PURPOSE
Understanding acute toxicities after whole-breast radiotherapy is important to inform patients, guide treatment decisions, and target supportive care. We evaluated patient-reported outcomes prospectively collected from a cohort of patients with breast cancer.
METHODS
We describe the maximal toxicity reported by 8,711 patients treated between 2012 and 2019 at 27 practices. Multivariable models identified characteristics associated with (1) breast pain, (2) bother from itching, stinging/burning, swelling, or hurting of the treated breast, and (3) fatigue within 7 days of completing whole-breast radiotherapy.
RESULTS
Moderate or severe breast pain was reported by 3,233 (37.1%): 1,282 (28.9%) of those receiving hypofractionation and 1,951 (45.7%) of those receiving conventional fractionation. Frequent bother from at least one breast symptom was reported by 4,424 (50.8%): 1,833 (41.3%) after hypofractionation and 2,591 (60.7%) after conventional fractionation. Severe fatigue was reported by 2,008 (23.1%): 843 (19.0%) after hypofractionation and 1,165 (27.3%) after conventional fractionation. Among patients receiving hypofractionated radiotherapy, younger age (P < .001), higher body mass index (BMI; P < .001), Black (P < .001) or other race (P = .002), smoking status (P < .001), larger breast volume (P = .002), lack of chemotherapy receipt (P = .004), receipt of boost treatment (P < .001), and treatment at a nonteaching center predicted breast pain. Among patients receiving conventionally fractionated radiotherapy, younger age (P < .001), higher BMI (P = .003), Black (P < .001) or other race (P = .002), diabetes (P = .001), smoking status (P < .001), and larger breast volume (P < .001) predicted breast pain.
CONCLUSION
In this large observational data set, substantial differences existed according to radiotherapy dose fractionation. Race-related differences in pain existed despite controlling for multiple other factors; additional research is needed to understand what drives these differences to target potentially modifiable factors. Intensifying supportive care may be appropriate for subgroups identified as being vulnerable to greater toxicity.
INTRODUCTION
Meta-analysis of randomized trials has demonstrated convincingly that radiation therapy provides substantial benefits in local control and modest improvements in survival for many patients with early-stage breast cancer.1 Radiotherapy is also known to cause both acute and late toxicity. Acute effects include fatigue and radiation dermatitis, and inflammatory symptoms may bother patients. Prior work2 has demonstrated that acute toxicity appears less frequently with the moderately hypofractionated schedules that are now guideline supported for most node-negative patients undergoing breast-conserving surgery.3 Nevertheless, nearly one half of all patients may experience grade 2 or greater acute toxicity even with these newer approaches.4
CONTEXT
Key Objective
To understand patient experiences with acute toxicity after whole-breast radiotherapy in a large multicenter cohort of patients with breast cancer treated in the United States between 2012 and 2019.
Knowledge Generated
We found that patients receiving moderately hypofractionated whole-breast radiotherapy reported considerably less acute toxicity. Race-related differences in pain experiences existed despite controlling for multiple other factors, with worse pain among women who were Black or whose race was defined as “other” (not White, Asian, or Black).
Relevance
These findings are useful to inform patients, guide treatment decisions, and target supportive care.
Patients often have fears about radiation-related toxicity.5-8 In one recent survey, 19% of patients with breast cancer felt they lacked sufficient information about the adverse effects to expect, and 32% indicated experiencing adverse effects that they wished they had known more about.9 Although some of this might be remediated by greater attention to physician-patient communication, the ability to fully inform patients is limited by gaps in the existing literature. Currently, there is a paucity of information that characterizes the experiences of radiation toxicity from the perspective of the patients themselves.10 Moreover, beyond the impact of fractionation, little is known about whether certain subgroups of patients (on the basis of treatment characteristics or underlying factors) may have higher risks of toxicity after whole-breast irradiation.
Because an understanding of acute patient-reported toxicities after breast radiotherapy would be valuable to inform patients, guide treatment decisions, and target supportive care interventions, we evaluated patient-reported outcomes in a statewide multicenter consortium, including prospectively collected data from a large cohort of women with breast cancer who received whole-breast radiotherapy after breast-conserving surgery.
METHODS
Data Collection and Sample
As part of a collaborative quality improvement initiative, the Michigan Radiation Oncology Quality Consortium (MROQC) prospectively collects clinical, dosimetric, and patient-reported outcomes data from women treated for breast cancer at 27 practices, together with information about facilities and providers.11 Eligible patients during the study period were those being treated with adjuvant whole-breast radiotherapy for nonmetastatic, unilateral breast cancer at an MROQC-participating institution.
