Abstract
Background:
In just over 2 years, tracking the COVID-19 pandemic through wastewater surveillance advanced from early reports of successful SARS-CoV-2 RNA detection in untreated wastewater to implementation of programs in at least 60 countries. Early wastewater monitoring efforts primarily originated in research laboratories and are now transitioning into more formal surveillance programs run in commercial and public health laboratories. A major challenge in this progression has been to simultaneously optimize methods and build scientific consensus while implementing surveillance programs, particularly during the rapidly changing landscape of the pandemic. Translating wastewater surveillance results for effective use by public health agencies also remains a key objective for the field.
Objectives:
We examined the evolution of wastewater surveillance to identify model collaborations and effective partnerships that have created rapid and sustained success. We propose needed areas of research and key roles academic researchers can play in the framework of wastewater surveillance to aid in the transition from early monitoring efforts to more formalized programs within the public health system.
Discussion:
Although wastewater surveillance has rapidly developed as a useful public health tool for tracking COVID-19, there remain technical challenges and open scientific questions that academic researchers are equipped to address. This includes validating methodology and backfilling important knowledge gaps, such as fate and transport of surveillance targets and epidemiological links to wastewater concentrations. Our experience in initiating and implementing wastewater surveillance programs in the United States has allowed us to reflect on key barriers and draw useful lessons on how to promote synergy between different areas of expertise. As wastewater surveillance programs are formalized, the working relationships developed between academic researchers, commercial and public health laboratories, and data users should promote knowledge co-development. We believe active involvement of academic researchers will contribute to building robust surveillance programs that will ultimately provide new insights into population health. https://doi.org/10.1289/EHP11519
Introduction
In the midst of the COVID-19 pandemic, SARS-CoV-2 wastewater surveillance has gained traction globally as a means to assess the occurrence of infections in communities. Shortly after the first reported detection of SARS-CoV-2 RNA in wastewater in the Netherlands,1,2 the virus was detected in untreated sewage in several countries, including the United States,3 Australia,4 and India,5 suggesting that the virus’ genetic material was sufficiently abundant in wastewater to provide an indication of community infections. Efforts shifted from basic detection to attempts to quantify and characterize the relationship between SARS-CoV-2 RNA in wastewater and associated COVID-19 clinical case data,6,7 and methodologies were refined to provide more quantitative grounding for wastewater measurements.8,9 Academic researchers in environmental engineering, environmental science, microbiology, environmental virology, and similar fields were heavily engaged in these efforts and disseminated early information to demonstrate the promise and challenges of wastewater surveillance for population-level assessments of infection. The research community used channels of communication such as published manuscripts and preprints, creation of a National Science Foundation-funded Research Coordination Network on Wastewater Surveillance for SARS-CoV-2, workshops (e.g., McClary-Gutierrez et al.,10 Lin et al.11), collaboratives, and informal communications, with knowledge shared freely across the scientific community.
As the pandemic progressed, wastewater surveillance efforts became more widespread, particularly in the United States, with many major cities, as well as some rural areas, implementing programs.12 We observed that research entities, including many universities, often led or participated in these efforts, in large part because of the need to overcome challenging method development alongside implementation of wastewater surveillance programs. This allowed for rapid implementation during a time of great need, but there were challenges in interpretation and potential actionability of the data. Academic researchers were not always experienced with how decision-making occurs within public health systems. Public health practitioners were also challenged with assessing a new data stream that was distinct from traditional disease metrics, such as tracking hospitalizations or clinical diagnostic test positivity rates. Researchers with expertise producing and interpreting environmental data and public health practitioners with expertise in outbreak response approached wastewater surveillance with distinctly different knowledge bases, different definitions for key terms such as variability and uncertainty, and at times different priorities (e.g., improving science-based measurements vs. implementing public health measures).13 We found close working partnerships were critical in bridging this gap, and wastewater surveillance has now matured into a useful tool for COVID-19 outbreak response and has been prioritized by the Centers for Disease Control and Prevention (CDC)14 and some states as part of future public health responses.
Here, we provide a perspective on the shifting role of researchers from primary data generators to supporting partners of wastewater surveillance programs. Our perspective is shaped by our experience as researchers implementing wastewater monitoring in six different states and forming partnerships with the public health sector to help establish wastewater surveillance as a public health tool. As the pandemic continues to evolve and home testing becomes more common,15 there is a need to sustain wastewater surveillance programs for SARS-CoV-2, as well as to further develop methods for other pathogens of concern.14 We believe that a robust wastewater surveillance system that can inform public health actions will require a foundation of sound science and ongoing collaborations between academic researchers and the public health sector to advance new technologies and knowledge in the field.
Discussion
Evolution of SARS-CoV-2 Wastewater Surveillance from Research to Practice
Many early SARS-CoV-2 wastewater surveillance efforts were initiated by academic researchers in collaboration with public health, wastewater, or municipal partners.3,7,16,17 We found the environmental microbiology capacity and experience of academic research labs positioned them to respond effectively to develop the analytical methods required for processing a complex sample matrix for wastewater monitoring. By developing methods and beginning regular sample processing, academic research labs were instrumental in starting wastewater surveillance programs in U.S. cities such as Houston, Texas,18 and San Francisco19 and San Diego,20 California, and statewide programs in Utah,21 Wisconsin,22 and Ohio.23 The structure of these partnerships varied, but in many instances, municipal utilities conducted sample collection and logistics, and academic partners managed the analytical burden, developing methodologies, processing samples, and providing data interpretation for public health partners, who then could use the information for decision-making.7,18,24 Such arrangements were pragmatic given the urgency and rapidly evolving nature of the pandemic. In other situations, public health, municipal utility, or other government agencies were responsible for some or all aspects of surveillance.25–28 Other research programs focused more strictly on method development and fundamental explorations that would be useful to the broader scientific community without explicitly identified partner recipients.8,9,29 Private companies have also emerged as providers of wastewater surveillance services, from providing only sample testing to also providing data analysis and reporting.30
Although wastewater surveillance at treatment plants can provide community-level insights into COVID-19 trends, another application is to monitor for SARS-CoV-2 RNA in wastewater collected from smaller areas or individual buildings. College campuses, for example, are particularly well-suited for this type of localized wastewater surveillance, with early efforts reported on at least 25 campuses in the United States.31 In many cases academic researchers were working within their own institutions to provide in-house expertise or analysis. In several cases, surveillance teams have been able to use wastewater data to reportedly avert COVID-19 outbreaks among populations in large residential halls.32–34 Efforts on university campuses have spanned the methodological gradient, from large-scale composite sampling and high-throughput automated analysis to greatly simplified passive sampling with qualitative detection.35–37 Universities have thus proven to be an important testbed for wastewater surveillance of SARS-CoV-2 infections among defined populations.
