Abstract
Aims
Pulmonary arterial hypertension (PAH) is a fatal disease without a cure. Previously, we found that transcription factor RUNX1-dependent haematopoietic transformation of endothelial progenitor cells may contribute to the pathogenesis of PAH. However, the therapeutic potential of RUNX1 inhibition to reverse established PAH remains unknown. In the current study, we aimed to determine whether RUNX1 inhibition was sufficient to reverse Sugen/hypoxia (SuHx)-induced pulmonary hypertension (PH) in rats. We also aimed to demonstrate possible mechanisms involved.
Methods and results
We administered a small molecule specific RUNX1 inhibitor Ro5-3335 before, during, and after the development of SuHx-PH in rats to investigate its therapeutic potential. We quantified lung macrophage recruitment and activation in vivo and in vitro in the presence or absence of the RUNX1 inhibitor. We generated conditional VE-cadherin-CreERT2; ZsGreen mice for labelling adult endothelium and lineage tracing in the SuHx-PH model. We also generated conditional Cdh5-CreERT2; Runx1(flox/flox) mice to delete Runx1 gene in adult endothelium and LysM-Cre; Runx1(flox/flox) mice to delete Runx1 gene in cells of myeloid lineage, and then subjected these mice to SuHx-PH induction. RUNX1 inhibition in vivo effectively prevented the development, blocked the progression, and reversed established SuHx-induced PH in rats. RUNX1 inhibition significantly dampened lung macrophage recruitment and activation. Furthermore, lineage tracing with the inducible VE-cadherin-CreERT2; ZsGreen mice demonstrated that a RUNX1-dependent endothelial to haematopoietic transformation occurred during the development of SuHx-PH. Finally, tissue-specific deletion of Runx1 gene either in adult endothelium or in cells of myeloid lineage prevented the mice from developing SuHx-PH, suggesting that RUNX1 is required for the development of PH.
Conclusion
By blocking RUNX1-dependent endothelial to haematopoietic transformation and pulmonary macrophage recruitment and activation, targeting RUNX1 may be as a novel treatment modality for pulmonary arterial hypertension.
Keywords: Endothelial cells, Macrophages, RUNX1, Pulmonary hypertension
1. Introduction
Pulmonary arterial hypertension (PAH) is a rare but progressive disease without a cure. The hallmark of PAH is the development of pulmonary vascular remodelling, which leads to elevated pulmonary vascular resistance, right heart failure, and ultimately death.1–3 Current therapies that consist of drugs with selective pulmonary vasodilatory properties can improve symptoms and delay disease progression, but their disease modifying effects are minimal.4,5 There is a critical need to develop novel treatments that target the underlying cause of the pulmonary vascular remodelling in PAH.
A strong association of PAH with dysregulated immunity and inflammation has been well-established. Considerable data have accrued to suggest that perivascular inflammatory responses mediated by bone marrow (BM)-derived macrophages play a primary role in the pathogenesis of PAH.6,7 Thus, evaluation of novel therapies for PAH that target both the origin and the activation of macrophages is warranted. Runt-related transcription factor 1 (RUNX1) is a member of the core-binding factor family of transcription factors that is indispensable for embryonic endothelial to haematopoietic transition (EHT) and the establishment of definitive haematopoiesis in vertebrates.8–10 RUNX1 partners with a constitutively expressed β subunit (core-binding factor β; CBFβ) to form a transcriptionally active heterodimer that can either activate or repress target gene expression.11 Mice with a homozygous knockout of Runx1 lack definitive haematopoiesis and are unable to survive past an early embryonic stage (Days 11.5–12.5).12 In our previous study, specific inhibition of RUNX1 blocked egress of BM-derived endothelial progenitor cells (EPCs) and attenuated the development of pulmonary hypertension (PH) in mice.13 We postulate that RUNX1 is required in the myeloid-skewed haematopoietic transformation of BM-derived EPCs during the development of PAH. In support of this hypothesis, we found that gene expression of Runx1 is increased in circulating CD34 + CD133 + EPCs isolated from peripheral blood of patients with PH.13
Macrophages that mediate inflammation have been classified as M1, whereas those that resolve inflammation as M2 macrophages.14 Our earlier studies showed that the drastic increases in production of pro-inflammatory cytokines (e.g. IL-1β, IL-6, and TNF-α) and chemokines (e.g. fractalkine/CX3CL1 and MCP-1/CCL2) attributed to M1 macrophages and M1 macrophage-treated endothelial cells (ECs) coincide with the elevated levels of the same cytokines and chemokines found in PAH patients.15 It is possible that M1 macrophages are the mediators of perivascular inflammation leading to pulmonary vascular remodelling. Interestingly, direct binding of RUNX1 to p50-NF-κB in macrophages was shown to trigger signals responsible for production of pro-inflammatory cytokines.16,17 Taken together, we hypothesize that RUNX1 plays a pivotal role in the production and activation of macrophages that lead to perivascular inflammation and vascular remodelling in PAH. To test this hypothesis, we inhibited RUNX1 activity via a small molecule specific inhibitor Ro5-333518 and examined its effect on overall macrophage production, recruitment, and activation, and the attenuation of PH in the Sugen/hypoxia (SuHx) rat model. Our objectives were to determine whether inhibition of RUNX1 was sufficient to reverse established PH and to also determine possible mechanisms involved. The current report is an extension of previously published work by our group which described that inhibition of RUNX1 prevents development of SuHx-induced PH in mice.13 In the present study, we now show that RUNX1 inhibition is not only effective in mice but also in rats, which is the more established model to investigate PAH. Furthermore, we show that inhibition of RUNX1 does not only prevent but also reverses SuHx-PH in rats. In addition, we generated transgenic mice with deletion of Runx1 gene in adult endothelium or in cells of myeloid linage. We found that the tissue-specific Runx1 deficiency protects the mice from developing SuHx-PH, indicating that RUNX1 is required for the development of PH. Overall, our study suggests that, by blocking RUNX1-dependent endothelial to haematopoietic transformation and pulmonary macrophage recruitment and activation, targeting RUNX1 may be as a novel treatment modality for PAH.
2. Methods
2.1 SuHx-PH model in rats
The SuHx-PH model in rats was carried out as published previously,19 which is also described in detail in Supplementary material online. Rats were anaesthetized via isoflurane inhalation (1–3%). For euthanasia, the inferior vena cava was transected and the anaesthetized rats were euthanized by exsanguination. All animal experiments were approved by the Lifespan Institutional Animal Care and Use Committee (IACUC) at Rhode Island Hospital. All procedures were performed conforming to the NIH Guide for the Care and Use of Laboratory Animals.
2.2 Study protocols of RUNX1 inhibition in vivo
A prevention, an intervention, and a reversal protocol were used in this study. In the disease prevention protocol, rats were given 20 mg/kg of Ro5-3335 in 100 µL of DMSO or 100 µL vehicle alone by subcutaneous injection every other day for three times at the beginning of SuHx treatment, and the development of PH was assessed 1 week after removal from hypoxia (Hx) (Figure 1A). In the disease intervention protocol, rats were given 20 mg/kg of Ro5-3335 in 100 µL of DMSO or 100 µL vehicle alone by subcutaneous injection every other day for three times 1 week after the beginning of SuHx treatment, and the development of PH was assessed 1 week after removal from Hx (Figure 3A). In the disease reversal protocol, rats were given 20 mg/kg of Ro5-3335 in 100 µL of DMSO or 100 µL vehicle alone by subcutaneous injection every other day for six times after the completion of SuHx treatment, and the development of PH was assessed 2 weeks after removal from Hx (Figure 4A). Bronchoalveolar lavage (BAL) fluid of the rats was collected by inserting a catheter in the trachea, through which 5 mL phosphate buffered saline (PBS) was instilled into the bronchioles and the BAL fluid was gently retracted. After centrifugation, the BAL supernatants were frozen at –20°C and used later to detect inflammatory cytokines. The cell pellets were resuspended in PBS and treated with the Red Blood Cell Lysis Buffer (MilliporeSigma, Burlington, MA) followed by twice PBS washes and filtration through a nylon mesh. Cells in the BAL fluid were fixed and permeabilized with LEUCOPERM (Bio-Rad, Hercules, CA) and stained with a mouse monoclonal antibody against rat CD68 conjugated with Alexa Fluor 488 (clone ED1, Bio-Rad) to identify rat macrophages. Flow cytometry analysis was performed on a 4-laser LSR II flow cytometer (BD Biosciences, San Diego, CA) and data were analysed with the FlowJo software (FlowJo, Ashland, OR).
