To the Editor:
Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have shown high efficacy in the prevention of coronavirus disease (COVID-19) (1). Allergic diseases, including allergic rhinitis (AR), asthma, and atopic dermatitis, are characterized by skewed type 2 immune responses and are estimated to affect 30–50% of the population globally (2). Recently, we have reported that after two doses, patients with AR displayed an enhanced humoral immune response to inactivated SARS-CoV-2 vaccines compared with healthy control samples, which was associated with an increase in type 2 follicular helper T (TFH2) cells in patients with AR (3). Allergen immunotherapy (AIT) is an effective disease-modifying treatment for allergic diseases by inducing immune tolerance and correcting or antagonizing skewed type 2 responses (4). A significant reduction of TFH2 cells and an increase of follicular regulatory T cells (TFR) are noted in patients with AR after AIT (5, 6). Thus, it is critical and interesting to understand whether AIT will influence the efficacy of SARS-CoV-2 vaccination in allergic patients.
A prospective observational trial (ClinicalTrials: NCT05009134) was conducted to compare the immunological response to inactivated SARS-CoV-2 vaccine in patients with AR with and without AIT. Thirty-three healthy subjects, 35 patients with AR without AIT, and 23 patients with AR receiving AIT for more than 1 year were enrolled from June 10, 2021, to December 15, 2021, at Tongji Hospital. Three groups of subjects were recruited simultaneously. All subjects had never been infected with SARS-CoV-2. All patients with AR had positive skin prick tests for Dermatophagoides pteronyssinus or D. farina. AIT was performed subcutaneously with semidepot house dust mite allergen extracts (Allergopharma GmbH) (5). All subjects received inactivated SARS-CoV-2 vaccine (WIBP-CorV, Sinopharm) on Days 0 and 30. Peripheral blood was taken on Days 0, 7, 30, 37, and 60 to analyze humoral immune responses to vaccination. One participant in the control group and two participants in the AR with AIT group were excluded because of loss to follow-up. Chemiluminescent immunoassay was performed to detect neutralizing antibodies against the receptor-binding domain (RBD) of the SARS-CoV-2 spike (S)1 protein (iFlash-2019-nCoV neutralization assay kit, YHLO Biotech Co), and IgG and IgM against the SARS-CoV-2 S and nucleocapsid (N) proteins (iFlash-SARS-CoV-2 IgM/IgG antibody test kit, YHLO Biotech Co) in plasma. B- and T-cell responses in peripheral blood were assessed by flow cytometry. This study was approved by the Ethics Committee of Tongji Hospital (TJ-IRB20210610), and written informed consent was obtained from each participant.
Control, AR without AIT, and AR with AIT groups were comparable in baseline demographic characteristics, including age (median and interquartile ranges, 26 years [24–38] for the control group, 27 years [23–38] for AR without AIT group, and 29 years [25–36] for AR with AIT group) and sex (female/male, 21/11 in control group, 22/13 in AR without AIT group, and 12/9 in AR with AIT group). SARS-CoV-2 vaccination elicited robust serological responses, showing markedly increased neutralizing antibody, IgG, and IgM after vaccination in all three groups (Figure 1) (3). Patients with AR without AIT displayed higher fold changes of neutralizing antibody on Days 30, 37, and 60, IgG on Days 30, 37, and 60, and IgM on Day 60 relative to the baseline concentrations at Day 0 than those in healthy control samples (Figure 1). However, interestingly, AIT reversed serological response to the SARS-CoV-2 vaccine in patients with AR, as reflected by the comparable changes of antibodies between patients with AR with AIT and healthy control samples (all P > 0.05) (Figure 1).
Figure 1.
