Abstract
Objectives:
Leprosy, or Hansen’s disease was a major public health problem in Japan in the early 20th century. Today, the number of new cases has decreased significantly. We aimed to investigate the trends of leprosy in Japan over the past 73 years and the challenges faced in recent years.
Methods:
We assessed the data on newly registered cases of leprosy from 1947 to 2020.
Results:
A total of 10,796 newly registered cases of leprosy were reported during the study period, of which 7573 were registered in mainland Japan, 2962 in Okinawa, and 250 were of foreign origin. Most autochthonous cases were born before 1950 in mainland Japan and before 1975 in Okinawa. The number of nonautochthonous cases surpassed that of autochthonous cases in 1992. Nonautochthonous cases originated from 26 countries, particularly Brazil and the Philippines. Three cases of antimicrobial resistance have been detected among nonautochthonous cases since 2004.
Conclusion:
Our data suggest that ongoing transmission of leprosy likely ceased in the 1940s in mainland Japan and in the 1970s in Okinawa. With the recent rise of nonautochthonous cases with globalization, continuous surveillance and efforts to maintain leprosy services within the country are necessary even after reaching the state of elimination.
Keywords: Birth year, Epidemiology, Hansen’s disease, Japan, Leprosy
Introduction
Leprosy, also known as Hansen’s disease, is a disease of the skin and peripheral nerves caused by Mycobacterium leprae, which can lead to life-long disfigurements if not treated early. It is classified as a neglected tropical disease by the World Health Organization (WHO) because it presents a largely hidden burden among poor communities with inadequate housing and sanitation, overcrowding, and limited access to basic health care. Worldwide, 200,000–250,000 new cases of leprosy are reported annually to the WHO, over 95% of which are from 23 global priority countries (World Health Organization [WHO], 2021a).
In many developed countries, leprosy has been considered eliminated. However, the issue remains, particularly due to migration related to globalization. In Japan, a few new cases of leprosy are recorded each year, most of which are imported or ‘nonautochthonous’ cases (Ishii et al., 2000; Koba et al., 2009). Recognizing this worldwide trend, the WHO began including data on nonautochthonous cases in its reporting in 2016 (World Health Organization, 2017).
Leprosy was a major public health concern in Japan in the early 20th century, with a prevalence of approximately 70 per 100,000 individuals in 1990 (Saikawa, 1981). The Government of Japan enacted the ‘Act of Leprosy Prevention’ in 1907, and hospitalization of patients at public sanatoriums was its main control strategy (Sato and Narita, 2003). After World War II, the country underwent exceptionally rapid economic growth, and the number of new cases decreased substantially (Ishii et al., 2000; Koba et al., 2009).
In this report, we present the patterns of newly diagnosed cases (both autochthonous and nonautochthonous) of leprosy in Japan, over a 73-year period. In the recent roadmap for neglected tropical diseases, the target for leprosy control changed from ‘elimination of leprosy as a public health problem’ to ‘elimination (interruption of transmission)’ (World Health Organization 2020). A collection of countries’ experiences, including ours, may provide the evidence to support in making important decisions to reflect this change. Further, we hope that the experiences and lessons of Japan can help devise policies for other countries with existing cases of leprosy and for those that have largely eliminated the disease but are faced with similar challenges as Japan.
Methods
Data source
Data on new leprosy cases were obtained from three sources: (i) the Ministry of Health and Welfare of Japan (1947–1973) (Ministry of Health and Welfare of Japan, 1975), (ii) the Okinawa Leprosy Prevention Association (1900–1998) (Saikawa, 1999), and (iii) an expert group (1964–1992, led by MO; 1993–2020, led by NI). Dermatologists and researchers from the Leprosy Research Center, a branch of the National Institute of Infectious Diseases, form the expert group on leprosy in Japan. Since 1964, this group has collected data through reporting from hospitals, clinics, and sanatoriums in Japan using a questionnaire. Okinawa was included in this dataset in 1974 after being ruled by the United States until 1972. For this study, we selected datasets for mainland Japan and Okinawa (Figure 1), depending on the availability and reliability of data. The data by the expert group were collected prospectively, with interactions with reporting physicians at times of ambiguity or missing data, and we regarded this dataset to be superior in quality over the other two datasets. For the overlapping years, the median differences between the datasets during the periods of 1964–1973 for mainland Japan and 1974–1998 for Okinawa were 15 (interquartile range [IQR] 25–75%, 13.25–38.25) cases and 1 (IQR 25–75%, 0.5–3) case, respectively. The population of Japan was obtained from a dataset issued by the Government of Japan (Statistics Bureau of Japan, 2022).
Figure 1.
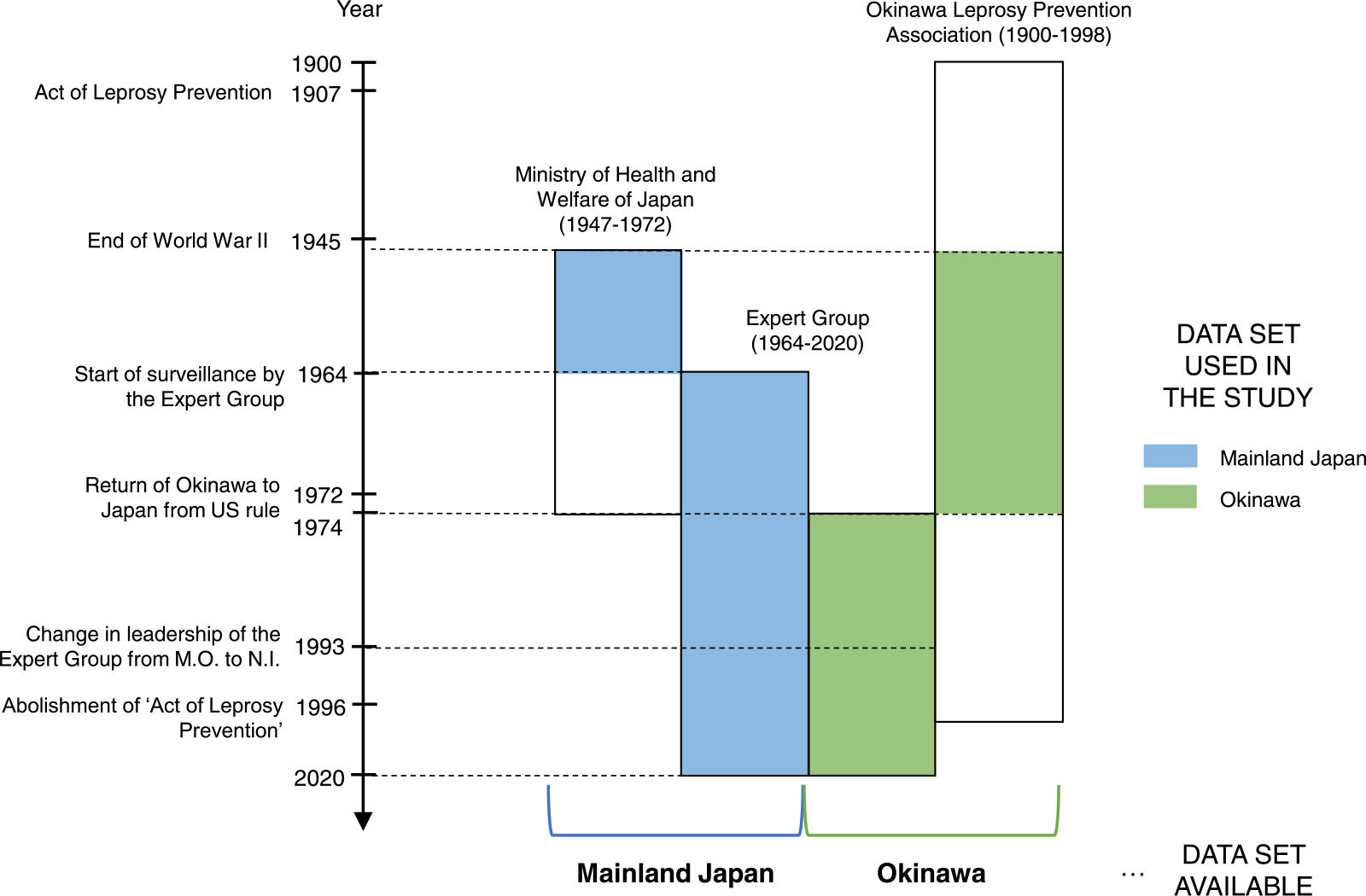
Data sources in the study.
