PURPOSE
Cardiovascular adverse events (CVAEs) can occur during proteasome inhibitor (PI) therapy. We conducted a prospective, observational, multi-institutional study to define risk factors and outcomes in patients with multiple myeloma (MM) receiving PIs.
PATIENTS AND METHODS
Patients with relapsed MM initiating carfilzomib- or bortezomib-based therapy underwent baseline assessments and repeated assessments at regular intervals over 6 months, including cardiac biomarkers (troponin I or T, brain natriuretic peptide [BNP], and N-terminal proBNP), ECG, and echocardiography. Monitoring occurred over 18 months for development of CVAEs.
RESULTS
Of 95 patients enrolled, 65 received carfilzomib and 30 received bortezomib, with median 25 months of follow-up. Sixty-four CVAEs occurred, with 55% grade 3 or greater in severity. CVAEs occurred in 51% of patients treated with carfilzomib and 17% of those treated with bortezomib (P = .002). Median time to first CVAE from treatment start was 31 days, and 86% occurred within the first 3 months. Patients receiving carfilzomib-based therapy with a baseline elevated BNP level higher than 100 pg/mL or N-terminal proBNP level higher than 125 pg/mL had increased risk for CVAE (odds ratio, 10.8; P < .001). Elevated natriuretic peptides occurring mid–first cycle of treatment with carfilzomib were associated with a substantially higher risk of CVAEs (odds ratio, 36.0; P < .001). Patients who experienced a CVAE had inferior progression-free survival (log-rank P = .01) and overall survival (log-rank P < .001). PI therapy was safely resumed in 89% of patients, although 41% required chemotherapy modifications.
CONCLUSION
CVAEs are common during PI therapy for relapsed MM, especially with carfilzomib, particularly within the first 3 months of therapy. CVAEs were associated with worse overall outcomes, but usually, discontinuation of therapy was not required. Natriuretic peptides were highly predictive of CVAEs; however, validation of this finding is necessary before uniform incorporation into the routine management of patients receiving carfilzomib.
INTRODUCTION
Patients with multiple myeloma (MM) are at high risk for cardiovascular (CV) disease, with studies reporting that more than 50% of patients have established CV disease.1-6 Development of proteasome inhibitors (PIs), including carfilzomib and bortezomib, has dramatically changed the therapeutic landscape in MM, improving progression-free survival (PFS) and overall survival (OS).7,8 As a result of improved longevity, CV complications have become more apparent in patients treated for MM.
Bortezomib is a boronic acid derivative and reversible nonselective inhibitor of proteasomes that blocks chymotrypsin-like activity at the 26S proteasome. This inhibition alters intracellular protein homeostasis, leading to accumulation of intracellular proteins, which results in cell-cycle arrest and ultimately apoptosis of cancer cells.9 Carfilzomib is an epoxyketone and, unlike bortezomib, is an irreversible potently selective inhibitor of proteasomes that blocks chymotrypsin-like activity of the 20S proteasome, which similarly leads to apoptosis of cancer cells.10 Both bortezomib and carfilzomib have been associated with CV adverse events (CVAEs); however, early data on carfilzomib showed a higher incidence of CV toxicity when compared with bortezomib.4,5,7 CVAEs, including hypertension (HTN), arrhythmia, heart failure (HF), ischemic heart disease, cardiomyopathy, thromboembolic events, pulmonary hypertension, and rarely sudden cardiac death, have been reported.2,3
Although there is an increased incidence of CVAEs with PIs, a detailed description of these events and associated risk factors is lacking. Furthermore, there is no validated protocol to help determine which patients are at highest risk of CV toxicity during therapy, nor is there management guidance for patients who experience a CVAE. We conducted a prospective, observational, multi-institutional study to define the CV risk factors, describe in detail the CVAEs, and evaluate outcomes in patients receiving carfilzomib or bortezomib for relapsed MM.
PATIENTS AND METHODS
Patients and Study Design
The PROTECT (Prospective Observation of Cardiac Safety With Proteasome Inhibitor) study was conducted at Vanderbilt University Medical Center (VUMC) and University of Pennsylvania Abramson Cancer Center (Penn) between September 2015 and March 2018. The primary objective was to prospectively quantitate the incidence of and determine risk factors for CV events in patients receiving bortezomib or carfilzomib for relapsed MM over a period of 18 months. Evaluation of PFS and OS were secondary objectives. This study was approved by the VUMC and Penn institutional review boards.
Patients with relapsed MM, defined according to the International Myeloma Working Group, initiating treatment with physician choice of carfilzomib- or bortezomib-based therapy were enrolled.11 Chemotherapy agents, dosages, and modifications were determined by the managing hematologist. Patients had received at least one prior line of chemotherapy. Exclusion criteria included symptomatic cardiac arrhythmia or New York Heart Association class 3 or 4 HF within 3 months before enrollment. Patients with light chain amyloidosis, assessed by clinical history, ECG, transthoracic echocardiography (TTE), albuminuria, troponin elevation, or end-organ biopsy or cardiac magnetic resonance imaging in select cases, were excluded.
Enrolled patients underwent baseline CV assessments, including traditional CV risk factors (family history, HTN, hyperlipidemia, diabetes, and tobacco use), cardiac biomarkers (troponin I or T, brain natriuretic peptide [BNP], or N-terminal proBNP [NTproBNP]), ECG, TTE, and cardiologist evaluation. VUMC performed troponin I and BNP, and Penn performed troponin T and NTproBNP, per institutional standards. TTE determination of significant change was defined as a quantitative left ventricular ejection fraction (LVEF) decline of 10% or greater from baseline to a value of less than 50%.12,13 CV assessments were performed at baseline and the start of each chemotherapy cycle (ie, every 3 or 4 weeks) for the first six cycles of PI-based therapy. Cardiac biomarkers were tested twice per cycle (day 1 and either day 8 or 15), ECG on day 1 of each cycle, cardiologist assessment on day 1 of each cycle, and TTE on day 1 of cycles two, four, and six. Thereafter, patients were clinically observed for development of CVAEs for an additional 12 months. CV assessments were performed at the time of any suspected CVAE.
Definitions
All CVAEs were graded according to Common Terminology Criteria for Adverse Events (version 4.03). CVAEs were adjudicated in real time by a cardiologist. Disease response and progression were defined according to the International Myeloma Working Group criteria.11 Definitions for CVAEs and high-risk MM are provided in the Appendix (online only).
