Abstract
The homeostasis of cellular activities is essential for the normal functioning of living organisms. Hence, the ability to regulate the fates of cells is of great significance for both fundamental chemical biology studies and therapeutic development. Despite the notable success of small-molecule drugs that normally acts on cellular protein functions, current clinical challenges have highlighted the use of macromolecules to tune cell function for improved therapeutic outcomes. As a class of hybrid biomacromolecules gaining rapidly increasing attention, protein conjugates have exhibited great potential as versatile tools to manipulate cell function for therapeutic applications, including cancer treatment, tissue engineering, and regenerative medicine. Therefore, recent progress in the design and assembly of protein conjugates used to regulate cell function is discussed in this review. The protein conjugates covered here are classified into three different categories based on their mechanisms of action and relevant applications: 1) regulation of intercellular interactions; 2) intervention in intracellular biological pathways; 3) termination of cell proliferation. Within each genre, a variety of protein conjugate scaffolds are discussed, which contain a diverse array of grafted molecules, such as lipids, oligonucleotides, synthetic polymers, and small molecules, with an emphasis on their conjugation methodologies and potential biomedical applications. While the current generation of protein conjugates is focused largely on delivery, the next generation is expected to address issues of site-specific conjugation, in vivo stability, controllability, target selectivity, and biocompatibility.
INTRODUCTION
As the basic structural and functional module of the human body, cells play essential roles in almost every aspect of physiological or pathological processes. The homeostasis of normal functions and roles of cells is closely associated with organism health1–5. Thus, the control and manipulation of cell function is a major focus of biomedical research and drug development.
Although small-molecule therapeutic agents have achieved extraordinary success in tuning cell functions for disease treatment, primarily by interfering with protein function, current challenges faced by modern medicine have highlighted the utility of macromolecules as tools to study and manage cell behavior. Indeed, therapeutic proteins constitute a rapidly expanding class of macromolecular research tools and clinically powerful therapeutics6–9. Over the last decade, there has been a rapidly growing interest in the development of protein bioconjugate based drugs due in part to their high level of clinical efficacy and usefulness as research tools. Conjugation of proteins with carbohydrates, lipids, oligonucleotides, synthetic polymers, and other small molecules has been shown to alter the behavior of the protein in definable ways, especially with regard to therapeutic payload delivery, allowing the protein conjugates to achieve better synergistic outcomes.
Among clinically approved protein bioconjugates, conjugates of antibodies or antibody-derived proteins have shown the greatest clinical and diagnostic success. This class of macromolecules consists not only of IgG proteins, but also Fab fragments, single chain antibodies (scFvs), nanobodies, and other immunoglobulin-derived molecules10. For example, antibody drug conjugates (ADC) have been shown to be potent and specific anti-cancer agents, as well as useful for target specific radio imaging11–13. The success of antibody-based conjugates has been largely attributed to the targeting ability of monoclonal antibodies as cargo carriers in concert with their ability to be modified with various functional payloads. Nevertheless, there are also some limitations associated with the antibody-based conjugates, one of which is the relatively laborious, time-consuming and costly preparation process of mammalian cell-based antibody expression and purification12,14,15. Alternatively, investigators have also constructed a variety of non-antibody-based protein scaffolds to serve as targeting domains of protein conjugates. These scaffolds exhibit multiple advantages over antibody-based proteins, including ease of preparation, high yield, high stability, and improved safety features9,16; thus, they have also been exploited to generate a large number of protein conjugates for the manipulation of cell functions.
In recent years, the toolbox of engineered non-antibody protein scaffolds has greatly expanded, including, for example, affibodies, affilins, avimers, atrimers, DARPins, and Fn3s9,16. Using these proteins as targeting elements, an array of different protein conjugates has been generated through diverse conjugation methods. Moreover, other proteins without targeting capabilities have also been developed to serve as functional building blocks for protein conjugate construction. For several of these proteins, their self-assembling properties or favorable biocompatibility has been used to facilitate the fabrication of new supramolecular structures, as well as reversible function when encountering a specific stimuli17–19. All these proteins possess unique functionalities that can be used to prepare new assemblies whose properties can be tailored to fulfill distinct objectives related to the programing of cellular activities.
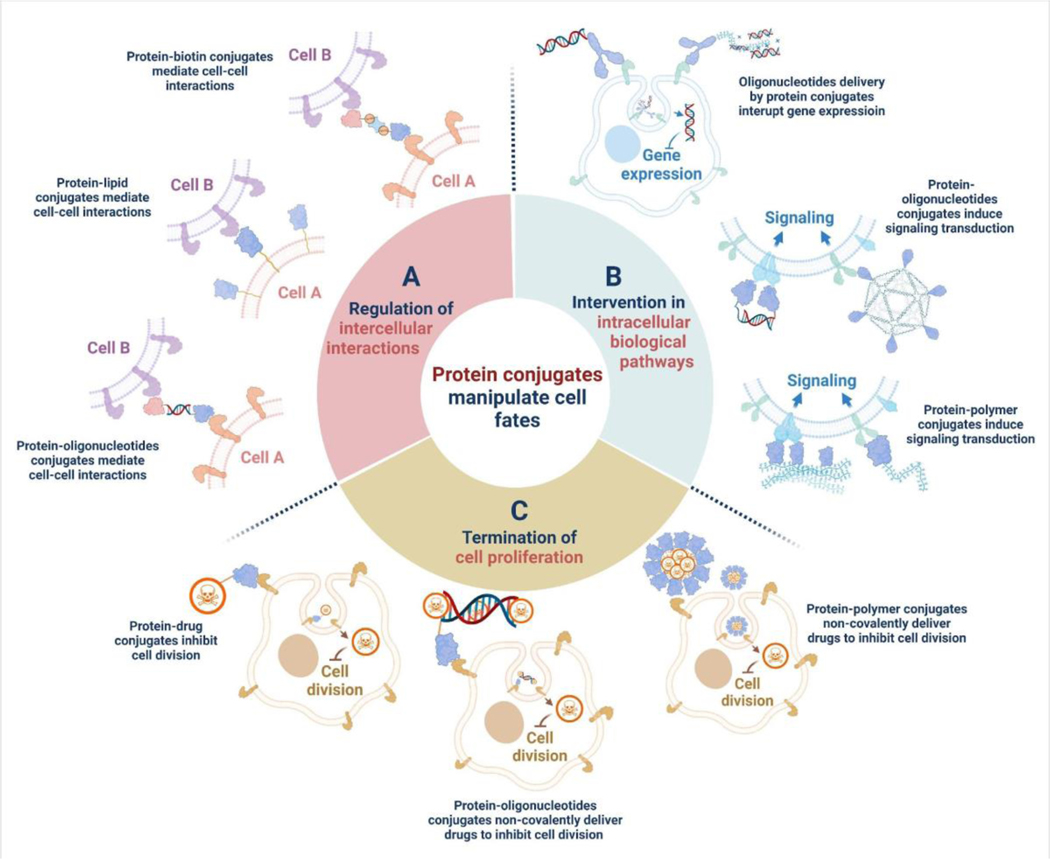
Here, different protein conjugates are classified into three major categories and reviewed based on their respective mechanisms to manipulate the fate of cells including 1) regulation of intercellular interactions, 2) intervention in intracellular biological pathways, and 3) termination of cell proliferation (Figure 1). Within each category, the chemical structures, conjugation methods, and specific applications of the protein conjugates are examined. Finally, a perspective of this research field is provided on the future development of therapeutic protein conjugates.
Figure 1. Manipulation of cell fates by protein conjugates.
Various types of protein conjugates have been developed as tools to manipulate cell fates by (A) regulation of intercellular interactions, (B) intervention in intracellular biological pathways, and (C) termination of cell proliferation. For each category, proteins were chemically conjugated with different functional molecules, including lipids, oligonucleotides, synthetic polymers, and other small molecules, for a wide range of biomedical applications.
REGULATION OF INTERCELLULAR INTERACTIONS
Cell-cell interactions are vital for all multicellular organisms and serve critical roles in numerous biological activities including cell differentiation, tissue development, neurotransmission, immune responses, and cancer progression20–25. Although these interactions are highly dynamic in duration and complicated in the composition of cell types and mechanisms, substantial efforts have been devoted to understanding them, and the insights gained from that work have greatly advanced our ability to manipulate them for clinical treatment26,27. For instance, chimeric antigen receptor (CAR) T therapy relies on a genetic engineering approach to modify the T cell surface with cancer-recognizing proteins that mediate their interactions with cancer cells. Albeit efficacious clinically, these CAR-T therapies are hindered by requisite genetic engineering methods, which result in several limitations including a time-consuming preparation process, high cost, safety issues, inconsistent transfection efficiency, and toxicity by potential un-regulated immune responses28,29. Additionally, these genetic engineering approaches are not amenable to some cell types, such as stem cells. Therefore, investigators have sought to develop non-genetic approaches for engineering cell-cell interactions28.
To program a pair of cell-cell interactions using non-genetic methodologies, the cell surface of the interacting pair needs to be decorated with engineered scaffolds that mediate cell-cell interactions through covalent bonding or non-covalent interactions. Thus, cell surface molecules, such as proteins, lipids, and polysaccharides, typically function as handles for non-genetic cell surface modifications. Accordingly, functional domains engaging these cell surface molecules were incorporated into the bioconjugates to induce cell-cell interactions. Here, diverse types of protein conjugates used to manipulate cell-cell interactions are described based on their chemical structures. In addition, the conjugation methodologies employed for their assembly and subsequent applications of these conjugates are also discussed.
Protein Conjugates to Regulate Intercellular Interactions
As previously noted, a number of different protein conjugates have been designed to mediate cell-cell communication through interactions with cell surface molecules, and these interactions can be generally divided into two categories according to their specificity or non-specificity. Cell surface antigens are unique biomarkers and can be specifically targeted by many types of binding molecules including proteins, oligonucleotides aptamers, and small-molecule ligands. Thus, these species can be integrated into the protein conjugates for specific cell surface binding. In contrast, plasma membrane lipids can non-specifically interact with hydrophobic moieties, such as lipids. Therefore, lipid molecules can also be conjugated to proteins and used to non-specifically tether functional proteins on the cell surface as a universal method for cell surface modifications and concomitant intercellular interactions. Moreover, protein conjugates containing reactive chemical moieties can also non-specifically react with natural chemical groups on the cell membrane or with bioorthogonal groups incorporated onto the cell surface via metabolic engineering.
Lipid insertion is a simple and direct method for cell surface modifications (Figure 2A). Protein-lipid conjugates can be generated via chemical reactions or enzymatic processes and intercalated into the lipid bilayer of the cell membrane through hydrophobic interactions. The protein components in the conjugates normally serve as binders of cell surface antigens to mediate cell-cell interactions. For example, palmitate-conjugated proteins have been generated by the Peacock group and used as “surrogate receptors” to mediate intercellular interactions since 198930,31. Others have adopted the same methodology to construct palmitate-protein G conjugates to functionalize the surface of mesenchymal stem cells (MSCs) or progenitor cells with antibodies for targeted cell delivery32–34. Meanwhile, chemokine receptor proteins or chemokine proteins were also conjugated to lipids and anchored on the surface of MSCs with the goal of enhancing their interactions with target tissues35,36. Moreover, targeting proteins such as single-domain antibodies were directly lipidated through enzymatic ligation by sortase A to regulate interactions between T cells and myeloid-derived suppressor cells37. Self-assembled targeting proteins were also developed and enzymatically lipidated by protein prenyltransferase to form multivalent protein-lipid conjugates for programming cell-cell interactions19. In addition, a non-targeting structural protein, gelatin, was also modified with cholesterol as a membrane anchor to enhance intercellular adhesion within cells of the same type18. Furthermore, other functional small molecules have also been conjugated to proteins to engineer cell-cell interactions (Figure 2B). For example, biotinylation, a commonly used strategy to modify proteins for various biomedical applications, was applied to antibodies and used to mediate cell-cell contacts with streptavidin as the bridging unit38.