This effort is institutional review board approved as a collaborative quality initiative; clinical information on all eligible patients is entered into the database, but patient participation in surveys is voluntary (with written consent documentation waived). Practices are provided with staff support, funded by Blue Cross Blue Shield of Michigan, to gather data on all patients treated with lumpectomy and unilateral whole-breast radiotherapy, regardless of insurer. Those practices that meet the quality benchmarks are provided with a “gold card” certification that eliminates the need for prior authorization for treatment if the patient is insured by Blue Cross Blue Shield of Michigan.
A total of 12,577 patients were treated with lumpectomy and whole-breast radiotherapy at MROQC sites and had data entered into the MROQC database between January 1, 2012, and September 30, 2019. We describe here the maximal toxicity reported by the 8,711 patients who provided survey responses within 7 days before or after the end of treatment and for whom we had sufficient data to determine dose fractionation and treatment fields.
Measures
Three primary predefined outcomes of interest were measured: (1) breast pain, (2) bother (related to itching, stinging/burning, swelling, or hurting of the treated breast), and (3) fatigue, defined using the maximum value recorded on any on-treatment weekly evaluation or on the end-of-treatment evaluation. Specifically, breast pain was assessed using an approved modification of the Brief Pain Inventory12 that asks patients to rate their pain during the last 24 hours at its worst, least, average, and “right now.” Breast pain was considered moderate or severe when the score on any one of those four items was ≥ 4 on the 10-point scale.
Bother was measured using a modified scaled measure adapted from the Skindex13 to include four symptoms of interest (itching of the skin of the treated breast, burning or stinging of the skin of the treated breast, swelling of the treated breast, and hurting of the treated breast). Patients were asked, “During the past week, how often have you been bothered by…” for each symptom, with response options of “never,” “rarely,” “sometimes,” “often,” or “all the time.” Bother was considered frequent when the score was “often” or “all the time” for any of the four subitems. Fatigue was measured as in prior work2 with a single item asking, “How often did you feel significant fatigue?” and was considered severe when rated as present “always” or “most of the time” (rather than “sometimes,” “rarely,” or “never”) over the past 4 weeks.
The patient characteristics analyzed included age (grouped as < 50 years, 50-59 years, 60-69 years, or ≥ 70 years), body mass index (BMI; grouped as < 18.5, 18.5 to < 25, 25 to < 30, 30 to < 35, 35 to < 40, and ≥ 40), race (defined by self-report where available and otherwise by clinician report, and grouped as White, Black, Asian, or other, with the “other” category including categories of American Indian/Alaska Native, Native Hawaiian or other Pacific Islander, Arab/Middle Eastern, or other),14 hypertension (yes or no), diabetes (yes or no), smoking status (never, former, or current smoker), and chemotherapy receipt (yes or no). Physical measures of body habitus included from treatment planning scans were separation distance from medial and lateral tangential beam entry (continuously measured in centimeters) and breast volume (continuously measured in cubic centimeters). Radiation treatment technique characteristics that were included were the use of a supraclavicular field (with or without additional regional fields) for radiotherapy (yes/no), fractionation (conventional fractionation v hypofractionation, defined as using a dose per fraction of 2.5 Gy or larger), and use of boost (yes/no).
Analytic Approach
We first described the outcomes of interest separately for patients treated with conventional fractionation and those treated with hypofractionation, given prior work suggesting that these two groups had substantially different rates of acute toxicity. Multilevel multivariable logistic regression models separately identified the patient-level individual and treatment characteristics associated with (1) breast pain, (2) a bother scale (related to itching, stinging/burning, swelling, or hurting of the treated breast), and (3) fatigue. Patients were clustered within institution, with institution associated as a random effect and whether the institution teaches residents/medical students as the sole institution-level covariable. Given that the use of a supraclavicular field was almost exclusive to patients receiving conventional fractionation, we constructed multivariable models of each of the three outcomes separately for conventionally fractionated cases and hypofractionated cases after excluding 15 hypofractionated cases in which a supraclavicular field was used. P values ≤ 5% were considered significant, and all analyses were conducted using the SAS System, version 9.4 (Cary, NC).
RESULTS
The characteristics of the study sample are reported for 4,268 conventionally fractionated and 4,443 hypofractionated cases in Table 1. Of patients receiving hypofractionation, 82.5% were White and 14.3% were Black; of patients receiving conventional fractionation, 77.0% were White and 19.1% were Black. Chemotherapy was known to have been received by 15.9% of those receiving hypofractionation and 44.3% of those receiving conventional fractionation.
TABLE 1.