Our dataset included the location (prefecture) of diagnosis and year of diagnosis (1947–2020); birth year, age at diagnosis, sex, and country of origin (1964–2020); and location of birth, prefecture of residence, characteristics of skin lesions, peripheral nerve damage, grade II disabilities, duration between onset and first consultation, family history of leprosy, laboratory test results (polymerase chain reaction [PCR], skin slit smear [SSS], histopathology, and antiphenolic glycolipid-I [PGL-I] antibody test), Ridley-Jopling and WHO classifications, treatment, and leprosy reactions (1993–2020). Antimicrobial resistance testing for multidrug therapy (MDT) has been performed at the National Institute of Infectious Diseases since 2004, wherein the data are also provided here. This protocol has been described previously (Mori et al., 2012).
Analysis
The data for mainland Japan and Okinawa were analyzed separately due to different endemicity trends. Okinawa, the southernmost part of Japan, consisting of over 40 inhabited and 110 uninhabited islands, was the last pocket of leprosy in the country. It constitutes approximately 1% of the total population. Based on the availability of data and to facilitate comparison, the study time-frame was divided into (i) 5-year blocks (for 1947–1950, 3 years) for comparison of newly registered cases, child and female cases, and incidence rates between 1947–2020; (ii) two periods (1964–1990 and 1991–2020) for comparison of the countries of origin of nonautochthonous cases; and (iii) 10-year blocks (for 1993–2000, 7 years) for the comparison of clinical presentations in newly registered cases between 1993–2020.
The mean incidence for a given time is the sum of the newly registered annual cases of leprosy divided by the sum of the yearly population for that time, presented as the number of cases per 100,000 individuals. Temporal changes in incidence were evaluated using univariate linear regression and the slope of the regression line (β) was tested for significance. The chi-square test and Z-test of proportions were used, as applicable. Statistical significance was set at P <0.05. All analyses were performed using JMP version 14SW (JMP, Cary, NC, USA). Patients aged <15 years were classified as child cases. An Okinawa or nonautochthonous case was defined as that involving an individual born in Okinawa or outside Japan, respectively. Newly registered cases were mapped by prefecture of residence from 1993 to 2020 using QGIS 3.16 software (Open Source Geospatial Foundation Project; http://qgis.osgeo.org).
Results
Between 1947 and 2020, a total of 10,796 newly registered cases of leprosy were reported in Japan, of which 7573 were registered in mainland Japan and 2962 in Okinawa, and 261 were of foreign origin (Table 1). Figure 2 shows the trend of these new cases. The total number of new cases peaked in 1949 (924 cases). In mainland Japan, the number of leprosy cases declined from over 500 annually during the post-World War II period (1947–1950) to under 100 annually by the mid-1960s. Okinawa reported over 100 new cases annually until the late 1960s and more cases than mainland Japan until the late 1990s. The decrease in the incidence of new cases was significant in both mainland Japan (β −6.294; P <0.001) and Okinawa (β −1.645; P <0.001). The last autochthonous child case was reported in 1990 in both mainland Japan (family history of lepromatous [LL] case) and Okinawa. Thereafter, there have only been reports of sporadic cases (one or two autochthonous cases) from mainland Japan and Okinawa since 2003 and 2002, respectively. The number of nonautochthonous cases surpassed that of the autochthonous cases in 1992. The dataset for each year is provided in Supplementary File 1.
Table 1.
Trends of Hansen’s disease in Japan by 5-year periods between 1947 and 2020.
| Period | Japan (ALL) | Mainland |
Okinawa |
Nonautochthonous | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| New cases (a+b+c) | New cases (a) | Child (<15 years) % | Female% | Mean incidence rate (/100,000 population) | New cases (b) | Child (<15 years) % | Female% | Mean incidence rate (/100,000 population) | New cases (c) | |
|
| ||||||||||
| 1947–1950 | 3121 | 2701 | NR | NR | 0.8361 | 420 | 14% | 35% | 17.9327 | NR |
| 1951–1955 | 2062 | 1792 | NR | NR | 0.4133 | 270 | 19% | 29% | 6.3454a | NR |
| 1956–1960 | 1927 | 1558 | NR | NR | 0.3398 | 369 | 17% | 36% | 8.6485 | NR |
| 1961–1965 | 1292 | 804 | NR | NR | 0.1680 | 477 | 21% | 32% | 10.3916 | 11b |
| 1966–1970 | 956 | 315 | 6% | 42% | 0.0623 | 623 | 23% | 40% | 13.1216 | 18 |
| 1971–1975 | 531 | 165 | 13% | 38% | 0.0304 | 352 | 18% | 41% | 7.1183 | 14 |
| 1976–1980 | 280 | 86 | 17% | 49% | 0.0149 | 188 | 7% | 43% | 3.4856 | 6 |
| 1981–1985 | 196 | 57 | 12% | 39% | 0.0095 | 132 | 5% | 41% | 2.3023 | 7 |
| 1986–1990 | 129 | 48 | 4% | 42% | 0.0078 | 71 | 1% | 31% | 1.1815 | 10 |
| 1991–1995 | 93 | 19 | 0% | 47% | 0.0030 | 28 | 0% | 46% | 0.4475 | 46 |
| 1996–2000 | 81 | 14 | 0% | 50% | 0.0022 | 17 | 0% | 35% | 0.2619 | 50 |
| 2001–2005 | 55 | 9 | 0% | 56% | 0.0014 | 8 | 0% | 25% | 0.1194 | 38 |
| 2006–2010 | 32 | 3 | 0% | 0% | 0.0005 | 2 | 0% | 50% | 0.0291 | 27 |
| 2011–2015 | 24 | 1 | 0% | 0% | 0.0002 | 4 | 0% | 50% | 0.0567 | 19 |
| 2016–2020 | 17 | 1 | 0% | 0% | 0.0002 | 1 | 0% | 100% | 0.0139 | 15 |
| Total | 10,796 | 7573 | 2962 | 261 | ||||||
NR, no report.
Calculated from population data of Okinawa from 1952–1955 due to unavailability of data for 1951.
Sum of cases for 1964 and 1965.
Figure 2.
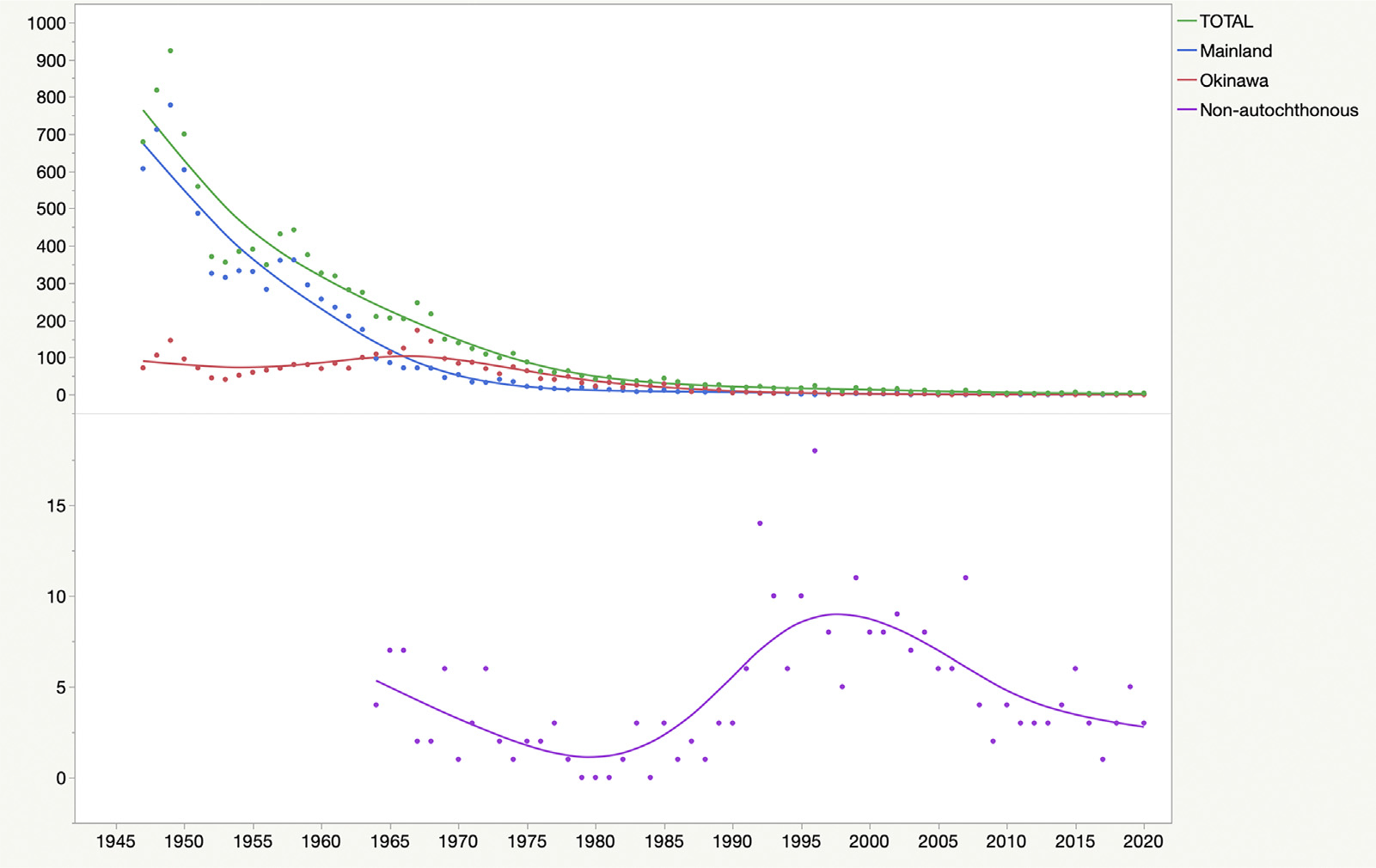
Trends of newly registered cases of leprosy in Japan, 1947–2020.
