Abstract
Intervertebral disc degeneration is a common cause of low back pain, the leading cause of disability worldwide. Appropriate preclinical models for intervertebral disc research are essential to achieving a better understanding of underlying pathophysiology and for the development, evaluation, and translation of more effective treatments. To this end, in vivo animal and ex vivo organ culture models are both widely used by spine researchers; however, the relative strengths and weaknesses of these two approaches are a source of ongoing controversy. In this article, members from the Spine and Preclinical Models Sections of the Orthopedic Research Society, including experts in both basic and translational spine research, present contrasting arguments in support of in vivo animal models versus ex vivo organ culture models for studies of the disc, supported by a comprehensive review of the relevant literature. The objective is to provide a deeper understanding of the respective advantages and limitations of these approaches, and advance the field toward a consensus with respect to appropriate model selection and implementation. We conclude that complementary use of several model types and leveraging the unique advantages of each is likely to result in the highest impact research in most instances.
Keywords: in vivo, intervertebral disc, models, organ culture, spine
1. INTRODUCTION
The prevalence of musculoskeletal conditions is growing worldwide, and low back pain (LBP) is significant among these as the leading cause of disability. 1 It is estimated that approximately 80% of adults are affected by LBP at some point in their lifetime. 2 LBP impacts individuals in both developed and developing countries alike, affects all age groups from children to the elderly, 2 , 3 and thus represents a significant burden for patients, health care systems, and the economies of many countries. Approximately 40% of LBP cases are attributable to degeneration of the intervertebral discs (IVDs), making this the most common cause of chronic LBP. 4 Intervertebral disc degeneration (IVDD) is a progressive, cell‐mediated cascade involving each of the IVD's three main anatomical regions: the central, proteoglycan‐rich nucleus pulposus (NP); the peripheral, fibrocartilaginous annulus fibrosus (AF), and the two cartilage endplates (CEPs) that interface with the adjacent vertebrae. The earliest manifestations of IVDD commonly occur in the NP, where proteoglycan loss compromises the distribution of loads leading to structural and mechanical derangement of the entire spinal motion segment. While IVDD commonly occurs with increasing age, risk factors for accelerating its progression include genetics, smoking, lifestyle, obesity, trauma, and mechanical stress. 5 , 6 , 7 IVDD may lead to LBP through direct compression of adjacent neural elements or by innervation of the IVD structures themselves, which combined with increased nerve sensitizing agents leads to increased pain. 8 , 9 , 10 , 11 , 12 , 13 , 14
The complexity of IVDD pathophysiology poses great challenges for effective long‐term treatment of associated LBP. 15 Current clinical treatments are predominantly focused on managing symptoms (e.g., alleviation of pain) rather than addressing underlying causes. These treatments may involve medications such as nonsteroidal anti‐inflammatory drugs (NSAIDs), which can address acute symptoms but carry the risk of increased internal bleeding during long‐term use. 16 For more severe symptoms, opioid‐based medications may be prescribed, 17 but they pose a serious risk of addiction, exacerbating the opioid epidemic and associated morbidities. 18 , 19 , 20 , 21 Where conservative treatments do not appear to modify disease progression, surgical interventions such as spinal fusion or total disc arthroplasty may be employed, but these fail to preserve disc structure or mechanical function long‐term and may result in progression of IVDD in adjacent levels. 15 Therefore, there is a significant clinical need for improved treatment options for patients suffering from IVDD and LBP that directly target the underlying causes.
The successful development, evaluation, and translation of new treatments for IVDD require the use of appropriate preclinical models that recapitulate the structural, functional, and biological characteristics of the clinical condition as closely as possible. Therefore, a deeper understanding of the benefits and limitations of various approaches to implementing currently available preclinical models is critical for advancing investigations of IVDD pathophysiology and treatment. Despite wide‐ranging attempts to develop both in vivo (large and small animal) and ex vivo (organ culture of viable postmortem tissue) models, controversies remain regarding the selection of appropriate models for IVD research.
The objective of this article is to contrast and debate the respective advantages and limitations of in vivo animal models versus ex vivo organ culture models for studies of IVDD and its treatment. To achieve this, we have leveraged the broad expertise of the members of two leading groups focused on basic and translational spine research—the Spine and Preclinical Models Sections of the Orthopedic Research Society (ORS)—coupled with a comprehensive review of the current scientific literature. We begin with arguments in support of in vivo animal and ex vivo organ culture models, respectively, for studies of IVDD and its treatment, and conclude with recommendations for incorporating these models into experimental designs to address specific research questions most effectively, with an emphasis on the complementary use of multiple models in order to generate the highest impact results.
2. ARGUMENTS IN SUPPORT OF IN VIVO MODELS
2.1. Introduction
Preclinical research studies are commonly conducted on the cervical, thoracic, lumbar, and caudal spines of research animals. Animal models have played a critical role in advancing understanding of the temporal evolution of IVDD, including how constitutive, environmental, or biomechanical risk factors may initiate, promote, or otherwise regulate degenerative changes, and how therapeutic strategies may ameliorate, resolve, or prevent IVDD. 22 Currently, in vivo studies of IVDD are conducted in small animals such as mice, rats, and rabbits; as well as larger animals such as dogs, pigs, goats, sheep, cows, and nonhuman primates. 23 However, given the complexity of human IVDD, a perfect animal model does not exist. 24 In this section, we outline key advantages that in vivo models have over ex vivo organ culture models, including pain evaluation, nutrition and blood supply, systemic effects related to the immune system, crosstalk with surrounding tissues, imaging, the requirements from regulatory agencies for clinical translation of new treatments for IVDD, and as prerequisites for clinical translation.
2.2. Pain evaluation
Pain can be defined as cortical interactions that initiate changes in behavior. 25 Pain behaviors may be influenced by physiological and immunological factors, cognition, and conduct. In human patients, LBP as a result of IVDD results in significant morbidity, preventing patients from completing their daily routine, removing individuals from the workforce, and resulting in stress, anxiety, and depression. 26 This pain is the main driver for patients seeking care, and a paramount factor in the diagnosis of IVDD. Importantly, studies have shown that IVDD does not always directly correlate with pain, and that IVDD may often be present in asymptomatic individuals. 27
While the direct connections between IVDD and pain remain complex, animal models have been and continue to be essential research tools for understanding physical and metabolic pathways of symptomatic IVDD (discogenic pain), and in the development of new therapeutics aimed at mitigating and preventing the onset of degeneration and pain. Put simply, only in vivo models can recreate the complex processes of pain resulting from disc degeneration, and permit assessments of behavioral and functional changes as outcome measures. This is not without its challenges, as each species has unique physical and behavioral manifestations of pain, and species‐specific, repeatable, and standardized pain scores must be used. 28 Among large animals, dogs provide an interesting model for discogenic pain as distinct and appreciable behavioral changes make these animals particularly valuable when assessing analgesics. 29 , 30 , 31 , 32 , 33 Nonetheless, the optimal way to measure pain in both preclinical models (and patients) is still the subject of extensive debate. Important aspects such as the nociceptive response generators, pain thresholds, and clinical and behavior manifestations need to be contemplated before selecting an animal model. 28 Validated methods of pain measurement include physical performance (e.g., grimace scales, lameness examinations, gait measurements), 34 behavioral changes (e.g., decreased burrowing and rearing), 35 , 36 and response to mechanical stimuli (e.g., hind‐paw mechanical hyperalgesia test). A recent study has shown that the Grimace scale (a subjective pain assessment method based on facial expressions) is highly reliable in mouse and rat models, and moderately reliable in rabbits, piglets, and sheep. 37
Large animal models have also led to the identification of molecular biomarkers of discogenic pain. 38 Biomarkers not only represent potentially powerful, noninvasive diagnostic tools for evaluating IVDD progression and response to therapeutic intervention, but also provide mechanistic insights into how local pathophysiological changes lead to the manifestation of clinical symptoms, informing the development of new therapies. This simply cannot be accomplished using ex vivo models where clinical manifestations of IVDD (e.g., pain) cannot be measured.
2.3. Nutrition and blood supply
The IVDs are largely avascular structures. During human development, blood vessels penetrate deep into the lamellar structure of the AF from around 35 weeks gestation. 39 , 40 Vessels then recede, and by the second decade of life remain only at the margins. At no point do blood vessels penetrate the central NP; instead, blood vessels terminate within the subchondral bone adjacent to the CEP. These locations—the AF margins and the vertebral endplates—are the sole sources of nutrition for cells within the IVD itself, with the latter considered the most important. 41 Physiological nutrition via these routes is therefore critical for IVD cell survival, and alterations to the adjacent vasculature that disrupt nutrient supply are considered to play an important role in the onset and progression of IVDD. Importantly, the role of vasculature in IVDD can only be investigated using in vivo animal models with an intact circulatory system and cannot be achieved using ex vivo organ culture models.
At a fundamental level, in vivo models have been used to establish mechanisms of nutrient flow into the IVD. For example, historically, in vivo large animal models were used to establish that vertebral endplate vasculature is the primary nutrient diffusion pathway into the IVD. 42 , 43 More recently, a rabbit model was used to demonstrate how alterations in microvasculature that occur with degeneration affect nutrient supply to the IVD. 44 In vivo models have also been essential for studies investigating how certain drugs impact the vasculature supplying nutrients to the IVD. For example, in vivo models have been used to show how vasoactive agents such as acetylcholine and nicotine, as well as cigarette smoking itself, may alter vasculature and nutrient supply to the IVD, implicating smoking in the etiology of IVDD. 45 , 46 , 47 , 48 In vivo models are also essential for evaluating the efficacy of therapeutic agents administered systemically to treat IVD pathologies, such as intravenous stem cells and antibiotics. 49 , 50
2.4. Long‐term evaluation
Irrespective of the factors initiating or driving IVDD, it is most often a long‐lasting process with changes in the cellular environment and the different structures of the IVD occurring over months or years, before leading to the gross structural and functional alterations that are associated with the manifestation of clinical symptoms. 51 As such, in vivo models have been important tools for elucidating the long‐term natural history of IVDD. 52 Furthermore, in vivo models are crucial for evaluating the long‐term efficacy of novel treatments for IVDD. 53 The primary goal of IVDD treatments is to both restore IVD function and structure, and alleviate painful symptoms. Acute toxicity and initial structural (e.g., an increase in cellularity and extracellular matrix [ECM] or IVD height) and functional changes can be assessed ex vivo and in vivo; however, potential therapeutic agents may have either a short half‐life or may diffuse out of the IVD, so their long‐term effects must be determined. Furthermore, initial treatment success may be diminished by the unfavorable degenerative environment of IVDD. In vivo models allow an observation period of several weeks (small animal models) to months or even years (large animal models), facilitating confirmation of sustained or permanent therapeutic effects. Furthermore, the same animal may be assessed over time using noninvasive, gold‐standard imaging techniques such as magnetic resonance imaging (MRI), radiographs, or computed tomography, increasing the clinical relevance of findings and reducing the number of experimental animals required. In addition, it is crucial to ensure both acute (i.e., toxicity) and long‐term safety (e.g., tumorgenicity) of novel biological treatments, which is only possible using in vivo models.
2.5. Systemic factors
A major advantage of using in vivo animal models for IVDD and LBP research is the ability to assess the contributions of systemic biological processes such as the immune system, or co‐morbidities such as diabetes or obesity on IVDD progression and treatment. Immune cell infiltration of mast cells, macrophages, neutrophils, and T lymphocytes has been identified in the painful human degenerate IVD following rupture of the AF or CEP 54 , 55 , 56 , 57 ; however, the mechanisms underlying the roles of these cells in IVDD are underexplored. The healthy IVD is largely avascular and immune‐privileged, yet with degeneration, there is evidence that these immune cells can infiltrate the disc from the bone marrow via lesions in the vertebral endplate and/or via aberrant blood vessel ingrowth into the endplate and AF. 58 In vivo animal models of IVDD and LBP are valuable tools with which to investigate the recruitment, invasion, and function of immune cells in pathological environments, which cannot be readily investigated ex vivo. For example, transgenic mice over‐expressing the pro‐inflammatory cytokine TNFα demonstrate increased infiltration of Tryptase‐expressing (mast) cells or CD68+ (macrophage) cells in IVD tissue regions associated with higher risks of herniation. 59 The increased presence of immune cells, specifically macrophages in herniated IVD tissue, has been corroborated using a novel in vivo mouse model of IVD herniation‐induced radiculopathy. 60 Green Fluorescent Protein (GFP) transgenic bone marrow chimeric mouse models of IVD injury have been used to determine the origin of M1 macrophages and demonstrated that following IVD injury, M1 macrophages are recruited specifically from outside the IVD. 61 A subsequent study verified these findings by demonstrating increased recruitment of macrophages to the dorsal region of the IVD together with neo‐innervation in an IVD injury model for up to 12 months. 62 These studies highlight the importance of in vivo models for investigating the role of the immune system in IVDD.
