Abstract
Nanomaterials possess superior advantages due to their special geometries, higher surface area, and unique mechanical, optical, and physicochemical properties. Their characteristics make them great contributors to the development of many technological and industrial sectors. Therefore, novel nanomaterials have an increasing interest in many research areas including biomedicine such as chronic inflammations, disease detection, drug delivery, and infections treatment. Their relevant role is, in many cases, associated with an effective catalytic application, either as a pure catalyst (acting as a nanozyme) or as a support for catalytically active materials (forming nanobiocatalysts). In this review, we analyze the construction of nanozymes and nanobiocatalyst by different existing forms of nanomaterials including carbon-based nanomaterials, metal-based nanomaterials, and polymer-based nanocomposites. Then, we examine successful examples of such nanomaterials employed in biomedical research. The role played by nanomaterials in catalytic applications is analyzed to identify possible research directions toward the development of the field and the achievement of real practicability.
Graphical Abstract
Keywords: Enzymes, Biocatalysis, Enzyme immobilization, Enzyme mimicking, Artificial enzymes, Nanomaterials
Introduction
Nanotechnology has played a fundamental role in the development of many industry sectors and science. The importance of nanomaterials –which are described as materials with at least one dimension below 100 nm—is based on their unique structural, chemical, optical, and mechanical properties [1]. In this manner, nanomaterials have offered great opportunities in different fields including energy, environmental science, food safety, transportation, electronics, and medicine [2]. Moreover, nanomaterials have been extensively used for the immobilization of a vast array of enzymes, and thus, overcome stability issues [3]. In addition, some nanomaterials termed “nanozymes” or “artificial enzymes” exhibit catalytic activities than those presented in natural enzymes but possess higher stability, ease of fabrication and storage, and better performances [4].
Nanobiocatalysts have emerged due to the innovative interaction between nanotechnology and biotechnology [1, 5]. This synergistic interaction occurs due to the superior physicochemical properties of nanomaterials and the remarkable catalytic activity of enzymes. In this manner, nanobiocatalysts might revolutionize the biomedical field due to the improved enzyme stability, efficiency, performance, and function [6].
Another nanomaterial-based approach is the construction of nanozymes, which are nanostructured artificial enzymes able to mimic the catalytic activity of natural enzymes [4, 7]. The great potential of nanozymes is associated with their higher catalytic stability, lower manufacturing/storage costs, and ease of modification in comparison to their natural counterparts [4].
In biomedical research, outstanding performances have been documented by advanced nano-systems, specifically by nanobiocatalysts and nanozymes. Some noteworthy applications include effective therapeutical effects on inflammatory diseases and cancer, innovative constructions of drug delivery systems, and designs for non-invasive clinical diagnosis, among others [8, 9].
There has been significant progress in understanding the mechanism of various diseases and how to treat them. For instance, cancer cells can adapt and survive allowing them to multiply; usually, barriers at the tissue and cellular level become a challenge for therapies. In this manner, nanomaterials as drug delivery tools can overcome such barriers. Furthermore, they can interact with enzymes or multiple targets to improve the therapeutic efficacy of current treatments since nanomaterials possess several advantages such as minimizing toxic side effects, resistance to chemotherapy, biocompatibility, selectivity, and antibacterial, antifungal, and antiviral properties, among others [10, 11].
In addition, new nanomedicine approaches have been studied to find strategies to treat Alzheimer's disease, which is a neurodegenerative condition that involves a combination of processes that ultimately lead to loss of synaptic integrity and effective neuronal connectivity. Studies have highlighted different pathological features such as amyloid β (Aβ) protein accumulation and deposition and neurofibrillary tangles (NFTs) in neurons resulting from over-phosphorylation of Tau protein together with processes of inflammation and oxidative stress [12, 13]. Nanomaterials increase the possibility of synergetic therapeutics, effective targeting, and diagnostic capabilities because both nanobiocatalysts and nanozymes have multimodal functionality throughout the brain [14].
On the other hand, inflammation has also been studied in recent years; it can be associated with several diseases, including pneumonia, rheumatoid arthritis, systemic lupus erythematosus, Crohn's disease, psoriasis, atherosclerosis, ischemic heart disease, and cancer [15]. Nanomaterials can regulate the expression of proinflammatory and anti-inflammatory molecules and target inflammatory sensors or macrophages through phagocytosis. Nanostructures have been designed as useful nanocarriers for diagnosis, therapy, monitoring, and balancing of inflammation immune homeostasis. The nanomaterials used as nano-vehicles are able to degrade and release active compounds in response to an inflammatory stimulus, such as reduced pH or a high level of reactive oxygen species; or even the nano-vehicle itself may possess intrinsic anti-inflammatory properties. Recently, research has focused on evading systemic clearance by the reticuloendothelial system (RES) or on improving tissue biodistribution by targeting inflammatory cell receptors [15, 16].
Diagnosis is another typical goal when using nanomaterials in the biomedical field. In this regard, researchers have evaluated the efficiency of biosensor platforms in which bimetallic nanomaterials have shown great potential to improve both the sensitivity and selectivity of electrochemical glucose biosensors. Furthermore, glucose oxidase (GOx) has been widely used with excellent results in terms of sensitivity and selectivity when integrated into nanostructured enzyme-based glucose sensors. Nowadays, the application of novel and non-toxic nanomaterials is vital for the control of diabetes [17].
In this review, we analyze the nanomaterial role in biomedical research by first examining different nanomaterial constructs and the immobilization methods which are fundamental for the preparation of nanobiocatalysts. Furthermore, we discuss in detail the potential in biomedical research of the most recent developments of nanobiocatalysts and nanozymes to finally identify the current challenges and recommendations to achieve greater advances and practical applicability.
Nanomaterials for the Construction of Nanobiocatalysts and Nanozymes
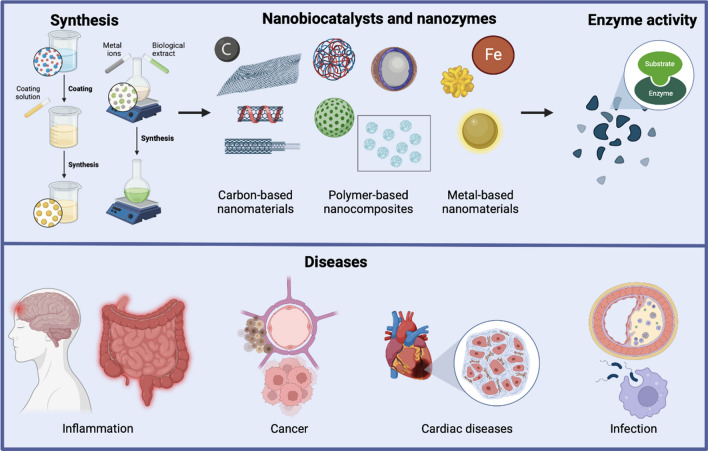
Materials in the nanoscale present different properties than those presented in the same material in their bulk form; typically, nanomaterials present superior properties and performances. In this manner, nanomaterials in different existing forms have been synthesized and evaluated in catalytic applications, showing great potential and excellent performances. Furthermore, nanomaterials behave differently in terms of their reactivity and other physicochemical properties, due to their higher surface area to volume ratio and extremely small size, which enables atoms/molecules to have a significant impact on material properties [18]. Carbon-based nanomaterials, metal-based nanomaterials, and polymer-based nanocomposites are some typical examples of nanostructured materials/hybrid materials forming nanobiocatalysts or acting as nanozymes. Moreover, the design of these nanomaterials represents a critical factor in the stability and optimal activity. In this manner, different nano-constructs can be formed such as hybrid nanoflowers, nanofibers, nanoporous substrates, nanotubes, magnetic nanoparticles, nanocomposites, and microspheres [6].
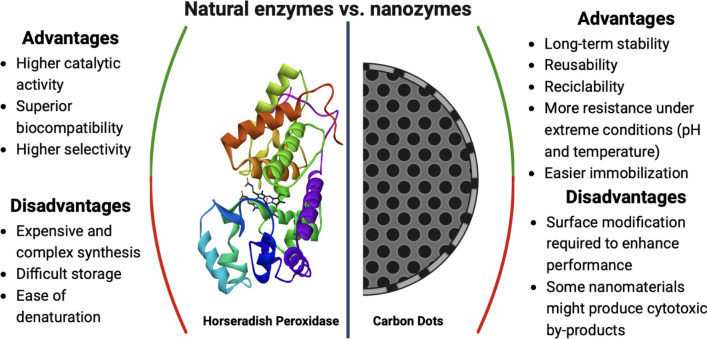
It is important to distinguish between nanobiocatalysts and nanozymes. Nanobiocatalysts, require the immobilization of enzymes to restrain enzyme molecules onto/within a support nanomaterial, and thus, enzymes can improve their activity and acquire other beneficial features [19]. In this manner, a nano-support constructed by an adequate protocol to achieve enhanced enzyme activity and reusability is highly desired in biocatalysis [20]. On the other hand, nanozymes are nanomaterials with intrinsic enzyme-like activities possessing advantageous features. Typically, natural enzymes are hard to purify, store, and recycle; in addition, they might easily lose their activity due to molecular denaturation or subunit depolymerization. In contrast, nanozymes are designed with better stability, high efficiency, the resistance to extreme conditions, and simple and low-cost production [21].
