Abstract
The pathologic features of cerebral Listeria monocytogenes infection strongly suggest that besides hematogenous spread, bacteria might also spread via a neural route. We propose that after snout infection of recombination activating gene 1 (RAG-1)-deficient mice, L. monocytogenes spreads to the brain via a neural route. The neural route of invasion is suggested by (i) the immunostaining of L. monocytogenes in the trigeminal ganglia (TG) and brain stem but not in other areas of the brain; (ii) the kinetics of bacterial loads in snout, TG, and brain; and (iii) the increased resistance of mice infected with a plcB bacterial mutant (unable to spread from cell to cell). Gamma interferon (IFN-γ) plays a protective role in neuroinvasion; inducible nitric oxide synthase (iNOS) accounts only partially for the protection, as shown by a comparison of the susceptibilities of IFN-γ receptor (IFN-γR)-deficient, iNOS-deficient, and wild-type mice to snout infection with L. monocytogenes. The dramatically enhanced susceptibility of RAG-1-deficient, IFN-γR gene-deficient mice indicated the overall importance of innate immune cells in the release of protective levels of IFN-γ. The source of IFN-γ appeared to be NK cells, as shown by use of RAG-1-deficient, γ-chain receptor gene-deficient mice; NK cells played a relevant protective role in neuroinvasion through a perforin-independent mechanism. In vitro evidence indicated that IFN-γ can directly induce bacteriostatic mechanisms in neural tissue.
Listeria monocytogenes is a gram-positive facultative intracellular bacterium which can cause severe infections in the nervous system of humans as well as domestic animals. L. monocytogenes can cause meningitis in immunocompromised humans, but after the first description by Eck (13), a number of cases of L. monocytogenes brain stem encephalitis (rhombencephalitis) also were reported. L. monocytogenes infection of trigeminal ganglia (TG) and nerves and inflammation in the brain stem that is most severe on the side of the affected trigeminal nerve in sheep have been described (10).
The pathways by which L. monocytogenes reaches the brain stem to cause rhombencephalitis have not been clarified. In general, hematogenous spread of L. monocytogenes to the nervous system via crossing of the mucosal barrier in the intestines is the accepted theory. The opinion that L. monocytogenes can pass through the blood-brain barrier is supported by experimental studies in vivo (7) and by the ability of L. monocytogenes to penetrate human endothelial cells in vitro (17, 37). However, the asymmetric bacterial load and pathology in the TG of infected sheep, goats, rabbits, and mice have indicated that the bacteria may spread along cranial nerves (5). Moreover, human patients have shown signs of progressive unilateral cranial nerve palsies followed by inflammation and the appearance of abscesses in the brain stem (2, 34). The hypothesis of a neural route of neuroinvasion was supported by the ultrastructural finding of L. monocytogenes in myelinated axons in naturally infected sheep (26) and by our previous observations of L. monocytogenes infections of neurons of dorsal root ganglia (DRG) in vitro (11).
We report here that L. monocytogenes spreads along the trigeminal nerve to the brain stem in genetically immunodeficient mice. We hypothesized that the immune responses controlling the neuronal dissemination of listerial infection might be qualitatively different from those active in the control of hematogenous infection with the bacteria. By using different knockout mice, we found that both innate and T- and/or B-cell-dependent immune mechanisms control the neural spread of bacteria. Innate gamma interferon (IFN-γ), apparently released by NK cells, but not NK-cell cytotoxicity, inhibited the spread of bacteria along this cranial nerve route. Inducible nitric oxide synthase (iNOS) activity accounted only partially for the IFN-γ-dependent protection. A direct bacteriostatic effect of IFN-γ-activated neural tissue on the protective effect of the cytokine is suggested.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were bred under specific-pathogen-free conditions. Mutant mouse strains without recombination activating gene 1 (RAG-1) or the genes for IFN-γ receptor (IFN-γR) (18), perforin (19), iNOS (23), and the common cytokine γ-chain receptor (γcR) (9) were generated by homologous recombination in embryonic stem cells and backcrossed with C57BL/6. Mice deficient in both RAG-1 and IFN-γR or RAG-1 and perforin were generated in our laboratory as recently described (28). RAG-1- and γcR-deficient mice were purchased from Taconic Farms (Germantown, N.Y.).
Bacteria.
L. monocytogenes wild-type (WT) strain EGD (BUG600, serotype 1/2a) and EGD ΔplcB2 (with a defective lecithinase) were grown in brain heart infusion (BHI) broth and on BHI agar (both from Difco Laboratories, Detroit, Mich.) at 37°C. For infection, bacteria were grown at 37°C in BHI broth to late exponential phase (optical density at 600 nm, 0.8), washed once with phosphate-buffered saline (PBS), suspended in 0.9% NaCl, and quantified on BHI agar plates. Bacterial suspensions were then fractioned and frozen at −70°C until used. L. monocytogenes (106 CFU) in 100 μl of PBS was injected into the snout (right side) of 6- to 8-week-old mice under methoxyflurane (Schering-Plough, Union, N.J.) anesthesia.
Quantification of L. monocytogenes load in organs of infected mice.
