotype of fresh bone marrow-derived hMSCs and hence, is not necessarily a negative side effect.[39]
One of the major issues with labeling cells with an exogenous agent such as SPIO is that the label persists following cell death. This may partially confound the interpretation of whether or not transplanted cells are alive,[40] and, upon cell death, if the SPIO label is transferred to infiltrating macrophages that clean up residual cellular debris and their content. Lepore et al. showed that Feridex®-labeled cells, but not unlabeled cells, induced influx of ED1-positive macrophages/microglia following intraparenchymal transplantation of glial-restriced precursor cells. Small numbers of these phagocytic cells took up iron from grafted cells in vivo, althoug the majority of Feridex® label was found in transplanted cells.[41] Using in vitro cell migration phantoms, others have found that transfer of SPIO nanoparticles to activated macrophages accounts for <10% of the total iron in labeled cells, dependent on the ratio of labeled cells:macrophages.[42]
4.2. Cell motility and migration
As with all exogenous labeling agents, the sensitivity of detection of labeled cells diminishes over time due to dilution of labeled cells amongst daughter cells. For SPIO-labeling, this will usually result in a gradual decrease of hypointense signal intensity and detection of labeled cells. When dividing, however, certain stem cells self-renew while generating a downstream lineage cell that eventuall will differentiate[43]; this phenomenon of asymmetric cell division can lead to a sharp decrease in labeled cell detectability (Figure 3).[44] Indeed, for SPIO-labeled neural stem cells transplanted in mouse brain, a pronounced mismatch was seen between the hypointense MR signal and the histologically determined cell distribution, with a surprisingly sharp cutoff rather than a gradual decrease of signal.[44]
Figure 3:

Symmetric vs. asymmetric cell division and dilution of SPIO particles among daughter cells. (A) In symmetrically dividing cells, the nanoparticles are distributed equally among daughter cells. (B) In asymmetrically dividing stem cells, the nanoparticles are not distributed equally. This can lead to a sharp decline in cell detectability. Progeny cells without particles are MR-invisible (solid crosses), while cells with only one particle are borderline detectable (dashed crosses). Asterisks represent parent cells that undergo self-renewal with an exact copy of itself, which is a hallmark feature of undifferentiated, noncommitted stem cells. (C-E) Intracerebroventricular transplantation of β-galactose-transduced C17.2 neural stem cells in mouse brain. Shown is a mismatch between high-resolution ex vivo MR images and histology, with a sharp boundary between MR-detectable and non-detectable cells as a result from asymmetric cell division. (C) Two weeks after transplantation, SPIO-labeled cells migrated vast distances toward the outer cortical layers of the cerebrum and olfactory bulb as revealed by anti-β-gal staining. This is in sharp contrast to the MRI pattern (D), which shows hypointense cells centered in and around the ventricles (site of transplantation), but not the cortical layers. (E) Merged histology/MR image, in which β-gal cells are visualized as red and MRI-hypointense cells are yellow, further illustrates this mismatch. Scale bar=1 mm. Panels Adapted/reproduced with permission.[44]2007, Wiley.
Schäfer et al. demonstrated that Ferucarbotran-labeling decreases MSC migration in vitro, which was transient with cells returning to unlabeled cell values following 2 passages in culture.[45] Colony formation was also significantly reduced, which persisted after 2 cell passages. No differences in cell differentiation into adipocytes, osteocytes, or chondrocytes was observed. The restoration of cell migration upon SPIO dilution through cell division is in agreement with studies by Cromer Berman et al., who found that SPIO-labeling resulted in decreased motility of neural stem cells and induced pluripotent stem cells in vivo when cells were transplanted into mouse brain.[46] Following exocytosis of SPIO by live cells, SPIO-depleted cells showed the farthest migration distance. This may confound a correct interpretation of cell migration on MRI, as those SPIO-depleted cells migrating the furthest distances will become undetectable.
What may cause such limited migration? Elegant studies on the viscoelasticity of labeled and unlabeled cells revealed that SPIO-labeling leads to cell “stiffening” within the first hours of uptake, returning to normal levels at 24 hours.[47] This early time frame of stiffening correlated well with the actin cytoskeleton and their role in intracellular transport of endosomes. Since cell motility is initiated by an actin-dependent protrusion of the cell’s leading edge, which is composed of armlike structures called lamellipodia and filopodia, actin cytoskeleton reorganization with actin bundling may interfere with cell motility.