A total of 261 nonautochthonous leprosy cases were registered between 1964 and 2020. The annual median number of cases was 3 (IQR 25–75%, 2–7). Brazil, the Philippines, and South Korea were the top three countries of origin, but the trends differed between the two periods (1964–1990 and 1991–2020) (Table 2).
Table 2.
Country of origin of nonautochthonous cases of leprosy in Japan, 1964–2020.
| 1964–1990 |
1991–2020 |
ALL |
||||||
|---|---|---|---|---|---|---|---|---|
| Country of origin | Case no. | % | Country of origin | Case no. | % | Country of origin | Case no. | % |
|
| ||||||||
| South Korea | 42 | 63.6% | Brazil | 85 | 43.6% | Brazil | 87 | 33.3% |
| Philippines | 7 | 10.6% | Philippines | 39 | 20.0% | Philippines | 46 | 17.6% |
| Viet Nam | 4 | 6.1% | Indonesia | 15 | 7.7% | South Korea | 45 | 17.2% |
| Taiwan R.O.C. | 3 | 4.5% | Nepal | 15 | 7.7% | Indonesia | 16 | 6.1% |
| Brazil | 2 | 3.0% | Bangladesh | 8 | 4.1% | Nepal | 15 | 5.7% |
| India | 2 | 3.0% | Myanmar | 6 | 3.1% | Bangladesh | 9 | 3.4% |
| Indonesia | 1 | 1.5% | Thailand | 4 | 2.1% | Myanmar | 6 | 2.3% |
| USA (Hawaii) | 1 | 1.5% | Paraguay | 3 | 1.5% | Viet Nam | 5 | 1.9% |
| Comoros | 1 | 1.5% | South Korea | 3 | 1.5% | Thailand | 4 | 1.5% |
| Bangladesh | 1 | 1.5% | Sri Lanka | 3 | 1.5% | India | 4 | 1.5% |
| French Polynesia | 1 | 1.5% | India | 2 | 1.0% | Paraguay | 3 | 1.1% |
| Kuwait | 1 | 1.5% | Micronesia | 2 | 1.0% | Sri Lanka | 3 | 1.1% |
| Timor-Leste | 1 | 0.5% | Taiwan R.O.C. | 3 | 1.1% | |||
| Viet Nam | 1 | 0.5% | Micronesia | 2 | 0.8% | |||
| Bolivia | 1 | 0.5% | USA (Hawaii) | 2 | 0.8% | |||
| Cambodia | 1 | 0.5% | Timor-Leste | 1 | 0.4% | |||
| China | 1 | 0.5% | Bolivia | 1 | 0.4% | |||
| Madagascar | 1 | 0.5% | Cambodia | 1 | 0.4% | |||
| Marshall Islands | 1 | 0.5% | China | 1 | 0.4% | |||
| Palau | 1 | 0.5% | Madagascar | 1 | 0.4% | |||
| USA (Hawaii) | 1 | 0.5% | Marshal Island | 1 | 0.4% | |||
| Tanzania | 1 | 0.5% | Palau | 1 | 0.4% | |||
| Sub-Total | 66 | Sub-Total | 195 | Tanzania | 1 | 0.4% | ||
| Comoros | 1 | 0.4% | ||||||
| French Polynesia | 1 | 0.4% | ||||||
| Kuwait | 1 | 0.4% | ||||||
| Total | 261 | |||||||
From 1993 to 2020, 260 new cases of leprosy were reported from 132 facilities (Table 3): 222 (85.4%) from 118 hospitals, 32 (12.3%) from eight sanatoriums, and six (2.3%) from private clinics. Among them, 220 (84.6%) were reported by dermatologists, 32 (12.3%) by doctors working in sanatoriums, and eight (3.1%) by other physicians. A total of 36 cases (13.8%) were from mainland Japan, 49 (18.9%) from Okinawa, and 175 (67.3%) were of foreign origin. There were no child cases among the three groups, except for a female aged 10 years from Madagascar, recorded in 1999. The median age of patients diagnosed from mainland Japan was >70 years, whereas for Okinawa, it gradually increased to 81 years (IQR 25–75%, 74–83) in the past decade. Two patients were in their 90s, with onsets between 1 year and 5 years before diagnosis. During the past three decades, the mean age for nonautochthonous cases was early 30s. Comparisons of the ages among the three groups are presented in Figure 3. More multibacillary (MB) than paucibacillary (PB) types were seen in all groups, with a statistical significance in the nonautochthonous group (chi-square test, P-value = 0.0085). Two (0.8%) patients had pure neuritic leprosy. Grade II disability was observed in 7.3% among the cases diagnosed between 2001 and 2020.
Table 3.
Clinical presentations of newly registered cases of leprosy in Japan, 1993–2020.
| Mainland |
Okinawa |
Nonautochthonous |
Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1993–2000 | 2001–2010 | 2011–2020 | 1993–2000 | 2001–2010 | 2011–2020 | 1993–2000 | 2001–2010 | 2011–2020 | 1993–2020 | ||