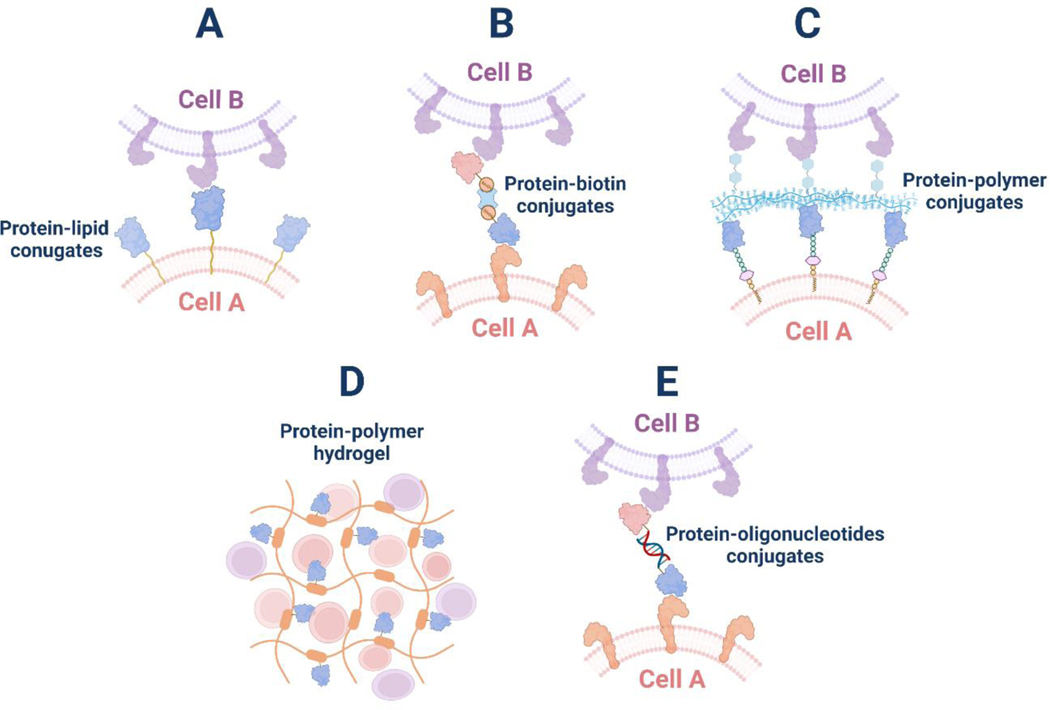
Figure 2. Different protein conjugates generated to regulate intercellular interactions.
(A) Protein-lipid conjugates have been used to modify cell surface and mediate cell-cell interactions. (B) Biotinylated proteins can induce cell-cell interactions through biotin-streptavidin bridging. (C) Protein-polysaccharide conjugates have been utilized to label the cell surface and mediate cell-cell interactions. (D) Synthetic polymers were modified with functional proteins to form hydrogels for the targeted delivery of cells to specific tissues and regulate their interactions. (E) Protein-oligonucleotides conjugates have been developed to program cell-cell interactions via oligonucleotides hybridization.
Polymers are also commonly used as building blocks in protein conjugates to regulate cell-cell interactions. For instance, hyaluronic acid (HA) has been frequently used as a liver-targeting molecule and can be incorporated into protein conjugates as a targeting domain. Accordingly, a hyaluronate-wheat germ agglutinin conjugate was generated to coat the MSC surface for liver-targeted stem cell delivery39 (Figure 2C). Although some of these natural polysaccharides can be used as targeting domains, in most cases, polymers were synthesized and grafted to the proteins for the fabrication of supramolecular structures, such as hydrogels, to encapsulate a group of cells and deliver them to specific tissues (Figure 2D). These hydrogel materials function as cell carriers, to not only maintain the cell-cell interactions within the network, but also to control the in-situ interactions of the encapsulated cells with target tissues at the injection site. In these hydrogel systems, proteins were typically conjugated to the polymers to provide supportive functionalities. For instance, insulin-like growth factor-1 (IGF-1) protein or its C domain fragment was chemically immobilized to a polymeric hydrogel for targeted delivery of cardiac progenitor cells or stem cells, where IGF-1 was shown to promote the survival, differentiation, and proliferation of therapeutic cells40–42. Other growth factors, such as vascular endothelial growth factor (VEGF), were also conjugated to hydrogels to play supportive roles for targeted cell delivery43. Moreover, the extracellular matrix (ECM) glycoprotein, fibronectin, was also coupled to hydrogels to facilitate cell adhesion and spreading44.
Although oligonucleotides have also been engineered as receptor binders for decades, they have generally been used alone to serve as bispecific cell engagers through hybridization or conjugated with lipids to induce cell-cell interactions, which have been reviewed by others45,46. In addition, oligonucleotides have been deployed as self-assembling frameworks for antibody-oligonucleotide conjugates to mediate cell-cell interactions (Figure 2E). Through hybridization with different antibody-oligonucleotides conjugates, bispecific or multispecific complexes can be formed as cell engagers for adoptive cell therapies47,48.
Conjugation Methods for Generating Protein Conjugates that Regulate Intercellular Interactions
The construction of protein bioconjugates relies on selective chemical reactions, generally with reactive groups present on amino acid side chains or other bioorthogonal groups incorporated into the proteins. Perhaps the most commonly used way to modify proteins is by reaction between primary amines and N-hydroxysuccinimide esters, which requires the appended molecules to be converted to their respective active esters for subsequent acylation. Palmitic acid has typically been activated as the N-hydroxysuccinimide (NHS) ester for the preparation of protein-lipid conjugates30–34, and 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine (DMPE) based lipids can also be conjugated to proteins using this strategy36. These methods have also been applied for the synthesis of protein-polymer conjugates40 or for biotinylation38. Additionally, maleimide-containing lipids have also been used to functionalize thiol groups present on cysteines35. Other functional lipids, such as cholesterol, have been coupled to proteins through the reaction between primary amines and cholesteryl chloroformate18. Moreover, aldehydes, carboxylic acids, and carbonyl diimidazole were also employed to react with amines in proteins to generate protein conjugates39,42,44.
Although effective and simple, such protein modification methods also have disadvantages with a major issue being that these reactions are not site-specific and therefore may produce heterogeneous conjugate products. Furthermore, if a nonspecific modification site is near the ligand-binding domain of the protein, the conjugated molecules may exhibit decreased function rendering them less useful for mediating cell-cell interactions31. To solve these problems, bioorthogonal groups, such as azide groups, have been used for click reactions with alkyne-containing molecules41, and aldehyde-containing unnatural amino acids have also been site-specifically incorporated into proteins to react with aminooxy-modified peptide nucleic acids (PNA)48. Moreover, highly efficient and site-specific enzymatic reactions have also been employed to synthesize protein conjugates. For instance, the ligation reaction catalyzed by sortase A has been used to construct protein-lipid conjugates37. The naturally occurring lipidation reaction, protein prenylation, has also been employed to synthesize protein-lipid conjugates for the regulation of cell-cell interactions19. Multiple biorthogonal functional groups for preparing more complex chimeric protein structures have been installed using this approach49. Compared with conventional chemical modifications, these enzymatic reactions are more efficient in their ability to generate homogenous site-specific conjugate products. They also avoid the use of toxic organic solvents, which is more compatible with biomedical applications involving mammalian cells.
Applications of Protein Conjugates that Regulate Intercellular Interactions
Although protein-conjugates were originally synthesized for fundamental studies of cell surface engineering and cell-cell interactions18,30,31, the great potential of these hybrid materials has inspired investigators to use them for cell-based therapies. One of the most common applications of protein conjugates in the field of cell-based therapy may be regenerative medicine, involving the delivery of stem cells or progenitor cells. Protein-lipid conjugates were made for the targeted delivery of MSCs to inflammatory human vascular endothelial cells34 or the delivery of chondrocytes to cartilage injury sites for cartilage repair32. Moreover, growth factor-conjugated hydrogels were synthesized to deliver adipose-derived MSCs for the treatment of acute kidney injury41, the treatment of limb ischemia42, and chondrogenesis50. Protein-HA conjugates were also generated for the systematic delivery of MSCs to the liver for liver disease treatment39. Furthermore, cancer immunotherapy greatly relies on the ability to control immune-cancer cell-cell interactions, which can be modulated by protein conjugates. To this end, various lipidated proteins and protein-oligonucleotides conjugates have been constructed to regulate cell-cell interactions between cytotoxic T cells and specific cancer cells, which were shown to induce cancer-specific killing by surface-engineered T cells19,37,47,48. Cell-based therapies have now demonstrated notable success in multiple clinical fields, including tissue engineering, regenerative medicine, and cancer immunotherapy51. These protein conjugates have highlighted their utility in controlling intercellular interactions of therapeutic cells and therefore have been regarded as valuable tools for biomedical applications.
INTERVENTION IN INTRACELLULAR BIOLOGICAL PATHWAYS
Apart from the regulation of intercellular interactions, protein conjugates can also be engineered to alter cell fates by manipulating intracellular biological pathways. These effects can be generated mainly by two means including the targeted intracellular delivery of functional oligonucleotides to change gene expression and the control of signal transduction through receptor binding and activation. For the first category, functional oligonucleotides can be either conjugated to a targeting protein to form protein-oligonucleotide conjugates or non-covalently carried by protein-polymer conjugates. Both approaches for oligonucleotide delivery aim to interfere with gene expression and finally disrupt the production of target proteins within the cell. Alternately, cellular signal transduction that is essential for normal cell function can be regulated by protein conjugates that act on surface receptor-mediated pathways. Although toxin delivery by protein-drug conjugates can also be regarded as an approach to block some intracellular biological pathways, in general the goal has been to broadly interrupt the essential pathways for cell survival, particularly cancer cells. Therefore, toxin delivery by protein conjugates is discussed in a subsequent section. In the next section, protein conjugates used for oligonucleotides delivery and surface receptor activation are reviewed with a focus on their structures, conjugation methodologies, and particular applications.
Protein Conjugates to Regulate Gene Expressions
It has been two decades since oligonucleotides were approved by FDA and used as targeted therapeutics, and since then, a wide variety of types of nucleic acid drugs have been developed, including antisense single-stranded DNA (ssDNA), small interfering RNA (siRNA), small hairpin RNA (shRNA), messenger RNA (mRNA), and plasmids52. Aside from lipid particles and virus-based vectors, engineered protein conjugates have also been commonly used to specifically deliver oligonucleotides to their target cells53.
As an emerging class of chimeric biomolecules, antibody-oligonucleotide conjugates (AOC) have been used for many bioanalytical applications, such as immuno-PCR (iPCR), proximity ligation assays (PLA), electrochemical proximity assays (ECPA), and microscopy54. For therapeutic purposes, functional oligonucleotides can be delivered by protein conjugates through covalent bonding or noncovalent interactions (Figure 3A). For instance, an siRNA designed to silence the peptidyl-prolyl cis-trans isomerase B (PPIB) gene has been conjugated to a variety of antibody-based proteins targeting different cancer-related antigens, including TENB2, Steap1, EtBr, NaPi2b, HER2, MUC6, and mesothelin55. The siRNA for the hypoxanthine-guanine phosphoribosyl transferase (HPRT) gene was also covalently conjugated to an anti-CD71 Fab for targeted delivery to cardiac and skeletal muscle56. Meanwhile, antisense oligonucleotides targeting the MAX dimerization protein 3 (MXD3) gene or FAM107A gene were also conjugated to different antibodies for anti-cancer gene therapies57,58. Alternatively, protamine or other positively charged polymers have been conjugated to various monoclonal antibodies to serve as carriers of negatively charged therapeutic oligonucleotides through electrostatic interactions. Antibodies targeting several common cancer-associated surface antigens, including HER2, EGFR, and PSMA, have been utilized to deliver siRNA payloads designed to knock down gene expression of essential cellular proteins59–65.