Statistical Analysis
Patient demographics and other disease characteristics were summarized using frequencies and relative frequencies for categorical variables; medians and interquartile ranges were computed for continuous variables. Groups were compared using χ2 tests for categorical variables and Wilcoxon rank sum tests for continuous variables. Duration of treatment, PFS, and OS were evaluated using the Kaplan-Meier method, and groups were compared with log-rank tests. Time to first CVAE was estimated by incidence function of CVAE in the presence of competing risk (death without CVAE) using the method of Fine and Gray (R package cmprsk; R software; https://www.R-project.org).14 Multivariable subdistribution regression for competing risk survival for first CVAE was conducted with R packages mstate and rms. We analyzed time to CVAE using subdistribution regression for competing risk survival with clustered data (R package crrSC). Covariates for multivariable analysis were selected a priori, and those included in the regression model were treatment, natriuretic peptide elevation at baseline (yes or no), natriuretic peptide increase at 2 or 3 weeks after treatment (yes or no), time from diagnosis to treatment, and traditional cardiac risk factors (low [one or none] v high [> one factor]). The significance level was a two-sided P value of .05. Analyses were performed using R software.
RESULTS
Baseline Characteristics
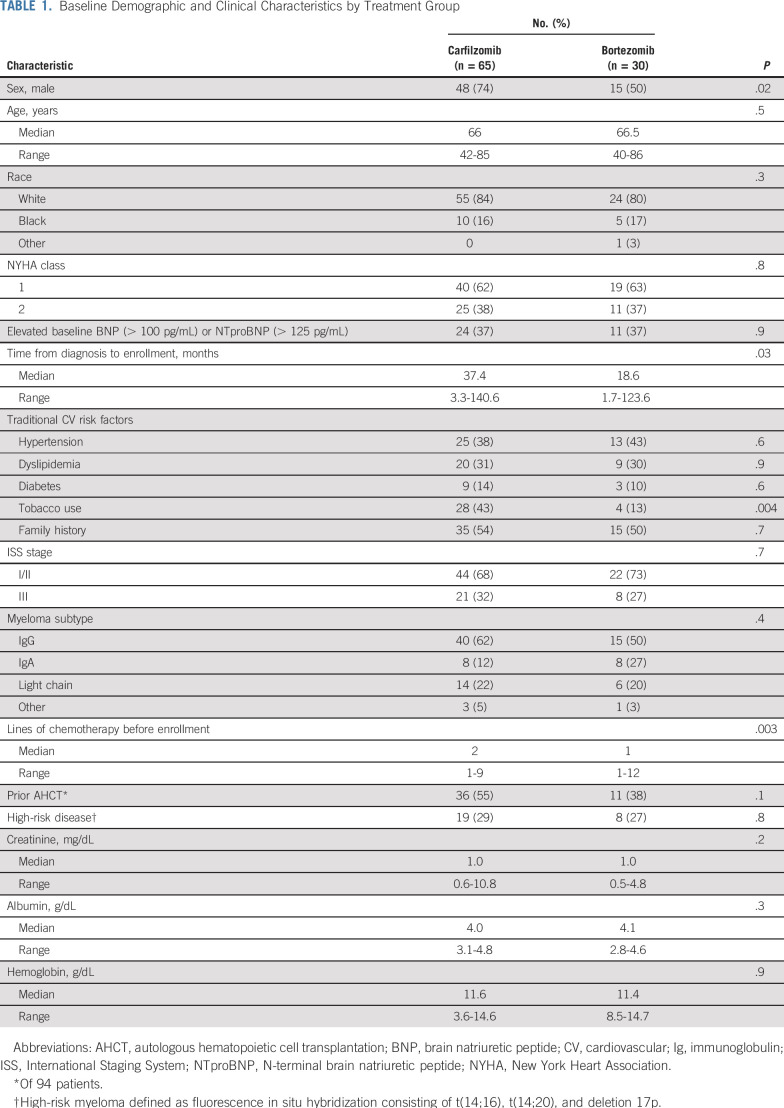
Ninety-five patients were enrolled, with 65 patients receiving carfilzomib-based therapy and 30 receiving bortezomib-based therapy. Median follow-up for survivors (n = 61) was 25 months (range, 3 to 41.8 months). Baseline characteristics are listed in Table 1. There was no difference between treatment groups in baseline natriuretic peptide levels or New York Heart Association HF class 1 or 2. Most patients receiving carfilzomib (60%) had previously received one or two prior lines of therapy, compared with 73% of the bortezomib group, who had received one prior line (P = .003). Patients receiving carfilzomib were more likely to have used tobacco (43% v 13%), to be men (74% v 50%), and to have enrolled in the study later after diagnosis (median, 37.4 v 18.6 months) compared with the bortezomib cohort. No other differences in baseline characteristics were observed between cohorts.
TABLE 1.
Baseline Demographic and Clinical Characteristics by Treatment Group
Cancer Treatments
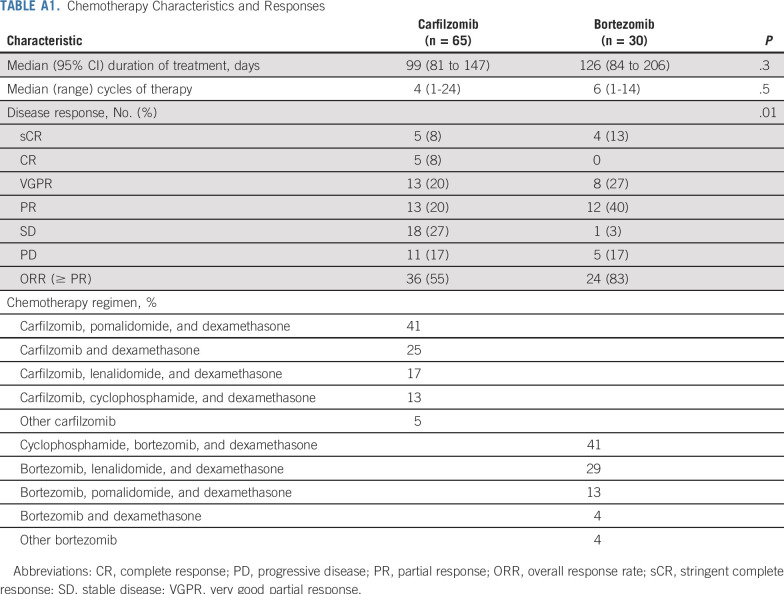
Chemotherapy regimens are listed in Appendix Table A1 (online only). The most common carfilzomib-based regimen was carfilzomib in combination with pomalidomide and dexamethasone. Fifty-one (79%) of 65 patients receiving carfilzomib-based therapy received a maximum dose of 27 mg/m2 of carfilzomib twice per week. Four patients (6%) received up to 56 mg/m2 of carfilzomib once per week, and 10 patients (15%) received up to 70 mg/m2 of carfilzomib once per week. As of September 1, 2018, 63 of the 65 patients had discontinued carfilzomib-based treatment for the following reasons: disease progression (n = 28; 44%), autologous hematopoietic cell transplantation (n = 12; 19%), change to maintenance chemotherapy (n = 11; 18%), other causes (n = 7; 11%), and CVAEs (n = 5; 8%). Median duration of treatment was 99 days (95% CI, 81 to 147 days).
