Abstract
Objective:
Since thoracic endovascular aortic repair (TEVAR) received U.S. Food and Drug Administration approval for the treatment of descending thoracic aneurysms in March 2005, excellent 30-day and midterm outcomes have been described. However, data on long-term outcomes are lacking with Medicare data suggesting that TEVAR has worse late survival compared with open descending repair. As such, the purpose of this study was to examine the long-term outcomes for on-label use of TEVAR for repair of descending thoracic aneurysms.
Methods:
Of 579 patients undergoing TEVAR between March 2005 and April 2016 at a single referral center for aortic surgery, 192 (33.2%) were performed for a descending thoracic aneurysm indication in accordance with the device instructions for use, including 106 fusiform (55.2%), 80 saccular (41.7%), and 6 with both saccular and fusiform (3.1%) aneurysms. All aneurysms were located distal to the left subclavian artery and proximal to the celiac axis, and hybrid procedures including arch or visceral debranching were excluded with the exception of left carotid-subclavian artery bypass. Aortic dissection and intramural hematoma as indications for TEVAR were also excluded. Primary 30-day and in-hospital outcomes included mortality, stroke, need for new permanent dialysis, and permanent paraparesis or paraplegia. Primary long-term outcomes included survival and rate of reintervention secondary to endoleak. The Kaplan-Meier method was used to estimate long-term overall and aorta-specific survivals.
Results:
The mean age was 71.1 ± 10.4 years. All aneurysms in this series were degenerative in nature and no patients with a connective tissue disorder were included. The mean aortic diameter was 5.9 ± 1.5 cm at time of intervention. Rates of 30-day and in-hospital mortality, stroke, permanent dialysis, and permanent paraparesis and paraplegia were 4.7%, 2.1%, 0.5%, and 0.5%, respectively. At a mean follow-up of 69 ± 44 months (range, 3–141 months), there were 68 late deaths (35.4%), two of which were due to aortic rupture. Overall and aorta-specific survivals at 141 months (11.8 years) were 45.7% and 96.2%, respectively. Endovascular reintervention was required in 14 patients (7.3%) owing to type I (n = 10), type II (n = 2), and type III (n = 2) endoleak, all of which subsequently resolved. No patient required open reintervention for any cause.
Conclusions:
Long-term (12-year) aorta-specific survival after on-label endovascular repair of degenerative descending thoracic aneurysms in nonsyndromic patients is excellent (96%) with sustained protection from rupture, and a low rate of reintervention owing to endoleak (7%). Endovascular repair should be considered the treatment of choice for this pathology.
After receiving U.S. Food and Drug Administration (FDA) approval for the treatment of descending thoracic aneurysms in March 2005,1–3 use of thoracic endovascular aortic repair (TEVAR) has increased steadily, and in many centers has largely replaced traditional open repair.4,5 Despite this initial approved indication, most studies to date have focused on other applications of TEVAR including aortic dissection, trauma, and hybrid procedures,6–9 and few studies address the outcomes of TEVAR for descending thoracic aortic aneurysm (DTAA), many of which report only short-term to mid-term results. Furthermore, subsequent reports with longer follow-up have suggested worse late survival for patients undergoing endovascular repair in lieu of open repair, challenging the appropriateness of TEVAR in open surgical candidates.10–12 Additional aspects of endovascular repair, such as endoleak, need for reintervention, and lifelong surveillance, have added to this controversy surrounding the use of TEVAR as an initial repair strategy for descending aneurysm.13 Similarly, long-term studies of endovascular and open repair of abdominal aortic aneurysms have shown a high rate of reintervention and no survival advantage for endovascular repair.14 Given this controversy, the purpose of this study was to report long-term outcomes of TEVAR for the indication of DTAA in patients treated and followed in accordance with U.S. FDA approval and device instructions for use (IFU).
METHODS
Patient selection.