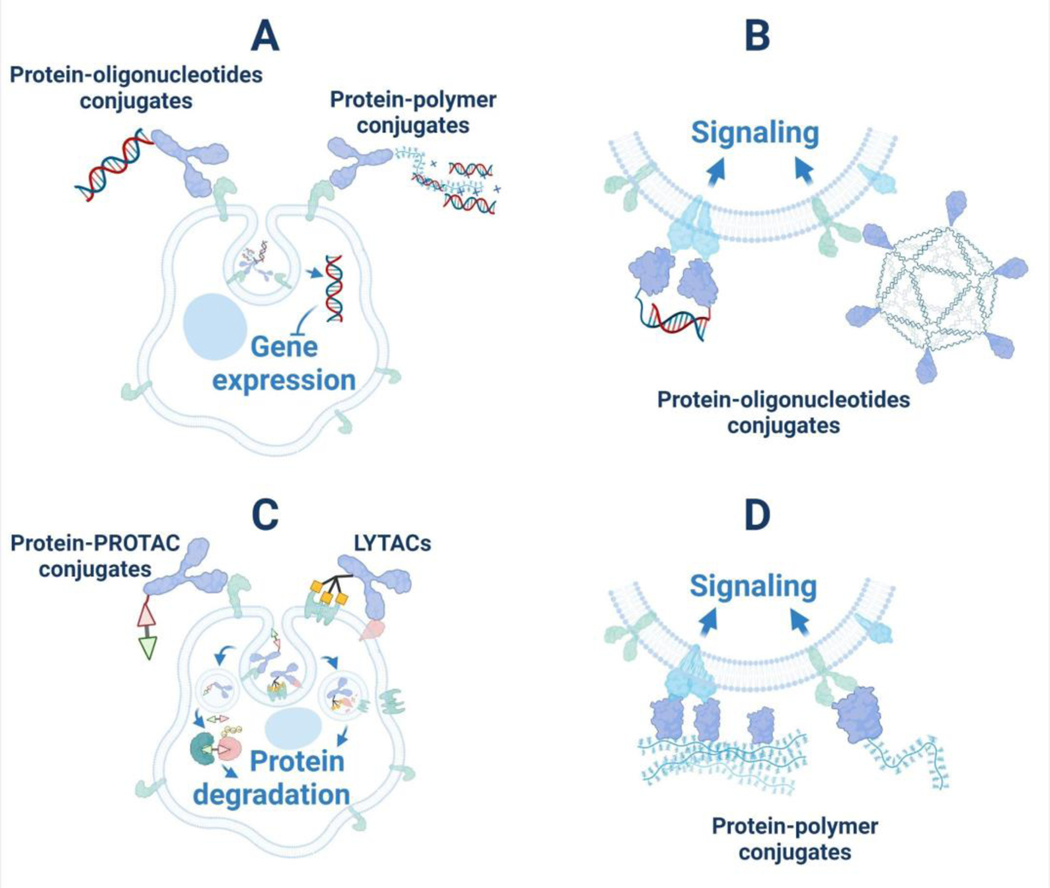
Figure 3. Different protein conjugates generated to intervene in intracellular biological pathways.
(A) Oligonucleotides covalently conjugated to proteins or non-covalently loaded onto positively charged protein-polymer conjugates for intracellular delivery to interrupt gene expression. (B) Protein-oligonucleotides synthesized to trigger cellular signaling by receptor binding and dimerization (or oligomerization). (C) Antibodies conjugated with small-molecule PROTACs or ligands for lysosome-targeting receptors to specifically degrade signaling proteins. (D) Functional proteins modified with synthetic polymers or conjugated to polymer-based hydrogels to activate receptor-mediated signaling of target cells or tissues.
In addition to antibody-based targeting domains, other protein scaffolds have also been employed for conjugation with therapeutic oligonucleotides. For example, several functional expression plasmids were non-covalently loaded onto biotinylated transferrin protein through biotin-streptavidin bridging and were specifically delivered to human erythroleukemia cells expressing transferrin receptors66. Human serum albumin was also used as a vehicle to deliver RGD-modified oligonucleotides to integrin-expressing tumor cells67. Moreover, CRISPR-Cas9–mediated precise genome editing has shown great potential for gene therapy. To improve the efficiency of such genome editing, site-specific Cas9-oligonucleotide conjugates were developed to recruit the donor DNA template to the target site and were shown to markedly increase homology-directed repair efficiency in both human cell culture and mouse zygotes68,69. Similarly, Cas12a-crRNA conjugates were also generated using bioorthogonal chemistry with site-specifically modified Cas12a proteins and 5’ chemically modified crRNA for genome editing in CAR-T cells70.
Protein Conjugates to Regulate Signaling Transductions
Signal transduction, or cell signaling, is the transmission of biomolecular signals from a cell’s exterior to its interior, which is mediated by cell surface receptors. Therefore, receptor-targeting proteins have often been utilized as activators of downstream receptor-mediated signaling pathways, and conjugation of additional functional fragments to those proteins can function to optimize their efficacy. For this purpose, protein-oligonucleotides conjugates have been produced to activate cell signaling pathways, where the oligonucleotides serve as the framework of nanostructures for the multivalent display of receptor-activating proteins (Figure 3B). For instance, the clinical vaccine immunogen eOD-GT8 protein was conjugated with DNA origami nanoparticles for B-cell receptor activation and the nanoparticles were shown to drive functional B-cell responses71. Similarly, bispecific or multispecific complexes were formed through the hybridization of antibody-oligonucleotides conjugates and shown to effectively activate T-cells for anti-cancer cytotoxcicity47,48. Moreover, the multimerization of protein-oligonucleotides can also be conducted on the cell surface and was demonstrated to effectively trigger the clustering of CD20 on B cells and activate downstream apoptosis signals72. In addition to oligonucleotides, photo-switchable molecules can also be incorporated into receptor-binding proteins to reversibly control their conformation, receptor binding and activation. For example, the photo-switchable reagent 3,3’-bis(sulfonato)-4,4’-bis(chloroacetamido)azobenzene (BSBCA) was conjugated to an anti-EGFR repebody for light-driven control of receptor binding, and downstream signaling73.
Moreover, recent success in small molecule proteolysis targeting chimeras (PROTAC) has also inspired the development of antibody-conjugated PROTACs for the site-specific delivery of the protein degradation molecules to desired tissues (Figure 3C). For example, several PROTAC molecules degrading bromodomain-containing protein 4 (BRD4) have been conjugated to the antibodies targeting a number of cancer-specific antigens, including C-lectin-like molecule 1 (CLL1), HER2, and six-transmembrane epithelial antigen of the prostate 1 (STEAP1)74–77. Meanwhile, estrogen receptor (ER) was also reported to be degraded by the anti-HER2 antibody-PROTAC conjugates as a potential treatment of ER-positive breast cancers78. Lysosome-targeting chimeras (LYTACs) are another emerging type of antibody-based conjugates that utilize the lysosome-trafficking receptors to degrade target proteins for the manipulation of cell signaling and have been reported to degrade several membrane-bound receptors, including EGFR, CD71, and HER279,80. These protein-degrading conjugates exerted selectivity for both cell surface antigens and intracellular protein targets, effectively disrupting the signaling transduction pathways controlled by the target proteins.
Functional proteins, especially growth factors, have also been frequently coupled to polymeric hydrogels to tune the cellular functions of local tissues for regenerative medicine (Figure 3D). Transforming growth factor beta-1 (TGF-β1) was covalently conjugated to chitosan hydrogels for the treatment of articular cartilage defects81. VEGF was also conjugated to a temperature-sensitive aliphatic polyester hydrogel for cardiac repair82. In another example, fibronectin functional domains were coupled to hyaluronan to stimulate adult human dermal fibroblast responses for wound healing83. Apart from tissue regeneration, protein-hydrogel conjugation has also been used to tune the immune response. For example, apoptosis-inducing anti-Fas monoclonal antibodies were conjugated to the surface of PEG-modified hydrogels to actively provide localized immunosuppression of autoreactive Fas-sensitive immune cells. This immunosuppression caused protective effects on transplanted pancreatic islet cells for the treatment of type I diabetes mellitus84. Other types of apoptosis-inducing protein ligands, such as TNF-related apoptosis-inducing ligand (TRAIL), were also used for conjugation with functional polymeric materials to trigger apoptosis of their cognate target cells85. Polymers can also be used to enhance the biodistribution and targeted localization of protein therapeutics with PEGylation being the most widely used modification method for this purpose. For instance, PEGylation was applied to modify bispecific fibronectin-based protein inhibitors of EGFR and IGF-1 for the inhibition of proliferative and survival signaling in cancer cells86, and the conjugated synthetic polymers greatly changed the pharmacokinetic properties of the modified proteins by increasing their hydrodynamic radius and preventing the uptake of the conjugates by macrophages, which consequently enhanced the therapeutic outcomes of the conjugates. Other functional materials, such as graphene oxide, have also been conjugated with proteins and applied to modulate cell fates87.
Conjugation Methods for Generating the Protein Conjugates that Intervene in Intracellular Biological Pathways
The preparation of most protein conjugates discussed in this section depends on endogenous nucleophilic functional groups including free thiols on cysteines and primary amines on lysines. Bifunctional cross-linkers, such as succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC)55,81, Sulfo-SMCC60–62, N-succinimidyl 4-(2-pyridyldithio)butanoate (SPDB)55,56, succinimidyl 3-(2-pyridyldithio)propionate (SPDP)67, and other reagents containing both NHS ester and maleimide moieties, have been used to link amine-containing molecules to solvent accessible cystines47,63,64,72,81. Moreover, enzymatically cleavable valine-citrulline-containing peptides or reducible disulfide bonds can also be integrated into these linkers for the intracellular release of oligonucleotides56. Maleimides85,86 or NHS esters82,84 can also be directly added onto polymer side chains during polymerization for subsequent coupling with functional proteins. Conversely, other functional groups can also be introduced into proteins using genetic methods that facilitate incorporation of unnatural amino acids. For example, an azide-functionalized amino acid was incorporated into recombinant Cas9 protein for conjugation with DBCO-modified oligonucleotides through strain-promoted click reaction68. Additionally, the noncanonical amino acid, p-acetylphenylalanine (pAcF), was incorporated into proteins and reacted with aminooxy-derivatized ligands of oligonucleotides47 or polymers59. These methods ensure the site-specific conjugation of the functional moiety to the target protein, although they require a relatively complicated process to produce the protein.
Applications of Protein Conjugates that Intervene in Intracellular Biological Pathways
As previously mentioned, protein conjugates can be used for gene therapies to alter gene expression in the target cells. To date, most protein conjugates targeting gene expression have been developed as potential cancer therapeutics. Based on the targeting proteins used for the assembly of such constructs, these conjugates can specifically deliver therapeutic oligonucleotides into different types of cancer cells including prostate carcinoma55,62, B cell acute lymphoblastic leukemia57, glioblastoma58, melanoma67, colon cancer60,61, breast cancer59,63,64, and others. Protein-oligonucleotide conjugates have also been constructed to silence myostatin and hypertrophy of the gastrocnemius for the regulation of muscle function and the treatment of muscular diseases56.