In contrast, Huang and Chen et al. showed that Ferucarbotran-labeling can actually stimulate hMSC proliferation and migration via diminishing intracellular H2O2 through peroxidase-like activity, which can affect the expression of cell cycle protein regulators. [48] Adding the iron chelator desferrioxamine reduced such stimulation. They suggested that this could be a result of the Fe-mediated Wnt signaling pathway, which regulates cell proliferation and migration.[49] Furthermore, while Resovist®-labeled endothelial progenitor cells showed no differences in proliferation and apoptosis as compared to unlabeled cells, their migration was significantly impaired with an increased cell adhesion.[50] This furthermore suggests that SPIO-labeling can remodel the architecture of the cytoskeleton. Soenen et al. reported that SPIO-labeling can inhibit cell proliferation, focal adhesion and maturation in a dose-dependent manner resulting from changes in the actin cytoskeleton and microtubuli network architectures.[51] Activated kinase and focal kinase levels were affected, and they suggested that high levels of peri-nuclear SPIO localization interfere with protein expression and mechanically/physcially block proper functiong of mature actin fibers, including the neurite outgrowth of magnetically labeled neural stem cells.[37]
4.3. Stem cell differentiation
Shortly after in vivo MRI cell tracking of SPIO-labeled MSCs was introduced,[52] the collaboration between the commercial provider of clinical-grade hMSCs at the time (Osiris Therapeutics, Inc.) and Johns Hopkins University demonstrated that downstream differentiation of Feridex®-labeling of hMSCs resulted in a complete inhibition of downstream cell differentiation into the chondrocytic lineage (Figure 4).[53] Inhibition was dose-dependent, and compared to unlabeled controls the labeled hMSCs exhibited an unaltered viability and proliferation, even for the chondrocytic pathway, and underwent normal adipogenic and osteogenic differentiation. The blocking of chondrogenic activity was mediated by Feridex®, rather than by the transfection agent poly-L-lysine (PLL) that was used, as incubation with PLL alone yielded similar results as for labeled cells.
Figure 4:

SPIO labeling of hMSCs inhibits chondrogenic differentiation. While SPIO-labeling does not affect labeled cells viability nor differentiation into adipocytes and osteocytes, labeled cells fail to produce a proper extracellular matrix that contains proteoglycans and collagen II. (A-C) hMSCs incubated with SPIO-PLL fail to generate (A) collagen II and (C) a safranin-O-positive extracellular matrix after 21 days of cell culture, with (B) Prussian Blue staining revealing SPIO-containing cells throughout the pellet. Partial inhibition of chondrogenesis in pellets made from 1:1 mixtures of SPIO-PLL-labeled and unlabeled hMSCs is shown in the next row. (D) Collagen II and (F) safranin O staining is limited to (E) unlabeled (Prussian Blue negative) cells. Chondrocytic differentiation is inhibited by SPIO and not PLL, as hMSCs exposed to PLL only (without SPIO) generate large amounts of (G) collagen II-rich and (I) safranin O-positive extracellular matrix, and (H) are unstained by PB. For comparison, hMSCs unexposed to either Feridex® or PLL are shown following staining for (J) collagen II, (L) safranin O, and (K) are unstained for PB. All pictures were taken at the same magnification. Bar in panel A represents 400 μm. Panels A-L reproduced with permission. [53a] 2004, Wiley.
This SPIO-mediated inhibition of chondrogenic differentiation was later confirmed by several other independent groups. Micropelleted chondrocyte differentiation cell studies of labeled and Feridex®-labeled hMSCs showed that labeled cells did not stain for Alcian Blue, i.e., did not a form a GAG extracellular matrix.[54] Similar results were obtained when Ferucarbotran (Resovist®) was used instead of Feridex®.[55] Chondrogenic gene expression (COL2A2, ACAN, SOX9, COL10, COMP) was significantly altered in a dose-dependent manner in Endorem® (aka Feridex®)-labeled cells, as were GAG and type II collagen staining.[56] In addition to lowering the concentration of Resovist® during incubation, chondrogenic differentiation could also be mitigated by shortening the incubation time.[57] Interestingly, it was recently demonstrated that SPIO-labeled hMSCs can recreate biogenic magnetic nanoparticles as a possible detoxifying mechanism, since this neosynthesis does not occur during chondrogenesis.[32]
Further evidence that the intracellular presence of iron atoms can be interfering with hMSC downstream cell differentiation was demonstrated by the observation that adding desferrioxamine as an iron-chelating agent mitigated Ferucarbotran-induced inhibition of osteogenesis, while activiating the signaling molecules β-catenin, a cancer/testis antigen, SSX, and matrix metalloproteinase 2 (MMP2) in a dose-dependent manner.[48b]. The authors also observed an increased mobilization/migration in culture (see Section 4.2. above), which they believe was responsible for the reduced osteogenic differentiation and is Fe-mediated by the Wnt signaling pathway. In this respect, for hMSCs, it has been previously reported that activation of the Wnt signaling pathway can enhance cell proliferation and mobilization while suppressing osteogenic differentiation.