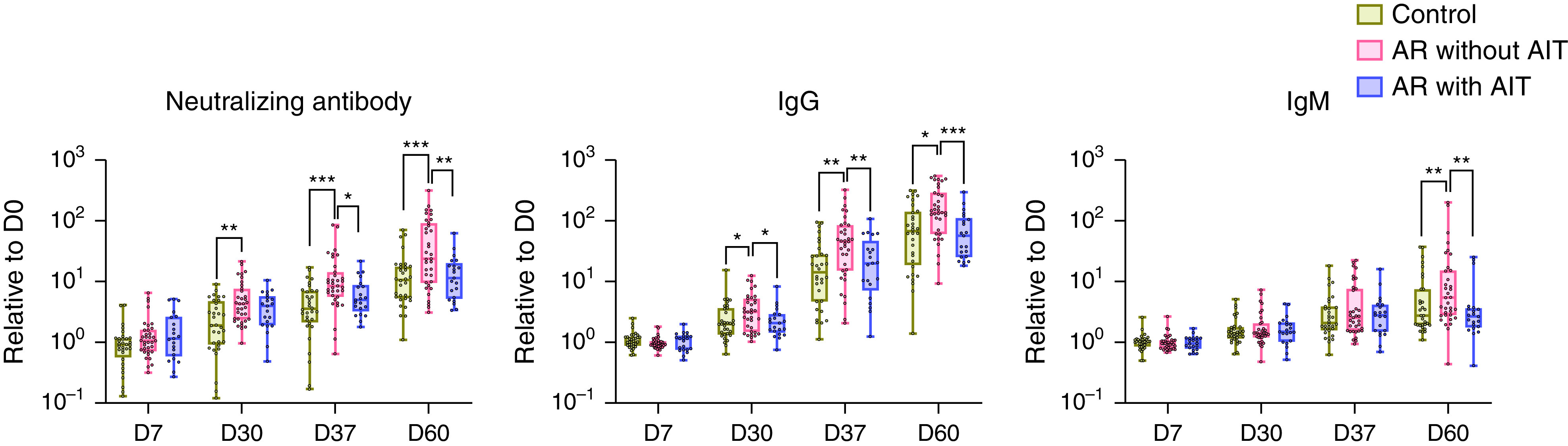
Reversed protective antibody responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine in patients with allergic rhinitis (AR) receiving allergen immunotherapy (AIT). Healthy subjects (n = 32), patients with AR without AIT (n = 35), and patients with AR receiving AIT for more than 1 year (n = 21) were enrolled and given inactivated SARS-CoV-2 vaccine on Days 0 and 30. Peripheral blood was collected on Days 0 (baseline), 7, 30, 37, and 60. Plasma-neutralizing antibodies against the receptor-binding domain of the SARS-CoV-2 S1 protein and IgG and IgM against the SARS-CoV-2 S and N proteins were measured by chemiluminescent immunoassay. Each dot represents one individual. Changes in antibody concentrations at the indicated time points are displayed as fold changes by normalizing to the baseline concentrations. Data are presented as median and interquartile range and analyzed by Mann-Whitney U test. *P < 0.05, **P < 0.01, and ***P < 0.001. D0 = Day 0; D7 = Day 7; D30 = Day 30; D37 = Day 37; D60 = Day 60.
We next assessed antigen-specific B-cell immune response to SARS-CoV-2 after vaccination (Figure 2). Higher frequencies of circulating RBD-specific memory B cells were found in patients with AR without AIT compared with those in patients with AR receiving AIT at Day 60, in which the RBD-specific memory B-cell frequencies were comparable to those in healthy control samples (P > 0.05) (Figure 2). TFH cells are critical for the generation of protective antibodies and long-lived humoral immunity after vaccination (7). We found that SARS-CoV-2 vaccination induced a robust expansion of circulating CXCR5+ICOShighPD-1high TFH cells at Day 37 (7 days after the second dose of vaccination) in all three groups. Notably, the increase of TFH cells in patients with AR without AIT as compared with healthy control samples was, again, reversed in AR with AIT group on Days 7, 37, and 60 (Figure 2), as reflected by no significant difference in changes of TFH and follicular regulatory T (TFR) cells between control and AR with AIT groups at all time points (all P > 0.05) (Figure 2).
Figure 2.