Carbon-Based Nanomaterials
Carbon nanomaterials have been reported effective in diverse catalytic applications, either as a pure catalyst or as a support for other active materials. For example, in biocatalytic applications, carbon materials are usually reported as nanozymes/enzyme-mimicking materials, or as supports for enzymes.
Carbon-based materials reported as nanozymes include carbon dots (CDs) and other similar materials like graphene quantum dots (GQDs). CDs can be defined as zero-dimensional materials with sizes generally below 10 nm; however, some studies report sizes of up to 60 nm [22, 23].
The properties of these materials are highly dependent on the precursor and the synthesis methods, which are grouped as bottom-up or top-down approaches [24]. Bottom-up methods are based on the polymerization and pyrolysis of molecular precursors, either conjugated or non-conjugated molecules. On the other hand, top-down methods are based on the fragmentation of bigger carbon materials, like carbon nanotubes or graphene sheets [23]. For cutting down these materials the most common techniques include chemical oxidation, arc discharge, laser treatment, or electrochemical methods [23, 25].
CDs have interesting characteristics like biocompatibility, easy surface functionalization, and fluorescence [26, 27]. Due to these characteristics, CDs have great potential in diverse applications like sensing, drug delivery, energy conversion and storage, and catalysis [28, 29].
Due to the nanozyme characteristics shown by CDs, with enzyme-like activity comparable to diverse enzymes, these materials have been recently evaluated in biocatalysis applications. For example, in a recent study, hemin@CDs hybrid nanozymes were evaluated for the peroxidase-like activity for sensing glucose, xanthine and H2O2 [30]. The authors employed 4-Aminoantipyrine (4-AAP) and phenol as substrates for the enzyme-like activity evaluation and reported that hemin@CDs catalyzed both in the presence of H2O2. The activity of hybrid nanozymes was much higher compared to hemin and CDs alone. This was probably because hemin acted as the active site; however, it has low water solubility. Interestingly, it significantly improved when combined with CDs, and thus, hemin dispersion increased and enhanced its activity. Also, this nanozyme was efficiently employed for the colorimetric detection of H2O2, xanthine, and glucose, with a low limit of detection of 0.11, 0.11, and 0.15 μM, respectively. The system was also useful for the fluorimetric detection of the same targets, with limits of detection of 0.15, 0.12, 0.15 μM, in the same order.
Another study showed CDs-based nanozyme with peroxidase-like activity used for the colorimetric detection of methylmercury (MeHg+) [31]. The authors employed a composite of noradrenaline-based CDs and Au nanoparticles (NA-CDs/AuNPs), and the peroxidase-like activity was evaluated using 3,3′,5,5′-tetramethylbenzidine (TMB) as substrate, in the presence of H2O2. The authors reported that the changes in the enzyme-like activity when MeHg+ was added corresponded to its degradation by the nanozyme. The authors reported a detection limit of 0.06 μg/L, which demonstrated that NA-CDs/AuNPs could be employed as a sensitive colorimetric detection probe.
Even though CDs with peroxidase-like activity are more reported for biocatalytic applications, there are studies where CDs mimicked the activity of other enzymes, like oxidase. For instance, single-atomic Fe doped CDs (SA Fe-CDs) were evaluated as oxidase-mimicking materials and their application in colorimetric and fluorescent assays for phosphate ions sensing [32]. SA Fe-CDs showed superior oxidase-like activity for the degradation of TMB compared to non-doped CDs; thus, the active sites are attributed to the homogeneously distributed Fe species. The authors compared changes in the activity of SA Fe-CDs during the time and no significant variations were observed.
Nanozymes formed by Cu and Cl co-doped CDs (Cu,Cl-CDs) were prepared to determine their oxidase-like and peroxidase-like activity to further evaluate their application for the colorimetric sensing of hydroquinone and H2O2 [33]. For the oxidase-like activity evaluation, three substrates were employed, TMB, o-Phenylenediamine (OPD), and 2,2′-Azinobis (3-ethylbenzothiazoline-6-sulfonic Acid Ammonium Salt) (ABTS). For the peroxidase-like activity, only TMB was employed as the substrate. The results showed that “Cu,Cl-CDs” have both, oxidase-like and peroxidase-like activities. Cu sites played a crucial role in the catalytic reaction, as Cl-CDs were unable to transform the substrates in both evaluations. For the colorimetric detection of hydroquinone using “Cu,Cl-CDs” the detection limit was 0.08 µM.
Several studies have shown that pure CDs (non-doped) have low enzyme-like activities for most enzymes in comparison with doped CDs or other CDs-based hybrid materials. However, some studies report successful results with non-doped CDs as enzyme-mimicking materials. For example, a study evaluated the oxidase-like activity of citric acid-derived CDs (CA-CDs) and their application in a fluorescent sensor for cystine detection [34]. The oxidase-like activity was evaluated using different concentrations of cystine, and CA-CDs showed high oxidase-like activity and better affinity than the natural enzyme. Also, the CA-CDs-based fluorescent sensor could detect cystine with a limit of detection of 0.036 µM.
CDs and other nanozymes have gained much attention in recent studies due to their superior characteristics compared to natural enzymes, like higher stability, faster synthesis methods, and easier storage (Fig. 1). These materials have shown great potential, with catalytic activity comparable to enzymes but showing higher effective life. However, it is still needed to optimize their synthesis parameters to reduce the use of metallic nanoparticles or doping processes to achieve enzyme-like activity levels similar to natural enzymes through simpler processes.
Fig. 1.
Advantages, disadvantages, and examples of natural enzymes and nanozymes
Metal-Based Nanomaterials
Magnetic nanoparticles (MNP) are widely investigated as support materials for enzyme immobilization due to their attractive properties such as large surface area, high enzyme loading, less toxicity, surface modification, recyclability, and uses in multiple cycles. In general, they are created by a magnetic core surrounded by a polymer shell, which should be non-toxic and eco-friendly as well [35].
MNP utilization for enzyme immobilization led to an increase in the yield in terms of biocatalysis, based on its high stability and recyclability. Several functional groups such as phosphate, thiolate, carboxylate, and amino groups are attached to metal nanoparticles and are responsible for the regular interactions with the enzyme. The co-factors are relevant to create a proper interaction with metal nanoparticles and allowing a high rate of immobilization [6].
Among the most reported materials are iron oxides (Fe3O4), alloy based (FePt, and CoPt3), pure metal (Fe and Co), and spinel-type ferromagnet (MgFe2O4, MnFe2O4, and CoFe2O4). Frequently the use of magnetite iron oxide nanoparticles (Fe3O4) has been influenced by their low toxicity and simple surface functionalization, which make it possible to control their morphology, shape, and size; important features in their applications. There is a large number of fabrication methods for Fe3O4 including thermal decomposition, co-precipitation, microemulsion, laser pyrolysis, electrochemical deposition, sonochemical, microwave method, etc. Moreover, the route of synthesis depends on the acquired shape of the nanomaterials [20].
For example, Touqeer et al., prepared iron oxide nanoparticles by solvothermal method; nanoparticles were functionalized with polydopamine (PDA) to facilitate the lipase immobilization for the production of biodiesel from waste cooking oil by enzymatic transesterification. The activity of the composite (Fe3O4-PDA-lipase) was not affected after the first four cycles and retained 25% of its activity after seven cycles. The nanoparticles were characterized by Fourier Transfer Infrared (FTIR), spectroscopy, X-ray diffraction (XRD) and transmission electron microscopy (TEM). Whereas the formation of the biodiesel was analyzed by FTIR and gas chromatography [36].
Cellulases are enzymes that convert lignocellulosic biomass into fermentable sugars for further ethanol production. The immobilization of these enzymes is gaining interest because of their stability and reusability while simultaneously improving fuel production. Kaur et al. reported the use of cellulases from A. fumigatus immobilized on magnetic nanoparticles by using a glutaraldehyde cross-linker. The immobilized enzyme retained 56.8% of its maximal activity after 6 h and 52.67% of rice straw saccharification efficiency. Electron microscopy and spectroscopy were used to confirm the immobilization [37].
Papain is a protease obtained from papaya latex and exhibits highly hydrolytic activity on proteins and peptides. This enzyme is very susceptible to denaturalization which means lower activity in an industrial process. The immobilization of papain on porous Fe3O4/SF nanoparticles has increased its catalytic efficiency. Nanoparticles were synthesized by a solvothermal procedure, and the crystal products were characterized by XRD. In addition, the enzyme can be separated from the solution using an external magnetic field and 70% of its activity was retained after 8 cycles [38].
Enzyme immobilization with applications in the pharmaceutical industry has gained relevance due to its biocompatibility, and easy recovery. Del Arco et al. described the functional and structural characterization of adenine phosphoribosyltransferase 2 from Thermus thermophilus HB8. The enzyme was immobilized onto glutaraldehyde-activated MagReSyn®Amine magnetic iron oxide porous microparticles for the synthesis of 6-aminopurine nucleosides-5´-monophosphate analogs. According to the catalyst experiments at pH 8.5 and 10.0, the activity recovery was 52% and 44% [39].
In literature, several research groups have implemented immobilized enzymes in cascade systems which consist of two or more stages to produce the target compounds. These sorts of systems try to mimic the biological systems at molecular, cellular, and in the whole organism. However, there is a need for optimized systems in terms of scalability and industrial applicability [40].