The most common bortezomib regimen was bortezomib in combination with cyclophosphamide and dexamethasone. Twenty-nine of 30 patients receiving bortezomib-based therapy stopped treatment for the following reasons: disease progression (n = 17; 59%), autologous hematopoietic cell transplantation (n = 4; 14%), change to maintenance chemotherapy (n = 3; 10%), other causes (n = 4; 14%), and a CVAE (n = 1; 3%). Median duration of bortezomib-based treatment was 126 days (95% CI, 84 to 206 days). Overall, patients received carfilzomib-based therapy for a median of four cycles, whereas those treated with bortezomib received a median of six cycles (P = .5).
CVAEs
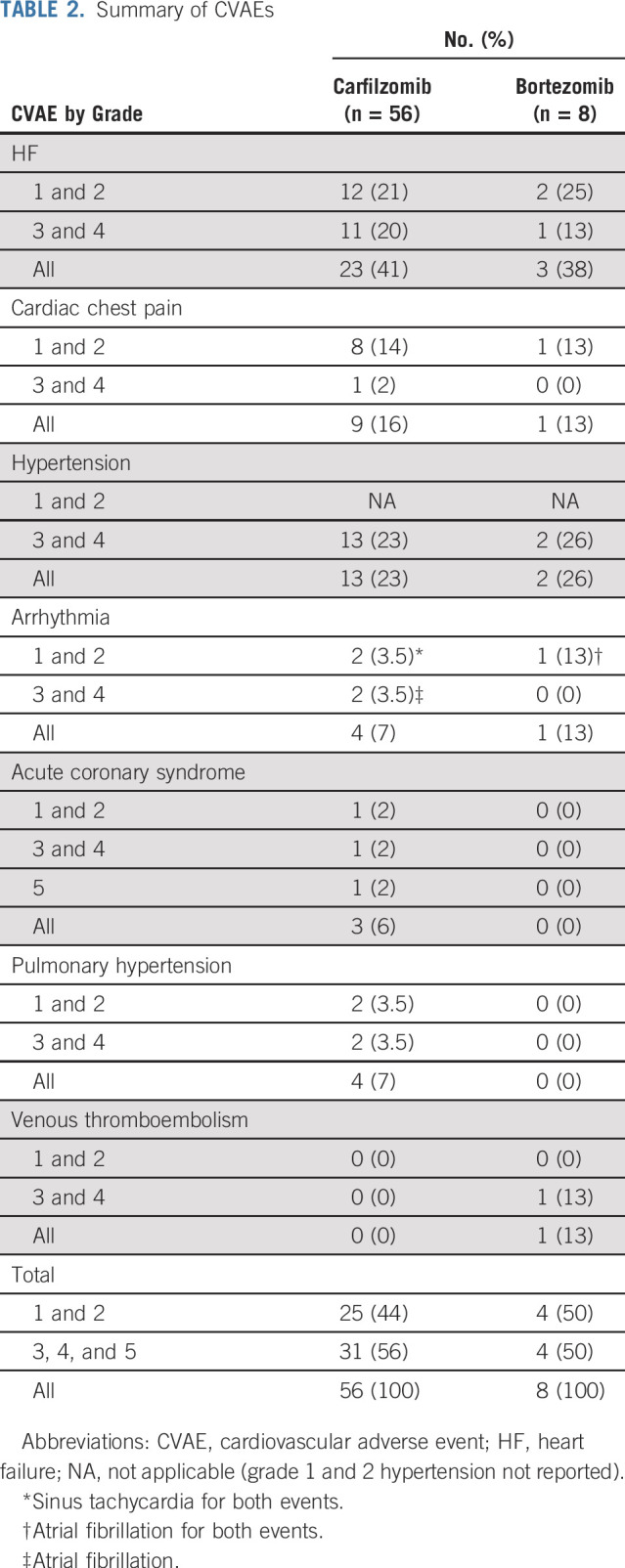
Table 2 lists the CVAEs. Patients receiving carfilzomib-based therapy had significantly higher rates of CVAEs (33 [50.7%] of 65) compared with patients receiving bortezomib (five [16.7%] of 30; P = .005). Most CVAEs were grade 2 or 3. Of the 64 CVAEs that occurred during the study, 56 (87.5%) happened during carfilzomib-based therapy; HF was the most common CVAE (n = 23), followed by grade 3 or 4 HTN (n = 13). Cardiac chest pain accounted for nine CVAEs and atrial fibrillation for two CVAEs. Acute coronary syndrome occurred three times during the study, with sudden cardiac death occurring in one patient after a myocardial infarction within 24 hours of carfilzomib infusion during the second week of treatment.
TABLE 2.
Summary of CVAEs
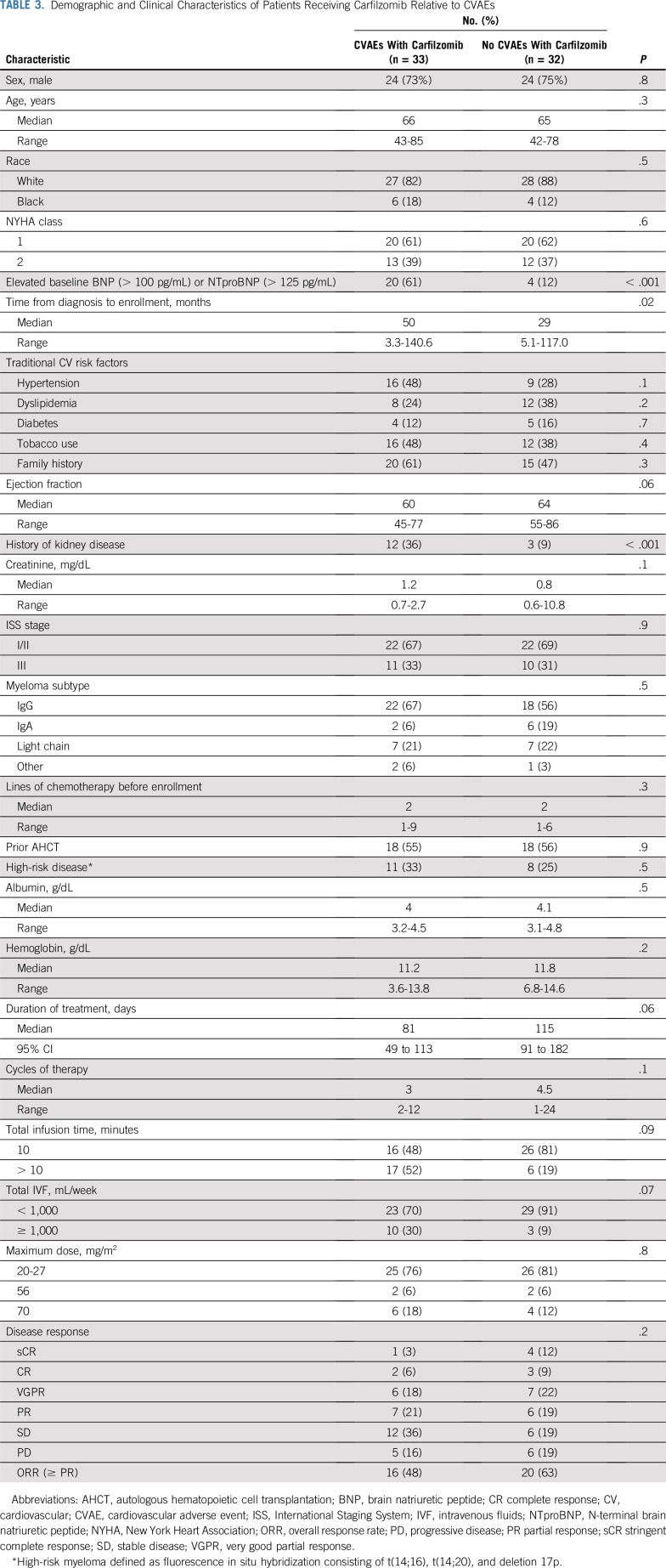
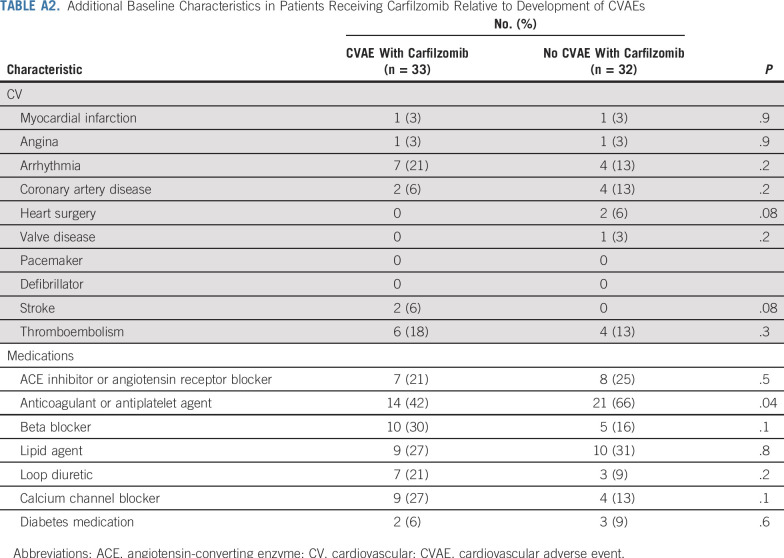
In Table 3 and Appendix Table A2 (online only), we compare characteristics of patients with and without CVAEs among those receiving carfilzomib. There was no difference in incidence of CVAEs for patients receiving 1,000 mL or more of intravenous normal saline per week with carfilzomib (76.9%) compared with less than 1,000 mL (44.2%) of normal saline per week (P = .07). No difference in rates of CVAEs was found in patients receiving a maximum of 27 mg/m2 (49.0%) compared with 56 mg/m2 or more (57.1%) of carfilzomib (P = .8). There was no difference in rate of CVAEs in patients receiving carfilzomib infusion over 10 minutes compared with longer than 10 minutes (P = .9). There was no difference in incidence of CVAEs for patients concurrently treated with immunomodulatory drugs versus no immunomodulatory drugs (P = .8). There was also no difference in early CVAEs and dexamethasone dose (P = .8).
TABLE 3.
Demographic and Clinical Characteristics of Patients Receiving Carfilzomib Relative to CVAEs
