Keywords: butyrate, intestinal epithelium, necrotizing enterocolitis, Notch1, short-chain fatty acids
Abstract
Single immunoglobulin interleukin-1-related receptor (SIGIRR), toll-interacting protein (TOLLIP), and A20 are major inhibitors of toll-like receptor (TLR) signaling induced postnatally in the neonatal intestine. Short-chain fatty acids (SCFAs), fermentation products of indigestible carbohydrates produced by symbiotic bacteria, inhibit intestinal inflammation. Herein, we investigated the mechanisms by which SCFAs regulate SIGIRR, A20, and TOLLIP expression and mitigate experimental necrotizing enterocolitis (NEC). Butyrate induced NOTCH activation by repressing sirtuin 1 (SIRT1)-mediated deacetylation of the Notch intracellular domain (NICD) in human intestinal epithelial cells (HIECs). Overexpression of NICD induced SIGIRR, A20, and TOLLIP expression. Chromatin immunoprecipitation revealed that butyrate-induced NICD binds to the SIGIRR, A20, and TOLLIP gene promoters. Notch1-shRNA suppressed butyrate-induced SIGIRR/A20 upregulation in mouse enteroids and HIEC. Flagellin (TLR5 agonist)-induced inflammation in HIEC was inhibited by butyrate in a SIGIRR-dependent manner. Neonatal mice fed butyrate had increased NICD, A20, SIGIRR, and TOLLIP expression in the ileal epithelium. Butyrate inhibited experimental NEC-induced intestinal apoptosis, cytokine expression, and histological injury. Our data suggest that SCFAs can regulate the expression of the major negative regulators of TLR signaling in the neonatal intestine through Notch1 and ameliorate experimental NEC. Enteral SCFAs supplementation in preterm infants provides a promising bacteria-free, therapeutic option for NEC.
NEW & NOTEWORTHY Short-chain fatty acids (SCFAs), such as propionate and butyrate, metabolites produced by symbiotic gut bacteria are known to be anti-inflammatory, but the mechanisms by which they protect against NEC are not fully understood. In this study, we reveal that SCFAs regulate intestinal inflammation by inducing the key TLR and IL1R inhibitors, SIGIRR and A20, through activation of the pluripotent transcriptional factor NOTCH1. Butyrate-mediated SIGIRR and A20 induction represses experimental NEC in the neonatal intestine.
INTRODUCTION
Soon after birth, the neonatal intestine is colonized with microbiota necessitating major changes in the intestinal mucosa that facilitate adaptation to the nonsterile environment (1–4). The intestinal epithelial cells (IECs) and mucin barrier serve as the first line of cellular defense against invading pathogens. Toll-lke receptors (TLRs) are innate immune receptors that recognize conserved structural motifs in bacteria and viruses, and contribute to antimicrobial host defense and intestinal homeostasis (5, 6). The establishment of symbiotic microbiota-host TLR relationships is key to gut homeostasis, but aberrant activation of TLR signaling, notably TLR4, has been implicated in mucosal injury and inflammation underlying necrotizing enterocolitis (NEC) and other diseases (7–9). Enteral supplementation with probiotics has been shown to reenforce mucosal immune responses and protect against inflammatory diseases in human and mouse models of disease, the molecular mechanisms underlying which are not fully understood (10–13).
Short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, fermentation products of dietary fiber and human milk oligosaccharides by gut bacteria, such as Bacteroidetes and Firmicutes in the intestinal lumen are known to be anti-inflammatory (14, 15). SCFAs maintain gut homeostasis in several ways including inhibiting enteric pathogen colonization by altering bacterial adherence (16), enhancing intestinal epithelial barrier function, and regulating the host mucosal immune system to prevent pathogen invasion (15, 17). In addition, SCFAs not only serve as a primary energy source for intestinal epithelial cells but also regulate stem cell turnover in intestinal epithelial crypts (18). However, mechanisms by which SCFA regulate intestinal epithelial TLR signaling tempering aberrant TLR activation in the neonatal intestine are not fully understood yet.