Through the activation of specific surface receptors, protein conjugates can also regulate the functions of immune cells for cancer immunotherapy47,48 and enable rational design of molecular vaccines71. Additionally, protein-conjugates have been used to directly target cancer-specific receptors to trigger apoptosis as a potential cancer treatment72,85,86. Protein-functionalized hydrogels have also been synthesized as possible regenerative medicines for cardiac repair82, wound healing83, and treatment of articular cartilage defects81. Protein-PROTAC conjugates have shown great potential as targeted therapeutics and are becoming an emerging type of ADC molecule for cancer treatment74–78.
TERMINATION OF CELL PROLIFERATION
The protein-conjugates used to terminate cell proliferation are virtually all protein-based delivery systems for cytotoxic drugs, which are designed to completely suppress cellular function and inhibit cell division. Therefore, such molecules are typically used for cancer therapies. As one of the most successful types of targeted cancer therapeutics, antibody-drug conjugates (ADCs) have been established and used as anticancer agents for almost four decades. The global antibody-drug conjugate market size was valued at USD 4.3 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 23.7% from 2021 to 2028, demonstrating the expanding therapeutic potential of ADC drugs. Since the history, development, applications, marketing, and future directions of ADCs have been comprehensively discussed in prior literature11–13,88, this section mainly focuses on non-antibody protein conjugates prepared for targeted toxin delivery. These conjugates involve several types of engineered protein scaffolds as targeting domains, with toxic drug payloads that were prepared either via covalent protein modification or by non-covalent interactions with a protein-conjugate that serves as the drug carrier.
Protein Conjugates to Terminate Cell proliferation by Toxin Delivery
In the last 20 years, more than a dozen of non-antibody-based protein scaffolds have been generated to target different cell surface receptors for research or theranostic purposes16. These macromolecules share several benefits over conventional IgG-derived proteins that are advantageous for the development of protein-drug conjugates for the treatment of corresponding cancers16 (Figure 4A). For instance, an affibody engineered to bind HER2 receptors was fused to an albumin-binding domain and conjugated with the cytotoxic maytansine derivative MC-DM1 to inhibit cell division of HER2-overexpressing cancer89,90. In related work, the photosensitizer, IR700, was conjugated to an affibody specific for the platelet-derived growth factor receptor β (PDGFRβ) for vascular-targeted photodynamic therapy of colorectal cancer91. Additionally, a small and soluble nanobody was developed to target CD38, and the highly potent antineoplastic agent, MMAE, was conjugated to that anti-CD38 nanobody for the treatment of multiple myeloma92. DARPin proteins constitute another emerging class of protein scaffolds that can be used for targeted cancer drug delivery. Accordingly, they have been engineered to exhibit a variety of molecular sizes and half-lives with the goal of specifically delivering the cytotoxic auristatin derivative, MMAF, to EpCAM-positive cancer cells93. Similarly, MMAF was also coupled to an EGFR-specific repebody for targeted cancer therapy94. Naturally existing proteins have also been explored for their ability to deliver conjugated toxins. For example, PEGylated conjugates of fibroblast growth factor 2 (FGF2) with hydrophilic auristatin were prepared for highly selective killing of cancer cells overexpressing fibroblast growth factor receptor 1 (FGFR1)95.
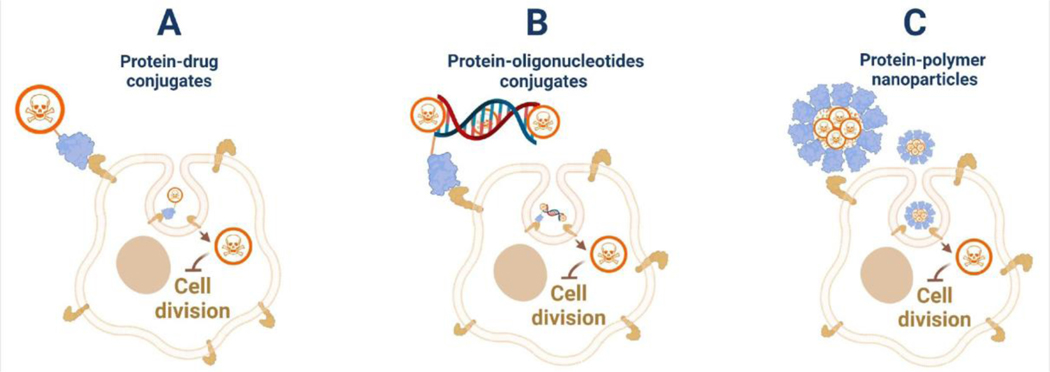
Figure 4. Different protein conjugates synthesized to terminate cell proliferation.
(A) Cytotoxic drugs have been conjugated to the antigen-specific proteins for targeted drug delivery. (B) Cytotoxic drugs can intercalate into oligonucleotides conjugated to targeting proteins for drug delivery. (C) Polymers can be coupled to proteins to form amphipathic conjugates that self-assemble into nanoparticles for the delivery of toxic drugs.
Rather than directly conjugating the drug payload to the targeting protein, the delivery of cytotoxic molecules can also be carried through non-covalent interactions. Since the chemotherapeutic agent, doxorubicin, can intercalate into double-stranded DNA, some protein-oligonucleotide conjugates have been developed for the targeted delivery of doxorubicin to cancer cells96,97 (Figure 4B). Radionuclides can also be loaded into protein-oligonucleotide conjugates for radiotherapy98. Protein-polymer conjugates have also been generated and self-assembled into nanoparticles for the encapsulation and delivery of cancer drugs17,99 (Figure 4C). For example, bovine serum albumin (BSA) is a commonly used protein scaffold for conjugation with hydrophobic polymers, and the resulting BSA-polymer nanoparticles have been used to deliver drugs, such as doxorubicin100 and camptothecin101. Likewise, silk sericin-polylactide protein-polymer conjugates have been constructed for the intracellular delivery of doxorubicin102. It is of note that protein-lipid conjugates can also be incorporated into liposomes as targeting elements to direct the liposomes to cancer cells for drug delivery purposes103,104.
Conjugation Methods for Generating Protein Conjugates that Terminate Cell Proliferation
For the conjugates covered in this section, the coupling reaction between maleimides and sulfhydryl groups on proteins was the most frequently used method to generate protein-drug conjugates. Various cancer chemotherapeutics, including mertansine and auristatin derivatives have been designed and synthesized to incorporate maleimide functional groups facilitating conjugation to cysteines to a cancer-specific targeting protein90,91,93,95. Similarly, primary amines on proteins can also be coupled with NHS ester-functionalized drugs91 or reacted with acrylic acid NHS ester for polymerization initialization to form protein-polymer conjugates as drug vehicles101. Bifunctional linkers, such as Sulfo-SMCC or succinimidyl-6-hydrazino-nicotinamide (S‐HyNic) have also been utilized to crosslink free amines on proteins to thiol-functionalized oligonucleotides96,97 or aldehyde-modified polymers102.
In addition to non-specific lysine and thiol coupling strategies, enzymatic reactions have also been employed as site-specific conjugation methods for the assembly of next-generation protein-drug conjugates using various enzymes94,105–108. For instance, farnesylation was used to incorporate an aldehyde-containing farnesyl analog into an anti-EGFR repebody, followed by subsequent ligation with aminooxy-containing MMAF94. Also, by first conjugating the maleimide-functionalized MMAE to a cysteine-containing short peptide, the cancer drug, MMAE, was linked to a CD38-targeting nanobody through sortase A catalyzed ligation92. Compared with non-specific reactions, these enzymatic methodologies can produce homogenous products with high efficiency, which is more desirable for industrial pharmaceutical production.
Applications of Protein Conjugates that Terminate Cell Proliferation
Cytotoxic drug delivery by protein conjugates is primarily employed for cancer treatment, and the specific application of each protein conjugate is largely driven by the specificity of the protein component in the conjugate to a cancer-specific antigen. Therefore, the target cancer type treated by the conjugates is largely dependent on the type and expression level of the corresponding antigen. Accordingly, anti-HER2 protein conjugates were used to treat HER2-positive ovarian cancer89,90 and anti-EpCAM protein conjugates were prepared to target EpCAM-overexpressing colon adenocarcinoma and breast cancer93. EGFR-overexpressing triple-negative breast cancer, epidermoid carcinoma, and lung adenocarcinoma can be targeted with the corresponding anti-EGFR protein-drug conjugates94,96,100. Other cancer-associated antigens, including PDGFRβ, CD38, and FGFR1 have also been targeted to treat colorectal cancer, myeloma, and lung carcinoma respectively91,92,95. For some protein-polymer based nanoparticles, no specific targeting elements were involved, and the localization of the drug at tumor tissues was dependent on the enhanced permeability and retention (EPR) effect or the pH-responsive property of the protein-polymer conjugates101,102.
Apart from cancer cells, protein-drug conjugates can also be applied to eliminate dysfunctional non-cancerous cells for therapeutic purposes. For instance, doxorubicin intercalated antibody-DNA conjugates were developed to specifically deplete Myo/Nog cells for the treatment of posterior capsule opacification (PCO) following cataract surgery because myofibroblasts emerge from Myo/Nog cells and can cause PCO in some adults and most children. The protein conjugates targeting G Protein-Coupled Receptor Kinase 1 (GRK1) were shown to effectively deliver doxorubicin to Myo/Nog cells without off-target effects97.
PERSPECTIVE
Albeit effective in numerous pre-clinical studies and some clinical applications, a number of hurdles to their expanded clinical application remain. Hence, the development of new methods for the preparation and use of protein-conjugates is of keen interest, especially with regard to the manipulation of cell fates. In this section, the desirability and challenge of optimizing protein conjugation preparation, functionality, and clinical application are discussed.