Patients with a baseline elevation in natriuretic peptides (BNP > 100 pg/mL or NTproBNP > 125 pg/mL [cutoff values for the testing assay]) before initiation of carfilzomib-based therapy experienced a significantly higher incidence of CVAEs (odds ratio, 10.8; 95% CI, 3.1 to 37.9; P < .001). In addition, patients receiving carfilzomib-based therapy who experienced an elevation in natriuretic peptides relative to baseline within the first 3 weeks of starting chemotherapy had a substantially higher risk of experiencing a clinical CVAE (odds ratio, 36.0; 95% CI, 4.4 to 296; P < .001).
In 14 patients with BNP elevations receiving carfilzomib, there were 21 events, with a median BNP increase at time of CVAE of 165 pg/mL (95% CI, 107 to 320 pg/mL) from baseline. Ten patients with NTproBNP elevations during carfilzomib treatment developed 18 CVAEs, and median NTproBNP increase was 5,925 pg/mL (95% CI, 673 to 10,454 pg/mL) from baseline. Natriuretic peptide levels returned to near-baseline values a median of 24.5 days (95% CI, 19 to 34.5 days) after a CVAE. Natriuretic peptides were found to be predictive for all CVAE categories (Table 2). Troponin I or T, ECG, and TTE (in terms of LVEF reduction) were not predictive of CVAEs.
Forty-eight percent of CVAEs (n = 31) were managed without chemotherapy modification, and patients were able to continue PI-based therapy. Forty-one percent of CVAEs (n = 26) required a dose delay and/or reduction before patients were able to resume PI-based therapy. Eleven percent of CVAEs (n = 7) required discontinuation of the PI-based therapy because of CV toxicity.
Time to CVAE: Competing Risk Survival Analyses
Most patients with CVAEs experienced only one CVAE (18 of 33 receiving carfilzomib; three of five receiving bortezomib). Eight patients had two CVAEs, six patients had three, and one patient had four with carfilzomib. A majority of CVAEs (86%) occurred within the first 3 months of treatment, with 32.8% occurring during the initial month of treatment. Among patients experiencing CVAEs, median time from start of treatment to first CVAE was 31 days (range, 0 to 151 days).
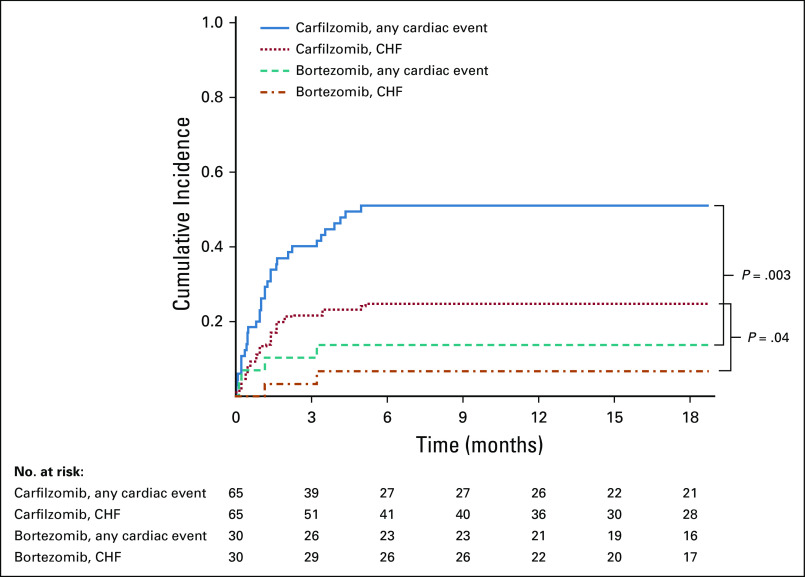
We analyzed the time to first CVAE using competing risk survival analysis. The cumulative incidence functions for first CVAE and for first HF event were significantly different (P = .003 and .04, respectively) between the two treatment groups (Fig 1), with higher incidence in the carfilzomib group.