Mice were sacrificed, and their brains (including the brain stems), right snouts, and right TG were dissected. The tissues were ground and lysed with sterile 0.6% Triton X-100 in PBS. The tissue lysates were diluted in sterile 0.9% NaCl, and 100 μl was plated on duplicate BHI agar plates. CFU from organs of individual mice were counted after overnight incubation at 37°C.
Immunostaining of tissue sections.
Mice anesthetized with 7% chloral hydrate were sacrificed and perfused with 4% formalin at different time after infection. The snouts, brain stems, and TG were dissected and snap frozen, and cryostat sections (12-μm thick) were cut. Special care was taken not to collect surrounding tissue together with TG. To visualize the bacteria, slides were incubated overnight with a rabbit polyclonal anti-Listeria antiserum (Difco) diluted 1:1,000 in PBS containing 1% bovine serum albumin (BSA) and 0.3% Triton X-100. After three 5-min washes with PBS, Cyomine dye 3 (Cy3)-conjugated donkey anti-rabbit immunoglobulin G (IgG) (Jackson Laboratories, West Grove, Pa.) diluted in PBS containing 1% BSA was added. After incubation with the secondary antibody, slides were extensively washed with PBS and mounted using polyphenylenediamine fluorescence mounting medium. When needed, slides were doubly stained by using a biotinylated rat anti-mouse Iab-monoclonal antibody (25-9-17; Pharmingen, San Diego, Calif.) and fluorescein-labeled streptavidin (Jackson Laboratories) simultaneously with the listerial staining. The sections were reviewed by use of a fluorescence microscope fitted with a charge-coupled device camera and image analysis software.
In vitro infections of primary DRG cultures.
DRG neurons were obtained from embryos (day 15 or 16 of gestation) of C57BL/6 mice, and DRG cultures were prepared as previously described (11). Briefly, DRG were dissociated by several passages through a Pasteur pipette, and the cells were seeded on plates containing glass coverslips. The coverslips had been precoated with collagen (In Vitrogen, Palo Alto, Calif.) and then Matrigel (Becton Dickinson, Bedford, Mass.). The DRG cell suspensions were grown in a culture medium based on serum-free Neurobasal medium containing B27 supplement, 5 mM l-glutamine, and 15 μg of gentamicin sulfate per ml (all from Gibco, Paisley, Scotland) and 1 ng of nerve growth factor (Sigma, St. Louis, Mo.) per ml. Two days later, the cell cultures (4 × 105 cells per well) were rinsed twice with prewarmed minimal essential medium (MEM; Gibco), and the bacterial suspension was added at a multiplicity of infection of 2 bacteria per eukaryotic cell. The cells were incubated for 1 h at 37°C in 5% CO2, washed once with MEM, and then cultured in 2 ml of culture medium containing 10 μg of gentamicin per ml (to kill extracellular bacteria) for 1, 6, or 24 h at 37°C in 5% CO2. The cultures were then washed and lysed with 0.6% Triton X-100 in PBS. The lysates were diluted in sterile 0.9% NaCl, and an aliquot was plated on a BHI agar plate. The colonies were counted after overnight incubation at 37°C. When needed, triplicate DRG cultures were pretreated with recombinant murine IFN-γ (Pharmingen) 24 h before infection. The cytokine was replaced after infection.
Infection of DRG cultures was also microscopically evaluated. For this purpose, cultures were washed, fixed, and stained with both rabbit anti-Listeria (Difco) and mouse anti-neuron-specific microtubule antibodies (TUJI) (Babco, Richmond, Calif.) antibodies. After being washed, slides were incubated with Cy2-conjugated donkey anti-mouse IgG and Cy3-conjugated donkey anti-rabbit IgG antibodies (both from Jackson Laboratories).
RESULTS
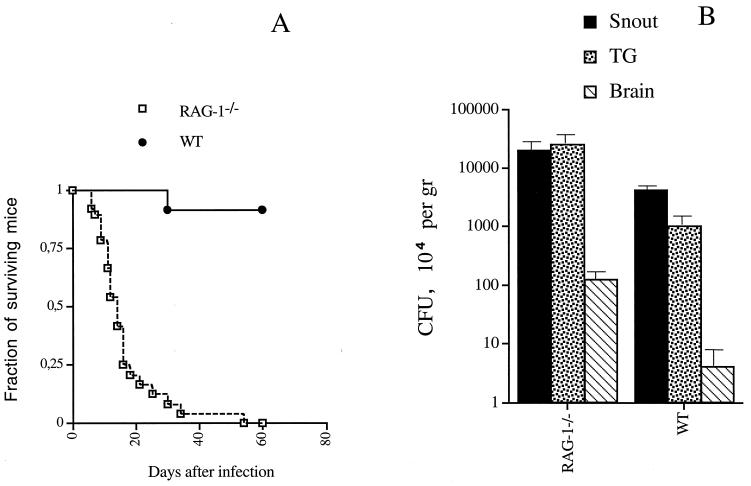
In an initial series of experiments, we examined if L. monocytogenes could spread to the brain stem along the trigeminal nerve. We assumed that the spread of the bacteria would be facilitated in immunodeficient mice and therefore used RAG-1-deficient mice, which lack B and T cells, in our investigations. After snout (where the trigeminal nerve innervates the vibrissae) infection with L. monocytogenes, all RAG-1-deficient mice died, whereas 11 of 12 WT mice survived (Fig. 1A). Differences in survival indicated that T and/or B cells control L. monocytogenes invasion, as bacteria were not detected in the TG or brains of infected WT mice (Fig. 1B).