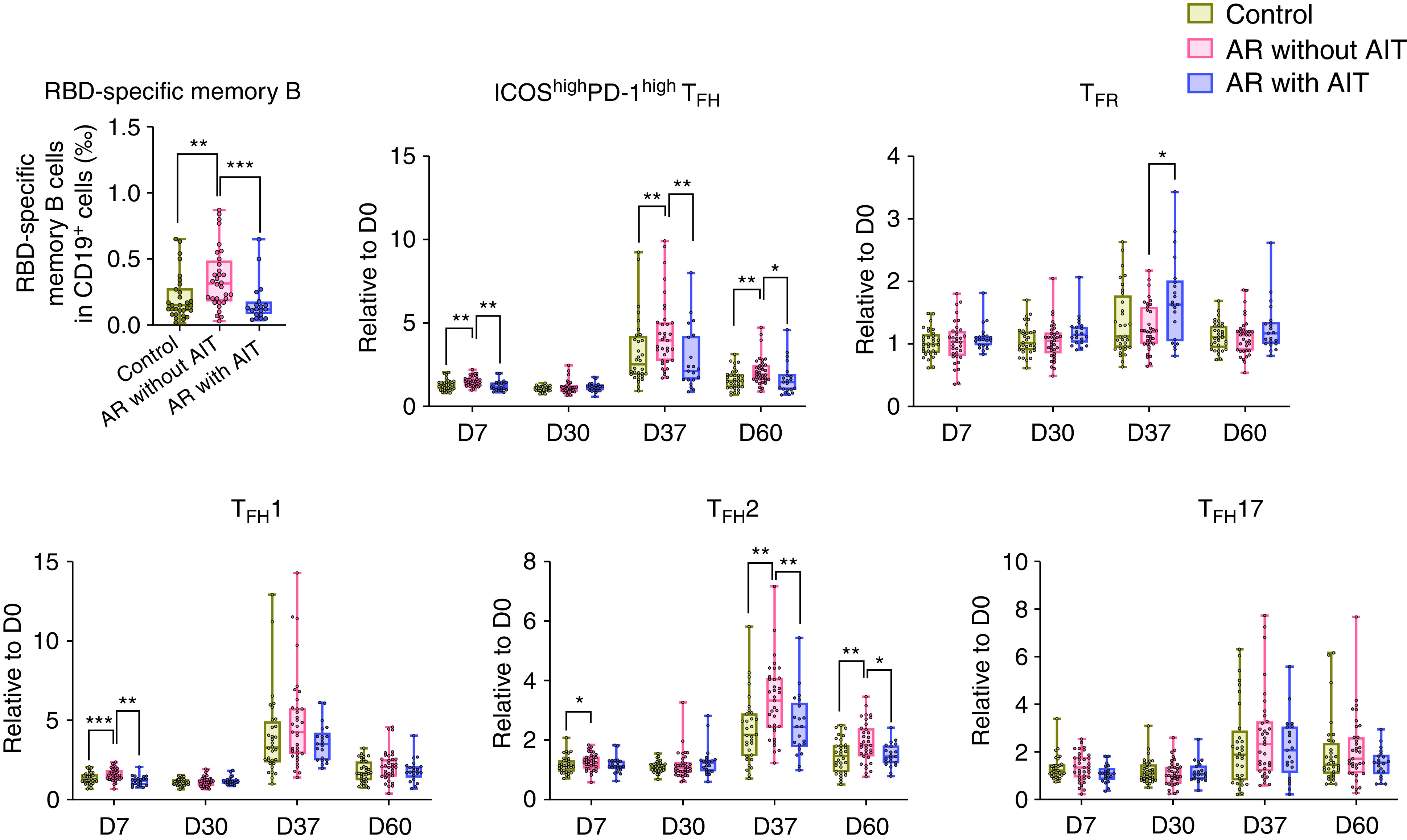
Reversed B- and T-cell responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine in patients with allergic rhinitis (AR) receiving allergen immunotherapy (AIT). Frequencies of circulating RBD-specific CD3−CD19+CD20+CD27+ memory B cells in healthy subjects (n = 32), patients with AR without AIT (n = 35), and patients with AR receiving AIT for more than 1 year (n = 21) on Day 60 were analyzed by flow cytometry. Frequencies of circulating CD4+CD45RAlowCXCR5+ICOShighPD-1highCD25−/low TFH cells, CD4+CD45RAlowCXCR5+CD25highCD127low TFR cells, and circulating ICOShighPD-1high TFH subsets (CXCR3+CCR6− TFH1, CXCR3−CCR6+ TFH17, and CXCR3−CCR6− TFH2) before and after vaccination were analyzed by flow cytometry. Each dot represents one individual. The changes of T cells in the indicated time points are displayed as fold changes by normalizing to the baseline amounts. Data are presented as median and interquartile range and analyzed by Mann-Whitney U test. *P < 0.05, **P < 0.01, and ***P < 0.001. D0 = Day 0; D7 = Day 7; D30 = Day 30; D37 = Day 37; D60 = Day 60; RBD = receptor-binding domain; TFH = follicular helper T; TFH1 = type 1 TFH; TFH17 = type 17 TFH; TFH2 = type 2 TFH; TFR = follicular regulatory T.