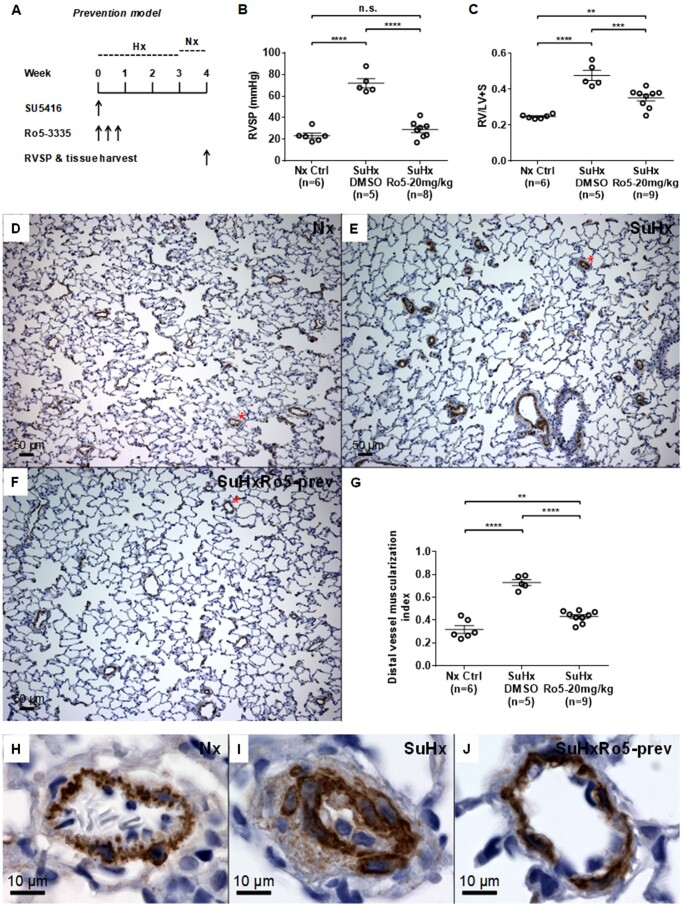
Figure 1.
Inhibition of RUNX1 in vivo prevents the development of Sugen/hypoxia-induced pulmonary hypertension (SuHx-PH) in rats. (A) Experimental protocol for prevention of SuHx-PH in rats shows administration of the RUNX1 inhibitor Ro5-3335 (Ro5) every other day for three times at the beginning of SuHx treatment. SU5416: VEGF receptor 2 antagonist Sugen 5416. (B and C) Right ventricular systolic pressure (RVSP) (B) and the Fulton’s index (right ventricle to left ventricle + septum, RV/LV+S ratio) (C) were measured 1 week after removal from 3 weeks of hypoxia (Hx). (D–F) Representative microscopic images of 100× magnification show immunohistochemical (IHC) staining in brown colour of α-smooth muscle actin (α-SMA) in the blood vessels from lungs of normoxia control (Nx Ctrl) rats (D), vehicle DMSO-treated SuHx rats (E), and 20 mg/kg Ro5-treated SuHx rats (F). (G) Muscularization of distal pulmonary vessels less than 50 µm in diameter was assessed by calculating the muscularization index defined as the total area of the vessel that stained positive for α-SMA divided by total cross-sectional area of the vessel. (H–J) Representative small blood vessels stained in brown colour of α-SMA, which are labelled with a red asterisk in (D–F), are shown in 600× magnification: (H) Nx Ctrl rats; (I) vehicle DMSO-treated SuHx rats; and (J) Ro5-treated SuHx rats. **P < 0.01, ***P < 0.001, ****P < 0.0001, n.s.: not significant, ordinary one-way ANOVA with multiple comparisons, n = number of animals in each experimental group.
Figure 3.
Inhibition of RUNX1 in vivo blocks the progression of SuHx-PH in rats. (A) Experimental protocol for intervention of SuHx-PH in rats shows administration of the RUNX1 inhibitor Ro5-3335 every other day for three times 1 week after the beginning of SuHx treatment. (B and C) RVSP (B) and RV/LV + S ratio (C) were measured 1 week after removal from 3 weeks of Hx. (D–F) Representative microscopic images of 100× magnification show IHC staining in brown colour of α-SMA in the blood vessels from lungs of Nx Ctrl rats (D), vehicle DMSO-treated SuHx rats (E) and Ro5-treated SuHx rats (F). (G) Muscularization of distal pulmonary vessels less than 50 µm in diameter was assessed by calculating the muscularization index. (H–J) Representative small blood vessels stained in brown colour of α-SMA, which are labelled with a red asterisk in (D–F), are shown in 600× magnification: (H) Nx Ctrl rats; (I) vehicle DMSO-treated SuHx rats; and (J) Ro5-treated SuHx rats. **P < 0.01, ***P < 0.001, ****P < 0.0001, n.s.: not significant, ordinary one-way ANOVA with multiple comparisons, n = number of animals in each experimental group.
Figure 4.
Inhibition of RUNX1 in vivo reverses established SuHx-PH in rats. (A) Experimental protocol for reversal of SuHx-PH in rats shows administration of the RUNX1 inhibitor Ro5-3335 every other day for six times after the completion of SuHx treatment. (B and C) RVSP (B) and RV/LV + S ratio (C) were measured 2 weeks after removal from 3 weeks of Hx. (D–F) Representative microscopic images of 100× magnification show IHC staining in brown colour of α-SMA in the blood vessels from lungs of Nx Ctrl rats (D), vehicle DMSO-treated SuHx rats (E) and Ro5-treated SuHx rats (F). (G) Muscularization of distal pulmonary vessels less than 50 µm in diameter was assessed by calculating the muscularization index. (H–J) Representative small blood vessels stained in brown colour of α-SMA, which are labelled with a red asterisk in (D–F), are shown in 600× magnification: (H) Nx Ctrl rats; (I) vehicle DMSO-treated SuHx rats; and (J) Ro5-treated SuHx rats. *P < 0.05, ***P < 0.001, ****P < 0.0001, n.s.: not significant, ordinary one-way ANOVA with multiple comparisons, n = number of animals in each experimental group.
2.3 Histologic analysis of lung sections
Histologic analysis of lung sections were carried out as described in Supplementary material online.
2.4 Inducibly labelling of ECs/EPCs in adult VE-cadherin-CreERT2; ZsGreen mice and lineage tracing in vivo under SuHx conditions
All mice (summarized in Supplementary material online, Table S1) were maintained in pathogen-free conditions and all procedures were approved by the Lifespan IACUC. The inducible endothelial-specific VE-cadherin-CreERT2 mice20,21 were obtained from Dr Iruela-Arispe (University of California, Los Angeles, CA), and genotyping for these mice was performed with the following primers: VE-cadherin common forward 5′-GCA GGC AGC TCA CAA AGG AAC AAT-3′; VE-cadherin reverse 5′-TGT CCT TGC TGA GTG ACA GTG GAA-3′; and VE-cadherin Cre reverse 5′-ATC ACT CGT TGC ATC GAC CGG TAA-3′. The Rosa26-LSL-ZsGreen reporter mice [B6.Cg-Gt(ROSA)26Sor(tm6)(CAG-ZsGreen1)Hze/J, JAX #007906] were purchased from the Jackson Laboratory (Bar Harbor, ME), and genotyping for these mice was performed according to the vendor’s protocol. Male VE-cadherin-CreERT2 mice were crossbred with female Rosa26-LSL-ZsGreen reporter mice to generate VE-cadherin-CreERT2; ZsGreen mice (see Supplementary material online, Figure S5A). Adult double transgenic mice of 10–13 weeks of age with correct genotype were used for lineage tracing experiments. Briefly, the inducible double transgenic mice were each given 2 mg of tamoxifen (Tam) (MilliporeSigma) dissolved in 100 µL of corn oil (MilliporeSigma) daily via intraperitoneal injection for 5 days followed by 1 week of incubation. Subsequent SuHx treatment consisted of 3 weekly subcutaneous injections of the Sugen VEGF receptor 2 inhibitor SU5416 at 20 mg/kg in 100 µL DMSO or vehicle alone. Concurrent with the SU5416 treatment, the double transgenic mice were exposed to Hx (8.5% O2) or normoxia (Nx) for 3 weeks, and 50 µL of peripheral blood samples were collected weekly by retro-orbital bleeding from each mouse (see Supplementary material online, Figure S5B). For anaesthesia during retro-orbital blood sampling, the mice were anaesthetized via intraperitoneal injection with ketamine (100 mg/kg) and xylazine (10 mg/kg). For euthanasia, the mice were euthanized via CO2 asphyxia followed by cervical dislocation. After red blood cell lysis, the rest of the cells in the blood samples were washed with PBS and stained with antibodies against mouse CD45 conjugated with Allophycocyanin (APC) (BioLegend, San Diego, CA). Flow cytometry analysis was performed on a 4-laser LSR II flow cytometer (BD Biosciences), and data were analysed with the FlowJo software.
