PURPOSE
Despite the tissue-agnostic approval of pembrolizumab in mismatch repair deficient (MMRD) solid tumors, important unanswered questions remain about the role of immune checkpoint blockade in mismatch repair–proficient (MMRP) and –deficient endometrial cancer (EC).
METHODS
This phase II study evaluated the PD-L1 inhibitor avelumab in two cohorts of patients with EC: (1) MMRD/POLE (polymerase ε) cohort, as defined by immunohistochemical (IHC) loss of expression of one or more mismatch repair (MMR) proteins and/or documented mutation in the exonuclease domain of POLE; and (2) MMRP cohort with normal IHC expression of all MMR proteins. Coprimary end points were objective response (OR) and progression-free survival at 6 months (PFS6). Avelumab 10 mg/kg intravenously was administered every 2 weeks until progression or unacceptable toxicity.
RESULTS
Thirty-three patients were enrolled. No patient with POLE-mutated tumor was enrolled in the MMRD cohort, and all MMRP tumors were not POLE-mutated. The MMRP cohort was closed at the first stage because of futility: Only one of 16 patients exhibited both OR and PFS6 responses. The MMRD cohort met the predefined primary end point of four ORs after accrual of only 17 patients; of 15 patients who initiated avelumab, four exhibited OR (one complete response, three partial responses; OR rate, 26.7%; 95% CI, 7.8% to 55.1%) and six (including all four ORs) PFS6 responses (PFS6, 40.0%; 95% CI, 16.3% to 66.7%), four of which are ongoing as of data cutoff date. Responses were observed in the absence of PD-L1 expression. IHC captured all cases of MMRD subsequently determined by polymerase chain reaction or genomically via targeted sequencing.
CONCLUSION
Avelumab exhibited promising activity in MMRD EC regardless of PD-L1 status. IHC for MMR assessment is a useful tool for patient selection. The activity of avelumab in MMRP/non-POLE–mutated ECs was low.
INTRODUCTION
Endometrial cancer (EC) affects more than 60,000 women in the United States every year.1 Although early-stage disease is associated with an excellent prognosis, long-term outcomes for patients with advanced stage or recurrent disease are poor.2,3 As such, EC represents an important unmet medical need and novel approaches to treatment are needed.
The Cancer Genome Atlas project4 identified two groups of ECs with high mutation frequency: an ultramutated group (approximately 7% of tumors) that harbored mutations in the exonuclease domain of polymerase ε (POLE), and a hypermutated group (approximately 28% of tumors) with microsatellite instability (MSI), the majority of which harbored MLH1 promoter hypermethylation. The ultramutated POLE group exhibited an extremely high mutation rate (≥ 200 mutations/Mb) with a unique nucleotide change spectrum of increased C→A transversion frequency, whereas the hypermutated MSI group exhibited mutation rates of approximately 18 mutations/Mb with variable length of DNA microsatellites as a result of underlying mismatch repair (MMR) deficiency (MMRD).5
Previous studies have shown that POLE and MSI ECs exhibit high neoantigen load, increased tumor-infiltrating lymphocytes (TILs) and high expression of PD-1 and PD-L1, suggesting that these tumors may be excellent candidates for immunotherapies targeting the PD-1/PD-L1 pathway.6,7 Therefore, we launched a phase II trial to assess the activity of the anti-PD-L1 antibody avelumab in patients with recurrent or persistent ECs stratified in two cohorts by mutational status: (1) a hypermutated cohort including MMRD and POLE-mutated ECs and (2) a hypomutated cohort including MMRP ECs. This study was activated 7 months before the anti–PD-1 antibody pembrolizumab received accelerated US Food and Drug Administration (FDA) approval for patients with MMRD solid tumors regardless of tissue of origin.8 Despite this tumor-agnostic approval, important questions remain in EC, including the correlation between MMRD and MMRP status and response to immune checkpoint blockade (ICB), the role of PD-L1 expression and prior lines of therapy, use of immunohistochemistry (IHC) or other assays to define MMR status, the response of POLE-mutated ECs to ICB, and mechanisms of de novo and acquired resistance.
METHODS
Study Design and Procedures
The primary end point of this investigator-initiated, nonrandomized, two-cohort, phase II study was the activity of avelumab as determined by the frequency of patients with progression-free survival (PFS) of at least 6 months (PFS6) after initiating therapy or had objective tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. Secondary end points included PFS, overall survival, and safety of avelumab for each cohort. Avelumab was administered on an outpatient basis at 10 mg/kg intravenously every 2 weeks until progression or unacceptable toxicity. Antitumor activity was assessed through radiologic tumor assessments conducted at baseline of starting therapy and every 8 weeks thereafter. The clinical trial was approved by the institutional review boards of all institutions (ClinicalTrials.gov identifier: NCT02912572). Merck KGaA/Pfizer Alliance provided avelumab and study funding.
Eligibility
Participants were classified into one of two cohorts of recurrent EC of any histology: (1) MMRD/POLE cohort including patients with MMRD EC, as determined by immunohistochemical complete loss of expression (absence of nuclear immunoreactivity) of one or more of the MMR genes MSH2, MSH6, MLH1, and PMS2 and/or POLE-mutated EC (ie, ECs known to harbor mutations in the exonuclease domain [amino acid residues 268 to 471] of POLE as determined by any Clinical Laboratory Improvement Amendments–approved assay); or (2) MMRP cohort including patients with MMRP EC, as determined by normal immunohistochemical nuclear expression of all MMR genes MSH2, MSH6, MLH1, and PMS2. Patients with ECs (as of the time of their entry in the study, their POLE mutation status was unknown but who were MMRP by IHC) were included in the MMRP cohort. Key inclusion criteria included measurable disease by RECIST 1.1, no upper limit of prior therapies but one or more prior chemotherapeutic regimen, Eastern Cooperative Oncology Group performance status ≤ 1, availability of a formalin-fixed paraffin-embedded block of cancer tissue from the original diagnosis specimen or a specimen of tissue from recurrent disease, and normal organ and marrow function. Key exclusion criteria included prior treatment with any ICB and known brain metastases.