Conjugation Methods
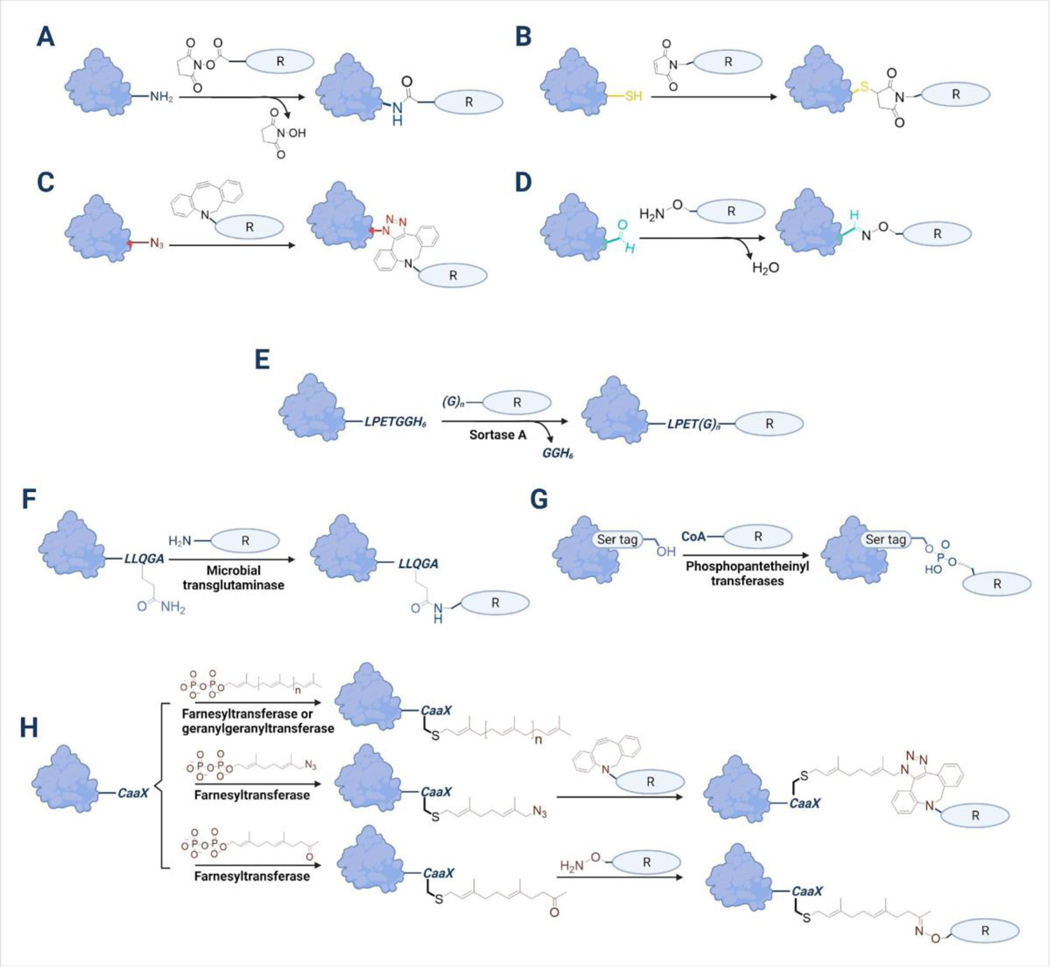
A hallmark of protein modification is the control of the quality of the final material by the conjugation methodology. Regardless of the conjugate composition, appropriate reactive groups on the protein are required for the attachment reaction. The most abundant reactive groups on proteins for conjugations are lysine and N-terminal primary amines. Hence, such amines have been widely used for linking lipids, oligonucleotides, polymers, and other molecules to proteins through reactions with NHS esters (Figure 5A). However, an obvious drawback of this nonspecific method is that it produces heterogeneous products, since specific lysine conjugation is difficult to achieve, resulting in difficulties in achieving product quality control. The coupling reaction between thiols (often obtained via disulfide reduction) and maleimides is another approach for preparing protein conjugates (Figure 5B). While single free thiols can be engineered into non-natural scaffold proteins, in antibodies they must be obtained by selective reduction of disulfides, a strategy that requires careful control of reaction conditions that may ultimately disrupt the structure and function of the protein, as well as enable the production of heterogeneous material, which is one of the major obstacles to the quality control of therapeutic antibody-drug conjugates. Another issue with these non-specific conjugation methods is the deactivation of functional proteins. For example, uncontrolled nonspecific conjugations may lead to protein precipitation or unfavored masking of the antigen-binding domains of antibodies or the active sites of enzymes31.
Figure 5. Different conjugation approaches to generate protein conjugates for the regulation of cell functions.
Most non-specific conjugations can be conducted by (A) reactions between primary amines and NHS esters or (B) reduced thiol coupling with maleimide-containing molecules. Unnatural amino acids were used to site-specifically incorporate (C) azide groups or (D) aldehyde groups into proteins for azide-alkyne cycloadditions or reactions with alkoxyamines respectively. Enzymatic reactions have also been employed as site-specific conjugation methods. (E) Sortase A was used to ligate functional peptides to proteins. (F) Microbial transglutaminase catalyzes the labeling of target proteins containing Q-tag sequences with lysine primary amine substrates. (G) Phosphopantetheinyl transferases modify serine residues with coenzyme A (CoA) derivatives. (H) Prenyltransferases including farnesyltransferase or geranylgeranyltransferase can be used to modify proteins with natural isoprenoids or their derivatives functionalized with reactive groups for corresponding coupling reactions.
To overcome these limitations, site-specific conjugation reactions were developed including the incorporation of unnatural amino acids that contain bioorthogonal groups into proteins48,59,68 (Figure 5C,D). Though viable, the preparation of such engineered proteins can be time-consuming and inefficient109. Therefore, various enzymatic reactions have also been exploited in recent years for site-specific protein functionalization110 (Figure 5E-H). These enzymes, including sortase A37, microbial transglutaminase108, phosphopantetheinyl transferase107, and prenyltransferase19,94, typically catalyze conjugations in aqueous solution with high efficiency, and require only that target proteins be genetically fused with a short tag for recognition. Moreover, due to the relaxed substrate tolerance of many of these enzymes, they can often be used to transfer functional groups such as azides or alkynes that can then be employed in a broad range of subsequent chemical conjugation reactions via bioorthogonal reactions94. Although current clinically investigated protein conjugates have been mainly constructed using non-specific modification methods, the promising potential of enzymatic conjugation approaches will likely be further employed in the development of next-generation therapeutic protein conjugates.
Functional Proteins
For most of the protein scaffolds discussed in this chapter, their role is typically to serve as targeting domains and bind specific cell surface receptors to either mediate cell-cell interactions, induce endocytosis, or trigger receptor-mediated signaling. These molecules include antibody-derived moieties or non-antibody proteins that have affinities for a variety of cell surface antigens. For many biomedical applications with these types of protein conjugates, a major concern is how to improve their selectivity to minimize off-target side effects, especially for the delivery of toxic drugs or gene-silencing oligonucleotides. One strategy is to fabricate multivalent scaffolds with multiple receptor-specific proteins to increase the avidity of the conjugates. The multivalent scaffolds can be formed either by modifying the single conjugate with multiple targeting proteins39 or through self-assembly processes to form supramolecular structures19,47,48,71. Moreover, different types of targeting domains can be incorporated into the same conjugate to generate bispecific or multi-specific constructs to enhance selectivity86. For protein-drug conjugates, such enhanced selectivity means greater accumulation of the drug at the tumor site and less premature drug release in normal tissues. It is expected that personalized protein-drug conjugates with multi-specificity and high selectivity can be developed based on analysis of the patient’s antigen type and expression level. Furthermore, engagement of multiple receptors may also be used to boost signal transduction upon receptor activation72, further emphasizing the significance of multivalency for designing protein conjugates.
On the other hand, temporospatial control over the functionalities of protein conjugates is also highly favorable for therapeutic purposes. For example, it would be highly beneficial if a protein conjugate can mediate reversible cell-cell interactions in a controllable manner because tunability has the potential to reduce the incidence and severity of potential adverse effects from cell-based therapies. In other words, the ability to “switch off” protein function can provide a higher level of safety and efficacy using protein conjugates compared with other approaches designed to mediate cell-cell interactions. Nevertheless, only a few protein conjugate systems to date have permitted temporal control over their function. For example, prenylated multivalent nanoring systems have been shown to mediate reversible cell-cell interactions, which can be abolished by the treatment with an FDA-approved drug, trimethoprim19. These studies have highlighted the significance of function reversibility and provided insights into the design of smart controllable systems. With the increasing need to optimize the selectivity of the protein conjugates for their targets, more efforts have been put into the development of multivalent protein conjugate constructs. The interplay between the avidity and selectivity of these multivalent constructs has also been investigated, providing insights into targeted drug delivery and regulation of cell-cell interactions111. Furthermore, when bispecific multivalent conjugates are used to engineer intercellular interactions, the ratio of the targeting elements can be tuned based on the relative number of the two types of corresponding receptors to induce optimal interactions19,112, and the concentration of the bispecific conjugates at their binding sites is another consideration for efficiently directing intercellular interactions according to the ternary complex model.
Functional Elements Conjugated to the Proteins
Next generation conjugates will likely depend on taking advantageous and compatible chemistry that can enhance conjugation synthesis. To regulate cell-cell interactions, protein-lipid conjugates need to be stable on the cell surface for a desired period of time. However, many protein-lipid conjugates employed for cell surface modifications and cell-cell interactions exhibit only short half-lives on cell surfaces ranging from mere minutes to a few hours. To increase the stability of protein-lipid conjugates on the cell surface, the hydrophobicity of the anchor can be enhanced for a stronger affinity with membrane lipids, or multivalent scaffolds can be constructed to increase the number of lipid anchors necessary to achieve greater stability19.
For protein-polymer conjugates used for hydrogels, the properties of the polymeric materials are of critical importance for biomedical applications. The polymeric materials should incorporate the necessary self-assembling and rheology characteristics, biocompatibility with tissues, and, if desired, controlled biodegradability113,114. In addition, especially for drug delivery applications, protein-polymer conjugates may need to be engineered to be responsive to defined physiological stimuli102. Likewise, photo-switchable linkers can also be rationally designed and incorporated into functional proteins to control their conformations and functionalities73.
Oligonucleotides conjugated to proteins can be designed with self-assembling properties for multivalent display of targeting proteins47,48,71,72, and their structure can also be chemically modified to obtain better in vivo stability towards enzymatic degradation72. It is likely that the combination of multiple functional molecules within the same protein conjugate may lead to synergistic effects, enhancing therapeutic efficacy.
CONCLUSION
Protein conjugates are versatile hybrid biomacromolecules that can be used to manipulate cell fate. Different functional materials have been conjugated to proteins for a plethora of biomedical applications, including regulation of intercellular interactions, intervention in intracellular biological pathways, and inhibition of cell proliferation. Collectively, the emergence of protein conjugate techniques and methodologies has demonstrated their promising potential for therapeutic application in the fields of cell-based immunotherapy, tissue engineering, regenerative medicine, targeted gene therapy, cancer drug delivery, and others. Although the clinical translation of these molecules remains challenging, studies employing these conjugates have accelerated the development of new conjugation methods and deepened our understanding of what constitutes favorable clinical properties for “ideal” conjugates. Overall, protein conjugates are poised to play increasingly significant roles in many biomedical applications well into the future.
ACKNOWLEDGMENTS
This work was supported by GM084152 (M.D.D.), GM141853 (M.D.D.), CA185627 (C.R.W.), CA247681 (C.R.W.), and NSF Grant ECCS-2025124 to the Minnesota Nano Center. Y.W. was funded by a Doctoral Dissertation Fellowship from the University of Minnesota.