Systemic inflammatory diseases such as obesity and diabetes demonstrate a strong association with IVDD and back pain, 63 , 64 and animal models (rodents in particular), demonstrating these disease phenotypes are useful tools to conduct mechanistic and therapeutic studies in which changes in whole IVD joint structure/function and pain behaviors can be investigated. Obesity and diabetes co‐exist and can be readily investigated simultaneously using in vivo animal models. Male and female leptin receptor‐deficient mice fed with a control (low fat) or high‐fat diet to mimic the effects of obesity and diabetes on disc health have been used to examine the effects of obesity and type‐2 diabetes on healthy intervertebral IVDs. 65 , 66 Sex‐dependent effects have been described, with only females developing diabetes and the most pronounced changes in IVD and bone structure, pointing toward a sex‐dependent role for leptin in the spine. 65
In a type‐2 diabetic rat model, several changes have been identified in the IVD joint compared to healthy control and obese rats. Specifically, decreases in the glycosaminoglycan (GAG) and water contents of the IVD, increases in mechanical stiffness, advanced glycation end‐products (AGEs) and catabolic markers, as well as increases in vertebral endplate thickness and decreased porosity were found, suggesting a reduction in nutrition to the IVD. 67 Similarly, AGE‐fed mice demonstrated age‐accelerated IVDD together with ectopic calcification of the spinal tissues and insulin resistance, highlighting a role for AGEs in promoting diabetes‐induced IVDD. 68 To further validate the role of AGEs in diabetes‐induced IVDD, diabetic mice were treated with oral anti‐inflammatory and anti‐AGE drugs. These drugs mitigated pathological effects observed on disc height, GAG content, and catabolic markers in diabetic mouse models, demonstrating broad clinical applications of anti‐AGE drugs on spinal health. 69 Together, these studies highlight the critical role of in vivo animal models in evaluating the effects of systemic co‐morbidities on IVDD progression and response to treatment.
2.6. Crosstalk with surrounding tissues
Investigating crosstalk with surrounding tissues is essential for a comprehensive understanding of IVDD progression and the development of LBP, and this is best achieved with the biological complexity inherent to in vivo models. For example, tissue crosstalk is important to consider when studying nociception. The dorsal root ganglion (DRG) has been suggested to interact with the NP in IVD herniation to elicit pathological consequences. This involves induction of pro‐inflammatory signaling pathways, 70 , 71 activation of microglia, 72 and modulation of the AMPK‐mTOR axis 73 in the DRG. Ex vivo co‐culture models may be able to model some tissue interactions. For example, a gene‐editing study using ex vivo co‐culture systems suggested that inflammatory signals from degenerative IVDs can sensitize nociceptive neurons, 74 and such sensitization can be manifested under mechanical stress, 75 suggesting that IVDD may play a role in pain sensitization. 76 However, even in ex vivo work, different results can be obtained depending on the study design. For example, differential effects of hypoxic stress on neurite outgrowth in DRGs were reported between the single cell and tissue levels. 77 Therefore, the study of neural activity in context of IVDD in vivo may yield contrasting results from those obtained ex vivo.
There are numerous examples of the importance of tissue crosstalk in IVDD pathophysiology. In a rabbit cornea implantation model, cartilaginous endplate explants may inhibit neovascularization while AF explants may promote it. 78 This implies compartmental crosstalk can craft the nutritional pathway of the discs. On the other hand, loss of vertebral bone integrity, for example, due to vertebroplasty 79 or bone loss in ovariectomized mice 80 may affect IVD health. Schmorl's nodes, an endplate defect, have been associated with IVDD, 81 and is consistent with findings that experimental injury to the endplate can initiate disc degeneration in large animal models. 82 IVD herniation may initiate at the endplate‐annulus interface in aged rats 83 and involves systemic TNF‐α upregulation. 59 These studies support that endplate and vertebral bone have major influences on IVD tissue homeostasis. At the cellular level, IVDD is associated with remodeling of the NP, which transitions into a fibrocartilaginous tissue composed of chondrocyte‐like and fibroblastic cells. NP ECM remodeling may in part be mediated by cell types originating in adjacent tissues. 84 , 85 , 86 , 87 Thorough interrogation of such dynamic cellular exchange among tissue compartments/systems requires in vivo models.
2.7. Physiologically relevant imaging
To ensure the physiological relevance of IVD imaging findings, it is important to consider tissue interaction. Imaging of whole IVD motion segments in live animals can better reflect the physiological status of the IVDs. For example, when performed in vivo, radiographic assessments of disc height (an important surrogate of IVDD progression and response to treatment) can be normalized to adjacent vertebral dimensions to account for variation across spine levels and individual animals. Moreover, in vivo imaging accounts for the mechanical constraints of para‐spinal tissues such as muscles and ligaments when evaluating IVD geometry. Sedation or anesthesia can be used to ensure proper positioning and muscle relaxation. 28 Animals with altered muscle activity such as GDF‐8 mouse mutants and botulinum toxin‐treated monkeys exhibit reduced IVD height. 88 Lastly, in vivo imaging permits long‐term, longitudinal imaging evaluations that cannot be achieved ex vivo.
2.8. Regulatory requirements and prerequisites for clinical translation
In vivo animal models provide superior preclinical platforms to address regulatory requirements and accelerate clinical translation by answering critical questions regarding both the safety and efficacy of novel IVDD treatments. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) oversee the approval process of any drug or medical device aimed at IVDD treatment, with the exception of human‐derived, minimally manipulated tissues. Preclinical studies must demonstrate that the benefits of the treatment outweigh its risks before approval for clinical use. Although animal testing is not required by the FDA, it is the most effective way to demonstrate the biological response in a living system and, therefore, is rarely excluded from the Investigational New Drug (IND) application process. The FDA has recognized this and has issued draft guidance to ensure such studies are rigorously conducted. 89 Also recognized is the need to refine, reduce and replace animal models in device and therapeutic testing where possible. 90 In most cases, preclinical animal study results are used to support an IND application and are followed by human clinical trials prior to FDA approval; however, in some specific instances, animal study results alone may be used for approval. This type of approval is covered by the FDA Animal Rule in situations where human efficacy trials may not be ethical or feasible. 91
Several preclinical in vivo animal models may be utilized in combination to satisfy regulatory requirements. For example, initial discovery of pathological mechanisms and screening of therapeutic targets may be carried out in rodent models that permit genetic manipulation, while subsequently, large animal models provide platforms for long‐term evaluation of safety and efficacy where IVD size and geometry are closer to that of humans. While organ culture models may also play a role in this process, ex vivo models are largely supportive of in vivo studies.
Intermixed with the FDA approval process is the concept of and strategy surrounding commercialization and translation to the clinic. Commercialization of a drug or device for the prevention or treatment of IVDD relies heavily on acceptance by medical physicians such as spine surgeons. A therapy could be groundbreaking with a high impact on affected patients but never realize its potential as a gold standard treatment if it is not considered sufficiently clinically relevant or if efficacy data is unconvincing. Preclinical in vivo animal models, and large animal models, in particular, are vital to the commercialization process of any groundbreaking therapy by more closely recapitulating the human condition, anatomy, IVD size and geometry, and life span. With respect to novel device development, large animal models mimic the surgical application requirements of such devices, providing practical feedback in the development of instrumentation and delivery systems, which may be as impactful to the overall success of the therapy as the therapy itself. If a surgeon cannot safely or consistently instrument an implant or deliver a therapy, then said therapy is irrelevant. Organ culture models do not provide this realistic, clinically relevant scenario. Additionally, advanced diagnostic imaging, specifically MRI, has grown to be the gold standard modality for assessing IVDD severity. As such, clinicians rely on MRI as an essential diagnostic tool for IVDD patients. Unlike organ culture models, MRI can be utilized in in vivo animal models to follow IVDD progression as well as to assess treatment efficacy, which is highly impactful with respect to the goal of achieving acceptance of therapies by clinicians and eventual commercialization. Ultimately, for a device or therapy to be useful, it must integrate seamlessly into the clinical environment, and leveraging clinically relevant in vivo animal models throughout the product development and translational process is the best way to achieve this.
Unfortunately, no model of IVDD mimics the human condition in all aspects. Despite their important role in the assessment for a new device or therapy, ethical considerations also impact the choice and use of in vivo models. For example, dog and primate models with spontaneously occurring IVDD closely translating to clinical findings in humans undergo increased public scrutiny making these models less accessible and more expensive. On the other hand, preclinical models utilizing livestock animals such as sheep, goats, and pigs are more widely accepted by the general public, although some are more limited for investigating human IVDD due to the retention of notochordal cells (pigs). There is evidence that animals that retain notochordal cell‐rich NPs, such as nonchondrodystrophic dogs, exhibit different biomechanical properties to animals that do not retain notochordal cells. 92 While organ culture models carry little ethical stigma, it is currently unusual for a therapy to move from benchtop to the affected patient via solely the use of organ culture models. Even if organ culture models were acceptable by regulatory agencies to provide safety and efficacy, it would be challenging to translate those results into the clinical situation without additional analysis in living systems.
3. ARGUMENTS IN SUPPORT OF ORGAN CULTURE MODELS
3.1. Introduction
Organ culture models are distinguished by the culture of whole multi‐tissue organs, under sterile conditions, over various periods of time from short‐term (hours or days) to longer‐term (weeks or months). In IVD research, organ culture models have been used for basic and translational studies for several decades. 93 In 1998, one of the first reports on long‐term IVD culture described the maintenance of entire rabbit IVDs embedded in alginate to preserve their structure and prevent excessive swelling. 94 In the ensuing years, methods have been advanced by the introduction of organ‐specific culture systems and bioreactors, with cultured IVDs originating from several different species including rodents, rabbits, large animals (e.g., sheep, goat, bovine), and humans. 93 , 95 , 96 , 97 , 98 , 99 , 100 , 101
Organ culture models for IVD research are popular for several reasons. First, the interaction between the IVD's tissue components is crucial for the functionality of the IVD, thus the culture of the whole organ is important for the study of the IVD in both healthy and diseased states. Second, whole organ culture means that the cells of the IVD, especially those of the NP, are naturally exposed to physiological nutrition, oxygen, pH, and hydrostatic pressure. Moreover, IVD tissues are characterized by a low cell density within an extensive ECM. Isolating the cells from this unique environment may alter their phenotype and behavior. Single‐cell cultures are therefore reduced from the true physiological environment, while three‐dimensional cell cultures and the use of specially tailored culture media are somewhat more representative in this respect. Third, the IVD with intact AF and CEP is considered a largely avascular, immune‐privileged organ; blood vessels and infiltrating immune cells are minimally present in the healthy IVD, and thus isolated whole organ studies are appropriate. Fourth, most of the existing in vivo animal models of IVDD still do not entirely recapitulate the pathophysiology of human IVDD, and their limitations must therefore be taken into account for addressing certain translational research questions. 23 , 52 Organ culture models can be precisely controlled in terms of the biochemical and biomechanical environment; they are flexible with respect to study design and, depending on the throughput of the specific model, are suitable as a screening platform. Moreover, the biological response, such as the production of cytokines, local inflammation, and structural changes, 102 can be directly attributed to the experimental variables with the appropriate control groups, due to fewer covariates compared to in vivo models. They avoid unnecessary use of animals by utilizing surplus tissue from donor animals or human cadavers.