|
| |||||||||||
| Number of cases | 21 | 11 | 2 | 35 | 11 | 5 | 76 | 65 | 34 | 260 | |
| Sex | Male | 10 | 6 | 2 | 22 | 8 | 2 | 56 | 51 | 21 | 178 |
| Female | 11 | 5 | 0 | 13 | 3 | 3 | 20 | 14 | 13 | 82 | |
| Male/female ratio | 0.9 | 1.2 | 0.0 | 1.7 | 2.7 | 0.7 | 2.8 | 3.6 | 1.6 | 2.2 | |
| Age | Median age (IQR 25%–75%) | 77 (69–80) | 73 (47–80) | 76 (75–76) | 61 (46–73.5) | 64 (46.5–70.5) | 81 (74–83) | 32 (25.8–39.3) | 31 (25–37) | 33 (27–45) | 36 (28–59.3) |
| Children younger than 15 years | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.3%) | 0 (0%) | 0 (0%) | 1 (0.4%) | |
| Ridley-Jopling classification | I | 0 (0%) | 1 (9.1%) | 0 (0%) | 2 (5.7%) | 0 (0%) | 0 (0%) | 2 (2.6%) | 2 (3.1%) | 0 (0%) | 7 (2.7%) |
| TT | 1 (4.8%) | 0 (0%) | 0 (0%) | 4 (11.4%) | 2 (18.2%) | 0 (0%) | 13 (17.1%) | 2 (3.1%) | 2 (5.9%) | 24 (9.2%) | |
| BT | 5 (23.8%) | 1 (9.1%) | 1 (50%) | 15 (42.9%) | 5 (45.5%) | 1 (20.0%) | 23 (30.3%) | 16 (24.6%) | 12 (35.3%) | 79 (30.4%) | |
| BB | 0 (0%) | 1 (9.1%) | 0 (0%) | 5 (14.3%) | 0 (0%) | 0 (0%) | 9 (11.8%) | 3 (4.6%) | 1 (2.9%) | 19 (7.3%) | |
| BL | 11 (52.4%) | 4 (36.4%) | 1 (50%) | 4 (11.4%) | 4 (36.4%) | 3 (60.0%) | 15 (19.7%) | 26 (40.0%) | 8 (23.5%) | 76 (29.2%) | |
| LL | 3 (14.3%) | 4 (36.4%) | 0 (0%) | 5 (14.3%) | 0 (0%) | 1 (20.0%) | 14 (18.4%) | 16 (24.6%) | 10 (29.4%) | 53 (20.4%) | |
| PNL | 1 (4.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2.9%) | 2 (0.8%) | |
| WHO classification | Multibacillary | 16 (76.2%) | 10 (90.9%) | 2 (100%) | 21 (60.0%) | 5 (45.5%) | 4 (80.0%) | 44 (57.9%) | 53 (81.5%) | 21 (61.8%) | 176 (67.7%) |
| Paucibacillary | 5 (23.8%) | 1 (9.1%) | 0 (0%) | 14 (40.0%) | 6 (54.5%) | 1 (20.0%) | 32 (42.1%) | 12 (18.5%) | 13 (38.2%) | 84 (32.3%) | |
| G2D | (+) | NA | 4 (36.4%) | 2 (100%) | NA | 1 (9.1%) | 0 (0%) | NA | 6 (9.2%) | 6 (17.6%) | 19 (7.3%) |
| Duration between onset and the first consultation | ≤1 year | 6 (28.6%) | 7 (63.6%) | 1 (50.0%) | 10 (28.6%) | 5 (45.5%) | 4 (80.0%) | 20 (26.3%) | 48 (73.8%) | 20 (58.8%) | 121 (46.5%) |
| 1–2 years | 4 (19.0%) | 0 (0%) | 0 (0%) | 9 (25.7%) | 1 (9.1%) | 0 (0%) | 14 (18.4%) | 1 (1.5%) | 0 (0%) | 29 (11.2%) | |
| 2–4 years | 2 (9.5%) | 1 (9.1%) | 0 (0%) | 5 (14.3%) | 2 (18.2%) | 1 (20.0%) | 17 (22.4%) | 12 (18.5%) | 6 (17.6%) | 46 (17.7%) | |
| 5–9 years | 1 (4.8%) | 2 (18.2%) | 1 (50.0%) | 3 (8.6%) | 2 (18.2%) | 0 (0%) | 4 (5.3%) | 2 (3.1%) | 3 (8.8%) | 18 (6.9%) | |
| ≥10 years | 5 (23.8%) | 1 (9.1%) | 0 (0%) | 4 (11.4%) | 1 (9.1%) | 0 (0%) | 5 (6.6%) | 2 (3.1%) | 4 (11.8%) | 22 (8.5%) | |
| No data | 3 (14.3%) | 0 (0%) | 0 (0%) | 4 (11.4%) | 0 (0%) | 0 (0%) | 16 (21.1%) | 0 (0%) | 1 (2.9%) | 24 (9.2%) | |
| Family history of leprosy | (+) | 1 (4.8%) | 1 (9.1%) | 0 (0%) | 4 (11.4%) | 2 (18.2%) | 1 (33.3%) | 9 (15.8%) | 12 (20.7%) | 2 (6.9%) | 32 (12.3%) |
| No data | 5 (23.8%) | 1 (9.1%) | 1 (50.0%) | 19 (54.3%) | 5 (45.5%) | 2 (40.0%) | 19 (25.0%) | 7 (10.8%) | 5 (14.7%) | 64 (24.6%) | |
I, indeterminate; TT, tuberculoid; BT, borderline tuberculoid; BB, borderline borderline; BL, borderline lepromatous; LL; lepromatous; PNL, pure neuritic leprosy; WHO, World Helath Organization; G2D, grade-II disabilities.
Figure 3.
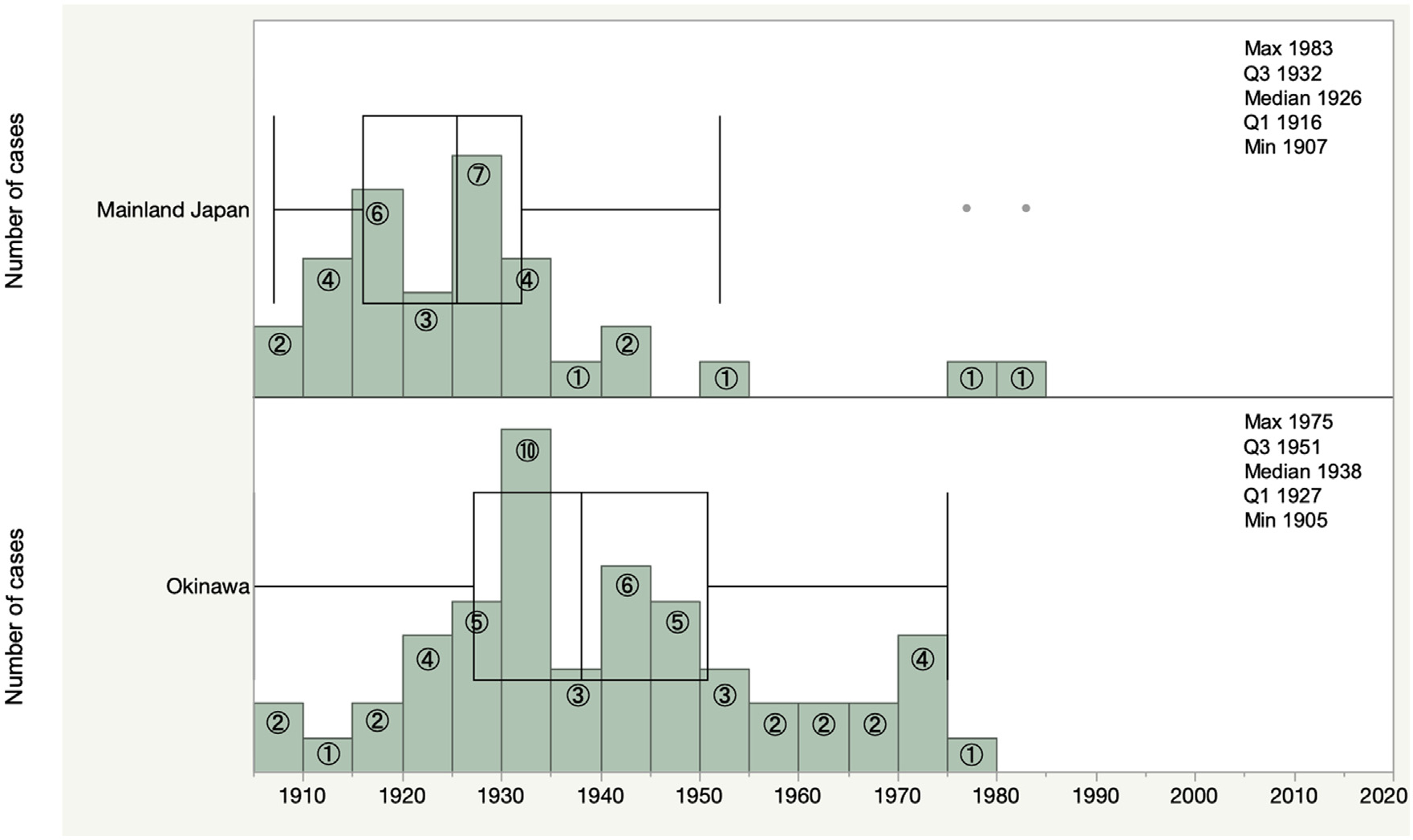
Years of birth of newly registered autochthonous cases of leprosy in Japan, 1993–2020.
Figure 4 presents birth years of newly diagnosed cases of leprosy between 1993 and 2020. All cases from mainland Japan were born before 1942, except for three cases. The detailed descriptions of these cases are provided in Supplementary File 2. For Okinawa, all cases were born before 1975.
Figure 4.