2.5 Genetic deletion of Runx1 in adult ECs/EPCs to prevent SuHx-PH in mice
We have previously used the constitutive VE-cadherin-Cre mice (JAX #006137) from the Jackson Laboratory and the inducible VE-cadherin-CreERT2 mice from Dr Iruela-Arispe’s lab (UCLA) (see Supplementary material online, Table S1). However, in order to most efficiently delete the Runx1 gene in adult ECs/EPCs, we have also purchased the Cdh5(PAC)-CreERT2 mouse developed by Dr Ralf Adams22 (Taconic #13073, Taconic Biosciences, Rensselaer, NY). Upon Tam induction, this mouse line has a significantly better VE-cadherin promoter driven Cre-mediated recombination in ECs/EPCs than the UCLA mouse line (Personal communication, 2018 Gordon Research Conference on Endothelial Cells). We have also established the Runx1(flox/flox) mouse colony (JAX #008772, Jackson Laboratory, Bar Harbor, ME) in our lab. We then crossbred them to generate Cdh5-CreERT2; Runx1(fl/fl) mice (Figure 5A). Mice with correct genotype were each treated with 2 mg of Tam daily via intraperitoneal injection for 5 days followed by 1 week of incubation to delete Runx1 gene in adult ECs/EPCs. Lung ECs from the mice treated with Tam were isolated via flow cytometry cell sorting,23 and the loss of Runx1 in ECs was verified per qRT–PCR with the following primers: forward 5′-CCT CCT TGA ACC ACT CCA CT-3′, and reverse 5′-CTG GAT CTG CCT GGC ATC-3′. GAPDH expression was assessed per qRT–PCR as an internal reference for quantification with the following primers: forward 5′-AAA AGC AAC TCC CAC TCT TC-3′ and reverse 5′-CCT GTT GCT GTA GCC GTA TT-3′. All PCR primers were synthesized by Integrated DNA Technologies (Coralville, IA). As a control experiment to test the endothelial specificity of Runx1 deletion, we also evaluated Runx1 gene expression in CD14+ monocytes isolated from BM of these mice following Tam induction. An anti-mouse CD14 antibody conjugated with APC was purchased from BioLegend and BM CD14+ cells were sorted on a BD Influx flow cytometry cell sorter (BD Biosciences). For SuHx-PH induction, similar to described above in Supplementary material online, Figure S5B, Tam was given daily via intraperitoneal injection for 5 days followed by 1 week of incubation before the 3 weeks SuHx treatment of the Cdh5-CreERT2; Runx1(fl/fl) mice. Development of PH in mice was determined by measurement of right ventricular systolic pressure (RVSP) and right ventricular (RV) hypertrophy [assessed by the Fulton’s index, i.e. RV to left ventricle + septum wet weight ratio (RV/LV + S)] as described in our previous study.13
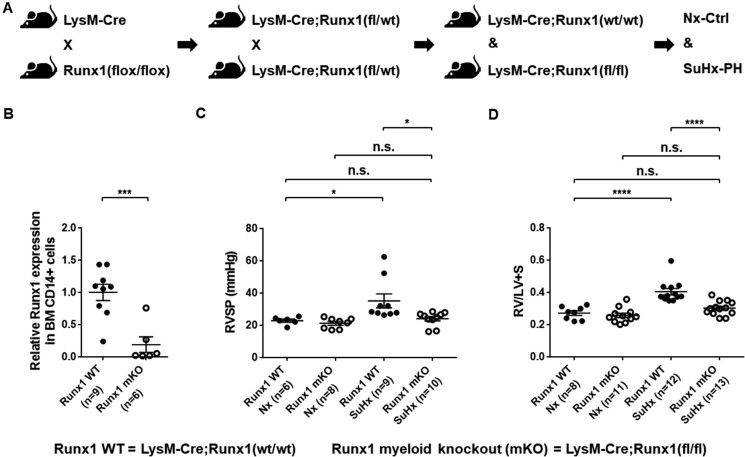
Figure 5.
Genetic deletion of Runx1 in adult ECs/EPCs prevents SuHx-PH development in mice. (A) Experimental design to crossbreed Cdh5(PAC)-CreERT2 and Runx1(flox/flox) mice to generate Cdh5-CreERT; Runx1(fl/fl) mice, which were then subjected to tamoxifen treatment followed by SuHx-PH induction. (B) In 10- to 13-week-old mice, the loss of Runx1 in lung endothelial cells (ECs) upon Tam induction was verified per qRT–PCR. No changes in Runx1 gene expression in BM-derived CD14+ cells were found, demonstrating the endothelial specificity of Runx1 deletion in these mice. (C and D) Under Nx conditions, Cdh5-CreERT2; Runx1(wt/wt) mice, Cdh5-CreERT2; Runx1(fl/fl) mice treated with corn oil, and Cdh5-CreERT2; Runx1(fl/fl) mice treated with Tam all exhibited normal RVSP (C) and RV/LV + S ratio (D). Under SuHx conditions, Cdh5-CreERT2; Runx1(wt/wt) mice and Cdh5-CreERT2; Runx1(fl/fl) mice treated with corn oil exhibited significantly elevated RVSP (C) and RV/LV + S ratio (D). When the Cdh5-CreERT2; Runx1(fl/fl) mice were treated with Tam and placed under SuHx conditions, they exhibited normal RVSP (C) and RV/LV + S ratio (D). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n.s.: not significant, unpaired two-tailed Student’s t-test (B) and ordinary one-way ANOVA with multiple comparisons (C and D), n = number of animals in each experimental group.
2.6 Genetic deletion of Runx1 in cells of myeloid lineage to prevent SuHx-PH in mice
LysM-Cre mice (JAX #004781) from the Jackson Laboratory allow for both specific and highly efficient Cre-mediated deletion of loxP-flanked target genes in myeloid cells: a deletion efficiency of 83–98% was determined in mature macrophages and near 100% in granulocytes.24,25 We crossbred the LysM-Cre mice with the Runx1(flox/flox) mice to generate LysM-Cre; Runx1(fl/fl) mice (Figure 6A). The loss of Runx1 in flow cytometry sorted BM-derived CD14+ monocytes was verified per qRT–PCR as described above. Subsequent SuHx treatment consisted of three weekly subcutaneous injections of the Sugen VEGF receptor 2 inhibitor SU5416 at 20 mg/kg in 100 µL DMSO. Concurrent with the SU5416 treatment, the transgenic mice were exposed to Hx (8.5% O2) for 3 weeks. Development of PH in mice was determined by measurement of RVSP and RV hypertrophy as described in our previous study.13
Figure 6.
Genetic deletion of Runx1 in cells of myeloid lineage prevents SuHx-PH development in mice. (A) Experimental design to crossbreed LysM-Cre and Runx1(flox/flox) mice to generate LysM-Cre; Runx1(fl/fl) mice, which were then subjected to SuHx-PH induction. (B) In 10- to 13-week-old mice, the loss of Runx1 in flow cytometry sorted BM-derived CD14+ monocytes was verified per qRT–PCR. (C and D) Under Nx conditions, LysM-Cre; Runx1(wt/wt) mice and LysM-Cre; Runx1(fl/fl) mice exhibited normal RVSP (C) and RV/LV + S ratio (D). Under SuHx conditions, LysM-Cre; Runx1(wt/wt) mice developed elevated RVSP (C) and RV/LV + S ratio (D), whereas the LysM-Cre; Runx1(fl/fl) mice maintained normal RVSP (C) and RV/LV + S ratio (D). *P < 0.05, ***P < 0.001, ****P < 0.0001, n.s.: not significant, unpaired two-tailed Student’s t-test (B) and ordinary one-way ANOVA with multiple comparisons (C and D), n = number of animals in each experimental group.
2.7 Macrophage polarization in vitro
Macrophage polarization in vitro was carried out as published previously,15 which is also described in detail in Supplementary material online.
2.8 Statistical analysis
Data are shown as the mean values ± standard error of the mean. The significance of difference was calculated with unpaired two-tailed Student’s t-test or one-way ANOVA when comparing more than two groups by using the GraphPad Prism programme (GraphPad Software, Inc., La Jolla, CA). P-values less than 0.05 were considered to be statistically significant.
3. Results
3.1 Inhibition of RUNX1 in vivo prevents the development of SuHx-PH in rats
Compared with Nx control rats, SuHx treatment caused severe PH in rats as evidenced by marked increases in RVSP, RV hypertrophy, and striking vascular remodelling in the lung.19 In the disease prevention model of the present study (Figure 1A), vehicle-treated SuHx rats developed elevated RVSP compared with Nx control rats (Figure 1B). RVSP in rats given the RUNX1 inhibitor Ro5-3335 (SuHxRo5) at the beginning of SuHx treatment was significantly lower than SuHx rats given vehicle alone, and was no different than in Nx control rats (Figure 1B). SuHx treatment also caused significant RV hypertrophy compared with the Nx rats as indicated by an increase in the Fulton’s index (RV/LV + S ratio) (Figure 1C). However, the Fulton’s index was lower in rats treated with the RUNX1 inhibitor than in SuHx rats given vehicle alone (Figure 1C). Compared with the Nx control rats (Figure 1D and H), SuHx rats developed marked vascular remodelling as indicated by an increase in muscularization of peripheral pulmonary vessels (Figure 1E and I), which was significantly reduced in rats treated with the RUNX1 inhibitor (Figure 1F and J). The distal pulmonary vessel (i.e. pulmonary arteriole) muscularization indexes in the prevention model are summarized in Figure 1G. Combined, these results suggest that inhibition of RUNX1 early on can effectively prevent the development of PH.
3.2 Inhibition of RUNX1 in vivo reduces macrophage recruitment in the lung of SuHx-treated rats
In the prevention model, we quantified the macrophages in the lung following immunohistochemical staining of CD68, which is a pan-macrophage marker for rats.26 Compared with the Nx rats (Figure 2A and E), the SuHx rats had significantly increased number of CD68+ macrophages (stained brown), many of which morphologically appeared to have been in an activated state as suggested by their expanded sizes and the formation of membrane blebs (Figure 2B and F). Treatment with the RUNX 1 inhibitor effectively prevented macrophage recruitment in the lungs of SuHx-treated rats (Figure 2C and G). The numbers of CD68+ macrophages in the prevention model are summarized in Figure 2D. These results suggest that inhibition of RUNX1 in vivo at the onset of the process can significantly reduce macrophage recruitment in the lung during SuHx induction of PH.