Sample Description: Stratified by Fractionation Scheme
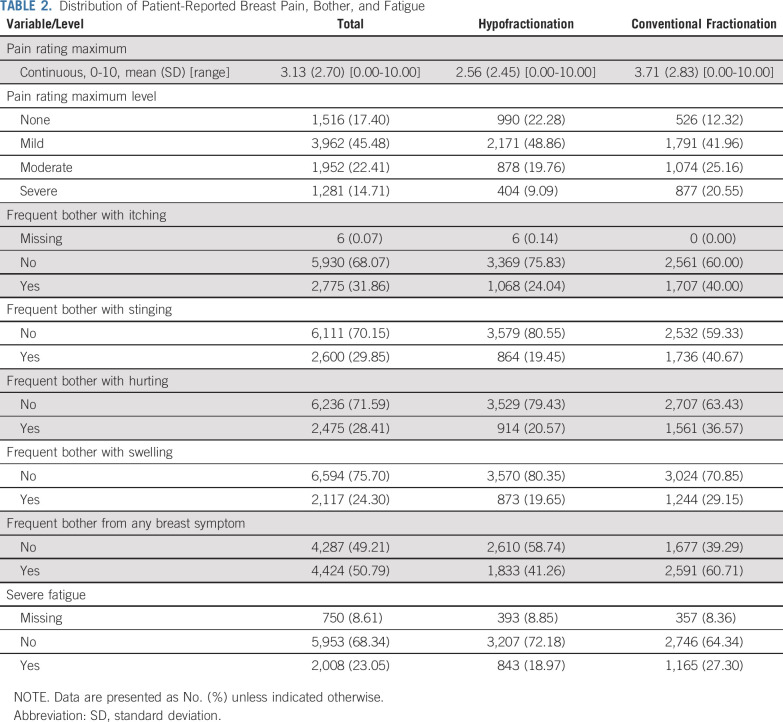
Table 2 lists the frequencies of the three main outcomes (and breakdown of the bother subitems) by fractionation. Moderate or severe breast pain was reported by 3,233 (37.1%): 1,282 (28.9%) of those receiving hypofractionation and 1,951 (45.7%) of those receiving conventional fractionation. Frequent bother from at least one breast symptom was reported by 4,424 (50.8%): 1,833 (41.3%) after hypofractionation and 2,591 (60.7%) after conventional fractionation. Severe fatigue was reported by 2,008 (23.1%): 843 (19.0%) after hypofractionation and 1,165 (27.3%) after conventional fractionation.
TABLE 2.
Distribution of Patient-Reported Breast Pain, Bother, and Fatigue
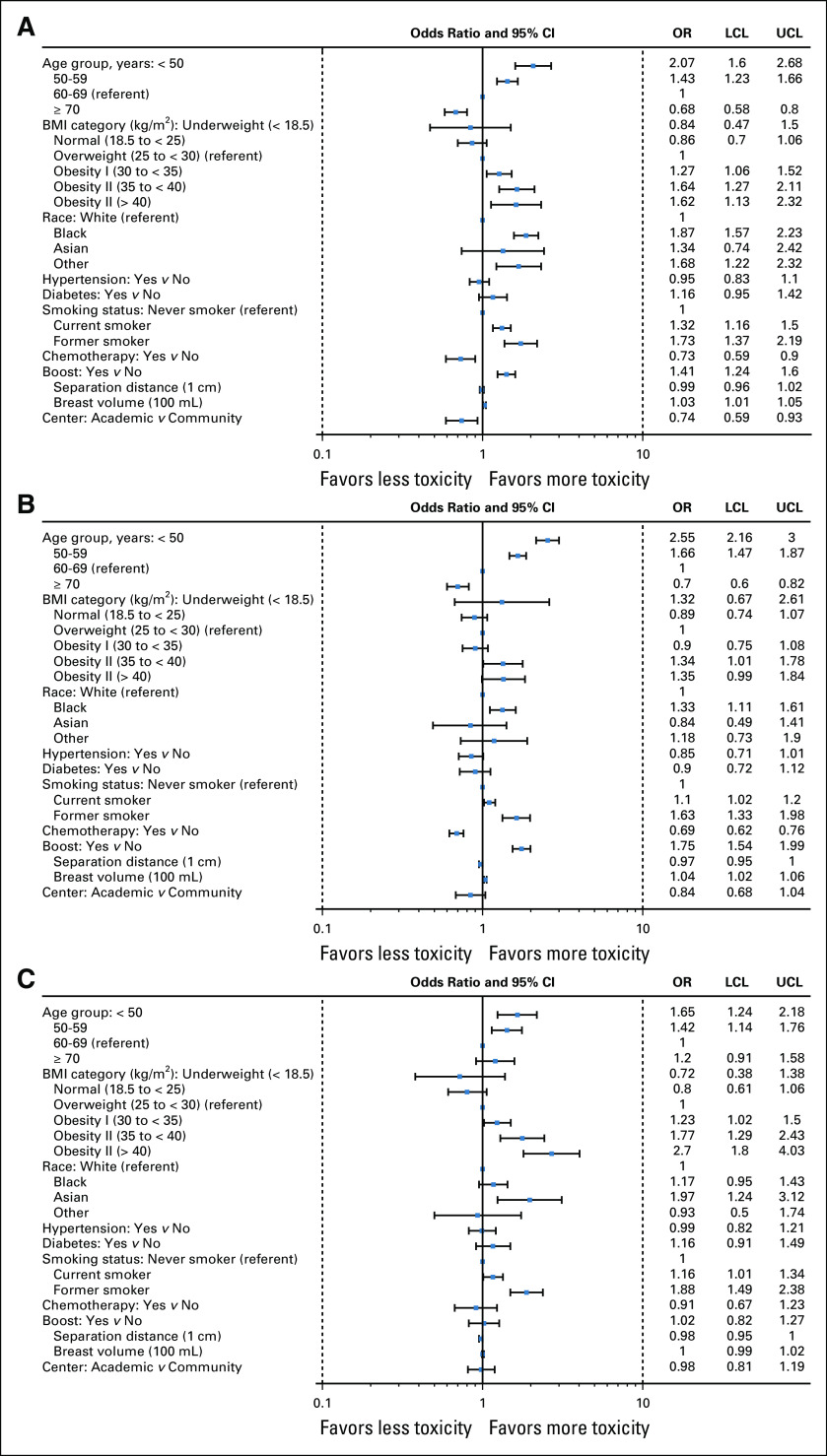
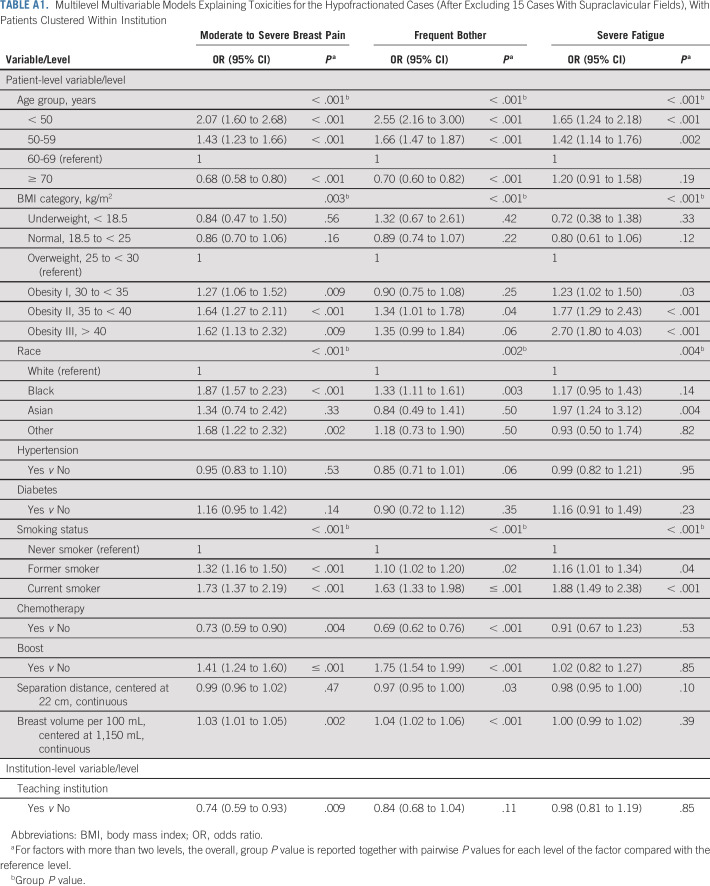
Figure 1 presents in its three panels the results of the multivariable models of the three outcomes of interest among patients receiving hypofractionated radiotherapy; detailed model results are presented in Appendix Table A1 (online only). Patient-level factors independently and significantly associated with moderate or severe breast pain were younger age (P < .001), higher BMI (P < .001), Black (P < .001) or other race (P = .002), former or current smoking status (P < .001), and larger breast volume (P = .002). Lack of receipt of chemotherapy (P = .004) and receipt of boost treatment (P < .001) also predicted breast pain. Treatment at a teaching center (P = .009) predicted less breast pain. Factors independently and significantly associated with frequent bother from breast symptoms were younger age (P < .001), higher BMI (P < .001), Black race (P = .002), former or current smoking status (P < .001), breast volume (P < .001), and separation distance (P = .04). Lack of receipt of chemotherapy (P < .001) and receipt of boost treatment (P < .001) also predicted bother from breast symptoms. Factors independently and significantly associated with severe fatigue were younger age (P < .001), higher BMI (P < .001), Asian race (P = .004), and former or current smoking status (P < .001). Treatment and dosimetric parameters were not independently associated with fatigue among patients treated with hypofractionation.
FIG 1.