Giannakopoulou et al., reported the preparation of a novel magnetic four-enzyme nanobiocatalyst by the co-immobilization of cellulase, β-glucosidase (bgl), glucose oxidase (GOx), and horseradish peroxidase (HRP) onto the surface of amino-functionalized magnetic nanoparticles (MNPs) employing glutaraldehyde. The co-immobilized enzymes retained up to 50% of their activity after 5 reaction cycles at 50 °C and remained active even after 24 days of incubation at 5 °C. The authors characterized the four-enzyme magnetic nanobiocatalyst by FTIR and X-ray Photoelectron Spectroscopy (XPS) [41].
Polymer-Based Nanocomposites
Composite materials are a type of tailored materials composed of two or more elements that can include polymeric, ceramic, or metallic materials, and any combination of them. In that same logic, nanocomposite materials are composite materials, with the addition of having at least one of their dimensions between 1 and 100 nm [42–44].
Nanocomposite materials are mainly composed of at least a matrix and a reinforcement or filler, which improve the properties of the matrix for certain applications such as physicochemical properties, mechanical properties, electrical properties, optical, and many more, depending on what conditions need to be met [45].
Based on the nature of the materials used as both matrix and reinforcement, there are countless options as to which combinations can be made. For example, hydrogels with nanoparticles as reinforcement, nanorod-reinforced nanofibers, and quantum dot-enhanced thin films, among others [46, 47].
These combinations of materials for the conformation of nanocomposites, have been proposed and applied recently as catalysts in the biomedical field, due to the possibility of synthesizing biocompatible, biodegradable, and non-toxic nanocomposites which form part of the larger group of nanomaterials known as nanocatalysts [48].
Nanobiocatalysts are formed by a support material holding enzymes, thus, they can be considered nanocomposite materials which usually use a metallic, ceramic, polymeric, and/or carbon-based matrix for the immobilization of the desired enzymes. These nanocomposites act as supports for the enzyme to reach the corresponding substrate without losing its activity or denaturalizing [6].
There is an increasing interest in the usage of biopolymeric materials as enzyme immobilizers, due to their tunability, biocompatibility, and biodegradability, as the properties of main importance in the biomedical and biotechnological fields, while also adding the enzymatic activity for applications such as bioremediation, wound debridement, personal care products, food-related applications, and many more, depending on the nature of the enzyme [49–51].
Due to their physicochemical properties such as biocompatibility and biodegradability, biopolymers have drawn much attention for biomedical applications in drug delivery and tissue engineering fields [52]. However, it is possible to improve the properties of chitosan, collagen, gelatin, and many other biopolymers by blending/reinforcing them with other materials to obtain polymeric-based composites. Such composite materials have tunable properties that make them more efficient than using each biopolymer on its own, from mechanical, to thermal, optical, and many more properties than can be enhanced [53].
Recent research has been performed to modify the properties of chitosan and other biopolymeric-base composite materials for a wide range of applications such as drug delivery, biomarking, wound healing, and tissue engineering [54]. Some examples of chitosan-based composites for wound healing include freeze-dried, hydrophilic hydrogels with antibacterial properties and no toxicity for their application as wound dressings [55]. Also, the addition of nanoparticles, such as silver nanoparticles, to the polymeric matrix of chitosan and gelatin has shown to improve antibacterial properties and stability of the nanocomposite, demonstrating to be an effective alternative to treat skin pathogens and allowing for proper wound healing. Furthermore, the morphological studies of the porosity for these sponges showed that as the addition of silver nanoparticles increased, so did the porosity of the material once chemically cross-linked with tannic acid which can be impactful in the water absorption of the material and thus its wound healing efficiency [56].
Gelatin-based hydrogels have also been combined with carbon-based materials such as graphene oxide for the elaboration of biocompatible hydrogels with collagenase for tissue engineering applications. In the work done by Piao and Chen [57], rheology studies showed the improvement in mechanical properties of the hydrogels as the concentration of gelatin increased, due to the higher amounts of cross-links between the polymer and the graphene oxide sheets.
Properties such as swelling due to water absorption, viscosity/ rheological behavior, porosity, and drug encapsulation/release kinetics are some other properties that are often analyzed for hydrogel/sponge-based nanocomposite materials, as well as the surface to volume ratio. Furthermore, whichever enzyme is added to these materials and its corresponding activity, can benefit from the rest of the properties of such materials, achieving a more complete multi-material construct that can tackle several challenges at once depending on the type of application [58].
Biopolymeric-based nanocomposites can also be given different morphologies such as microspheres, nanoparticles, and nanofibers. In this regard, nanofibrous beads from bacterial cellulose were successfully synthesized by using alginate as a template to control the bead formation, with the addition of lipase for its immobilization and potential application in the biomedical, pharmaceutical, and biocatalytic fields. The beads showed a good crystallinity index of 0.7 and high retention capacity of water. Another important test in this study was the bacterial cellulose nanofiber growth on the template surface, which increased as the incubation time of G. xylinus was increased up to 36 h, covering the whole surface of the bead with cellulose nanofibers [59].
In general, nanocomposite materials applied as nanobiocatalysts in the biomedical field through the immobilization/encapsulation of enzymes such as collagenase, lipase, gelatinase, and many more, are proving to be more efficient for drug delivery, tissue engineering, and wound healing applications as research moves forward towards a more nanotechnologically focused global market [60]. There is a wide array of possible combinations between polymeric matrices such as hydrogels, nanofibers, microspheres, and many other materials such as nanoparticles, enzymes, and pharmaceutical compounds, which makes nanobiocatalyst composite materials great systems for the biomedical field [61].
Enzyme Immobilization Methods in Nanomaterials
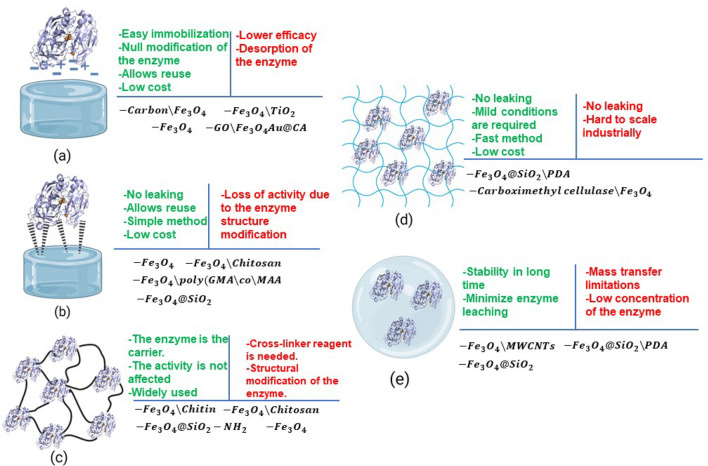
The application of enzymes as nanobiocatalyst is usually limited to some problems related to the implementation of the free form of the enzyme, including lack of long-term operation, stability, inability to recyclability, low scalability, and high production and separation costs [62, 63]. Thus, research regarding solving these limitations has been developed over the past 10 years, in which it has been established that the immobilization enzymes over nanomaterials might provide a proper matrix with enhanced properties, such as higher thermal, pH, and storage stability, reusability, and higher enzymatic activity [64, 65]. As depicted in Fig. 2, these immobilization methods exhibit various characteristics, advantages, and disadvantages.
Fig. 2.
Immobilization methods a physical adsorption, b covalent binding, c cross-link, d entrapment and e encapsulation; and its advantages (green), disadvantages (red) and magnetic materials implemented in literature for enzyme immobilization. Reprinted from [65] with permission from Elsevier
Adsorption
Immobilization of enzymes in nanomaterials by physical adsorption (Fig. 1a) is the simplest and fastest method. This method might be performed by weak ionic bonds (van de Waals forces) or physical adsorption (adsorbed in surface pores). Thus, the most common nanomaterials implemented for this application are cation and anion resins, activated charcoal, silica gel, alumina, controlled pore glass, ceramics, and carbon-based nanomaterials [65, 66]. For example, physical adsorption is the most attractive approach for enzyme immobilization into these nanomaterials with high surface area, since it maintains the native structure of the enzyme, which provides many advantages like convenient operation, low operation cost, and repeatable use [67]. However, physical adsorption might not be functional for long-term operation, since enzymes easily present desorption from the host matrix [68]. Another important advantage attributed to this method, is that no additional coupling agents and modification processes are required, instead of it, adsorption conditions are convenient for the preservation of the initial catalytic properties of the enzymes. Since the weak binding forces can be affected by changes in pH, temperature, or ionic strength, a leaching effect may be presented [66, 69].
Covalent Immobilization
The covalent immobilization method (Fig. 2b) is based on the chemical activation of functional groups in the support matrix and the subsequent reaction with functional groups of the enzyme [70]. The covalent process to immobilize enzymes into nanomaterials consists of two main stages: (1) Activation of nanomaterials’ surface by multifunctional reagents (e.g., glutaraldehyde, carbidiimide); and (2) formation of covalent bindings between the activated support and functional groups of the enzymes, usually amino, carboxylic, phenolic, sulfhydryl, thiol, imidazole, and hydroxyl groups [65, 71]. The formation of covalent bonds provides a strong binding effect between the enzymes and the host matrix, thus preventing the leakage of the enzyme from the nanomaterial and increment stability, which means that the enzyme has resistance to the effects of temperature, pH, and different ambient conditions. However, due to the modification of the structure of the enzyme when the covalent bond is formed, active sites of the enzyme might be deactivated, and the catalytic properties decrease [66]. Moreover, compared to the adsorption method, covalent immobilization requires longer incubation times and complex and harsh conditions [68, 71].