[58] In addition to inhibition of osteogenesis alone, one study has reported that both osteogenesis and adipogenesis is reduced in SPIO-labeled adipose-derived stem cells[59] and another one both osteogenesis and chondrogenesis.[60] The latter study revealed an upregulation of secreted growth factors in SPIO-labeled cells, including amphiregulin, heparin-binding epidermal growth factor, and vascular endothelial growth factor, which play critical roles in maintaining MSC stemness and differentiation, and proliferation.[61] It should be noted that interference with stem cell differentiation below a level of cytotoxicity is not just limited to SPIO nanoparticles, but has been seen for other preparations, including silica nanoparticles, gold nanoparticles, carbon nanoparticles, and quantum dots,[62], to name a few, which is beyond the scope of this perspective. The take home message here is that proper magnetic labeling for specific stem cell downstream differentiation applications will need to be optimized and personalized on a case-by-case basis.[63]
4.4. Genetic changes
The most comprehensive study on gene expression profiles of Feridex®-labeled vs. unlabeled neural stem cells has been performed by Kedziorek et al.[64] Relative to unlabeled cells, less than 1% of genes (49 total) exhibited greater than 2-fold difference in expression in response to labeling. This included early downregulation of transferrin receptor 1 (Tfrc) and heme oxygenase 1 (Hmox1) expression, whereas genes involved in lysosomal function (Sulf1) and detoxification (Clu, Cp, Gstm2, Mgst1) were upregulated at later time points. Though these genes showed changes in expression over time, the overall extent was limited. Gene ontology analysis demonstrated that genes encoding zinc-binding proteins were enriched after labeling, genes involved with the apoptosis/programmed cell death pathway did not display increased expression. Despite the early cellular genetic response to maintain iron homeostasis, overall neural stem cell gene expression remained largely unaltered following SPIO-labeling. Others have confirmed upregulation of the transferrin receptor in MSCs,[65] although these findings have been disputed.[66] Since this receptor only binds extracellular elemental iron bound to transferrin, other candidates involved with iron homeostasis deserve further attention, such as perhaps divalent metal transporter-1, which binds and transports elemental iron from fused endosomes/lysosomes (containing biodegrading SPIO particles) into the cytoplasm.
More focused studies demonstrated that, in agreement with the inhibition of chondrogenesis described above in Section 4.3, as compared to unlabeled cells SPIO-labeled chondrocytes exhibited a significantly reduced gene expression of the chondrogenic transcription factor sox9 and cartilage extracellular matrix protein collagen type 2, while the expression of the extracellular cartilage matrix protein aggrecan was increased. Chondrogenic gene expression (COL2A2, ACAN, SOX9, COL10, COMP) was significantly altered in a dose-dependent manner.[56] Foldager et al. also studied the chondrocyte-specific genes aggrecan, collagen type 2, and sox9 and showed that all were affected by SPIO- labeling in a dose-dependent manner, without any changes in cell proliferation.[67]
5. In vivo MRI of unlabeled cells
Is magnetic labeling of transplanted cells always necessary for detection by MRI or MPI? For MPI there is no choice, as the signal is derived only from the superparamagnetic moment that flips direction as near-zero field. For MRI, it was recently demonstrated that an innate property of hMSCs, i.e., high expression of high-mannose N-linked glycans on the cell surface, can be exploited as a biomarker using mannose-weighted chemical exchange saturation transfer (CEST) MRI (Figure 5).[68]
Figure 5:

Detection of unlabeled hMSCs with MRI. Unlike other cells, hMSCs have an abundant expression of high-mannose N-linked glycans on the cell membrane, that can generate signal on MANw CEST MRI. Shown are T2-weighted (A,C) and MANw CEST MR (B,D) images for hMSCs (A,B) and (as representative example for other cells) human glial restrictor precursor cells (C,D). Panels A-D reproduced with permission.[68] 2022, Nature Publishing Group.
hMSCs xenografted into mouse brain could be clearly distinguished from the surrounding host tissue, with an excellent agreement between the areas of CEST MRI contrast and mannose-specific fluorescent lectin staining. When cells were differentiated in the osteogenic cell lineage, the loss of HM N-linked glycan expression correlated with a lower CEST MRI signal intensity. Such label-free imaging of hMSCs may facilitate the development and testing of cell therapies, but has so far not yet been demonstrated for other cell types.
It should be mentioned that transgene labeling methods have been developed aiming to avoid the use of SPIO-labeling. This includes the use of CEST MRI reporter genes,[69] DMT-1,[70] and (apo)ferritin.[71] However, none of these marker proteins can be detected by MPI. For the iron-based reporters, although the iron accumulates in the protective iron storage protein ferritin, cells still need to take up excess iron beyond normal levels, which may induce some of the reported side effects outlined above. In addition, the use of viral vectors for non-transient gene transduction requires careful consideration for clinical translation.