This study was reviewed and approved by the Institutional Review Board of Duke University Medical Center, and the need for individual patient consent was waived. A retrospective review was performed using a prospectively maintained database from a referral aortic center (Duke University Medical Center, Durham, NC) to identify all adult patients undergoing TEVAR between March 2005 and April 2016. Of 579 patients undergoing TEVAR during this interval, 192 (33.2%) underwent TEVAR for the indication of DTAA, of which there were 107 fusiform (55.2%), 80 saccular (41.7%), and 6 with both saccular and fusiform aneurysms (3.1%). All patients in this series underwent on-label TEVAR in accordance with the device (IFU). All aneurysms were located distal to the left subclavian artery and proximal to the celiac axis. Left subclavian artery revascularization was performed for previously described indications,15 including the presence of a dominant left vertebral artery, left vertebral artery arising directly from the aortic arch, patent pedicled left internal mammary artery bypass graft, patent left upper extremity hemodialysis access graft or fistula, patients at high risk for spinal cord ischemia (eg, long pavement zones or prior abdominal aortic aneurysm repair), and evidence of left arm ischemia intraoperatively after left subclavian artery ostial coverage with the endograft. Hybrid procedures including arch or visceral debranching were excluded, with the exception of left carotid-subclavian artery bypass. Patients undergoing TEVAR for the indications of dissection and intramural hematoma were likewise excluded, as were patients with endograft landing zone in Dacron replaced aorta.16
TEVAR procedures.
All patient procedures and management were part of routine clinical care as determined by the clinical care team. Patient selection for TEVAR, techniques of device delivery and deployment, and postoperative surveillance have been previously described.17–19 Preoperative planning of endograft procedures was performed using the TeraRecon system (TeraRecon Inc, San Mateo, Calif) with centerline measurements of flow lumen diameter by computed tomography angiography to assess landing zones as well as iliofemoral access vessels. All computed tomography angiograms included the base of the neck to allow assessment of the common carotid and vertebral arteries. Routine assessment of the circle of Willis or internal carotid arteries was not performed. Routine postoperative surveillance consisted of computed tomography angiography at 1, 6, and 12 months after TEVAR and annually thereafter. In addition, 3-month follow-up assessment and imaging was obtained in patients with an endoleak identified at 1 month, if the decision for initial endoleak observation was made. All follow-up assessments were done at the Duke University Center for Aortic Surgery, and a dedicated nurse practitioner contacted all patients to ensure clinical follow-up appointments were maintained.
Analysis.
Clinical events and complications were defined in accordance with the Society of Thoracic Surgeons Adult Cardiac Surgery Database definitions (available at www.sts.org). Survival status and follow-up intervals were determined using a combination of the electronic medical record and publicly available data. Patient and procedural characteristics were reported using percentages for categorical variables and medians for continuous variables, unless otherwise specified. Primary 30-day and in-hospital outcomes included mortality, stroke, need for new permanent dialysis, and permanent paraparesis and paraplegia. Primary long-term outcomes included survival and rate of reintervention secondary to endoleak. Long-term overall and aorta-specific survival were estimated using the Kaplan-Meier method. Statistical analysis was completed using SPSS v23 (IBM, Armonk NY).
RESULTS
In total, 192 patients underwent on-label TEVAR for isolated DTAA during the study period. The mean age was 71.1 ± 10.4 years (Table I). All aneurysms were attributed to degenerative atherosclerotic disease without suspicion for connective tissue disorder. Hypertension, hyperlipidemia, and tobacco use were the most common comorbidities in this cohort. Prior aortic surgery had been performed in 79 patients (41.1%; Table I). The mean aortic diameter at the time of intervention was 5.9 ± 1.5 cm, with fusiform aneurysms being the most common morphology (55.2%; n = 106; Table II). Seventy-five percent of cases were performed electively. Of the 25% of cases (n = 48) performed urgently or emergently, 10 presented with aortic rupture. The remainder presented with either symptomatic aneurysms or radiographic evidence of impending rupture. The use of various endovascular devices is outlined in Table II. Most patients required two endografts to fully exclude the aneurysmal aortic segment (range, 1–5 endografts). The average length of thoracic aorta covered by the endografts was 23 ± 13 cm overall and was shorter for saccular aneurysms (18 ± 12 cm for saccular vs 27 ± 9 cm for fusiform). Partial or complete left subclavian artery coverage occurred in 88 patients (45.8%), of whom 12 (13.6%) underwent concomitant revascularization via left carotid-subclavian artery bypass for previously described indications.15 The incidence of left subclavian coverage was similar in the elective (48% of cases with left subclavian coverage) and nonelective settings (38%). Celiac artery coverage did not occur in any patient, either intentionally or unintentionally. Prophylactic cerebrospinal fluid (CSF) drainage was likewise used selectively in 24 patients (12.5%) for previously published indications.20
Table I.