Figure 2.
Inhibition of RUNX1 in vivo reduces macrophage recruitment in the lung of SuHx-treated rats. (A–C) Representative microscopic images of 100× magnification show IHC staining in dark brown colour of CD68+ macrophages in the lung of Nx rats (A), vehicle DMSO-treated SuHx rats (B), and Ro5-treated SuHx rats (C). (D) Three representative images of 100× magnification of the staining were taken for each sample and the number of CD68+ macrophages in each image was manually counted. A summary of the numbers of CD68+ macrophages per microscopic field is shown. (E–G) Representative macrophages stained in dark brown colour for CD68, which are labelled with a red asterisk in (A–C), are shown in 600× magnification: (E) Nx Ctrl rats; (F) vehicle DMSO-treated SuHx rats; and (G) Ro5-treated SuHx rats. ***P < 0.001, ****P < 0.0001, n.s.: not significant, ordinary one-way ANOVA with multiple comparisons, n = number of animals in each experimental group.
3.3 Inhibition of RUNX1 in vivo blocks the progression of SuHx-PH in rats
In the disease intervention model of the current study (Figure 3A), SuHx treatment caused a significantly elevated RVSP in rats compared with Nx control rats (Figure 3B). However, when rats received the RUNX1 inhibitor Ro5-3335 1 week after the onset of SuHx induction of PH, their average RVSP was significantly lower than that of the SuHx rats treated only with the vehicle, and had no difference compared with the Nx control rats (Figure 3B). Compared with the Nx control rats, SuHx treatment led to a higher Fulton’s index suggesting the development of RV hypertrophy (Figure 3C). However, compared with the vehicle-treated SuHx rats, the degree of RV hypertrophy was significantly diminished when rats were treated with the RUNX1 inhibitor (Figure 3C). Compared with the Nx control rats (Figure 3D and H), the SuHx rats developed marked vascular remodelling as indicated by the α-SMA staining in brown colour (Figure 3E and I), which was significantly inhibited when the rats were treated with the RUNX1 inhibitor (Figure 3F and J). The distal pulmonary vessel (i.e. pulmonary arteriole) muscularization indexes in the intervention model are summarized in Figure 3G. Combined, these results suggest that inhibition of RUNX1 in vivo during disease development can effectively block the progression of SuHx-PH.
3.4 Inhibition of RUNX1 in vivo reverses established SuHx-PH in rats
In the reversal model, rats received extended RUNX1 inhibition after SuHx-induced PH had already established (Figure 4A). SuHx rats treated with vehicle alone developed elevated RVSP compared with Nx control rats (Figure 4B). Treatment with the RUNX1 inhibitor Ro5-3335 significantly reduced RVSP in SuHxRo5 rats when compared with the vehicle-treated SuHx rats, and reversed RVSP to the level comparable to that of the Nx control rats (Figure 4B). SuHx induction of PH resulted in prominent RV hypertrophy in the SuHx rats compared with the Nx control rats as indicated by the Fulton’s index (Figure 4C). However, the severity of RV hypertrophy was significantly attenuated in rats that were given the RUNX1 inhibitor when compared with the vehicle-treated SuHx rats (Figure 4C). Compared with the Nx control rats (Figure 4D and H), the SuHx rats developed marked vascular remodelling as indicated by the α-SMA staining in brown colour (Figure 4E and I), which was significantly reversed when the rats were treated with the RUNX1 inhibitor (Figure 4F and J). The distal pulmonary vessel (i.e. pulmonary arteriole) muscularization indexes in the reversal model are summarized in Figure 4G. In addition, we also demonstrated that there were no effects of Ro5-3335 on RVSP and RV/LV + S ratio in normal rats after a 2-week long treatment (see Supplementary material online, Figure S1A–C). Combined, these results suggest that inhibition of RUNX1 in vivo after the completion of SuHx induction of PH can effectively reverse established disease.
3.5 Inhibition of RUNX1 in vivo depletes alveolar macrophages in rats
Alveolar macrophages in the BAL consist of resident alveolar macrophages derived from yolk sac precursors of foetal monocytes and BM monocyte-derived macrophages following inflammatory insults.27 In the reversal model, rats received extended RUNX1 inhibition after SuHx-induced PH had already established. Flow cytometry analysis showed that in Nx control rats CD68+ macrophages accounted for an average 75.3 ± 2.0%, but in Nx rats treated with the RUNX1 inhibitor, CD68+ macrophages were almost completely depleted and accounted for an average 1.6 ± 0.3% of the cellular content of the BAL (see Supplementary material online, Figure S1D). In SuHx rats, CD68+ macrophages accounted for an average 64.9 ± 6.5% of the cellular content of the BAL, which is comparable with that in the Nx rats. SuHx rats that received RUNX1 inhibition in our reversal protocol had significantly fewer CD68+ macrophages (56.4 ± 8.5%) than that of the Nx rats, but the alveolar macrophage number was not significantly lower than that of the SuHx-PH rats (see Supplementary material online, Figure S1D).
3.6 Inhibition of RUNX1 in vivo has no effect on pulmonary arteriole density in rats
We counted all distal pulmonary vessels less than 50 µm in diameter that were stained α-SMA+ in the above three study protocols and then compared the numbers between SuHx treated rats with and without RUNX1 inhibition. Our results suggest that inhibition of RUNX1 in vivo has no effect on pulmonary arteriole density among SuHx treated rats (see Supplementary material online, Figure S2).
3.7 No immediate vasodilatory improvement of SuHx-PH in mice by the RUNX1 inhibitor Ro5-3335
To investigate if the RUNX1 inhibitor Ro5-3335 may alleviate SuHx-PH in rodent models through pulmonary vasodilatory properties, we injected C57BL/6J mice with 20 mg/kg Ro5-3335 following 3 weeks of SuHx-PH induction and assessed RVSP and RV hypertrophy at 1 h or 3 h after Ro5-3335 injection (see Supplementary material online, Figure S3A). As shown in Supplementary material online, Figure S3B and C, no reductions of RVSP or RV hypertrophy were seen at these two time points following Ro5-3335 injection. These results, which show no immediate vasodilatory improvement of SuHx-PH in mice by the RUNX1 inhibitor Ro5-3335, suggest that Ro5-3335 does not act as a vessel dilator.
3.8 Inducibly labelling of adult ECs/EPCs in mice and lineage tracing demonstrates haematopoietic transformation of ECs in vivo
We generated the VE-cadherin-CreERT2; ZsGreen double transgenic mice (see Supplementary material online, Figure S5A and Table S1), which were treated with 2 mg of Tam daily for 5 days followed by 1 week of incubation before SuHx-PH induction (see Supplementary material online, Figure S5B). Our results of adult lung endothelium labelling upon Tam induction in these mice are demonstrated and quantified in Supplementary material online, Figure S4A–G, which suggested an age-dependent labelling efficiency similar with published results.20,21 In Cre+/–ZsG+/– mice, there is an extremely low level (e.g. 0.032%) of background of CD45+ZsG+ cells upon Tam induction, compared with Cre+/–ZsG+/– mice without Tam induction or Cre+/–ZsG–/– mice with Tam induction (see Supplementary material online, Figure S5C). However, when a Tam induced Cre+/–ZsG+/– mouse (#917) was treated with SuHx, the CD45+ZsG+ population in the PB of this mouse increased greater than 10-fold from the initial 0.074% at W0 to 0.32% at W1 and to 0.80% at W2 before returning to the background level at W3 (see Supplementary material online, Figure S5D). Another Tam-induced Cre+/–ZsG+/– mouse (#977) also produced a distinct CD45+ZsG+ population (0.2%) at W1 compared with the initial 0% background (see Supplementary material online, Figure S5D). The generation of CD45+ZsG+ new blood cells by ZsG-labelled adult endothelium in the VE-cadherin-CreERT2; ZsGreen double transgenic mice is quantitatively summarized in Supplementary material online, Figure S6A. The significant difference in CD45+ cells that express ZsG before (W0) and after SuHx treatment (W1) in these mice would have to derive from ECs/EPCs in adulthood. The exhaustion of ZsG-labelled progenitor cells may be the reason for the disappearance of CD45+ZsG+ cells at W3. The time course of expression for CD45+ZsG+ cells in the Tam-inducible model over 3 weeks is similar to that in the constitutive model.13 In our first control experiment, Tam-induced Cre+/–ZsG+/– mice were placed in Nx instead of SuHx for 3 weeks, and there was no development of a CD45+ZsG+ population in the PB of these mice (see Supplementary material online, Figures S5E and S6B). In our second control experiment, Cre+/–ZsG+/– mice were injected with vehicle corn oil without Tam followed by SuHx induction of PH, and there were no CD45+ZsG+ cells in the PB of these mice throughout the 3 weeks of SuHx treatment (see Supplementary material online, Figures S5F and S6C). More importantly, we demonstrated that the emergence of CD45+ZsG+ cells is RUNX1-dependent, as the RUNX1 inhibitor Ro5-3335 blocked the development of any such cells (see Supplementary material online, Figure S6D and E). This RUNX1-dependent transformation is reminiscent of that in the constitutive model which we reported earlier.13 Combined, our lineage tracing experiments, in both constitutive and inducible mouse models, suggest that under pathological conditions, such as Hx or induction of PH, haematopoietic transformation of adult endothelium may occur in a RUNX1-dependent manner.