6. Conclusions and future outlook
While MRI cell tracking has been succesfully pursued for some 30 years, MPI cell tracking is still in its infancy. The dozen or so clinical MRI studies prove that that SPIO-labeling of cells is safe for the patient, but under certain circumstances the labeling may affect the fate and function of cells. Hence, it is imperative to perform detailed in vitro „toxicity“ studies for each specific labeled cell scenario. As there are currently many new SPIO formulations being developed that are optimized for MRI, such testing will become even more important. Current reports on negative findings following cell labeling are often anecdotal, and very greatly among different laboratories due to differences in cell preparation, cell passage, SPIO formulation, SPIO coating, SPIO incubation doses, and the use of adjunct (e.g., transfection) agents. Overall guidelines for the minimal requirements of testing SPIO-labeled cells are lacking, and a more structured and robust approach for comparing labeled vs. unlabelled cells will be needed to further advance the field and clinical translation.
Acknowledgements
J.W.M.B. is funded by grants from the National Institutes of Health (P41 EB024495, R01 EB023647, R01 EB030376, R01 CA257557, UH3 EB028904, and S10 OD026740), the Maryland Stem Cell Research Fund (MSCRFD-5416), the National Multiple Sclerosis Society (RFA-2104-37460), Philips Medical Systems Inc., and NovaDip Biosciences S.A. C.W. is partially supported by the Pearl and Yueh-Heng Yang foundation. The figures in this manuscript were created with BioRender software (https://biorender.com).
Biographies

Jeff W.M. Bulte is a Professor of Radiology, Oncology, Biomedical Engineering, and Chemical & Biomolecular Engineering at the Johns Hopkins University School of Medicine. He is the inaugural Radiology Director of Scientific Communications, and serves as Director of Cellular Imaging in the Johns Hopkins Institute for Cell Engineering. He is a Fellow and Gold Medal awardee of the ISMRM, a Fellow of WMIS, AIMBE, and IAMBE, and a Distinguished Investigator of the Academy of Radiology Research. His research interest is the development of new imaging agents and theranostics as applied to molecular and cellular imaging, with particular emphasis on in vivo cell tracking and regenerative medicine.

Chao Wang is a postdoctoral fellow in the Russell H. Morgan Department of Radiology and Radiological Science, the Johns Hopkins University School of Medicine, Baltimore, U.S. He received his Ph.D. in 2019 from the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, China. Currently, his main scientific interests include the synthesis and characterization of bionanomaterials, nanotoxicology, multi-modal cell tracking, and applying nanoparticles for cancer therapy.

Ali Shakeri-Zadeh is an Assistant Professor of Radiology at the Johns Hopkins University School of Medicine. His research is focused on developing multifunctional nanostructures for theranostic purposes. Using multifunctional nanoplatforms he has applied different imaging modalities for treatment planning of nanoparticle-assisted cancer therapy. For the past three years, he has been developing multimodal in vivo tracking of stem cells using CT, MRI, and MPI. His current research interest is to combine nanotheranostic labeling of stem cells with image-guided therapy to develop new precision-based personalized methods for treating cancer and neurodegenerative diseases.
Footnotes
Conflict of interest statement
J.W.M.B. is a paid consultant to NovaDip Biosciences SA and SuperBranche. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. C.W. and A.S-Z. have nothing to disclose.
References
- [1].Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT, et al. , N Engl J Med 1987, 316, 889. [DOI] [PubMed] [Google Scholar]