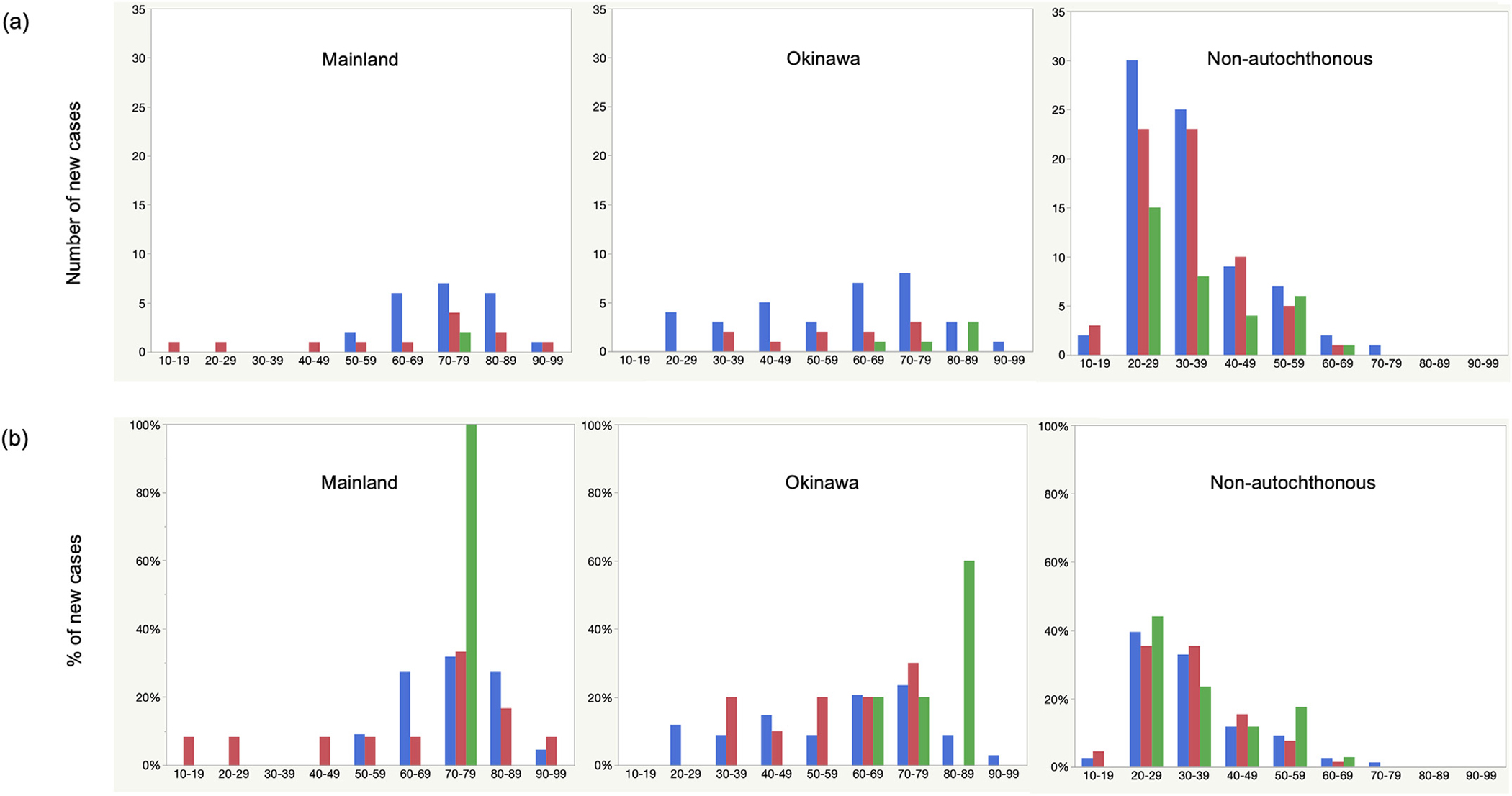
Numbers (a) and percentages (b) of newly diagnosed leprosy cases by age groups in Japan, 1993–2020. In blue, 1993–2000; in red, 2001–2010; in green, 2011–2020.
Figure 5 shows the geographical distributions of all cases by the prefecture of residence and autochthonous cases by the prefecture of birth, during the period between 1993 and 2020. For those with missing residences (16 cases, 6.2%), the institutional address where they were diagnosed was used. Cases were concentrated in Okinawa (52 cases, 20.0 %), with one case of foreign origin. The major industrial zones of Japan include prefectures of Tokyo (capital city), Kanagawa, Saitama, and Chiba in the Kanto region and Aichi, Mie, Osaka, and Hyogo. Prefectures from these zones, together with Shizuoka, where there are large Brazilian migrant communities, reported 114 (43.8%) cumulative cases, among which, 102 (89.5%) cases were of foreign origin. When autochthonous cases were mapped out by their prefecture of birth, the concentration of cases was seen in Kagoshima prefecture (six cases as opposed to one case by the prefecture of residence), besides Okinawa. The prefecture includes Amami-oshima, which is a string of islands geographically a part of the Okinawa archipelago but historically belongs to Kagoshima prefecture, and was also the last endemic site for leprosy in Japan. No case was reported from the northern part of the country for both autochthonous and nonautochthonous cases.
Figure 5.
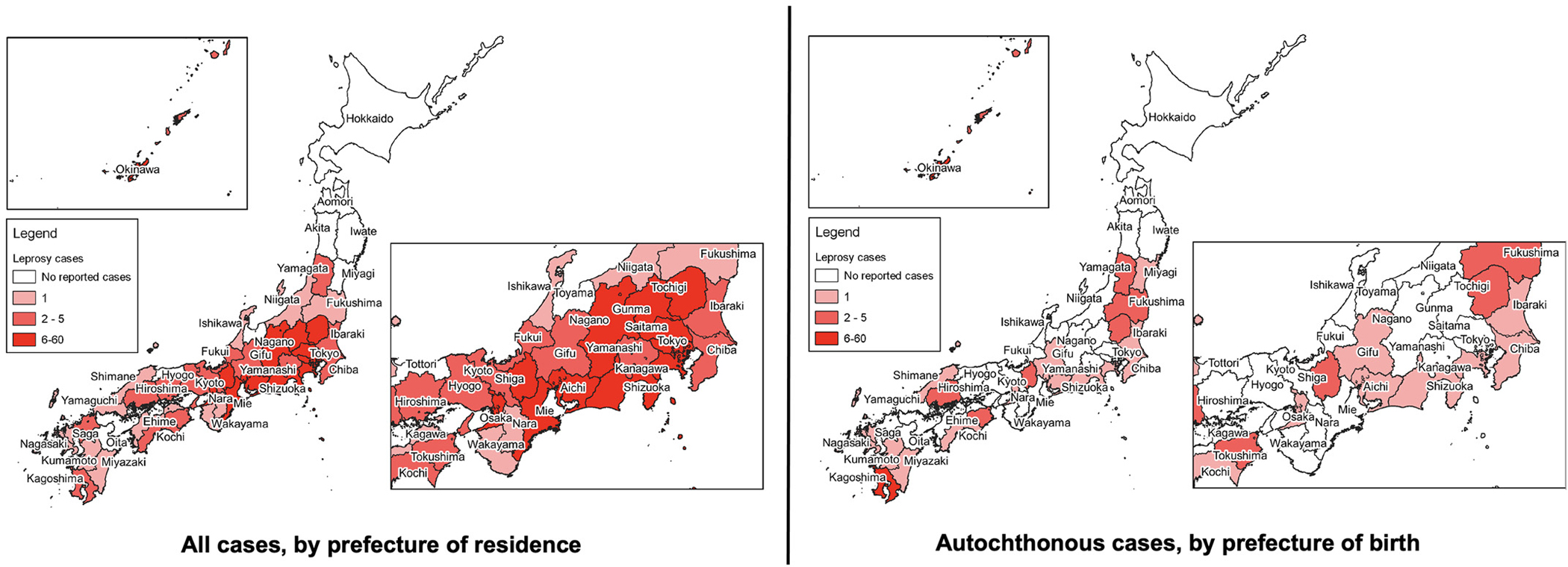
Distribution of newly registered cases of leprosy in Japan, 1993–2020.
A total of 86 of 96 (88.7%) MB cases were PCR-positive, as were 26 (59.1%) of the PB cases (chi-square test, P <0.0001). The sensitivity of PCR was compared against SSS and histopathology and was found to be significantly greater than the two tests (Z-test of proportion, P <0.0001) in PB but not in MB type. In terms of disease spectrum, PCR was positive in all spectra, with the highest observed for borderline (100%), followed by LL (96.9%), and the least (55.6%) for tuberculoid subgroups (Table 4). The sensitivity for both SSS and acid-fast bacilli in histopathology increased consistently as the spectrum moved from indeterminate to LL subgroups. Anti-PGL-I antibody was positive in 40.1% of the cases, with a higher positive proportion seen in borderline lepromatous and LL subgroups.
Table 4.
Percentage positivity (sensitivity) of laboratory tests performed on cases of leprosy in Japan, 1993–2020.
| (a) | Total | Multibacillary | P-value | Paucibacillary | P-value |
|
| |||||
| Polymerase chain reaction (positive: negative) (n = 141) | 112: 29 (79.4%) | 86: 11 (88.7%) | - | 26: 18 (59.1%) | - |
| AFB in slit-skin smear (positive: negative) (n = 211) | 129: 82 (61.1%) | 129: 21 (86.0%) | 0.5433 | 0: 61 (0%) | < 0.0001 |
| AFB in histopathology (positive: negative) (n = 230) | 154: 76 (67.0%) | 147: 15 (90.7%) | 0.5896 | 7: 61 (10.3%) | < 0.0001 |
| Anti-PGL-I antibody (n = 110) | 45: 65 (40.1%) | 41: 37 (52.6%) | <0.0001 | 4: 28 (12.5%) | < 0.0001 |
|
| |||||
| (b) Spectrum of leprosy | Polymerase chain reaction (positive: negative) (n = 141) | AFB in slit-skin smear (present: absent) (n = 211) | AFB in histopathology (present: absent) (n = 230) | Anti-PGL-I antibody (positive: negative) (n = 110) | |
|
| |||||
| Indeterminate | 3: 1 (75.0%) | 0: 5 (0%) | 0: 6 (0%) | 0: 4 (0%) | |
| Tuberculoid | 5: 3 (62.5%) | 0: 14 (0%) | 0: 13 (0%) | 0: 5 (0%) | |
| Borderline tuberculoid | 25: 16 (61.0%) | 14: 48 (22.6%) | 28: 46 (37.8%) | 5: 28 (15.2%) | |
| Borderline borderline | 6: 0 (100%) | 10: 3 (76.9%) | 13: 2 (86.7%) | 0: 2 (0%) | |
| Borderline lepromatous | 42: 7 (85.7%) | 62: 8 (88.6%) | 66: 6 (91.7%) | 22: 18 (55.0%) | |
| Lepromatous | 31: 1 (96.9%) | 43: 2 (95.6%) | 47: 2 (95.2%) | 18: 7 (72.0%) | |
| Pure neuritic leprosy | 0: 1 (0%) | 0: 2 (0%) | 0: 1 (0%) | 0: 1 (0%) | |
AFB, acid-fast bacilli; PGL-I, phenolic glycolipid-I.