Finally, organ culture models have the advantage of a favorable cost–benefit profile. The design, development, manufacturing, and set‐up of custom organ culture systems and bioreactor devices may be initially cost‐intensive; however, once the method is established, numerous different studies can be performed in a standardized manner, ensuring reproducibility. For example, it has been estimated that the expenses for the set‐up of an IVD bioreactor system capable of culturing and loading four large animal IVDs simultaneously, are approximately equal to the costs of one typical large animal (e.g., sheep) study, involving 10 animals in total in Switzerland. 103 Moreover, in vivo studies, especially large animal studies, require a significant contribution from highly trained professionals (e.g., veterinary surgeons) and specialized animal facilities (e.g., surgical suites, animal care, and monitoring) that necessitate significantly more specialized infrastructure investment than organ culture models. These factors make in vivo models less accessible to diverse sets of researchers worldwide. Given the vast burden of LBP due to IVDD, rapid and rigorous research can be more easily achieved with organ culture models.
3.2. Addressing the “3Rs”: Reduce, Refine, Replace
Importantly, IVD organ culture models address the 3Rs principle (Reduce, Refine, Replace) of animal testing, especially if the IVDs originate from animals that are not specifically euthanized for research purposes. The number of live animals in preclinical research can be reduced by evaluating new therapies, such as molecular, 104 cellular, 105 or biomaterial‐based approaches, 106 under organ culture conditions prior to planning an in vivo study. In this respect, prescreening of treatment formulations in an organ culture model may help to rule out sub‐optimal or ineffective methods, thereby avoiding unnecessary live animal studies. 107 Many questions on the interaction between the treatment and the host tissue can reliably be addressed with organ cultures, 103 given the avascular nature of the IVD. As such, only an optimized method with satisfactory organ culture results would ultimately be studied in vivo to provide the safety data required for regulatory approvals, which is currently required. With the continuous advancement of complexity of organ culture models, the complete replacement of live animals in preclinical IVD research may be possible in the future. The implementation of physiological mechanical loading in specific bioreactors, and the co‐culture of IVDs with other cell types further expand the application of IVD culture models. In addition, the possibility of using whole human IVDs for research, which are naturally degenerated, reflects a model environment of unequaled physiological relevance, 108 as species differences are a well‐known shortcoming when working with animal IVDs, 23 and methods of IVDD induction do not fully mimic the pathophysiology of human IVDD.
In relation to the 3R principles of animal research, the institutional regulatory processes for obtaining study permission are negligible if IVDs for organ culture are obtained from animals that are euthanized for other purposes, such as porcine, bovine, caprine, or ovine IVDs that are sourced from animals used as a source of meat. Accordingly, there are no further regulatory requirements for the ethical use of such tissues for research. The administrative and veterinary efforts required for approval of an in vivo study are thus not necessary for organ culture experiments, which saves significant time and institutional resources. On the other hand, the availability of human whole IVDs is still very limited and subject to ethical regulations. Access to whole human IVDs is rarely obtainable from surgical specimens and thus cadaveric materials are required; however, given the avascular nature of the IVD and in the experience of the authors, living IVDs can be sourced from cadaveric material for prolonged periods after death for up to 1 week.
Organ culture models allow for higher throughput analysis of disease‐simulating or therapeutic agents, including crosstalk between the disease state and therapy. One major advantage of organ culture models is the ability to examine ECM‐related changes (integrity and content) sooner than through in vivo models. This is of paramount importance given that the IVDD phenotype is often defined by ECM degradation. For example, organ culture models exposed to inflammatory cytokines exhibit GAG loss within 1–2 weeks. 109 Such effects in animal models require evaluation over weeks and months, 23 in part because the severity of the degenerative stimuli in vivo is limited by the number of injections and volume (e.g., injection of catabolic enzymes or cytokines). The allocation of IVD tissue from multiple spinal levels to organ culture groups facilitates increased sample size per group, the inclusion of both positive and negative controls, and evaluation of factors at multiple time points while bypassing the use of extensive live animals. These advantages are also paramount for enhancing rigor and reproducibility of experiments using organ culture models.
Thus, in this section, we present arguments outlining key features that make organ culture models more advantageous compared to in vivo animal models for IVD research, including the capability to use both human and animal IVDs, controllable physical and biochemical environments (i.e., nutrition, mechanical loading, and immune and inflammatory factors), flexible model types (i.e., diabetes, rapid degeneration, etc.), the ability to study IVDD mechanisms and crosstalk between tissue structures, the ability for both short and long‐term evaluation with numerous time points, and improved imaging outcomes compared to in vivo imaging. Furthermore, we address regulatory concerns, and question the need for in vivo models as a prerequisite for clinical translation.
3.3. Species differences
Organ culture models can employ either nonhuman animal or primary human tissues. Several species differences that differentiate human versus animal IVDs are highlighted below, such as size limitations when using small rodent models, and the presence of notochordal cells in some animals (i.e., porcine, mouse), whereas notochordal cells are not present in the skeletally mature adult human IVD. 52 , 110 As the clinical prevalence of LBP is in humans, the use of human tissue in organ culture may offer the most immediately relevant insights compared to animal models.
3.4. Molecular mechanisms of pain evaluation
Evaluation of pain as an outcome measure in studying therapeutics for IVDD is critical. While in vivo models may be useful for studying behavioral characteristics, the translatability of pain behaviors assessed in animal models, especially small rodents, to the human condition requires further validation. 111 Furthermore, the induction of IVDD using AF puncture in animal models does not necessarily recapitulate the initiating mechanisms of IVDD in humans. Nevertheless, there are many similarities in the degenerative changes in IVD structure and chronicity of inflammatory and pain‐associated cytokines. 36 , 62 , 102 In humans, LBP in the presence of an intact degenerate IVD is associated with nerve ingrowth and neurotrophic factor release. In other cases, following AF or CEP rupture, exposure of local nerves to disc material, released factors, and induction of inflammatory responses become important. These pathophysiological mechanisms of pain are not fully replicated in all in vivo models of LBP, which, combined with limited validated methodologies to accurately measure pain in such models, limits the relevance of investigation in vivo. Meanwhile, pain‐related molecular factors can be studied in organ culture models; for example, neurotrophic factor expression, which reduces the need to provoke pain behavior in animal models, in alignment with the 3Rs. 77 In addition, these cellular and signaling mechanisms in organ culture models can be deterministically attributed to the IVD, and the results are specific to the biology of the IVD.
3.5. Biochemical environment
Due to its largely avascular nature, the environment of the IVD is characterized by hypoxia, acidic pH, and low nutrient supply. Additionally, the consumption of glucose and oxygen, and the production of lactate by the IVD cells are interdependent. There is, however, great variation in the reported intra‐discal oxygen and nutrient concentrations in vivo. The reason for this variation is the complex regulation of metabolites as a combination of nutrient supply, access, and demand, whereby the latter depends on the individual IVD cell density and activity. In an experimental study, oxygen concentrations were measured in IVDs of patients during discography or spine surgery. 112 The levels ranged from 5 to 150 mmHg (~0.7%–20% O2) in the center of the NP, whereby no correlation with age or degeneration state was found. While the in situ measurements are challenging, different numerical models have calculated the concentration gradients of oxygen, lactate, and glucose within the IVD. Most studies estimate oxygen concentrations between 0.3 and 1.1 kPa (~0.3%–1.1% O2) in the center of the IVD 113 , 114 ; while glucose concentrations of around 1–2 mM were predicted for the IVD center, with levels of less than 1 mM in degenerated IVD or due to endplate calcification. 113 , 115 , 116 Finally, high lactate levels are correlated with a low intradiscal pH. There are only a few reports on in vivo pH levels; pH values of ~6.7 and ~6.9 were measured in lumbar IVDs from patients with severe and moderate LBP, respectively. 117 Interestingly, these values lie between the values for IVDs with impermeable endplates and IVDs with 50% permeable endplates as predicted from numerical models, 118 stressing the importance of the endplate permeability for IVD metabolism.
Organ culture models should mimic in vivo human conditions as closely as possible. Studies show that physiological glucose, oxygen, and pH levels can be reproduced in organ culture systems to simulate healthy and degenerate IVD conditions. This implies a balance between sufficient nutrition to maintain cell viability and activity, while avoiding supra‐physiological levels of nutrients and oxygen. Interestingly, around 70% of previous organ culture experiments have been carried out under high glucose (4.5 g/L or 25 mM) medium conditions. 119 Computational and experimental models show that high glucose media results in glucose levels between ~5–15 mM in the center of an organ‐cultured bovine caudal IVD, depending on the size of the IVD. 119 In general, these high glucose conditions are referred to as a “physiological” culture environment. Indeed, a significant drop in cell viability by 40%–50% has been observed in both NP and AF of ovine IVDs cultured in low glucose media containing 2 g/L (11 mM) glucose compared to the standard high glucose (4.5 g/L) condition. 120 The reduction in cell viability was evident after 7 days and was stable until 21 days of culture under simulated physiological loading conditions in a bioreactor. Moreover, limited glucose culture can be implemented as a degeneration organ culture model, simulating compromised nutrition in combination with high‐frequency loading, which showed additive effects on cell death. 121 Studies with bovine IVDs confirmed the findings from ovine explants, demonstrating a decrease in AF and NP cell viability under low glucose (2 g/L) medium and high‐frequency loading conditions. 104 , 122 Meanwhile, low glucose concentration is viable for culturing human cells due to the low cell concentration, further contributing to the clinical advantage of human organ culture. 123 , 124 , 125 , 126
In view of the physiological blood glucose level of approximately 5.5 mM, the level of 25 mM necessary to keep the IVD cells viable seems highly supra‐physiological. In fact, high blood glucose levels in vivo have been shown to be detrimental to IVD homeostasis. Similarly, the predicted physiological intradiscal in vivo glucose levels are 5–10 times lower than the computed and measured ex vivo levels (see above). 113 , 115 , 116 , 119 This discrepancy may result from differences between the ex vivo and in vivo situations, such as the absence of capillaries in the IVD explants, the different mechanical loading, and osmotic pressure conditions.
Several studies have shown that low oxygen concentrations of 1%–5% are beneficial for the maintenance of the NP cell phenotype. 127 , 128 Most reported IVD organ culture experiments have been conducted under normal oxygen conditions externally, implying 20%–21% oxygen tension to the outer regions of the disc. According to computed or experimental data, this would correspond to an approximate oxygen tension of 1%–5% in the center of a bovine IVD, 119 which is similar to the in vivo oxygen tension. The removal of the CEP significantly alters the diffusion into the center of the IVD. Therefore, oxygen levels of 1%–5% are in line with the physiological levels that are known to promote the phenotype and function of IVD cells, and this can be reproduced using organ culture models that retain the CEP.
The experimentally determined and predicted pH values of standard cultured bovine IVD organ cultures have been reported to range between ~6.7 and ~6.9; hence, they are quite consistent with measured values from patients. 117 An increase in oxygen concentration and pH level was however predicted in a numerical model when dynamic axial compression was applied to the disc, 118 emphasizing the importance of mechanical bioreactors for culture of whole IVD organs.
IVD cell nutrition equally depends on the diffusion of nutrients through the CEP and/or the AF. In most ex vivo organ cultures, the vertebral bone part is removed, whereas the CEP is maintained. Care should be taken to clean the CEP from blood clots and debris to facilitate the diffusion of molecules into and out of the IVD, 93 since the central endplate region has been recognized as the major area of nutrient exchange. 129 In this context, the species‐ and age‐related differences in the CEP thickness and the presence of a growth plate in young animals need to be considered, as these parameters can markedly influence the diffusion rate. There are also organ culture systems where the bony endplate and some vertebral bones are maintained as well. These cultures require a special preparation that ensures the preservation of both the bony structure and long‐term IVD cell viability. 130 Furthermore, it has been suggested that nutrient exchange through the AF plays a more prominent role in organ‐cultured IVDs, because of the increased lateral surface area surrounding the AF which permits more nutrient transport through the periphery compared to the in vivo situation. 119
Taken together, by varying the glucose concentration, oxygen tension, pH, and nutrient transport, various metabolic states can be induced in organ‐cultured IVDs, which may represent different degrees or types of degeneration. Current numerical models provide a relevant indication of the intra‐discal nutrient gradients under defined circumstances. More experimental and clinical data are required to adjust each organ model to a particular clinical situation. Importantly, however, in ex vivo organ culture, there is consistency and control over all these biochemical influences, which are poorly controlled in in vivo models: levels can be measured and maintained in a predictable fashion, removing confounding factors from studies.