- [2].Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. , N Engl J Med 1988, 319, 1676. [DOI] [PubMed] [Google Scholar]
- [3].Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN, The Lancet Oncology 2014, 15, e257. [DOI] [PubMed] [Google Scholar]
- [4].Gill S, Maus MV, Porter DL, Blood Rev 2016, 30, 157. [DOI] [PubMed] [Google Scholar]
- [5].Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J, eBioMedicine 2020, 59, 102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bulte JWM, Daldrup-Link HE, Radiology 2018, 289, 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM, Science 1998, 282, 1145. [DOI] [PubMed] [Google Scholar]
- [8].Wagers AJ, Weissman IL, Cell 2004, 116, 639. [DOI] [PubMed] [Google Scholar]
- [9].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S, Cell 2007, 131, 861. [DOI] [PubMed] [Google Scholar]
- [10].a) Fisher B, Packard BS, Read EJ, Carrasquillo JA, Carter CS, Topalian SL, Yang JC, Yolles P, Larson SM, Rosenberg SA, J Clin Oncol 1989, 7, 250; [DOI] [PubMed] [Google Scholar]; b) Pockaj BA, Sherry RM, Wei JP, Yannelli JR, Carter CS, Leitman SF, Carasquillo JA, Steinberg SM, Rosenberg SA, Yang JC, Cancer 1994, 73, 1731. [DOI] [PubMed] [Google Scholar]
- [11].Kurpisz M, Czepczynski R, Grygielska B, Majewski M, Fiszer D, Jerzykowska O, Sowinski J, Siminiak T, Int J Cardiol 2007, 121, 194. [DOI] [PubMed] [Google Scholar]
- [12].a) Ahrens ET, Bulte JW, Nat Rev Immunol 2013, 13, 755; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bulte JWM, Shakeri-Zadeh A, Mol Imaging Biol 2022, 24, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Bulte JW, Walczak P, Gleich B, Weizenecker J, Markov DE, Aerts HC, Boeve H, Borgert J, Kuhn M, Proc SPIE Int Soc Opt Eng 2011, 7965, 79650z; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sehl OC, Gevaert JJ, Melo KP, Knier NN, Foster PJ, Tomography 2020, 6, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Avugadda SK, Wickramasinghe S, Niculaes D, Ju M, Lak A, Silvestri N, Nitti S, Roy I, Samia ACS, Pellegrino T, Nanomaterials (Basel) 2020, 11; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lu C, Han L, Wang J, Wan J, Song G, Rao J, Chem Soc Rev 2021, 50, 8102. [DOI] [PubMed] [Google Scholar]
- [15].a) Bulte JW, Hoekstra Y, Kamman RL, Magin RL, Webb AG, Briggs RW, Go KG, Hulstaert CE, Miltenyi S, The TH, et al. , Magn Reson Med 1992, 25, 148; [DOI] [PubMed] [Google Scholar]; b) Bulte JW, Ma LD, Magin RL, Kamman RL, Hulstaert CE, Go KG, The TH, de Leij L, Magn Reson Med 1993, 29, 32; [DOI] [PubMed] [Google Scholar]; c) Yeh TC, Zhang W, Ildstad ST, Ho C, Magn Reson Med 1993, 30, 617; [DOI] [PubMed] [Google Scholar]; d) Bulte JW, Laughlin PG, Jordan EK, Tran VA, Vymazal J, Frank JA, Acad Radiol 1996, 3 Suppl 2, S301; [DOI] [PubMed] [Google Scholar]; e) Ahrens ET, Feili-Hariri M, Xu H, Genove G, Morel PA, Magn Reson Med 2003, 49, 1006; [DOI] [PubMed] [Google Scholar]; f) Long CM, van Laarhoven HW, Bulte JW, Levitsky HI, Cancer Res 2009, 69, 3180; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Dekaban GA, Hamilton AM, Fink CA, Au B, de Chickera SN, Ribot EJ, Foster PJ, Wiley Interdiscip Rev Nanomed Nanobiotechnol 2013, 5, 469. [DOI] [PubMed] [Google Scholar]
- [16].de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJ, Bonenkamp JJ, Boezeman JB, Adema GJ, Bulte JW, Scheenen TW, Punt CJ, Heerschap A, Figdor CG, Nat Biotechnol 2005, 23, 1407. [DOI] [PubMed] [Google Scholar]
- [17].a) Karmarkar PV, Kraitchman DL, Izbudak I, Hofmann LV, Amado LC, Fritzges D, Young R, Pittenger M, Bulte JW, Atalar E, Magn Reson Med 2004, 51, 1163; [DOI] [PubMed] [Google Scholar]; b) Janowski M, Lyczek A, Engels C, Xu J, Lukomska B, Bulte JW, Walczak P, J Cereb Blood Flow Metab 2013, 33, 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gleich B, Weizenecker J, Nature 2005, 435, 1214. [DOI] [PubMed] [Google Scholar]