Results of three multivariable models of patient-reported acute toxicity experiences among 4,428 patients treated with hypofractionated whole-breast radiation therapy (and without supraclavicular fields). (A) Moderate or severe breast pain. (B) Frequent bother from breast symptoms. (C) Severe fatigue. BMI, body mass index; CL, confidence limit; LCL, Lower Confidence Limit; OR, odds ratio; UCL, Upper Confidence Limit.
Figure 2 presents in its three panels the results of the multivariable models of the three outcomes of interest among patients receiving conventionally fractionated radiotherapy detailed model results are presented in Appendix Table A2 (online only). Factors independently and significantly associated with moderate or severe breast pain were younger age (P < .001), higher BMI (P = .003), Black (P < .001) or other race (P = .002), diabetes (P = .001), current or former smoking status (P < .001), and larger breast volume (P < .001). Factors independently and significantly associated with frequent bother from breast symptoms were younger age (P < .001), Black (P = .003) or other race (P = .004), hypertension (P < .001), diabetes (P < .001), current or former smoking status (P < .001), breast volume (P < .001), and separation distance (P = .01). Lack of receipt of chemotherapy (P = .007) and use of a supraclavicular field (P = .006) but not boost also predicted bother from breast symptoms among patients receiving conventional fractionation. Factors independently and significantly associated with severe fatigue were younger age (P < .001), higher BMI (P = .02), diabetes (P = .003), former smoking status (P = .006), and separation distance (P = .011). Treatment and dosimetric parameters were not significantly independently associated with fatigue among patients treated with conventional fractionation. Figure 3 presents the frequencies of the three main outcomes by race for conventional and hypofractionated cases, respectively.
FIG 2.
Results of three multivariable models of patient-reported acute toxicity experiences among 4,268 patients treated with conventionally fractionated whole-breast radiation therapy (either with or without supraclavicular fields). (A) Moderate or severe breast pain. (B) Frequent bother from breast symptoms. (C) Severe fatigue. BMI, body mass index; CL, confidence limit; iSCV, Irradiated Supraclavicular Lymph Nodes; LCL, Lower Confidence Limit; OR, odds ratio; UCL, Upper Confidence Limit.
FIG 3.
Frequency of patient-reported acute toxicity after breast radiotherapy, by fractionation and race.
DISCUSSION
This large multicenter study quantifies patient-reported experiences of pain, bother from breast symptoms, and fatigue within 7 days of completing modern whole-breast radiotherapy for breast cancer. This information is critically important to inform patients who desire realistic information about the likelihood of acute treatment-related toxicity in their own individual circumstances. Several important insights about patient and treatment factors associated with toxicity emerged. Not only did outcomes differ by radiotherapy approach, including dose fractionation, boost treatment, and regional nodal irradiation, but acute toxicity also varied by body habitus, age, race, smoking behavior, and comorbidities, with most factors consistent regardless of which fractionation schedule was used.
Radiotherapy approaches that were associated with higher acute toxicity included conventional fractionation, boost radiotherapy among those receiving hypofractionation, and regional nodal irradiation among those receiving conventional fractionation. The substantial differences observed according to fractionation approach are consistent with the findings of smaller prior reports and provide additional support for efforts to ensure that all women for whom evidence exists to support the use of moderate hypofractionation are provided this option for treatment.14,15 The observation that toxicity was higher in patients who received boost radiotherapy, but only among those receiving hypofractionated whole-breast radiotherapy, is intriguing. This may reflect a true causal impact of boost treatment in increasing toxicity in that setting, although it is also possible that the additional week of observation in patients receiving boost dose drives the difference observed, if acute toxicity tends to manifest primarily after 3 weeks, as is plausible given the transit time of the basal layer of the epidermis. Additional research, including evaluation of patient experiences soon after completion of radiotherapy, as is now being collected on a standard basis by the MROQC, will be important in developing a more definitive understanding of this observation. For now, this observation suggests that efforts to delineate which patients derive meaningful benefit from boost radiotherapy are important. Finally, the observation of increased bother from breast symptoms among patients who received regional nodal irradiation (who in this analytic data set were all treated with conventional fractionation) suggests that discussion of acute toxicity is relevant to include when guiding patients for whom the indications for regional nodal irradiation are ambiguous. It also heightens the need for enrollment in trials such as NRG B-5116 and NCIC MA-39,17 which seek to identify patients in whom regional nodal irradiation can safely be omitted, either because of excellent response to neoadjuvant therapy in the case of B-51 or because of inherently favorable biologic features in the case of MA-39.