Table 5 presents the different regimens of drugs used in treating leprosy in Japan. A total of 150 (69.4%) cases received MDT. Resistance testing against MDT (rifampicin, dapsone, and quinolone) was performed for 56 PCR-positive cases diagnosed between 2004 and 2020 (Table 6). All drug-resistant cases were nonautochthonous, MB type, with one case each coming from Brazil, South Korea, and the Philippines. They were registered as all newly diagnosed cases without previous family history and treatment for leprosy (Supplementary File 3).
Table 5.
Treatment of leprosy cases in Japan, 1993–2020.
| No. of drugs | Single drug | n | Two drugs | n | Three drugs | n | Four drugs | n | Five drugs | n |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Regimen | DDS | 5 | DDS+RFP(MDT/PB) | 34 | DDS+RFP+CLF (MDT/MB) | 116 | MDT+OFLX | 4 | MDT+OFLX+CAM | 1 |
| RFP | 2 | DDS+SPFX | 1 | DDS+RFP+OFLX | 7 | MDT+CAM | 1 | |||
| CLF | 1 | DDS+OFLX | 1 | DDS+RFP+LVFX | 3 | MDT+LVFX | 14 | |||
| SPFX | 2 | CLF+SPFX | 1 | DDS+CLF+SPFX | 1 | MDT+SPFX | 2 | |||
| OFLX | 1 | RFP+CLF | 1 | DDS+CLF+LVFX | 1 | MDT+MINO | 2 | |||
| LVFX | 1 | DDS+MINO | 1 | DDS+CLF+CAM | 3 | DDS+RFP+OFLX+SPFX | 3 | |||
| DDS+CLF | 2 | DDS+RFP+SPFX | 2 | DDS+CLF+SPFX+CAM | 1 | |||||
| RFP+CLF+OFLX | 1 | DDS+RFP+OFLX+MINO | 2 | |||||||
| OFLX+MINO+CAM+LVFX | 1 | |||||||||
|
| ||||||||||
| Total number, by regimen (%) | 12 (5.5%) | 40 (18.4%) | 134 (61.8%) | 30 (13.9%) | 1 (0.5%) | |||||
|
| ||||||||||
| Total number (%) | 216 (100%) | |||||||||
CAM, clarithromycin; CLF, clofazimine; DDS, dapsone; LVFX, levofloxacin; MB, multibacillary; MDT, multidrug therapy; MINO, minocycline; OFLX; ofloxacin; PB, paucibacillary; RFP, rifampicin; SPFX, sparfloxacin.
Table 6.
Resistance to multidrug therapy in cases of leprosy diagnosed in Japan, 2004–2020.
| Country | No. of polymerase chain reaction (+) samples tested for mutation | Mutation |
|||
|---|---|---|---|---|---|
| No mutation | fol P (dapsone) | rpo B (rifampicin) | gyr A (quinolones) | ||
|
| |||||
| Bangladesh | 1 | 1 | 0 | 0 | 0 |
| Brazil | 14 | 13 | 1 | 0 | 0 |
| Cambodia | 0 | 0 | 0 | 0 | 0 |
| East Timor | 1 | 1 | 0 | 0 | 0 |
| Indonesia | 7 | 7 | 0 | 0 | 0 |
| Japan | 7 | 7 | 0 | 0 | 0 |
| South Korea | 1 | 0 | 1 | 0 | 0 |
| Myanmar | 1 | 1 | 0 | 0 | 0 |
| Nepal | 10 | 10 | 0 | 0 | 0 |
| Philippines | 12 | 11 | 0 | 0 | 1 |
| Sri Lanka | 0 | 0 | 0 | 0 | 0 |
| Tanzania | 0 | 0 | 0 | 0 | 0 |
| Thailand | 1 | 1 | 0 | 0 | 0 |
| Viet Nam | 1 | 1 | 0 | 0 | 0 |
| Total | 56 | 53 | 2 | 0 | 1 |
| % | 100% | 94.6% | 3.6% | 0.0% | 1.8% |
Type 1 leprosy reactions were reported in 30 (11.5%) cases, and type 2 reactions or erythema nodosum leprosum were reported in 24 (9.2%) cases. A total of 12 (4.6%) patients presented with symptoms compatible with reactions but were not classified. Six patients received thalidomide for erythema nodosum leprosum treatment.
Discussion
Our dataset is one of the most detailed longitudinal nationwide datasets for new cases of leprosy in recent years, and we observed a steep decline in cases in Japan over the 73-year study period. Japan has reached elimination status for leprosy, as currently defined by less than one case per 100,000 population sometime in the first half of the 20th century in mainland Japan and in the early 1990s in Okinawa. However, we are still experiencing cases due to globalization and intercontinental migration, which are bringing in new challenges. This is a challenge to the country and to the affected individuals because diagnosis tend to get delayed owing to the physicians’ limited awareness and knowledge of the disease. In the long run, nonautochthonous cases may potentially pose a challenge for transmission of leprosy within Japan unless we maintain some form of surveillance system in place.
It was exceptionally interesting to observe the continuous steep decline of number of new leprosy cases in Japan over this study period as the country has undergone a very rapid development from the 1950s to 1970s. During this time, the gross domestic product of Japan grew almost by 30 folds, which is currently the third largest in the world (National Economic Indicators, 2021). Leprosy is commonly known to be related to poor hygiene and nutrition as well as socioeconomic conditions (Dwivedi et al, 2019; Pescarini et al., 2018; Saikawa, 1981). Multiple factors contribute to this decline, and it is almost impossible to identify specific causal factors. Besides improvements in living conditions, it is possible that the strict segregation mandated by the government contributed, to some extent, to reducing disease transmission among the general public before effective microbial treatment became readily available (Saikawa, 1981). In contrast with mainland Japan, Okinawa experienced a delay in case decline, most likely due to a lag in leprosy control measures, geographic characteristics (many islands), strong stigma against patients with leprosy, and slow improvement of water and sewage systems (Koba et al., 2009).
Because infection by M. leprae is known to happen at a very early stage of life, birth years of cases of leprosy may potentially provide evidence for continued infection transmission as well as an estimation for when transmission cessation occurred (Feldman and Sturdivant, 1975; Koba et al., 2009). We found that most autochthonous cases in mainland Japan were born before the 1940s. and estimate that the ongoing transmission ceased in mainland Japan sometime during the 1940s. We define cessation of ongoing transmission as the absence of new cases by birth year for a period of at least 5 years. Of the three sporadic cases born after 1950, two cases were individuals living in proximity to Okinawa (Kagoshima and Nagasaki prefectures), and they may have exhibited the Okinawa pattern more. The third case was a Japanese-Brazilian who was the first generation born in Japan with a family history of leprosy. In Okinawa, no cases have been reported in patients born after 1975, and thus, the ongoing transmission highly likely ceased in the 1970s. Interestingly, a report by Nagao in 1985 analyzed new cases of leprosy against their birth years by different locations in the Miyako Islands of Okinawa and found that all cases reported in 1975–1984 were born before the 1970s (Nagao, 1985). Moreover, a study in the United States showed a decrease in overall incidence rates by successive birth cohorts between 1855–1970 (Feldman and Sturdivant, 1975). Notably, there is a lack of epidemiological reports, which include birth years in the reporting of leprosy in recent years. Because it provides valuable insights into the status of transmission of leprosy within a defined area, we suggest that this needs to be revisited as one indicator to assess transmission in the current epidemiological data collection for leprosy.
Japan, as an archipelago, is an interesting country to investigate the transmission as well as the incubation period of leprosy. The genomic analysis of M. leprae in our cases has proven the circulation of leprosy in Japan has been through in-country infections (Benjak et al., 2018). The median incubation period of leprosy is estimated at 2–5 and 8–12 years for PB and MB types, respectively, but can be longer than 20 years in some patients (Fine, 1982; Diniz and Maciel, 2018; Taggart et al., 2022). The cases in our older adult population, some aged over 90 years, is surprising because this indicates that the incubation period can be longer than previously known.