- [19].Bulte JWM, Adv Drug Deliv Rev 2019, 138, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bulte JW, Walczak P, Janowski M, Krishnan KM, Arami H, Halkola A, Gleich B, Rahmer J, Tomography 2015, 1, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].a) Zheng B, von See MP, Yu E, Gunel B, Lu K, Vazin T, Schaffer DV, Goodwill PW, Conolly SM, Theranostics 2016, 6, 291; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nejadnik H, Pandit P, Lenkov O, Lahiji AP, Yerneni K, Daldrup-Link HE, Mol Imaging Biol 2019, 21, 465. [Google Scholar]
- [22].Rivera-Rodriguez A, Hoang-Minh LB, Chiu-Lam A, Sarna N, Marrero-Morales L, Mitchell DA, Rinaldi-Ramos CM, Nanotheranostics 2021, 5, 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gevaert JJ, Fink C, Dikeakos JD, Dekaban GA, Foster PJ, Mol Imaging Biol 2022, DOI: 10.1007/s11307-022-01738-w. [DOI] [PubMed] [Google Scholar]
- [24].a) Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, Zywicke H, Miller B, van Gelderen P, Moskowitz BM, Duncan ID, Frank JA, Nat Biotechnol 2001, 19, 1141; [DOI] [PubMed] [Google Scholar]; b) Frank JA, Zywicke H, Jordan EK, Mitchell J, Lewis BK, Miller B, Bryant LH Jr., Bulte JW, Acad Radiol 2002, 9 Suppl 2, S484; [DOI] [PubMed] [Google Scholar]; c) Arbab AS, Bashaw LA, Miller BR, Jordan EK, Bulte JW, Frank JA, Transplantation 2003, 76, 1123. [DOI] [PubMed] [Google Scholar]
- [25].a) Walczak P, Kedziorek DA, Gilad AA, Lin S, Bulte JW, Magn Reson Med 2005, 54, 769; [DOI] [PubMed] [Google Scholar]; b) Walczak P, Ruiz-Cabello J, Kedziorek DA, Gilad AA, Lin S, Barnett B, Qin L, Levitsky H, Bulte JW, Nanomedicine 2006, 2, 89; [DOI] [PubMed] [Google Scholar]; c) Engberink RD, van der Pol SM, Walczak P, van der Toorn A, Viergever MA, Dijkstra CD, Bulte JW, de Vries HE, Blezer EL, Mol Imaging 2010, 9, 268. [PMC free article] [PubMed] [Google Scholar]
- [26].a) Qiu B, Xie D, Walczak P, Li X, Ruiz-Cabello J, Minoshima S, Bulte JW, Yang X, Magn Reson Med 2010, 63, 1437; [DOI] [PubMed] [Google Scholar]; b) Xie D, Qiu B, Walczak P, Li X, Ruiz-Cabello J, Minoshima S, Bulte JW, Yang X, NMR Biomed 2010, 23, 480. [DOI] [PubMed] [Google Scholar]
- [27].Bulte JW, Zhang S, van Gelderen P, Herynek V, Jordan EK, Duncan ID, Frank JA, Proc Natl Acad Sci U S A 1999, 96, 15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Van de Walle A, Kolosnjaj-Tabi J, Lalatonne Y, Wilhelm C, Acc Chem Res 2020, 53, 2212. [DOI] [PubMed] [Google Scholar]
- [29].a) Arbab AS, Wilson LB, Ashari P, Jordan EK, Lewis BK, Frank JA, NMR Biomed 2005, 18, 383; [DOI] [PubMed] [Google Scholar]; b) Mazuel F, Espinosa A, Luciani N, Reffay M, Le Borgne R, Motte L, Desboeufs K, Michel A, Pellegrino T, Lalatonne Y, Wilhelm C, ACS Nano 2016, 10, 7627. [DOI] [PubMed] [Google Scholar]
- [30].Weissleder R, Stark DD, Engelstad BL, Bacon BR, Compton CC, White DL, Jacobs P, Lewis J, AJR Am J Roentgenol 1989, 152, 167. [DOI] [PubMed] [Google Scholar]
- [31].Van de Walle A, Fromain A, Plan Sangnier A, Curcio A, Lenglet L, Motte L, Lalatonne Y, Wilhelm C, Nano Res 2020, 13, 467. [Google Scholar]
- [32].a) Curcio A, Van de Walle A, Serrano A, Preveral S, Pechoux C, Pignol D, Menguy N, Lefevre CT, Espinosa A, Wilhelm C, ACS Nano 2020, 14, 1406; [DOI] [PubMed] [Google Scholar]; b) Van de Walle A, Plan Sangnier A, Abou-Hassan A, Curcio A, Hemadi M, Menguy N, Lalatonne Y, Luciani N, Wilhelm C, Proc Natl Acad Sci U S A 2019, 116, 4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bulte JW, Douglas T, Mann S, Frankel RB, Moskowitz BM, Brooks RA, Baumgarner CD, Vymazal J, Strub MP, Frank JA, J Magn Reson Imaging 1994, 4, 497. [DOI] [PubMed] [Google Scholar]
- [34].Sarkar A, Sil PC, Food Chem Toxicol 2014, 71, 106. [DOI] [PubMed] [Google Scholar]
- [35].Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, Kalish H, Frank JA, Radiology 2003, 229, 838. [DOI] [PubMed] [Google Scholar]