FIG 1.
Cumulative incidence curves for time to first cardiac event (all grades) estimated using Fine and Gray method for competing risk survival analysis. CHF, congestive heart failure.
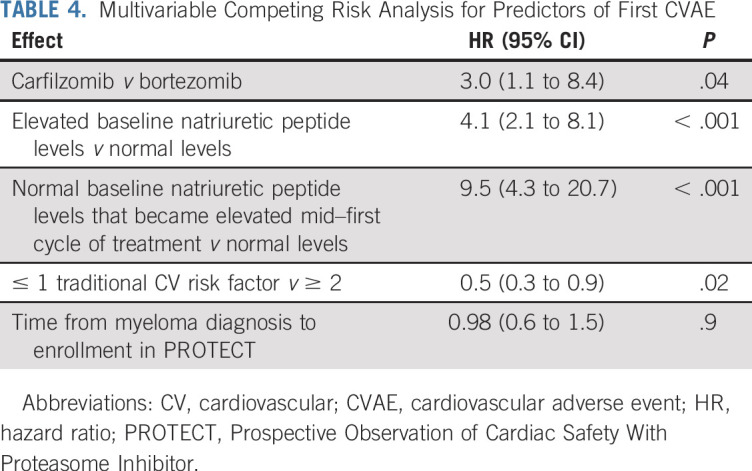
Multivariable subdistribution regression analysis was performed for time to first CVAE. The estimated adjusted hazard ratios (HRs) are listed in Table 4. Carfilzomib-based therapy was associated with a higher risk of CVAEs compared with bortezomib-based therapy (HR, 3.0; 95% CI, 1.1 to 8.4; P = .04). Elevated baseline natriuretic peptide levels were associated with higher risk of CVAEs (HR, 4.1; 95% CI, 2.1 to 8.1; P < .001). Baseline natriuretic peptide levels that became elevated during cycle one of chemotherapy were associated with higher risk of CVAEs compared with persistently normal natriuretic peptide values (HR, 9.5; 95% CI, 4.3 to 20.7; P < .001). Patients with zero or one baseline traditional CV risk factor had a lower risk of CVAEs (HR, 0.5; 95% CI, 0.3 to 0.9; P = .02). No evidence of increased risk of CVAEs on the basis of time from myeloma diagnosis to enrollment in PROTECT was found (P = .9).
TABLE 4.
Multivariable Competing Risk Analysis for Predictors of First CVAE
PFS and OS
Patients who experienced a CVAE had significantly inferior PFS (log-rank P = .01) and OS (log-rank P < .001) compared with patients not experiencing a CVAE (Figs 2A and 2B). Median PFS in patients experiencing a CVAE was 10.4 months (95% CI, 3.3 to 14.5 months) compared with 28.4 months (95% CI, 10.6 to not reached) for those without a CVAE. Median OS for patients experiencing a CVAE was 18.1 months (95% CI, 11.6 to not reached). Median OS for patients not experiencing a CVAE had not been reached at the time of analysis. In total, there were 34 patient deaths, with a majority (88%) resulting from myeloma disease progression. There was one sudden cardiac death within 24 hours after carfilzomib infusion. One patient died as a result of progressive heart failure, one as a result of complications of graft-versus-host disease, and one as a result of respiratory failure.
FIG 2.
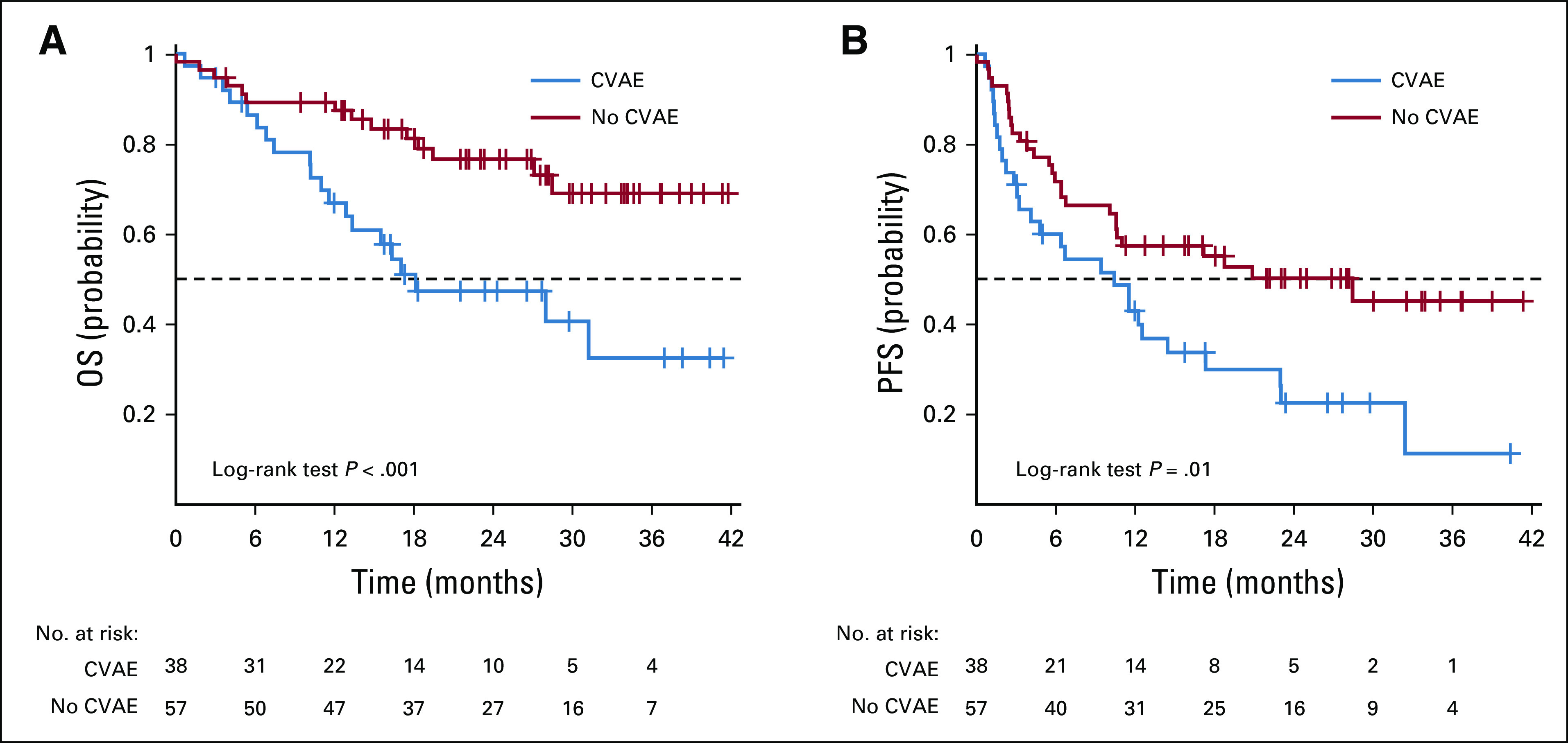
Kaplan-Meier curves of (A) overall survival (OS) and (B) progression-free survival (PFS) according to occurrence of cardiovascular adverse event (CVAE) versus no CVAE.