Biomarker Evaluation
POLE mutation status and tumor mutational burden (TMB) were determined from archival, formalin-fixed tissues, using targeted-panel next-generation sequencing (Oncopanel; Dana Farber Cancer Institute, Boston, MA) performed at Dana-Farber Cancer Institute.9-11 MMRD status was determined by IHC, by polymerase chain reaction (PCR) and genomically by assessment of the mutational signature as determined by Oncopanel. IHC was performed for CD4, CD8 and PD-L1 on formalin-fixed paraffin-embedded tissue samples (Data Supplement).
Statistical Analysis
Statistical considerations were developed for coprimary objectives of objective response rate (ORR) and rate of PFS6, with a two-stage design that allowed for early stopping for futility for each cohort. A two-stage test was constructed using the method of Sill et al,12 with the goal of stopping early for futility to limit patient exposure to an inactive agent while restricting the probabilities of type I and type II errors to approximately 10% and 15%, respectively. For the coprimary end points, a true ORR of 5% or less and a rate of PFS6 of 10% or less would not be of clinical interest (null hypothesis: πOR ≤ 5% and πPFS6 ≤ 10%), whereas an improvement to a 20% ORR or 30% PFS6 rate would warrant additional investigation of avelumab. In the first stage, 16 patients would be enrolled. If there were two or more objective responses (ORs) or two or more patients who were progression free at 6 months in any of the two cohorts, accrual would continue to the second stage, where an additional 19 patients would be enrolled in these cohorts. Overall, if at least four treated patients with an OR or at least eight patients who were progression free at 6 months were observed, avelumab would be considered worthy of additional study in the corresponding cohort.
RESULTS
Patients
The study was activated on November 2, 2016, approximately 7 months before pembrolizumab received accelerated FDA approval for patients with MMRD solid tumors regardless of tissue of origin. The MMRP cohort was closed after the first stage because of futility after accrual of 16 patients. The MMRD/POLE cohort continued to the second stage and was closed to accrual on September 14, 2018, because the primary end point of four OR was met with a final accrual of 17 patients, approximately 4 months after pembrolizumab had received FDA approval. A total of 33 patients were enrolled in both cohorts; 31 patients (15 in the MMRD/POLE cohort and 16 in the MMRP cohort) initiated protocol treatment (two patients in the MMRD/POLE cohort did not initiate protocol treatment and were excluded from all analyses).
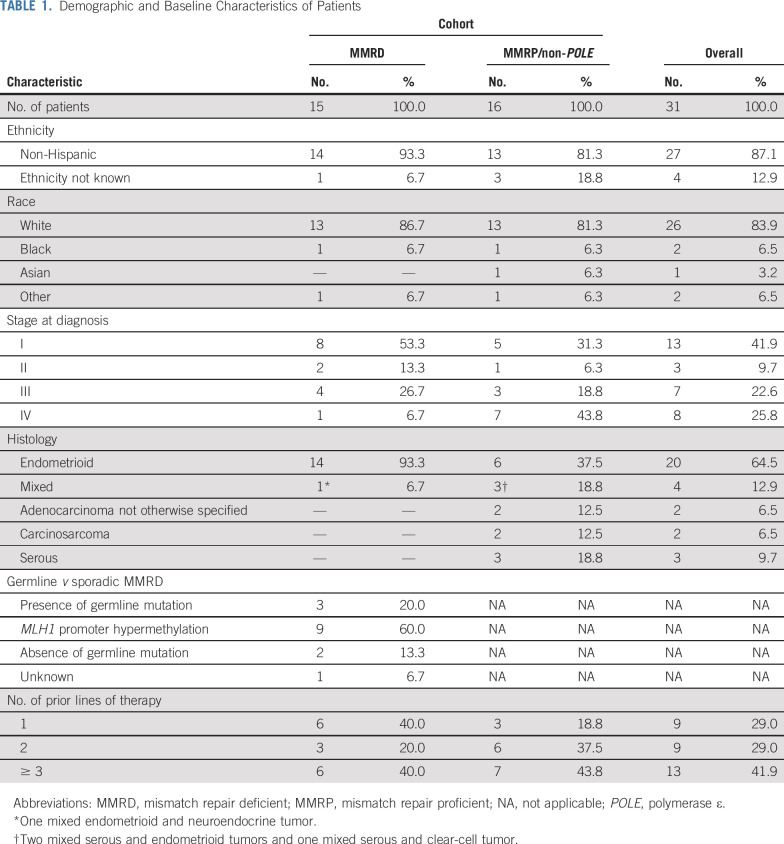
All patients enrolled in the MMRD/POLE cohort were MMRD by IHC; no patient in the MMRD/POLE cohort had a documented POLE mutation, so this cohort is henceforth referred to as the MMRD cohort in this article. All patients in the MMRP cohort were subsequently assessed for presence of POLE mutations and no patient harbored a POLE mutation; this cohort is henceforth referred to as the MMRP/non-POLE cohort. Patient characteristics are provided in Table 1.