Cross-Linking Immobilization
The cross-linking immobilization method (Fig. 2c) is characterized by the implementation of bifunctional reagents to promote the self-immobilization of the enzymes by the formation of covalent cross-linkages named cross-linked enzyme aggregates (CLEAs) Commonly, glutaraldehyde, dialdehydes diiminoesters, diisocyanates, and diamines activated by carbodiimide, which are the most common bifunctional reagents used in cross-link immobilization, react with amine groups of the enzyme proteins to create a three-dimensional complex structure [65, 66]. Under this type of immobilization, the complex enzyme-support formed is resistant to the effects of pH and temperature, improving reusability. However, large quantities of the enzyme are required to obtain the CLEAs and the enzyme might lose activity after the immobilization [68].
Entrapment and Encapsulation
Entrapment (Fig. 2d) and encapsulation (Fig. 2e) are similar immobilization methods that consist in a two-stage process in which the enzyme is mixed with a monomer solution that will be processed for polymerization and the enzyme is finally confined/entrapped within the polymer network without chemical interactions [65]. Indeed, the encapsulation method can be considered a type of entrapment immobilization, with the difference that, in encapsulation, a semipermeable membrane is used to separate the immobilized particles from the external environment [69, 71]. These methods provide significant protection from the external environmental conditions to the enzymes, which enhance stability. Moreover, by these immobilization methods, the denaturation of the enzyme is considerably decreased [63]. However, some disadvantages of these methods are the mass transfer resistance limitations, which means that substrates have problems getting into the polymer matrix and leaking enzymes due to variable pore size [66, 70].
Role of Nanomaterials in Different Biomedical Applications
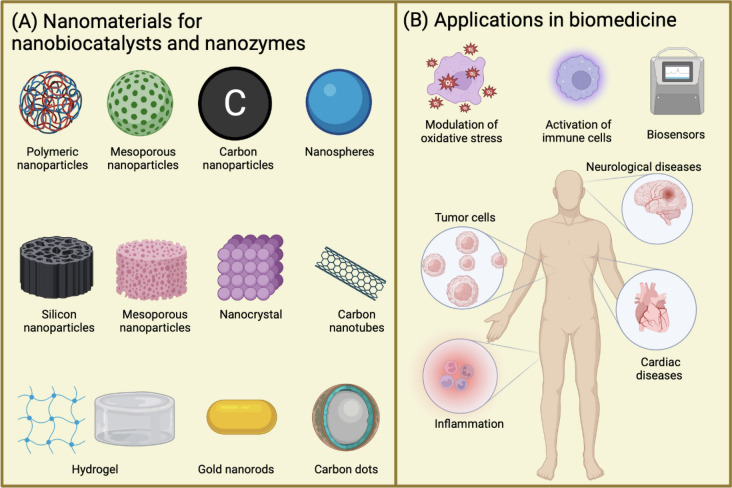
In the biomedical field, nanotechnology has tremendous ongoing research with outstanding achievements that suggest great potential for the next years. The fundamental role that nanomaterials are playing in biomedicine is related to their unique characteristics. For example, nanomaterials used as supports to immobilize enzymes provide more robust, stable, and efficient nanobiocatalysts since they possess higher surface area to volume ratio [3]. On the other hand, researchers have reported enzyme-mimicking activities in some nanomaterials. Such nanozymes or artificial enzymes present advantageous features in comparison to their natural counterparts including higher catalytic stability, ease of modification, and cost-efficiency [7]. In this manner, the development of nanobiocatalysts and nanozymes has contributed to great research advancements in different biomedical applications such as neuropathological treatments, immunity, bacterial resistance, alternative therapies for cancer, and others with enormous potential and future perspectives (Fig. 3) [4].
Fig. 3.
Nanomaterials in biomedicine. A Nanomaterials used for the immobilization of enzymes and/or used as nanozymes. B Applications of nanozymes and nanobiocatalysts the biomedical field
Nanobiocatalysts
Nanobiocatalysts are formed by the synergistic interaction of nanomaterials and enzymes. Enzymes are immobilized on materials at the nanoscale to obtain tailored properties like enhanced reusability, stability, and enzyme efficiency. The role of the nanomaterials is frequently related to their physicochemical properties such as larger surface areas, mechanical properties, special geometries, thermal properties, biocompatibility, or magnetic properties [3]. Nanobiocatalysts have fulfilled expectations in different fields including medical therapies or treatments. For example, nanobiocatalysts have shown multifaceted activities for antitumor detection and therapy due to increased stability in the tumor microenvironment.
Hu et al. constructed a synergistic system formed by liquid metal nanoparticles and glucose oxidase termed as “LM@GOX”. It was used for a combination therapy, using two therapeutic modes: starvation and photothermal for tumor therapy. Interestingly, the catalytic efficiency could be photo-controlled since glucose oxidase exhibited an enhanced enzymatic activity with increased temperatures, with optimal values in the range of 43–60 °C. During the photothermal therapy, it was observed several effects such as a decrease of adenosine triphosphate (ATP) and heat shock proteins levels, increase on enzymatic activity, accelerated blood flow, and relieved hypoxia situation in tumor tissue. Also, the authors mentioned that the acidic environment formed in the catalysis of glucose oxidase, could further degraded the liquid metal nanoparticles having great potential in biomedical applications [72].
In the immunology field, chronic inflammation has been extensively studied because it is mediated by multiple diseases. Currently, some drugs reduce and help mitigate inflammation; however, they are often not as effective due to absorption mechanisms in the body [73]. Polymeric nanoparticles loaded with drugs offer several favorable biological properties, such as biocompatibility and mucoadhesiveness [74]. For instance, Zhang et al. prepared a novel nanomaterial containing uricase (UOx) and catalase (CAT) into zeolitic imidazolate framework-8 (ZIF-8) coated with a neutrophil membrane to treat a type of inflammatory arthritis. Both in vitro and in vivo results showed an enzymatic system with high specificity and the potential to degrade up to 90% of uric acid; in addition, inflammation was reduced, and high therapeutic efficiency was achieved [75].
Another example of the employment of nanocomposites in biomedicine was reported by Ali et al. In their study, doxorubicin-conjugated graphene oxide and zinc ferrite hybrid nanocomposites (GO-ZnFe2O4/DOX) were presented as drug delivery systems with great potential for in-vitro theranostic applications. In addition, synthesized nanocomposites exhibited good performances for magnetic resonance imaging diagnosis. The results demonstrated a pH dependence in which the maximum release of the drug was under a pH value of 7.4. Finally, the studies are tested in HeLa cells with potential cytotoxicity results [76].
Nanobiocatalysts have been employed to design systems for the biodetection of different analytes and compounds since nanomaterials possess exceptional optical and electrochemical characteristics. As a representative example, He et al. encapsulated glucose oxidase and horseradish peroxidase in DNA nanoflowers for the detection of human papillomavirus (HPV). The nanoflowers were synthesized by a one-pot rolling circle amplification method. Then, they were captured as signaling molecules by sensitive hydrogel. HPV16 E6 and E7 oncogenes were used as targets DNA sequences, which caused the disruption of the crosslinking structure of the DNA hydrogel, releasing DNA nanoflowers encapsulating glucose oxidase and horseradish peroxidase, which subsequently catalyzed the oxidation of tetramethylbenzidine and glucose generating a significant electrical signal. The biosensor presented several advantages like affordability, sensitivity, and rapidity; biosensor required a detection time of 25 min, which is lower to other techniques analyzing the same target [77].
Furthermore, nanobiocatalysts can be used for the design of diverse biomaterials for clinical applications such as in infections replacing antibiotics. As a representative example of this application, Soares et al. prepared a hybrid nano-biomaterial composed of zinc oxide nanoparticles, chitosan support, and the immobilized papain. The nanoparticles were synthesized by the co-precipitation method and because of this design was obtained a nano-triangular structure with a size of 150 nm. The proteolytic activity of papain, in vitro analyses, demonstrated a decrease in the activation of phagocytic cells but did not induce toxicity. This innovative nano-biomaterial can be used for future treatments for wound healing and psoriasis [78]. Moreover, research on nanobiocatalysts aimed to assist in different applications related to nanomedicine are included in Table 1.
Table 1.