Throughout the evolution of COVID-19 wastewater surveillance thus far, a progressively larger group of public health practitioners have used wastewater data.14 In the early stages of the pandemic, some members of the public health community were hesitant regarding the use wastewater data for decision-making because such data was unfamiliar and its performance characteristics were largely unknown.13,38–40 Efforts to define uncertainty in the wastewater data, as well as to understand its relationship to clinical case data,22,41 continue to build confidence and improve its potential for application. In addition, as practitioners gained experience and a larger knowledge base was generated, deploying a national wastewater surveillance system for COVID-19 seemed increasingly feasible.30,42
As states implement their programs, contributions of their data to such a system offers a nationwide comparison of wastewater surveillance data and a community of practitioners to share methods and experience. Critical to these efforts has been funding to states to establish programs (J. Meiman, personal communication). In September 2020, the CDC launched the National Wastewater Surveillance System (NWSS) in the United States and, on 4 February 2022, wastewater data were officially incorporated into the agency’s COVID-19 Data Dashboard.43 Globally, countries such as the Netherlands, UK, Austria, Australia, Canada, Pakistan, Malawi, and Spain (to name a few) have national and regional dashboards showing wastewater surveillance results.44 There is an increasing number of examples showing that, if performed in the context of a well-organized and well-integrated effort, wastewater surveillance can aid public health responses.44,45
Looking Back: Lessons Learned
In our experience, the most important aspect of successful wastewater surveillance programs thus far has been the development of multi-stakeholder partnerships founded in active collaboration and communication throughout the process. As researchers in academic laboratories, we have historically worked with a broad array of partners in university administration, municipalities, industry, and public health laboratories; these foundational relationships allowed us to quickly expand or initiate new collaborations to start wastewater surveillance programs. Our partnerships have taken many forms, and here we discuss some of the lessons learned from these different types of collaborations.
One effective model for cooperation involved direct partnerships between academic researchers and public health laboratories, many of which were members of the Association of Public Health Laboratories. These partnerships, by nature, extended to the environmental health or public health departments that the laboratories served and allowed wastewater surveillance efforts to tap into existing frameworks for communication and data transfer within the public health system. Researchers brought specific technical and scientific expertise that helped implement virus detection in the complex matrix of wastewater, which added to the capacity of public health laboratories to expand testing to include untreated sewage. We found that environmental health experts within public health departments were often effective liaisons between the data producers and data end users because of their familiarity with both environmental measurements and clinical data.13 Some state public health laboratories are housed within universities and thus have existing collaborative research with academic units; examples include the Wisconsin State Laboratory of Hygiene within the University of Wisconsin-Madison and the Illinois Department of Public Health Laboratory housed within the University of Illinois Chicago School of Occupational and Environmental Health. With this model, academic researchers, students, and postdoctoral scholars were able to use their existing expertise to focus on thoroughly investigating scientific and technical questions without detracting from the routine analysis pipeline. In addition, public health laboratories had the capacity to scale up and optimize methods using high-throughput platforms. Ongoing exchange of information allowed each partner to stay apprised of the latest developments in the field more easily. Collective troubleshooting and sample exchange for cross-validation were also beneficial for accelerated data interpretation.
It is noteworthy that several of the seven states that were the first and are currently the largest participants in the NWSS had academic research/public health laboratory partnerships early in the pandemic.46 In Wisconsin, a partnership between a research laboratory and the state public health laboratory, also part of a university, enabled the implementation of a statewide wastewater surveillance program47 with 72 wastewater treatment plants (WWTPs) in August 2020.22 The statewide wastewater surveillance program in Illinois is similarly organized, with a partnership between a university, and city and state public health entities. The Illinois program began in October 2020 with 7 WWTPs in the City of Chicago48 and expanded statewide in May 2021 with 65 WWTPs in 49 counties.49 The Ohio and Utah statewide programs are additional examples of this type of partnership.14
There were also specific challenges associated with these types of partnerships. In our own experience, wastewater surveillance programs were less sustainable if sample processing was scaled up in academic labs without a long-term plan to transfer processing to a production lab or public health laboratory that had capacity to do scheduled, frequent analysis on a long-term basis. Further, some public health agencies were less receptive to using wastewater data or did not have the personnel or infrastructure capacity to implement SARS-CoV-2 wastewater surveillance within their public health system.13,50
Academic researchers also collaborated with other types of laboratories to build capacity for surveillance programs, including municipal wastewater utility laboratories and commercial laboratories capable of rapid, high-throughput sample processing.28,41 In both cases, academic researchers were involved in methods development, data analysis, and troubleshooting, but routine analysis was conducted by a dedicated staff of analysts using agency or company staffing logistics (i.e., shifts, overtime policies) that are not often established in academic labs. We found that engaging such laboratory partners (whether municipal or commercial) from the beginning of protocol optimization allowed academic partners to train analysts as needed and incorporate feedback from analysts and scientists who offered important perspectives into practical and scientific considerations for routine sample processing. Working directly with municipal laboratories associated with wastewater utilities offers the logistical benefits of using existing sample collection and transport structures and having direct access to wastewater data (e.g., wastewater flow rates and characteristics) critical for troubleshooting and data interpretation. Partnering with commercial laboratories offered the benefit of using existing equipment, leveraging expertise of personnel, and applying existing high-throughput sample processing strategies.
Finally, some academic researchers generated data in their academic laboratories without a specific data end user identified.8,29 Although in these instances the resulting data were not used in real time, findings from these efforts have been critical in establishing the scientific basis for wastewater surveillance, including proof-of-concept and method development, and will be important for retrospective analysis in local areas moving forward. All of these early frameworks offered a platform for cooperative research to refine and validate methods.
In our experience, surveillance programs were less likely to aid in public health response to COVID-19 if WWTP partners or public health end users were not identified at the beginning of the effort. Further, providing surveillance data without access to expertise to explain the context, scientific basis, limitations, and interpretations did not promote continued demand for or application of the data by its end users.30 We conclude that regardless of whether or not public health practitioners are directly involved in data generation, close collaboration among researchers, public health agencies, and laboratories is critical for interpretating wastewater data in a public health context and building confidence in wastewater surveillance programs.
Looking Forward: Role of Academic Researchers in Wastewater Surveillance
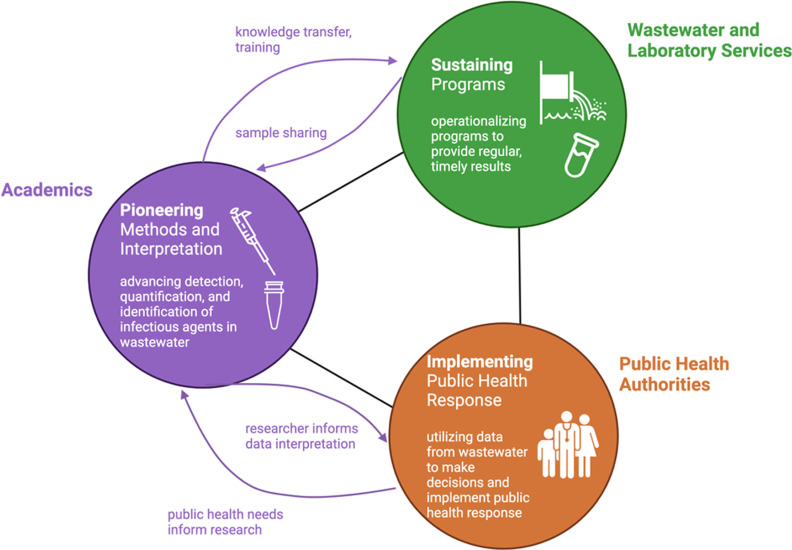
The evolution of wastewater surveillance during the COVID-19 pandemic has demonstrated the utility of these programs during disease outbreaks and underscored the need for dynamic, collaborative program structures to respond to rapidly changing circumstances. As wastewater surveillance programs become integrated into public health systems, continued access to scientific expertise in engineering and microbiology will be crucial in establishing wastewater surveillance as an effective, institutionalized public health tool. In our view, there are three interconnected activities that are essential for the support of effective wastewater surveillance systems, namely a) pioneering new capabilities, b) sustaining surveillance efforts, and c) integrating surveillance data for effective public health response (Figure 1). Academic researchers can play an important role in contributing to each of these activities.