Patient characteristics
| Variable | % (No.) or mean ± SD (N = 192) |
|---|---|
|
| |
| Age, years | 71.1 ± 10.4 |
| Male gender | 55.7 (107) |
| White race | 71.9 (138) |
| BMI, kg/m2 | 27.8 ± 5.6 |
| Hypertension | 90.6 (174) |
| Hyperlipidemia | 74.5 (143) |
| History of tobacco use | 67.2 (129) |
| Diabetes mellitus | 21.9 (42) |
| Coronary artery disease | 38.5 (74) |
| History of stroke | 10.9 (21) |
| PAD | 33.9 (65) |
| COPD | 32.8 (63) |
| CKD | 20.3 (39) |
| Connective tissue disorder | 0.0 (0) |
| Prior aortic surgery | 41.1 (79) |
| ASA score, median | 3 (2–4) |
ASA, American Society of Anesthesiologists; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; PAD, peripheral arterial disease.
Table II.
Operative characteristics
| Variable | % (No.) (N = 192) |
|---|---|
|
| |
| Procedure status | |
| Elective | 75.0 (144) |
| Urgent or emergent | 25.0 (48) |
| Indication for TEVAR | |
| DTA, fusiform | 55.2 (106) |
| DTA, saccular | 41.7 (80) |
| DTA, fusiform and saccular | 3.1 (6) |
| Access vessel | |
| Femoral | 82.8 (159) |
| Iliac | 17.2 (33) |
| Infrarenal aorta | 0.5 (1) |
| Other | 1.0 (2) |
| Left subclavian artery coverage | 45.8 (88) |
| Maximum aortic diameter, cm | 5.9 ± 1.5 |
| Type of endograft | |
| Gore TAG/C-TAGa | 57.8 (111) |
| Medtronic Talent/Valiantb | 24.0 (46) |
| Cook Zenith TX2/Alpha Thoracicc | 16.1 (31) |
| Bolton Relayd | 1.6 (3) |
| Medtronic Aneurxb | 0.5 (1) |
| Endografts used, median | 2 (1–5) |
| Intraoperative CSF drainage | 12.5 (24) |
CSF, Cerebrospinal fluid; DTA, descending thoracic aneurysm; TEVAR, thoracic endovascular aortic repair.
W. L. Gore and Associates, Flagstaff, Ariz.
Medtronic, Minneapolis, Minn.
Cook, Bloomington, Ind.
Bolton Medical, Inc, Sunrise, Fla.