Recent advancements on nanobiocatalysts developed for different applications in biomedicine
| Nanomaterial | Immobilized-enzyme | Application | Main findings | Reference |
|---|---|---|---|---|
| Liquid metal nanoparticles | Glucose oxidase | Starvation/ photothermal therapy | A significant inhibition of the solid tumor growth under Near-Infrared Light | [72] |
| Poly (amide amine) (PAMAM) nanoparticles | Hyaluronidase | Biocatalyst and therapeutics | Appreciable thermostability, pH tolerance and enhanced enzyme performance | [79] |
| Electrospun polyvinyl alcohol/Chitosan nanofibers | Urease | Drug delivery systems | Preparation by electrospinning technique and removal of urea from artificial blood serum | [8] |
| Multi-walled carbon nanotubes | Transglutaminase | Hydrogel scaffolds designing | Immobilization efficiency, satisfactory reusability properties, biocompatible, and bioconjugation properties | [80] |
| Copper hybrid nanoflowers embedded with amine | Glucose oxidase | Antibacterial activity | Broad-spectrum antibacterial properties and possible use for the fabrication of antibacterial gauzes | [81] |
| Iron nanoparticles coated with graphene's layers supported on multi-walled carbon nanotubes | Catalase | Biosensor | Hybrid nano biomaterial presenting long-term stability and high sensitivity | [82] |
| Zinc oxide and silicon nanowires | Glucose oxidase | Glucose biosensor | High sensibility, long-term stability, and reproducibility. Compatible with microelectronics processing to future large-scale commercial production | [83] |
| Organic–inorganic hybrid nanoflower | Cholesterol oxidase | Cholesterol biosensor | Excellent selectivity, precision, and sensibility to detect diverse levels of cholesterol in human blood samples | [84] |
| Multi-walled carbon nanotubes | Microbial Transglutaminase | Application in hydrogel scaffolds design | Good cross-linking efficiency in gelatin hydrogel scaffold. Non-toxic and biocompatible strategy with good reusability. Potential for tissue engineering | [80] |
| Poly lactic-coglycolic acid particles and polyethylene glycol polymers with click nucleic acids (PEG-CNA-PLGA) | Cytosine deaminase | Drug delivery systems | Potential use as a co-delivery system for chemotherapy agents. It is an innovate strategy by the incorporation of click nucleic acids (CAN) | [85] |
Nanozymes
Due to the intrinsic enzyme-mimicking properties of some nanomaterials, they can substitute natural enzymes to overcome disadvantages such as high instability and costly manufacturing/storage. In this manner, nanozymes are suitable candidates for multiple applications in biomedicine showing great selectivity, sensitivity, cost-efficiency, stability, and ease of fabrication [4, 7].
In this context, catalytic cancer treatment using nanozymes have shown excellent potential since nanozymes do not only imitate the catalytic activity in biological reactions, but they can also develop their performance more efficiently than natural enzymes. In this manner, they have been widely studied and employed for the construction of innovative designs aimed to suppress tumor activity. In this respect, Zeng et al. reported the design of a PEGylated Cu-doped polypyrrol nanozyme (CuPP) through a facile synthesis using CuCl2 as an oxidizing catalyst. Interestingly, the prepared nanozyme exhibited catalytic activity of three different enzymes: catalase (CAT), peroxidase (POD), and glutathione peroxidase (GPx). The nanoenzyme was able to act following a photothermal strategy, inducing oxidative stress that interrupted redox homeostasis. The authors reported a highly efficient antitumor effect and active adaptative immune response that reprogrammed M1 and M2 macrophages [86].
On the other hand, reactive oxygen species (ROS) play an essential role in regulating various physiological functions of living organisms. However, excessive accumulation can promote neurodegenerative diseases due to neuroinflammation as an immune response. Ren et al. reported an alternative method by directing nanozymes to the mitochondrial of the microglial, and not to neurons as documented in previous studies. They intended to eliminate amyloid beta (Aβ) more efficiently using functionalized molybdenum disulfide quantum dots. The synthesized nanozyme can mimic the activity of superoxide dismutase (SOD) and CAT, showing a reduction in neurotoxicity and elimination Aβ aggregates in mice. Therefore, nanozyme could change the polarization of the microglia from the proinflammatory M1 phenotype to the anti-inflammatory M2 phenotype. In this manner, phagocytosis was activated, and the inflammation produced by M1 was mitigated [87].
Similarly, metabolic diseases like diabetes can also be treated with nanozymes. For instance, iron oxide nanoparticles (Fe3O4NPs) with peroxidase-like and catalase-like activity have displayed therapeutic effects on hyperinsulinemia and hyperglycemia. The study was performed using Drosophila as a genetic model and a high-sugar diet model for type 2 diabetes. The nanozyme stimulated the activity of adenosine 5′-monophosphate-activated protein kinase (AMPK) in the liver, muscle and fat tissues. In addition, it decreased blood sugar levels, and improves glucose and insulin tolerance in the leptin-deficient (ob/ob) diabetic animal model. Findings indicate that the peroxidase-like and catalase-like activities of the nanozyme generate physiological levels of lysosomal ROS for AMPK activation and increase the glucose uptake capacity in diverse metabolically active cells and insulin resistant cell models. The authors concluded that the prepared nanozyme exhibited great potential in diabetes treatment due to their multiple beneficial effects like AMPK stimulation, insulin-resistance enhancement, and glycemic control [88]
In addition, nanozymes can also be used in biosensors providing great selectivity and sensitivity through simple techniques. Su et al., developed a nanozyme formed by carbon dots co-doped with Co and N elements. Nanozymes exhibited peroxidase-like enzymatic activity in a neutral environment in addition of their fluorescence properties. This novel system had a multi-mode approach (radiometric fluorescence imaging and colorimetric) to detect cholesterol and xanthine in both solution and human serum samples. Among the identified advantages of the system is included a reduced detection time, ease of operation, and improved analytical performance [89]. Different nanozymes have been developed and successfully employed for diverse biomedical applications (Table 2).
Table 2.
Recent advancements on nanozymes employed for different applications in biomedicine
| Nanozyme | Enzyme activity | Application | Main findings | Reference |
|---|---|---|---|---|
| Two-dimensional vanadium carbide Mxene nanoenzyme | SOD, CAT, POD, GPx, TPx, and HPO | Therapeutic effects on inflammatory and neurodegenerative diseases | Cytoprotection against oxidative stress and high biocompatibility | [9] |
| Selenium-polydopamine nanozyme | SOD and CAT | Therapy in Alzheimer's disease | Significant antioxidant activity, neuroprotection effects, and enhance cognitive ability | [90] |
| Platinum loaded CoSn(OH)6 nanocubes | POD | Detection of dopamine | Appreciable thermostability, excellent stability and sensitive colorimetric platform | [91] |
| Melanin-like nanoparticles | Oxidase | Therapeutic effects | Nanocarriers, antioxidant activity, and potential therapy option for COVID-19 | [92] |
| Chitosan-Modified Fe3O4 Nanozymes | POD, CAT, and SOD | Antibacterial effect | Stimulated the production of ROS in drug-sensitive and drug-resistant bacteria | [93] |
| Carbon dots confined in N-doped carbon | POD | Detection of cancer | Potential non-invasively clinical diagnosis and early-stage detection | [94] |
| Bimetallic phosphide nanoparticle | POD and GPX | Cancer therapy | Activate the immune response, good biocompatibility, and significant oxidative damage of the cancer cells | [95] |
SOD Superoxide dismutase, CAT catalase, POD peroxidase, GPx glutathione peroxidase, TPx thiol peroxidase, HPO haloperoxidase, ROS reactive oxygen species
Current Challenges and Recommendations
Nowadays, nanotechnology has been a critical factor in the progress and development of many research areas including biomedicine. In this manner, nanostructured materials have shown excellent potential either as nanozymes or as nano-supports for enzymes. The special geometries, small particle sizes, and high surface-to-volume ratio, among other unique properties presented in nanomaterials, have been associated with their outstanding performances. Despite the advancements achieved in understanding their role, there are still some challenges to overcome.
Nanobiocatalysts formed by the employment of nanomaterials to immobilize enzymes usually improve catalytic activity and other features like reusability and recyclability; however, there is a need to deeply understand the interaction enzyme-nanomaterial and to propose adequate and sustainable protocols to optimize the catalytic activity and reusability [20]. Some of the challenges faced by nanozymes applied in biomedicine are associated with the fact that the catalytic mechanism of many nanomaterials with enzyme-like activity has not been fully understood. In addition, the development of sustainable synthesis methods and the control of parameters are required to maximize the production yield and enhance performance. For instance, the proposal of biological strategies with superior results in comparison to classical synthesis methods will represent benefits in terms of cost-effectiveness and eco-friendliness [18].
Research on the application of nanomaterials for specific applications either in therapy or diagnosis should continue to improve through the design of innovative, effective, and non-toxic nano-systems [17]. Innovative strategies should be explored. For instance, 3D printing techniques for enzyme immobilization could produce customized, flexible, and complex structures/geometries at low cost [96]. 3D printing strategies have been applied for the printing of nanomaterials aimed to assist in biomedical applications such as tissue regeneration [97]. In this manner, further research might be directed toward the customized synthesis of nanomaterials with enzyme-like activities and nanomaterials with specific geometries or structures able to maximize the enzyme load and catalytic activity.
Conclusion
Nanotechnology has revolutionized many technological and industrial sectors due to the many advantages and tunable performances of materials at the nanoscale. For instance, the higher surface area to volume ratio is fundamental in applications related to the immobilization of enzymes, since it allows higher enzyme load and thus higher catalytic activity. In this manner, nanobiocatalysts with higher stability, better performances, and ease of recovery can be constructed. Moreover, some novel nanomaterials can mimic the catalytic functions of natural enzymes; such nanozymes possess advantages like higher stability, superior catalytic activity, and low-cost fabrication. Thus, nanomaterials have contributed significantly to the development of different fields including biomedical research. Engineered nanomaterials are considerably contributing to modern biomedicine from different perspectives and applications such as bioimaging, drug delivery, biosensing, and so on. In this review, the role played by nanomaterials either as nanozymes or nanobiocatalysts has been analyzed by exploring their most recent advances for biomedical applications. Nanobiocatalysts have demonstrated outstanding potential with versatile applications; however, further efforts are still needed to understand in detail the nanomaterial-enzyme interaction. Similarly, nanozymes have shown obvious advantages over natural enzymes; however, some challenges need to be addressed in terms of biosafety and a deep understanding of the relationship between the catalytic activity and the structure of nanozymes.