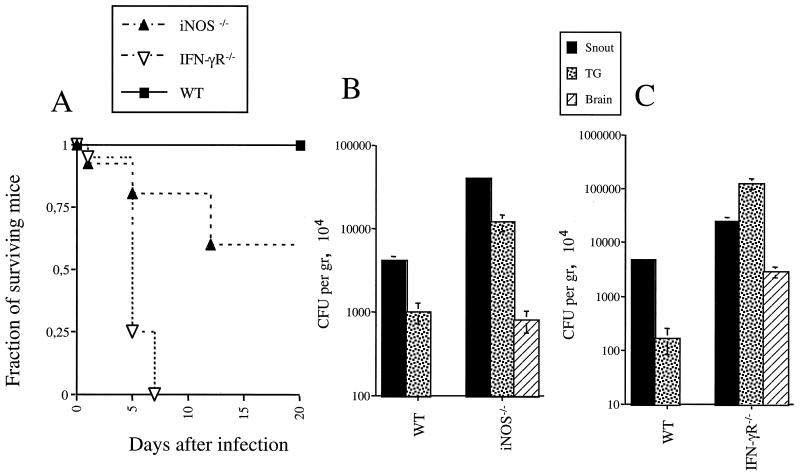
FIG. 1.
Survival of (A) and bacterial loads in (B) RAG-1-deficient (RAG-1−/−) and WT mice after snout infection with 106 CFU of L. monocytogenes. (A) Survival of 20 RAG-1-deficient and 12 WT mice infected in the snout with 106 CFU of L. monocytogenes. Differences between the groups were significant (P, <0.05, as determined by a Wilcoxon U test after a Kaplan-Meier survival analysis). (B) L. monocytogenes CFU in the snouts, TG, and brains of 10 RAG-1-deficient and 6 WT mice 5 days after snout infection. Bacterial load was expressed as mean CFU per gram of tissue ± standard error of the mean. Differences for the same tissues relative to results for the WT group were significant for all tissues (P, <0.05, as determined by the Student t test).
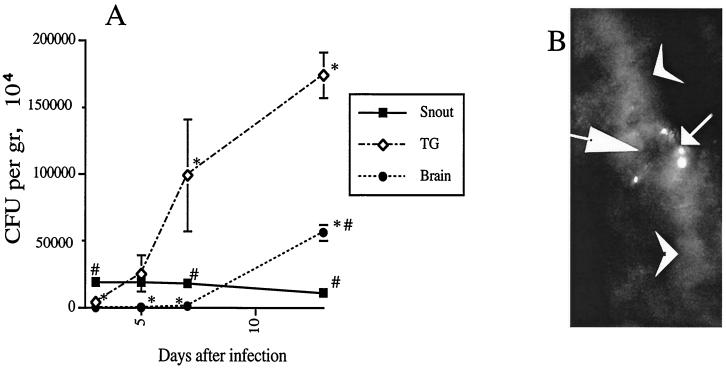
We reasoned that a neural route of L. monocytogenes infection should be reflected in the kinetics of bacterial load in the inoculation site, TG, and brains of infected RAG-1-deficient mice. We observed a sequential appearance of bacteria in the snout, TG, and brain. This observation supports that L. monocytogenes can spread via the trigeminal nerve (Fig. 2A).
FIG. 2.
Experimental evidence for a neural route of listerial neuroinvaison. (A) Kinetics of bacterial load in RAG-1-deficient mice (6 to 10 mice per time point) in snout, TG, and brain after snout infection with L. monocytogenes. Error bars indicate the standard error of the mean. Asterisks and number signs indicate that differences in bacterial loads for snouts or TG at a given time point were significant (P < 0.05 [Student t test]). (B) Immunostaining of L. monocytogenes in TG obtained from RAG-1-deficient mice 7 days after snout infection. Immunostaining was performed using a rabbit antilisterial polyclonal anti serum as described in Materials and Methods. Note the listerial staining in the neuron cell body (small arrow). Large arrow, neuron nucleus; arrowheads, neurites. Magnification, ×120.
In order to obtain direct evidence that L. monocytogenes could infect TG neurons in vivo, sections of TG from infected RAG-1-deficient mice were stained with L. monocytogenes-specific antibodies. L. monocytogenes could be observed in sections of the right TG (the injected side) as early as 7 days after infection and were localized in the cytoplasm of nerve cell bodies (Fig. 2B). No bacteria could be detected in sections from the left TG. At later times after infection (13 days after infection), clusters of bacteria were observed in the brain stems of RAG-1-deficient mice (data not shown), but no bacteria could be found in sections from the rest of the brain.