Several patient characteristics also correlated with the patient-reported acute toxicity outcomes measured here. One risk factor was larger body habitus (as measured by BMI and by dosimetric parameters of separation distance and breast volume). The higher rates of bother from breast symptoms and pain in these patients likely relate to skin and soft tissue reactions that develop in these patients. These reactions result from the physical properties of megavoltage beams used for modern radiotherapy administration that make obtaining dose homogeneity more challenging in larger patients, together with the “auto-bolus effect,” which increases skin dose in skin folds, where there may also be additional damage because of friction. The association of larger habitus with fatigue is intriguing; research should investigate whether a greater volume of irradiated tissue leads to higher levels of inflammatory cytokine release and helps identify targets for prevention and management of treatment-related fatigue. These findings should also motivate ongoing research to identify best dosimetric practices and whether alternative techniques, including partial breast irradiation, which has recently been found to result in minimal if any differences in disease control,18-20 may be particularly useful in this patient population. They also suggest the importance of exploring how to improve supportive care, perhaps by including additional nursing visits beyond the routine once-per-week physician examinations standard in radiation oncology practice, to address the substantial symptoms these patients experience.
Other groups of patients who may benefit from greater supportive care as they undergo radiation after breast-conserving surgery are those of a younger age and Black race. Whether the increased rates of toxicity reported in these patient groups reflect inherent biologic or socially constructed socioeconomic differences of the patients themselves or differences in provider and/or patient behavior merits additional attention. There is some reason to believe that biologic differences may explain some of the differences observed according to race; prior studies have suggested that the frequency of genes involved in inflammation, wound repair, and fibrotic response to radiation vary by race, although these studies have focused primarily on genes related to late toxicity.21,22 There is also reason to fear that social differences may explain other aspects of the differences observed, especially if patients who are Black or younger have, for example, less secure finances to acquire supportive medications and topical therapies.
Differences in provider behavior may also play a role in explaining the race-related differences observed herein. A litany of worrisome studies have shown providers to be less sensitive to the pain of Black patients and less likely to prescribe pain medication to them.23-31 One recent study revealed that “false beliefs about biological differences between blacks and whites (e.g., ‘black people’s skin is thicker than white people’s skin’)”32(p 4296) were endorsed not only by White laypersons but also by one half of a sample of White medical students and residents. Moreover, those who endorsed the false beliefs were found to demonstrate racial bias in the accuracy of their pain treatment recommendations.32 Additional research is necessary to determine the extent to which differences detected in the current study reflect differences in provider beliefs and behaviors and how best to mitigate bias in care delivery. In any case, this study substantially advances the understanding of how race does indeed seem to relate to the experience of acute toxicity of whole-breast radiotherapy, given that prior research has been limited to much smaller cohorts yielding mixed findings.33,34 Of great importance, however, is that these findings should not be taken as a reason to dissuade Blacks from receiving breast conservation as an approach to breast cancer management; rather, they should motivate efforts to optimize supportive care.
In addition, observations of higher toxicity among smokers and those with the comorbidities of hypertension and diabetes also merit note. Whether these might be modifiable by smoking cessation or by tighter medical management of the underlying comorbidities are important questions for additional research. Intensifying supportive management is also important for these patient groups.
Finally, the observation that pain among patients treated with hypofractionation was lower when they received treatment at a teaching facility merits additional investigation. Future research should seek to identify which aspects of care, such as differences in treatment planning or toxicity management, at the academic institutions in this study might explain why patients treated in those settings had less pain. In this way, best practices to minimize pain could be generalized.
Although this study has the strengths of prospectively collecting patient-reported outcomes from a large number of individuals in a multicenter setting reflecting modern real-world practice in the United States, it also has several limitations. First, as in any observational study, associations cannot be taken to imply causation. Unmeasured confounding factors may exist. Second, although a high proportion of all eligible patients treated during the study period participated, selection effects may also have biased our findings. Third, because virtually no patients received regional nodal irradiation in combination with hypofractionation, we were unable to evaluate whether supraclavicular fields increase toxicity among patients treated with hypofractionation. Fourth, all data in the current study reflect patient self-report and may differ from physician-reported toxicity; although some consider patient-reported outcomes to be the gold standard, some might consider such data to be subjective.35 We nevertheless believe that the patient’s perspective provides irreplaceable information that other patients would value as a reference for what they might expect to experience. Fifth, differences in pain or fatigue among patient groups may have existed before radiation. Finally, we lacked information on when symptoms subsided.
In this large observational study of patient-reported toxicities after whole-breast radiotherapy, substantial differences existed not only according to radiotherapy dose fractionation but also according to a number of other patient personal and treatment characteristics. Of particular concern, race-related differences in breast pain and bother existed despite controlling for multiple other factors, including age, body habitus, comorbidities, and treatment characteristics. Additional research is needed to understand the factors that drive these and other differences detected in the current study, to target those that are potentially modifiable. Intensification of supportive care may also be appropriate for subgroups identified as being vulnerable to greater toxicity. Clinical trials must recruit diverse patients to ensure that they adequately capture toxicity experiences.