Rates of 30-day and in-hospital mortality, stroke, permanent dialysis, and permanent paraparesis or paraplegia were 4.7% (n = 9), 2.1% (n = 4), 0.5% (n = 1), and 0.5% (n = 1), respectively. Notably, 30-day and in-hospital survival was 90% amongst the 10 patients presenting with aortic rupture. Of the four strokes, all were embolic in nature secondary to atheroemboli from the aortic arch and involved the anterior and posterior circulation distributions. The single case of permanent spinal cord ischemia was a 75-year-old woman treated with TEVAR very early in the series for a 10-cm descending aneurysm that had developed proximal to a prior open distal descending thoracic aneurysm repair and required iliac conduit owing to small access vessels. Further, her left subclavian artery was covered but not bypassed, as our current left subclavian revascularization protocol had not been established at that time. She developed delayed postoperative paraparesis on postoperative day 1 in the setting of hypotension from retroperitoneal bleeding. This was managed with blood pressure augmentation and CSF drain placement with near full motor strength recovery by discharge and complete recovery in follow-up. She survived for another 5 years after her TEVAR procedure before dying of nonaortic causes. This patient would have received left subclavian revascularization under the current protocol, which may have prevented the spinal cord ischemia. Delayed transient paraparesis or paraplegia occurred in five patients, all in the setting of postoperative hypotension, and each of whom responded to pharmacologic intervention with blood pressure augmentation and without placement of a lumbar drain.
The median duration of stay was 3 days (interquartile range, 2–5 days). Survival status was 100% complete, and 85% of patients had complete clinical follow-up. At a mean follow-up of 69 ± 44 months (range, 3–141 months), there were 68 late deaths (35.4%). Of 77 total deaths in the cohort, six were due to aortic disease including aortic rupture in two patients. Of the two ruptures, one was due to technical error in a patient very early in the series who underwent emergent TEVAR for a leaking descending aneurysm. The case was done before fixed imaging was available at our institution, and the completion angiogram was interpreted as showing no endoleak. However, the patient ruptured postoperatively in the intensive care unit and autopsy demonstrated the distal stent graft landed short of the intended distal seal zone, which supports the now generally accepted standard of fixed imaging for complex endovascular interventions. The second patient developed a type I endoleak 4 years after TEVAR for a 6.3-cm descending aneurysm. At the time of endoleak diagnosis, the patient was 91 years old and elected to forego reintervention. The patient later died of thoracic aortic rupture.
Overall and aorta-specific survivals at 141 months (11.8 years) were 45.7% and 96.2%, respectively (Fig). During long-term follow-up, endovascular reintervention was required in 14 patients (7.3%) owing to type I (n = 10), type II (n = 2), and type III (n = 2) endoleak. All endoleaks subsequently resolved, and no patients required open reintervention for any cause. There were no significant distal migrations of deployed endografts.
Fig.
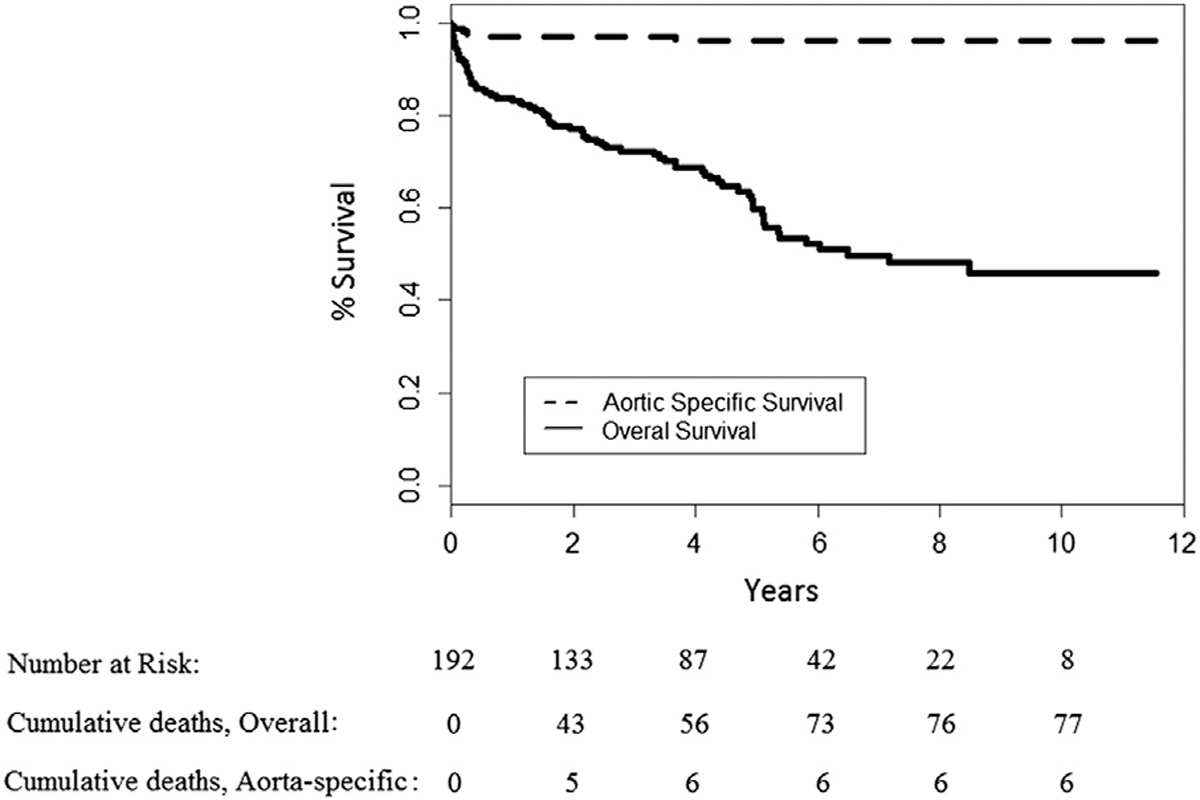
Long-term overall and aorta-specific survival.
DISCUSSION