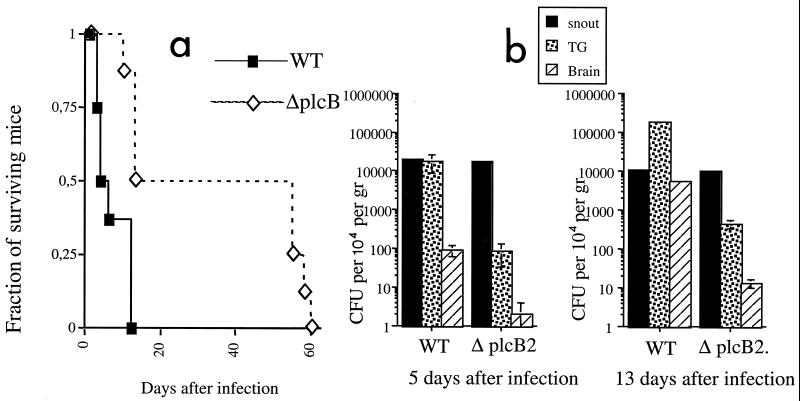
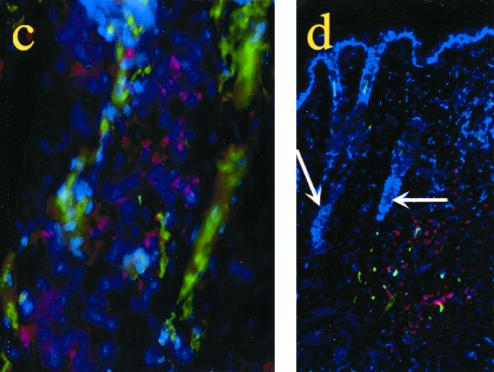
The plcB gene product, lecithinase, is involved in bacterial spread from cell to cell. We examined if the plcB gene product participated in L. monocytogenes neuroinvasion. RAG-1-deficient mice infected with the ΔplcB2 strain had a dramatically increased survival time compared with those infected with the WT strain (Figure 3a). This result suggested that L. monocytogenes propagates mainly from cell to cell, rather than via systemic dissemination. Extended survival in mice infected with ΔplcB2 was related to lower levels of bacteria in the TG and brain but not in the snout compared to the levels in those infected with the WT strain (Fig. 3b). This result indicated that TG axons in the snout are not the first cellular targets infected by L. monocytogenes. In accordance with this observation, listeriae were stained in major histocompatibility complex class II-expressing mononuclear infiltrates, sometimes in the proximity of TG-innervated vibrissae (Fig. 3c and d).
FIG. 3.
Cell-to cell spread of listeria is involved in neuroinvasion. (a) Survival of RAG-1-deficient mice infected in the snout with 106 CFU of WT strain EGD) (n = 12 mice) or ΔpclB2 (n = 8 mice). Differences between the groups were significant (P < 0.05 [Wilcoxon U test after a Kaplan-Meier analysis]). (b) Mean CFU in snouts, TG, and brains of RAG-1-deficient mice 5 and 13 days after snout infection six mice per group). Error bars indicate the standard error of the mean. Differences for the same tissues relative to results for the WT group were significant for TG and brain (P < 0.05 [Student t test]). No differences between the groups were recorded for snout CFU. (c and d) Immunostaining of L. monocytogenes in the snouts of RAG-1-deficient mice 5 days after infection. Listeria-positive cells (red) and MHC class II-positive cells (green) were stained in snout sections using specific antibodies followed by fluorochrome-labeled secondary antibodies as described in Materials and Methods. Listeria staining was not observed in TG-innervated vibrissae (arrows) but was present in the surrounding interstitia. Nuclei (blue) were stained with 4′, 6′-diamidino-2-phenylindole (DAPI). Magnifications: C, ×600; d, ×160.
Our results also indicate that TG neurons can be infected by L. monocytogenes and suggest that this bacterium can use the trigeminal nerve route to enter the brain stem.
We next studied the role of IFN-γ in the control of L. monocytogenes neuroinvasion. IFN-γR gene-deficient infected mice died earlier than WT control mice and showed higher bacterial loads in the snout, TG, and brain (Fig. 4). This result shows that IFN-γ inhibits the spread of L. monocytogenes to the nervous system. IFN-γ-dependent induction of iNOS activity is a major microbicidal mechanism in different bacterial and parasitic infections. iNOS-deficient mice showed higher susceptibility to infection with L. monocytogenes than WT mice but survived longer than IFN-γR gene-deficient mice (Fig. 4). This result indicates that NO accounts only partially for the IFN-γ-mediated control of L. monocytogenes cerebral infection.
FIG. 4.
Role of IFN-γ and iNOS in resistance of mice to snout infection with L. monocytogenes. (A) WT, iNOS-deficient (iNOS−/−), and IFN-γR-deficient (IFN-γR−/−) mice (10 per group) were infected, and mortality was recorded. Differences between WT and iNOS-deficient groups were significant (P < 0.05 [Wilcoxon U test after a Kaplan-Meier survival analysis]). (B) Listerial CFU were measured in snouts, TG, and brains of WT and iNOS-deficient mice 5 days after infection with L. monocytogenes. Differences in bacterial loads for the same tissues in WT mice were significant (P < 0.05 [Student t test]). No bacteria were detected in the brains of WT mice in this experiment. Error bars indicate the standard error of the mean. (C) Listerial CFU were measured in snouts, TG, and brains of WT and IFN-γR-deficient mice 3 days after infection with L. monocytogenes. Differences in bacterial loads for the same tissues in WT mice were significant (P < 0.05 [Student t test]). Less than 10 bacteria were detected in the brains of WT mice in this experiment.