Single immunoglobulin interleukin-1-related receptor (SIGIRR) is an orphan receptor that inhibits TLR signaling by competitively binding to myeloid differentiation primary response gene 88 (MYD88), the primary TLR adapter, through its TIR domain to inhibit TLR-MYD88 signal transduction (19, 20). Loss of SIGIRR function renders the intestine more vulnerable to inflammation-associated diseases, such as NEC (21, 22) and colonic tumors (23). A20, a ubiquitin-editing enzyme, also imposes repression on TLR and IL1R signaling through terminating TRAF2 and TRAF6-mediated NFκB activity (24, 25). Interestingly, A20 and SIGRR expression in the neonatal intestine is developmentally regulated and temporally associated with the colonization of symbiotic bacteria (26) and elevated SCFAs concentration (27). Expression of both A20 and SIGIRR is decreased in NEC, whereas TLR4 and downstream cytokines are elevated (28). We have recently shown that loss of SIGIRR function in intestinal epithelium results in loss of immune tolerance to mucosal bacteria and induces TLR hyperresponsiveness and spontaneous inflammation in the neonatal intestine (2). We have previously demonstrated that the administration of probiotic Lactobacillus rhamnosus (LGG) protects against intestinal TLR-mediated injury through activating A20 and SIGIRR (13). How probiotics induce expression of TLR signaling inhibitors has not been investigated yet, although it is known that LGG can produce SCFAs, such as propionate and acetate (29). Studies in humans have shown that preterm infants who are more vulnerable to NEC have lower concentrations of intestinal SCFAs when compared with full-term neonates (27, 30). We therefore hypothesized that SCFAs will suppress experimental NEC by inducing SIGIRR and A20 expression in the intestinal epithelium.
MATERIALS AND METHODS
Ethical Approval
Care of mice before and during experimental procedures was conducted in accordance with the policies at the University of Missouri-Kansas City Lab Animal Resource Center and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Protocols had prior approval from the University of Missouri-Kansas City Institutional Animal Care and Use Committee. Investigators understand the ethical principles and work described here complies with the animal ethics checklist published in the Journal.
Mouse Studies
For butyrate treatment, DOL5 C57BL/6J (B6) pups were orally administrated the 100 µL of 400 mM sodium butyrate (Sigma, Cat. No. B5887) via oral gavage twice a day for 3 days. At DOL8, the pups were euthanized for IEC isolation. For experimental NEC model, DOL5 B6 pups were randomly assigned into three groups: breastmilk fed controls (control), NEC, and NEC with enteral butyrate (NEC+ butyrate). From DOL5, seven pups were pretreated with either butyrate (100 µL of 400 mM sodium butyrate twice a day for 3 days) or vehicle as per their assigned groups. Starting at DOL8 to DOL10, pups in the NEC groups underwent a modified NEC protocol as previously described (13), and NEC + butyrate group received 100 µL 400 mM butyrate per day besides NEC induction. Mice were euthanized on DOL11.
Cell Culture
Human intestinal epithelial cell (HIEC-6) was purchased from ATCC (No. CRL-3266) and cultured as per manufacturer’s instruction. HIEC-6 is a normal cell line isolated by thermolysin treatment of a human fetal small intestine. HIEC-6 is not tumorigenic and retains its nature in passage (31). Human embryonic kidney HEK293 cells expressing TLR4 (293/TLR4, No. 293-htlr4a, Invivogen) were cultured in DMEM containing 10% FBS and antibiotic-antimycotic (Thermo Fisher). HIEC transduced by lentiviral particles expressing Notch1 shRNA, Notch2 shRNA, or control shRNA were passaged two times to eliminate the effect of lentivirus. HIECs were transfected with pcDNA3 vectors carrying wild-type NICD, NICD 14KR mutant (32) (a kind gift from Dr. Michael Potente at Max Planck Institute for Heart and Lung Research) or FLAG SIRT1(No. 1791, Addgene) using Lipofectamine 3000 according to manufacturer’s protocol. HIEC transduced by lentiviral particles expressing SIGIRR shRNA or scramble shRNA were passaged two times to eliminate the effect of lentivirus. Two days after transfection, HIECs were lysed for mRNA and protein expression analysis. Normal human colon epithelial cell line (FHC) was purchased from ATCC (no. CRL-1831) and cultured as per manufacturer’s instruction. L-WRN (CRL-3276) cell lines were obtained from American Type Culture Collection (ATCC) and cultured in DMEM with 10% FBS. L-WRN conditioned media was made as previously described and used for enteroids culture (33).