Acknowledgements
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT) and Tecnologico de Monterrey, Mexico under Sistema Nacional de Investigadores (SNI) program awarded to Roberto Parra-Saldívar (CVU: 35753).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Angel M. Villalba-Rodríguez and Lidia Yaritza Martínez Zamudio contributed equally as First author.
Contributor Information
Reyna Berenice González-González, Email: reyna.g@tec.mx.
Roberto Parra-Saldívar, Email: r.parra@tec.mx.
References
- 1.Araújo RG, González-González RB, Martinez-Ruiz M, et al. Expanding the scope of nanobiocatalysis and nanosensing: applications of nanomaterial constructs. ACS Omega. 2022;7:32863–32876. doi: 10.1021/acsomega.2c03155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muhammad ID. A comparative study of research and development related to nanotechnology in Egypt, Nigeria and South Africa. Technol Soc. 2022 doi: 10.1016/j.techsoc.2022.101888. [DOI] [Google Scholar]
- 3.Fazal Nawaz A, Zafar S, Laraib Fatim S, et al (2021) Use of nanomaterials for the immobilization of industrially important enzymes. J Nanotechnol Res. 10.26502/jnr.2688-85210023
- 4.Lopez-Cantu DO, González-González RB, Sharma A, et al. Bioactive material-based nanozymes with multifunctional attributes for biomedicine: Expanding antioxidant therapeutics for neuroprotection, cancer, and anti-inflammatory pathologies. Coord Chem Rev. 2022;469:214685. doi: 10.1016/j.ccr.2022.214685. [DOI] [Google Scholar]
- 5.de Jesús R-A, Mancera-Andrade EI, Patiño MBG, et al. Nanobiocatalysis: nanostructured materials—a minireview. Biocatalysis. 2016;2:1–24. doi: 10.1515/boca-2016-0001. [DOI] [Google Scholar]
- 6.Reshmy R, Philip E, Sirohi R, et al. Nanobiocatalysts: advancements and applications in enzyme technology. Bioresour Technol. 2021;337:125491. doi: 10.1016/j.biortech.2021.125491. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Cantu DO, González-González RB, Melchor-Martínez EM, et al. Enzyme-mimicking capacities of carbon-dots nanozymes: properties, catalytic mechanism, and applications—a review. Int J Biol Macromol. 2022;194:676–687. doi: 10.1016/j.ijbiomac.2021.11.112. [DOI] [PubMed] [Google Scholar]
- 8.Kutlu N, İspirli Doğaç Y, Deveci İ, Teke M. Urease immobilized electrospun PVA/chitosan nanofibers with improved stability and reusability characteristics: an application for removal of urea from artificial blood serum. Prep Biochem Biotechnol. 2020;50:425–437. doi: 10.1080/10826068.2019.1679175. [DOI] [PubMed] [Google Scholar]
- 9.Feng W, Han X, Hu H, et al. 2D vanadium carbide MXenzyme to alleviate ROS-mediated inflammatory and neurodegenerative diseases. Nat Commun. 2021 doi: 10.1038/s41467-021-22278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan MI, Zahra Q ul A, Batool F, et al. Trends in Nanotechnology to improve therapeutic efficacy across special structures. OpenNano. 2022;7:100049. doi: 10.1016/J.ONANO.2022.100049. [DOI] [Google Scholar]
- 11.Khan MI, Batool F, Ali R, et al. Tailoring radiotherapies and nanotechnology for targeted treatment of solid tumors. Coord Chem Rev. 2022;472:214757. doi: 10.1016/J.CCR.2022.214757. [DOI] [Google Scholar]
- 12.Zeng H, Qi Y, Zhang Z, et al. Nanomaterials toward the treatment of Alzheimer’s disease: recent advances and future trends. Chin Chem Lett. 2021;32:1857–1868. doi: 10.1016/J.CCLET.2021.01.014. [DOI] [Google Scholar]
- 13.Carneiro P, Morais S, Pereira MC. Nanomaterials towards biosensing of Alzheimer’s disease biomarkers. Nanomaterials. 2019;9:1–23. doi: 10.3390/nano9121663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripathi P, Shukla P, Bieberich E. Theranostic applications of nanomaterials in Alzheimer’s disease: a multifunctional approach. Curr Pharm Des. 2022;28:116–132. doi: 10.2174/1381612827666211122153946. [DOI] [PubMed] [Google Scholar]
- 15.Jin K, Luo Z, Zhang B, Pang Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B. 2018;8:23–33. doi: 10.1016/J.APSB.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan H, Shao D, Lao YH, et al. Engineering Cell Membrane-Based Nanotherapeutics to Target Inflammation. Adv Sci. 2019;6:1900605. doi: 10.1002/advs.201900605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannan P, Maduraiveeran G. Bimetallic nanomaterials-based electrochemical biosensor platforms for clinical applications. Micromachines (Basel) 2022;13:76. doi: 10.3390/mi13010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalil AT, Iqbal J, Shah A, et al. The bio–nano interface as an emerging trend in assembling multi-functional metal nanoparticles. Nanoscience. 2021;7:1–24. doi: 10.1039/9781839163791-00001. [DOI] [Google Scholar]
- 19.Gkantzou E, Chatzikonstantinou AV, Fotiadou R, et al. Trends in the development of innovative nanobiocatalysts and their application in biocatalytic transformations. Biotechnol Adv. 2021;51:107738. doi: 10.1016/J.BIOTECHADV.2021.107738. [DOI] [PubMed] [Google Scholar]
- 20.Bilal M, Iqbal HMN, Adil SF, et al. Surface-coated magnetic nanostructured materials for robust bio-catalysis and biomedical applications-a review. J Adv Res. 2022;38:157–177. doi: 10.1016/J.JARE.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang R, Yan X, Fan K. Nanozymes inspired by natural enzymes. Acc Mater Res. 2021;2:534–547. doi: 10.1021/accountsmr.1c00074. [DOI] [Google Scholar]
- 22.Liu ML, Chen B, Li CM, Huang CZ. Carbon dots: synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019;21:449–471. doi: 10.1039/C8GC02736F. [DOI] [Google Scholar]
- 23.Sagbas S, Sahiner N. Carbon dots: preparation, properties, and application. Nanocarb Compos. 2019 doi: 10.1016/B978-0-08-102509-3.00022-5. [DOI] [Google Scholar]
- 24.Ge G, Li L, Wang D, et al. Carbon dots: synthesis, properties and biomedical applications. J Mater Chem B. 2021;9:6553–6575. doi: 10.1039/D1TB01077H. [DOI] [PubMed] [Google Scholar]
- 25.Döring A, Ushakova E, Rogach AL. Chiral carbon dots: synthesis, optical properties, and emerging applications. Light. 2022;11(1):1–23. doi: 10.1038/s41377-022-00764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozhukil-Valappil M, Pillai V, Alwarappan S. Spotlighting graphene quantum dots and beyond: synthesis, properties and sensing applications. Appl Mater Today. 2017;9:350–371. doi: 10.1016/J.APMT.2017.09.002. [DOI] [Google Scholar]
- 27.Tabish TA, Zhang S. Graphene quantum dots: syntheses, properties, and biological applications. Comprehen Nanosci Nanotechnol. 2016;1–5:171–192. doi: 10.1016/B978-0-12-803581-8.04133-3. [DOI] [Google Scholar]
- 28.Wang B, Lu S. The light of carbon dots: from mechanism to applications. Matter. 2022;5:110–149. doi: 10.1016/J.MATT.2021.10.016. [DOI] [Google Scholar]
- 29.Kang Z, Lee ST. Carbon dots: advances in nanocarbon applications. Nanoscale. 2019;11:19214–19224. doi: 10.1039/C9NR05647E. [DOI] [PubMed] [Google Scholar]
- 30.Su L, Cai Y, Wang L, et al. Hemin@carbon dot hybrid nanozymes with peroxidase mimicking properties for dual (colorimetric and fluorometric) sensing of hydrogen peroxide, glucose and xanthine. Microchim Acta. 2020;187:1–11. doi: 10.1007/S00604-019-4103-4/TABLES/3. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Li H, Li K, et al. Specific colorimetric detection of methylmercury based on peroxidase-like activity regulation of carbon dots/Au NPs nanozyme. J Hazard Mater. 2023;441:129919. doi: 10.1016/J.JHAZMAT.2022.129919. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Ding S, Lyu Z, et al. Single-atomic iron doped carbon dots with both photoluminescence and oxidase-like activity. Small. 2022;18:2203001. doi: 10.1002/SMLL.202203001. [DOI] [PubMed] [Google Scholar]
- 33.Zhao N, Song J, Zhao L. Metallic deep eutectic solvents-assisted synthesis of Cu, Cl-doped carbon dots as oxidase-like and peroxidase-like nanozyme for colorimetric assay of hydroquinone and H2O2. Colloids Surf A. 2022;648:129390. doi: 10.1016/J.COLSURFA.2022.129390. [DOI] [Google Scholar]