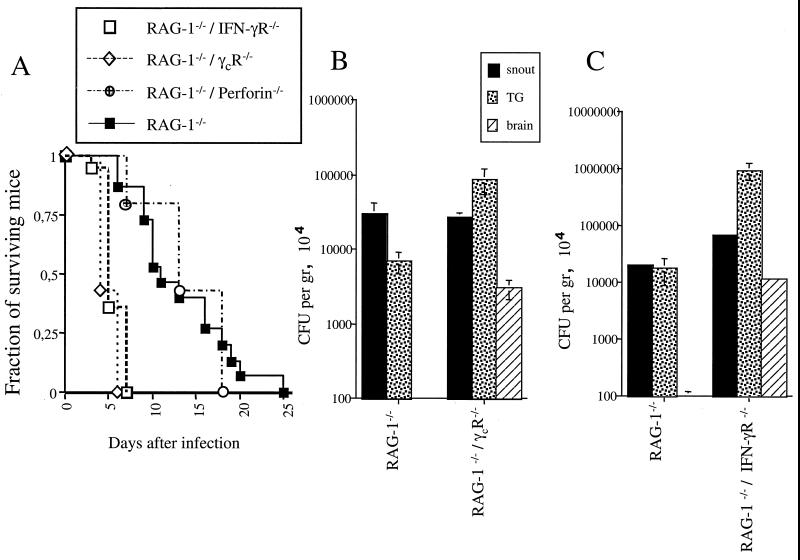
A protective role for the innate release of IFN-γ was suggested by the higher susceptibility to infection of IFN-γR gene-deficient mice than of RAG-1-deficient mice (Fig. 1 and 4). The higher susceptibility of RAG-1-deficient, IFN-γR gene-deficient mice than of RAG-1-deficient mice demonstrates the relevance of the innate release of IFN-γ in the control of cerebral infection with L. monocytogenes (Fig. 5). Moreover, RAG-1-deficient, IFN-γR gene-deficient mice and IFN-γR gene-deficient mice showed similar levels of resistance to infection (Fig. 4 and 5).
FIG. 5.
Role of innate IFN-γ and perforin in resistance of mice to snout infection with L. monocytogenes. (A) Survival curves for various mice after snout infection with L. monocytogenes. Mortality was recorded after infection of at least 10 mice per group with 106 CFU of L. monocytogenes. Differences in mortality between RAG-1-deficient (RAG-1−/−), IFN-γR-deficient (IFN-γR−/−) mice or RAG-1-deficient, γcR-deficient (γcR−/−) mice and RAG-1-deficient mice or RAG-1-deficient, perforin-deficient (perforin −/−) mice were significant (P < 0.05 [Kaplan-Meier survival analysis]). Differences in mortality between RAG-1-deficient, γcR-deficient mice and RAG-1-deficient, IFN-γR-deficient, infected mice were not significant Differences in mortality between RAG-1-deficient and RAG-1-deficient, perforin-deficient mice were not significant. (B) Listerial CFU were measured in snouts, TG, and brains of RAG-1-deficient and RAG-1-(RAG-1−/−) deficient, γcR-deficient mice 5 days after infection with L. monocytogenes. Error bars indicate the standard error of the mean. Differences in bacterial loads between RAG-1-deficient, γcR-deficient mice and RAG-1-deficient mice (same tissues) were significant (P < 0.05 [Student t test]). (C) Bacterial loads were also measured in RAG-1-deficient and RAG-1-deficient, IFN-γR-deficient mice 3 days after infection with L. monocytogenes. Differences in bacterial loads between TG and brain tissues from RAG-1-deficient, IFN-γR-deficient and RAG-1-deficient mice were significant (P < 0.05 [Student t test]).
NK cells are believed to provide the initial burst of IFN-γ in several protozoan and bacterial infections. In order to study the involvement of NK cells in the control of L. monocytogenes cerebral infection, RAG-1-deficient, γcR gene-deficient mice, which have a complete blockade in NK cell development due to the lack of the common γcR and no B and T cells, were used. RAG-1-deficient, γcR gene-deficient mice were more susceptible to snout infection with L. monocytogenes than were RAG-1-deficient mice (Fig. 5). Thus, NK cells are of importance in the control of cerebral infection with L. monocytogenes. RAG-1-deficient, γcR gene-deficient mice showed susceptibility to infection similar to that of RAG-1-deficient, IFN-γR gene-deficient mice (Fig. 5). Furthermore, RAG-1-deficient, γcR gene-deficient mice showed dramatically lower levels of IFN-γ mRNA in the snout 3 days after infection with L. monocytogenes than did RAG-1-deficient mice (data not shown). NK cells might also mediate the control of infection due to their cytotoxic ability, which is mainly mediated by perforin. Compared to RAG-1-deficient control mice, RAG-1-deficient, perforin gene-deficient mice showed similar susceptibility to infection, indicating that NK-cell-mediated cytotoxicity plays no relevant role in the control of neuroinvasion by L. monocytogenes.