DISCUSSION
In our study, CV toxicities were common, particularly with carfilzomib-based therapy, with just more than half of all patients receiving carfilzomib experiencing a CVAE. Many studies have demonstrated a modest increase in CVAEs with carfilzomib but not to this degree. Importantly, predictors of CVAEs have been lacking.3,15-22 This study, conducted with multicenter hematology and cardiology collaboration, prospectively demonstrates that monitoring patients receiving carfilzomib-based therapy with natriuretic peptides is highly predictive for development of CVAEs. Because a majority of CVAEs (86%) occurred within the first 3 months of therapy, measuring natriuretic peptides levels using either BNP or NTproBNP seems to provide clinical benefit. These data are consistent with other CV studies demonstrating that baseline elevations in natriuretic peptides are predictive of CVAEs.23,24 Obtaining natriuretic peptide levels before initiation of carfilzomib therapy and monitoring these levels on day 1 of each cycle and midcycle for at least the first 3 months of therapy are suggested; however, this recommendation should be validated before uniformly incorporating this strategy into routine clinical practice. Longer monitoring is appropriate if clinically indicated. Patients with elevated natriuretic peptides before initiation of therapy or who develop elevated levels during treatment are at highest risk for CVAEs.
In our study, no changes were made based solely on laboratory abnormalities, and therefore, it is difficult to recommend modifications of chemotherapy on the basis of abnormalities in natriuretic peptides alone. This observation was previously highlighted by Atrash et al3 in a descriptive retrospective single-institution cohort of 130 patients, of whom 29 (22%) experienced a CVAE. Similar to our study, many patients experienced BNP elevations, although elevations did not always correlate with clinical symptoms or hospitalizations. However, should an elevation in natriuretic peptides occur, these patients should be monitored more closely. This will allow for earlier, modifiable practice-based adjustments, including reduction in intravenous fluid volume, use of diuretics, prolongation of infusion time of carfilzomib, and addition and optimization of other CV medications, particularly antihypertensives. Patients with multiple CV risk factors, particularly those with a history of CVAEs and/or elevated baseline natriuretic peptides, should be referred for a comprehensive cardiac evaluation. Such patients are at highest risk of CVAEs with carfilzomib-based therapy, and optimization of CV therapy seems to improve overall care, allow continuation of potentially lifesaving cancer treatment, and affect severity or development of CVAEs.
Similar to our findings, the ENDEAVOR (ClinicalTrials.gov identifier: NCT1900231) trial cardiac substudy demonstrated that serial TTEs have limited value for predicting or demonstrating CVAEs.7,25 In fact, a vast majority patients experiencing HF with PI-based therapy had HF with preserved ejection fraction, which makes detection by TTE difficult.26,27 In our study, patients experienced signs and symptoms of HF with normal or near-normal LVEF, which did not readily change despite an HF CVAE. Other tests, including ECG and troponin, were not predictive of CVAEs and are not recommended as part of routine management unless clinically indicated.
In this study, a majority of CVAEs were transient, and natriuretic peptides returned to near-baseline values a median of 24.5 days from peak, which may suggest a vascular endothelial phenomenon. The underlying CVAE mechanism is likely multifactorial, and more investigation is necessary. Transient endothelial dysfunction, cardiac myocyte oxidative stress abnormalities, and cardiorenal syndrome are areas of investigation.28-30 Reports of thrombotic microangiopathy provide additional evidence of a systemic PI-induced endothelial dysfunction.31
In this study, 17% of patients receiving bortezomib-based therapy experienced a CVAE. This rate is similar to previously published reports and consistent with a low rate of cardiac toxicity with the agent.32 However, the incidence of CVAEs with carfilzomib-based therapy was higher than that in prior reports.2,33,34 A prospective study design, frequent biomarker assessment, and specific evaluation for CVAEs as the primary end point may explain this discrepancy. In addition, patients receiving carfilzomib were more heavily pretreated, which may have predisposed patients to more CVAEs. In most cases, CVAEs were manageable, and patients were able to resume therapy. Patients experiencing a CVAE had inferior PFS and OS compared with patients without a CVAE and often required dose reductions or delays.
Limitations of the study include the absence of standardized treatments. Although not standardized, triplet therapy consistent with contemporary myeloma management was administered to most patients. As such, there was a wide dosing range for carfilzomib relative to bortezomib. This study did not demonstrate a difference in CVAEs according to carfilzomib dose level; however, the lack of standardized dosing may have affected this finding. Similarly, the most common chemotherapeutic partner with carfilzomib was pomalidomide, whereas cyclophosphamide was most commonly used with bortezomib. In this study, no difference in outcome was observed based on partner agent; however, immunomodulatory drugs can result in endothelial toxicity, which may have affected these observations.35
In addition, the enrollment goal was 65 patients in each cohort. This was achieved with carfilzomib but not bortezomib. Bortezomib is no longer a first-line choice for relapsed myeloma, and therefore, the enrollment goal was not achieved. However, because of the high CVAE rate with carfilzomib, the study remained adequately powered to evaluate for differences in outcome between cohorts.
In summary, CVAEs, particularly with carfilzomib-based therapy, were common and did not usually require discontinuation of therapy after cardiac optimization; however, they were associated with inferior PFS and OS. Natriuretic peptide testing and detailed CV medical history are highly predictive of carfilzomib-based CVAEs and should be considered as part of management for this treatment.
Supplementary Material
Appendix
Cardiovascular Adverse Events
Symptomatic heart failure (HF) was graded as symptoms with mild to moderate activity or exertion (grade 2), at rest or minimal exertion requiring intervention (grade 3), or life-threatening consequences requiring urgent intervention (grade 4). HF was demonstrated by at least one item in two of the following three categories: symptoms of HF (paroxysmal nocturnal dyspnea, shortness of breath, swelling, orthopnea, or weight gain), physical examination findings (presence of jugular venous distension, lung crackles, edema, or S3 heart sound), and diagnostic testing (pulmonary edema on chest x-ray or brain natriuretic peptide > 100 pg/mL or N-terminal pro–brain natriuretic peptide > 125 pg/mL; Maisel AS, et al: N Engl J Med 347:161-167, 2002).
Acute coronary syndrome was graded as symptomatic, progressive angina with normal cardiac enzymes and hemodynamically stable (grade 2); symptomatic, unstable angina and/or acute myocardial infarction with abnormal cardiac enzymes and hemodynamically stable (grade 3); or symptomatic, unstable angina and/or acute myocardial infarction with abnormal cardiac enzymes and hemodynamically unstable (grade 4). Patients were required to have two of the three following: troponin elevation, chest pain, and ECG changes. Sudden cardiac death was defined as death within 24 hours of proteasome inhibitor therapy without obvious cancer progression.