TABLE 1.
Demographic and Baseline Characteristics of Patients
Antitumor Activity
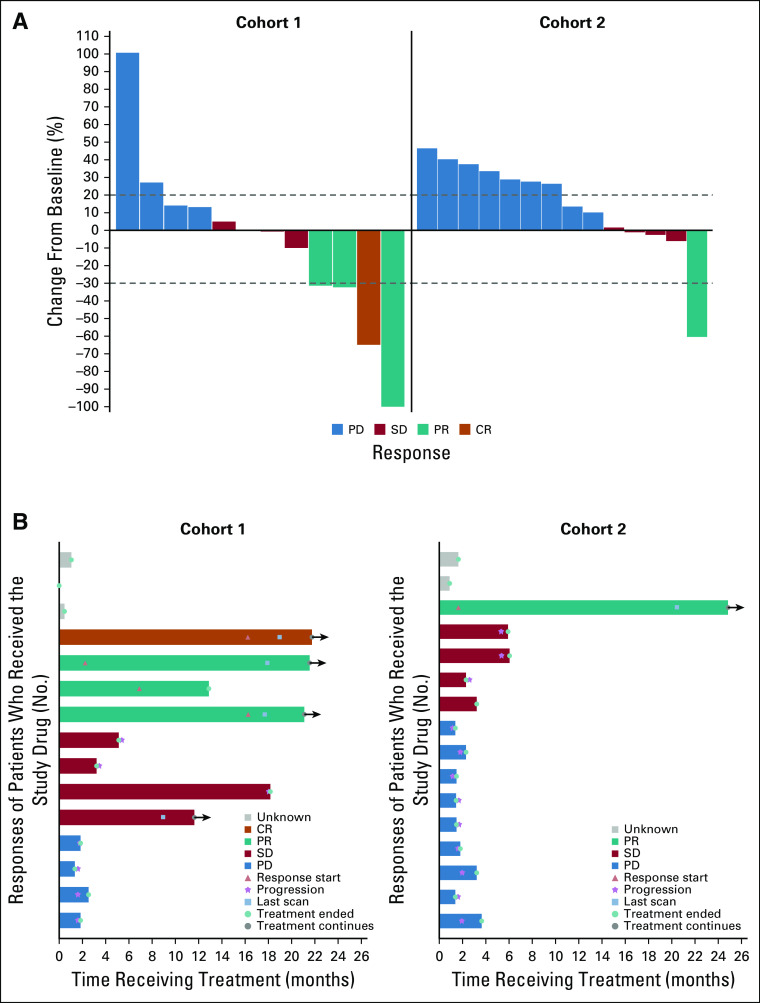
Of the 31 analysis patients, there were five ORs: four in the MMRD cohort (one complete response and three partial responses [PRs]) and one PR in the MMRP/non-POLE cohort (Fig 1A). All responses were confirmed. Four patients in the MMRD cohort and four patients in the MMRP/non-POLE cohort had stable disease of any duration as the best response (Table 2). Overall, the exact binomial-confirmed ORR in the MMRD cohort was 26.7% (95% CI, 7.8% to 55.1%) and 6.25% (95% CI, 0.16% to 30.2%) in the MMRP/non-POLE cohort.
FIG 1.
Antitumor activity of avelumab in the MMRD and MMRP/non-POLE cohorts. (A) Best change in target lesions from baseline. Five patients were excluded because of missing lesion-diameter values at follow-up. (B) Treatment duration for patients in both cohorts. CR, complete response; MMRD, mismatch repair deficient; MMRP, mismatch repair proficient; PD, progressive disease; POLE, polymerase ε; PR, partial response; SD, stable disease.
TABLE 2.
Confirmed Objective Response and PFS6 in MMRD and MMRP/non-POLE Cohorts
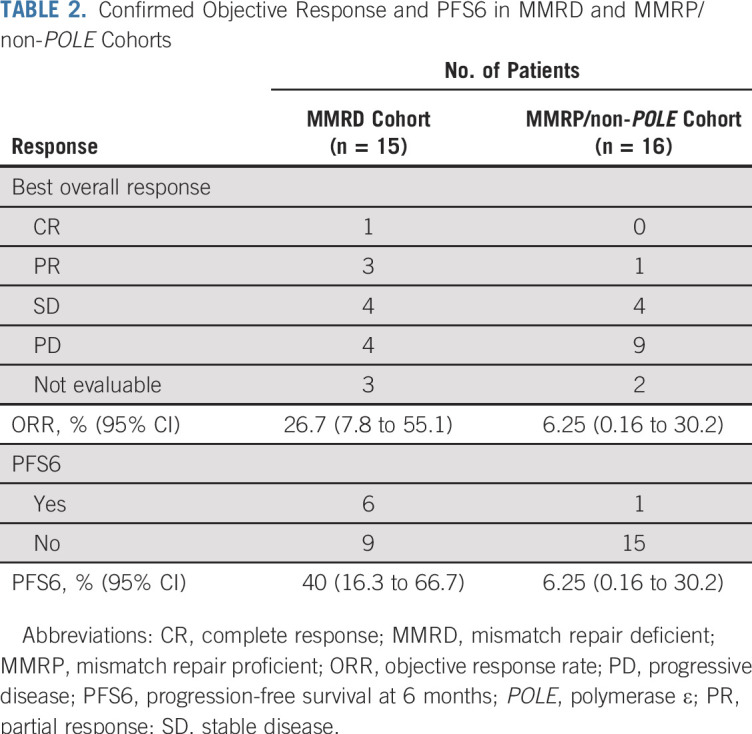
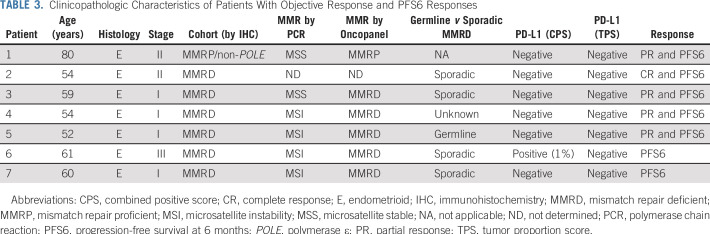
PFS6 was a coprimary end point; there were seven patients who exhibited PFS6 (six in the MMRD, including the four patients with ORs, and one in the MMRP/non-POLE cohort [the same patient who had a PR]; Table 2). Clinicopathologic characteristics of patients with OR and PFS6 responses are presented in Table 3. PFS6 was 40.0% (95% CI, 16.3% to 66.7%) in the MMRD and 6.25% (95% CI, 0.16% to 30.2%) in the MMRP/non-POLE cohorts. Five patients (four in the MMRD and one in the MMRP/non-POLE cohort) were still receiving the protocol treatment at the data cutoff date (Fig 1B).