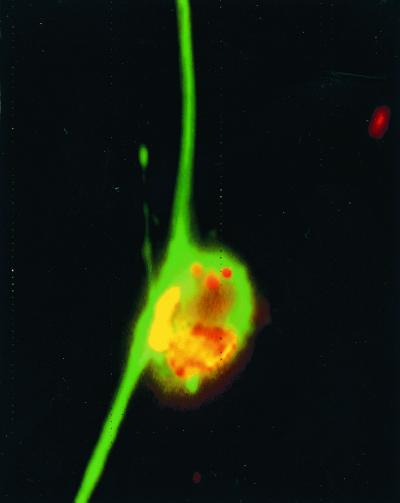
In order to examine whether IFN-γ can directly affect the growth of L. monocytogenes in nerve tissue, DRG cultures were coincubated with the cytokine before and during infection with L. monocytogenes. Coculturing with IFN-γ diminished the growth of intracellular bacteria (Table 1), which were observed to infect both neurons and Schwann cells (Fig. 6).
TABLE 1.
Effect of IFN-γ on the intracellular growth of L. monocytogenes in neural tissuea
| Time (h) after infection | CFU (104/ml of lysate) of L. monocytogenes in samples treated with IFN-γ at the indicated U/ml:
|
||
|---|---|---|---|
| 0 | 5 | 50 | |
| 1 | 4.4 | 3.1 | 1.1b |
| 6 | 9.3 | 4.4 | 1.1b |
| 24 | 25 | 17 | 3.5b |
DRG cell cultures were incubated with recombinant IFN-γ 24 h before infection with L. monocytogenes at a multiplicity of infection of 2 bacterium per DRG cell. One hour after infection, cells were washed, gentamicin was added, and recombinant IFN-γ was replaced. Cells were washed and lysed 1, 6, or 24 h after infection, and L. monocytogenes CFU in the lysates were quantified. All conditions were used in triplicate. The results of one of three representative experiments are shown.
Differences compared with the untreated group were significant (P, <0.05, as determined by the Student t test).
FIG. 6.
In vitro infection of neurons and Schwann cells with L. monocytogenes. DRG cultures were infected with L. monocytogenes and cultured for 24 h in the presence of gentamicin. Cultures were stained with anti-L. monocytogenes and anti-neuron-specific tubulin antibodies and visualized using Cy3 (bacteria in red or yellow)- or Cy2 (neurons in green)-labeled secondary antibodies. Magnification, ×400.
DISCUSSION
The present study suggests that L. monocytogenes can spread to the brain stem along the trigeminal nerve. This observation correlates with what has been described for natural infections in sheep, where trigeminal neuritis has been described for the majority of afflicted animals, suggesting that an ascending infection along this nerve is important in the pathogenesis of encephalitis (10). Also, signs of clinical involvement of the trigeminal nerve precede the symptoms of brain stem infection in human listerial rhombencephalitis. However, other cranial nerves, i.e., the facial, abducens, glossopharyngeal, and vagal nerves, may also serve as portals of entry of the bacterium into the brain stem (2).
Cytosolically replicating intracellular bacteria seem to possess, in general, the ability to spread from the primary infected cell to neighboring cells. For Listeria, this latter property depends on the ability to polymerize cellular actin at one bacterial cell pole by means of the specific bacterial surface protein ActA (16). The plcB gene product, lecithinase, causes lysis of the double membrane of bacterium-containing vacuoles (secondary phagosome). These vacuoles appear when L. monocytogenes has budded from one cell and has been taken up via endocytosis by another cell (31). Thus, PlcB also allows the bacteria to spread from cell to cell. Previous studies have shown that mutation of the plcB gene results in reduced virulence by blocking cell-to-cell spread (29, 36). Moreover, PlcB has been shown to be an important virulence factor in murine cerebral listeriosis (29). In the present study, we found that RAG-1-deficient mice infected with ΔplcB2 mutants survived significantly longer than those infected with WT L. monocytogenes.
On the basis of the direct evidence of unilateral TG listerial localization and the sequential appearance of bacteria in the snout, TG, and brain, we suggest that after inoculation in the snout, L. monocytogenes invades the central nervous system through cell-to-cell spread in the trigeminal system. The smaller numbers of ΔplcB2 mutants in the TG and brain, but not in the snout, than of WT bacteria suggest that infection of TG is secondary to that of mononuclear inflammatory cells in the snout. Accordingly, in vitro infection of neurons is facilitated by cell-to-cell spread from infected macrophages (12).
Our data indicate that the protective immune responses in this model of snout infection are similar to those seen after parenteral listerial infection. The production of IFN-γ in the early phase of infection by L. monocytogenes has been shown to be crucial for the activation of macrophage effector functions required to limit bacterial growth (3, 8, 35). We demonstrate that iNOS participates in resistance to snout infection by L. monocytogenes, as has been shown during parenteral listerial infection (6, 24, 30). However, iNOS, which is transcriptionally induced by IFN-γ, accounts only partially for the protection conferred by IFN-γ during snout infection by listeriae.