- [36].Carenza E, Barcelo V, Morancho A, Levander L, Boada C, Laromaine A, Roig A, Montaner J, Rosell A, Nanomedicine 2014, 10, 225. [DOI] [PubMed] [Google Scholar]
- [37].Soenen SJ, Himmelreich U, Nuytten N, De Cuyper M, Biomaterials 2011, 32, 195. [DOI] [PubMed] [Google Scholar]
- [38].Lewinski N, Colvin V, Drezek R, Small 2008, 4, 26. [DOI] [PubMed] [Google Scholar]
- [39].Kim HS, Oh SY, Joo HJ, Son KR, Song IC, Moon WK, NMR in Biomedicine 2010, 23, 514. [DOI] [PubMed] [Google Scholar]
- [40].Berman SC, Galpoththawela C, Gilad AA, Bulte JW, Walczak P, Magn Reson Med 2011, 65, 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lepore AC, Walczak P, Rao MS, Fischer I, Bulte JW, Exp Neurol 2006, 201, 49. [DOI] [PubMed] [Google Scholar]
- [42].Pawelczyk E, Arbab AS, Chaudhry A, Balakumaran A, Robey PG, Frank JA, Stem Cells 2008, 26, 1366. [DOI] [PubMed] [Google Scholar]
- [43].Knoblich JA, Cell 2008, 132, 583. [DOI] [PubMed] [Google Scholar]
- [44].Walczak P, Kedziorek DA, Gilad AA, Barnett BP, Bulte JW, Magn Reson Med 2007, 58, 261. [DOI] [PubMed] [Google Scholar]
- [45].Schäfer R, Kehlbach R, Müller M, Bantleon R, Kluba T, Ayturan M, Siegel G, Wolburg H, Northoff H, Dietz K, Cytotherapy 2009, 11, 68. [DOI] [PubMed] [Google Scholar]
- [46].Cromer Berman SM, Kshitiz C. J. Wang, Orukari I, Levchenko A, Bulte JW, Walczak P, Magn Reson Med 2013, 69, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Perez JE, Fage F, Pereira D, Abou-Hassan A, Asnacios S, Asnacios A, Wilhelm C, Journal of Nanobiotechnology 2021, 19, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].a) Huang D-M, Hsiao J-K, Chen Y-C, Chien L-Y, Yao M, Chen Y-K, Ko B-S, Hsu S-C, Tai L-A, Cheng H-Y, Biomaterials 2009, 30, 3645; [DOI] [PubMed] [Google Scholar]; b) Chen Y-C, Hsiao J-K, Liu H-M, Lai I-Y, Yao M, Hsu S-C, Ko B-S, Chen Y-C, Yang C-S, Huang D-M, Toxicology and applied pharmacology 2010, 245, 272. [DOI] [PubMed] [Google Scholar]
- [49].a) Moon RT, Bowerman B, Boutros M, Perrimon N, Science 2002, 296, 1644; [DOI] [PubMed] [Google Scholar]; b) Logan CY, Nusse R, Annu Rev Cell Dev Biol 2004, 20, 781; [DOI] [PubMed] [Google Scholar]; c) Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J, Angiogenesis 2005, 8, 43. [DOI] [PubMed] [Google Scholar]
- [50].Yang J-X, Tang W-L, Wang X-X, Cytotherapy 2010, 12, 251. [DOI] [PubMed] [Google Scholar]
- [51].Soenen SJ, Nuytten N, De Meyer SF, De Smedt SC, De Cuyper M, Small 2010, 6, 832. [DOI] [PubMed] [Google Scholar]
- [52].Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, Hare JM, Bulte JW, Circulation 2003, 107, 2290. [DOI] [PubMed] [Google Scholar]
- [53].a) Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW, NMR Biomed 2004, 17, 513; [DOI] [PubMed] [Google Scholar]; b) Bulte JW, Kraitchman DL, Mackay AM, Pittenger MF, Blood 2004, 104, 3410. [DOI] [PubMed] [Google Scholar]
- [54].Reddy AM, Kwak BK, Shim HJ, Ahn C, Cho SH, Kim BJ, Jeong SY, Hwang SJ, Yuk SH, Contrast Media Mol Imaging 2009, 4, 118. [DOI] [PubMed] [Google Scholar]
- [55].Boddington SE, Sutton EJ, Henning TD, Nedopil AJ, Sennino B, Kim A, Daldrup-Link HE, Molecular Imaging and Biology 2011, 13, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Roeder E, Henrionnet C, Goebel JC, Gambier N, Beuf O, Grenier D, Chen B, Vuissoz PA, Gillet P, Pinzano A, PLoS One 2014, 9, e98451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Henning TD, Sutton EJ, Kim A, Golovko D, Horvai A, Ackerman L, Sennino B, McDonald D, Lotz J, Daldrup-Link HE, Contrast Media Mol Imaging 2009, 4, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].a) Boland GM, Perkins G, Hall DJ, Tuan RS, J Cell Biochem 2004, 93, 1210; [DOI] [PubMed] [Google Scholar]; b) Schambony A, Kunz M, Gradl D, Differentiation 2004, 72, 307; [DOI] [PubMed] [Google Scholar]; c) Neth P, Ciccarella M, Egea V, Hoelters J, Jochum M, Ries C, Stem Cells 2006, 24, 1892. [DOI] [PubMed] [Google Scholar]