TABLE 3.
Clinicopathologic Characteristics of Patients With Objective Response and PFS6 Responses
In the MMRD cohort, one of three patients with germline MMR mutation had a PR, four of 11 patients with sporadic MMRD (either MLH1 promoter hypermethylation or negative germline testing) had either a PR or PFS6 response, and one patient with MMRD of unknown germline versus sporadic etiology had a PR. All four ORs and five of six PFS6 responses in the MMRD cohort were observed in patients with at least three lines of prior therapy (Fisher’s exact test P = .011).
With a median follow-up of 18.6 (range, 4.4 to 22.2) months, the median PFS was 4.4 months (95% CI, 1.7 months to not reached) for the MMRD cohort, and 1.9 (95% CI, 1.6 to 2.8) months for the MMRP/non-POLE cohort (Fig 2). Median overall survival for the MMRP/non-POLE cohort was 6.6 (95% CI, 2.0 to 10.2) months; the median survival for the MMRD cohort has not been reached yet (Fig 2).
FIG 2.
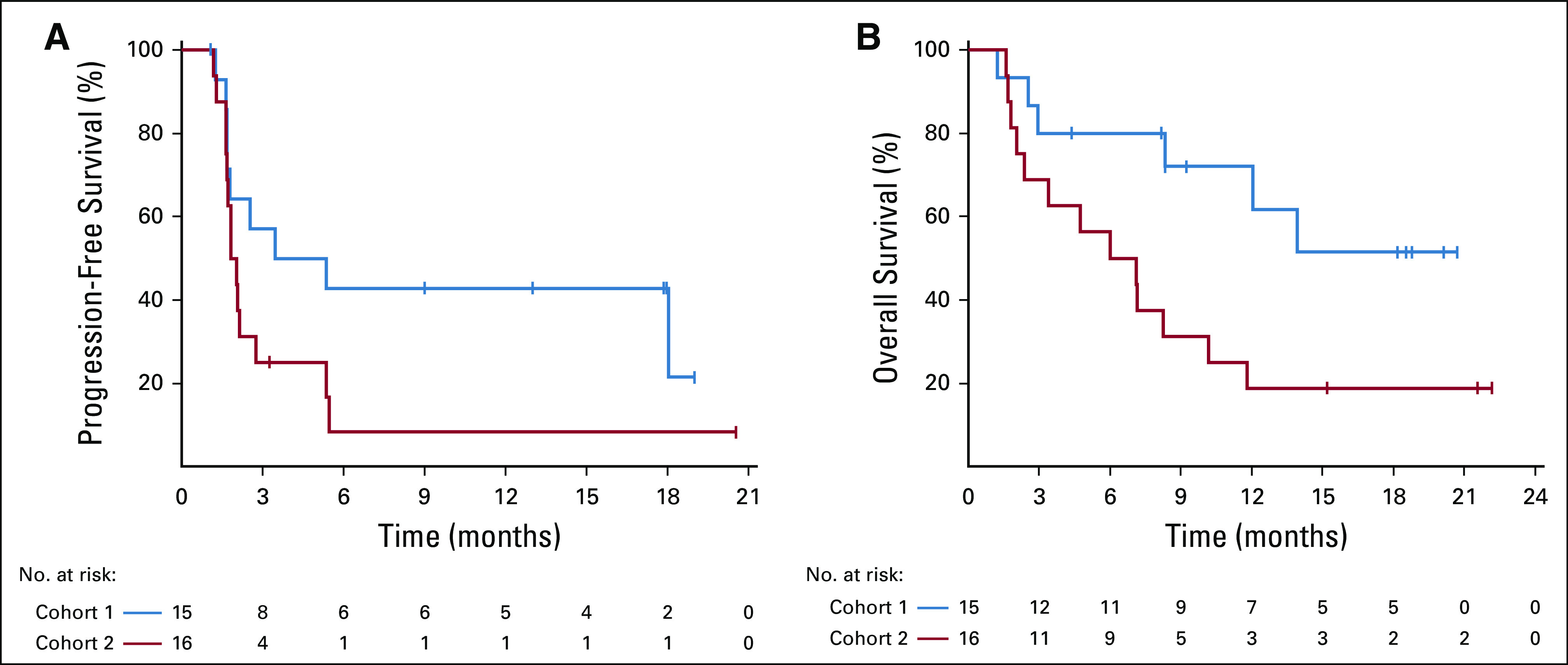
Graphs of (A) progression-free survival and (B) overall survival in the mismatch repair deficient and mismatch repair proficient/non-POLE cohorts.