Isolation of Mouse Small Intestine Epithelial Cells
Mouse small intestine epithelial cells were isolated as previously reported (2). Briefly, 5 cm terminal ileum was flushed with cold PBS, opened longitudinally, and cut into 1-cm pieces, followed by incubation in 5 mL HBSS (Ca2+, Mg2+ free) with 2% FBS, 5 mM EDTA, and 1 mM DTT in a 37°C shaker for 30 min. Cells were collected by passing supernatant through a 100-µm cell strainer, and spinning at 300 g for 5 min. Isolated cells were confirmed positive for epithelial cell markers, E-Cadherin, and EpCAM by immunofluorescence.
Enteroids Culture
To maximize the lentivirus transduction efficiency, we used a two-dimensional (2-D) enteroids culture method (34). Crypts were isolated as previously described (35). Briefly, terminal ileum was opened longitudinally and washed with PBS. To dissociate the crypts, tissues were incubated in 10 mM EDTA in DMEM with 10% FBS on ice for 15 min, washed in PBS, and transferred into 5 mM EDTA in PBS for an additional 30 min of incubation at 4°C. Samples were then filtered out by a 70-mm cell strainer. Purified crypts were seeded on Matrigel-coated plate and enteroids were maintained in 50% L-WRN conditioned media and 50% advanced DMEM/F-12 containing 20% FBS, 1X penicillin-streptomycin, 1X l-glutamine, 1% gentamicin, 0.2% amphotericin B, 0.05 mM N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES), 10 µM Y-27632, and 50 ng/mL EGF. The isolated crypt grew on the Matrigel basement membrane and form monolayer. The enteroids were transduced with lentiviral Notch1 shRNA or control shRNA for 5 days followed by 5 mM butyrate treatment for 2 days.
Chromatin Immunoprecipitation
The chromatin immunoprecipitation (ChIP) experiment was performed as previously described (2). Briefly, HIECs were fixed with 1% formaldehyde for 10 min. The Pierce Magnetic ChIP Kit and ChIP grade Notch1 antibody (Cell Signaling, No. 3608) were used according to the manufacturer’s instructions. ChIP products were analyzed by quantitative real-time PCR. The sequences of primers were designed to target RBPJ binding sites on promoter according to the prediction of JASPAR 2018 and listed as follows: SIGIRR-f, 5′- CCTGTGTTTGCTCCTTATTTGG-3′; SIGIRR-r, 5′- AAGGGACTATGGCATCTTGTG-3′; A20-f, 5′- GGGTGAGTGTTGTTCTGATTCC-3′; A20-r, 5′- TTTGGTCCCCATCTCCATTG-3′; TOLLIP-f, 5′- GCCAGTCCACAGAGTAAAGTG-3′; TOLLIP-r, 5′- ACATATAAACAAGCGTACCCAGG-3′; HEY2-f, 5′- CGCAGGGGTTAGCAAGATTG-3′; HEY2-r, 5′- TGGTACCCCAGAGCAGC-3′.
Quantitative Real-Time PCR
Total RNA was isolated using TRIzol (Thermo Fisher). cDNA was synthesized using a cDNA reverse transcription kit (Bio-Rad). For the quantification of gene amplification, real-time PCR was performed using a ViiA7 or Quantstudio 3 (Applied Biosystems). The sequences of gene-specific primers are given in supplemental information. 18S rRNA or GAPDH was used as endogenous normalization control.