By using RAG-1-deficient, IFN-γR-deficient mice, we have found that IFN-γ is necessary for innate resistance to infection with L. monocytogenes. This finding expands the results obtained after anti-IFN-γ antibody administration to SCID mice systemically infected with L. monocytogenes (32). Adaptive immune mechanisms, including perforin-mediated lysis (20) and TNF-α secretion by CD8+T cells (4, 21), and a role for antibodies (14) have been shown to participate in resistance to primary infection with L. monocytogenes. However, the similar susceptibilities of IFN-γR-deficient mice and RAG-1-deficient, IFN-γR-deficient mice suggest that, in the absence of endogenous IFN-γ, IFN-γ-independent adaptive immune mechanisms are of minor importance in resistance to primary listerial infection.
The role of NK cells in resistance to Listeria has been extensively demonstrated (1, 33, 35). In this report, we show that (i) NK-cell-deficient RAG-1-deficient, γcR-deficient mice show dramatically enhanced susceptibility, similar to that of RAG-1-deficient, IFN-γR-deficient mice, and (ii) the levels of IFN-γ transcripts are reduced in the snouts of these mice. On the other hand, perforin-dependent NK-cell cytotoxicity does not seem to play a relevant role in the control of L. monocytogenes neuroinvasion. Thus, NK-cell-mediated protection is probably achieved through the initial burst of IFN-γ, as shown during parenteral infections with L. monocytogenes (1).
Our present experiments suggest that IFN-γ can induce anti listerial activity in neural cells (neurons and Schwann cells) as well. In support of this notion, the occurrence of IFN-γR on subsets of sensory neurons and physiological responses after receptor triggering have been described (25, 27). Thus, similar to the finding that IFN-γ induces antiviral mechanisms of neurons (15, 22), our results suggest that this cytokine can also exert an antilisterial effect on neural cells.
In summary, we provide data suggesting a neural route of invasion by L. monocytogenes. Innate IFN-γ release by NK cells, but not NK-cell cytotoxicity, plays a dominant role in the control of neuroinvasion. We suggest that IFN-γ-dependent bactericidal mechanisms are present in neural tissues.
ACKNOWLEDGMENTS
This work was supported by a grant (711.1213/97) from SJFR, by Amgen, by the Karolinska Institute, by a grant (9502025) from the Danish Biotechnological Research and Development Programme of the Danish Research Councils, and by a grant (9701274) from the Danish Agricultural and Veterinary Research Council.
We are grateful to P. Cossart (Unite des Interactions Bacteries-Cellules, Institut Pasteur, Paris, France) and T. Chakraborty (Institut für Medizinische Mikrobiologie, Justus-Liebig-Universität Gießen, Gießen, Germany) for their kind gifts of the L. monocytogenes strains.
REFERENCES
- 1.Andersson A, Dai W J, Di Santo J P, Brombacher F. Early IFN-gamma production and innate immunity during Listeria monocytogenes infection in the absence of NK cells. J Immunol. 1998;161:5600–5606. [PubMed] [Google Scholar]
- 2.Armstrong R W, Fung P C. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review. Clin Infect Dis. 1993;16:689–702. doi: 10.1093/clind/16.5.689. [DOI] [PubMed] [Google Scholar]
- 3.Bancroft G J, Kelly J P. Macrophage activation and innate resistance to infection in SCID mice. Immunobiology. 1994;191:424–431. doi: 10.1016/S0171-2985(11)80448-1. [DOI] [PubMed] [Google Scholar]
- 4.Bancroft G J, Sheehan K C, Schreiber R D, Unanue E R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989;143:127–130. [PubMed] [Google Scholar]
- 5.Barlow R M, McGorum B. Ovine listerial encephalitis: analysis, hypothesis and synthesis. Vet Rec. 1985;116:233–236. doi: 10.1136/vr.116.9.233. [DOI] [PubMed] [Google Scholar]
- 6.Beckerman K P, Rogers H W, Corbett J A, Schreiber R D, McDaniel M L, Unanue E R. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol. 1993;150:888–895. [PubMed] [Google Scholar]
- 7.Blanot S, Joly M M, Vilde F, Jaubert F, Clement O, Frija G, Berche P. A gerbil model for rhombencephalitis due to Listeria monocytogenes. Microb Pathog. 1997;23:39–48. doi: 10.1006/mpat.1997.0131. [DOI] [PubMed] [Google Scholar]
- 8.Buchmeier N A, Schreiber R D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci USA. 1985;82:7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao X, Shores E W, Hu-Li J, Anver M R, Kelsall B L, Russell S M, Drago J, Noguchi M, Grinberg A, Bloom E T, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 10.Charlton K M, Garcia M M. Spontaneous listeric encephalitis and neuritis in sheep. Light microscopic studies. Vet Pathol. 1977;14:297–313. doi: 10.1177/030098587701400401. [DOI] [PubMed] [Google Scholar]
- 11.Dons L, Weclewicz K, Jin Y, Bindseil E, Olsen J E, Kristensson K. Rat dorsal root ganglia neurons as a model for Listeria monocytogenes infections in culture. Med Microbiol Immunol. 1999;188:15–21. doi: 10.1007/s004300050100. [DOI] [PubMed] [Google Scholar]