Safety
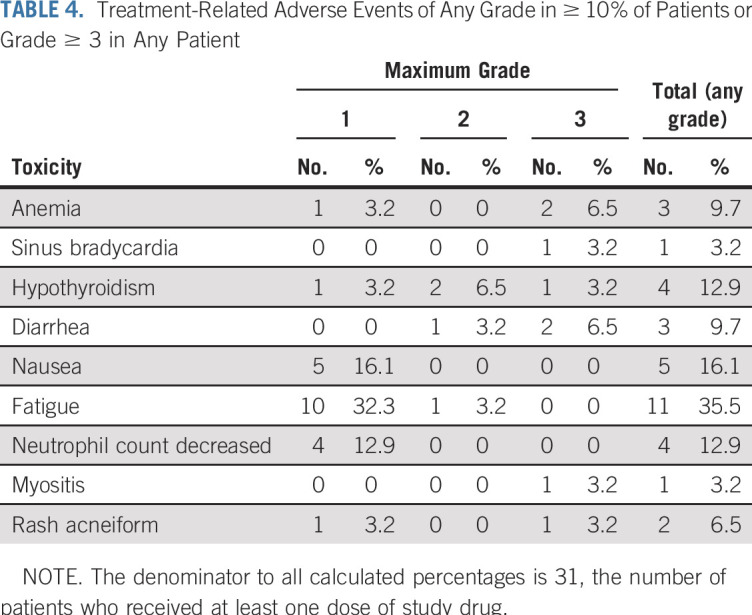
Of the 31 patients who initiated protocol treatment, 22 (71%) had a treatment-related adverse event of any grade. Six patients (19.4%) reported treatment-related grade 3 toxicities; there were no treatment-related grade 4 and grade 5 toxicities in either cohort (Table 4; Data Supplement).
TABLE 4.
Treatment-Related Adverse Events of Any Grade in ≥ 10% of Patients or Grade ≥ 3 in Any Patient
Biomarker Analyses
PD-L1 status was assessed in 25 patients (12 in the MMRD cohort and 13 in the MMRP/non-POLE cohort). Based on a PD-L1 expression tumor proportion score (TPS) of ≥ 1%, seven of 25 tumors (28%) were PD-L1 positive, including four (33.3%) MMRD and three (23.1%) MMRP/non-POLE tumors. Among the tumors with known PD-L1 status, all ORs and five of the six PFS6 responses in both cohorts were observed in PD-L1–negative tumors by TPS and combined positive score (CPS). One PFS6 response was observed in an MMRD tumor, which was a PD-L1–negative tumor by TPS but positive by CPS.
All 16 patients enrolled in the MMRP/non-POLE cohort were MMRP by IHC; 13 of these patients (including the sole avelumab responder) had their MMR status also assessed by PCR and all were determined to be microsatellite stable (MSS). Furthermore, 15 of the 16 patients in the MMRP/non-POLE cohort (including the sole avelumab responder) had their MMR status assessed genomically by Oncopanel and all, again, were determined to be MMRP. These data suggest that IHC did not miss a case of MMRD determined by PCR and Oncopanel.
In the MMRD cohort, all 15 patients were MMRD by IHC; 12 of these had their MMR status also assessed by PCR and Oncopanel. Three of 12 tumors were MSS by PCR and one of these had a PR to avelumab (ie, PCR missed one avelumab response). Two of 12 tumors were MMRP by Oncopanel, with none having an avelumab response. Overall, in MMRD tumors determined by Oncopanel, ORR and PFS6 were 30% and 50%, respectively, and in MMRD tumors determined by PCR, ORR and PFS6 were 22.2% and 44%, respectively.
TMB and TILs did not correlate with response to avelumab (Data Supplement). However, analysis of the MMRD tumors that did not respond to avelumab, revealed three that each harbored two mutations of JAK1 or B2M, possibly reflecting biallelic inactivation of these genes, which have been associated with resistance to ICB13 (Data Supplement). Specifically, one tumor harbored the hotspot JAK1 frameshift deletion K860fs and a JAK1 missense mutation L1071P toward the end of the kinase domain (exon 23), another tumor harbored the same hotspot JAK1 frameshift deletion K860fs and a missense mutation JAK1 Q750R on the pseudokinase domain (exon 16), and the third tumor harbored two previously reported B2M mutations: a B2M c.68-2A>G splice site mutation, and a p.M1? mutation changing the start codon.