Western Blot Analysis
The Western blot analysis was performed as described previously (2). In brief, cells were washed once with ice‐cold PBS and suspended in RIPA lysis buffer (Pierce) containing a cocktail of protease inhibitors and phosphatase inhibitors. Mouse tissues in RIPA buffer containing protease and phosphatase inhibitors were homogenized by a bullet blender (Midwest Scientific). The concentration of the crude protein was measured with a bicinchoninic acid (BCA) Protein Assay Kit (Thermo Fisher). Equal amounts of cell lysates were loaded and separated by SDS‐PAGE, and gels were blotted to PVDF membranes. Membranes with blotted proteins were incubated with primary antibodies, washed, and incubated with peroxidase‐conjugated secondary antibodies. Reactive proteins were revealed with an enhanced chemiluminescence detection system and visualized on the imaging system iBright FL1000 (Thermo Fisher). Densitometry was performed using ImageJ software and changes were normalized to β-actin (ACTB) or the corresponding nonphosphorylated antibody. The primary antibodies used for Western blot are diluted at 1:1,000 and listed as follows: anti-A20 (Cell Signaling, No. 5630), anti-SIGIRR (Abcam, No. ab177937), anti-NICD (Cell Signaling, No. 4147), anti-β-actin (Sigma, No. A2228), anti-ADAM17 (GeneTex, No. GTX101358), anti-p-P65 (Cell Signaling, No. 3033), anti-P65 (Cell Signaling, No. 6956), anti-p-IKKβ [(Cell Signaling, No. 2694), anti-IKKβ (Cell Signaling, No. 2678)]. These commercial antibodies have been validated for Western blotting as shown in their respective company information pages.
Detection of Acetylated NICD
HIECs were treated for 6 h in the presence or absence of butyrate. Lysates were clarified using IP Lysis Buffer (Thermo Fisher), protease inhibitors, and phosphatase inhibitors (Sigma). The Protein A magnetic beads (Bio-Rad) were washed in lysis buffer and conjugated with the rabbit anti-NICD antibody (Cell Signaling) overnight with rotation at 4°C. After conjugation, the beads were gently washed with lysis buffer and the same concentration of the sample was added to the beads, typically 500–600 ng. The samples and conjugated beads were rotated at room temperature for 60 min, at which point the beads were washed gently with lysis buffer, reconstituted in 2× Laemmli, and boiled for 7 min. Western blotting was performed with an antibody targeting acetylated lysine (Cell Signaling) (36).
Immunostaining
Paraffin sections of the terminal ileum were cut in 4-µm thickness. Sections were deparaffinized with xylene and graded series of alcohol. Antigen retrieval was performed using citrate buffer for 20 min at 95°C. After being rinsed several with PBS, sections were blocked with Power Block Universal Blocking Reagent for 1 h and then stained overnight at 4°C with primary anti-Notch1 (LSbio, LS-C114369-100 1:100). After being washed with PBS, slides were stained with secondary antibody conjugated with Alexa488 (Invitrogen, 1:100) for 1 h at room temperature. After being treated with secondary antibodies, slides were washed several times with PBS, counterstained with 4′6′-diamidino-2-phenylindole (DAPI), and mounted with FLUORO-GEL (Electron Microscopy Science 1798510). Images were acquired using X25/oil objective with a Zeiss Inverted LSM 510 meta laser scanning confocal microscope. Red-green-blue images were assembled using ImageJ software.
Data Analysis
Data are presented as means ± SD. Error bars reflect variation between biological replicates. P < 0.05 was considered significant. For cell culture experiments, data from a minimum of three independent experiments with adequate technical replicates were used for quantification. All animal data were obtained in littermate controls. For animal experiments, a minimum of four animals were used for each experimental group. RNA quantification and PCR results had two to three technical replicates. Statistical analysis was done using GraphPad Prism 9.0 (San Diego, CA). For all data, we initially examined whether the distribution of data was Gaussian using the D'Agostino-Pearson omnibus normality test. Comparisons between two groups were made by one-sample, two-tailed Student’s t test for parametric or nonparametric data. Comparisons between three or more groups were analyzed by one-way ANOVA and post hoc Tukey tests or Student’s t test for multiple comparisons.