Figure 1.
Framework for building robust and adaptive wastewater surveillance programs. In addition to undertaking basic research that underpins the basis of wastewater surveillance, researchers have a critical role to play in continuous methods development, technology transfer, research that informs data context and interpretation, and training the next generation of professionals.
Pioneering new capabilities.
Even as wastewater surveillance becomes more routine, infectious agents by their nature are constantly evolving. Researchers are uniquely poised to contribute to the future of wastewater surveillance by taking on a primary role in pioneering new capabilities and methods for detection, quantification, and identification of infectious agents in wastewater, as well as interpretation of the relationship between resulting data and health outcomes (Table 1). Thus far, the COVID-19 pandemic has demonstrated that both existing public health metrics and novel techniques are critical for our ability to respond to and stay ahead of emerging public health crises. A clear example of this has been in the identification and rapid roll-out of new assays for identifying SARS-CoV-2 variants of concern. Researchers designed new assays for the SARS-CoV-2 Omicron variant within a few days of its identification, and wastewater surveillance programs in four states (California, Colorado, New York, and Texas) were able to use these assays and sequencing-based approaches to detect evidence of the Omicron variant within days of the first clinical cases, demonstrating in some areas that the variant was already spreading in the community before clinical cases were identified.51 More recently, retrospective analysis of wastewater revealed positive detection of polio in three counties in New York, following reports of a single case of identification of one clinical case of paralytic poliomyelitis in an unvaccinated person.52
Table 1.
Pioneering activities for strategic engagement of academic researchers to advance wastewater surveillance.
| Pioneering research activity | Published examples |
|---|---|
| Surveillance of new SARS-CoV-2 variants of concern and lineages | Wastewater surveillance of alpha variant (B.1.1.7) via spike protein mutations detected in wastewater in the United Kingdom87 |
| Screening for alpha (B.1.1.7), beta (B.1.351), and gamma (P.1) variants of concern via RT-qPCR allelic discrimination assays88 | |
| Rapid response wastewater surveillance of Omicron variant (B.1.1.529) throughout the United States51,83,89 | |
| Genomic sequencing of SARS-CoV-2 from wastewater to monitor variants of concern (B.1.1.7, B.1.351, B.1.617.2) in municipalities across Europe90 | |
| Wastewater surveillance of SARS-CoV-2 lineages via deep sequencing of the receptor binding domain91 | |
| Method development, refinement, and optimization | Kit-free RNA extraction method for wastewater surveillance of SARS-CoV-224 |
| Rapid, high-throughput wastewater testing via automated concentration and extraction (Karthikeyan et al.)35 | |
| Monitoring of SARS-CoV-2 via wastewater settled solids7,41 | |
| Biosensors for near real-time wastewater surveillance92 | |
| Development of resource-efficient methods for accessible wastewater surveillance | Passive sampling and RT-LAMP for building-level wastewater surveillance37 |
| Membrane-based RT-LAMP for wastewater surveillance of SARS-CoV-275 | |
| Paper-based testing devices for wastewater surveillance | |
| Biological analyte fate and transport in wastewater systems | SARS-CoV-2 accumulation in biofilms in wastewater collection systems93 |
| Enhanced decay of SARS-CoV-2 RNA in sewers with biofilms94 | |
| Partitioning of enveloped and unenveloped viruses to suspended solids70 | |
| Wastewater surveillance of other infectious agents | Respiratory syncytial virus surveillance via wastewater settled solids63 |
| Deep sequencing of wastewater for enterovirus surveillance95 | |
| Influenzae wastewater surveillance64 | |
| Modeling and quantitative analysis | Distributed-lag time-series model to relate SARS-CoV-2 RNA counts in sewage and COVID-19 cases6 |
| Susceptible–exposed–infected–recovered model to relate SARS-CoV-2 RNA counts in wastewater and prevalence of COVID-1996 |
Note: RT-LAMP, reverse transcription-loop-mediated isothermal amplification; RT-qPCR, reverse transcription polymerase chain reaction.
As new tests are brought online, or existing methods are improved, academic researchers can contribute their extensive expertise working with wastewater samples. There has been a rapid adoption of what were previously research methods as routine assays, and many of the complications of working with wastewater may be underappreciated. Specialized expertise within the academic community can support optimization, validation, and standardization; the latter being critical for the transition of these methods to public health laboratories. Researchers working side by side with laboratories implementing methods can help shed light on unexpected results and provide expertise on wastewater conveyance systems and the complicated matrix of wastewater, which will accelerate troubleshooting efforts and validation of methods for new targets.
Before SARS-CoV-2, wastewater surveillance was used to study the epidemiology of a variety of infectious diseases, although these were most often fecal–oral pathogens. Most famously, wastewater has been used to study the epidemiology of poliovirus and other enteroviruses,53–56 but many other infectious agents have been surveilled via wastewater, including Vibrio cholerae,57 hepatitis E,58 Cryptosporidium,59 group A rotavirus,60 norovirus,61 and Giardia,62 to name a few. The recent success of wastewater surveillance for SARS-CoV-2 is now rekindling interest in using wastewater to examine the epidemiology of other respiratory viruses, such as respiratory syncytial virus63 and influenza A.64 Recent concerns of the community spread of monkey pox and polio, both of which have been found in wastewater in communities with reported cases,52,65,66 highlights the utility of an operational surveillance system that can pivot to respond to new threats. In our opinion, expanding the wastewater surveillance paradigm to include a diversity of infectious agents, along with a fundamental characterization and mechanistic understanding of these pathogens’ fate and transport in wastewater, will continue to be a crucial pioneering contribution of research teams.
As the COVID-19 pandemic enters an endemic phase and infections in the community decrease, more sensitive methods will be needed. Early in the COVID-19 pandemic, researchers found wastewater solids to be an efficient and methodologically convenient SARS-CoV-2 sample type.6,7,67,68 Other research teams have also made use of suspended solids as a highly sensitive approach to wastewater surveillance, even during periods of low transmission or after mass vaccination (e.g., on a university campus).41,69 Sensitive methods will also be needed for new infectious disease targets that may not be as prevalent as SARS-CoV-2. Although solids have been found to be useful for SARS-CoV-2 and could also be useful for other targets, other viruses, or bacterial or fungal targets may behave differently in wastewater depending on their morphology and structure,70 and so ongoing method development is important for new pathogen targets.
In the future, scaling-up wastewater surveillance programs and efforts to make them more widely accessible will also require the development of methods that are cost efficient and less resource intensive. Various research teams have led efforts to develop passive sampling techniques, such as the Moore swab, for economical sampling of wastewater with superior performance to grab sampling.71–73 In addition, researchers have also initiated efforts to develop molecular testing techniques that do not require expensive quantitative polymerase chain reaction (qPCR) equipment, such as loop-mediated isothermal amplification,74 and have coupled these with passive samplers and electronegative membranes to allow rapid wastewater testing.37,75 Researchers have also proposed paper-based testing devices and biosensors as future analytical platforms for near real-time surveillance of infectious agents in wastewater, although as of yet no proof of concept has been published.76 It is abundantly clear that researchers have a strategic role to play in the development of efficient and scalable wastewater surveillance methodologies.