3.6. Mechanical loading
Another major advantage of organ culture models over in vivo animal models is the ability to control mechanical loading at the tissue level, and even present models with the desired mechanical properties and level of tissue damage to mimic physiological or disease conditions and relevant forces. Most animal models, with the exception of primates, are quadrupeds, which may differ in load transfer throughout the spine compared to bipedal humans. In addition, the sizes and geometries of animal IVDs exhibit differences compared to human IVDs as highlighted previously, which may confound the ability to study IVDD under physiological human conditions. The use of organ culture permits researchers precise control of the mechanical forces presented to the IVD, including physiological and injurious loading similar to that experienced by the human spine. The human IVD is normally exposed to multimodal loading (compression, tension, shear, HP, and osmotic pressure) ranging up to 4× body weight. 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 Organ culture models have provided significant insights into the response of the IVD to loading. Zonal biological responses have been observed that depend on tissue location, magnitude, and frequency of loading. 118 , 139 , 140 , 141 , 142 , 143 , 144 , 145 A maintenance stimulus of approximately 0.1–0.5 Hz applied at moderate stress levels (e.g., 0.2–0.5 MPa) promotes steady‐state IVD metabolic responses. Compressive loading above this level (e.g., high‐frequency loading) or below this level (e.g., static loading) typically results in remodeling or degeneration. Occupational exposures to high‐frequency vibration can also cause LBP 146 and IVDD. 147 , 148 Lying in recumbency promotes rehydration, increasing IVD height and volume, and normalization of intradiscal hydrostatic pressure, 149 which can be simulated in organ culture with diurnal loading profiles. Exercise can be beneficial for the IVD, with specific moderate‐frequency exercise protocols providing the greatest improvement in IVD material properties. 150 These loading factors can be simulated in organ cultures with the use of dynamic mechanical loading profiles. Indeed, dynamic loading is favorable for promoting mechanotransduction in IVD cells and for maintaining physiological nutrition, whereby at least a diurnal cycle, representing daily IVD compression and decompression (recovery, or swelling) can be applied to organ cultures of isolated whole IVDs. 93 , 118 , 151 Advanced bioreactor systems will allow researchers to apply controlled multiaxial loading to the IVD under long‐term culture conditions. 152 In contrast, the application of controlled, physiological loading using in vivo models is extremely challenging, and has only been successfully accomplished in rodents and rabbits. 153 , 154 , 155
3.7. Systemic effects
Since the healthy IVD is a largely avascular, immune‐privileged organ, unless structural defects expose it to the systemic environment of the body, infiltrating immune cells generally do not penetrate the intact healthy IVD, and thus isolated studies within an organ culture setting are physiologically appropriate. Furthermore, the influence of systemic co‐morbidities such as diabetes can be investigated at a mechanistic level, for example by identifying influences of increased glucose or the presence of damage‐associated molecular patterns (DAMPS) without compounding factors such as obesity and poor circulation, which occur in vivo and manifest differently in different animal models. Thus, where systemic effects such as inflammation and diabetes are described as advantages of in vivo models in the presence of immune cell migration/infiltration, in an intact IVD, this has little relevance or appropriateness; however, organ culture models have a unique advantage with respect to assessing specific mechanisms, by controlling the presence of specific immune cells to simulate interactions of the local or systemic immune system with rupture or disease. 156 , 157 Other specific soluble factors such as catabolic enzymes and cytokines, and DAMPs, can also be simulated with organ culture models, 158 , 159 , 160 in addition to environmental factors (e.g., glucose) which allows for a more precise mechanistic evaluation than in vivo.
3.8. Tissue‐specific responses and cross‐talk
A key argument for in vivo, as opposed to organ culture studies, is that tissue cross‐talk cannot be investigated in organ culture studies. On the contrary, organ culture models allow for the disambiguation of different tissue types within the IVD and surrounding bone structures and muscles, providing the capability for studying tissue‐specific responses. They can also be co‐cultured in the presence of multiple associated tissues, enabling carefully controlled tissue crosstalk investigations to be undertaken. The dissection of tissue‐specific roles and interactions cannot be studied easily in in vivo models. While using co‐culture systems, specific cross‐talk investigations can be investigated, where IVDs complete with CEPs can be maintained within a loaded bioreactor improving nutrient flow and maintenance of IVD and bone cell viability. IVDs could also potentially be co‐cultured with muscle, ligament, nerve, and fat to investigate tissue cross‐talk in a controlled environment, enabling mechanistic interactions between these tissues to be understood.
3.9. Rapid degeneration models
In vivo models generally require long‐term time points (anywhere from weeks to months) in order to generate IVDD comparable to the human condition. In comparison, rapid degeneration can be induced in organ culture models, which allows the study of IVDD under accelerated conditions, thus reducing the time needed for respective studies. For example, using enzyme induction of degeneration in a large animal goat or sheep model, 3 months is required for induction of degeneration, 161 while a similar degeneration process can be induced within 1 week using organ cultures of enzyme degradation followed by physiological loading. 162
3.10. Imaging
While imaging, such as micro‐CT (with the use of contrast agents) and MRI can be conducted in vivo or ex vivo; the resolution and fidelity of the acquired data are typically superior in the ex vivo scenario where the surrounding tissues are removed and thus do not obscure the IVD. Additional advantages of ex vivo imaging include the ability to conduct longer imaging sessions (thereby improving the signal‐to‐noise ratio), the lack of motion artifacts from breathing, and not having to handle and administer anesthesia. The improvement in resolution and imaging quality enables more sophisticated biochemical and detailed structural analyses of the IVD and enables more mechanistic studies to be conducted. Likewise, parallel, clinically relevant imaging parameters, such as IVD hydration, IVD height index, and bone parameters, can also be obtained from the higher‐resolution ex vivo imaging. 97 , 163 , 164 , 165 Another advantage in terms of imaging organ culture models is the application of molecular imaging to track changes in the biological activity of cells in organ culture over time (e.g., cell metabolism). One example of this is the use of fluorescence molecular tomography (FMT) which is capable of retrieving the 3D bio‐distribution of fluorescent molecular markers noninvasively, thus offering higher molecular sensitivity than microCT or MRI. 166 One key feature of FMT is the use of near‐infrared (NIR) fluorescence probes, which have been shown to be the most effective for deep tissue imaging. In the NIR spectral range, the attenuation of living tissue is minimal, allowing the use of sufficient laser power for fluorescence excitation and detection without causing tissue damage under prolonged illumination. Moreover, molecular‐based imaging findings can also be coupled with analyses of changes in the culture media, to inform coupled in situ and surrounding microenvironmental changes.
3.11. Regulatory requirements and clinical translation
A major argument put forward by in vivo model proponents involves the regulatory requirements for in vivo animal evaluations prior to human clinical trials. However, numerous studies have shown critical differences between animals and humans, and not just solely in the spine field. With the further development of highly functional and systemically controlled organ culture systems, the use of animals could be reduced, and regulatory pathways limited to more ethical and clinically relevant ex vivo human organ culture testing.
4. APPROPRIATE MODEL SELECTION
It is clear that the selection of a model system for any project must be driven by the research question. Just as an inappropriate sample size can invalidate the results of a project, so too can the use of an inappropriate model system. Therefore, an understanding of the strengths and weaknesses of the various ex vivo organ culture and in vivo models available is a critical step in study design. The relative strengths of in vivo animal and ex vivo organ culture models, as outlined in the preceding sections and summarized in Figure 1, are not necessarily universal; again, they are driven by the question that is being asked and the outcome measures that best answer that question. A particularly obvious example would be that it would not be possible to test a new spinal implant intended for human use in a rat or rabbit, but it would be achievable in a sheep, pig, or calf model. When selecting among the available options, in this case, outcome measures are particularly important: what do you need to sample, how often, and over what period of time?
FIGURE 1.
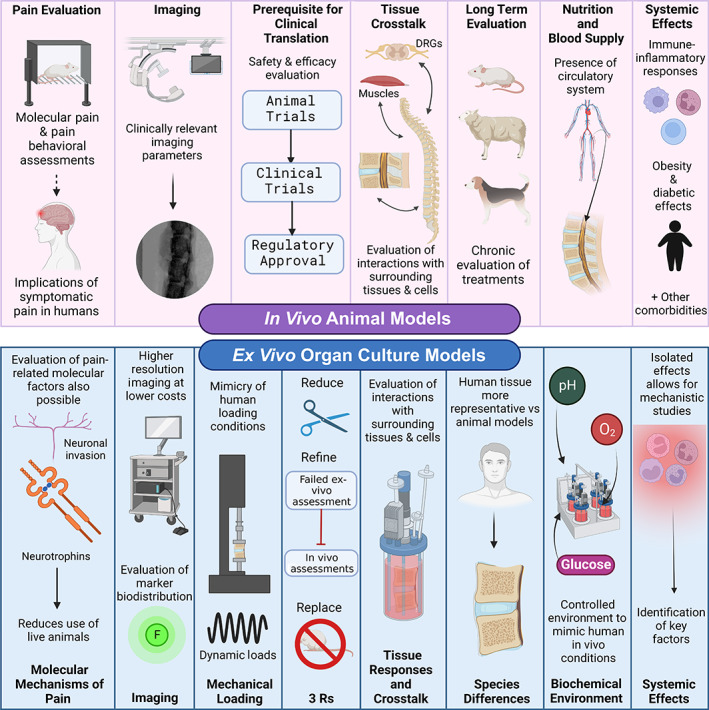
Summary of the respective advantages of in vivo animal versus ex vivo organ culture models for studies of the intervertebral disc. Figure created using BioRender.com by Shirley N. Tang with license to publish
Both organ culture and in vivo models have their limitations, but both also play a vital role in the overall successful understanding of the disease process and the development of potentially life‐changing therapies for human patients. The types of available in vivo animal models used for spine research have ranged from non‐mammalian vertebrates (such as zebrafish) to small mammals (such as rodents) to large mammals (such as dogs and livestock). With the increasing complexity of the model, there is more likely to be translation to human disease; however, the increased complexity may complicate the mechanistic understanding across multiple tissues. Furthermore, the cost of larger models is higher, both in dollars and potentially in negative public perception. In general, single‐cell organisms, invertebrates, and non‐mammalian vertebrates have the most utility in investigating the cellular or molecular basis of disease. Non‐mammalian vertebrates and some rodents are amenable to genetic manipulation, allowing for the creation of genetic models that display a particular phenotype (which may include susceptibility or resistance to disease). This type of manipulation is not currently possible in most large mammalian models, but these species are highly useful for the study of naturally occurring and induced models of disease. It should be noted that modern gene‐editing technology is making genetic manipulation of larger animals more feasible. 167 No animal model can perfectly recapitulate human disease, and the ability to use cadaveric human tissue in organ culture must be considered a potential advantage of that approach. Organ culture models may offer the benefit of systematic control of the biomechanics and metabolics of the experimental system that more closely mimic the human condition.
There are many advantages to naturally occurring models of disease. Because they are closest to the “real mechanism,” they can give the best insight into disease biology and the best evaluation of diagnostics and therapeutics. Furthermore, if companion animals can be used—for example, when studying IVDD in dogs—then researchers may be able to recruit client‐owned cases, which may reduce costs and reduce unnecessary animal usage. Such investigations are also of dual benefit, with advances made for the treatment of the species being studied as well as potential translational benefits to humans. However, there are also possible disadvantages to studying naturally occurring diseases, including the fact that variables beyond the researchers' control may affect results (such as genetic diversity within highly outbred species), and appropriate cases may be difficult to find.