3.9 Genetic deletion of Runx1 in adult ECs/EPCs prevents SuHx-PH development in mice
In order to conditionally delete Runx1 gene in adult ECs/EPCs, we crossbred Cdh5(PAC)-CreERT2 and Runx1(flox/flox) mice to generate the Cdh5-CreERT2; Runx1(fl/fl) mice (Figure 5A and Supplementary material online, Table S1). At 10–13 weeks of age, these inducible double transgenic mice were each treated with 2 mg of Tam daily for 5 days followed by 1 week of incubation before SuHx-PH induction (Figure 5A). The loss of Runx1 in mouse lung ECs upon Tam induction was verified per qRT–PCR (Figure 5B). In a control experiment to demonstrate the endothelial specificity of Runx1 deletion in the Cdh5-CreERT2; Runx1(fl/fl) mice, we flow-sorted CD14+ monocytes from BM of these mice following tam induction. In comparison with controls, no changes of Runx1 gene expression were found in the BM-derived CD14+ cells, indicating that endothelial deletion of Runx1 did not affect Runx1 expression in myeloid cells in this mouse model (Figure 5B). Under Nx conditions, Cdh5-CreERT2; Runx1(wt/wt) mice, Cdh5-CreERT2; Runx1(fl/fl) mice treated with corn oil, and Cdh5-CreERT2; Runx1(fl/fl) mice treated with Tam all exhibited normal RVSP and RV/LV + S ratio (Figure 5C and D). Under SuHx conditions, Cdh5-CreERT2; Runx1(wt/wt) mice and Cdh5-CreERT2; Runx1(fl/fl) mice treated with corn oil exhibited significantly elevated RVSP and RV/LV + S ratio (Figure 5C and D). However, when the Cdh5-CreERT2; Runx1(fl/fl) mice were treated with Tam and placed under SuHx conditions, they exhibited normal RVSP and RV/LV + S ratio, which were significantly less than those of the Cdh5-CreERT2; Runx1(fl/fl) mice treated with corn oil and placed under SuHx conditions, and were comparable with those values of the Cdh5-CreERT2; Runx1(fl/fl) mice treated with Tam but placed under Nx conditions (Figure 5C and D). These results suggest that genetic deletion of Runx1 in adult ECs/EPCs prevents SuHx-PH development in mice.
3.10 Genetic deletion of Runx1 in cells of myeloid lineage prevents SuHx-PH development in mice
We crossbred LysM-Cre and Runx1(flox/flox) mice to generate the LysM-Cre; Runx1(fl/fl) mice (Figure 6A and Supplementary material online, Table S1). In 10- to 13-week-old mice, the loss of Runx1 in flow cytometry sorted BM-derived CD14+ monocytes was verified per qRT–PCR (Figure 6B). Under Nx conditions, LysM-Cre; Runx1(wt/wt) mice and LysM-Cre; Runx1(fl/fl) mice exhibited normal RVSP and RV/LV + S ratio (Figure 6C and D). Under SuHx conditions, LysM-Cre; Runx1(wt/wt) mice developed elevated RVSP and RV/LV + S ratio. However, under SuHx conditions, the LysM-Cre; Runx1(fl/fl) mice maintained normal RVSP and RV/LV + S ratio, which were significantly less than those of the LysM-Cre; Runx1(wt/wt) mice under SuHx conditions, and were comparable with those values of the LysM-Cre; Runx1(fl/fl) mice kept in Nx (Figure 6C and D). These results suggest that genetic deletion of Runx1 in cells of myeloid lineage prevents SuHx-PH development in mice.
3.11 Inhibition of RUNX1 dampens macrophage activation in vitro
We used different sets of cell surface markers (CD14+CD80+ and CD68+CD80+) to quantify M1-polarized activation of human bone marrow mononuclear cells (BMMNCs). Compared with untreated cells, LPS and IFN-γ treatment drastically increased the expression of CD14, CD68, and CD80 in BMMNCs. Co-incubation of BMMNCs with the RUNX1 inhibitor Ro5-3335, but not with vehicle alone, decreased M1-polarized activation in a dose-dependent manner (see Supplementary material online, Figure S7A and B). We also used different sets of cell surface markers (CD163+CD206+ and CD11b+CD206+) to indicate M2-polarized activation of BMMNCs. Compared with untreated cells, IL-4 and IL-13 treatment increased the expression of CD11b, CD163, and CD206 in BMMNCs. Co-incubation of BMMNCs with the RUNX1 inhibitor Ro5-3335 had little effect on M2-polarized activation, although a non-significant trend towards reduced M2 macrophages was seen at the highest dose tested (see Supplementary material online, Figure S7C and D). Similarly, compared with untreated cells, M1-polarized activation of human THP1 cells drastically increased the expression of CD14, CD80, and HLA-DR. Co-incubation of THP1 cells with Ro5-3335, but not with vehicle alone, decreased M1-polarized activation in a dose-dependent manner (see Supplementary material online, Figure S7E and F). Combined, these results indicate that inhibition of RUNX1 can effectively dampen overall activation of macrophages.
4. Discussion
Perivascular infiltration by macrophages is well described in PAH and increasingly recognized as a major pathogenic component of pulmonary vascular remodelling.6,28–33 Several studies have focused on the role of perivascular macrophages infiltrating pulmonary arterioles. CD68+ macrophages are prominent in advanced obliterative plexiform lesions observed in experimental and clinical PH,33 and Florentin et al.34 have recently reported that inflammatory macrophage expansion in PH depends upon mobilization of blood-borne monocytes. Blocking CD68+ macrophage-derived leukotriene B4 prevents endothelial injury and reverses PH in animal models of the disease.35 However, a recently completed multi-centre prospective clinical trial of the leukotriene B4 inhibitor (Ubenimex) was not successful,36 nor was another trial of the IL-1β receptor blocker anakinra.37 These failures suggest that blocking one or more pro-inflammatory mediators will not be sufficient to impede PAH development. Hence, our goal of the current study was to reverse established PH by: (i) reducing the overall macrophage production through blocking RUNX1-dependent myeloid-skewed haematopoiesis; and (ii) inhibiting RUNX1-dependent macrophage recruitment and activation.
In the present study, we used conditional labelling of adult endothelium and lineage tracing to demonstrate that during the induction of PH, a small proportion of adult ECs/EPCs undergo haematopoietic transformation to yield cells of myeloid lineage and that this phenomenon is mediated by RUNX1. Inhibition of RUNX1 was effective at preventing such transformation, reducing macrophage recruitment in the lung and the classical pro-inflammatory activation. Furthermore, tissue-specific deletion of Runx1 gene either in adult endothelium or in cells of myeloid lineage prevented the mice from developing SuHx-PH, suggesting that RUNX1 is required for the development of PH. Finally, in the rat model of SuHx-PH, administration of a RUNX1 inhibitor markedly attenuated RVSP, RV hypertrophy, and vascular remodelling in three separate protocols demonstrating that this approach was effective at preventing the disease, halting its progression and reversing established PH. Our additional experiments indicate that the RUNX1 inhibitor Ro5-3335 does not act as a vessel dilator in the short term. Combined, these findings extend our previously published observations of RUNX1 inhibition in murine models of PH and suggest that targeting RUNX1 may be an effective approach to treating PAH.
The importance of RUNX1-mediated haematopoietic transformation of ECs/EPCs in PH has been questioned due to the small number of myeloid cells that can be traced to endothelial lineage and to previous studies suggesting this transition does not occur in adult animals.38 In our recent studies, we bred VE-cadherin-Cre; ZsGreen mice, and subjected these constitutive transgenic mice to SuHx induction of PH.13 However, a small number of the haemangioblasts are known to have VE-cadherin promoter activity during early development of the embryo,39 which may have resulted in labelling of some haematopoietic cells with ZsG, and made it difficult to distinguish de novo CD45+ cells that derived from the endothelium in adulthood. To eliminate this background in lineage tracing, we acquired the inducible VE-cadherin-CreERT2 mice. These mice have no constitutive expression of ZsG in embryonic haemangioblasts meaning that the generation of CD45+ZsG+ new blood cells in the VE-cadherin-CreERT2; ZsGreen double transgenic mice would have to derive from ECs/EPCs in adulthood. Importantly, CD45+ZsG+ were nearly undetectable in adult control mice, but increased approximately 10-fold within 2 weeks of starting SuHx and this increase was completely blocked by inhibition of RUNX1. Nonetheless, the number of myeloid cells that could be traced to endothelial lineage is most likely limited by the labelling efficiency of the inducible VE-cadherin-CreERT2; ZsGreen double transgenic mice used in the experiments. Our ability to characterize CD45+ZsG+ cells was very much limited due to the minuscule output of these cells after the inducible VE-cadherin-CreERT2; ZsGreen mice were treated with Tam and placed under SuHx conditions. Several studies suggest that the haemogenic endothelium is not as transient in nature as previously thought and may exist beyond the embryonic period as an untapped haematopoietic reservoir.40–42 Our lineage tracing experiments, in both constitutive13 and inducible mouse models in this study, suggest that under pathological conditions, haematopoietic transformation of ECs/EPCs may reactivate and contribute to adult haematopoiesis in a RUNX1-dependent manner.