RESULTS
SCFAs Induce Expression of TLR Inhibitors in HIEC
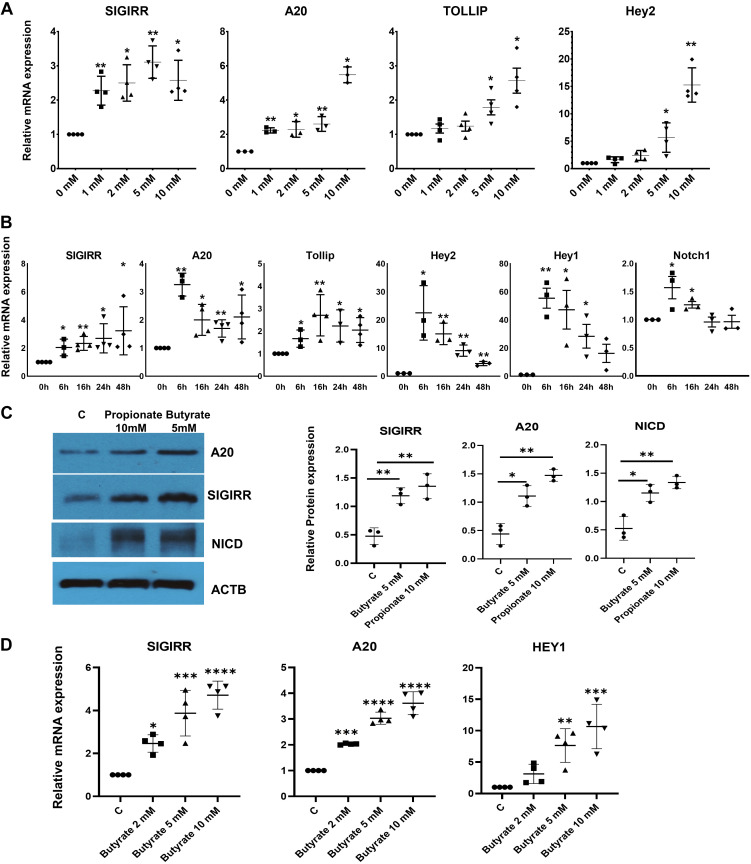
To determine whether SCFAs promote the expression of genes that repress TLR signaling in intestinal epithelium, human intestinal epithelial cells (HIECs) were treated with sodium butyrate at different doses for 48 h. Quantitative PCR (qPCR) revealed that the TLR-negative regulators, SIGIRR, A20, and toll-interacting protein (TOLLIP) were significantly elevated in a dose-dependent manner in HIEC treated with butyrate (Fig. 1A). Based on induction efficacy and estimated concentration in human intestine (27, 37), we chose to use butyrate at 5 mM for all cell culture experiments. To identify optimal treatment windows, HIECs were treated with 5 mM butyrate for varying times. Butyrate promoted A20, SIGIRR, and TOLLIP expression after 6 h and maintain the expression at a high level for at least 48 h (Fig. 1B). Next, we confirmed that butyrate could enhance A20 and SIGIRR expression at the protein level by Western blotting (Fig. 1C). Propionate, one of the common short chain fatty acids, showed comparable effect on induction of A20 and SIGIRR expression albeit at a higher concentration (Fig. 1C). This finding was confirmed in human colon epithelial cells. Treatment of fetal human colonic epithelial cells induced SIGIRR and A20 expression in a dose-dependent manner at 16 h (Fig. 1D). These data suggest that SCFAs promote TLR negative regulators expression in human intestinal epithelial cells.
Figure 1.