Supplementary Material
ACKNOWLEDGMENT
We acknowledge the helpful, formal discussion of the preliminary findings of this work at the 2019 San Antonio Breast Cancer Symposium by Karen Hoffman, MD, MHSc, MPH, Lori Pierce, of MD Anderson Cancer Center.
Appendix
TABLE A1.
Multilevel Multivariable Models Explaining Toxicities for the Hypofractionated Cases (After Excluding 15 Cases With Supraclavicular Fields), With Patients Clustered Within Institution
TABLE A2.
Multilevel Multivariable Models Explaining Toxicities for the Conventionally Fractionated Cases, With Patients Clustered Within Institution
SUPPORT
Supported by Blue Cross Blue Shield of Michigan and the Blue Care Network of Michigan as part of the Blue Cross Blue Shield of Michigan Value Partnerships Program.
AUTHOR CONTRIBUTIONS
Conception and design: Reshma Jagsi, Kent A. Griffith, Frank Vicini, Jacob Burmeister, Michael Dominello, Inga Grills, Jeffrey D. Radawski, Eleanor Walker
Financial support: Reshma Jagsi
Administrative support: Reshma Jagsi
Provision of study material or patients: Reshma Jagsi, Jeffrey D. Radawski, Lori Pierce
Collection and assembly of data: Reshma Jagsi, Frank Vicini, Inga Grills, James A. Hayman, Eleanor Walker, Lori Pierce
Data analysis and interpretation: Reshma Jagsi, Kent A. Griffith, Frank Vicini, Thomas Boike, Jacob Burmeister, Michael Dominello, Inga Grills, James A. Hayman, Jean M. Moran, Peter Paximadis, Eleanor Walker, Lori Pierce
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Toward Improving Patients’ Experiences of Acute Toxicity From Breast Radiotherapy: Insights From the Analysis of Patient-Reported Outcomes in a Large Multicenter Cohort
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Reshma Jagsi
Employment: University of Michigan
Stock and Other Ownership Interests: Equity Quotient
Consulting or Advisory Role: Amgen, Vizient
Research Funding: AbbVie (Inst)
Expert Testimony: Baptist Health/Dressman Benziger Lavalle Law
Travel, Accommodations, Expenses: Amgen
Other Relationship: JAMA Oncology Editorial Board
Open Payments Link: https://openpaymentsdata.cms.gov/physician/373670/summary
Frank Vicini
Employment: 21st Century Oncology
Consulting or Advisory Role: ImpediMed
Travel, Accommodations, Expenses: PreludeDX-DCISionRT, Concure Oncology
Thomas Boike
Employment: MHP Radiation Oncology
Jacob Burmeister
Research Funding: Varian Medical Systems, Novocure
Jean M. Moran
Research Funding: Varian Medical Systems
Patents, Royalties, Other Intellectual Property: Patent pending
Travel, Accommodations, Expenses: Sun Nuclear Corporation
Peter Paximadis
Travel, Accommodations, Expenses: Zeiss
Lori Pierce
Stock and Other Ownership Interests: PFS Genomics
Patents, Royalties, Other Intellectual Property: UpToDate, PFS Genomics
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1250431/summary
REFERENCES
- 1.Darby S, McGale P, Correa C, et al. : Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378:1707-1716, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jagsi R, Griffith KA, Boike TP, et al. : Differences in the acute toxic effects of breast radiotherapy by fractionation schedule: Comparative analysis of physician-assessed and patient-reported outcomes in a large multicenter cohort. JAMA Oncol 1:918-930, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Bellon JR, Blitzblau R, et al. : Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol 8:145-152, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Shaitelman SF, Schlembach PJ, Arzu I, et al. : Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: A randomized clinical trial. JAMA Oncol 1:931-941, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaverdian N, Wang X, Hegde JV, et al. : The patient’s perspective on breast radiotherapy: Initial fears and expectations versus reality. Cancer 124:1673-1681, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Gillan C, Abrams D, Harnett N, et al. : Fears and misperceptions of radiation therapy: Sources and impact on decision-making and anxiety. J Cancer Educ 29:289-295, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Nold RJ, Beamer RL, Helmer SD, et al. : Factors influencing a woman’s choice to undergo breast-conserving surgery versus modified radical mastectomy. Am J Surg 180:413-418, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Hammick M, Tutt A, Tait DM: Knowledge and perception regarding radiotherapy and radiation in patients receiving radiotherapy: A qualitative study. Eur J Cancer Care (Engl) 7:103-112, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Shaverdian N, Yeboa DN, Gardner L, et al. : Nationwide survey of patients’ perspectives regarding their radiation and multidisciplinary cancer treatment experiences. J Oncol Pract 15:e1010-e1017, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Lam E, Yee C, Wong G, et al. : A systematic review and meta-analysis of clinician-reported versus patient-reported outcomes of radiation dermatitis. Breast 50:125-134, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran JM, Feng M, Benedetti LA, et al. : Development of a model web-based system to support a statewide quality consortium in radiation oncology. Pract Radiat Oncol 7:e205-e213, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Cleeland CS, Ryan KM: Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore 23:129-138, 1994. https://www.ncbi.nlm.nih.gov/pubmed/8080219 [PubMed]