In contrast, experimentally induced models have the advantage of enhanced reproducibility of the intervention/injury, in as many animals as needed and when they are needed. The downside is that induced disease may not exactly recapitulate natural disease and therefore response to therapeutics might not translate perfectly. Furthermore, there are significant costs and ethical concerns to navigate. When considering induced models of disease for spine research, surgical models are most common; however, other methods of inducing disease may be considered, including genetic manipulation, dietary manipulation, and chemically induced disease. 23
There is no single “gold standard” model for IVD research precisely because different research questions lend themselves to different approaches. Thus, the path to selecting the right model starts with the research question. This will lead to the outcomes of interest, and the selection of the methods that will be used to measure them. These, in turn, will drive the selection of a specific model. In some cases, the elimination of clearly unsuitable models may be the easiest first step. From there, the strengths and weaknesses of potentially suitable options can be weighed. It may turn out that two models could answer your question equally well, and in this case, other factors such as cost and convenience will certainly play a role. It is possible (even likely) that a broad research question cannot be answered effectively by a single model, and that multiple models must be used in sequence or simultaneously to address different aspects of the question. Indeed, complementary use of several IVD model types and leveraging the unique advantages of each is likely to result in the highest impact research in most instances. For example, taking the development of a novel biologic for IVDD treatment as a general case study, a study may commence by establishing and characterizing an IVDD phenotype in a naturally occurring or transgenic rodent model, and identifying a putative therapeutic target. Subsequently, potential therapeutic agents could be screened in organ culture models under controlled experimental conditions and utilizing cadaveric human discs to confirm relevance to the human condition. Short‐term safety and efficacy studies could then be undertaken in rodent or rabbit models, followed by longer‐term studies in large animal models using gold‐standard clinically relevant outcome measures. As access to such a wide array of model systems may be beyond the capabilities of a single laboratory, financially and/or logistically, such studies could be undertaken through collaborations across laboratories and institutions.
In conclusion, in this article, we debate the relative advantages of in vivo animal and ex vivo organ culture models for studies of the IVD. In doing so we also identify their respective limitations, and the continued need to strive for improved experimental platforms in order to achieve the best possible treatment outcomes for LBP patients. Many reviews of different IVD model systems are available in the published literature, 23 , 93 , 168 , 169 , 170 , 171 and these can serve as valuable resources for researchers seeking the best model system for their research question. Consideration should also be given to the development and use of standardized outcome measures for various models, 172 , 173 which makes comparing results across studies easier and more valuable.
AUTHOR CONTRIBUTIONS
Lachlan J. Smith conceived the original idea for the manuscript. Andres F. Bonilla and Shirley N. Tang led the drafting of the in vivo and organ culture subsections respectively. All authors provided conceptual input, drafted sections of the manuscript, and reviewed and approved the completed document prior to submission.
CONFLICT OF INTEREST
Lachlan J. Smith, Sibylle Grad, Victor Leung, Christine L. Le Maitre, and Devina Purmessur are members of the JOR Spine Advisory Review Board. The authors have no other relevant conflicts to declare.
ACKNOWLEDGMENTS
This article was made possible by funding from the National Institute of Health (NIH) R01AR074441, R01AR077678, and P30AR074992 supporting Dr Simon Y. Tang; NIH R01AR069668, R01AR077760, and R21AR080516 supporting Dr Nadeen O. Chahine; NIH R01AR077435 and R21AR077261, and Department of Veteran's Affairs I01RX001321 supporting Dr Lachlan J. Smith; AO Spine and Swiss National Science Foundation Grant 189915 supporting Dr Sibylle Grad; Research Grant Council of Hong Kong GRF17126820 supporting Dr Victor Leung; and the Fulbright—ICETEX Pasaporte a la Ciencia program supporting Andres F. Bonilla.
Tang, S. N. , Bonilla, A. F. , Chahine, N. O. , Colbath, A. C. , Easley, J. T. , Grad, S. , Haglund, L. , Le Maitre, C. L. , Leung, V. , McCoy, A. M. , Purmessur, D. , Tang, S. Y. , Zeiter, S. , & Smith, L. J. (2022). Controversies in spine research: Organ culture versus in vivo models for studies of the intervertebral disc. JOR Spine, 5(4), e1235. 10.1002/jsp2.1235
Shirley N. Tang and Andres F. Bonilla contributed equally to this study.
Funding information Fulbright ‐ ICETEX Pasaporte a la Ciencia Program; National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant/Award Numbers: P30AR074992, R01AR069668, R01AR074441, R01AR077435, R01AR077678, R01AR077760, R21AR077261, R21AR080516; Research Grant Council of Hong Kong, Grant/Award Number: GRF17126820; Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung, Grant/Award Number: 189915; U.S. Department of Veterans Affairs, Grant/Award Number: I01RX001321
REFERENCES
- 1. De David CN, Deligne LDMC, Da Silva RS, et al. The burden of low back pain in Brazil: estimates from the global burden of disease 2017 study. Popul Health Metr. 2020;18:1‐10. doi: 10.1186/S12963-020-00205-4/FIGURES/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low Back pain. Arch Intern Med. 2009;169:251‐258. doi: 10.1001/archinternmed.2008.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391:2356‐2367. [DOI] [PubMed] [Google Scholar]
- 4. Zhang YG, Guo TM, Guo X, Wu SX. Clinical diagnosis for discogenic low back pain. Int J Biol Sci. 2009;5:647‐658. doi: 10.7150/IJBS.5.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chou L, Brady SRE, Urquhart DM, et al. The association between obesity and low back pain and disability is affected by mood disorders: a population‐based, cross‐sectional study of men. Medicine. 2016;95:e3367. doi: 10.1097/MD.0000000000003367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oichi T, Taniguchi Y, Oshima Y, Tanaka S, Saito T. Pathomechanism of intervertebral disc degeneration. JOR Spine. 2020;3:e1076. doi: 10.1002/JSP2.1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shiri R, Falah‐Hassani K, Heliövaara M, et al. Risk factors for low back pain: a population‐based longitudinal study. Arthritis Care Res. 2019;71:290‐299. doi: 10.1002/ACR.23710 [DOI] [PubMed] [Google Scholar]
- 8. Freemont AJA, Peacock TE, Goupille P, Hoyland JAJ, O'Brien J, Jayson MIV. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178‐181. doi: 10.1016/S0140-6736(97)02135-1 [DOI] [PubMed] [Google Scholar]
- 9. Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10:R99. doi: 10.1186/ar2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Binch ALA, Cole AA, Breakwell LM, et al. Nerves are more abundant than blood vessels in the degenerate human intervertebral disc. Arthritis Res Ther. 2015;17:370. doi: 10.1186/s13075-015-0889-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Binch LA, Cole AA, Breakwell LM, et al. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res Ther. 2014;16:416. doi: 10.1186/s13075-014-0416-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freemont AJ, Watkins A, Le Maitre C, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286‐292. doi: 10.1002/path.1108 [DOI] [PubMed] [Google Scholar]
- 13. Richardson SM, Doyle P, Minogue BM, Gnanalingham K, Hoyland JA. Increased expression of matrix metalloproteinase‐10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res Ther. 2009;11:R126. doi: 10.1186/ar2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olmarker K. Radicular pain—recent pathophysiologic concepts and therapeutic implications. Der Schmerz. 2001;15:425‐429. doi: 10.1007/s004820100027 [DOI] [PubMed] [Google Scholar]
- 15. Alizadeh R, Sharifzadeh SR. Pathogenesis, etiology and treatment of failed back surgery syndrome. Neurochirurgie. 2021;68:426‐431. doi: 10.1016/J.NEUCHI.2021.09.005 [DOI] [PubMed] [Google Scholar]
- 16. Mokhtare M, Mohammad Valizadeh S, Emadian O, Valizadeh SM. Lower gastrointestinal bleeding due to non‐steroid anti‐inflammatory drug‐induced colopathy case report and literature review. Middle East J Dig Dis. 2013;5:107‐111. [PMC free article] [PubMed] [Google Scholar]
- 17. Ravalli S, Musumeci G. New horizons of knowledge in intervertebral disc disease. J Invest Surg. 2021;34:912‐913. doi: 10.1080/08941939.2019.1708998 [DOI] [PubMed] [Google Scholar]
- 18. Lyden J, Binswanger IA. The United States opioid epidemic. Semin Perinatol. 2019;43:123‐131. doi: 10.1053/J.SEMPERI.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silverman LI, Heaton W, Farhang N, et al. Perspectives on the treatment of lumbar disc degeneration: the value proposition for a cell‐based therapy, immunomodulatory properties of discogenic cells and the associated clinical evaluation strategy. Front Surg. 2020;7:554382. doi: 10.3389/FSURG.2020.554382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foster NEN, Anema JR, Cherkin D, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018;391:2368‐2383. [DOI] [PubMed] [Google Scholar]
- 21. Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350:g6380. doi: 10.1136/bmj.g6380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin L, Balian G, Li XJ. Animal models for disc degeneration—an update. Histol Histopathol. 2018;33:543‐554. doi: 10.14670/HH-11-910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fusellier M, Clouet J, Gauthier O, Tryfonidou M, Le Visage C, Guicheux J. Degenerative lumbar disc disease: in vivo data support the rationale for the selection of appropriate animal models. Eur Cell Mater. 2020;39:18‐47. doi: 10.22203/eCM.v039a02 [DOI] [PubMed] [Google Scholar]
- 24. Reitmaier S, Graichen F, Shirazi‐Adl A, Schmidt H. Separate the sheep from the goats: use and limitations of large animal models in intervertebral disc research. J Bone Jt Surg ‐ Am Vol. 2017;99:e102. doi: 10.2106/JBJS.17.00172 [DOI] [PubMed] [Google Scholar]
- 25. Shi C, Qiu S, Riester SM, et al. Animal models for studying the etiology and treatment of low back pain. J Orthop Res. 2018;36:1305‐1312. doi: 10.1002/JOR.23741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andersson GBJ. Epidemiological features of chronic low‐back pain. Lancet. 1999;354:581‐585. doi: 10.1016/S0140-6736(99)01312-4 [DOI] [PubMed] [Google Scholar]
- 27. Lotz JC. Animal models of intervertebral disc degeneration. Spine. 2004;29:2742‐2750. doi: 10.1097/01.brs.0000146498.04628.f9 [DOI] [PubMed] [Google Scholar]
- 28. Lee NN, Salzer E, Bach FC, et al. A comprehensive tool box for large animal studies of intervertebral disc degeneration. JOR Spine. 2021;4:e1162. doi: 10.1002/jsp2.1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Testa B, Reid J, Scott ME, Murison PJ, Bell AM. The short form of the Glasgow composite measure pain scale in post‐operative analgesia studies in dogs: a scoping review. Front Vet sci. 2021;8:1084. doi: 10.3389/FVETS.2021.751949/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujii K, Yamazaki M, Kang JD, et al. Discogenic back pain: literature review of definition, diagnosis, and treatment. JBMR Plus. 2019;3:e10180. doi: 10.1002/jbm4.10180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pelled G, Salas MM, Han P, et al. Intradiscal quantitative chemical exchange saturation transfer MRI signal correlates with discogenic pain in human patients. Sci Rep. 2021;11:19195. doi: 10.1038/S41598-021-97672-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen YT, Cotter A, Isaac Z. Back pain: discogenic. Clinical Guide to Musculoskeletal Medicine. Springer; 2022. [Google Scholar]