SCFAs enhance A20 and SIGIRR expression in association with activated Notch intracellular domain (NICD) in HIECs. A: expression of A20, SIGRR, TOLLIP, and HEY2, a Notch downstream target in HIECs treated with sodium butyrate for 48 h at different concentration as indicated, was quantified by real-time PCR. n = 4. B: gene expression was quantified by real-time PCR in HIECs treated with 5 mM butyrate for different times. n = 3. C: HIECs were treated with 5 mM butyrate or 10 mM propionate for 16 h, and cell lysates were analyzed by Western blotting using the indicated antibodies. n = 3. Densitometry quantification is shown graphically. D: gene expression was quantified by real-time PCR in colon epithelial cells treated for 16 h at indicated concentrations. n = 4. Data shown as means ± SD, *P < 0.05, **P < 0.01, ***P < 0.005. ****P < 0.001. HIEC, human intestinal epithelial cell; NICD, Notch intracellular domain; SIGIRR, single immunoglobulin interleukin-1-related receptor; TOLLIP, toll-interacting protein.
Butyrate-Mediated Expression of SIGIRR, A20, and TOLLIP is Transcriptionally Regulated by Notch Intracellular Domain and Is Modulated by Sirtuin 1
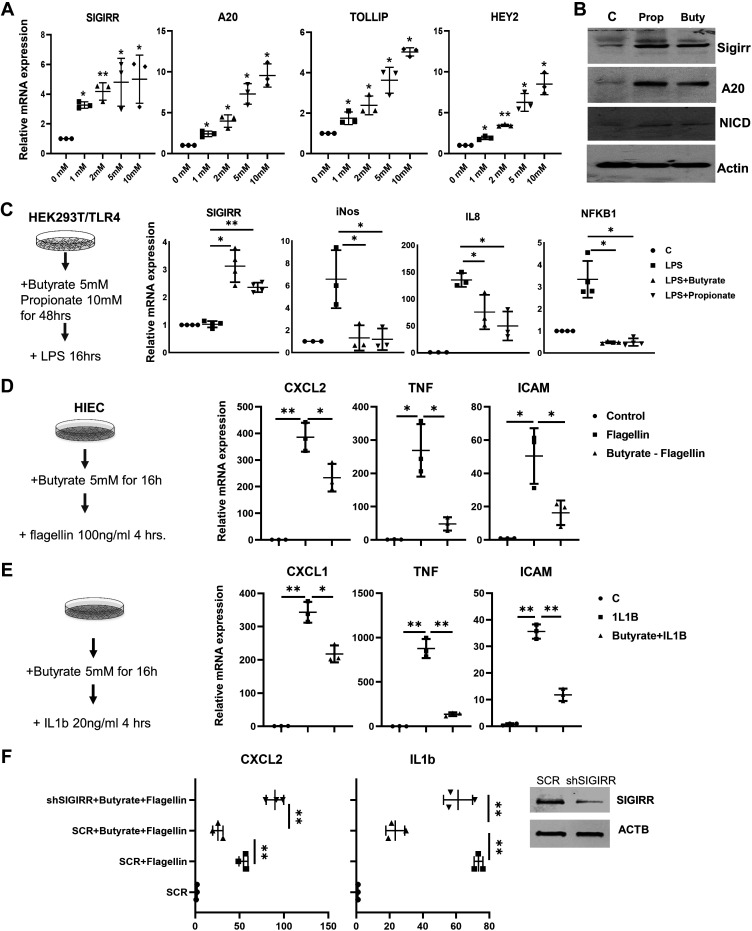
Notch1, a well-studied membrane receptor, forms a transcriptional activator complex with recombination signal binding protein for immunoglobulin kappa J region (RBPJ) through its cleavage product, NICD, is known to be involved in intestinal homeostasis (38) and inflammation regulation (39). Butyrate has been shown to be able to activate Notch1 signaling in pheochromocytoma cells(40). Therefore, we investigated whether Notch-mediated SCFA-induced SIGIRR and A20 expression in HIEC. HEY2 and HEY1, Notch downstream targets, were greatly induced by butyrate (Fig. 1, A–B and D and Fig. 4, A and B). Although NOTCH1 mRNA was marginally induced, NICD, the transcriptionally active form of Notch1 accumulated significantly in propionate or butyrate-treated cells (Fig. 1C and Fig. 4B), indicating that SCFAs activate Notch1 signaling by stabilizing NICD.
Figure 4.