Outside of laboratory method development, there remains a critical need to continue advancing modeling and data analysis methods for wastewater surveillance applications. Early efforts during the COVID-19 pandemic relied on relatively simple correlation analyses between SARS-CoV-2 RNA concentrations and COVID-19 cases or hospitalizations in a community. As research efforts continue to develop numerical techniques for surveillance of SARS-CoV-2 and other targets,77,78 attention to predictive modeling techniques and integration of wastewater data into other epidemiological data analyses will remain critical. Further, retrospective analyses of the volumes of data that have been collected during the COVID-19 pandemic may reveal important insights that will inform our response to future pandemics.
Sustaining surveillance efforts.
Similar to other public or environmental health monitoring efforts, effective wastewater surveillance requires frequent sample collection and analysis. Many researchers have determined that a minimum frequency of weekly or twice per week sampling is needed for useful applications of the data7,22 and daily samples with 24-h turnaround are ideal in some use cases (A. Boehm, personal communication). Despite the efforts that academic research labs have undertaken to initiate wastewater surveillance programs during the pandemic and the value that these efforts have had in technical training and education,79 it is also clear that academic research labs cannot be expected to sustain the day-to-day sampling, analysis, and monitoring required by full-fledged, long-term surveillance systems—nor should they. Research labs typically work on novel solutions to both new and long-standing challenges and, in academic settings, are meant to provide a training ground for students and early career scientists to develop their critical thinking, experimental research, and professional skills. Public health labs and commercial labs are, on the other hand, well suited for routine analyses and data production given their high-throughput capacity, technical expertise, and existing personnel management structures.
In instances where academic labs have taken active roles in generating wastewater surveillance data during the first years of the COVID-19 pandemic, these responsibilities will need to switch to either the end users of the data (public health labs/municipalities) or to other high-production entities (commercial labs). This necessary shift from routine monitoring in research labs to professional labs does not, however, imply that researchers will not have a critical role to play in the sustainability of surveillance efforts. Wastewater surveillance is a highly interdisciplinary area, and the professional skillsets required to sustain these efforts are widely distributed across a range of fields, including environmental engineering, microbiology, molecular biology, statistics, epidemiology, data management, public policy, and data communication, among others (Table 2). We believe that researchers can play a key role in transferring and combining these skillsets in the professional world through training, sharing expertise, and technology transfer of new methods.
Table 2.
Examples of disciplines and skills required for effective wastewater surveillance programs.
| Disciplines | Skills |
|---|---|
| Environmental engineering |
|
| |
| |
| Environmental microbiology and molecular biology |
|
| |
| |
| Statistics and data science |
|
| |
| |
| Public health and public policy |
|
| |
| |
| Communication |
|
| |
|
Staffing wastewater surveillance programs will require specialized skillsets. For example, wastewater as a matrix is highly complex and unique in comparison to the types of sample materials typically worked with in clinical laboratories (e.g., blood or fecal specimens), and methods for optimal wastewater processing remain unstandardized. Because of this, we assert that one of the most critical roles for researchers is training the next generation of professionals who will in turn sustain long-term wastewater surveillance efforts as they enter the workforce. By professionalizing the discipline of wastewater surveillance through formal and informal educational and training programs, researchers can also benefit from permanent relationships with monitoring programs and professional labs that allow rapid identification of new research questions and areas for improvement.
Integrating surveillance data for effective public health response.
An important goal of wastewater surveillance programs is to monitor outbreaks to inform public health actions. As wastewater surveillance programs expanded to achieve this goal, we found through our own involvement that close partnerships and knowledge sharing between academics involved in project implementation and public health practitioners in a position to use data for pandemic response were essential. Because the field progressed quickly, there was a significant need for researchers to act as conduits to public health agencies to provide information on the scientific basis for the measurements, the limitations and uncertainty, and how that data could be contextualized and interpreted.
In Houston, wastewater surveillance efforts have been led by public health experts, and results have been used to target testing, vaccination, and educational resources toward parts of the city with a particularly high COVID-19 burden identified via wastewater samples.80,81 In several cases, wastewater testing conducted on campuses by academic institutions has been used by those institutions to implement additional targeted testing and other responsive measures to protect public health.32,35,82 Citywide testing can also inform decisions such as mask mandates and hospital staffing and resource forecasting as a new outbreak begins.14 As the benefits of wastewater surveillance are demonstrated, localities hesitant to use or continue wastewater surveillance may become less so13,50; therefore, ongoing dissemination of these successes is critical.
The novelty of wastewater monitoring and ongoing adaptations to meet evolving needs during the COVID-19 outbreak and beyond can be aided by researcher–practitioner partnerships that facilitate changes to ongoing programs and enable the development of public health guidelines based on these conclusions. We believe it is important that practitioners and researchers should acknowledge that what is knowable from wastewater surveillance has and will continue to change as the technology develops. One key example is the surveillance of SARS-CoV-2 variants in wastewater. Although technical challenges originally raised concerns about the feasibility of this use case, variant tracking through wastewater has proven valuable to provide indicators of variants in circulation—in some cases ahead of clinical data—despite important technical caveats.51,83,84 Researchers who have pioneered new techniques have an important role to guide these advances as they are incorporated into regular operation of wastewater surveillance programs.
As wastewater surveillance is professionalized and other methods of tracking COVID-19 outbreaks relax, we assert that data from wastewater will have an even greater role to play in guiding responses. In the United States, free testing programs have been discontinued in some places and there is increased reliance on at-home tests (which are often not reported to traditional disease surveillance systems).15,85 Wastewater surveillance is therefore less duplicative of other sources of information on outbreaks and has a significant lead on other indicators, such as hospitalizations,6,14,68,86 for which reporting will likely continue to be robust. We feel it is critically important that researchers continue to facilitate the interpretation and integration of this new data stream into public health response to outbreaks.
Conclusions
The academic research community launched wastewater surveillance as a largely ad hoc grassroots effort in the face of a global crisis, which in the United States has evolved into the NWSS. Similar efforts emerged in tandem worldwide.12 In our opinion, academic research laboratories can and should continue to contribute to these efforts by offering their strengths in pioneering new methods, transferring knowledge and expertise to support data interpretations, and training the next generation of professionals who will work in the frontline agencies involved in wastewater surveillance. Further, academic researchers can contribute to modeling and synthesizing the large volumes of data generated during the first years of the COVID-19 pandemic, which will be critical for understanding future pandemics.
The science and methods behind wastewater surveillance have made steady progress in just over 2 years, but as a field, we believe it is premature to codify and scale a single method or approach. It is clear there is much work yet to be done. We feel the rich diversity of methods developed and investigative approaches that spurred the progress to date should continue in academic research labs. Researchers can also contribute to evaluating options for standard methods. As academic researchers shift their efforts toward a more investigative and supporting role, they likely will have increased bandwidth to tackle important underpinning questions that will make wastewater surveillance, as a population health metric, a more useful tool for the public health community.
The pandemic has prompted many academic researchers to partner with commercial, municipal, and public health laboratories and to deliver data and, importantly, key interpretations of that data, to the public health sector. We encourage researchers to maintain these connections. There is a distinct advantage for advancing the field if researchers are closely tied to actual surveillance programs79 because this provides improved access to samples and data and makes new findings rapidly available for advancing wastewater surveillance. Importantly, we believe that two-way communication with public health laboratories and practitioners will foster stakeholder-driven research in academics’ programs (Figure 1).