DISCUSSION
In this investigator-initiated, phase II study, avelumab activity in the MMRD cohort met the prespecified criteria to be considered worthy of additional investigation. The ORR and PFS6 were 26.7% and 40%, respectively, with four patients (including three of four with PR and four of six with PFS6) continuing protocol treatment as of the data cutoff date. Importantly, IHC identified MMRD in the responders to avelumab without the need to assess MMRD by more expensive and time-consuming molecular diagnostic approaches. In addition, responses were observed regardless of PD-L1 expression, suggesting that response to anti–PD-L1 antibodies such as avelumab is not dependent on demonstrating PD-L1 expression in the tumor microenvironment. Response to avelumab was observed in patients who had received multiple prior lines of therapy and those with either somatic or germline origin of MMRD. Conversely, avelumab did not demonstrate enough activity to be worthy of additional evaluation among ECs determined to be MMRP by IHC and not POLE mutated. Avelumab demonstrated an acceptable safety profile, consistent with previous studies in other solid malignancies, and no unexpected toxicities.14-16
Despite our intention to enroll patients with ECs with POLE mutations, no tumor harbored a documented POLE mutation. This is likely because POLE-mutated tumors represent only approximately 7% of all ECs at initial diagnosis, recur rarely, and are associated with excellent prognosis, likely as a result of their high immunogenicity.4,6,7 Several isolated case reports have subsequently shown that POLE-mutated ECs respond extremely well to immunotherapies targeting the PD-1 pathway.6,7,17 However, prospective evaluation of POLE-mutated tumors for response to ICB has never been performed, to our knowledge, although admittedly, this evaluation may be more feasible in a tissue-agnostic manner. Nonetheless, despite their rarity, recurrent POLE-mutated ECs do exist (one of the three PRs to pembrolizumab in the KEYNOTE 028 study was subsequently found to be in a POLE-mutated tumor18) and POLE mutation status should be an important consideration in immunotherapy studies conducted in EC.
In this study, IHC was uniformly used to determine MMRD status for formal eligibility assessment and formal assignment of all patients to one of the two study cohorts. IHC was chosen because it is inexpensive, has a quick turnaround, and is a standard test performed routinely as part of the initial evaluation of patients newly diagnosed with EC in the United States. MMRD status was also retrospectively assessed by PCR and genomically using Oncopanel; importantly, this analysis showed IHC did not miss any case of MMRD determined by PCR and Oncopanel. Conversely, PCR missed one case of an avelumab PR (patient 3, Table 3), which was determined to be MMRD by IHC and Oncopanel. However, two tumors defined as MMRD by IHC were subsequently determined to be MMRP by Oncopanel, with none of these tumors responding to avelumab indicating that IHC may be less specific than Oncopanel in correlating with avelumab response. Our study had neither the intent nor the power to detect differences in the avelumab response between tumors defined as MMRD via IHC, PCR, and Oncopanel assays; nonetheless, the results suggest the observed avelumab response may be different depending on the assay used to define MMR status.
The data reported here for avelumab are generally consistent with those of other reported studies of ICB in advanced EC, although direct comparisons are hindered by study-specific differences in eligibility criteria, patient characteristics, sizes, designs, and use of different assays to define MMR status. In the KEYNOTE-02818 study of pembrolizumab in PD-L1–positive EC, there was one PR among 18 patients with MSS status (by PCR) and one PR in an MSS tumor, which was subsequently found to harbor a POLE mutation. Overall, pembrolizumab ORR was 5.6% among patients with MSS, non-POLE-mutated ECs. In ECs determined to be MMRD by IHC or PCR, pembrolizumab demonstrated an ORR of 36%, as reported in the FDA package insert.18a In a preliminary report of the anti–PD-1 antibody TSR-042 in EC,19 TSR-042 also showed good activity against microsatellite instability-high ECs defined genomically by next-generation sequencing; the response of TSR-042 among MSS ECs was 20.3%, although no information about POLE mutation status was available for these patients.
Previously reported biomarkers of response to ICB, including PD-L1 expression, number of TILs, and TMB20-22 did not correlate with avelumab response. Interestingly, most responses were observed in patients with PD-L1–negative tumors by TPS and CPS, although it is important to acknowledge limitations in the interpretation of PD-L1 staining, including the absence of any validated PD-L1 IHC assay in EC and the fact that PD-L1 staining was assessed in archival samples that, in certain cases, were obtained several years before initiation of avelumab therapy.23
Interestingly, we found mutations suggesting possible biallelic inactivation of B2M and JAK1 in three MMRD tumors that did not respond to avelumab, thereby providing a possible explanation for the de novo resistance of these tumors to avelumab. Although loss-of-function mutations in genes involved in the interferon-receptor signaling pathway (JAK1 and JAK2) and in antigen presentation (B2M, leading to loss of surface expression of major histocompatibility complex class I) confer resistance to ICB in melanoma, this has not been previously demonstrated in the context of EC and never, to our knowledge, as a mechanism of de novo resistance.13 In this regard, hotspot-truncating JAK1 mutations, including the JAK1 frameshift deletion K860fs, have been reported to occur de novo in EC, especially in MMRD ECs, and have been functionally characterized to abrogate interferon-γ signaling and contribute to tumor immune evasion.24-26 The precise role of these mutations in de novo and acquired resistance to ICB in MMRD EC will need to be formally evaluated in a larger cohort of patients.
The finding that all ORs and all but one PFS6 responses were observed in patients with three or more lines of therapy is intriguing and requires independent validation. A similar trend was observed in the KEYNOTE-100 study of pembrolizumab in ovarian cancer.27 Ample evidence indicates that cytotoxic chemotherapy, radiation therapy, and targeted therapies have immunomodulatory effects and may prime tumors to respond to ICB via several mechanisms, including activation of type I interferon response and upregulation of PD-L1.28-31 Whether number, type, duration, or intensity of prior therapies may affect future response to ICB is an important question that warrants additional investigation.