Symptomatic arrhythmias included atrial fibrillation and atrial flutter graded as symptomatic but not requiring urgent medical intervention (grade 2) or symptomatic and incompletely controlled medically with medical management or requiring device implantation or ablation (grade 3). Grade 2 sinus tachycardia was defined as symptomatic tachycardia not requiring urgent medical intervention. Arrhythmias were confirmed by ECG. No grade 4 arrhythmias occurred during the study.
Venous thromboembolic events were determined by imaging. These were defined as symptomatic thrombosis requiring medical intervention (grade 3). No other thromboembolic grades occurred during the study.
Pulmonary hypertension (HTN) was graded by moderate dyspnea with cough requiring evaluation by cardiac catheterization and medical intervention (grade 2) or severe symptoms associated with hypoxemia, right heart failure, and new oxygen requirement (grade 3). No grade 1 or 4 pulmonary HTN events occurred during the study. Pulmonary HTN was further defined as tricuspid regurgitation velocity of more than 3 m/s on transthoracic echocardiography.
Grade 3 HTN was defined as systolic blood pressure of 160 mm Hg or greater or diastolic blood pressure of 100 mm Hg or greater requiring more than one drug or more intensive therapy than previously used. Grade 4 HTN was defined as life-threatening consequences requiring urgent intervention. Only grade 3 and 4 HTN were recorded as cardiovascular adverse events, and all HTN events were confirmed by repeat blood pressure measurements at least 10 minutes apart. Grade 1 and 2 HTN were not reported, because these events were common and of no clinical significance relative to the study.
Cardiac chest pain was without ECG changes or troponin elevation and defined as mild pain (grade 1), moderate pain that limited activities of daily living (grade 2), or pain at rest, limiting self-care activities of daily living (grade 3). Cardiac chest pain events were only recorded if medical treatment was required and symptoms resolved with cardiac-specific intervention.
High-Risk Myeloma
High-risk myeloma was defined as chromosomal abnormalities detected by conventional cytogenetics or fluorescence in situ hybridization consisting of t(14;16), t(14;20), and deletion 17p (Mikhael JR, et al: Mayo Clin Proc 88:360-376, 2013 [Erratum: Mayo Clin Proc 88:777, 2013]; Rajkumar SV: Am J Hematol 87:78-88, 2012).
TABLE A1.
Chemotherapy Characteristics and Responses
TABLE A2.
Additional Baseline Characteristics in Patients Receiving Carfilzomib Relative to Development of CVAEs
Footnotes
Clinical trial information: NCT02178579.
Presented at the 59th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 9-12, 2017.
AUTHOR CONTRIBUTIONS
Conception and design: Robert F. Cornell, Daniel Lenihan
Financial support: Daniel Lenihan
Administrative support: Michael R. Savona, Daniel Lenihan
Provision of study materials or patients: Brendan M. Weiss, Adam D. Cohen, Brian G. Engelhardt, Alfred L. Garfall, Stacey A. Goodman, Adetola A. Kassim, Javid Moslehi, Michael R. Savona, David Slosky, Amanda Smith, Edward A. Stadtmauer, Dan T. Vogl, Daniel Lenihan
Collection and assembly of data: Robert F. Cornell, Bonnie Ky, Brendan M. Weiss, Deepak K. Gupta, Adam D. Cohen, Alfred L. Garfall, Shelton Lacy Harrell, Trafina Jadhav, Rupal O’Quinn, Michael R. Savona, David Slosky, Amanda Smith, Edward A. Stadtmauer, Dan T. Vogl, Daniel Lenihan
Data analysis and interpretation: Robert F. Cornell, Bonnie Ky, Brendan M. Weiss, Cherie N. Dahm, Deepak K. Gupta, Liping Du, Joseph R. Carver, Adam D. Cohen, Brian G. Engelhardt, Stacey A. Goodman, Shelton Lacy Harrell, Adetola A. Kassim, Madan Jagasia, Javid Moslehi, Michael R. Savona, David Slosky, Edward A. Stadtmauer, Dan T. Vogl, Adam Waxman, Daniel Lenihan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prospective Study of Cardiac Events During Proteasome Inhibitor Therapy for Relapsed Multiple Myeloma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Robert F. Cornell
Honoraria: Takeda Pharmaceuticals, Karyopharm Therapeutics
Consulting or Advisory Role: Takeda Pharmaceuticals, Karyopharm Therapeutics
Research Funding: Takeda Pharmaceuticals
Bonnie Ky
Honoraria: UpToDate
Consulting or Advisory Role: Roche, Bristol-Myers Squibb, Mateon Therapeutics, Gilead Sciences, Bioinvent, American College of Cardiology
Speakers’ Bureau: Bristol-Myers Squibb
Research Funding: Pfizer, Roche, Singulex
Patents, Royalties, Other Intellectual Property: Patent on use of neuregulin-1b as biomarker in heart failure; pending patent application on use of immunoglobulin E as biomarker of cardiotoxicity
Brendan M. Weiss
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson
Honoraria: Novartis
Research Funding: Janssen Research & Development (Inst), GlaxoSmithKline (Inst), Prothena (Inst)
Deepak K. Gupta
Research Funding: Astellas Pharma
Patents, Royalties, Other Intellectual Property: Patent pending for new heart failure biomarkers
Joseph R. Carver
Consulting or Advisory Role: Boehringer Ingelheim
Adam D. Cohen
Consulting or Advisory Role: Celgene, GlaxoSmithKline, Bristol-Myers Squibb, Seattle Genetics, Janssen Oncology, Oncopeptides, Kite Pharma, Takeda Pharmaceuticals, Seattle Genetics
Research Funding: Bristol-Myers Squibb, Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patents related to CAR T cells and biomarkers of cytokine release syndrome
Expert Testimony: Janssen Oncology
Travel, Accommodations, Expenses: Celgene, Bristol-Myers Squibb, GlaxoSmithKline, Takeda Pharmaceuticals
Alfred L. Garfall
Consulting or Advisory Role: Kite Pharma, Surface Oncology
Research Funding: Amgen (Inst), Novartis (Inst), Tmunity Therapeutics (Inst)
Madan Jagasia
Honoraria: Incyte, Mallinckrodt, Genentech
Consulting or Advisory Role: Incyte, Kadmon
Research Funding: Mallinckrodt, Janssen Oncology
Travel, Accommodations, Expenses: Incyte, Mallinckrodt, Kadmon
Javid Moslehi
Consulting or Advisory Role: Pfizer, Novartis, Bristol-Myers Squibb, Myokardia, Deciphera, Audentes Therapeutics, AstraZeneca
Research Funding: Pfizer, Bristol-Myers Squibb
Rupal O’Quinn
Consulting or Advisory Role: Bracco Diagnostics, Guidepoint Global
Travel, Accommodations, Expenses: Bracco Diagnostics
Michael R. Savona
Stock and Other Ownership Interests: Karyopharm Therapeutics
Consulting or Advisory Role: Karyopharm Therapeutics, Celgene, TG Therapeutics, Merck
Research Funding: Sunesis Pharmaceuticals (Inst), TG Therapeutics (Inst), Astex Pharmaceuticals (Inst), Incyte (Inst), Takeda Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Product license Boehringer Ingelheim (minority owner)
Edward A. Stadtmauer
Consulting or Advisory Role: Celgene, Takeda Pharmaceuticals, Novartis, TEVA Pharmaceuticals Industries, Janssen, Amgen, Sanofi
Dan T. Vogl
Consulting or Advisory Role: Celgene, Amgen, Karyopharm Therapeutics, TEVA Pharmaceuticals Industries, Janssen, Active Biotech, Takeda Pharmaceuticals, Karyopharm Therapeutics
Daniel Lenihan