Academic researchers will therefore need to make concerted efforts to develop relationships outside of their traditional disciplinary silos. Such efforts are not necessarily motivated by conventional metrics of academic success (i.e., publication of peer-reviewed literature), underscoring the need for new ways to incentivize the continued involvement of researchers in wastewater surveillance programs. Paramount to further advances in the field is funding to pursue research to address the most relevant stakeholder-driven questions. We argue that relationships are the impact-limiting ingredient for establishing a new complex public health monitoring system, and it will be important for researchers to stay embedded in the process.
Our proposed model for working partnerships is one in which researchers provide training and consulting, as well as transfer new knowledge from their research programs. The global pandemic organically grew a new type of hands-on academic/public health partnership that accelerated implementation of wastewater surveillance as a public health measure, and we feel strongly that we should build upon this success.
Acknowledgments
We thank A. Boehm for insightful discussions. This work was funded through a grant from the Alfred P. Sloan Foundation (to S.L.M., A.I.S., and K.B.).
References
- 1.Medema G, Heijnen L, Elsinga G, Italiaander R, Brouwer A. 2020. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ Sci Technol Lett 7(7):511–516, 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- 2.Lodder W, de Roda Husman AM. 2020. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol Hepatol 5(6):533–534, PMID: , 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherchan SP, Shahin S, Ward LM, Tandukar S, Aw TG, Schmitz B, et al. 2020. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci Total Environ 743:140621, PMID: , 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed W, Angel N, Edson J, Bibby K, Bivins A, O’Brien JW, et al. 2020. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ 728:138764, PMID: , 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar M, Patel AK, Shah AV, Raval J, Rajpara N, Joshi M, et al. 2020. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci Total Environ 746:141326, PMID: , 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peccia J, Zulli A, Brackney DE, Grubaugh ND, Kaplan EH, Casanovas-Massana A, et al. 2020. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat Biotechnol 38(10):1164–1167, PMID: , 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham KE, Loeb SK, Wolfe MK, Catoe D, Sinnott-Armstrong N, Kim S, et al. 2021. SARS-CoV-2 RNA in wastewater settled solids is associated with COVID-19 cases in a large urban sewershed. Environ Sci Technol 55(1):488–498, PMID: , 10.1021/acs.est.0c06191. [DOI] [PubMed] [Google Scholar]
- 8.Philo SE, Keim EK, Swanstrom R, Ong AQW, Burnor EA, Kossik AL, et al. 2021. A comparison of SARS-CoV-2 wastewater concentration methods for environmental surveillance. Sci Total Environ 760:144215, PMID: , 10.1016/j.scitotenv.2020.144215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pecson BM, Darby E, Haas CN, Amha YM, Bartolo M, Danielson R, et al. 2021. Reproducibility and sensitivity of 36 methods to quantify the SARS-CoV-2 genetic signal in raw wastewater: findings from an interlaboratory methods evaluation in the U.S. Environ Sci (Camb) 7(3):504–520, PMID: , 10.1039/D0EW00946F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClary-Gutierrez JS, Aanderud ZT, Al-Faliti M, Duvallet C, Gonzalez R, Guzman J, et al. 2021. Standardizing data reporting in the research community to enhance the utility of open data for SARS-CoV-2 wastewater surveillance. Environ Sci (Camb) 7(9):1545–1551, PMID: , 10.1039/D1EW00235J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin NJ, Servetas SL, Jackson SA, Lippa KA, Parratt KH, Mattson P, et al. 2022. Report on the DHS/NIST Workshop on Standards for an Enduring Capability in Wastewater Surveillance for Public Health (SWWS Workshop). NIST SP 1279. Gaithersburg, MD: National Institute of Standards and Technology. https://nvlpubs.nist.gov/nistpubs/SpecialPublications/NIST.SP.1279.pdf [accessed 5 December 2022]. [Google Scholar]
- 12.Naughton CC, Roman FA Jr, Alvarado AGF, Tariqi AQ, Deeming MA, Bibby K, et al. 2021. Show us the data: global COVID-19 wastewater monitoring efforts, equity, and gaps. medRxiv. Preprint posted online March 17, 2021. 10.1101/2021.03.14.21253564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClary-Gutierrez JS, Mattioli MC, Marcenac P, Silverman AI, Boehm AB, Bibby K, et al. 2021. SARS-CoV-2 wastewater surveillance for public health action. Emerg Infect Dis 27(9):1–8, PMID: , 10.3201/eid2709.210753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirby AE, Walters MS, Jennings WC, Fugitt R, LaCross N, Mattioli M, et al. 2021. Using wastewater surveillance data to support the COVID-19 Response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep 70(36):1242–1244, PMID: , 10.15585/mmwr.mm7036a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritchey MD, Rosenblum HG, Guercio KD, Humbard M, Santos S, Hall J, et al. 2022. COVID-19 self-test data: challenges and opportunities—United States, October 31, 2021–June 11, 2022. MMWR Morb Mortal Wkly Rep 71(32):1005–1010, PMID: , 10.15585/mmwr.mm7132a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bivins A, North D, Ahmad A, Ahmed W, Alm E, Been F, et al. 2020. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. Environ Sci Technol 54(13):7754–7757, PMID: , 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- 17.Randazzo W, Truchado P, Cuevas-Ferrando E, Simón P, Allende A, Sánchez G. 2020. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res 181:115942, PMID: , 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaTurner ZW, Zong DM, Kalvapalle P, Gamas KR, Terwillinger A, Crosby T, et al. 2021. Evaluating recovery, cost, and throughput of different concentration methods for SARS-CoV-2 wastewater-based epidemiology. Water Res 197:117043, PMID: , 10.1016/j.watres.2021.117043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenwald HD, Kennedy LC, Hinkle A, Whitney ON, Fan VB, Crits-Christoph A, et al. 2021. Tools for interpretation of wastewater SARS-CoV-2 temporal and spatial trends demonstrated with data collected in the San Francisco Bay Area. Water Res X 12:100111, PMID: , 10.1016/j.wroa.2021.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karthikeyan S, Ronquillo N, Belda-Ferre P, Alvarado D, Javidi T, Longhurst CA, et al. 2021. High-throughput wastewater SARS-CoV-2 detection enables forecasting of community infection dynamics in San Diego County. mSystems 6(2):e00045-21, PMID: , 10.1128/mSystems.00045-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weidhaas J, Aanderud ZT, Roper DK, VanDerslice J, Gaddis EB, Ostermiller J, et al. 2021. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci Total Environ 775:145790, PMID: , 10.1016/j.scitotenv.2021.145790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng S, Roguet A, McClary-Gutierrez JS, Newton RJ, Kloczko N, Meiman JG, et al. 2021. Evaluation of sampling, analysis, and normalization methods for SARS-CoV-2 concentrations in wastewater to assess COVID-19 burdens in Wisconsin communities. ACS EST Water 1(8):1955–1965, 10.1021/acsestwater.1c00160. [DOI] [Google Scholar]