Since homozygous Runx1 knockout mice exhibit an embryonic lethal phenotype,12 additional experiments with genetic deletion of Runx1 in adult endothelium or in cells of myeloid lineage were performed in this study after the more superior Cdh5(PAC)-CreERT2 mouse developed by Dr Ralf Adams22 became available to our lab. Importantly, our control experiments testing Runx1 gene expression in the BM-derived CD14+ monocytes of Tam-induced Cdh5-CreERT2; Runx1(fl/fl) mice indicate that endothelial deletion of Runx1 did not affect Runx1 expression in myeloid cells in this mouse model. A causative role for RUNX1 in PH is then strongly supported by the demonstration that that tissue-specific knockout of Runx1 in adult ECs/EPCs or in cells of myeloid lineage prevents SuHx-PH development in mice, and that inhibition of RUNX1 is sufficient to both prevent and reverse PH in mice and rat models of PH. Thus, the ability of RUNX1 to induce myeloid cell production through haematopoietic transformation of ECs/EPCs and to promote pro-inflammatory activation of monocytes/macrophages underscores its importance in the pathogenesis of PAH. Recently, several large scale patient studies utilizing whole genome sequencing in cohorts from Europe, the USA, and Japan have independently identified mutations in the Sox17 enhancer region associated with PAH.43–47 However, the mechanisms with which Sox17 mutations lead to PAH is not understood. Notably, SOX17 can directly bind Runx1 gene to repress haematopoietic cell fate during embryonic EHT,48 and small perturbations in SOX17 levels are accompanied by concomitantly increased levels of RUNX1 driving haemogenic endothelium towards a haematopoietic fate.49 Importantly, SOX17 was found to function as a switch that directs cell fate of CD34+ progenitors towards an endothelial vs. haematopoietic lineage.50,51 Thus, it is possible that loss of repression of Runx1 by SOX17 in ECs/EPCs facilitates myeloid-skewed haematopoietic transformation serving as the origin of inflammatory cells seen in PAH.
As a master-regulator transcription factor implicated in diverse signalling pathways and cellular mechanisms during normal development and disease, RUNX1 has recently also been proposed as a therapeutic target for cardiovascular disease.52 McCarroll et al.53 conducted a study using mice with an inducible cardiomyocyte-specific Runx1 deficiency, and provided evidence that Runx1 deficiency protects against adverse cardiac remodelling after myocardial infarction. This finding was later corroborated by other investigators as well.54,55 Hence, novel therapies targeting RUNX1 may be efficacious by impacting on multiple downstream signalling pathways to mitigate adverse cardiac remodelling. Although transcription factors were thought as unlikely and challenging druggable targets in the past, transcription factor drivers of cancer have been directly targeted using small molecule inhibitors.56 The compound Ro5-3335 and its analog Ro24-7429 were initially developed as anti-HIV drugs that block HIV gene expression by inhibiting Tat-mediated transactivation.57–59 In 2012, Cunningham et al.18 first reported that Ro5-3335 was able to directly interact with RUNX1 and its heterodimeric partner CBFβ, repress RUNX1/CBFβ-dependent transactivation in reporter assays, and repress RUNX1-dependent haematopoiesis in zebrafish embryos. The investigators concluded that Ro5-3335 interacts with both RUNX1 and CBFβ, with stronger affinity for the former. However, Ro5-3335 does not disrupt RUNX1–CBFβ interaction completely, but changes the conformation of their complex or increases the distance between RUNX1 and CBFβ in the complex.18 Ro5-3335 was subsequently used to inhibit RUNX1 in a variety of disease processes including in retinal angiogenesis60 and acute myeloid leukaemia,61 and to suppress macrophage-induced inflammation in septic shock.16 Studies on pharmacokinetics and pharmacodynamics of Ro5-3335 have been conducted, and long-term administration of the compound is well-tolerated in mice.18 In another series of studies, Illendula et al.62 synthesized a library of analogs of compound AI-4-57 and characterized their activity as small molecule inhibitors of RUNX-CBFβ binding. The compound AI-4-57 was initially discovered to bind to the CBFβ portion of the CBFβ-SMMHC fusion protein and inhibits its binding to the Runt domain of RUNX proteins.63 AI-4-57 and its derivatives may also be tested as candidates for RUNX1 inhibition in SuHx-PH rats in future studies. In summary, by blocking RUNX1-dependent myeloid-skewed haematopoiesis and macrophage recruitment and activation (Figure 7), inhibition of RUNX1 prevents and reverses PH in the SuHx-PH model in rats. It may be possible to use RUNX1 inhibitors to evaluate the role of RUNX1-dependent aberrant haematopoiesis and macrophage activation in patients with PAH. Thus, targeting RUNX1 may be a novel therapeutic approach in the treatment of PAH.
Figure 7.

Tentative mechanisms with which targeting the haematopoietic transcription factor RUNX1 can be an effective novel treatment modality for PAH. Our model of pathogenesis of PAH (black arrows in clockwise) and proposed inhibition of RUNX1-dependent pathogenic processes indicated in red. Specific RUNX1 inhibition by a small molecule compound Ro5-3335 has the potential to block both the aberrant myeloid-skewed haematopoiesis and macrophage recruitment and activation, thus significantly impede disease development and progression.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
E.-M.J. and M.P.: Substantial contributions to study conception and design, analysis and interpretation of data, drafting and revision and final approval of manuscript, and agreement to be accountable for all aspects of the work. E.-Y.S., K.Q.W., M.D.T., S.W., and M.S.D.: Contributions to study design, acquisition, analysis, and interpretation of data, revision, and final approval of manuscript. P.M.D., A.M.R., C.E.V., P.J.Q., and J.R.K.: Contributions to interpretation of data and revision and final approval of manuscript. O.D.L.: Substantial contributions to study conception and design, data acquisition, analysis and interpretation of data, revision and final approval of manuscript, and agreement to be accountable for all aspects of the work.
Supplementary Material
Acknowledgements
We wish to thank the Flow Cytometry Core of the COBRE Center for Stem Cells and Aging. We also thank Ginny Hovanesian for microscope imaging and Dongfang Yang for immunohistochemical staining.
Funding
This work was supported in part by grants from the National Institutes of Health P20 GM119943 (O.D.L. and P.J.Q.), P20 GM103652 (O.D.L.), R01 HL141268 (C.E.V.), and T32 HL116249 (E.-Y.S., O.D.L., and P.J.Q.), by the American Heart Association Transformational Project Award 18TPA34110329 (O.D.L.), the Rhode Island Foundation Medical Research Fund Nr. 5219_20200595 (E.-M.J. and O.D.L.), and an Academic Assessment Research Award from the Brown Physicians, Inc. (O.D.L.).
Contributor Information
Euy-Myoung Jeong, Division of Hematology/Oncology, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA.
Mandy Pereira, Division of Hematology/Oncology, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA; Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA.
Eui-Young So, Division of Hematology/Oncology, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA.
Keith Q Wu, Division of Hematology/Oncology, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA.
Michael Del Tatto, Division of Hematology/Oncology, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA.
Sicheng Wen, Division of Hematology/Oncology, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA.
Mark S Dooner, Division of Hematology/Oncology, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA.
Patrycja M Dubielecka, Division of Hematology/Oncology, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA.
Anthony M Reginato, Division of Rheumatology, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA.
Corey E Ventetuolo, Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA.
Peter J Quesenberry, Division of Hematology/Oncology, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA.
James R Klinger, Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA.
Olin D Liang, Division of Hematology/Oncology, Department of Medicine, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI 02903, USA.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Translational perspective
RUNX1 inhibition in vivo reversed pulmonary hypertension and improved vascular remodelling in the Sugen/hypoxia-induced pulmonary hypertension model in rats. These findings suggest that, by blocking RUNX1-dependent endothelial to haematopoietic transformation and pulmonary macrophage recruitment and activation, targeting RUNX1 may be as a novel treatment modality for pulmonary arterial hypertension.