In our study, data on family history were missing in several cases (24.6%). Moreover, we are unsure of the credibility of these data because patients and their families tend to hide their history of the disease due to fear of stigma. The higher rate of missing data for Okinawa, where stigma is more apparent, demonstrates this tendency. However, assuming the data are accurate, there was no family history of leprosy in the two oldest cases, as also most of the older cases were diagnosed in 1993–2020. This shows that when the duration from infection to onset is long, it becomes more challenging to trace the point or source of transmission. This is corroborated by recent reports of autochthonous cases in Europe and the Americas (Beauvillain et al., 2021; Naidu et al., 2021; Rendini and Levis, 2017). In contrast, earlier studies report that being a family contact of a patient plays an increasingly important role in disease transmission when closer to elimination (Koba et al., 2009). Our sporadic cases indeed tended to have a family history of leprosy. Furthermore, the scenario of our Japanese-Brazilian case born in Japan raised a question of how to better define an ‘autochthonous’ case. The working definition we used in our study for ‘autochthonous’ was being born in Japan. However, with globalization, the place of birth less frequently reflects race or origin, and the differentiation between autochthonous and nonautochthonous is becoming more obscure, thus making the task complex. In addition, studies have highlighted the possibility of international migration as a source of transmission (Rendini and Levis, 2017). We have not yet seen a case in Japan in which we suspect infection happened from a nonautochthonous case outside of the same household, but caution is needed as the country continues to experience import of new cases from abroad.
Two factors seemingly affect the number of nonautochthonous cases diagnosed in Japan: the size of the migratory population and the endemicity of leprosy in the country of origin. From 1964 to 1990, cases of leprosy from South Korea were the highest, which probably reflected both the number of the South Koreans in Japan and the endemicity of the disease in South Korea during that period. Leprosy was endemic in South Korea until the late 1970s to the 1980s (Chae, 2020). To date, following India, Brazil has been the second most endemic country with leprosy (World Health Organization [WHO], 2021a). Notably, cases from Brazil during 1991–2020 accounted for 43.6% of the total new registered cases of leprosy among patients of foreign origin during that period. The number of residents from Brazil in Japan started to increase in the 1980s and declined during the financial crisis in 2007–2008 (Immigration Services Agency of Japan, 2021); new cases of leprosy from Brazil also declined considerably since 2007. The population of Filipinos surpassed Brazilians in 2012 (Immigration Services Agency of Japan, 2021), and we are currently seeing more cases in this population. Demographically, most nonautochthonous cases were of working age at the time of diagnosis. This is also reflected in the geographical distribution of the cases, as demonstrated by the increased concentration of cases around Japan’s industrial zones. Similar geographical patterns of leprosy distribution within countries have been previously reported (Suárez-García et al., 2020; Souza et al., 2019).
The male-to-female ratio during the period of decreasing leprosy incidence differs largely across countries (Chen et al., 2007; Feldman and Sturdivant, 1975; Hambridge et al., 2021; Irgens and Skjaerven, 1985; Irgens et al., 1990). In our recent autochthonous cases, we observed more female patients, especially those from Okinawa, which we believe is attributed to higher life expectancy in women (87.74 years) than in men (81.64 years) in Japan (Ministry of Health, 2022). The male-to-female ratio was high among our nonautochthonous cases, which reflects the migratory population of the young male workforce in Japan.
An increasing proportion of MB types have been described in other populations with declining leprosy incidence (Chen et al., 2007; Feldman and Sturdivant, 1975; Hambridge et al., 2021; Irgens et al., 1990; Irgens and Skjaerven, 1985); this pattern was also observed in our autochthonous cases, both from mainland Japan and Okinawa.
We observed that PCR was superior to other methods for diagnosis confirmation. However, no differences were observed in the diagnosis of MB types between SSS, histopathology, and PCR. Because PCR is not always available in resource-limited settings where leprosy is endemic, SSS should be the mainstay of diagnostics for MB type leprosy (Banerjee et al., 2011). For the PB type of leprosy, our results for SSS were not surprising because this is the characteristic of the PB type but, again, emphasized the importance of other methods in its diagnosis. The use of PCR where available and the development of novel field-friendly diagnostic tools, especially for diagnosing the PB type of leprosy, should be encouraged. The effectiveness of the sole use of the anti-PGL-I antibody test was not observed, supporting previous studies (Richardus et al., 2017).
Unfortunately, treatment duration and outcomes of the cases were not collected; however, patients with severe disease (e.g., high bacterial index and numerous skin lesions) were treated for up to 3 years. In Japan, we have our own guidelines for managing leprosy, wherein it is recommended that patients are treated until their bacterial index turns negative or until their active skin lesions disappear (Goto et al., 2013). It also lists alternative treatments when there are contraindications to using the standard MDT, and therefore, we observed some extent of heterogeneity in the types of treatment used. Worldwide, MDT has been used to treat M. leprae infection, with no change in its regimen for decades since 1981 (Smith et al., 2017). There is an ongoing discussion regarding the need for MDT alternatives for cases with adverse events or antimicrobial resistance (World Health Organization [WHO], 2021b). Some antimicrobials listed in this report may support the selection of the candidates.
Monitoring for antimicrobial resistance at our reference center is ongoing as well as in other reference centers in different regions. Although these cases were registered as new, we are uncertain if they were primary or secondary resistance because most often, patients tend to hide their disease status back in their home country. Dapsone resistance has been previously reported from untreated and/or relapse cases of leprosy in Brazil and in South Korea, with varying rates of 1.2–12.7% and 19.2–34%, respectively (Cambau et al., 2018; Andrade et al., 2022; You et al., 2005; Lee et al., 2001; Wu et al., 2022). In contrast, although some efforts have been made for antimicrobial resistance surveillance in the Philippines, as far as the studies show, quinolone resistance has never been reported from the country previously (Cambau et al., 2018; Matsuoka et al., 2007). This is alarming and highlights the importance of continued monitoring of antimicrobial-resistant cases worldwide.
Our study has a few limitations. First, our dataset was obtained from three sources with differences in data collection, which may introduce bias. However, it was important that we integrated these datasets to observe the trend over a longer period. Although we did select one dataset over another, the discrepancies between the overlapping years were not substantial as was described. Second, the efforts to collect data on newly diagnosed leprosy cases have been led by our expert group throughout the years; however, not all physicians in the country are aware of this reporting system. Although the surveillance covers the whole country, we cannot rule out the possibility of unreported cases because leprosy is not on the list of mandatory infectious diseases to be reported to the Government of Japan.
Conclusion
As demonstrated in this study, we no longer have any ongoing transmission of leprosy in Japan; however, we are still challenged with new cases as we embrace globalization. It is necessary that even after reaching the state of elimination, countries maintain diligence in understanding the status of leprosy within the country. Raising awareness and education about the disease, including reporting, to physicians must be continued to retain and build the capacity for continued leprosy services. This also includes the laboratory capacity to diagnose the disease. It is only when efforts from countries with all stages of leprosy endemicity are made that we can further leprosy control and reach our target of zero leprosy worldwide.
Supplementary Material
Acknowledgments
In memory of Dr. Seigo Hazama (Emeritus Director of the National Ohshima Seisho Sanatorium). The authors would like to thank him for his immense contributions to the epidemiological survey on new cases of leprosy in Japan from 1964 to 1980. The authors would also like to thank Ms. Kayo Shinozaki (Leprosy Research Center, National Institute of Infectious Diseases) for her support in integrating and organizing our dataset.
Funding
This research was supported by the Japan Agency for Medical Research and Development (grant number JP21fk0108610) and the National Institutes of Health (grant number 1R21TW011860-01).
Footnotes
Declaration of competing interest
All authors have no competing interests to declare.
Ethical considerations
Ethical approval was obtained from the ethical committees of the National Institute of Infectious Diseases (No.880), School of Tropical Medicine and Global Health of Nagasaki University (NU_TMGH_2020_110_1), National Sanatorium Tamazenshoen (01–01), and the University of the Ryukyus (No. 1680).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.ijid.2022.10.027.