- 33. Thompson K, Moore S, Tang S, Wiet M, Purmessur D. The chondrodystrophic dog: a clinically relevant intermediate‐sized animal model for the study of intervertebral disc‐associated spinal pain. JOR Spine. 2018;1:1‐13. doi: 10.1002/jsp2.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Millecamps M, Czerminski JT, Mathieu AP, Stone LS. Behavioral signs of axial low back pain and motor impairment correlate with the severity of intervertebral disc degeneration in a mouse model. Spine J. 2015;15:2524‐2537. doi: 10.1016/J.SPINEE.2015.08.055 [DOI] [PubMed] [Google Scholar]
- 35. Lai A, Moon A, Purmessur D, et al. Assessment of functional and behavioral changes sensitive to painful disc degeneration. J Orthop Res. 2015;33:755‐764. doi: 10.1002/jor.22833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leimer EM, Gayoso MG, Jing L, Tang SY, Gupta MC, Setton LA. Behavioral compensations and neuronal remodeling in a rodent model of chronic intervertebral disc degeneration. Sci Rep. 2019;9:1‐10. doi: 10.1038/s41598-019-39657-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Evangelista MC, Monteiro BP, Steagall PV. Measurement properties of grimace scales for pain assessment in non‐human mammals. Pain. 2021;163:e697‐e714. doi: 10.1097/j.pain.0000000000002474 [DOI] [PubMed] [Google Scholar]
- 38. Willems N, Tellegen AR, Bergknut N, et al. Inflammatory profiles in canine intervertebral disc degeneration. BMC Vet Res. 2016;12:10. doi: 10.1186/s12917-016-0635-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nerlich AG, Schaaf R, Wälchli B, Boos N. Temporo‐spatial distribution of blood vessels in human lumbar intervertebral discs. Eur Spine J. 2007;16:547‐555. doi: 10.1007/S00586-006-0213-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rudert M, Tillmann B. Detection of lymph and blood vessels in the human intervertebral disc by histochemical and immunohistochemical methods. Ann Anat. 1993;175:237‐242. doi: 10.1016/S0940-9602(11)80009-9 [DOI] [PubMed] [Google Scholar]
- 41. Grunhagen T, Shirazi‐Adl A, Fairbank JCT, Urban JPG. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42:465‐477. doi: 10.1016/J.OCL.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 42. Ogata K, Whiteside LA. 1980 Volvo award winner in basic science. Nutritional pathways of the intervertebral disc. An experimental study using hydrogen washout technique. Spine. 1981;6(3):211‐216. [PubMed] [Google Scholar]
- 43. Van Der Werf M, Lezuo P, Maissen O, Van Donkelaar CC, Ito K. Inhibition of vertebral endplate perfusion results in decreased intervertebral disc intranuclear diffusive transport. J Anat. 2007;211:769‐774. doi: 10.1111/J.1469-7580.2007.00816.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ashinsky BG, Bonnevie ED, Mandalapu SA, et al. Intervertebral disc degeneration is associated with aberrant endplate remodeling and reduced small molecule transport. J Bone Miner Res. 2020;35:1572‐1581. doi: 10.1002/JBMR.4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holm S, Nachemson A. Nutrition of the intervertebral disc: acute effects of cigarette smoking: an experimental animal study. Ups J Med Sci. 1988;93:91‐99. doi: 10.1517/03009734000000042 [DOI] [PubMed] [Google Scholar]
- 46. Iwahashi M, Matsuzaki H, Tokuhashi Y, Wakabayashi K, Uematsu Y. Mechanism of intervertebral disc degeneration caused by nicotine in rabbits to explicate intervertebral disc disorders caused by smoking. Spine. 2002;27:1396‐1401. doi: 10.1097/00007632-200207010-00005 [DOI] [PubMed] [Google Scholar]
- 47. Wallace AL, Wyatt BC, McCarthy D, Hughes SP. Humoral regulation of blood flow in the vertebral endplate. Spine. 1994;19:1324‐1328. doi: 10.1097/00007632-199406000-00004 [DOI] [PubMed] [Google Scholar]
- 48. Gullbrand SE, Peterson J, Mastropolo R, et al. Drug‐induced changes to the vertebral endplate vasculature affect transport into the intervertebral disc in vivo. J Orthop Res. 2014;32:1694‐1700. doi: 10.1002/JOR.22716 [DOI] [PubMed] [Google Scholar]
- 49. Cunha C, Almeida CR, Almeida MI, et al. Systemic delivery of bone marrow mesenchymal stem cells for in situ intervertebral disc regeneration. Stem Cells Transl Med. 2017;6:1029‐1039. doi: 10.5966/SCTM.2016-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Walters R, Rahmat R, Fraser R, Moore R. Preventing and treating discitis: cephazolin penetration in ovine lumbar intervertebral disc. Eur Spine J. 2006;15:1397‐1403. doi: 10.1007/S00586-006-0144-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Urban JPGG, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120‐130. doi: 10.1186/ar629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2‐19. doi: 10.1007/s00586-007-0414-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Daly C, Ghosh P, Jenkin G, Oehme D, Goldschlager T. A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int. 2016;2016:1‐14. doi: 10.1155/2016/5952165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang W, Nie L, Wang Y, et al. CCL20 secretion from the nucleus pulposus improves the recruitment of CCR6‐expressing Th17 cells to degenerated IVD tissues. PLoS One. 2013;8:e66286. doi: 10.1371/JOURNAL.PONE.0066286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nakazawa KR, Walter BA, Laudier DM, et al. Accumulation and localization of macrophage phenotypes with human intervertebral disc degeneration. Spine J. 2018;18:343‐356. doi: 10.1016/j.spinee.2017.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wiet MG, Piscioneri A, Khan SN, Ballinger MN, Hoyland JA, Purmessur D. Mast cell‐intervertebral disc cell interactions regulate inflammation, catabolism and angiogenesis in discogenic back pain. Sci Rep. 2017;7:12492. doi: 10.1038/s41598-017-12666-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peng B, Hao J, Hou S, et al. Possible pathogenesis of painful intervertebral disc degeneration. Spine. 2006;31:560‐566. doi: 10.1097/01.BRS.0000201324.45537.46 [DOI] [PubMed] [Google Scholar]
- 58. Ye F, Lyu F, Wang H, Zheng Z. The involvement of immune system in intervertebral disc herniation and degeneration. JOR Spine. 2022;5:e1196. doi: 10.1002/jsp2.1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gorth DJ, Shapiro IM, Risbud MV. Transgenic mice overexpressing human TNF‐α experience early onset spontaneous intervertebral disc herniation in the absence of overt degeneration. Cell Death Dis. 2019;10:7. doi: 10.1038/s41419-018-1246-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jin L, Xiao L, Ding M, et al. Heterogeneous macrophages contribute to the pathology of disc herniation induced radiculopathy. Spine J. 2022;22:677‐689. doi: 10.1016/j.spinee.2021.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kawakubo A, Uchida K, Miyagi M, et al. Investigation of resident and recruited macrophages following disc injury in mice. J Orthop Res. 2020;38:1703‐1709. doi: 10.1002/JOR.24590 [DOI] [PubMed] [Google Scholar]
- 62. Lee S, Millecamps M, Foster DZ, Stone LS. Long‐term histological analysis of innervation and macrophage infiltration in a mouse model of intervertebral disc injury‐induced low back pain. J Orthop Res. 2020;38:1238‐1247. doi: 10.1002/jor.24560 [DOI] [PubMed] [Google Scholar]
- 63. Mahmoud M, Kokozidou M, Auffarth A, Schulze‐Tanzil G. The relationship between diabetes mellitus type II and intervertebral disc degeneration in diabetic rodent models: a systematic and comprehensive review. Cell. 2020;9:2208. doi: 10.3390/CELLS9102208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ruiz‐Fernández C, Francisco V, Pino J, et al. Molecular relationships among obesity, inflammation and intervertebral disc degeneration: are adipokines the common link? Int J Mol Sci. 2019;20:2030. doi: 10.3390/IJMS20082030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Natelson DM, Lai A, Krishnamoorthy D, Hoy RC, Iatridis JC, Illien‐Jünger S. Leptin signaling and the intervertebral disc: sex dependent effects of leptin receptor deficiency and Western diet on the spine in a type 2 diabetes mouse model. PLoS One. 2020;15:e0227527. doi: 10.1371/journal.pone.0227527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lintz M, Walk RE, Tang SY, Bonassar LJ. The degenerative impact of hyperglycemia on the structure and mechanics of developing murine intervertebral discs. JOR Spine. 2022;5:e1191. doi: 10.1002/jsp2.1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fields AJ, Berg‐Johansen B, Metz LN, et al. Alterations in intervertebral disc composition, matrix homeostasis and biomechanical behavior in the UCD‐T2DM rat model of type 2 diabetes. J Orthop Res. 2015;33:738‐746. doi: 10.1002/JOR.22807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Svenja IJ, Young L, Sheeraz AQ, et al. Chronic ingestion of advanced glycation end products induces degenerative spinal changes and hypertrophy in aging pre‐diabetic mice. PLoS One. 2015;10:e0116625. doi: 10.1371/JOURNAL.PONE.0116625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Illien‐Junger S, Grosjean F, Laudier DM, Vlassara H, Striker GE, Iatridis JC. Combined anti‐inflammatory and anti‐AGE drug treatments have a protective effect on intervertebral discs in mice with diabetes. PLoS One. 2013;8:e64302. doi: 10.1371/JOURNAL.PONE.0064302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xie L, Zhao Z, Chen Z, et al. Melatonin alleviates radiculopathy against apoptosis and NLRP3 inflammasomes via the parkin‐mediated mitophagy pathway. Spine. 2021;46:E859‐E868. doi: 10.1097/BRS.0000000000003942 [DOI] [PubMed] [Google Scholar]
- 71. Huang SJ, Yan JQ, Luo H, Zhou LY, Luo JG. IL‐33/ST2 signaling contributes to radicular pain by modulating MAPK and NF‐ΚB activation and inflammatory mediator expression in the spinal cord in rat models of noncompressive lumber disk herniation. J Neuroinflammation. 2018;15:1‐12. doi: 10.1186/S12974-017-1021-4/FIGURES/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang X, Wang W, Liu X, et al. Bone mesenchymal stem cells attenuate radicular pain by inhibiting microglial activation in a rat noncompressive disk herniation model. Cell Tissue Res. 2018;374:99‐110. doi: 10.1007/S00441-018-2855-5 [DOI] [PubMed] [Google Scholar]
- 73. Liu Y, Li J, Li H, et al. AMP‐activated protein kinase activation in dorsal root ganglion suppresses mTOR/p70S6K signaling and alleviates painful radiculopathies in lumbar disc herniation rat model. Spine. 2019;44:E865‐E872. doi: 10.1097/BRS.0000000000003005 [DOI] [PubMed] [Google Scholar]
- 74. Stover JD, Farhang N, Lawrence B, Bowles RD. Multiplex epigenome editing of dorsal root ganglion neuron receptors abolishes redundant interleukin 6, tumor necrosis factor alpha, and interleukin 1β signaling by the degenerative intervertebral disc. Hum Gene Ther. 2019;30:1147‐1160. doi: 10.1089/HUM.2019.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stover JD, Lawrence B, Bowles RD. Degenerative IVD conditioned media and acidic pH sensitize sensory neurons to cyclic tensile strain. J Orthop Res. 2021;39:1192‐1203. doi: 10.1002/JOR.24682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ma J, Stefanoska D, Grad S, Alini M, Peroglio M. Direct and intervertebral DiscMediated sensitization of dorsal root ganglion neurons by hypoxia and low pH. Neurospine. 2020;17:42‐59. doi: 10.14245/ns.2040052.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ma J, Stefanoska D, Stone LS, et al. Hypoxic stress enhances extension and branching of dorsal root ganglion neuronal outgrowth. JOR Spine. 2020;3:e1090. doi: 10.1002/JSP2.1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Carreon LY, Ito T, Yamada M, Uchiyama S, Takahashi HE. Neovascularization induced by annulus and its inhibition by cartilage endplate. Its role in disc absorption. Spine. 1997;22:1429‐1434. doi: 10.1097/00007632-199707010-00001 [DOI] [PubMed] [Google Scholar]