REFERENCES
- (1).Goldring MB; Marcu KB Cartilage Homeostasis in Health and Rheumatic Diseases. Arthritis Res. Ther 2009, 11 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Honda K; Littman DR The Microbiota in Adaptive Immune Homeostasis and Disease. Nature 2016, 535 (7610), 75–84. [DOI] [PubMed] [Google Scholar]
- (3).Kotas ME; Medzhitov R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160 (5), 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wynn TA; Chawla A; Pollard JW Macrophage Biology in Development, Homeostasis and Disease. Nature 2013, 496 (7446), 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Brestoff JR; Artis D. Immune Regulation of Metabolic Homeostasis in Health and Disease. Cell 2015, 161 (1), 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Kintzing JR; Filsinger Interrante MV; Cochran JR Emerging Strategies for Developing Next-Generation Protein Therapeutics for Cancer Treatment. Trends Pharmacol. Sci 2016, 37 (12), 993–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Carter PJ Introduction to Current and Future Protein Therapeutics: A Protein Engineering Perspective. Exp. Cell Res 2011, 317 (9), 1261–1269. [DOI] [PubMed] [Google Scholar]
- (8).Leader B; Baca QJ; Golan DE Protein Therapeutics: A Summary and Pharmacological Classification. Nat. Rev. Drug Discov 2008, 7 (1), 21–39. [DOI] [PubMed] [Google Scholar]
- (9).Simeon R; Chen Z. In Vitro-Engineered Non-Antibody Protein Therapeutics. Protein Cell 2018, 9 (1), 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Spiess C; Zhai Q; Carter PJ Alternative Molecular Formats and Therapeutic Applications for Bispecific Antibodies. Mol. Immunol 2015, 67 (2), 95–106. [DOI] [PubMed] [Google Scholar]
- (11).Walko CM; West HJ Antibody Drug Conjugates for Cancer Treatment. JAMA Oncol. 2019, 5 (11), 1648. [DOI] [PubMed] [Google Scholar]
- (12).Beck A; Goetsch L; Dumontet C; Corvaïa N. Strategies and Challenges for the next Generation of Antibody-Drug Conjugates. Nat. Rev. Drug Discov 2017, 16 (5), 315–337. [DOI] [PubMed] [Google Scholar]
- (13).Thomas A; Teicher BA; Hassan R. Antibody–Drug Conjugates for Cancer Therapy. Lancet Oncol. 2016, 17 (6), e254–e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Samaranayake H; Wirth T; Schenkwein D; Räty JK; Ylä-Herttuala S. Challenges in Monoclonal Antibody-Based Therapies. Ann. Med 2009, 41 (5), 322–331. [DOI] [PubMed] [Google Scholar]
- (15).Liu JKH The History of Monoclonal Antibody Development - Progress, Remaining Challenges and Future Innovations. Ann. Med. Surg 2014, 3 (4), 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Vazquez-Lombardi R; Phan TG; Zimmermann C; Lowe D; Jermutus L; Christ D. Challenges and Opportunities for Non-Antibody Scaffold Drugs. Drug Discov. Today 2015, 20 (10), 1271–1283. [DOI] [PubMed] [Google Scholar]
- (17).Liu Q; Tian J; Liu J; Zhu M; Gao Z; Hu X; Midgley AC; Wu J; Wang X; Kong D; Zhuang J; Liu J; Yan X; Huang X. Modular Assembly of Tumor-Penetrating and Oligomeric Nanozyme Based on Intrinsically Self-Assembling Protein Nanocages. Adv. Mater 2021, 33 (39). [DOI] [PubMed] [Google Scholar]
- (18).Ushiyama A; Ono M; Kataoka-Hamai C; Taguchi T; Kaizuka Y. Induction of Intermembrane Adhesion by Incorporation of Synthetic Adhesive Molecules into Cell Membranes. Langmuir 2015, 31 (6), 1988–1998. [DOI] [PubMed] [Google Scholar]
- (19).Wang Y; Kilic O; Csizmar CM; Ashok S; Hougland JL; Distefano MD; Wagner CR Engineering Reversible Cell-Cell Interactions Using Enzymatically Lipidated Chemically Self-Assembled Nanorings. Chem. Sci 2021, 12 (1), 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Levine JF; Stockdale FE Cell-Cell Interactions Promote Mammary Epithelial Cell Differentiation. J. Cell Biol 1985, 100 (5), 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Sharma A; Shiras A. Cancer Stem Cell-Vascular Endothelial Cell Interactions in Glioblastoma. Biochem. Biophys. Res. Commun 2016, 473 (3), 688–692. [DOI] [PubMed] [Google Scholar]
- (22).Kramer RH; Nicolson GL Interactions of Tumor Cells with Vascular Endothelial Cell Monolayers: A Model for Metastatic Invasion. Proc. Natl. Acad. Sci. U. S. A 1979, 76 (11), 5704–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Kim J; Denu RA; Dollar BA; Escalante LE; Kuether JP; Callander NS; Asimakopoulos F; Hematti P. Macrophages and Mesenchymal Stromal Cells Support Survival and Proliferation of Multiple Myeloma Cells. Br. J. Haematol 2012, 158 (3), 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Akins MR; Biederer T. Cell-Cell Interactions in Synaptogenesis. Curr. Opin. Neurobiol 2006, 16 (1), 83–89. [DOI] [PubMed] [Google Scholar]
- (25).Parekkadan B; Berdichevsky Y; Irimia D; Leeder A; Yarmush G; Toner M; Levine JB; Yarmush ML Cell-Cell Interaction Modulates Neuroectodermal Specification of Embryonic Stem Cells. Neurosci. Lett 2008, 438 (2), 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hawkins RE; Gilham DE; Debets R; Eshhar Z; Taylor N; Abken H; Schumacher TN Development of Adoptive Cell Therapy for Cancer: A Clinical Perspective. Hum. Gene Ther 2010, 21 (6), 665–672. [DOI] [PubMed] [Google Scholar]
- (27).June CH Adoptive T Cell Therapy for Cancer in the Clinic. J. Clin. Invest 2007, 117 (6), 1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Csizmar CM; Petersburg JR; Wagner CR Programming Cell-Cell Interactions through Non-Genetic Membrane Engineering. Cell Chem. Biol 2018, 25 (8), 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Sterner RC; Sterner RM CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kim SA; Peacock JS The Use of Palmitate-Conjugated Protein A for Coating Cells with Artificial Receptors Which Facilitate Intercellular Interactions. J. Immunol. Methods 1993, 158 (1), 57–65. [DOI] [PubMed] [Google Scholar]
- (31).Colsky AS; Peacock JS Palmitate-Derivatized Antibodies Can Function as Surrogate Receptors for Mediating Specific Cell-Cell Interactions. J. Immunol. Methods 1989, 124 (2), 179–187. [DOI] [PubMed] [Google Scholar]
- (32).Dennis JE; Cohen N; Goldberg VM; Caplan AI Targeted Delivery of Progenitor Cells for Cartilage Repair. J. Orthop. Res 2004, 22 (4), 735–741. [DOI] [PubMed] [Google Scholar]
- (33).Lo CY; Antonopoulos A; Dell A; Haslam SM; Lee T; Neelamegham S. The Use of Surface Immobilization of P-Selectin Glycoprotein Ligand-1 on Mesenchymal Stem Cells to Facilitate Selectin Mediated Cell Tethering and Rolling. Biomaterials 2013, 34 (33), 8213–8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Ko IK; Kean TJ; Dennis JE Targeting Mesenchymal Stem Cells to Activated Endothelial Cells. Biomaterials 2009, 30 (22), 3702–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Won YW; Patel AN; Bull DA Cell Surface Engineering to Enhance Mesenchymal Stem Cell Migration toward an SDF-1 Gradient. Biomaterials 2014, 35 (21), 5627–5635. [DOI] [PubMed] [Google Scholar]
- (36).Lim KS; Lee DY; Valencia GM; Won YW; Bull DA Cell Surface-Engineering to Embed Targeting Ligands or Tracking Agents on the Cell Membrane. Biochem. Biophys. Res. Commun 2017, 482 (4), 1042–1047. [DOI] [PubMed] [Google Scholar]
- (37).Wöll S; Bachran C; Schiller S; Swee LK; Scherließ R. Sortase-A Mediated Chemoenzymatic Lipidation of Single-Domain Antibodies for Cell Membrane Engineering. Eur. J. Pharm. Biopharm 2020, 153 (March), 121–129. [DOI] [PubMed] [Google Scholar]
- (38).Koyfman AY; Braun GB; Reich NO Cell-Targeted Self-Assembled DNA Nanostructures. J. Am. Chem. Soc 2009, 131 (40), 14237–14239. [DOI] [PubMed] [Google Scholar]
- (39).Kim YS; Kong WH; Kim H; Hahn SK Targeted Systemic Mesenchymal Stem Cell Delivery Using Hyaluronate–Wheat Germ Agglutinin Conjugate. Biomaterials 2016, 106, 217–227. [DOI] [PubMed] [Google Scholar]
- (40).Li Z; Xu Y; Li H; Guan J. Immobilization of Insulin-like Growth Factor-1 onto Thermosensitive Hydrogels to Enhance Cardiac Progenitor Cell Survival and Differentiation under Ischemic Conditions. Sci. China Chem 2014, 57 (4), 568–578. [Google Scholar]
- (41).Feng G; Zhang J; Li Y; Nie Y; Zhu D; Wang R; Liu J; Gao J; Liu N; He N; Du W; Tao H; Che Y; Xu Y; Kong D; Zhao Q; Li Z. IGF-1 C Domain-Modified Hydrogel Enhances Cell Therapy for AKI. J. Am. Soc. Nephrol 2016, 27 (8), 2357–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Wang X; Zhang J; Cui W; Fang Y; Li L; Ji S; Mao D; Ke T; Yao X; Ding D; Feng G; Kong D. Composite Hydrogel Modified by IGF-1C Domain Improves Stem Cell Therapy for Limb Ischemia. ACS Appl. Mater. Interfaces 2018, 10 (5), 4481–4493. [DOI] [PubMed] [Google Scholar]
- (43).Yin Nina, Hana Yongming, Xu Hanlin, Gao Yisen, Yi Tao, Yao Jiale, Li Dong; Dejun Cheng, Z. C. VEGF-Conjugated Alginate Hydrogel Prompt Angiogenesis and Improve Pancreatic Islet Engraftment and Function in Type 1 Diabetes. Mater. Sci. Eng. C 2016, 59, 958–964. [DOI] [PubMed] [Google Scholar]
- (44).Millon LE; Padavan DT; Hamilton AM; Boughner DR; Wan W. Exploring Cell Compatibility of a Fibronectin-Functionalized Physically Crosslinked Poly(Vinyl Alcohol) Hydrogel. J. Biomed. Mater. Res. - Part B Appl. Biomater 2012, 100 B (1), 1–10. [DOI] [PubMed] [Google Scholar]
- (45).Vandghanooni S; Eskandani M; Barar J; Omidi Y. Bispecific Therapeutic Aptamers for Targeted Therapy of Cancer: A Review on Cellular Perspective. J. Mol. Med 2018, 96 (9), 885–902. [DOI] [PubMed] [Google Scholar]
- (46).Lin M; Zhang J; Wan H; Yan C; Xia F. Rationally Designed Multivalent Aptamers Targeting Cell Surface for Biomedical Applications. ACS Appl. Mater. Interfaces 2021, 13 (8), 9369–9389. [DOI] [PubMed] [Google Scholar]