- 23.Ai Y, Davis A, Jones D, Lemeshow S, Tu H, He F, et al. 2021. Wastewater SARS-CoV-2 monitoring as a community-level COVID-19 trend tracker and variants in Ohio, United States. Sci Total Environ 801:149757, PMID: , 10.1016/j.scitotenv.2021.149757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitney ON, Kennedy LC, Fan VB, Hinkle A, Kantor R, Greenwald H, et al. 2021. Sewage, salt, silica, and SARS-CoV-2 (4S): an economical kit-free method for direct capture of SARS-CoV-2 RNA from wastewater. Environ Sci Technol 55(8):4880–4888, PMID: , 10.1021/acs.est.0c08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez R, Curtis K, Bivins A, Bibby K, Weir MH, Yetka K, et al. 2020. COVID-19 surveillance in southeastern Virginia using wastewater-based epidemiology. Water Res 186:116296, PMID: , 10.1016/j.watres.2020.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerrity D, Papp K, Stoker M, Sims A, Frehner W. 2021. Early-pandemic wastewater surveillance of SARS-CoV-2 in Southern Nevada: methodology, occurrence, and incidence/prevalence considerations. Water Res X 10:100086, PMID: , 10.1016/j.wroa.2020.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagarkar M, Keely SP, Jahne M, Wheaton E, Hart C, Smith B, et al. 2022. SARS-CoV-2 monitoring at three sewersheds of different scales and complexity demonstrates distinctive relationships between wastewater measurements and COVID-19 case data. Sci Total Environ 816:151534, PMID: , 10.1016/j.scitotenv.2021.151534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoar C, Chauvin F, Clare A, McGibbon H, Castro E, Patinella S, et al. 2022. Monitoring SARS-CoV-2 in wastewater during New York City’s second wave of COVID-19: sewershed-level trends and relationships to publicly available clinical testing data. Environ Sci Water Res Technol 8:1021–1035, 10.1039/D1EW00747E. [DOI] [Google Scholar]
- 29.Bivins A, North D, Wu Z, Shaffer M, Ahmed W, Bibby K. 2021. Within- and between-day variability of SARS-CoV-2 RNA in municipal wastewater during periods of varying COVID-19 prevalence and positivity. ACS EST Water 1(9):2097–2108, 10.1021/acsestwater.1c00178. [DOI] [Google Scholar]
- 30.Keshaviah A, Hu XC, Henry M. 2021. Developing a flexible national wastewater surveillance system for COVID-19 and beyond. Environ Health Perspect 129(4):45002, PMID: , 10.1289/EHP8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris-Lovett S, Nelson KL, Beamer P, Bischel HN, Bivins A, Bruder A, et al. 2021. Wastewater surveillance for SARS-CoV-2 on college campuses: initial efforts, lessons learned, and research needs. Int J Environ Res Public Health 18(9):4455, PMID: , 10.3390/ijerph18094455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betancourt WQ, Schmitz BW, Innes GK, Prasek SM, Progreba Brown KM, Stark ER, et al. 2021. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. Sci Total Environ 779:146408, PMID: , 10.1016/j.scitotenv.2021.146408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooks YM, Gryskwicz B, Sheehan S, Piers S, Mahale P, McNeil S, et al. 2021. Detection of SARS-CoV-2 in wastewater at residential college, Maine, USA, August–November 2020. Emerg Infect Dis 27(12):3111–3114, PMID: , 10.3201/eid2712.211199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corchis-Scott R, Geng Q, Seth R, Ray R, Beg M, Biswas N, et al. 2021. Averting an outbreak of SARS-CoV-2 in a university residence hall through wastewater surveillance. Microbiol Spectr 9(2):e0079221, PMID: , 10.1128/Spectrum.00792-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karthikeyan S, Nguyen A, McDonald D, Zong Y, Ronquillo N, Ren J, et al. 2021. Rapid, large-scale wastewater surveillance and automated reporting system enable early detection of nearly 85% of COVID-19 cases on a university campus. mSystems 6(4):e0079321, PMID: , 10.1128/mSystems.00793-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeves K, Leibig J, Feula A, Saldi T, Lasda E, Johnson W, et al. 2021. High-resolution within-sewer SARS-CoV-2 surveillance facilitates informed intervention. medRxiv. Preprint posted online May 26, 2021. 10.1101/2021.05.24.21257632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bivins A, Lott M, Shaffer M, Wu Z, North D, Lipp EK, et al. 2022. Building-level wastewater surveillance using tampon swabs and RT-LAMP for rapid SARS-CoV-2 RNA detection. Environ Sci Water Res Technol 8(1):173–183, 10.1039/D1EW00496D. [DOI] [Google Scholar]
- 38.Water Research Foundation. 2020. Wastewater Surveillance of the COVID-19 Genetic Signal in Sewersheds: Recommendations from Global Experts. Alexandria, VA: Water Research Foundation. [Google Scholar]
- 39.Ahmed W, Bivins A, Bertsch PM, Bibby K, Choi PM, Farkas K, et al. 2020. Surveillance of SARS-CoV-2 RNA in wastewater: methods optimization and quality control are crucial for generating reliable public health information. Curr Opin Environ Sci Health 17:82–93, PMID: , 10.1016/j.coesh.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed W, Simpson SL, Bertsch PM, Bibby K, Bivins A, Blackall LL, et al. 2022. Minimizing errors in RT-PCR detection and quantification of SARS-CoV-2 RNA for wastewater surveillance. Sci Total Environ 805:149877, PMID: , 10.1016/j.scitotenv.2021.149877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolfe MK, Archana A, Catoe D, Coffman MM, Dorevich S, Graham KE, et al. 2021. Scaling of SARS-CoV-2 RNA in settled solids from multiple wastewater treatment plants to compare incidence rates of laboratory-confirmed COVID-19 in their sewersheds. Environ Sci Technol Lett 8(5):398–404, 10.1021/acs.estlett.1c00184. [DOI] [PubMed] [Google Scholar]
- 42.Sharara N, Endo N, Duvallet C, Ghaeli N, Matus M, Heussner J, et al. 2021. Wastewater network infrastructure in public health: applications and learnings from the COVID-19 pandemic. PLOS Glob Public Health 1(12):e0000061, PMID: , 10.1371/journal.pgph.0000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CDC (Centers for Disease Control and Prevention). 2022. Press release for COVID-19 Wastewater Surveillance dashboard. https://covid.cdc.gov/covid-data-tracker/#wastewater-surveillance [accessed 2 September 2022].
- 44.Global Water Pathogens Project. 2022. Wastewater Sphere [website]. https://sphere.waterpathogens.org/ [accessed 5 December 2022].
- 45.Safford HR, Shapiro K, Bischel HN. 2022. Wastewater analysis can be a powerful public health tool—if it’s done sensibly. Proc Natl Acad Sci USA (6):e2119600119, PMID: , 10.1073/pnas.2119600119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wastewater surveillance: a new public health tool to understand COVID-19’s spread in a community. 2022. https://www.cdc.gov/healthywater/surveillance/wastewater-surveillance/wastewater-surveillance.html [accessed 5 December 2022].
- 47.State of Wisconsin. 2022. Wisconsin Coronavirus Wastewater Monitoring Network [dashboard]. Last revised 16 September 2022. https://www.dhs.wisconsin.gov/covid-19/wastewater.htm [accessed 5 December 2022].
- 48.City of Chicago. 2022. COVID-19 Wastewater Surveillance. https://www.chicago.gov/city/en/sites/covid-19/home/covid-19-wastewater-surveillance.html [accessed 5 December 2022].