References
- 1. Farber HW, Loscalzo J.. Pulmonary arterial hypertension. N Engl J Med 2004;351:1655–1665. [DOI] [PubMed] [Google Scholar]
- 2. Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A.. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004;43:5S–12S. [DOI] [PubMed] [Google Scholar]
- 3. Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R.. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34–D41. [DOI] [PubMed] [Google Scholar]
- 4. McLaughlin VV, Shah SJ, Souza R, Humbert M.. Management of pulmonary arterial hypertension. J Am Coll Cardiol 2015;65:1976–1997. [DOI] [PubMed] [Google Scholar]
- 5. Ventetuolo CE, Klinger JR.. WHO Group 1 pulmonary arterial hypertension: current and investigative therapies. Prog Cardiovasc Dis 2012;55:89–103. [DOI] [PubMed] [Google Scholar]
- 6. Rabinovitch M, Guignabert C, Humbert M, Nicolls MR.. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 2014;115:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nicolls MR, Voelkel NF.. The roles of immunity in the prevention and evolution of pulmonary arterial hypertension. Am J Respir Crit Care Med 2017;195:1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Swiers G, de Bruijn M, Speck NA.. Hematopoietic stem cell emergence in the conceptus and the role of Runx1. Int J Dev Biol 2010;54:1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lichtinger M, Ingram R, Hannah R, Muller D, Clarke D, Assi SA, Lie ALM, Noailles L, Vijayabaskar MS, Wu M, Tenen DG, Westhead DR, Kouskoff V, Lacaud G, Gottgens B, Bonifer C.. RUNX1 reshapes the epigenetic landscape at the onset of haematopoiesis. EMBO J 2012;31:4318–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA.. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 2009;457:887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levanon D, Groner Y.. Structure and regulated expression of mammalian RUNX genes. Oncogene 2004;23:4211–4219. [DOI] [PubMed] [Google Scholar]
- 12. Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA.. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci U S A 1996;93:3444–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang OD, So EY, Egan PC, Goldberg LR, Aliotta JM, Wu KQ, Dubielecka PM, Ventetuolo CE, Reginato AM, Quesenberry PJ, Klinger JR.. Endothelial to haematopoietic transition contributes to pulmonary arterial hypertension. Cardiovasc Res 2017;113:1560–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol 2012;32:463–488. [DOI] [PubMed] [Google Scholar]
- 15. Wu KQ, Muratore CS, So EY, Sun C, Dubielecka PM, Reginato AM, Liang OD.. M1 macrophage-induced endothelial-to-mesenchymal transition promotes infantile hemangioma regression. Am J Pathol 2017;187:2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luo MC, Zhou SY, Feng DY, Xiao J, Li WY, Xu CD, Wang HY, Zhou T.. Runt-related transcription factor 1 (RUNX1) binds to p50 in macrophages and enhances TLR4-triggered inflammation and septic shock. J Biol Chem 2016;291:22011–22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tugal D, Liao X, Jain MK.. Transcriptional control of macrophage polarization. Arterioscler Thromb Vasc Biol 2013;33:1135–1144. [DOI] [PubMed] [Google Scholar]
- 18. Cunningham L, Finckbeiner S, Hyde RK, Southall N, Marugan J, Yedavalli VR, Dehdashti SJ, Reinhold WC, Alemu L, Zhao L, Yeh JR, Sood R, Pommier Y, Austin CP, Jeang KT, Zheng W, Liu P.. Identification of benzodiazepine Ro5-3335 as an inhibitor of CBF leukemia through quantitative high throughput screen against RUNX1-CBFbeta interaction. Proc Natl Acad Sci U S A 2012;109:14592–14597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klinger JR, Pereira M, Del Tatto M, Brodsky AS, Wu KQ, Dooner MS, Borgovan T, Wen S, Goldberg LR, Aliotta JM, Ventetuolo CE, Quesenberry PJ, Liang OD.. Mesenchymal stem cell extracellular vesicles reverse Sugen/hypoxia pulmonary hypertension in rats. Am J Respir Cell Mol Biol 2020;62:577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML.. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 2008;3:625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML.. VE-cadherin-CreERT2 transgenic mouse: a model for inducible recombination in the endothelium. Dev Dyn 2006;235:3413–3422. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH.. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 2010;465:483–486. [DOI] [PubMed] [Google Scholar]
- 23. van Beijnum JR, Rousch M, Castermans K, van der Linden E, Griffioen AW.. Isolation of endothelial cells from fresh tissues. Nat Protoc 2008;3:1085–1091. [DOI] [PubMed] [Google Scholar]
- 24. Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I.. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 1999;8:265–277. [DOI] [PubMed] [Google Scholar]
- 25. Abram CL, Roberge GL, Hu Y, Lowell CA.. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J Immunol Methods 2014;408:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Damoiseaux JG, Dopp EA, Calame W, Chao D, MacPherson GG, Dijkstra CD.. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology 1994;83:140–147. [PMC free article] [PubMed] [Google Scholar]
- 27. Hu G, Christman JW.. Editorial: alveolar macrophages in lung inflammation and resolution. Front Immunol 2019;10:2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El Chami H, Hassoun PM.. Immune and inflammatory mechanisms in pulmonary arterial hypertension. Prog Cardiovasc Dis 2012;55:218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tuder RM, Groves B, Badesch DB, Voelkel NF.. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 30. Sahara M, Sata M, Morita T, Nakamura K, Hirata Y, Nagai R.. Diverse contribution of bone marrow-derived cells to vascular remodeling associated with pulmonary arterial hypertension and arterial neointimal formation. Circulation 2007;115:509–517. [DOI] [PubMed] [Google Scholar]
- 31. Burke DL, Frid MG, Kunrath CL, Karoor V, Anwar A, Wagner BD, Strassheim D, Stenmark KR.. Sustained hypoxia promotes the development of a pulmonary artery-specific chronic inflammatory microenvironment. Am J Physiol Lung Cell Mol Physiol 2009;297:L238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S.. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation 2011;123:1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, Klussmann S, Ghofrani HA, Weissmann N, Klepetko W, Banat GA, Seeger W, Grimminger F, Schermuly RT.. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012;186:897–908. [DOI] [PubMed] [Google Scholar]
- 34. Florentin J, Coppin E, Vasamsetti SB, Zhao J, Tai YY, Tang Y, Zhang Y, Watson A, Sembrat J, Rojas M, Vargas SO, Chan SY, Dutta P.. Inflammatory macrophage expansion in pulmonary hypertension depends upon mobilization of blood-borne monocytes. J Immunol 2018;200:3612–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tian W, Jiang X, Tamosiuniene R, Sung YK, Qian J, Dhillon G, Gera L, Farkas L, Rabinovitch M, Zamanian RT, Inayathullah M, Fridlib M, Rajadas J, Peters-Golden M, Voelkel NF, Nicolls MR.. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med 2013;5:200ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Voelkel NF, Peters-Golden M.. A new treatment for severe pulmonary arterial hypertension based on an old idea: inhibition of 5-lipoxygenase. Pulm Circ 2020;10:2045894019882635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trankle CR, Canada JM, Kadariya D, Markley R, De Chazal HM, Pinson J, Fox A, Van Tassell BW, Abbate A, Grinnan D.. IL-1 blockade reduces inflammation in pulmonary arterial hypertension and right ventricular failure: a single-arm, open-label, phase IB/II pilot study. Am J Respir Crit Care Med 2019;199:381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hirschi KK. Hemogenic endothelium during development and beyond. Blood 2012;119:4823–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML.. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn 2006;235:759–767. [DOI] [PubMed] [Google Scholar]
- 40. Pelosi E, Valtieri M, Coppola S, Botta R, Gabbianelli M, Lulli V, Marziali G, Masella B, Muller R, Sgadari C, Testa U, Bonanno G, Peschle C.. Identification of the hemangioblast in postnatal life. Blood 2002;100:3203–3208. [DOI] [PubMed] [Google Scholar]