Although TEVAR has been applied to and studied extensively for the indications of dissection, trauma, and hybrid repair, results of its use for the original on-label indication of isolated DTAA are less prevalent. Short-term and mid-term results have demonstrated the feasibility of TEVAR in this setting; however, long-term results are lacking. Furthermore, Medicare data have suggested worse late survival for patients undergoing TEVAR as compared with open surgery and challenged the use of endovascular repair for patients who are candidates for open surgery and expected to have longer survival.10 The results of the presents study, however, clearly demonstrate that endovascular repair of DTAAs can be achieved with excellent short-term and long-term outcomes. Specifically, the procedure appears to be durable with a late aorta-specific survival of 96%, suggesting few patients treated with TEVAR die from their aortic pathology, which is the primary goal of therapy. Rates of endovascular reintervention for endoleak were likewise low (7.3%), and no patient required open reintervention. Furthermore, these results were achieved despite the advanced age and multiple comorbidities of the patient cohort, as well as the significant number (25.0%) of urgent or emergent cases.
As detailed in a recent Cochrane review of thoracic stent graft versus surgery for thoracic aneurysm, there are no randomized controlled trials of open versus endovascular repair of descending thoracic aneurysms. However, based upon the nonrandomized data available, the authors of the Cochrane review concluded that TEVAR may be a good alternative to open repair, although true benefit cannot be established in the absence of randomized controlled trials. The authors acknowledged that such randomized controlled trials are unlikely to ever be performed and also called for high-quality studies demonstrating benefit, given the generally short-term outcomes available in the literature. The current study seems to answer this call, demonstrating very low rates of perioperative morbidity and mortality and sustained long-term freedom from aorta-related mortality in patients with DTAA treated with TEVAR in accordance with the original device IFU. The results also support the recommendations of the most recent (2016) multispecialty consensus guidelines to address this topic, namely the Joint Position Statement on Open and Endovascular Surgery for Thoracic Aortic Disease from the Canadian Cardiovascular Society, Canadian Society of Cardiac Surgeons, and Canadian Society for Vascular Surgery, which recommended endovascular repair of DTAAs for patients with suitable anatomy, adequate endograft landing zones, and absence of a connective tissue disorder (strong recommendation, medium-quality evidence).21
The long-term success of TEVAR is likely multifactorial, although several specific aspects merit mention. First, the technology available to clinicians has evolved substantially since its introduction in 2005. These advances have included improvements in the design of earlier generation endografts as well as subsequent FDA approval of newer devices. Many of these newer devices are lower profile, thereby reducing the incidence of peripheral vascular complications, as well as more conformable with better aortic wall apposition in hostile anatomies such as tightly angulated aortic arches. However, the data also highlight the importance of patient selection, especially with regard to device IFU. All patients in the current series were treated on label with adequate (≥2 cm) proximal and distal landing zones and no hybrid repairs. Reports of TEVAR demonstrating less optimal outcomes frequently have included patients treated outside the IFU,12 a scenario in which the results of endovascular repair are less predictable. Finally, as has been demonstrated in multiple studies, the results of TEVAR are likely similar to other thoracic aortic operations22,23 in that patients treated in centers with a dedicated high-volume multidisciplinary team and including standardized protocols for preoperative imaging, device sizing, patient risk stratification, intraoperative and early postoperative management, as well as lifelong clinical and imaging surveillance follow-up are likely to have the best outcomes. The importance of team experience cannot be overstated, and the results of the current study demonstrate evidence for ongoing quality improvement within our own institution with decreases in 30-day and in-hospital mortality and morbidity, as well as improved aorta-specific survival, as compared with our previously reported mid-term results of TEVAR for DTAA from 2005 to 2009.17