On the basis of the expression of chemokine receptors CXCR3 and CCR6, human circulating TFH cells can be divided into TFH1, TFH2, and TFH17 cells (7). Marked expansion of TFH1 and TFH2 cells, but not TFH17 cells, was noted on Day 37 in all three groups (Figure 2). We have recently reported that increased TFH2 cells were associated with an enhanced humoral immune response to SARS-CoV-2 vaccines in patients with AR (3). Here, we observed a significant decrease in changes of TFH2 cells in patients with AR with AIT compared with those without AIT on Days 37 and 60 relative to the baseline concentrations at Day 0, and there was no difference in the changes of TFH2 cells between control and AR with AIT group (all P > 0.05) (Figure 2). We also noted a temporary increase of change of TFH1 cells on Day 7 in patients with AR without AIT compared with patients with AR with AIT and control samples (Figure 2). Fold changes of TFH1 and TFH17 cells were comparable between control and AR with AIT group at all the time points (all P > 0.05) (Figure 2). Collectively, these results indicate that AIT may reduce the humoral immune responses to inactive SARS-CoV-2 vaccine in patients with AR; however, the humoral immune responses in patients with AR undergoing AIT are not compromised in comparison to healthy control samples. The prospective design in this study allowed us to measure serological and cellular response to SARS-CoV-2 simultaneously, both supporting the above conclusion. Several previous studies have evaluated the effect of biologic therapies targeting type 2 responses on antibody response to SARS-CoV-2 infection or vaccination. Ungar and colleagues reported lower antibody concentrations after COVID-19 infection in patients with atopic dermatitis treated with dupilumab compared with those receiving systemic or limited/no therapies (8). Similarly, Bhalla and colleagues described in a case report that a dupilumab-treated patient with asthma had blunted IgG and IgM antibodies to SARS-CoV-2 S protein and RBD after SARS-CoV-2 infection in comparison with two patients with asthma without dupilumab treatment (9). Recently, Runnstrom and colleagues have observed lower antibody concentrations after SARS-CoV-2 mRNA vaccination in patients with severe asthma or atopic dermatitis on biologics targeting type 2 responses than those in healthy adults (10). Their data, together with ours, suggest that type 2 response-modifying treatments may decrease the immune response to SARS-CoV-2 vaccination.
In summary, by extending our previous findings (3), we revealed that AIT may reverse the enhanced humoral response to SARS-CoV-2 vaccination in patients with AR to degrees comparable to healthy control samples. The change of TFH2 cells may underline these phenomena. Nevertheless, our study is limited by small sample size, and further studies with longer follow-up are required to confirm our findings. In addition, whether AIT can change immune responses to other SARS-CoV-2 vaccines, such as mRNA vaccines, deserves further investigation.
Acknowledgments
Acknowledgment
The authors thank Lin Yang, Zhao-Qin Lian, Meng-Chen Wang, and Yin Wang for blood sample collection and Li-Ming Cheng for plasma antibody detection.