- (47).Pan L; Cao C; Run C; Zhou L; Chou JJ DNA-Mediated Assembly of Multispecific Antibodies for T Cell Engaging and Tumor Killing. Adv. Sci 2020, 7 (2), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Kazane SA; Axup JY; Kim CH; Ciobanu M; Wold ED; Barluenga S; Hutchins BA; Schultz PG; Winssinger N; Smider VV Self-Assembled Antibody Multimers through Peptide Nucleic Acid Conjugation. J. Am. Chem. Soc 2013, 135 (1), 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Rashidian M; Kumarapperuma SC; Gabrielse K; Fegan A; Wagner CR; Distefano MD Simultaneous Dual Protein Labeling Using a Triorthogonal Reagent. J. Am. Chem. Soc 2013, 135 (44), 16388–16396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Jung HH; Park K; Han DK Preparation of TGF-Β1-Conjugated Biodegradable Pluronic F127 Hydrogel and Its Application with Adipose-Derived Stem Cells. J. Control. Release 2010, 147 (1), 84–91. [DOI] [PubMed] [Google Scholar]
- (51).Golchin A; Farahany TZ Biological Products: Cellular Therapy and FDA Approved Products. Stem Cell Rev. Reports 2019, 15 (2), 166–175. [DOI] [PubMed] [Google Scholar]
- (52).Kulkarni JA; Witzigmann D; Thomson SB; Chen S; Leavitt BR; Cullis PR; van der Meel R. The Current Landscape of Nucleic Acid Therapeutics. Nat. Nanotechnol 2021, 16 (6), 630–643. [DOI] [PubMed] [Google Scholar]
- (53).Juliano RL The Delivery of Therapeutic Oligonucleotides. Nucleic Acids Res. 2016, 44 (14), 6518–6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Maerle AV; Simonova MA; Pivovarov VD; Voronina DV; Drobyazina PE; Trofimov DY; Alekseev LP; Zavriev SK; Ryazantsev DY Development of the Covalent Antibody-DNA Conjugates Technology for Detection of IgE and IgM Antibodies by Immuno-PCR. PLoS One 2019, 14 (1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Cuellar TL; Barnes D; Nelson C; Tanguay J; Yu SF; Wen X; Scales SJ; Gesch J; Davis D; Van Brabant Smith A; Leake D; Vandlen R; Siebel CW Systematic Evaluation of Antibody-Mediated siRNA Delivery Using an Industrial Platform of THIOMAB-siRNA Conjugates. Nucleic Acids Res. 2015, 43 (2), 1189–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Sugo T; Terada M; Oikawa T; Miyata K; Nishimura S; Kenjo E; Ogasawara-Shimizu M; Makita Y; Imaichi S; Murata S; Otake K; Kikuchi K; Teratani M; Masuda Y; Kamei T; Takagahara S; Ikeda S; Ohtaki T; Matsumoto H. Development of Antibody-siRNA Conjugate Targeted to Cardiac and Skeletal Muscles. J. Control. Release 2016, 237, 1–13. [DOI] [PubMed] [Google Scholar]
- (57).Satake N; Duong C; Yoshida S; Oestergaard M; Chen C; Peralta R; Guo S; Seth PP; Li Y; Beckett L; Chung J; Nolta J; Nitin N; Tuscano JM Novel Targeted Therapy for Precursor B-Cell Acute Lymphoblastic Leukemia: Anti-CD22 Antibody-MXD3 Antisense Oligonucleotide Conjugate. Mol. Med 2016, 22 (13), 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Arnold AE; Malek-Adamian E; Le PU; Meng A; Martínez-Montero S; Petrecca K; Damha MJ; Shoichet MS Antibody-Antisense Oligonucleotide Conjugate Downregulates a Key Gene in Glioblastoma Stem Cells. Mol. Ther. - Nucleic Acids 2018, 11 (June), 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Lu H; Wang D; Kazane S; Javahishvili T; Tian F; Song F; Sellers A; Barnett B; Schultz PG Site-Specific Antibody-Polymer Conjugates for siRNA Delivery. J. Am. Chem. Soc 2013, 135 (37), 13885–13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Bäumer S; Bäumer N; Appel N; Terheyden L; Fremerey J; Schelhaas S; Wardelmann E; Buchholz F; Berdel WE; Müller-Tidow C. Antibody-Mediated Delivery of Anti-KRAS-siRNA in Vivo Overcomes Therapy Resistance in Colon Cancer. Clin. Cancer Res 2015, 21 (6), 1383–1394. [DOI] [PubMed] [Google Scholar]
- (61).Bäumer N; Appel N; Terheyden L; Buchholz F; Rossig C; Müller-Tidow C; Berdel WE; Bäumer S. Antibody-Coupled siRNA as an Efficient Method for in Vivo MRNA Knockdown. Nat. Protoc 2016, 11 (1), 22–36. [DOI] [PubMed] [Google Scholar]
- (62).Shi SJ; Wang LJ; Han DH; Wu JH; Jiao D; Zhang KL; Chen JW; Li Y; Yang F; Zhang JL; Zheng GX; Yang AG; Zhao AZ; Qin WJ; Wen WH Therapeutic Effects of Human Monoclonal PSMA Antibody-Mediated TRIM24 siRNA Delivery in PSMA-Positive Castration-Resistant Prostate Cancer. Theranostics 2019, 9 (5), 1247–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Reshadmanesh A; Rahbarizadeh F; Ahmadvand D; Jafari Iri Sofla F. Evaluation of Cellular and Transcriptional Targeting of Breast Cancer Stem Cells via Anti-HER2 Nanobody Conjugated PAMAM Dendrimers. Artif. Cells, Nanomedicine Biotechnol 2018, 46 (sup3), S105–S115. [DOI] [PubMed] [Google Scholar]
- (64).Jafari Iri Sofla F; Rahbarizadeh F; Ahmadvand D; Nomani A; Rahimi Jamnani F. Specific Gene Delivery Mediated by Poly(Ethylene Glycol)-Grafted Polyamidoamine Dendrimer Modified with a Novel HER2-Targeting Nanobody. J. Bioact. Compat. Polym 2015, 30 (2), 129–144. [Google Scholar]
- (65).Dovgan I; Koniev O; Kolodych S; Wagner A. Antibody-Oligonucleotide Conjugates as Therapeutic, Imaging, and Detection Agents. Bioconjug. Chem 2019, 30 (10), 2483–2501. [DOI] [PubMed] [Google Scholar]
- (66).Sato Y; Yamauchi N; Takahashi M; Sasaki K; Fukaura J; Neda H; Fujii S; Hirayama M; Itoh Y; Koshita Y; Kogawa K; Kato J; Sakamaki S; Niitsu Y. In Vivo Gene Delivery to Tumor Cells by Transferrin‐streptavidin‐DNA Conjugate. FASEB J. 2000, 14 (13), 2108–2018. [DOI] [PubMed] [Google Scholar]
- (67).Ming X; Carver K; Wu L. Albumin-Based Nanoconjugates for Targeted Delivery of Therapeutic Oligonucleotides. Biomaterials 2013, 34 (32), 7939–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Ling X; Xie B; Gao X; Chang L; Zheng W; Chen H; Huang Y; Tan L; Li M; Liu T. Improving the Efficiency of Precise Genome Editing with Site-Specific Cas9-Oligonucleotide Conjugates. Sci. Adv 2020, 6 (15), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Ling X; Gao X; Chang L; Chen H; Shi X; Liu T. Rational Design of Minimum CRISPR Guide RNA by Site-Specific Cas9-RNA Conjugation. Chem. Commun 2020, 56 (54), 7515–7518. [DOI] [PubMed] [Google Scholar]
- (70).Ling X; Chang L; Chen H; Zhang B; Hu J; Ling X; Chang L; Chen H; Gao X; Yin J; Zuo Y; Huang Y; Zhang B; Hu J. Technology Improving the Efficiency of CRISPR-Cas12a-Based Genome Editing with Site-Specific Covalent Cas12a-CrRNA Conjugates. Mol. Cell 2021, 81 (22), 4747–4756.e7. [DOI] [PubMed] [Google Scholar]
- (71).Veneziano R; Moyer TJ; Stone MB; Wamhoff EC; Read BJ; Mukherjee S; Shepherd TR; Das J; Schief WR; Irvine DJ; Bathe M. Role of Nanoscale Antigen Organization on B-Cell Activation Probed Using DNA Origami. Nat. Nanotechnol 2020, 15 (8), 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Li L; Wang J; Li Y; Radford DC; Yang J; Kopeček J. Broadening and Enhancing Functions of Antibodies by Self-Assembling Multimerization at Cell Surface. ACS Nano 2019, 13 (10), 11422–11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Heu W; Choi JM; Kyeong H-H; Choi Y; Kim HY; Kim H-S Repeat Module-Based Rational Design of a Photoswitchable Protein for Light-Driven Control of Biological Processes. Angew. Chemie 2018, 130 (34), 11025–11029. [DOI] [PubMed] [Google Scholar]
- (74).Dragovich PS; Pillow TH; Blake RA; Sadowsky JD; Adaligil E; Adhikari P; Chen J; Corr N; Dela Cruz-Chuh J; Del Rosario G; Fullerton A; Hartman SJ; Jiang F; Kaufman S; Kleinheinz T; Kozak KR; Liu L; Lu Y; Mulvihill MM; Murray JM; O’Donohue A; Rowntree RK; Sawyer WS; Staben LR; Wai J; Wang J; Wei B; Wei W; Xu Z; Yao H; Yu SF; Zhang D; Zhang H; Zhang S; Zhao Y; Zhou H; Zhu X. Antibody-Mediated Delivery of Chimeric BRD4 Degraders. Part 2: Improvement of in Vitro Antiproliferation Activity and in Vivo Antitumor Efficacy. J. Med. Chem 2021, 64 (5), 2576–2607. [DOI] [PubMed] [Google Scholar]
- (75).Pillow TH; Adhikari P; Blake RA; Chen J; Del Rosario G; Deshmukh G; Figueroa I; Gascoigne KE; Kamath AV; Kaufman S; Kleinheinz T; Kozak KR; Latifi B; Leipold DD; Sing Li C; Li R; Mulvihill MM; O’Donohue A; Rowntree RK; Sadowsky JD; Wai J; Wang X; Wu C; Xu Z; Yao H; Yu SF; Zhang D; Zang R; Zhang H; Zhou H; Zhu X; Dragovich PS Antibody Conjugation of a Chimeric BET Degrader Enables in Vivo Activity. ChemMedChem 2020, 15 (1), 17–25. [DOI] [PubMed] [Google Scholar]
- (76).Dragovich PS; Pillow TH; Blake RA; Sadowsky JD; Adaligil E; Adhikari P; Bhakta S; Blaquiere N; Chen J; Dela Cruz-Chuh J; Gascoigne KE; Hartman SJ; He M; Kaufman S; Kleinheinz T; Kozak KR; Liu L; Liu L; Liu Q; Lu Y; Meng F; Mulvihill MM; O’Donohue A; Rowntree RK; Staben LR; Staben ST; Wai J; Wang J; Wei B; Wilson C; Xin J; Xu Z; Yao H; Zhang D; Zhang H; Zhou H; Zhu X. Antibody-Mediated Delivery of Chimeric BRD4 Degraders. Part 1: Exploration of Antibody Linker, Payload Loading, and Payload Molecular Properties. J. Med. Chem 2021, 64 (5), 2534–2575. [DOI] [PubMed] [Google Scholar]
- (77).Maneiro M; Forte N; Shchepinova MM; Kounde CS; Chudasama V; Baker JR; Tate EW Antibody-PROTAC Conjugates Enable HER2-Dependent Targeted Protein Degradation of BRD4. ACS Chem. Biol 2020, 15 (6), 1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Dragovich PS; Adhikari P; Blake RA; Blaquiere N; Chen J; Cheng YX; den Besten W; Han J; Hartman SJ; He J; He M; Rei Ingalla E; Kamath AV; Kleinheinz T; Lai T; Leipold DD; Li CS; Liu Q; J.; Y.; Meng F; Meng L; Ng C; Peng K; Lewis Phillips G; Pillow TH; Rowntree RK; Sadowsky JD; Sampath D; Staben L; Staben ST; Wai J; Wan K; Wang X; Wei BQ; Wertz IE; Xin J; Xu K; Yao H; Zang R; Zhang D; Zhou H; Zhao Y. Antibody-Mediated Delivery of Chimeric Protein Degraders Which Target Estrogen Receptor Alpha (ERα). Bioorganic Med. Chem. Lett 2020, 30 (4), 126907. [DOI] [PubMed] [Google Scholar]