Additional aspects of our practice that merit further discussion include the use of left subclavian artery revascularization and prophylactic lumbar CSF drainage. As mentioned, we reserve subclavian revascularization for specific clinical settings, with left carotid-subclavian artery bypass being our preferred method of revascularization. Although 46% of this cohort had either partial or complete left subclavian artery coverage, only 13.6% underwent revascularization. Although this rate seems to be lower than comparable rates in the recent literature,24 the vast majority of patients in whom revascularization was not performed had only partial left subclavian artery coverage with some preserved antegrade flow in the left subclavian. We have found partial coverage to be effective in many patients to attain proximal seal yet avoid the need for concomitant revascularization. Our practice, especially in the latter years of the series, is to revascularize nearly all patients in whom the left subclavian is fully covered with no preservation of antegrade flow in accordance with published guidelines from the Society for Vascular Surgery.25 Furthermore, our overall rates of stroke and spinal cord injury, 2.1% and 0.5%, respectively, are lower than commonly reported in the literature despite this lower incidence of revascularization, which seems to support our current practice.15 Finally, a recent metaanalysis24 demonstrated no difference in the incidences of stroke, spinal cord injury, or death among those who did and did not undergo left subclavian artery revascularization with TEVAR, which is likewise concordant with our current results as well as our previously published findings with regard to the use of a selective left subclavian revascularization strategy.15
With regard to CSF drainage, prior work from our institution has demonstrated that prophylactic lumbar drain placement was not associated with a lower risk of spinal cord ischemia and was associated with drain complications in 11% of patients who had one placed.20 All of our isolated descending thoracic TEVAR patients are managed postoperatively with permissive hypertension, including stopping all antihypertensive medications for 30 days postoperatively unless the cuff systolic blood pressure consistently exceeds 160 mm Hg, and we feel this intervention is much more important for augmenting spinal cord perfusion than any minor incremental benefit of CSF drainage. As again demonstrated in the current series, the risk of permanent spinal cord injury after isolated descending thoracic aortic TEVAR is quite low, and we do not feel this small risk justifies the liberal use of CSF drainage and the potentially devastating complications associated with this intervention. As such, our practice includes restricted use of prophylactic lumbar drainage, which has consistently yielded a low incidence of permanent spinal cord injury.
Limitations of the current study are several. Although data collection occurred prospectively, the current study is observational and the analysis was performed retrospectively. It is thus limited by the potential for unobserved confounding variables. Second, all of the devices currently FDA approved for a descending aneurysm indication were not used in equal numbers, although the devices all performed well, and we have no reason to believe the results would differ with a more even distribution of device type use. Finally, the results presented represent outcomes in a high-volume referral center and may not be generalizable. Regardless, the potential for excellent long-term outcomes of TEVAR for patients with DTAA treated within the IFU of the variously available endovascular devices is clearly demonstrated in the present study, thereby mitigating some of the controversy surrounding the choice of open versus endovascular repair for this pathology.