- 12.Dramsi S, Levi S, Triller A, Cossart P. Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread: an in vitro study. Infect Immun. 1998;66:4461–4468. doi: 10.1128/iai.66.9.4461-4468.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eck H. Encephalomyelitis listeria apostematosa. Schweiz Med Wochenschr. 1957;87:210–214. [PubMed] [Google Scholar]
- 14.Edelson B T, Cossart P, Unanue E R. Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J Immunol. 1999;163:4087–4090. [PubMed] [Google Scholar]
- 15.Geiger K D, Nash T C, Sawyer S, Krahl T, Patstone G, Reed J C, Krajewski S, Dalton D, Buchmeier M J, Sarvetnick N. Interferon-gamma protects against herpes simplex virus type 1-mediated neuronal death. Virology. 1997;238:189–197. doi: 10.1006/viro.1997.8841. [DOI] [PubMed] [Google Scholar]
- 16.Goebel W, Kuhn M. Bacterial replication in the host cell cytosol. Curr Opin Microbiol. 2000;3:49–53. doi: 10.1016/s1369-5274(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 17.Greiffenberg L, Goebel W, Kim K S, Weiglein I, Bubert A, Engelbrecht F, Stins M, Kuhn M. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect Immun. 1998;66:5260–5267. doi: 10.1128/iai.66.11.5260-5267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang S, Hendricks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R, Aguet M. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 19.Kagi D, Lederman B, Burki K, Seiler P, Odermatt B, Olsen K, Poldack E, Zinkernagel R, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 20.Kagi D, Ledermann B, Burki K, Hengartner H, Zinkernagel R M. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur J Immunol. 1994;24:3068–3072. doi: 10.1002/eji.1830241223. [DOI] [PubMed] [Google Scholar]
- 21.Kagi D, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 22.Komatsu T, Bi Z, Reiss C S. Interferon-gamma induced type I nitric oxide synthase activity inhibits viral replication in neurons. J Neuroimmunol. 1996;68:101–108. doi: 10.1016/0165-5728(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 23.Laubach V, Sheeley E, Smithies O, Sherman P. Mice lacking inducible nitric oxide synthase are not resistant to LPS-induced death. Proc Natl Acad Sci USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie Q W, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 25.Neumann H, Schmidt H, Wilharm E, Behrens L, Wekerle H. Interferon gamma gene expression in sensory neurons: evidence for autocrine gene regulation. J Exp Med. 1997;186:2023–2031. doi: 10.1084/jem.186.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otter A, Blakemore W F. Observation on the presence of Listeria monocytogenes in axons. Acta Microbiol Hung. 1989;36:125–131. [PubMed] [Google Scholar]
- 27.Robertson B, Xu X J, Hao J X, Wiesenfeld-Hallin Z, Mhlanga J, Grant G, Kristensson K. Interferon-gamma receptors in nociceptive pathways: role in neuropathic pain-related behaviour. Neuroreport. 1997;8:1311–1316. doi: 10.1097/00001756-199703240-00050. [DOI] [PubMed] [Google Scholar]
- 28.Rottenberg M E, Gigliotti Rothfuchs A, Gigliotti D, Ceausu M, Une C, Levitsky V, Wigzell H. Regulation and role of IFN-gamma in the innate resistance to infection with Chlamydia pneumoniae. J Immunol. 2000;164:4812–4818. doi: 10.4049/jimmunol.164.9.4812. [DOI] [PubMed] [Google Scholar]
- 29.Schluter D, Domann E, Buck C, Hain T, Hof H, Chakraborty T, Deckert-Schluter M. Phosphatidylcholine-specific phospholipase C from Listeria monocytogenes is an important virulence factor in murine cerebral listeriosis. Infect Immun. 1998;66:5930–5938. doi: 10.1128/iai.66.12.5930-5938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiloh M U, MacMicking J D, Nicholson S, Brause J E, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 31.Songer J G. Bacterial phospholipases and their role in virulence. Trends Microbiol. 1997;5:156–161. doi: 10.1016/S0966-842X(97)01005-6. [DOI] [PubMed] [Google Scholar]
- 32.Tripp C S, Gately M K, Hakimi J, Ling P, Unanue E R. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-gamma. J Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- 33.Tripp C S, Wolf S F, Unanue E R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uldry P A, Kuntzer T, Bogousslavsky J, Regli F, Miklossy J, Bille J, Francioli P, Janzer R. Early symptoms and outcome of Listeria monocytogenes rhombencephalitis: 14 adult cases. J Neurol. 1993;240:235–242. doi: 10.1007/BF00818711. [DOI] [PubMed] [Google Scholar]
- 35.Unanue E R. Macrophages, NK cells and neutrophils in the cytokine loop of Listeria resistance. Res Immunol. 1996;147:499–505. doi: 10.1016/s0923-2494(97)85214-5. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez-Boland J A, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson S L, Drevets D A. Listeria monocytogenes infection and activation of human brain microvascular endothelial cells. J Infect Dis. 1998;178:1658–1666. doi: 10.1086/314490. [DOI] [PubMed] [Google Scholar]