- (79).Ahn G; Banik SM; Miller CL; Riley NM; Cochran JR; Bertozzi CR LYTACs That Engage the Asialoglycoprotein Receptor for Targeted Protein Degradation . Nat. Chem. Biol 2021, 17 (9), 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Banik SM; Pedram K; Wisnovsky S; Ahn G; Riley NM; Bertozzi CR Lysosome-Targeting Chimaeras for Degradation of Extracellular Proteins. Nature 2020, 584 (7820), 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Choi B; Kim S; Fan J; Kowalski T; Petrigliano F; Evseenko D; Lee M. Covalently Conjugated Transforming Growth Factor-Β1 in Modular Chitosan Hydrogels for the Effective Treatment of Articular Cartilage Defects. Biomater. Sci 2015, 3 (5), 742–752. [DOI] [PubMed] [Google Scholar]
- (82).Wu J; Zeng F; Huang XP; Chung JCY; Konecny F; Weisel RD; Li RK Infarct Stabilization and Cardiac Repair with a VEGF-Conjugated, Injectable Hydrogel. Biomaterials 2011, 32 (2), 579–586. [DOI] [PubMed] [Google Scholar]
- (83).Ghosh K; Ren XD; Shu XZ; Prestwich GD; Clark RAF Fibronectin Functional Domains Coupled to Hyaluronan Stimulate Adult Human Dermal Fibroblast Responses Critical for Wound Healing. Tissue Eng. 2006, 12 (3), 601–613. [DOI] [PubMed] [Google Scholar]
- (84).Cheung CY; Anseth KS Synthesis of Immunoisolation Barriers That Provide Localized Immunosuppression for Encapsulated Pancreatic Islets. Bioconjug. Chem. 2006, 17 (4), 1036–1042. [DOI] [PubMed] [Google Scholar]
- (85).Wheatley MA; Cochran MC; Eisenbrey JR; Oum KL Cellular Signal Transduction Can Be Induced by TRAIL Conjugated to Microcapsules. J. Biomed. Mater. Res. - Part A 2012, 100 A (10), 2602–2611. [DOI] [PubMed] [Google Scholar]
- (86).Emanuel SL; Engle LJ; Chao G; Zhu RR; Cao C; Lin Z; Yamniuk A; Hosbach J; Brown J; Fitzpatrick E; Gokemeijer J; Morin P; Morse B; Carvajal IM; Fabrizio D; Wright MC; Das Gupta R; Gosselin M; Cataldo D; Ryseck RP; Doyle ML; Wong TW; Camphausen RT; Cload ST; Marsh HN; Gottardis MM; Furfine ES A Fibronectin Scaffold Approach to Bispecific Inhibitors of Epidermal Growth Factor Receptor and Insulin-like Growth Factor-I Receptor. MAbs 2011, 3 (1), 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Eckhart KE; Schmidt SJ; Starvaggi FA; Wolf ME; Vickery WM; Sydlik SA Peptide- and Protein-Graphene Oxide Conjugate Materials for Controlling Mesenchymal Stem Cell Fate. Regen. Eng. Transl. Med 2021, 7 (4), 460–484. [Google Scholar]
- (88).Khongorzul P; Ling CJ; Khan FU; Ihsan AU; Zhang J. Antibody-Drug Conjugates: A Comprehensive Review. Mol. Cancer Res 2020, 18 (1), 3–19. [DOI] [PubMed] [Google Scholar]
- (89).Ding H; Altai M; Rinne SS; Vorobyeva A; Tolmachev V; Gräslund T; Orlova A. Incorporation of a Hydrophilic Spacer Reduces Hepatic Uptake of Her2-Targeting Affibody–Dm1 Drug Conjugates. Cancers (Basel). 2019, 11 (8), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Xu T; Ding H; Vorobyeva A; Oroujeni M; Orlova A; Tolmachev V; Gräslund T. Drug Conjugates Based on a Monovalent Affibody Targeting Vector Can Efficiently Eradicate HER2 Positive Human Tumors in an Experimental Mouse Model. Cancers (Basel). 2021, 13 (1), 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Shi Q; Tao Z; Yang H; Fan Q; Wei D; Wan L; Lu X. PDGFRβ-Specific Affibody-Directed Delivery of a Photosensitizer, IR700, Is Efficient for Vascular-Targeted Photodynamic Therapy of Colorectal Cancer. Drug Deliv. 2017, 24 (1), 1818–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Chen YJ; Deng QW; Wang L; Guo XC; Yang JY; Li T; Xu Z; Lee HC; Zhao YJ GALA Peptide Improves the Potency of Nanobody-Drug Conjugates by Lipid-Induced Helix Formation. Chem. Commun 2021, 57 (12), 1434–1437. [DOI] [PubMed] [Google Scholar]
- (93).Brandl F; Busslinger S; Zangemeister-Wittke U; Plückthun A. Optimizing the Anti-Tumor Efficacy of Protein-Drug Conjugates by Engineering the Molecular Size and Half-Life. J. Control. Release 2020, 327 (July), 186–197. [DOI] [PubMed] [Google Scholar]
- (94).Lee JJ; Choi H-JJ; Yun M; Kang Y; Jung J-EE; Ryu Y; Kim TY; Cha YJ; Cho H-SS; Min J-JJ; Chung C-WW; Kim H-SS Enzymatic Prenylation and Oxime Ligation for the Synthesis of Stable and Homogeneous Protein-Drug Conjugates for Targeted Therapy. Angew. Chemie 2015, 127 (41), 12188–12192. [DOI] [PubMed] [Google Scholar]
- (95).Krzyscik MA; Zakrzewska M; Otlewski J. Site-Specific, Stoichiometric-Controlled, PEGylated Conjugates of Fibroblast Growth Factor 2 (FGF2) with Hydrophilic Auristatin y for Highly Selective Killing of Cancer Cells Overproducing Fibroblast Growth Factor Receptor 1 (FGFR1). Mol. Pharm 2020, 17 (7), 2734–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Setyawati MI; Kutty RV; Leong DT DNA Nanostructures Carrying Stoichiometrically Definable Antibodies. Small 2016, 12 (40), 5601–5611. [DOI] [PubMed] [Google Scholar]
- (97).Gerhart J; Greenbaum M; Casta L; Clemente A; Mathers K; Getts R; George-Weinstein M. Antibody-Conjugated, DNA-Based Nanocarriers Intercalated with Doxorubicin Eliminate Myofibroblasts in Explants of Human Lens Tissue. J. Pharmacol. Exp. Ther 2017, 361 (1), 60–67. [DOI] [PubMed] [Google Scholar]
- (98).Westerlund K; Altai M; Mitran B; Konijnenberg M; Oroujeni M; Atterby C; De Jong M; Orlova A; Mattsson J; Micke P; Karlströmy AE; Tolmachevy V. Radionuclide Therapy of HER2-Expressing Human Xenografts Using Affibody-Based Peptide Nucleic Acid-Mediated Pretargeting: In Vivo Proof of Principle. J. Nucl. Med 2018, 59 (7), 1092–1098. [DOI] [PubMed] [Google Scholar]
- (99).Jiang Y; Stenzel M. Drug Delivery Vehicles Based on Albumin–Polymer Conjugates. Macromol. Biosci. 2016, 791–802. [DOI] [PubMed]
- (100).Liu Z; Dong C; Wang X; Wang H; Li W; Tan J; Chang J. Self-Assembled Biodegradable Protein-Polymer Vesicle as a Tumor-Targeted Nanocarrier. ACS Appl. Mater. Interfaces 2014, 6 (4), 2393–2400. [DOI] [PubMed] [Google Scholar]
- (101).Ge J; Neofytou E; Lei J; Beygui RE; Zare RN Protein-Polymer Hybrid Nanoparticles for Drug Delivery. Small 2012, 8 (23), 3573–3578. [DOI] [PubMed] [Google Scholar]
- (102).Boonpavanitchakul K; Bast LK; Bruns N; Magaraphan R. Silk Sericin-Polylactide Protein-Polymer Conjugates as Biodegradable Amphiphilic Materials and Their Application in Drug Release Systems. Bioconjug. Chem 2020, 31 (10), 2312–2324. [DOI] [PubMed] [Google Scholar]
- (103).Belfiore L; Saunders DN; Ranson M; Thurecht KJ; Storm G; Vine KL Towards Clinical Translation of Ligand-Functionalized Liposomes in Targeted Cancer Therapy: Challenges and Opportunities. J. Control. Release 2018, 277 (March), 1–13. [DOI] [PubMed] [Google Scholar]
- (104).Mojarad-Jabali S; Farshbaf M; Walker PR; Hemmati S; Fatahi Y; Zakeri-Milani P; Sarfraz M; Valizadeh H. An Update on Actively Targeted Liposomes in Advanced Drug Delivery to Glioma. Int. J. Pharm 2021, 602 (April), 120645. [DOI] [PubMed] [Google Scholar]
- (105).Falck G; Müller K. Enzyme-Based Labeling Strategies for Antibody–Drug Conjugates and Antibody Mimetics. Antibodies 2018, 7 (1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Lieser RM; Yur D; Sullivan MO; Chen W. Site-Specific Bioconjugation Approaches for Enhanced Delivery of Protein Therapeutics and Protein Drug Carriers. Bioconjug. Chem 2020, 31 (10), 2272–2282. [DOI] [PubMed] [Google Scholar]
- (107).Grünewald J; Klock HE; Cellitti SE; Bursulaya B; McMullan D; Jones DH; Chiu HP; Wang X; Patterson P; Zhou H; Vance J; Nigoghossian E; Tong H; Daniel D; Mallet W; Ou W; Uno T; Brock A; Lesley SA; Geierstanger BH Efficient Preparation of Site-Specific Antibody-Drug Conjugates Using Phosphopantetheinyl Transferases. Bioconjug. Chem 2015, 26 (12), 2554–2562. [DOI] [PubMed] [Google Scholar]
- (108).Strop P; Tran TT; Dorywalska M; Delaria K; Dushin R; Wong OK; Ho WH; Zhou D; Wu A; Kraynov E; Aschenbrenner L; Han B; O’Donnell CJ; Pons J; Rajpal A; Shelton DL; Liu SH RN927C, a Site-Specific Trop-2 Antibody-Drug Conjugate (ADC) with Enhanced Stability, Is Highly Efficacious in Preclinical Solid Tumor Models. Mol. Cancer Ther 2016, 15 (11), 2698–2708. [DOI] [PubMed] [Google Scholar]
- (109).Hallam TJ; Wold E; Wahl A; Smider VV Antibody Conjugates with Unnatural Amino Acids. Mol. Pharm 2015, 12 (6), 1848–1862. [DOI] [PubMed] [Google Scholar]
- (110).Zhang Y; Park KY; Suazo KF; Distefano MD Recent Progress in Enzymatic Protein Labelling Techniques and Their Applications. Chem. Soc. Rev 2018, 47 (24), 9106–9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Csizmar CM; Petersburg JR; Perry TJ; Rozumalski L; Hackel BJ; Wagner CR Multivalent Ligand Binding to Cell Membrane Antigens: Defining the Interplay of Affinity, Valency, and Expression Density. J. Am. Chem. Soc 2019, 141 (1), 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Kilic O; De Souza MRM; Almotlak AA; Wang Y; Siegfried JM; Distefano MD; Wagner CR Anti-EGFR Fibronectin Bispecific Chemically Self-Assembling Nanorings (CSANs) Induce Potent T Cell-Mediated Antitumor Responses and Downregulation of EGFR Signaling and PD-1/PD-L1 Expression. J. Med. Chem 2020, 63 (18), 10091–10532. [DOI] [PubMed] [Google Scholar]
- (113).Kaith BS; Singh A; Sharma AK; Sud D. Hydrogels: Synthesis, Classification, Properties and Potential Applications—A Brief Review. J. Polym. Environ 2021, 29 (12), 3827–3841. [Google Scholar]
- (114).Mondal S; Das S; Nandi AK A Review on Recent Advances in Polymer and Peptide Hydrogels. Soft Matter 2020, 16 (6), 1404–1454. [DOI] [PubMed] [Google Scholar]