- 41. Wu X, Lensch MW, Wylie-Sears J, Daley GQ, Bischoff J.. Hemogenic endothelial progenitor cells isolated from human umbilical cord blood. Stem Cells 2007;25:2770–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pelosi E, Castelli G, Martin-Padura I, Bordoni V, Santoro S, Conigliaro A, Cerio AM, De Santis Puzzonia M, Marighetti P, Biffoni M, Alonzi T, Amicone L, Alcalay M, Bertolini F, Testa U, Tripodi M.. Human haemato-endothelial precursors: cord blood CD34+ cells produce haemogenic endothelium. PLoS One 2012;7:e51109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Graf S, Haimel M, Bleda M, Hadinnapola C, Southgate L, Li W, Hodgson J, Liu B, Salmon RM, Southwood M, Machado RD, Martin JM, Treacy CM, Yates K, Daugherty LC, Shamardina O, Whitehorn D, Holden S, Aldred M, Bogaard HJ, Church C, Coghlan G, Condliffe R, Corris PA, Danesino C, Eyries M, Gall H, Ghio S, Ghofrani HA, Gibbs JSR, Girerd B, Houweling AC, Howard L, Humbert M, Kiely DG, Kovacs G, MacKenzie Ross RV, Moledina S, Montani D, Newnham M, Olschewski A, Olschewski H, Peacock AJ, Pepke-Zaba J, Prokopenko I, Rhodes CJ, Scelsi L, Seeger W, Soubrier F, Stein DF, Suntharalingam J, Swietlik EM, Toshner MR, van Heel DA, Vonk Noordegraaf A, Waisfisz Q, Wharton J, Wort SJ, Ouwehand WH, Soranzo N, Lawrie A, Upton PD, Wilkins MR, Trembath RC, Morrell NW.. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun 2018;9:1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hiraide T, Kataoka M, Suzuki H, Aimi Y, Chiba T, Kanekura K, Satoh T, Fukuda K, Gamou S, Kosaki K.. SOX17 mutations in Japanese patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2018;198:1231–1233. [DOI] [PubMed] [Google Scholar]
- 45. Zhu N, Welch CL, Wang J, Allen PM, Gonzaga-Jauregui C, Ma L, King AK, Krishnan U, Rosenzweig EB, Ivy DD, Austin ED, Hamid R, Pauciulo MW, Lutz KA, Nichols WC, Reid JG, Overton JD, Baras A, Dewey FE, Shen Y, Chung WK.. Rare variants in SOX17 are associated with pulmonary arterial hypertension with congenital heart disease. Genome Med 2018;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rhodes CJ, Batai K, Bleda M, Haimel M, Southgate L, Germain M, Pauciulo MW, Hadinnapola C, Aman J, Girerd B, Arora A, Knight J, Hanscombe KB, Karnes JH, Kaakinen M, Gall H, Ulrich A, Harbaum L, Cebola I, Ferrer J, Lutz K, Swietlik EM, Ahmad F, Amouyel P, Archer SL, Argula R, Austin ED, Badesch D, Bakshi S, Barnett C, Benza R, Bhatt N, Bogaard HJ, Burger CD, Chakinala M, Church C, Coghlan JG, Condliffe R, Corris PA, Danesino C, Debette S, Elliott CG, Elwing J, Eyries M, Fortin T, Franke A, Frantz RP, Frost A, Garcia JGN, Ghio S, Ghofrani HA, Gibbs JSR, Harley J, He H, Hill NS, Hirsch R, Houweling AC, Howard LS, Ivy D, Kiely DG, Klinger J, Kovacs G, Lahm T, Laudes M, Machado RD, Ross RVM, Marsolo K, Martin LJ, Moledina S, Montani D, Nathan SD, Newnham M, Olschewski A, Olschewski H, Oudiz RJ, Ouwehand WH, Peacock AJ, Pepke-Zaba J, Rehman Z, Robbins I, Roden DM, Rosenzweig EB, Saydain G, Scelsi L, Schilz R, Seeger W, Shaffer CM, Simms RW, Simon M, Sitbon O, Suntharalingam J, Tang H, Tchourbanov AY, Thenappan T, Torres F, Toshner MR, Treacy CM, Vonk Noordegraaf A, Waisfisz Q, Walsworth AK, Walter RE, Wharton J, White RJ, Wilt J, Wort SJ, Yung D, Lawrie A, Humbert M, Soubrier F, Tregouet DA, Prokopenko I, Kittles R, Graf S, Nichols WC, Trembath RC, Desai AA, Morrell NW, Wilkins MR;Consortium UNBRD, Consortium UPCS, Consortium UPB . Genetic determinants of risk in pulmonary arterial hypertension: international genome-wide association studies and meta-analysis. Lancet Respir Med 2019;7:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhu N, Swietlik EM, Welch CL, Pauciulo MW, Hagen JJ, Zhou X, Guo Y, Karten J, Pandya D, Tilly T, Lutz KA, Martin JM, Treacy CM, Rosenzweig EB, Krishnan U, Coleman AW, Gonzaga-Jauregui C, Lawrie A, Trembath RC, Wilkins MR, Heritable PAH, Morrell NW, Shen Y, Graf S, Nichols WC, Chung WK;Regeneron Genetics Center, PAH Biobank Enrolling Centers’ Investigators, NIHR BioResource for Translational Research – Rare Diseases, National Cohort Study of Idiopathic and Heritable PAH . Rare variant analysis of 4241 pulmonary arterial hypertension cases from an international consortium implicates FBLN2, PDGFD, and rare de novo variants in PAH. Genome Med 2021;13:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lizama CO, Hawkins JS, Schmitt CE, Bos FL, Zape JP, Cautivo KM, Borges Pinto H, Rhyner AM, Yu H, Donohoe ME, Wythe JD, Zovein AC.. Repression of arterial genes in hemogenic endothelium is sufficient for haematopoietic fate acquisition. Nat Commun 2015;6:7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bos FL, Hawkins JS, Zovein AC.. Single-cell resolution of morphological changes in hemogenic endothelium. Development 2015;142:2719–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang L, Jambusaria A, Hong Z, Marsboom G, Toth PT, Herbert BS, Malik AB, Rehman J.. SOX17 regulates conversion of human fibroblasts into endothelial cells and erythroblasts by dedifferentiation into CD34(+) progenitor cells. Circulation 2017;135:2505–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakajima-Takagi Y, Osawa M, Oshima M, Takagi H, Miyagi S, Endoh M, Endo TA, Takayama N, Eto K, Toyoda T, Koseki H, Nakauchi H, Iwama A.. Role of SOX17 in hematopoietic development from human embryonic stem cells. Blood 2013;121:447–458. [DOI] [PubMed] [Google Scholar]
- 52. Riddell A, McBride M, Braun T, Nicklin SA, Cameron E, Loughrey CM, Martin TP.. RUNX1: an emerging therapeutic target for cardiovascular disease. Cardiovasc Res 2020;116:1410–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McCarroll CS, He W, Foote K, Bradley A, McGlynn K, Vidler F, Nixon C, Nather K, Fattah C, Riddell A, Bowman P, Elliott EB, Bell M, Hawksby C, MacKenzie SM, Morrison LJ, Terry A, Blyth K, Smith GL, McBride MW, Kubin T, Braun T, Nicklin SA, Cameron ER, Loughrey CM.. Runx1 deficiency protects against adverse cardiac remodeling after myocardial infarction. Circulation 2018;137:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li X, Zhang S, Wa M, Liu Z, Hu S.. MicroRNA-101 protects against cardiac remodeling following myocardial infarction via downregulation of runt-related transcription factor 1. J Am Heart Assoc 2019;8:e013112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Koth J, Wang X, Killen AC, Stockdale WT, Potts HG, Jefferson A, Bonkhofer F, Riley PR, Patient RK, Gottgens B, Mommersteeg MTM.. Runx1 promotes scar deposition and inhibits myocardial proliferation and survival during zebrafish heart regeneration. Development 2020;147:dev186569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lambert M, Jambon S, Depauw S, David-Cordonnier MH.. Targeting transcription factors for cancer treatment. Molecules 2018;23:1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Haubrich RH, Flexner C, Lederman MM, Hirsch M, Pettinelli CP, Ginsberg R, Lietman P, Hamzeh FM, Spector SA, Richman DD;AIDS Clinical Trials Group 213 Team . A randomized trial of the activity and safety of Ro 24-7429 (Tat antagonist) versus nucleoside for human immunodeficiency virus infection. J Infect Dis 1995;172:1246–1252. [DOI] [PubMed] [Google Scholar]
- 58. Kira T, Hashimoto K, Baba M, Okamoto T, Shigeta S.. 2-Glycineamide-5-chlorophenyl 2-pyrryl ketone, a non-benzodiazepin Tat antagonist, is effective against acute and chronic HIV-1 infections in vitro. Antiviral Res 1996;32:55–62. [DOI] [PubMed] [Google Scholar]
- 59. Shahabuddin M, Volsky B, Hsu MC, Volsky DJ.. Restoration of cell surface CD4 expression in human immunodeficiency virus type 1-infected cells by treatment with a Tat antagonist. J Virol 1992;66:6802–6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lam JD, Oh DJ, Wong LL, Amarnani D, Park-Windhol C, Sanchez AV, Cardona-Velez J, McGuone D, Stemmer-Rachamimov AO, Eliott D, Bielenberg DR, van Zyl T, Shen L, Gai X, D’Amore PA, Kim LA, Arboleda-Velasquez JF.. Identification of RUNX1 as a mediator of aberrant retinal angiogenesis. Diabetes 2017;66:1950–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Richter LE, Wang Y, Becker ME, Coburn RA, Williams JT, Amador C, Hyde RK.. HDAC1 is a required cofactor of CBFbeta-SMMHC and a potential therapeutic target in inversion 16 acute myeloid leukemia. Mol Cancer Res 2019;17:1241–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Illendula A, Gilmour J, Grembecka J, Tirumala VSS, Boulton A, Kuntimaddi A, Schmidt C, Wang L, Pulikkan JA, Zong H, Parlak M, Kuscu C, Pickin A, Zhou Y, Gao Y, Mishra L, Adli M, Castilla LH, Rajewski RA, Janes KA, Guzman ML, Bonifer C, Bushweller JH.. Small molecule inhibitor of CBFbeta-RUNX binding for RUNX transcription factor driven cancers. EBioMedicine 2016;8:117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Illendula A, Pulikkan JA, Zong H, Grembecka J, Xue L, Sen S, Zhou Y, Boulton A, Kuntimaddi A, Gao Y, Rajewski RA, Guzman ML, Castilla LH, Bushweller JH.. Chemical biology. A small-molecule inhibitor of the aberrant transcription factor CBFbeta-SMMHC delays leukemia in mice. Science 2015;347:779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Translational perspective
RUNX1 inhibition in vivo reversed pulmonary hypertension and improved vascular remodelling in the Sugen/hypoxia-induced pulmonary hypertension model in rats. These findings suggest that, by blocking RUNX1-dependent endothelial to haematopoietic transformation and pulmonary macrophage recruitment and activation, targeting RUNX1 may be as a novel treatment modality for pulmonary arterial hypertension.