CONCLUSIONS
Long-term (12-year) aorta-specific survival after on-label endovascular repair of degenerative descending thoracic aneurysms in non-syndromic patients is excellent with a low rate of device migration, reintervention owing to endoleak, and endovascular repair should be considered the treatment of choice for this pathology.
ARTICLE HIGHLIGHTS.
• Type of Research:
Single-center, retrospective cohort study
• Take Home Message:
Thoracic endovascular aortic repair was performed in 192 patients with degenerative disease with an early mortality, stroke, and permanent paraparesis or paraplegia rate of 4.7%, 2.1%, and 0.5%, respectively. Overall and aorta-specific survivals at 12 years were 45.7% and 96.2%, respectively. There was no conversion; 7.3% required endovascular reintervention with excellent result.
• Recommendation:
This study suggests that thoracic endovascular aortic repair for degenerative descending thoracic aortic aneurysms is safe, effective, and durable at 12 years of follow-up.
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Author conflict of interest: none.
Presented at the Forty-first Annual Meeting of the Southern Association for Vascular Surgery, Naples, Fla, January 18–21, 2017.
REFERENCES
- 1.Makaroun MS, Dillavou ED, Kee ST, Sicard G, Chaikof E, Bavaria J, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg 2005;41:1–9. [DOI] [PubMed] [Google Scholar]
- 2.Bavaria JE, Appoo JJ, Makaroun MS, Verter J, Yu ZF, Mitchell RS. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg 2007;133:369–77. [DOI] [PubMed] [Google Scholar]
- 3.Makaroun MS, Dillavou ED, Wheatley GH, Cambria RP. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J Vasc Surg 2008;47:912–8. [DOI] [PubMed] [Google Scholar]
- 4.Patterson BO, Thompson MM. The MOTHER Multicenter Registry: an overview and discussion of a registry that may help us understand how and to what degree various factors influence the risk of complications after TEVAR. Endovascular Today 2012:10–4. [Google Scholar]
- 5.Leurs LJ, Bell R, Degrieck Y, Thomas S, Hobo R, Lundbom J. Endovascular treatment of thoracic aortic diseases: combined experience from the EUROSTAR and United Kingdom Thoracic Endograft registries. J Vasc Surg 2004;40:670–9. [DOI] [PubMed] [Google Scholar]
- 6.Jonker FH, Trimarchi S, Verhagen HJ, Moll FL, Sumpio BE, Muhs BE. Meta-analysis of open versus endovascular repair for ruptured descending thoracic aortic aneurysm. J Vasc Surg 2010;51:1026–32. [DOI] [PubMed] [Google Scholar]
- 7.Patel HJ, Williams DM, Upchurch GR Jr, Shillingford MS, Dasika NL, Proctor MC, et al. Long-term results from a 12-year experience with endovascular therapy for thoracic aortic disease. Ann Thorac Surg 2006;82:2147–53. [DOI] [PubMed] [Google Scholar]
- 8.Steuer J, Eriksson MO, Nyman R, Bjorck M, Wanhainen A. Early and long-term outcome after thoracic endovascular aortic repair (TEVAR) for acute complicated type B aortic dissection. Eur J Vasc Endovasc Surg 2011;41:318–23. [DOI] [PubMed] [Google Scholar]
- 9.Thrumurthy SG, Karthikesalingam A, Patterson BO, Holt PJ, Hinchliffe RJ, Loftus IM, et al. A systematic review of midterm outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg 2011;42:632–47. [DOI] [PubMed] [Google Scholar]
- 10.Goodney PP, Travis L, Lucas FL, Fillinger MF, Goodman DC, Cronenwett JL, et al. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the Medicare population. Circulation 2011;124:2661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geisbusch P, Hoffmann S, Kotelis D, Able T, Hyhlik-Durr A, Bockler D. Reinterventions during midterm follow-up after endovascular treatment of thoracic aortic disease. J Vasc Surg 2011;53:1528–33. [DOI] [PubMed] [Google Scholar]