In conclusion, our study findings indicate MMRD by IHC correlates with response to avelumab in EC, with responses observed in patients who lacked PD-L1 expression and in those with multiple prior lines of therapy and either somatic or germline origin of the MMRD. Our findings support routine use of IHC to determine MMRD status in EC when considering treatment with ICB. Conversely, avelumab did not demonstrate activity worthy of additional evaluation in MMRP/non-POLE-mutated ECs, suggesting that alternative approaches are needed. In this regard, a clinical trial of avelumab plus the PARP inhibitor talazoparib in MMRP/non-POLE–mutated ECs is currently ongoing (ClinicalTrials.gov identifier: NCT02912572).
Supplementary Material
Footnotes
Supported by Merck KGaA/Pfizer Alliance.
Clinical Trial Information: NCT02912572
AUTHOR CONTRIBUTIONS
Conception and design: Panagiotis A. Konstantinopoulos, Joyce F. Liu, Jeffrey J. Ishizuka, Elizabeth Stover, Kathryn P. Gray, Ursula A. Matulonis
Administrative support: Panagiotis A. Konstantinopoulos, Heather Crowe, Neal I. Lindeman, Jennifer Curtis, Ursula A. Matulonis
Provision of study material or patients: Panagiotis A. Konstantinopoulos, Joyce F. Liu, Carolyn Krasner, Mary Buss, Whitfield B. Growdon, Susana Campos, Neal I. Lindeman, Elizabeth Stover, Susan Schumer, Alexi A. Wright, Gini F. Fleming, Ursula A. Matulonis
Collection and assembly of data: Panagiotis A. Konstantinopoulos, Joyce F. Liu, Carolyn Krasner, Mary Buss, Whitfield B. Growdon, Heather Crowe, Susana Campos, Neal I. Lindeman, Sarah Hill, Jennifer Curtis, Susan Schumer, Alexi A. Wright, Roxanne Quinn, Christin Whalen, Richard T. Penson, Ursula A. Matulonis
Data analysis and interpretation: Panagiotis A. Konstantinopoulos, Weixiu Luo, Joyce F. Liu, Doga C. Gulhan, Jeffrey J. Ishizuka, Allison A. Gockley, Neal I. Lindeman, Elizabeth Stover, Alexi A. Wright, Kathryn P. Gray, Richard T. Penson, Stephen A. Cannistra, Gini F. Fleming, Ursula A. Matulonis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Study of Avelumab in Patients With Mismatch Repair Deficient and Mismatch Repair Proficient Recurrent/Persistent Endometrial Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Panagiotis A. Konstantinopoulos
Consulting or Advisory Role: Merck, Vertex, AstraZeneca, Pfizer/EMD Serono, Tesaro
Research Funding: Pfizer (Inst), Eli Lilly (Inst)
Joyce F. Liu
Consulting or Advisory Role: Tesaro, Mersana, Clovis Oncology
Research Funding: Roche (Inst), AstraZeneca (Inst), Boston Biomedical (Inst), Atara Biotherapeutics (Inst), Acetylon (Inst), Bristol-Myers Squibb (Inst), Agenus (Inst), CytomX Therapeutics (Inst), Regeneron (Inst), Tesaro (Inst), Clovis Oncology (Inst), Surface Oncology (Inst), 2X Oncology (Inst), Vigeo Therapeutics (Inst), Aravive (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Doga C. Gulhan
Patents, Royalties, Other Intellectual Property: A provisional patent application is being drafted regarding an algorithm developed by the author for which a coversheet provisional has been filed on September 24, 2018 titled “Computational method to identify mutational signatures from sequencing data” (Inst)
Carolyn Krasner
Research Funding: Cerulean Pharma
Jeffrey J. Ishizuka
Stock and Other Ownership Interests: Jounce Therapeutics
Consulting or Advisory Role: Rheos Therapeutics, Tango Therapeutics, Two River
Patents, Royalties, Other Intellectual Property: I am an inventor on a patent on approaches targeting dsRNA sensing, titled “Modulating dsRNA Editing, Sensing, and Metabolism to Increase Tumor Immunity and Improve the Efficacy of Cancer Immunotherapy and/or Modulators of Intratumoral Interferon”
Mary Buss
Honoraria: UpToDate
Elizabeth Stover
Patents, Royalties, Other Intellectual Property: Patent pending
Alexi A. Wright
Honoraria: CVS Health Care
Kathryn P. Gray
Stock and Other Ownership Interests: DVAX, ArQL
Richard T. Penson
Honoraria: AbbVie, AstraZeneca, Clovis Oncology, Eisai, Roche, Johnson & Johnson, Mersana Therapeutics, Newlink Genetics, Sutro Biopharma, Tesaro, Vascular Biogenics
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, Baxalta, Care4ward, Clovis Oncology, Eisai, Genentech, Merck, Mersana, Tesaro, Vascular Biogenics, Sutro, Janssen Oncology
Research Funding: Array BioPharma (Inst), AstraZeneca (Inst), Cerulean Pharma (Inst), Eisai (Inst), Genentech (Inst), Regeneron (Inst), Sanofi (Inst), Tesaro (Inst), Vascular Biogenics (Inst)
Patents, Royalties, Other Intellectual Property: BMJ, Blackwell Publishing, UpToDate
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, Genentech, Tesaro, Vascular Biogenics
Other Relationship: AbbVie
Stephen A. Cannistra
Research Funding: Merck (Inst), Tesaro (Inst), AstraZeneca (Inst), Clovis (Inst), Bristol-Myers Squibb (Inst)
Gini F. Fleming
Research Funding: Corcept Therapeutics (Inst), AbbVie (Inst), Genentech (Inst), Tesaro (Inst), Syndax (Inst), Forty Seven (Inst), Iovance Biotherapeutics (Inst), Syros Pharmaceuticals (Inst), Astex Pharmaceuticals (Inst), Merck (Inst), Sanofi (Inst), Sermonix Pharmaceuticals (Inst)
Ursula A. Matulonis
Consulting or Advisory Role: Merck, Geneos, 2X Oncology, Myriad Genetics, Fujifilm, Mersana, Immunogen, Novartis, Tesaro, Syndax
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2019. CA Cancer J Clin 69:7-34, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Aghajanian C, Sill MW, Darcy KM, et al. : Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: A Gynecologic Oncology Group study. J Clin Oncol 29:2259-2265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez EA, Brady WE, Walker JL, et al. : Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 129:22-27, 2013 [DOI] [PubMed] [Google Scholar]
