Objective:
A novel automated auscultatory upper arm-cuff blood pressure (BP) monitor (InBody BPBIO480KV) for office use was developed. An electronic stethoscope embedded in the device cuff records the Korotkoff sounds, which are audible to the user and graphically displayed during cuff deflation. Automated BP measurements are provided, while allowing the user to assess the Korotkoff sounds. The device accuracy was tested using the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Universal Standard (ISO 81060-2:2018) and its Amendment 1.2020-01.
Methods:
Participants were recruited to fulfil the age, sex, BP, arm circumference and cuff distribution criteria of the Universal Standard in general population using the same arm sequential measurement method. Three cuffs of the test device were used for arm circumference 23–28, 28–35 and 33–42 cm.
Results:
Data from 85 individuals were analysed [mean age 57.3 ± 15.0 (SD) years, 53 men, arm circumference 23–42 cm]. For validation criterion 1, the mean ± SD of the differences between the test device and reference BP readings (N = 255) was 0.3 ± 5.5/0.6 ± 4.7 mmHg (systolic/diastolic; threshold ≤5 ± 8 mmHg). For criterion 2, the SD of the averaged BP differences per individual (N = 85) was 3.76/3.61 mmHg (systolic/diastolic; threshold ≤6.95/6.91 mmHg).
Conclusion:
The InBody BPBIO480KV device for office use, which provides automated auscultatory measurements while reproducing and displaying the Korotkoff sounds, comfortably fulfilled the AAMI/ESH/ISO Universal Standard requirements in general population and can be recommended for clinical use. The assessment of Korotkoff sounds by healthcare professionals for evaluating the quality of automated measurements requires further evaluation.
Keywords: accuracy, auscultatory, blood pressure measurement, clinic, validation
INTRODUCTION
Automated electronic blood pressure (BP) monitors are currently recommended as the ‘preferred’ devices for BP measurement by healthcare professionals in the office, mainly because they are devoid of the human observer biases and errors, which are known limitations of the manual auscultatory method [1,2]. However, the automated oscillometric technique, which has been developed to replicate the manual auscultatory BP measurements without observer-related issues and is currently being widely used for office, ambulatory, and home BP measurements, has other sources of error, and even properly validated oscillometric devices might not be accurate in some individuals [3,4]. Thus, the manual auscultatory method remains the gold standard method against which any novel technology for BP measurement must be tested [5–7].
The development of automated auscultatory (microphonic) BP monitors, which simulate the gold standard measurement but avoid the observer error and bias, appears to be an attractive alternative. This study assessed the BP measurement accuracy of a novel professional automated auscultatory upper arm cuff BP monitor InBody BPBIO480KV (InBody Co., Ltd, Seoul, Korea) developed for measuring BP by healthcare professionals in the office, according to the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Universal Standard (ISO 81060–2:2018) and its Amendment 1.2020–01 [5–8].
METHODS
The practical recommendations for performing and reporting validation studies according to the AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) and its Amendment 1.2020-01 were strictly followed [5–8]. The study protocol was approved by the hospital scientific committee. All participants signed informed consent for study participation.