Footnotes
Supported by the National Natural Science Foundation of China (NSFC) grants 82130030 and 8192010801 (Z.L.) and 82101198 (Y.Y.) and the Leader Fellowship from Australian National Health and Medical Research Council grant GNT2009554 (D.Y.).
Author Contributions: Y.Y. planned and performed most experiments with major support from A.H. and Y.-K.D. Z.-Z.W., N.W., Y.L., and H.-Y.Z. collected and processed blood samples. Z.-Z.W. and R.-F.Z. collected clinical data. Z.L., D.Y., and Y.Y. designed the study and supervised the project.
Originally Published in Press as DOI: 10.1164/rccm.202203-0608LE on June 1, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Singer BD, Chotirmall SH, Leither LM, Meldrum OW, Joudi AM, Seam N, et al. Update in COVID-19 2021 Am J Respir Crit Care Med [online ahead of print] 20 April 2022; DOI: 10.1164/rccm.202202-0277UP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yao Y, Chen CL, Yu D, Liu Z. Roles of follicular helper and regulatory T cells in allergic diseases and allergen immunotherapy. Allergy . 2021;76:456–470. doi: 10.1111/all.14639. [DOI] [PubMed] [Google Scholar]
- 3. Yao Y, Wang ZZ, Huang A, Liu Y, Wang N, Wang ZC, et al. TFH 2 cells associate with enhanced humoral immunity to SARS-CoV-2 inactivated vaccine in patients with allergic rhinitis. Clin Transl Med . 2022;12:e717. doi: 10.1002/ctm2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shamji MH, Sharif H, Layhadi JA, Zhu R, Kishore U, Renz H. Diverse immune mechanisms of allergen immunotherapy for allergic rhinitis with and without asthma. J Allergy Clin Immunol . 2022;149:791–801. doi: 10.1016/j.jaci.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 5. Yao Y, Wang ZC, Wang N, Zhou PC, Chen CL, Song J, et al. Allergen immunotherapy improves defective follicular regulatory T cells in patients with allergic rhinitis. J Allergy Clin Immunol . 2019;144:118–128. doi: 10.1016/j.jaci.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 6. Sharif H, Acharya S, Dhondalay GKR, Varricchi G, Krasner-Macleod S, Laisuan W, et al. Altered chromatin landscape in circulating T follicular helper and regulatory cells following grass pollen subcutaneous and sublingual immunotherapy. J Allergy Clin Immunol . 2021;147:663–676. doi: 10.1016/j.jaci.2020.10.035. [DOI] [PubMed] [Google Scholar]
- 7. Yao Y, Chen Z, Zhang H, Chen C, Zeng M, Yunis J, et al. Selenium-GPX4 axis protects follicular helper T cells from ferroptosis. Nat Immunol . 2021;22:1127–1139. doi: 10.1038/s41590-021-00996-0. [DOI] [PubMed] [Google Scholar]
- 8. Ungar B, Lavin L, Golant AK, Gontzes A, David E, Estrada YD, et al. The impact of dupilumab treatment on severe acute respiratory syndrome coronavirus 2-coronavirus disease 2019 antibody responses in patients with atopic dermatitis. Ann Allergy Asthma Immunol . 2022;128:734–736. doi: 10.1016/j.anai.2022.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhalla A, Mukherjee M, Radford K, Nazy I, Kjarsgaard M, Bowdish DME, et al. Dupilumab, severe asthma airway responses, and SARS-CoV-2 serology. Allergy . 2021;76:957–958. doi: 10.1111/all.14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Runnstrom MC, Morrison-Porter A, Ravindran M, Quehl H, Ramonell RP, Woodruff M, et al. Reduced COVID-19 vaccine response in patients treated with biologic therapies for asthma. Am J Respir Crit Care Med . 2022;205:1243–1245. doi: 10.1164/rccm.202111-2496LE. [DOI] [PMC free article] [PubMed] [Google Scholar]