- 49.Owen C, Wright-Foulkes D, Alvarez P, Delgado H, Durance E, Wells G, et al. 2022. Persistence and discharge of SARS-CoV-2 RNA in Chicago-area water reclamation plants. FEMS Microbes 3:xtac015, 10.1093/femsmc/xtac015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keshaviah A, Karmali RN, Vohra D, Huffman T, Hu XC, Diamond MB. 2022. The Role of Wastewater Data in Pandemic Management. Washington, DC: Mathematica. https://www.rockefellerfoundation.org/wp-content/uploads/2022/04/The-Role-of-Wastewater-Data-in-Pandemic-Management-Survey-Research-Brief-Final.pdf [accessed 5 December 2022]. [Google Scholar]
- 51.Kirby AE, Welsh RM, Marsh ZA, Yu AT, Vugia DJ, Boehm AB, et al. 2022. Notes from the field: early evidence of the SARS-CoV-2 B.1.1.529 (Omicron) variant in community wastewater—United States, November–December 2021. MMWR Morb Mortal Wkly Rep 71(3):103–105, PMID: , 10.15585/mmwr.mm7103a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Link-Gelles R, Lutterloh E, Ruppert PS, Backenson PB, St George K, Rosenberg ES, et al. 2022. Public health response to a case of paralytic poliomyelitis in an unvaccinated person and detection of poliovirus in wastewater—New York, June–August 2022. Morb Mortal Wkly Rep 71(33):1065–1068. https://www.cdc.gov/mmwr/volumes/71/wr/mm7133e2.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozere RL, Faulkner R, Van Rooyen CE. 1961. Enteroviruses in sewage and epidemic poliomyelitis in eastern Canada. Can Med Assoc J 85(27):1419–1424, PMID: . [PMC free article] [PubMed] [Google Scholar]
- 54.Peigue-Lafeuille H, Laveran H, Auberger M, Chambon M, Trimolet M, Beytout D. 1985. Evaluation of 4 years of surveillance of enteroviruses in sewage. Correlation with human pathology. [In French.] Rev Epidemiol Sante Publique 33(6):445–451, PMID: . [PubMed] [Google Scholar]
- 55.Bondarenko VI, Zadorozhnaia VI, Siniak LI, Prikhod’ko EF, Stoletniaia M, Trifonova NA, et al. 1991. The epidemiological surveillance of poliomyelitis in the Ukraine. [In Russian.] Zh Mikrobiol Epidemiol Immunobiol 7:38–41, PMID: . [PubMed] [Google Scholar]
- 56.Manor Y, Handsher R, Halmut T, Neuman M, Bobrov A, Rudich H, et al. 1999. Detection of poliovirus circulation by environmental surveillance in the absence of clinical cases in Israel and the Palestinian Authority. J Clin Microbiol 37(6):1670–1675, PMID: , 10.1128/JCM.37.6.1670-1675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madico G, Checkley W, Gilman RH, Bravo N, Cabrera L, Calderon M, et al. 1996. Active surveillance for Vibrio cholerae O1 and vibriophages in sewage water as a potential tool to predict cholera outbreaks. J Clin Microbiol 34(12):2968–2972, PMID: , 10.1128/jcm.34.12.2968-2972.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pina S, Jofre J, Emerson SU, Purcell RH, Girones R. 1998. Characterization of a strain of infectious hepatitis E virus isolated from sewage in an area where hepatitis E is not endemic. Appl Environ Microbiol 64(11):4485–4488, PMID: , 10.1128/AEM.64.11.4485-4488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou L, Singh A, Jiang J, Xiao L. 2003. Molecular surveillance of Cryptosporidium spp. in raw wastewater in Milwaukee: implications for understanding outbreak occurrence and transmission dynamics. J Clin Microbiol 41(11):5254–5257, PMID: , 10.1128/JCM.41.11.5254-5257.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sedmak G, Bina D, MacDonald J. 2003. Assessment of an enterovirus sewage surveillance system by comparison of clinical isolates with sewage isolates from Milwaukee, Wisconsin, collected August 1994 to December 2002. Appl Environ Microbiol 69(12):7181–7187, PMID: , 10.1128/AEM.69.12.7181-7187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villena C, El-Senousy WM, Abad FX, Pintó RM, Bosch A. 2003. Group A rotavirus in sewage samples from Barcelona and Cairo: emergence of unusual genotypes. Appl Environ Microbiol 69(7):3919–3923, PMID: , 10.1128/AEM.69.7.3919-3923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robertson LJ, Forberg T, Gjerde BK. 2008. Giardia cysts in sewage influent in Bergen, Norway 15–23 months after an extensive waterborne outbreak of giardiasis. J Appl Microbiol 104(4):1147–1152, PMID: , 10.1111/j.1365-2672.2007.03630.x. [DOI] [PubMed] [Google Scholar]
- 63.Hughes B, Duong D, White BJ, Wigginton KR, Chan EMG, Wolfe MK, et al. 2022. Respiratory syncytial virus (RSV) RNA in wastewater settled solids reflects RSV clinical positivity rates. Environ Sci Technol Lett 9(2):173–178, 10.1021/acs.estlett.1c00963. [DOI] [Google Scholar]
- 64.Wolfe MK, Duong D, Bakker KM, Ammerman M, Mortenson L, Hughes B, et al. 2022. Wastewater-based detection of an influenza outbreak. medRxiv. Preprint posted online February 19, 2022. 10.1101/2022.02.15.22271027. [DOI] [Google Scholar]
- 65.Nelson B. 2022. What poo tells us: wastewater surveillance comes of age amid covid, monkeypox, and polio. BMJ 378:o1869, PMID: , 10.1136/bmj.o1869. [DOI] [PubMed] [Google Scholar]
- 66.Wolfe MK, Duong D, Hughes B, Chan-Herur V, White BJ, Boehm AB. 2022. Detection of monkeypox viral DNA in a routine wastewater monitoring program. medRxiv. Preprint posted online July 26 2022. 10.1101/2022.07.25.22278043. [DOI] [Google Scholar]
- 67.Kim S, Kennedy LC, Wolfe MK, Criddle CS, Duong DH, Topol A, et al. 2022. SARS-CoV-2 RNA is enriched by orders of magnitude in primary settled solids relative to liquid wastewater at publicly owned treatment works. Environ Sci (Camb) 8(4):757–770, PMID: , 10.1039/d1ew00826a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D’Aoust PM, Graber TE, Mercier E, Montpetit D, Alexandrov I, Neault N, et al. 2021. Catching a resurgence: increase in SARS-CoV-2 viral RNA identified in wastewater 48 h before COVID-19 clinical tests and 96 h before hospitalizations. Sci Total Environ 770:145319, PMID: , 10.1016/j.scitotenv.2021.145319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bivins A, Bibby K. 2021. Wastewater surveillance during mass COVID-19 vaccination on a college campus. Environ Sci Technol Lett 8(9):792–798, 10.1021/acs.estlett.1c00519. [DOI] [PubMed] [Google Scholar]
- 70.Ye Y, Ellenberg RM, Graham KE, Wigginton KR. 2016. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ Sci Technol 50(10):5077–5085, PMID: , 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- 71.Liu P, Ibaraki M, VanTassell J, Geith K, Cavallo M, Kann R, et al. 2022. A sensitive, simple, and low-cost method for COVID-19 wastewater surveillance at an institutional level. Sci Total Environ 807(pt 3):151047, PMID: , 10.1016/j.scitotenv.2021.151047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Habtewold J, McCarthy D, McBean E, Law I, Goodridge L, Habash M, et al. 2022. Passive sampling, a practical method for wastewater-based surveillance of SARS-CoV-2. Environ Res 204(pt B):112058, PMID: , 10.1016/j.envres.2021.112058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hayes EK, Sweeney CL, Fuller M, Erjavec GB, Stoddart AK, Gagnon GA. 2022. Operational constraints of detecting SARS-CoV-2 on passive samplers using electronegative filters: a kinetic and equilibrium analysis. ACS EST Water 2(11):1910–1920, 10.1021/acsestwater.1c00441. [DOI] [PubMed] [Google Scholar]