Test device
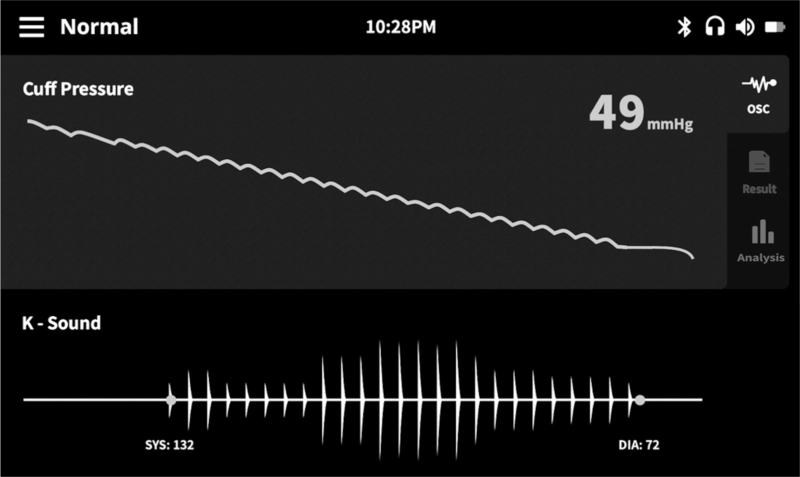
The InBody BPBIO480KV is a fully automated auscultatory device developed for professional use in the office (Fig. 1). An electronic stethoscope embedded in the device's cuff records the Korotkoff sounds, which are audible to the user during cuff deflation (function can be switched off) and are graphically displayed on the device screen (Fig. 2). At the end of the deflation, the device automatically interprets the Korotkoff sounds and reports SBP and DBP values at a 0–300 mmHg range using a proprietary algorithm. It also provides automated measurement of pulse rate at a 30–240 beats/min range. It is powered by a rechargeable 7.26 V, 2600 mAh, lithium-ion battery and AC Adaptor (50/60 hz, DC 12 V, 1.2 A). The total arm circumference range intended by the manufacturer for use is 23–42 cm. Three cuffs were used with the device: small (‘cuff 1’, specified for arm circumference 23–28 cm), medium (‘cuff 2’, for arm circumference 28–35 cm) and large (‘cuff 3’ for arm circumference 33–42 cm). The dimensions of the device are 200 (L) × 180 (W) × 210 (H) mm and its weight is 2.28 kg (without battery). The manufacturer recommends maintenance check every 12 months as it is currently recommended for professional BP measuring devices [1] and is mainly intended for checking the device cuffs, tubes, and connections, rather than the performance of the proprietary algorithm for automated BP measurement, which is not affected by use.
FIGURE 1.
InBody BPBIO480KV automated auscultatory device and cuff with embedded electronic stethoscope.
FIGURE 2.
InBody BPBIO480KV screen with visual display of Korotkoff sounds during cuff deflation. SBP and DBP points are automatically marked with dots.
Participants
According to the AAMI/ESH/ISO Universal Standard for a general population validation study at least 85 individuals aged older than 12 years are required [5–8]. Individuals were recruited from patients attending the outpatient hypertension clinic and from hospital staff.
Validation setting and team
The study was conducted in a BP measurement research lab. Each validation session was conducted by a supervisor and two trained observers who were physicians experienced in BP measurement research and were standardized for their agreement in BP measurement before the study initiation [7]. Five observers participated who rotated according to their availability.
Reference blood pressure measurement
A standard mercury sphygmomanometer (Baumanometer, WA Baum Co. Inc., New York, USA), which was calibrated before the study initiation, was used for simultaneous reference auscultatory BP measurements by two observers (adjacent position of the observers, blinded from each other's readings with a partition, and one of them manually inflated/deflated the cuff with deflation rate 2–3 mmHg/s) using a dual-head teaching stethoscope (3M Littmann Classic II SE, St Paul, Minnesota, USA). Three cuffs with inflatable bladder dimensions 12 × 23, 14 × 28 and 16 × 33 cm were used so that the length would cover 75–100% of the individual participants’ mid-arm circumference and the width 37–50% [7].
Procedure
The same arm sequential method was applied with two entry BP measurements (reference R0 and test device T0) followed by four reference measurements (R1, R2, R3 and R4) taken alternately with three test device measurements (T1, T2 and T3) [5–7]. All measurements were taken on the left arm. The observers were blinded to each other's readings and the test device results. The supervisor documented the test device measurements in the study forms and checked the observers’ measurements. In case of SBP or DBP disagreement larger than 4 mmHg between the observers, the measurement was discarded, and additional pairs of measurements were performed [7]. A maximum of eight pairs of BP determinations was allowed after which the participant was excluded.
Analysis
The AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) and the ISO Amendment 1.2020-01 requirements were strictly followed [5–8]. Each of the reference BP measurements (R0–R4) was the average of the simultaneous readings of the two observers. Measurements R0 and T0 were not used in the analysis. Each of the test device measurements was compared against the average of the previous and next reference BP reading. Differences were calculated by subtracting the reference from the test device BP measurement. The mean BP difference (test versus reference device) and its SD were calculated.