- 13.Chren M-M, Lasek RJ, Sahay AP, et al. : Measurement properties of Skindex-16: A brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg 5:105-110, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Laucis AM, Jagsi R, Griffith KA, et al. : The role of facility variation on racial disparities in use of hypofractionated whole breast radiation therapy. Int J Radiat Oncol Biol Phys 107:949-958, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Jagsi R: Hypofractionated whole breast radiotherapy: Adapting to the evidence. JAMA Oncol 1:144-145, 2015 [DOI] [PubMed] [Google Scholar]
- 16.ClinicalTrials.gov:A Randomized Phase III Clinical Trial Evaluating Post-Mastectomy Chestwall and Regional Nodal XRT and Post-Lumpectomy Regional Nodal XRT in Patients With Positive Axillary Nodes Before Neoadjuvant Chemotherapy Who Convert to Pathologically Negative Axillary Nodes After Neoadjuvant Chemotherapy. ClinicalTrials.gov identifier: NCT01872975. Updated August 26, 2019. https://clinicaltrials.gov/ct2/show/NCT01872975
- 17.ClinicalTrials.gov: TAILOR RT. A randomized trial of regional radiotherapy in biomarker low risk node positive breast cancer. ClinicalTrials.gov identifier: NCT03488693. https://clinicaltrials.gov/ct2/show/NCT03488693
- 18.Coles CE, Griffin CL, Kirby AM, et al. : Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-Year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 390:1048-1060, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vicini FA, Cecchini RS, White JR, et al. : Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: A randomised, phase 3, equivalence trial. Lancet 394:2155-2164, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livi L, Meattini I, Marrazzo L, et al. : Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-Year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer 51:451-463, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Alam A, Mukhopadhyay ND, Ning Y, et al. : A preliminary study on racial differences in HMOX1, NFE2L2, and TGFβ1 gene polymorphisms and radiation-induced late normal tissue toxicity. Int J Radiat Oncol Biol Phys 93:436-443, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossberg AJ, Lei X, Xu T, et al. : Association of transforming growth factor β polymorphism C-509T with radiation-induced fibrosis among patients with early-stage breast cancer: A secondary analysis of a randomized clinical trial. JAMA Oncol 4:1751-1757, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson KO, Green CR, Payne R: Racial and ethnic disparities in pain: Causes and consequences of unequal care. J Pain 10:1187-1204, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Bonham VL: Race, ethnicity, and pain treatment: Striving to understand the causes and solutions to the disparities in pain treatment. J Law Med Ethics 29:52-68, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Cintron A, Morrison RS: Pain and race in the United States: A systematic review. J Palliat Med 9:1454-1473, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Cleeland CS, Gonin R, Baez L, et al. : Pain and treatment of pain in minority patients with cancer. The Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Ann Intern Med 127:813-816, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Freeman HP, Payne R: Racial injustice in health care. N Engl J Med 342:1045-1047, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Green CR, Anderson KO, Baker TA, et al. : The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Med 4:277-294, 2003. [Erratum: Pain Med 6:99, 2005] [DOI] [PubMed] [Google Scholar]
- 29.Shavers VL, Bakos A, Sheppard VB: Race, ethnicity, and pain among the U.S. adult population. J Health Care Poor Underserved 21:177-220, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Institute of Medicine . Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: The National Academies Press, 2003. doi:10.17226/12875 [PubMed] [Google Scholar]
- 31.Todd KH, Deaton C, D’Adamo AP, et al. : Race and analgesic practice. Ann Emerg Med 35:11-16, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Hoffman KM, Trawalter S, Axt JR, et al. : Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci USA 113:4296-4301, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright JL, Takita C, Reis IM, et al. : Prospective evaluation of radiation-induced skin toxicity in a race/ethnically diverse breast cancer population. Cancer Med 5:454-464, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright JL, Takita C, Reis IM, et al. : Racial variations in radiation-induced skin toxicity severity: Data from a prospective cohort receiving postmastectomy radiation. Int J Radiat Oncol Biol Phys 90:335-343, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Hamilton DF, Giesinger JM, Giesinger K: It is merely subjective opinion that patient-reported outcome measures are not objective tools. Bone Joint Res 6:665-666, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]