- 74.Amoah ID, Mthethwa NP, Pillay L, Deepnarain N, Pillay K, Awolusi OO, et al. 2021. RT-LAMP: a cheaper, simpler and faster alternative for the detection of SARS-CoV-2 in wastewater. Food Environ Virol 13(4):447–456, PMID: , 10.1007/s12560-021-09489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu Y, Wu X, Gu A, Dobelle L, Cid CA, Li J, et al. 2022. Membrane-based in-gel loop-mediated isothermal amplification (mgLAMP) system for SARS-CoV-2 quantification in environmental waters. Environ Sci Technol 56(2):862–873, PMID: , 10.1021/acs.est.1c04623. [DOI] [PubMed] [Google Scholar]
- 76.El-Sherif DM, Abouzid M, Gaballah MS, Ahmed AA, Adeel M, Sheta SM. 2022. New approach in SARS-CoV-2 surveillance using biosensor technology: a review. Environ Sci Pollut Res Int 29(2):1677–1695, PMID: , 10.1007/s11356-021-17096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Proverbio D, Kemp F, Magni S, Ogorzaly L, Cauchie HM, Gonçalves J, et al. 2022. Model-based assessment of COVID-19 epidemic dynamics by wastewater analysis. Sci Total Environ 827:154235, PMID: , 10.1016/j.scitotenv.2022.154235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arabzadeh R, Grünbacher DM, Insam H, Kreuzinger N, Markt R, Rauch W. 2021. Data filtering methods for SARS-CoV-2 wastewater surveillance. Water Sci Technol 84(6):1324–1339, PMID: , 10.2166/wst.2021.343. [DOI] [PubMed] [Google Scholar]
- 79.Delgado Vela J, McClary-Gutierrez JS, Al-Faliti M, Allan V, Arts P, Barbero R, et al. 2022. Impact of disaster research on the development of early career researchers: lessons learned from the wastewater monitoring pandemic response efforts. Environ Sci Technol 56(8):4724–4727, PMID: , 10.1021/acs.est.2c01583. [DOI] [PubMed] [Google Scholar]
- 80.Houston Health Department. Houston Wastewater Epidemiology. https://hou-wastewater-epi.org/ [accessed 5 December 2022].
- 81.Stadler LB, Ensor KB, Clark JR, Kalvapalle P, LaTurner ZW, Mojica L, et al. 2020. Wastewater analysis of SARS-CoV-2 as a predictive metric of positivity rate for a major metropolis. medRxiv. Preprint posted online November 6, 2020. 10.1101/2020.11.04.20226191. [DOI] [Google Scholar]
- 82.Scott LC, Aubee A, Babahaji L, Vigil K, Tims S, Aw TG. 2021. Targeted wastewater surveillance of SARS-CoV-2 on a university campus for COVID-19 outbreak detection and mitigation. Environ Res 200:111374, PMID: , 10.1016/j.envres.2021.111374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolfe M, Hughes B, Duong D, Chan-Herur V, Wigginton KR, White BJ, et al. 2022. Detection of SARS-CoV-2 variants Mu, Beta, Gamma, Lambda, Delta, Alpha, and Omicron in wastewater settled solids using mutation-specific assays is associated with regional detection of variants in clinical samples. Appl Environ Microbiol 88(8):e0004522, PMID: , 10.1128/aem.00045-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schussman MK, Roguet A, Schmoldt A, Dinan B, McLellan SL. 2022. Wastewater surveillance using ddPCR accurately tracked Omicron emergence due to altered N1 probe binding efficiency. Environ Sci Water Res Technol 8(10):2190–2195, 10.1039/D2EW00194B. [DOI] [Google Scholar]
- 85.Rader B, Gertz A, Iuliano AD, Gilmer M, Wronski L, Astley CM, et al. 2022. Use of at-home COVID-19 tests—United States, August 23, 2021–March 12, 2022. MMWR Morb Mortal Wkly Rep 71(13):489–494, PMID: , 10.15585/mmwr.mm7113e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saguti F, Magnil E, Enache L, Churqui MP, Johansson A, Lumley D, et al. 2021. Surveillance of wastewater revealed peaks of SARS-CoV-2 preceding those of hospitalized patients with COVID-19. Water Res 189:116620, PMID: , 10.1016/j.watres.2020.116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carcereny A, Garcia-Pedemonte D, Martínez-Velázquez A, Quer J, Garcia-Cehic D, Gregori J, et al. 2022. Dynamics of SARS-CoV-2 Alpha (B.1.1.7) Variant spread: the wastewater surveillance approach. Environ Res 208:112720, PMID: , 10.1016/j.envres.2022.112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peterson SW, Lidder R, Daigle J, Wonitowy Q, Dueck C, Nagasawa A, et al. 2022. RT-qPCR detection of SARS-CoV-2 mutations S 69–70 del, S N501Y and N D3L associated with variants of concern in Canadian wastewater samples. Sci Total Environ 810:151283, PMID: , 10.1016/j.scitotenv.2021.151283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu AT, Hughes B, Wolfe MK, Leon T, Duong D, Rabe A, et al. 2022. Estimating relative abundance of 2 SARS-CoV-2 variants through Wastewater surveillance at 2 large metropolitan sites, United States. Emerg Infect Dis 28(5):940–970, PMID: , 10.3201/eid2805.212488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agrawal S, Orschler L, Schubert S, Zachmann K, Heijnen L, Tavazzi S, et al. 2022. Prevalence and circulation patterns of SARS-CoV-2 variants in European sewage mirror clinical data of 54 European cities. Water Res 214:118162, PMID: , 10.1016/j.watres.2022.118162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smyth DS, Trujillo M, Gregory DA, Cheung K, Gao A, Graham M, et al. 2022. Tracking cryptic SARS-CoV-2 lineages detected in NYC wastewater. Nat Commun 13(1):635, PMID: , 10.1038/s41467-022-28246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mao K, Zhang H, Yang Z. 2020. Can a paper-based device trace COVID-19 sources with wastewater-based epidemiology? Environ Sci Technol 54(7):3733–3735, PMID: , 10.1021/acs.est.0c01174. [DOI] [PubMed] [Google Scholar]
- 93.Morales Medina WR, D’Elia S, Fahrenfeld NL. 2022. Accumulation of SARS-CoV-2 RNA in sewer biofilms. ACS EST Water 2(11):1844–1851, 10.1021/acsestwater.1c00345. [DOI] [Google Scholar]
- 94.Shi J, Li X, Zhang S, Sharma E, Sivakumar M, Sherchan SP, et al. 2022. Enhanced decay of coronaviruses in sewers with domestic wastewater. Sci Total Environ 813:151919, PMID: , 10.1016/j.scitotenv.2021.151919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bisseux M, Debroas D, Mirand A, Archimbaud C, Peigue-Lafeuille H, Bailly JL, et al. 2020. Monitoring of enterovirus diversity in wastewater by ultra-deep sequencing: an effective complementary tool for clinical enterovirus surveillance. Water Res 169:115246, PMID: , 10.1016/j.watres.2019.115246. [DOI] [PubMed] [Google Scholar]
- 96.McMahan CS, Self S, Rennert L, Kalbaugh C, Kriebel D, Graves D, et al. 2021. COVID-19 wastewater epidemiology: a model to estimate infected populations. Lancet Planet Health 5(12):e874–e881, PMID: , 10.1016/S2542-5196(21)00230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]