RESULTS
A total of 108 individuals were recruited, of whom 13 were excluded because of reference BP variability (larger than 12/8 mmHg for systolic/diastolic), 5 because of inaudible/uncertain Korotkoff sounds in reference measurements, 3 because of arrhythmia and two because of patient distress. Eighty-five individuals were analysed, all providing three pairs of BP comparisons. The mean age of the participants was 57.3 ± 15.0 (SD) years (range 23–80), 53 were male individuals (62.4%), arm circumference 32.1 ± 5.1 cm (range 23–42), entry SBP (R0) 129.0 ± 19.9 mmHg (range 90–187) and DBP 79.4 ± 15.3 mmHg (range 57–132). The Universal Standard requirements for age, gender, BP, arm size and cuff distribution were fulfilled. The mean BP difference between the simultaneous observers’ reference measurements was −0.1 ± 1.5 (SD)/0.1 ± 1.7 mmHg (systolic/diastolic, range −4 to 4 mmHg). In three of the R1–R4 readings with inter-observer disagreement larger than 4 mmHg, pairs of reference/test BP measurements were repeated.
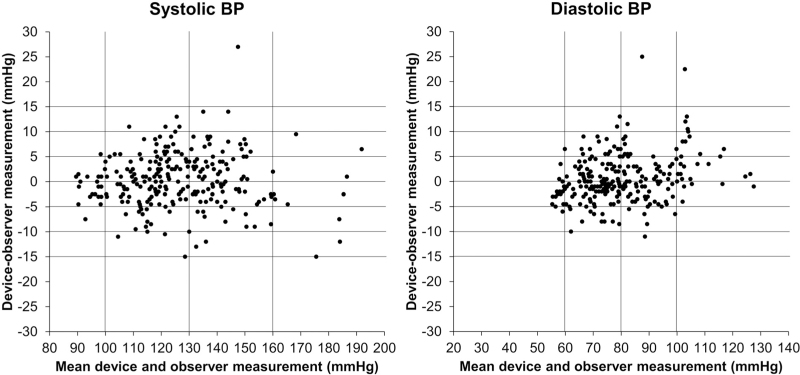
Of the SBP readings, 10% were 100 mmHg or less, 5.9% were at least 160 mmHg and 23.5% at least 140 mmHg. Of the DBP readings, 6.2% were 60 mmHg or less, 10.3% were at least 100 mmHg and 32.6% at least 85 mmHg. The cuff 1 of the test device was used in 25 (29.4%) participants, the cuff 2 in 30 (35.3%) and the cuff 3 in 30 (35.3%). Participants with arm circumference within each quarter of the total arm circumference range were 24 (28.2%), 22 (25.9%), 22 (25.9%) and 17 (20%) from the lowest to the highest quartile and within the lowest/highest octile were 11 (12.9%)/9 (10.6%), respectively. The validation analysis is shown in Table 1. Both the criteria 1 and 2 met the ‘pass’ requirements for SBP and DBP. Criterion 1 was easily fulfilled when measurements taken using each one of the cuffs were analysed separately (cuff 1: test-reference SBP/DBP 0.1 ± 4.6/0.7 ± 5.1 mmHg; cuff 2: 0.0 ± 5.9/0.4 ± 5.1 mmHg and cuff 3: 0.7 ± 5.7/0.6 ± 4.0 mmHg). Standardized Bland–Altman scatterplots of the test-reference BP differences against their average showed no impact of the SBP level on the device accuracy and a tendency towards larger average error in DBP greater than 100 mmHg (Fig. 3). Moreover, scatterplots of test-reference BP differences according to the participants’ arm circumference showed similar accuracy across the range of arm size included in the study (Fig. 4).
TABLE 1.