- 34.Lin Z, Zeng Q, Deng Q, et al. Citric acid-derived carbon dots as excellent cysteine oxidase mimics for cysteine sensing. Sens Actuators B Chem. 2022;359:131563. doi: 10.1016/J.SNB.2022.131563. [DOI] [Google Scholar]
- 35.Bilal M, Iqbal HMN. Chemical, physical, and biological coordination: an interplay between materials and enzymes as potential platforms for immobilization. Coord Chem Rev. 2019;388:1–23. doi: 10.1016/j.ccr.2019.02.024. [DOI] [Google Scholar]
- 36.Touqeer T, Mumtaz MW, Mukhtar H, et al. Fe3O4-PDA-lipase as surface functionalized nano biocatalyst for the production of biodiesel using waste cooking oil as feedstock: characterization and process optimization. Energies (Basel) 2020;13:177. doi: 10.3390/en13010177. [DOI] [Google Scholar]
- 37.Kaur P, Sachdeva Taggar M, Kalia A. Characterization of magnetic nanoparticle-immobilized cellulases for enzymatic saccharification of rice straw. Biomass Convers Biorefin. 2021;11:955–969. doi: 10.1007/s13399-020-00628-x/Published. [DOI] [Google Scholar]
- 38.Sheng W, Xi Y, Zhang L, et al. Enhanced activity and stability of papain by covalent immobilization on porous magnetic nanoparticles. Int J Biol Macromol. 2018;114:143–148. doi: 10.1016/j.ijbiomac.2018.03.088. [DOI] [PubMed] [Google Scholar]
- 39.del Arco J, Pérez E, Naitow H, et al. Structural and functional characterization of thermostable biocatalysts for the synthesis of 6-aminopurine nucleoside-5″-monophospate analogues. Bioresour Technol. 2019;276:244–252. doi: 10.1016/j.biortech.2018.12.120. [DOI] [PubMed] [Google Scholar]
- 40.Lau ECHT, Yiu HHP, et al. Chapter 11: enzyme immobilization on magnetic nanoparticle supports for enhanced separation and recycling of catalysts. In: Castro GR, Nadda AK, Nguyen TA, et al., editors. Nanomaterials for biocatalysis. Elsevier; 2022. pp. 301–321. [Google Scholar]
- 41.Giannakopoulou A, Patila M, Spyrou K, et al. Development of a four-enzyme magnetic nanobiocatalyst for multi-step cascade reactions. Catalysts. 2019;9:995. doi: 10.3390/catal9120995. [DOI] [Google Scholar]
- 42.Pelegrino TM, Seabra BA. Chitosan-based nanomaterials for skin regeneration. AIMS Med Sci. 2017;4:352–381. doi: 10.3934/medsci.2017.3.352. [DOI] [Google Scholar]
- 43.Jacob J, Haponiuk JT, Thomas S, Gopi S. Biopolymer based nanomaterials in drug delivery systems: a review. Mater Today Chem. 2018;9:43–55. doi: 10.1016/j.mtchem.2018.05.002. [DOI] [Google Scholar]
- 44.Fu S, Sun Z, Huang P, et al. Some basic aspects of polymer nanocomposites: a critical review. Nano Mater Sci. 2019;1:2–30. doi: 10.1016/j.nanoms.2019.02.006. [DOI] [Google Scholar]
- 45.Giliopoulos D, Zamboulis A, Giannakoudakis D, et al. Polymer/metal organic framework (MOF) nanocomposites for biomedical applications. Molecules. 2020;25:185. doi: 10.3390/molecules25010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koosha M, Mirzadeh H, Shokrgozar MA, Farokhi M. Nanoclay-reinforced electrospun chitosan/PVA nanocomposite nanofibers for biomedical applications. RSC Adv. 2015;5:10479–10487. doi: 10.1039/C4RA13972K. [DOI] [Google Scholar]
- 47.Wahid F, Zhao XJ, Jia SR, et al. Nanocomposite hydrogels as multifunctional systems for biomedical applications: current state and perspectives. Compos B Eng. 2020;200:108208. doi: 10.1016/j.compositesb.2020.108208. [DOI] [Google Scholar]
- 48.Heidarizadeh M, Doustkhah E, Rostamnia S, et al. Dithiocarbamate to modify magnetic graphene oxide nanocomposite (Fe3O4-GO): a new strategy for covalent enzyme (lipase) immobilization to fabrication a new nanobiocatalyst for enzymatic hydrolysis of PNPD. Int J Biol Macromol. 2017;101:696–702. doi: 10.1016/j.ijbiomac.2017.03.152. [DOI] [PubMed] [Google Scholar]
- 49.Sunar K, Kumar U, Deshmukh SK. Chapter 12: recent applications of enzymes in personal care products. In: Dhillon GS, Kaur S, editors. Agro-industrial wastes as feedstock for enzyme production. San Diego: Academic Press; 2016. pp. 279–298. [Google Scholar]
- 50.Villalba-Rodríguez AM, Martínez-González S, Sosa-Hernández JE, et al. Nanoclay/polymer-based hydrogels and enzyme-loaded nanostructures for wound healing applications. Gels. 2021;7:59. doi: 10.3390/gels7020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohammadi ZB, Zhang F, Kharazmi MS, Jafari SM. Nano-biocatalysts for food applications; immobilized enzymes within different nanostructures. Crit Rev Food Sci Nutr. 2022 doi: 10.1080/10408398.2022.2092719. [DOI] [PubMed] [Google Scholar]
- 52.Kumar S, Sarita NM, et al. Recent advances and remaining challenges for polymeric nanocomposites in healthcare applications. Prog Polym Sci. 2018;80:1–38. doi: 10.1016/j.progpolymsci.2018.03.001. [DOI] [Google Scholar]
- 53.Notario B, Pinto J, Rodriguez-Perez MA. Nanoporous polymeric materials: a new class of materials with enhanced properties. Prog Mater Sci. 2016;78–79:93–139. doi: 10.1016/j.pmatsci.2016.02.002. [DOI] [Google Scholar]
- 54.Elgadir MA, Uddin MS, Ferdosh S, et al. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: a review. J Food Drug Anal. 2015;23:619–629. doi: 10.1016/j.jfda.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu S, Bi S, Yan D, et al. Preparation of composite hydroxybutyl chitosan sponge and its role in promoting wound healing. Carbohydr Polym. 2018;184:154–163. doi: 10.1016/j.carbpol.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 56.Ye H, Cheng J, Yu K. In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int J Biol Macromol. 2019;121:633–642. doi: 10.1016/j.ijbiomac.2018.10.056. [DOI] [PubMed] [Google Scholar]
- 57.Piao Y, Chen B. One-pot synthesis and characterization of reduced graphene oxide–gelatin nanocomposite hydrogels. RSC Adv. 2016;6:6171–6181. doi: 10.1039/C5RA20674J. [DOI] [Google Scholar]
- 58.Pathania D, Kumari S (2020) Nanocomposites based on biopolymer for biomedical and antibacterial applications. In: Adapting 2D nanomaterials for advanced applications. American Chemical Society, pp 375–391
- 59.Kim JH, Park S, Kim H, et al. Alginate/bacterial cellulose nanocomposite beads prepared using Gluconacetobacter xylinus and their application in lipase immobilization. Carbohydr Polym. 2017;157:137–145. doi: 10.1016/j.carbpol.2016.09.074. [DOI] [PubMed] [Google Scholar]
- 60.Sharma G, Thakur B, Naushad M, et al. Applications of nanocomposite hydrogels for biomedical engineering and environmental protection. Environ Chem Lett. 2018;16:113–146. doi: 10.1007/s10311-017-0671-x. [DOI] [Google Scholar]
- 61.Bilal M, Ashraf SS, Ferreira LFR, et al. Nanostructured materials as a host matrix to develop robust peroxidases-based nanobiocatalytic systems. Int J Biol Macromol. 2020;162:1906–1923. doi: 10.1016/j.ijbiomac.2020.08.122. [DOI] [PubMed] [Google Scholar]
- 62.Alvarado-Ramírez L, Rostro-Alanis M, Rodríguez-Rodríguez J, et al. Exploring current tendencies in techniques and materials for immobilization of laccases—a review. Int J Biol Macromol. 2021;181:683–696. doi: 10.1016/j.ijbiomac.2021.03.175. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen HH, Kim M. An overview of techniques in enzyme immobilization. Appl Sci Converg Technol. 2017;26:157–163. doi: 10.5757/asct.2017.26.6.157. [DOI] [Google Scholar]
- 64.Darwesh OM, Matter IA, Eida MF. Development of peroxidase enzyme immobilized magnetic nanoparticles for bioremediation of textile wastewater dye. J Environ Chem Eng. 2019;7:102805. doi: 10.1016/j.jece.2018.11.049. [DOI] [Google Scholar]
- 65.Martínez SAH, Melchor-Martínez EM, Hernández JAR, et al. Magnetic nanomaterials assisted nanobiocatalysis systems and their applications in biofuels production. Fuel. 2022 doi: 10.1016/j.fuel.2021.122927. [DOI] [Google Scholar]