- 12.Lee WA, Daniels MJ, Beaver TM, Klodell CT, Raghinaru DE, Hess PJ Jr. Late outcomes of a single-center experience of 400 consecutive thoracic endovascular aortic repairs. Circulation 2011;123:2938–45. [DOI] [PubMed] [Google Scholar]
- 13.Abraha I, Romagnoli C, Montedori A, Cirocchi R. Thoracic stent graft versus surgery for thoracic aneurysm. Cochrane Database Syst Rev 2016:Cd006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1881–9. [DOI] [PubMed] [Google Scholar]
- 15.Lee TC, Andersen ND, Williams JB, Bhattacharya SD, McCann RL, Hughes GC. Results with a selective revascularization strategy for left subclavian artery coverage during thoracic endovascular aortic repair. Ann Thorac Surg 2011;92:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganapathi AM, Andersen ND, Hanna JM, Gaca JG, McCann RL, Hughes GC. Comparison of attachment site endoleak rates in Dacron versus native aorta landing zones after thoracic endovascular aortic repair. J Vasc Surg 2014;59:921–9. [DOI] [PubMed] [Google Scholar]
- 17.Hughes GC, Lee SM, Daneshmand MA, Bhattacharya SD, Williams JB, Tucker SW Jr, et al. Endovascular repair of descending thoracic aneurysms: results with “on-label” application in the post Food and Drug Administration approval era. Ann Thorac Surg 2010;90:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah AA, Barfield ME, Andersen ND, Williams JB, Shah JA, Hanna JM, et al. Results of thoracic endovascular aortic repair 6 years after United States Food and Drug Administration approval. Ann Thorac Surg 2012;94:1394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes GC, Daneshmand MA, Swaminathan M, Nienaber JJ, Bush EL, Husain AH, et al. “Real world” thoracic endografting: results with the Gore TAG device 2 years after U.S. FDA approval. Ann Thorac Surg 2008;86:1530–7. [DOI] [PubMed] [Google Scholar]
- 20.Hanna JM, Andersen ND, Aziz H, Shah AA, McCann RL, Hughes GC. Results with selective preoperative lumbar drain placement for thoracic endovascular aortic repair. Ann Thorac Surg 2013;95:1968–74. [DOI] [PubMed] [Google Scholar]
- 21.Appoo JJ, Bozinovski J, Chu MW, El-Hamamsy I, Forbes TL, Moon M, et al. Canadian Cardiovascular Society/Canadian Society of Cardiac Surgeons/Canadian Society for Vascular Surgery Joint Position Statement on Open and Endovascular Surgery for Thoracic Aortic Disease. Can J Cardiol 2016;32:703–13. [DOI] [PubMed] [Google Scholar]
- 22.Andersen ND, Ganapathi AM, Hanna JM, Williams JB, Gaca JG, Hughes GC. Outcomes of acute type a dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. J Am Coll Cardiol 2014;63:1796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller DC. Another meiosis in the specialty of cardiovascular and thoracic surgery: birth of the purebred “thoracic aortic surgeon”? J Am Coll Cardiol 2014;63:1804–6. [DOI] [PubMed] [Google Scholar]
- 24.Bradshaw RJ, Ahanchi SS, Powell O, Larion S, Brandt C, Soult MC, et al. Left subclavian artery revascularization in zone 2 thoracic endovascular aortic repair is associated with lower stroke risk across all aortic diseases. J Vasc Surg 2017;65:1270–9. [DOI] [PubMed] [Google Scholar]
- 25.Matsumura JS, Rizvi AZ. Left subclavian artery revascularization: Society for Vascular Surgery Practice Guidelines. J Vasc Surg 2010;52(4 Suppl):65s–70s. [DOI] [PubMed] [Google Scholar]