- [59].Fan J, Tan Y, Jie L, Wu X, Yu R, Zhang M, Stem Cell Res Ther 2013, 4, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chang YK, Liu YP, Ho JH, Hsu SC, Lee OK, Journal of Orthopaedic Research 2012, 30, 1499. [DOI] [PubMed] [Google Scholar]
- [61].a) Qin L, Tamasi J, Raggatt L, Li X, Feyen JH, Lee DC, Dicicco-Bloom E, Partridge NC, J Biol Chem 2005, 280, 3974; [DOI] [PubMed] [Google Scholar]; b) Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, Scarpa A, Dazzi F, Pizzolo G, Vinante F, Blood 2005, 106, 59; [DOI] [PubMed] [Google Scholar]; c) Huang Z, Ren PG, Ma T, Smith RL, Goodman SB, Cytokine 2010, 51, 305. [DOI] [PubMed] [Google Scholar]
- [62].a) Park MV, Annema W, Salvati A, Lesniak A, Elsaesser A, Barnes C, McKerr G, Howard CV, Lynch I, Dawson KA, Toxicology and applied pharmacology 2009, 240, 108; [DOI] [PubMed] [Google Scholar]; b) Zhou X, Yuan L, Wu C, Luo G, Deng J, Mao Z, Rsc Advances 2018, 8, 17656; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Abdal Dayem A, Lee SB, Cho S-G, Nanomaterials 2018, 8, 761; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Liu X, Yang Z, Sun J, Ma T, Hua F, Shen Z, International Journal of Nanomedicine 2019, 14, 3875; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Han SB, Kim JK, Lee G, Kim DH, Advanced Biosystems 2020, 4, 2000247. [DOI] [PubMed] [Google Scholar]
- [63].a) Fayol D, Luciani N, Lartigue L, Gazeau F, Wilhelm C, Advanced healthcare materials 2013, 2, 313; [DOI] [PubMed] [Google Scholar]; b) Van de Walle A, Perez JE, Abou-Hassan A, Hémadi M, Luciani N, Wilhelm C, Materials Today Nano 2020, 11, 100084. [Google Scholar]
- [64].Kedziorek DA, Muja N, Walczak P, Ruiz-Cabello J, Gilad AA, Jie CC, Bulte JW, Magn Reson Med 2010, 63, 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].a) Pawelczyk E, Arbab AS, Pandit S, Hu E, Frank JA, NMR Biomed 2006, 19, 581; [DOI] [PubMed] [Google Scholar]; b) Schäfer R, Kehlbach R, Wiskirchen J, Bantleon R, Pintaske J, Brehm BR, Gerber A, Wolburg H, Claussen CD, Northoff H, Radiology 2007, 244, 514. [DOI] [PubMed] [Google Scholar]
- [66].Pawelczyk E, Frank JA, Radiology 2008, 247, 913; author reply 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Foldager CB, Pedersen M, Ringgaard S, Bünger C, Lind M, Journal of Magnetic Resonance Imaging 2011, 33, 724. [DOI] [PubMed] [Google Scholar]
- [68].Yuan Y, Wang C, Kuddannaya S, Zhang J, Arifin DR, Han Z, Walczak P, Liu G, Bulte JWM, Nat Biomed Eng 2022, 6, 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].a) Airan RD, Bar-Shir A, Liu G, Pelled G, McMahon MT, van Zijl PC, Bulte JW, Gilad AA, Magn Reson Med 2012, 68, 1919; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bar-Shir A, Alon L, Korrer MJ, Lim HS, Yadav NN, Kato Y, Pathak AP, Bulte JWM, Gilad AA, Magn Reson Med 2018, 79, 1010; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Bar-Shir A, Liang Y, Chan KW, Gilad AA, Bulte JW, Chem Commun (Camb) 2015, 51, 4869; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Bar-Shir A, Liu G, Chan KW, Oskolkov N, Song X, Yadav NN, Walczak P, McMahon MT, van Zijl PC, Bulte JW, Gilad AA, ACS Chem Biol 2014, 9, 134; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Bar-Shir A, Liu G, Greenberg MM, Bulte JW, Gilad AA, Nat Protoc 2013, 8, 2380; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Gilad AA, McMahon MT, Walczak P, Winnard PT Jr., Raman V, van Laarhoven HW, Skoglund CM, Bulte JW, van Zijl PC, Nat Biotechnol 2007, 25, 217. [DOI] [PubMed] [Google Scholar]
- [70].Bartelle BB, Szulc KU, Suero-Abreu GA, Rodriguez JJ, Turnbull DH, Magn Reson Med 2013, 70, 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].a) Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET, Nat Med 2005, 11, 450; [DOI] [PubMed] [Google Scholar]; b) Cohen B, Dafni H, Meir G, Harmelin A, Neeman M, Neoplasia 2005, 7, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]