- 66.Liu D-M, Chen J, Shi Y-P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC Trends Anal Chem. 2018;102:332–342. doi: 10.1016/j.trac.2018.03.011. [DOI] [Google Scholar]
- 67.Bilal M, Anh Nguyen T, Iqbal HMN. Multifunctional carbon nanotubes and their derived nano-constructs for enzyme immobilization—a paradigm shift in biocatalyst design. Coord Chem Rev. 2020;422:213475. doi: 10.1016/j.ccr.2020.213475. [DOI] [Google Scholar]
- 68.Zhou W, Zhang W, Cai Y. Laccase immobilization for water purification: a comprehensive review. Chem Eng J. 2021;403:126272. doi: 10.1016/j.cej.2020.126272. [DOI] [Google Scholar]
- 69.Rodríguez-Delgado MM, Alemán-Nava GS, Rodríguez-Delgado JM, et al. Laccase-based biosensors for detection of phenolic compounds. TrAC Trends Anal Chem. 2015;74:21–45. doi: 10.1016/j.trac.2015.05.008. [DOI] [Google Scholar]
- 70.Lyu X, Gonzalez R, Horton A, Li T. Immobilization of enzymes by polymeric materials. Catalysts. 2021;11:1211. doi: 10.3390/catal11101211. [DOI] [Google Scholar]
- 71.Somu P, Narayanasamy S, Gomez LA, et al. Immobilization of enzymes for bioremediation: a future remedial and mitigating strategy. Environ Res. 2022 doi: 10.1016/j.envres.2022.113411. [DOI] [PubMed] [Google Scholar]
- 72.Hu JJ, Liu MD, Gao F, et al. Photo-controlled liquid metal nanoparticle-enzyme for starvation/photothermal therapy of tumor by win-win cooperation. Biomaterials. 2019 doi: 10.1016/j.biomaterials.2019.119303. [DOI] [PubMed] [Google Scholar]
- 73.Pahwa R, Goyal A, Jialal I. Chronic inflammation. Pathobiol Hum Dis. 2022 doi: 10.1016/B978-0-12-386456-7.01808-6. [DOI] [Google Scholar]
- 74.Kost OA, Beznos OV, Davydova NG, et al. Superoxide dismutase 1 nanozyme for treatment of eye inflammation. Oxid Med Cell Longev. 2016 doi: 10.1155/2016/5194239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L, Zhang C, Zhuang ZN, et al. Bio-inspired nanoenzyme for metabolic reprogramming and anti-inflammatory treatment of hyperuricemia and gout. Sci China Chem. 2021;64:616–628. doi: 10.1007/s11426-020-9923-9. [DOI] [Google Scholar]
- 76.Ali R, Aziz MH, Gao S, et al. Graphene oxide/zinc ferrite nanocomposite loaded with doxorubicin as a potential theranostic mediu in cancer therapy and magnetic resonance imaging. Ceram Int. 2022;48:10741–10750. doi: 10.1016/j.ceramint.2021.12.290. [DOI] [Google Scholar]
- 77.He H, Cheng L, He Y, et al. Dual-enzyme cascade amplification electrochemical biosensor for human papillomavirus based on DNA nanoflower structure. Sens Actuators B Chem. 2022 doi: 10.1016/j.snb.2022.132532. [DOI] [Google Scholar]
- 78.Soares AMBF, Gonçalves LMO, Ferreira RDS, et al. Immobilization of papain enzyme on a hybrid support containing zinc oxide nanoparticles and chitosan for clinical applications. Carbohydr Polym. 2020;243:116498. doi: 10.1016/J.CARBPOL.2020.116498. [DOI] [PubMed] [Google Scholar]
- 79.Soozanipour A, Taheri-Kafrani A, Razmjou A, Asadnia M. Hyaluronidase enzyme conjugated polyamidoamine dendrimer: an efficient and stable nanobiocatalyst for enzymatic degradation of hyaluronic acid. J Mol Liq. 2022;349:118111. doi: 10.1016/J.MOLLIQ.2021.118111. [DOI] [Google Scholar]
- 80.Fatima SW, Barua S, Sardar M, Khare SK. Immobilization of Transglutaminase on multi-walled carbon nanotubes and its application as bioinspired hydrogel scaffolds. Int J Biol Macromol. 2020;163:1747–1758. doi: 10.1016/J.IJBIOMAC.2020.09.091. [DOI] [PubMed] [Google Scholar]
- 81.Lee I, Cheon HJ, Adhikari MD, et al. Glucose oxidase-copper hybrid nanoflowers embedded with magnetic nanoparticles as an effective antibacterial agent. Int J Biol Macromol. 2020;155:1520–1531. doi: 10.1016/J.IJBIOMAC.2019.11.129. [DOI] [PubMed] [Google Scholar]
- 82.Soto D, Alzate M, Gallego J, Orozco J. Hybrid nanomaterial/catalase-modified electrode for hydrogen peroxide sensing. J Electroanal Chem. 2021;880:114826. doi: 10.1016/J.JELECHEM.2020.114826. [DOI] [Google Scholar]
- 83.Miao F, Lu X, Tao B, et al. Glucose oxidase immobilization platform based on ZnO nanowires supported by silicon nanowires for glucose biosensing. Microelectron Eng. 2016;149:153–158. doi: 10.1016/j.mee.2015.10.011. [DOI] [Google Scholar]
- 84.Chung M, Jang YJ, Kim MI. Convenient colorimetric detection of cholesterol using multi-enzyme co-incorporated organic-inorganic hybrid nanoflowers. J Nanosci Nanotechnol. 2018;18:6555–6561. doi: 10.1166/jnn.2018.15697. [DOI] [PubMed] [Google Scholar]
- 85.Harguindey A, Roy S, Harris AW, et al. Click nucleic acid mediated loading of prodrug activating enzymes in PEG-PLGA nanoparticles for combination chemotherapy. Biomacromol. 2019;20:1683–1690. doi: 10.1021/acs.biomac.9b00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeng W, Yu M, Chen T, et al. Polypyrrole nanoenzymes as tumor microenvironment modulators to reprogram macrophage and potentiate immunotherapy. Adv Sci. 2022 doi: 10.1002/advs.202201703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ren C, Li D, Zhou Q, Hu X. Mitochondria-targeted TPP-MoS2 with dual enzyme activity provides efficient neuroprotection through M1/M2 microglial polarization in an Alzheimer’s disease model. Biomaterials. 2020 doi: 10.1016/j.biomaterials.2019.119752. [DOI] [PubMed] [Google Scholar]
- 88.Zhou Y, Liu C, Yu Y, et al. An organelle-specific nanozyme for diabetes care in genetically or diet-induced models. Adv Mater. 2020;32:2003708. doi: 10.1002/ADMA.202003708. [DOI] [PubMed] [Google Scholar]
- 89.Su L, Qin S, Cai Y, et al. Co, N-doped carbon dot nanozymes with acid pH-independence and substrate selectivity for biosensing and bioimaging. Sens Actuators B Chem. 2022 doi: 10.1016/j.snb.2021.131150. [DOI] [Google Scholar]
- 90.Gong Y, Huang A, Guo X, et al. Selenium-core nanozymes dynamically regulates Aβ & neuroinflammation circulation: augmenting repair of nervous damage. Chem Eng J. 2021;418:129345. doi: 10.1016/J.CEJ.2021.129345. [DOI] [Google Scholar]
- 91.Liu H, He YL, Li N, et al. Organic-inorganic composite nanorods as an excellent mimicking peroxidases for colorimetric detection and evaluation of antioxidant. ACS Appl Bio Mater. 2020;3:2499–2506. doi: 10.1021/acsabm.0c00198. [DOI] [PubMed] [Google Scholar]
- 92.Lou X-F, Wang C, Zhang J-C, et al. A melanin-like nanoenzyme for acute lung injury therapy via suppressing oxidative and endoplasmic reticulum stress response melanin-like nanoenzyme for acute lung injury therapy via suppressing oxidative and endoplasmic reticulum stress response. Pharmaceutics. 2021;13:1850. doi: 10.3390/pharmaceutics13111850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wenjun W, Ziman W, Peiru S, et al. Antibacterial effect of chitosan-modified Fe3O4 nanozymes on Acinetobacter baumannii. J Microbiol Biotechnol. 2022;32:263–267. doi: 10.4014/JMB.2107.07046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Z, Liu W, Ni P, et al. Carbon dots confined in N-doped carbon as peroxidase-like nanozyme for detection of gastric cancer relevant D-amino acids. Chem Eng J. 2022;428:131396. doi: 10.1016/J.CEJ.2021.131396. [DOI] [Google Scholar]
- 95.Zhao A, She J, Manoj D, et al. Functionalized graphene fiber modified by dual nanoenzyme: towards high-performance flexible nanohybrid microelectrode for electrochemical sensing in live cancer cells. Sens Actuators B Chem. 2020;310:127861. doi: 10.1016/J.SNB.2020.127861. [DOI] [Google Scholar]
- 96.Shao Y, Liao Z, Gao B, He B. Emerging 3D printing strategies for enzyme immobilization: materials, methods, and applications. ACS Omega. 2022;7(14):11530–11543. doi: 10.1021/acsomega.2c00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O'Brien CM, Holmes B, Faucett S, Zhang LG. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng Part B Rev. 2015;21(1):103–114. doi: 10.1089/ten.TEB.2014.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]