Consulting or Advisory Role: Roche/Genentech, Bristol-Myers Squibb, Prothena, ION Pharma, Takeda Pharmaceuticals, Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Raab MS, Podar K, Breitkreutz I, et al. : Multiple myeloma. Lancet 374:324-339, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Siegel D, Martin T, Nooka A, et al. : Integrated safety profile of single-agent carfilzomib: Experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica 98:1753-1761, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atrash S, Tullos A, Panozzo S, et al. : Cardiac complications in relapsed and refractory multiple myeloma patients treated with carfilzomib. Blood Cancer J 5:e272, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Garcia D, Cornell RF, et al. : Cardiovascular and thrombotic complications of novel multiple myeloma therapies: A review. JAMA Oncol 3:980-988, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Fradley MG, Groarke JD, Laubach J, et al. : Recurrent cardiotoxicity potentiated by the interaction of proteasome inhibitor and immunomodulatory therapy for the treatment of multiple myeloma. Br J Haematol 180:271-275, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Chen JH, Lenihan DJ, Phillips SE, et al. : Cardiac events during treatment with proteasome inhibitor therapy for multiple myeloma. Cardiooncology 3:4, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimopoulos MA, Goldschmidt H, Niesvizky R, et al. : Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): An interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol 18:1327-1337, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Avet-Loiseau H, Bahlis NJ, Chng WJ, et al. : Ixazomib significantly prolongs progression-free survival in high-risk relapsed/refractory myeloma patients. Blood 130:2610-2618, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Accardi F, Toscani D, Bolzoni M, et al. : Mechanism of action of bortezomib and the new proteasome inhibitors on myeloma cells and the bone microenvironment: Impact on myeloma-induced alterations of bone remodeling. BioMed Res Int 2015:172458, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pautasso C, Bringhen S, Cerrato C, et al. : The mechanism of action, pharmacokinetics, and clinical efficacy of carfilzomib for the treatment of multiple myeloma. Expert Opin Drug Metab Toxicol 9:1371-1379, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Paiva B, Anderson KC, et al. : International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17:e328-e346, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Narayan HK, Finkelman B, French B, et al. : Detailed echocardiographic phenotyping in breast cancer patients: Associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation 135:1397-1412, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardinale D, Colombo A, Sandri MT, et al. : Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 114:2474-2481, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 15.Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. : Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv 1:449-454, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikhael J: Management of carfilzomib-associated cardiac adverse events. Clin Lymphoma Myeloma Leuk 16:241-245, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Chari A, Hajje D: Case series discussion of cardiac and vascular events following carfilzomib treatment: Possible mechanism, screening, and monitoring. BMC Cancer 14:915, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavazzoni M, Lombardi CM, Vizzardi E, et al. : Irreversible proteasome inhibition with carfilzomib as first line therapy in patients with newly diagnosed multiple myeloma: Early in vivo cardiovascular effects. Eur J Pharmacol 838:85-90, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Mangla A, Paydary K, Liu J, et al. : Carfilzomib-associated cardiovascular adverse events in a non-Caucasian cohort of patients with multiple myeloma: A real-world experience. Hematol Oncol 36:715-717, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Waxman AJ, Clasen S, Hwang WT, et al. : Carfilzomib-associated cardiovascular adverse events: A systematic review and meta-analysis. JAMA Oncol 4:e174519, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakubowiak AJ, DeCara JM, Mezzi K: Cardiovascular events during carfilzomib therapy for relapsed myeloma: Practical management aspects from two case studies. Hematology 22:585-591, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. : Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 372:142-152, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Ndumele CE, Matsushita K, Sang Y, et al. : N-terminal pro-brain natriuretic peptide and heart failure risk among individuals with and without obesity: The atherosclerosis risk in communities (ARIC) study. Circulation 133:631-638, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anand IS, Fisher LD, Chiang YT, et al. : Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the valsartan heart failure trial (Val-HeFT). Circulation 107:1278-1283, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Chari A, Stewart AK, Russell SD, et al. : Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv 2:1633-1644, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam CSP, Voors AA, de Boer RA, et al. : Heart failure with preserved ejection fraction: From mechanisms to therapies. Eur Heart J 39:2780-2792, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Sharma K, Kass DA: Heart failure with preserved ejection fraction: Mechanisms, clinical features, and therapies. Circ Res 115:79-96, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imam F, Al-Harbi NO, Al-Harbia MM, et al. : Rutin attenuates carfilzomib-induced cardiotoxicity through inhibition of NF-κB, hypertrophic gene expression and oxidative stress. Cardiovasc Toxicol 17:58-66, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Hasinoff BB, Patel D, Wu X: Molecular mechanisms of the cardiotoxicity of the proteasomal-targeted drugs bortezomib and carfilzomib. Cardiovasc Toxicol 17:237-250, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal A, Luthi J, Belohlavek M, et al. : Carfilzomib and the cardiorenal system in myeloma: An endothelial effect? Blood Cancer J 6:e384, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yui JC, Van Keer J, Weiss BM, et al. : Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol 91:E348-E352, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Laubach JP, Moslehi JJ, Francis SA, et al. : A retrospective analysis of 3954 patients in phase 2/3 trials of bortezomib for the treatment of multiple myeloma: Towards providing a benchmark for the cardiac safety profile of proteasome inhibition in multiple myeloma. Br J Haematol 178:547-560, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimopoulos MA, Moreau P, Palumbo A, et al. : Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol 17:27-38, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Wang M, Cheng J: Overview and management of cardiac and pulmonary adverse events in patients with relapsed and/or refractory multiple myeloma treated with single-agent carfilzomib. Oncology (Williston Park) 27:24-30, 2013. (suppl 3) [PubMed] [Google Scholar]
- 35.Valsami S, Leikauf MS, Madon J, et al. : Immunomodulatory drugs (thalidomide and lenalidomide) up-regulate endothelial tissue factor in vitro resulting in an acquired hypercoagulable state. Blood 112:1893-1893, 2008 [Google Scholar]