- 79. Feng Z, Chen L, Hu X, Yang G, Chen Z, Wang Y. Vertebral augmentation can induce early signs of degeneration in the adjacent intervertebral disc: evidence from a rabbit model. Spine. 2018;43:E1195‐E1203. doi: 10.1097/BRS.0000000000002666 [DOI] [PubMed] [Google Scholar]
- 80. Xiao ZF, He JB, Su GY, et al. Osteoporosis of the vertebra and osteochondral remodeling of the endplate causes intervertebral disc degeneration in ovariectomized mice. Arthritis Res Ther. 2018;20:207. doi: 10.1186/S13075-018-1701-1/FIGURES/10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Samartzis D, Mok FPS, Karppinen J, Fong DYT, Luk KDK, Cheung KMC. Classification of Schmorl's nodes of the lumbar spine and association with disc degeneration: a large‐scale population‐based MRI study. Osteoarthr Cartil. 2016;24:1753‐1760. doi: 10.1016/J.JOCA.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 82. Cinotti G, Della RC, Romeo S, Vittur F, Toffanin R, Trasimeni G. Degenerative changes of porcine intervertebral disc induced by vertebral endplate injuries. Spine. 2005;30:174‐180. doi: 10.1097/01.BRS.0000150530.48957.76 [DOI] [PubMed] [Google Scholar]
- 83. Kuga N, Kawabuchi M. Histology of intervertebral disc protrusion: an experimental study using an aged rat model. Spine. 2001;26:E379‐E384. doi: 10.1097/00007632-200109010-00005 [DOI] [PubMed] [Google Scholar]
- 84. Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine. 2003;28:982‐990. doi: 10.1097/01.BRS.0000061986.03886.4F [DOI] [PubMed] [Google Scholar]
- 85. Kim KW, Ha KY, Park JB, Woo YK, Chung HN, An HS. Expressions of membrane‐type I matrix metalloproteinase, Ki‐67 protein, and type II collagen by chondrocytes migrating from cartilage endplate into nucleus pulposus in rat intervertebral discs: a cartilage endplate‐fracture model using an intervertebral disc organ culture. Spine. 2005;30:1373‐1378. doi: 10.1097/01.BRS.0000166155.48168.0E [DOI] [PubMed] [Google Scholar]
- 86. Au TYK, Lam TK, Peng Y, et al. Transformation of resident notochord‐descendent nucleus pulposus cells in mouse injury‐induced fibrotic intervertebral discs. Aging Cell. 2020;19:e13254. doi: 10.1111/ACEL.13254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Xiong CJ, Huang B, Zhou Y, et al. Macrophage migration inhibitory factor inhibits the migration of cartilage end plate‐derived stem cells by reacting with CD74. PLoS One. 2012;7:e43984. doi: 10.1371/JOURNAL.PONE.0043984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Han SK, Lee Y, Hong JJ, et al. In vivo study of paraspinal muscle weakness using botulinum toxin in one primate model. Clin Biomech. 2018;53:1‐6. doi: 10.1016/J.CLINBIOMECH.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 89. United States Food and Drug Administration . General Considerations for Animal Studies for Medical Devices. US FDA; 2015. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/general‐considerations‐animal‐studies‐medical‐devices [Google Scholar]
- 90. Hampshire VA, Gilbert SH. Refinement, reduction, and replacement (3R) strategies in preclinical testing of medical devices. Toxicol Pathol. 2019;47:329‐338. doi: 10.1177/0192623318797289 [DOI] [PubMed] [Google Scholar]
- 91. United States Food and Drug Administration . Animal Rule Approvals. US FDA; 2002. https://www.fda.gov/drugs/nda-and-bla-approvals/animal-rule-approvals [Google Scholar]
- 92. Smolders LA, Kingma I, Bergknut N, et al. Biomechanical assessment of the effects of decompressive surgery in non‐chondrodystrophic and chondrodystrophic canine multisegmented lumbar spines. Eur Spine J. 2012;21:1692‐1699. doi: 10.1007/S00586-012-2285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pfannkuche JJ, Guo W, Cui S, et al. Intervertebral disc organ culture for the investigation of disc pathology and regeneration—benefits, limitations, and future directions of bioreactors. Connect Tissue Res. 2020;61:304‐321. doi: 10.1080/03008207.2019.1665652 [DOI] [PubMed] [Google Scholar]
- 94. Chiba K, Andersson GBJ, Masuda K, Momohara S, Williams JM, Thonar EJMA. A new culture system to study the metabolism of the intervertebral disc in vitro. Spine. 1998;23:1821‐1828. doi: 10.1097/00007632-199809010-00002 [DOI] [PubMed] [Google Scholar]
- 95. Gantenbein B, Illien‐Jünger S, Chan S, et al. Organ culture bioreactors—platforms to study human intervertebral disc degeneration and regenerative therapy. Curr Stem Cell Res Ther. 2015;10:339‐352. doi: 10.2174/1574888x10666150312102948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pelle DW, Peacock JD, Schmidt CL, et al. Genetic and functional studies of the intervertebral disc: a novel murine intervertebral disc model. PLoS One. 2014;9:e112454. doi: 10.1371/JOURNAL.PONE.0112454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liu JW, Lin KH, Weber C, et al. An in vitro organ culture model of the murine intervertebral disc. J Vis Exp. 2017;2017:55437. doi: 10.3791/55437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Seol D, Choe H, Ramakrishnan PS, et al. Organ culture stability of the intervertebral disc: rat versus rabbit. J Orthop Res. 2013;31:838‐846. doi: 10.1002/JOR.22285 [DOI] [PubMed] [Google Scholar]
- 99. Gantenbein B, Grünhagen T, Lee CR, Van Donkelaar CC, Alini M, Ito K. An in vitro organ culturing system for intervertebral disc explants with vertebral endplates: a feasibility study with ovine caudal discs. Spine. 2006;31:2665‐2673. doi: 10.1097/01.BRS.0000244620.15386.DF [DOI] [PubMed] [Google Scholar]
- 100. Lee CR, Iatridis JC, Poveda L, Alini M. In vitro organ culture of the bovine intervertebral disc: effects of vertebral endplate and potential for mechanobiology studies. Spine. 2006;31:515‐522. doi: 10.1097/01.brs.0000201302.59050.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Walter BAB, Illien‐Jünger S, Nasser PR, Hecht ACA, Iatridis JC, May PW. Development and validation of a bioreactor system for dynamic loading and mechanical characterization of whole human intervertebral discs in organ culture. J Biomech. 2014;47:2095‐2101. doi: 10.1016/j.jbiomech.2014.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Abraham AC, Liu JW, Tang SY. Longitudinal changes in the structure and inflammatory response of the intervertebral disc due to stab injury in a murine organ culture model. J Orthop Res. 2016;34:1431‐1438. doi: 10.1002/JOR.23325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Peroglio M, Gaspar D, Zeugolis DI, Alini M. Relevance of bioreactors and whole tissue cultures for the translation of new therapies to humans. J Orthop Res. 2018;36:10‐21. doi: 10.1002/JOR.23655 [DOI] [PubMed] [Google Scholar]
- 104. Li Z, Gehlen Y, Heizmann F, et al. Preclinical ex‐vivo testing of anti‐inflammatory drugs in a bovine intervertebral degenerative disc model. Front Bioeng Biotechnol. 2020;8:583. doi: 10.3389/FBIOE.2020.00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Peroglio M, Douma LS, Caprez TS, et al. Intervertebral disc response to stem cell treatment is conditioned by disc state and cell carrier: an ex vivo study. J Orthop Transl. 2017;9:43‐51. doi: 10.1016/J.JOT.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Guo W, Douma L, Hu MH, et al. Hyaluronic acid‐based interpenetrating network hydrogel as a cell carrier for nucleus pulposus repair. Carbohydr Polym. 2022;277:118828. doi: 10.1016/J.CARBPOL.2021.118828 [DOI] [PubMed] [Google Scholar]
- 107. Zhou Z, Zeiter S, Schmid T, et al. Effect of the CCL5‐releasing fibrin gel for intervertebral disc regeneration. Cartilage. 2020;11:169‐180. doi: 10.1177/1947603518764263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rosenzweig DH, Gawri R, Moir J, et al. Dynamic loading, matrix maintenance and cell injection therapy of human intervertebral discs cultured in a bioreactor. Eur Cell Mater. 2016;31:26‐39. doi: 10.22203/ECM.V031A03 [DOI] [PubMed] [Google Scholar]
- 109. Furtwängler T, Chan SCW, Bahrenberg G, Richards PJ, Gantenbein‐Ritter B. Assessment of the matrix degenerative effects of MMP‐3, ADAMTS‐4, and HTRA1, injected into a bovine intervertebral disc organ culture model. Spine. 2013;38:E1377‐E1387. doi: 10.1097/BRS.0b013e31829ffde8 [DOI] [PubMed] [Google Scholar]
- 110. Rodrigues‐Pinto R, Richardson SM, Hoyland JA, et al. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell‐based tissue regeneration therapies for disc degeneration. Eur Spine J. 2014;23(9):1803‐1814. [DOI] [PubMed] [Google Scholar]
- 111. Tang SN, Walter BA, Heimann MK, et al. In vivo mouse intervertebral disc degeneration models and their utility as translational models of clinical discogenic back pain: a comparative review. Front Pain Res. 2022;3:e894651. doi: 10.3389/fpain.2022.894651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bartels EM, Fairbank JCT, Winlove CP, Urban JPG. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine. 1998;23:1‐8. doi: 10.1097/00007632-199801010-00001 [DOI] [PubMed] [Google Scholar]
- 113. Mokhbi Soukane D, Shirazi‐Adl A, Urban JPG. Computation of coupled diffusion of oxygen, glucose and lactic acid in an intervertebral disc. J Biomech. 2007;40:2645‐2654. doi: 10.1016/j.jbiomech.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 114. Holm S, Maroudas A, Urban JPG, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101‐119. doi: 10.3109/03008208109152130 [DOI] [PubMed] [Google Scholar]
- 115. Jackson AR, Huang CYC, Brown MD, Yong WG. 3D finite element analysis of nutrient distributions and cell viability in the intervertebral disc: effects of deformation and degeneration. J Biomech Eng. 2011;133:091006. doi: 10.1115/1.4004944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jackson AR, Huang C‐Y, Gu WY. Effect of endplate calcification and mechanical deformation on the distribution of glucose in intervertebral disc: a 3D finite element study. Comput Methods Biomech Biomed Engin. 2011;14:195‐204. doi: 10.1080/10255842.2010.535815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Nachemson A. Intradiscal measurements of pH in patients with lumbar rhizopathies. Acta Orthop Scand. 1969;40:23‐42. doi: 10.3109/17453676908989482 [DOI] [PubMed] [Google Scholar]
- 118. Huang CY, Gu WY. Effects of mechanical compression on metabolism and distribution of oxygen and lactate in intervertebral disc. J Biomech. 2008;41:1184‐1196. doi: 10.1016/J.JBIOMECH.2008.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. McDonnell EE, Buckley CT. Investigating the physiological relevance of ex vivo disc organ culture nutrient microenvironments using in silico modeling and experimental validation. JOR Spine. 2021;4:e1141. doi: 10.1002/jsp2.1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Jünger S, Gantenbein‐Ritter B, Lezuo P, Alini M, Ferguson SJ, Ito K. Effect of limited nutrition on in situ intervertebral disc cells under simulated‐physiological loading. Spine. 2009;34:1264‐1271. doi: 10.1097/BRS.0B013E3181A0193D [DOI] [PubMed] [Google Scholar]
- 121. Illien‐Jünger S, Gantenbein‐Ritter B, Grad S, et al. The combined effects of limited nutrition and high‐frequency loading on intervertebral discs with endplates. Spine. 2010;35:1744‐1752. doi: 10.1097/BRS.0B013E3181C48019 [DOI] [PubMed] [Google Scholar]
- 122. Lang G, Liu Y, Geries J, et al. An intervertebral disc whole organ culture system to investigate proinflammatory and degenerative disc disease condition. J Tissue Eng Regen Med. 2018;12:e2051‐e2061. doi: 10.1002/TERM.2636 [DOI] [PubMed] [Google Scholar]
- 123. Parolin M, Gawri R, Mwale F, et al. Development of a whole disc organ culture system to study human intervertebral disc. Evid Based Spine Care J. 2010;1:67‐68. doi: 10.1055/s-0028-1100919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gawri R, Moir J, Ouellet J, et al. Physiological loading can restore the proteoglycan content in a model of early IVD degeneration. PLoS One. 2014;9:e101233. doi: 10.1371/journal.pone.0101233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Haglund L, Moir J, Beckman L, et al. Development of a bioreactor for axially loaded intervertebral disc organ culture. Tissue Eng Part C Methods. 2011;17:1011‐1019. doi: 10.1089/ten.tec.2011.0025 [DOI] [PubMed] [Google Scholar]