- 4. doi: 10.1038/nature12113. Kandoth C, Schultz N, Cherniack AD, et al: Integrated genomic characterization of endometrial carcinoma. Nature 497:67-73, 2013 [Erratum: Nature 500:242, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guillotin D, Martin SA: Exploiting DNA mismatch repair deficiency as a therapeutic strategy. Exp Cell Res 329:110-115, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Howitt BE, Shukla SA, Sholl LM, et al. : Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol 1:1319-1323, 2015 [DOI] [PubMed] [Google Scholar]
- 7.van Gool IC, Eggink FA, Freeman-Mills L, et al. : POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res 21:3347-3355, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le DT, Uram JN, Wang H, et al. : PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509-2520, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sholl LM, Do K, Shivdasani P, et al. : Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight 1:e87062, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagle N, Berger MF, Davis MJ, et al. : High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov 2:82-93, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia EP, Minkovsky A, Jia Y, et al. : Validation of OncoPanel: A targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med 141:751-758, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Sill MW, Rubinstein L, Litwin S, et al. : A method for utilizing co-primary efficacy outcome measures to screen regimens for activity in two-stage phase II clinical trials. Clin Trials 9:385-395, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. : Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 375:819-829, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlesi F, Vansteenkiste J, Spigel D, et al. : Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): An open-label, randomised, phase 3 study. Lancet Oncol 19:1468-1479, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Disis ML, Taylor MH, Kelly K, et al. : Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: Phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol 5:393, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan R, Thomas A, Nemunaitis JJ, et al. : Efficacy and safety of avelumab treatment in patients with advanced unresectable mesothelioma: Phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol 5:351-357, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veneris JT, Lee EK, Goebel EA, et al. : Diagnosis and management of a recurrent polymerase-epsilon (POLE)-mutated endometrial cancer. Gynecol Oncol 153:471-478, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Ott PA, Bang YJ, Berton-Rigaud D, et al. : Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: Results from the KEYNOTE-028 Study. J Clin Oncol 35:2535-2541, 2017 [DOI] [PubMed] [Google Scholar]
- 18a. US Food and Drug Administration: KEYTRUDA (pembrolizuman) powder. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125514Orig1s054lbl.pdf.
- 19. Oaknin A, Duska LR, Sullivan RJ, et al. Preliminary safety, efficacy, and pharmacokinetic/pharmacodynamic characterization from GARNET, a phase I/II clinical trial of the anti–PD-1 monoclonal antibody, TSR-042, in patients with recurrent or advanced MSI-h and MSS endometrial cancer. Gynecol Oncol 154:17, 2019 (suppl 1) [Google Scholar]
- 20.Sabari JK, Leonardi GC, Shu CA, et al. : PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol 29:2085-2091, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Duan J, Cai S, et al. : Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol 5:696-702, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voong KR, Feliciano J, Becker D, et al. : Beyond PD-L1 testing-emerging biomarkers for immunotherapy in non-small cell lung cancer. Ann Transl Med 5:376, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cottrell TR, Taube JM: PD-L1 and emerging biomarkers in immune checkpoint blockade therapy. Cancer J 24:41-46, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albacker LA, Wu J, Smith P, et al. : Loss of function JAK1 mutations occur at high frequency in cancers with microsatellite instability and are suggestive of immune evasion. PLoS One 12:e0176181, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Y, Zhang Y, Liu RZ, et al. : JAK1 truncating mutations in gynecologic cancer define new role of cancer-associated protein tyrosine kinase aberrations. Sci Rep 3:3042, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stelloo E, Versluis MA, Nijman HW, et al. : Microsatellite instability derived JAK1 frameshift mutations are associated with tumor immune evasion in endometrioid endometrial cancer. Oncotarget 7:39885-39893, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matulonis UA, Shapira-Frommer R, Santin A, et al: Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Interim results from the phase 2 KEYNOTE-100 study. J Clin Oncol 36:5511, 2018 (15 suppl) [DOI] [PubMed]
- 28.Coffelt SB, de Visser KE: Immune-mediated mechanisms influencing the efficacy of anticancer therapies. Trends Immunol 36:198-216, 2015 [DOI] [PubMed] [Google Scholar]
- 29. Ding L, Kim HJ, Wang Q, et al: PARP inhibition elicits STING-dependent antitumor immunity in Brca1-deficient ovarian cancer. Cell Rep 25:2972-2980.e5, 2018. [DOI] [PMC free article] [PubMed]
- 30.Galluzzi L, Buqué A, Kepp O, et al. : Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28:690-714, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Zitvogel L, Galluzzi L, Smyth MJ, et al. : Mechanism of action of conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 39:74-88, 2013 [DOI] [PubMed] [Google Scholar]