Device accuracy according to criteria 1 and 2 of the Universal Standard
| Achieved | |||
| Pass requirement | SBP | DBP | |
| Criterion 1 (255 BP pairs) | |||
| Mean BP difference (mmHg) | ≤5 | 0.3 | 0.6 |
| SD (mmHg) | ≤8 | 5.5 | 4.7 |
| Pass | Pass | ||
| Criterion 2 (85 individuals) | |||
| SD (mmHg, SBP/DBP) | ≤6.95/6.91 | 3.76 | 3.61 |
| Pass | Pass | ||
| Result | Pass | ||
FIGURE 3.
Standardized Bland–Altman scatterplots of the test-reference blood pressure differences against their average. Data from [5–7].
FIGURE 4.
Scatterplots of test-reference blood pressure differences against the participants’ arm circumference [8]. Vertical lines indicate borders for each of the three cuffs.
DISCUSSION
This study validated a novel automated BP monitor InBody BPBIO480KV for use by healthcare professionals in the office or clinic using the AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) and its Amendment 1.2020-01 [5–8]. The test device comfortably passed all the accuracy criteria of the Universal Standard in the general population (Table 1). The arm size and the SBP level had no impact on the device accuracy, whereas the average DBP error tended to be larger at high DBP levels. However, this finding is based on few readings and requires further investigation, as well as investigation of the device accuracy in special populations such as children, pregnancy, and atrial fibrillation [5–7]. There were no issues with the device use and the implementation of the validation process.
The correct positioning of the cuff with the microphone placed over the brachial artery is important in this auscultatory device, yet healthcare professionals are familiar in palpating the brachial artery when performing manual auscultatory BP measurements. Correct fitting of the cuff in participants with large arm is an additional issue, yet this general population study included individuals with arm circumference up to 42 cm, as larger ones are regarded as special population requiring separate validation [5–7].
This professional BP monitor does not use the classic oscillometric principle as most automated electronic devices but processes digitized and recorded auscultatory signals and provides automated algorithmic determination of systolic and diastolic pressure points [9,10]. The feature of the device to provide audible and visible Korotkoff sounds to the user during cuff deflation is challenging and potentially advantageous [9,10]. As with classic manual auscultatory BP measurement during which the observer not only identifies SBP and DBP but also evaluates the quality and reliability of the BP readings by hearing the Korotkoff sounds, the InBody BPBIO480KV device allows the healthcare professionals to assess the quality of BP readings by hearing and also viewing the clarity, intensity and rhythmicity of Korotkoff sounds, while the automated measurement avoids observer errors and biases.
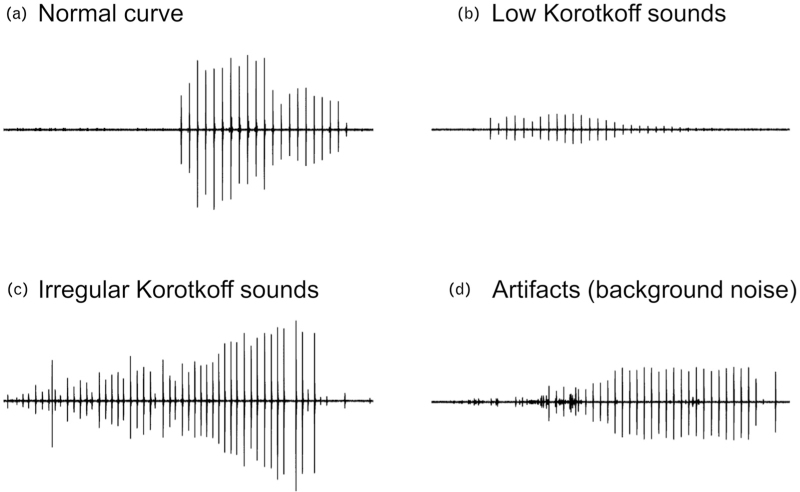
In the process for the development of the InBody BPBIO480KV device and the execution of the validation study, the research team performed more than 1000 automated BP measurements in more than 200 individuals. The auditory reproduction and visual display of Korotkoff sounds may reveal cases with atypical and potentially inaccurate BP information, via assessing the signal quality, for example, low signal amplitude or sound intensity, aberrant signal morphology, interference of extreme background noise or other signal artifact, presence of auscultatory gap or irregular rhythm (Fig. 5). Thus, as in clinical practice healthcare professionals may discard some of their manual auscultatory measurements, which they regard as having low quality, the users of this automated auscultatory BP monitor may detect and discard some questionable readings, which is not possible with automated oscillometric devices. The selection of ‘good’ test device BP readings was not allowed in this formal validation study and raises the possibility of observer's bias in clinical practice where signal issues might be more frequent than in this standardized lab test. However, it might be argued that the device accuracy might have been superior had such an approach been applied by the study investigators aiming to discard problematic readings.