References
- Andrade ESN, Brandao JG, Silva JSD, Coriolano CRF, Rosa PS, Moraes MO, et al. Antimicrobial Resistance among Leprosy Patients in Brazil: Real-World Data Based on the National Surveillance Plan. Antimicrob Agents Chemother 2022;66(5):e0217021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Biswas N, Kanti Das N, Sil A, Ghosh P, Hasanoor Raja AH, et al. Diagnosing leprosy: revisiting the role of the slit-skin smear with critical analysis of the applicability of polymerase chain reaction in diagnosis. Int J Dermatol 2011;50:1522–7. [DOI] [PubMed] [Google Scholar]
- Beauvillain Q, Lok C, Joachim C, Hamdad F, Lafabregue E, Attencourt C, et al. Autochthonous leprosy in Europe: a case report and literature review. Int J Infect Dis 2021;110:111–13. [DOI] [PubMed] [Google Scholar]
- Benjak A, Avanzi C, Singh P, Loiseau C, Girma S, Busso P, et al. Phylogenomics and antimicrobial resistance of the leprosy bacillus Mycobacterium leprae. Nat Commun 2018;9:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabinet Office Government of Japan. National Economic Indicators. Annual Fiscal Economic Report 2021. Tokyo: Government of Japan; 2021. p. 260–281 (in Japanese). [Google Scholar]
- Cambau E, Saunderson P, Matsuoka M, Cole ST, Kai M, Suffys P, et al. Antimicrobial resistance in leprosy: results of the first prospective open survey conducted by a WHO surveillance network for the period 2009–15. Clini Microbio Infect 2018;24(12):1305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae GT. Modern history of Hansen’s disease in Korea. Infect Chemother 2020;52:647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zheng Y, Zheng M, Wang D. Rapid survey on case detection of leprosy in a low endemic situation, Zhucheng County, Shandong Province, The People’s Republic of China. Lepr Rev 2007;78:65–9. [PubMed] [Google Scholar]
- Diniz LM, Maciel LB. Leprosy: clinical and epidemiological study in patients above 60 years in Espirito Santo State - Brazil. An Bras Dermatol 2018;93(6):824–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi VP, Banerjee A, Das I, Saha A, Dutta M, Bhardwaj B, Biswas S, et al. Diet and nutrition: an important risk factor in leprosy. Microb Pathog 2019;137 103714. [DOI] [PubMed] [Google Scholar]
- Feldman RA, Sturdivant M. Leprosy in Louisiana, 1855–1970. An epidemiologic study of long-term trends. Am J Epidemiol 1975;102:303–10. [DOI] [PubMed] [Google Scholar]
- Fine PE. Leprosy: the epidemiology of a slow bacterium. Epidemiol Rev 1982;4:161–88. [DOI] [PubMed] [Google Scholar]
- Goto M, Nogami R, Okano Y, Gidoh M, Yotsu R. Guidelines for the treatment of Hansen’s disease in Japan (third edition) (in Japanese). Nihon Hansenbyo Gakkai Zasshi 2013;82(3):143–84. [DOI] [PubMed] [Google Scholar]
- Hambridge T, Nanjan Chandran SL, Geluk A, Saunderson P, Richardus JH. Mycobacterium leprae transmission characteristics during the declining stages of leprosy incidence: a systematic review. PLoS Negl Trop Dis 2021;15 e0 0 09436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irgens LM, Skjaerven R. Secular trends in age at onset, sex ratio, and type index in leprosy observed during declining incidence rates. Am J Epidemiol 1985;122:695–705. [DOI] [PubMed] [Google Scholar]
- Immigration Services Agency of JapanMinistry of Justice. Statistic data of foreign residents in Japan. 2022. https://isa.go.jp/en/policies/statistics/index.html (Accessed 12 September 2022).
- Irgens LM, Melo Caeiro F, Lechat MF. Leprosy in Portugal 1946–80: epidemiologic patterns observed during declining incidence rates. Lepr Rev 1990;61:32–49. [DOI] [PubMed] [Google Scholar]
- Ishii N, Onoda M, Sugita Y, Tomoda M, Ozaki M. Survey of newly diagnosed leprosy patients in native and foreign residents of Japan. Int J Lepr Other Mycobact Dis 2000;68:172–6. [PubMed] [Google Scholar]
- Koba A, Ishii N, Mori S, Fine PE. The decline of leprosy in Japan: patterns and trends 1964–2008. Lepr Rev 2009;80:432–40. [PubMed] [Google Scholar]
- Ministry of Health and Welfare of Japan. History of National Sanatorium (Hansen’s Disease). Tokyo: Government of Japan; 1975. (in Japanese). [Google Scholar]
- Lee SB, Kim SK, Chae GT, Shin HK, Kim JP, Ko YH, et al. The prevalence of folP1 mutations associated with clinical resistance to dapsone, in Mycobacterium leprae isolates from South Korea. Ann Trop Med Parasitol 2001;95(4):429–432. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Budiawan T, Aye KS, Kyaw K, Tan EV, Cruz ED, et al. The frequency of drug resistance mutations in Mycobacterium leprae isolates in untreated and relapsed leprosy patients from Myanmar, Indonesia and the Philippines. Lepr Rev 2007;78(4):343–52. [PubMed] [Google Scholar]
- Ministry of Health, Labour and Welfare Overview of the 2020 Life Chart (Simplified) (in Japanese). 2022. https://www.mhlw.go.jp/toukei/saikin/hw/life/life20/index.html (Accessed 19 September 2022).
- Mori S, Yotsu RR, Suzuki K, Makino M, Ishii N. Present situation of leprosy in Japan, 2006–2010: analysis of drug resistance in new registered and relapsed cases by molecular biological methods. J Dermatol Sci 2012;67:192–4. [DOI] [PubMed] [Google Scholar]
- Nagao E. Nansei-shoto no rai no genjyo to shorai. Jpn J Lepr 1985;54:111–13 (in Japanese). [Google Scholar]
- Naidu P, Sharma R, Kanji JN, Marks V, King A. Autochthonous North American leprosy: a second case in Canada. Infect Dis Rep 2021;13:917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescarini JM, Strina A, Nery JS, Skalinski LM, Andrade KVF, Penna MLF, et al. Socioeconomic risk markers of leprosy in high-burden countries: a systematic review and meta-analysis. PLoS Negl Trop Dis 2018;12 e0006622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendini T, Levis W. Autochthonous leprosy without armadillo exposure, Eastern United States. Emerg Infect Dis 2017;23:1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardus RA, van der Zwet K, van Hooij A, Wilson L, Oskam L, Faber R, et al. Longitudinal assessment of anti-PGL-I serology in contacts of leprosy patients in Bangladesh. PLoS Negl Trop Dis 2017;11 e0006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikawa K The effect of rapid socio-economic development on the frequency of leprosy in a population. Lepr Rev 1981;52(1):167–75 Suppl. [DOI] [PubMed] [Google Scholar]
- Saikawa K. Changes in Hansen’s disease policy. Naha-shi: Okinawa Leprosy Prevention Association, 1999. (in Japanese). [Google Scholar]
- Sato H, Narita M. Politics of leprosy segregation in Japan: the emergence, transformation and abolition of the patient segregation policy. Soc Sci Med 2003;56:2529–39. [DOI] [PubMed] [Google Scholar]
- Smith CS, Aerts A, Saunderson P, Kawuma J, Kita E, Virmond M. Multidrug therapy for leprosy: a game changer on the path to elimination. Lancet Infect Dis 2017;17(9):e293–7. [DOI] [PubMed] [Google Scholar]
- Souza CDF, Luna CF, Magalhães MAFM. Leprosy transmission in Bahia, 2001–2015: modeling based on Jointpoint regression and spatial scan statistics. Epidemiol Serv Saude 2019;28(1) e2018065. [DOI] [PubMed] [Google Scholar]
- Statistics Bureau of Japan e-Stat: Summary of Population of Japan (in Japanese). 2022. https://www.stat.go.jp/data/jinsui/2.html#series (Accessed 8 September 2022).
- Suárez-García I, Gómez-Barroso D, Fine PEM. Autochthonous leprosy in Spain: has the transmission of Mycobacterium leprae stopped? PLoS Negl Trop Dis 2020;14 e0008611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart M, Kelly A, Stell R, Chu E. Multibacillary leprosy with an incubation period exceeding 50 years. BMJ Case Rep 2022;15(7):e250835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You EY, Kang TJ, Kim SK, Lee SB, Chae GT. Mutations in genes related to drug resistance in Mycobacterium leprae isolates from leprosy patients in Korea. J Infect 2005;50(1):6–11. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global leprosy (Hansen’s disease). Update 2020: impact of COVID-19 on global leprosy control. Weekly Epidemiological Record. Geneva: World Health Organization; 2021a;96:421–44. [Google Scholar]
- World Health Organization. Proceedings of the from the technical advisory group for leprosy meeting. Geneva: World Health Organization, 2021b. [Google Scholar]
- World Health Organization. Global leprosy update, 2016: accelerating reduction of disease burden. Wkly Epidemiol Rec 2017;92:501–19. [PubMed] [Google Scholar]
- World Health Organization. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization, 2020. [Google Scholar]
- Wu Z, Wang C, Wang Z, Shi Y, Jiang H, Wang H. Risk factors for dapsone resistance in leprosy patients: a systematic meta-analysis. J Glob Antimicrob Reist 2022;30:459–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