- 126. Gawri R, Mwale F, Ouellet J, et al. Development of an organ culture system for long‐term survival of the intact human intervertebral disc. Spine. 2011;36:1835‐1842. doi: 10.1097/BRS.0b013e3181f81314 [DOI] [PubMed] [Google Scholar]
- 127. Gorth DJ, Lothstein KE, Chiaro JA, et al. Hypoxic regulation of functional extracellular matrix elaboration by nucleus pulposus cells in long‐term agarose culture. J Orthop Res. 2015;33:747‐754. doi: 10.1002/JOR.22821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yang SH, Hu MH, Sun YH, Lin FH. Differential phenotypic behaviors of human degenerative nucleus pulposus cells under normoxic and hypoxic conditions: influence of oxygen concentration during isolation, expansion, and cultivation. Spine J. 2013;13:1590‐1596. doi: 10.1016/J.SPINEE.2013.05.025 [DOI] [PubMed] [Google Scholar]
- 129. Wu Y, Cisewski SE, Wegner N, et al. Region and strain‐dependent diffusivities of glucose and lactate in healthy human cartilage endplate. J Biomech. 2016;49:2756‐2762. doi: 10.1016/J.JBIOMECH.2016.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Grant M, Epure LM, Salem O, et al. Development of a large animal long‐term intervertebral disc organ culture model that includes the bony vertebrae for ex vivo studies. Tissue Eng Part C Methods. 2016;22:636‐643. doi: 10.1089/ten.TEC.2016.0049 [DOI] [PubMed] [Google Scholar]
- 131. Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine. 1999;24:755‐762. doi: 10.1097/00007632-199904150-00005 [DOI] [PubMed] [Google Scholar]
- 132. Nachemson AL. Disc pressure measurements. Spine. 1981;6:93‐97. doi: 10.1097/00007632-198101000-00020 [DOI] [PubMed] [Google Scholar]
- 133. Baer AE, Laursen TA, Guilak F, Setton LA. The micromechanical environment of intervertebral disc cells determined by a finite deformation, anisotropic, and biphasic finite element model. J Biomech Eng. 2003;125:1‐11. doi: 10.1115/1.1532790 [DOI] [PubMed] [Google Scholar]
- 134. Best BA, Guilak F, Setton LA, et al. Compressive mechanical properties of the human annulus fibrosus and their relationship to biochemical composition. Spine. 1994;19:212‐221. doi: 10.1097/00007632-199401001-00017 [DOI] [PubMed] [Google Scholar]
- 135. Gu WY, Yao H. Effects of hydration and fixed charge density on fluid transport in charged hydrated soft tissues. Ann Biomed Eng. 2003;31:1162‐1170. doi: 10.1114/1.1615576 [DOI] [PubMed] [Google Scholar]
- 136. Iatridis JC, Setton LA, Foster RJ, Rawlins BA, Weidenbaum M, Mow VC. Degeneration affects the anisotropic and nonlinear behaviors of human annulus fibrosus in compression. J Biomech. 1998;31:535‐544. doi: 10.1016/S0021-9290(98)00046-3 [DOI] [PubMed] [Google Scholar]
- 137. Kwan MK, Lai WM, Mow VC. A finite deformation theory for cartilage and other soft hydrated connective tissues—I. Equilibrium results. J Biomech. 1990;23:145‐155. doi: 10.1016/0021-9290(90)90348-7 [DOI] [PubMed] [Google Scholar]
- 138. Setton LA, Zhu W, Weidenbaum M, Ratcliffe A, Mow VC. Compressive properties of the cartilaginous end‐plate of the baboon lumbar spine. J Orthop Res. 1993;11:228‐239. doi: 10.1002/jor.1100110210 [DOI] [PubMed] [Google Scholar]
- 139. Le Maitre CL, Frain J, Fotheringham AP, Freemont AJ, Hoyland JA. Human cells derived from degenerate intervertebral discs respond differently to those derived from non‐degenerate intervertebral discs following application of dynamic hydrostatic pressure. Biorheology. 2008;45:563‐575. doi: 10.3233/BIR-2008-0498 [DOI] [PubMed] [Google Scholar]
- 140. Le Maitre CL, Frain J, Millward‐Sadler J, Fotheringham AP, Freemont AJ, Hoyland JA. Altered integrin mechanotransduction in human nucleus pulposus cells derived from degenerated discs. Arthritis Rheum. 2009;60:460‐469. doi: 10.1002/art.24248 [DOI] [PubMed] [Google Scholar]
- 141. Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Jt Surg. 2006;88:52‐57. doi: 10.2106/JBJS.F.00001 [DOI] [PubMed] [Google Scholar]
- 142. Gu W, Zhu Q, Gao X, Brown MD. Simulation of the progression of intervertebral disc degeneration due to decreased nutritional supply. Spine. 2014;39:E1411‐E1417. doi: 10.1097/BRS.0000000000000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Salvatierra JC, Yuan TY, Fernando H, et al. Difference in energy metabolism of annulus fibrosus and nucleus pulposus cells of the intervertebral disc. Cell Mol Bioeng. 2011;4:302‐310. doi: 10.1007/s12195-011-0164-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Travascio F, Jackson AR, Brown MD, Gu WY. Relationship between solute transport properties and tissue morphology in human annulus fibrosus. J Orthop Res. 2009;27:1625‐1630. doi: 10.1002/jor.20927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Yao H, Gu WY. Physical signals and solute transport in human intervertebral disc during compressive stress relaxation: 3D finite element analysis. Biorheology. 2006;43:323‐335. [PubMed] [Google Scholar]
- 146. Wilder D, Pope M. Epidemiological and aetiological aspects of low back pain in vibration environments—an update. Clin Biomech. 1996;11:61‐73. doi: 10.1016/0268-0033(95)00039-9 [DOI] [PubMed] [Google Scholar]
- 147. McCann MR, Veras MA, Yeung C, et al. Whole‐body vibration of mice induces progressive degeneration of intervertebral discs associated with increased expression of Il‐1β and multiple matrix degrading enzymes. Osteoarthr Cartil. 2017;25:779‐789. doi: 10.1016/j.joca.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 148. McCann MR, Patel P, Pest MA, et al. Repeated exposure to high‐frequency low‐amplitude vibration induces degeneration of murine intervertebral discs and knee joints. Arthritis Rheumatol. 2015;67:2164‐2175. doi: 10.1002/ART.39154/ABSTRACT [DOI] [PubMed] [Google Scholar]
- 149. Belavy DL, Quittner M, Ling Y, Connell D, Rantalainen T. Cervical and thoracic intervertebral disc hydration increases with recumbency: a study in 101 healthy volunteers. Spine J. 2018;18:314‐320. doi: 10.1016/j.spinee.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 150. Belavý DL, Quittner MJ, Ridgers N, Ling Y, Connell D, Rantalainen T. Running exercise strengthens the intervertebral disc. Sci Rep. 2017;7:45975. doi: 10.1038/srep45975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ferguson SJ, Ito K, Nolte LP. Fluid flow and convective transport of solutes within the intervertebral disc. J Biomech. 2004;37:213‐221. doi: 10.1016/S0021-9290(03)00250-1 [DOI] [PubMed] [Google Scholar]
- 152. Šećerović A, Ristaniemi A, Cui S, et al. Toward the next generation of spine bioreactors: validation of an ex vivo intervertebral disc organ model and customized specimen holder for multiaxial loading. ACS Biomater Sci Eng. 2022;8:3969‐3976. doi: 10.1021/acsbiomaterials.2c00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Gullbrand SE, Peterson J, Mastropolo R, et al. Low rate loading‐induced convection enhances net transport into the intervertebral disc in vivo. Spine J. 2015;15:1028‐1033. doi: 10.1016/J.SPINEE.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 154. Mac Lean JJ, Lee CR, Alini M, Iatridis JC. The effects of short‐term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res. 2005;23:1120‐1127. doi: 10.1016/j.orthres.2005.01.020 [DOI] [PubMed] [Google Scholar]
- 155. Walsh AJL, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37:329‐337. doi: 10.1016/S0021-9290(03)00290-2 [DOI] [PubMed] [Google Scholar]
- 156. Li X‐C, Luo S‐J, Fan W, Zhou T‐L, Huang C‐M, Wang M‐S. M2 macrophage‐conditioned medium inhibits intervertebral disc degeneration in a tumor necrosis factor‐α‐rich environment. J Orthop Res. 2022;40:2488‐2501. doi: 10.1002/jor.25292 [DOI] [PubMed] [Google Scholar]
- 157. Silva AJ, Ferreira JR, Cunha C, et al. Macrophages down‐regulate gene expression of intervertebral disc degenerative markers under a pro‐inflammatory microenvironment. Front Immunol. 2019;10:1508. doi: 10.3389/fimmu.2019.01508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Walter BA, Likhitpanichkul M, Illien‐Junger S, Roughley PJ, Hecht AC, Iatridis JC. TNFα transport induced by dynamic loading alters biomechanics of intact intervertebral discs. PLoS One. 2015;10:e0118358. doi: 10.1371/journal.pone.0118358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Du J, Pfannkuche J‐J, Lang G, et al. Proinflammatory intervertebral disc cell and organ culture models induced by tumor necrosis factor alpha. JOR Spine. 2020;3:e1104. doi: 10.1002/jsp2.1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Chooi WH, Chan SCW, Gantenbein B, Chan BP. Loading‐induced heat‐shock response in bovine intervertebral disc organ culture. PLoS One. 2016;11:e0161615. doi: 10.1371/journal.pone.0161615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Gullbrand SE, Malhotra NR, Schaer TP, et al. A large animal model that recapitulates the spectrum of human intervertebral disc degeneration. Osteoarthr Cartil. 2017;25:146‐156. doi: 10.1016/j.joca.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Rustenburg CME, Snuggs JW, Emanuel KSK, et al. Modelling the catabolic environment of the moderately degenerated disc with a caprine ex vivo loaded disc culture system. Eur Cell Mater. 2020;40:21‐37. doi: 10.22203/eCM.v040a02 [DOI] [PubMed] [Google Scholar]
- 163. Lin KH, Wu Q, Leib DJ, Tang SY. A novel technique for the contrast‐enhanced microCT imaging of murine intervertebral discs. J Mech Behav Biomed Mater. 2016;63:66‐74. doi: 10.1016/j.jmbbm.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lin KH, Tang SY. The quantitative structural and compositional analyses of degenerating intervertebral discs using magnetic resonance imaging and contrast‐enhanced micro‐computed tomography. Ann Biomed Eng. 2017;45:2626‐2634. doi: 10.1007/s10439-017-1891-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Walk RE, Tang SY. In vivo contrast‐enhanced microCT for the monitoring of mouse thoracic, lumbar, and coccygeal intervertebral discs. JOR Spine. 2019;2:e1058. doi: 10.1002/jsp2.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ren W, Cui S, Alini M, et al. Noninvasive multimodal fluorescence and magnetic resonance imaging of whole‐organ intervertebral discs. Biomed Opt Express. 2021;12:3214‐3227. doi: 10.1364/BOE.421205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hay AN, Farrell K, Leeth CM, Lee K. Use of genome editing techniques to produce transgenic farm animals. Adv Exp Med Biol. 2022;1354:279‐297. doi: 10.1007/978-3-030-85686-1_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Joyce K, Sakai D, Pandit A. Preclinical models of vertebral osteomyelitis and associated infections: current models and recommendations for study design. JOR Spine. 2021;4:e1142. doi: 10.1002/JSP2.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lv X, Xu J, Jiang J, Wu P, Tan R, Wang B. Genetic animal models of scoliosis: a systematical review. Bone. 2021;152:116075. doi: 10.1016/J.BONE.2021.116075 [DOI] [PubMed] [Google Scholar]
- 170. Lyu F‐J, Cui H, Pan H, et al. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Res. 2021;9:1‐14. doi: 10.1038/s41413-020-00125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Goel SA, Varghese V, Demir T. Animal models of spinal injury for studying back pain and SCI. J Clin Orthop Trauma. 2020;11:816‐821. doi: 10.1016/J.JCOT.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Lai A, Gansau J, Gullbrand SE, et al. Development of a standardized histopathology scoring system for intervertebral disc degeneration in rat models: an initiative of the ORS spine section. JOR Spine. 2021;4:e1150. doi: 10.1002/JSP2.1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Gullbrand SE, Ashinsky BG, Lai A, et al. Development of a standardized histopathology scoring system for intervertebral disc degeneration and regeneration in rabbit models—an initiative of the ORS spine section. JOR Spine. 2021;4:1‐12. doi: 10.1002/jsp2.1147 [DOI] [PMC free article] [PubMed] [Google Scholar]