FIGURE 5.
Visual assessment of signal quality via display of Korotkoff sound curves on the device screen showing a normal curve morphology (a) and other curves showing issues which might affect the measurement accuracy (b–d).
Interestingly, greater BP measurement accuracy has been previously demonstrated when Korotkoff sounds were viewed on screen rather than listened to via a stethoscope [10]. Whether the reproduction and display of Korotkoff sounds provided by this novel device is really useful for healthcare professionals in identifying BP readings which are not reliable, as they do with manual auscultatory BP measurement, needs further investigation in a future study. Another issue with the automated oscillometric devices is that they may have different accuracy in some special populations, such as children and pregnant women, and separate validation is required [5–7]. Whether automated auscultatory devices as the InBody BPBIO480KV are devoid of this issue of the oscillometric ones, also deserves further investigation.
ACKNOWLEDGEMENTS
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: 1711138442, KMDF_PR_20200901_0212).
Source of funding: The study was funded by InBody, Seoul, Korea with a grant by the Korea Medical Device Development Fund to the University of Athens Special Account for Research Grants.
Conflicts of interest
G.S. received consulting fees by InBody.
Footnotes
Abbreviations: AAMI, Association for the Advancement of Medical Instrumentation; BP, blood pressure; ESH, European Society of Hypertension; ISO, International Organization for Standardization
REFERENCES
- 1.Stergiou GS, Palatini P, Parati G, O’Brien E, Januszewicz A, Lurbe E, et al. European Society of Hypertension Council and the European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens 2021; 39:1293–1302. [DOI] [PubMed] [Google Scholar]
- 2.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020; 75:1334–1357. [DOI] [PubMed] [Google Scholar]
- 3.Sharman JE, Tan I, Stergiou GS, Lombardi C, Saladini F, Butlin M, et al. Automated ’oscillometric’ blood pressure measuring devices: how they work and what they measure. J Hum Hypertens 2022; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stergiou GS, Lourida P, Tzamouranis D, Baibas NM. Unreliable oscillometric blood pressure measurement: prevalence, repeatability and characteristics of the phenomenon. J Hum Hypertens 2009; 23:794–800. [DOI] [PubMed] [Google Scholar]
- 5.Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens 2018; 36:472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. International Organization for Standardization. ISO 81060-2:2018. Noninvasive sphygmomanometers: part 2: clinical investigation of intermittent automated measurement type. Available at: https://www.iso.org/standard/73339.html. [Accessed 15 November 2022] [Google Scholar]
- 7.Stergiou GS, Palatini P, Asmar R, Ioannidis JP, Kollias A, Lacy P, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring Recommendations and Practical Guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J Hypertens 2019; 37:459–466. [DOI] [PubMed] [Google Scholar]
- 8. International Organization for Standardization. ISO 81060-2:2018/AMD 1:2020. Noninvasive sphygmomanometers: part 2: clinical investigation of intermittent automated measurement type - Amendment 1. Available at: https://www.iso.org/standard/75432.html. [Accessed 2 July 2022] [Google Scholar]
- 9.Celler BG, Le P, Basilakis J, Ambikairajah E. Improving the quality and accuracy of noninvasive blood pressure measurement by visual inspection and automated signal processing of the Korotkoff sounds. Physiol Meas 2017; 38:1006–1022. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Chee Y, Kim I, Karpettas N, Kollias A, Atkins N, et al. High-fidelity digital recording and playback sphygmomanometry system: device description and proof of concept. Blood Press Monit 2015; 20:266–272. [DOI] [PubMed] [Google Scholar]