SCFAs suppress TLR- and IL1R-mediated inflammation in cell culture. A: expression of A20, SIGRR, TOLLIP, and HEY2 in 293/TLR4 treated with sodium butyrate for 48 h at different concentration as indicated was quantified by real-time PCR. n = 3. B: 293/TLR4 were treated with 5 mM butyrate or 10 mM propionate for 48 h, and cell lysates were analyzed by Western blotting using the indicated antibodies. n = 3. C: SCFAs suppressed TLR4-mediated inflammation in HEK293. 293/TLR4 were stimulated with 100 ng/mL LPS, a TLR4 agonist for 16 h after 48-h treatment with 5 mM butyrate or 10 mM propionate. SIGIRR, IL8, NFκB1, and iNOS expression were analyzed by real-time PCR. n = 4. D: butyrate inhibits flagellin-induced inflammatory gene expression. HIECs were treated with 5 mM butyrate for 16 h and then were stimulated with flagellin, a TLR5 agonist. Proinflammatory cytokines and inflammatory maker expression were quantified by real-time PCR. n = 3. E: butyrate reduced IL1R-mediated inflammation. HIECs were treated with 5 mM butyrate for 16 h and then were stimulated with IL1β, an IL1R agonist. Proinflammatory cytokines and inflammatory maker expression were quantified by real-time PCR. n = 3. F: SIGIRR is involved in butyrate-mediated inflammation suppression. HIECs stably expressing lentiviral SIGIRR shRNA or scramble shRNA (SCR) were treated with 5 mM butyrate for 16 h, followed by flagellin at 100 ng/mL for 8 h. Proinflammatory cytokines expression were quantified by real-time PCR. *P < 0.05. n = 3. Data shown as means ± SD, *P < 0.05, **P < 0.01. HIEC, human intestinal epithelial cell; SCFA, short-chain fatty acid; SIGIRR, single immunoglobulin interleukin-1-related receptor; TOLLIP, toll-interacting protein; TLR, toll-like receptor.
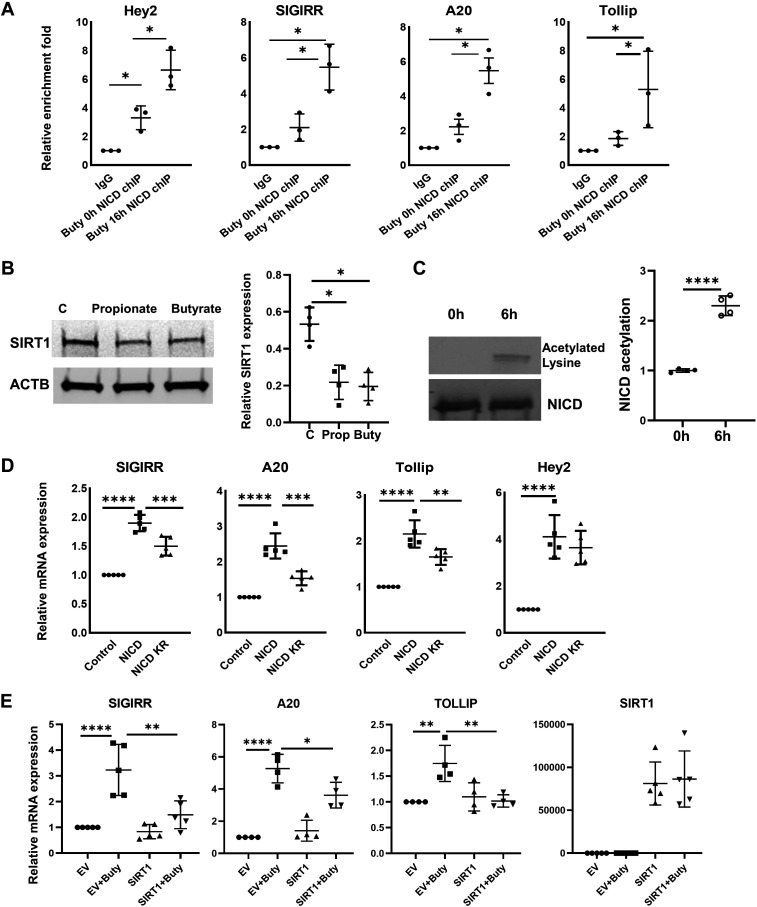
The 2-kb promoters of SIGIRR, A20, and TOLLIP were predicted to possess multiple RBPJ binding sites by JASPAR (http://jaspar.genereg.net/). The binding of NICD/RBPJ complex on promoters of A20, SIGIRR at baseline was evident by chromatin immunoprecipitation (ChIP) assay using Notch1-specific antibody followed by qPCR with primers targeting the specific binding sites (Fig. 2A). More importantly, butyrate treatment greatly enhanced the binding affinity of NICD on A20, SIGIRR, and TOLLIP promoters (Fig. 2A). SIRT1, an nicotinamide adenine dinucleotide-dependent lysine deacetylase, has been shown to deacetylate lysine residues on NICD and destabilize it (32). We therefore postulated that butyrate may inhibit SIRT1, and thereby enhance NICD accumulation. We observed that SIRT1 expression was downregulated by propionate and butyrate treatment (Fig. 2B). Acetylated NICD was significantly elevated in butyrate-treated HIECs (Fig. 2C). To determine whether Notch1 functions as a transcriptional activator for TLR negative regulators, HIECs were transfected with NICD for 48 h, and then lysates were used for gene expression analysis. NICD overexpression greatly increased SIGIRR, A20, and TOLLIP mRNA accompanied by Notch signaling target, HEY2. However, acetylation-deficient NICD mutant 14KR (32) was less effective in inducing expression of SIGIRR, A20, and TOLLIP compared with wild-type NICD in HIECs (Fig. 2D). To clarify the role of SIRT1 in butyrate-induced SIGIRR, A20, and TOLLIP expression, HIECs were transfected with SIRT1 for 48 h followed by 8 h butyrate treatment. Forced expression of SIRT1 greatly suppressed butyrate-mediated upregulation of SIGIRR, A20, and TOLLIP (Fig. 2E), suggesting that SIRT1-mediated deacetylation regulates butyrate-induced NICD. Taken together, SIGIRR, A20, and TOLLIP are SIRT1-dependent Notch downstream targets in intestinal epithelial cells after butyrate treatment.
Figure 2.
NICD transcriptionally regulates expression of TLR-negative regulator. A: ChIP-qPCR assay was performed using anti-NICD antibody to analyze the recruitment of NICD on promoters of SIGIRR, A20, and HEY2 in HIECs with 5 mM butyrate treatment, and values were quantified against IgG controls. n = 3. B: whole cell lysate of HIECs with 5 mM butyrate or 10 mM propionate was immunoblotted for SIRT1 and β-actin. Densitometry quantification is shown graphically. n = 4. C: NICD acetylation in HIECs with butyrate treatment. NICD was immunoprecipitated by NICD antibody from HIECs with 6 h of treatment of butyrate, then analyzed by Western blotting with NICD antibody and acetylated lysine antibody. Densitometry quantification is shown graphically. n = 3. D: gene expression was quantified by real-time PCR in HIECs transfected with pCDNA3 empty vector (control), pCDNA3-NICD, or pCDNA3 NICD 14KR. n = 5. E: overexpression of SIRT1 in HIEC suppress butyrate-induced expression of SIGIRR, A20, and TOLLIP. HIECs were transfected with pcDNA3-empty vector (EV) or FLAG-SIRT1(Addgene) for 48 h and then treated with butyrate at 5 mM for 8 h. Gene expression were quantified by real-time PCR. n = 4. Data shown as means ± SD, *P < 0.05, **P < 0.01, ***P < 0.005. ****P < 0.001. ChIP, chromatin immunoprecipitation; HIEC, human intestinal epithelial cell; NICD, Notch intracellular domain; SIGIRR, single immunoglobulin interleukin-1-related receptor; TOLLIP, toll-interacting protein; TLR, toll-like receptor.
Notch1 Is Required for Butyrate-Mediated SIGIRR and A20 Expression
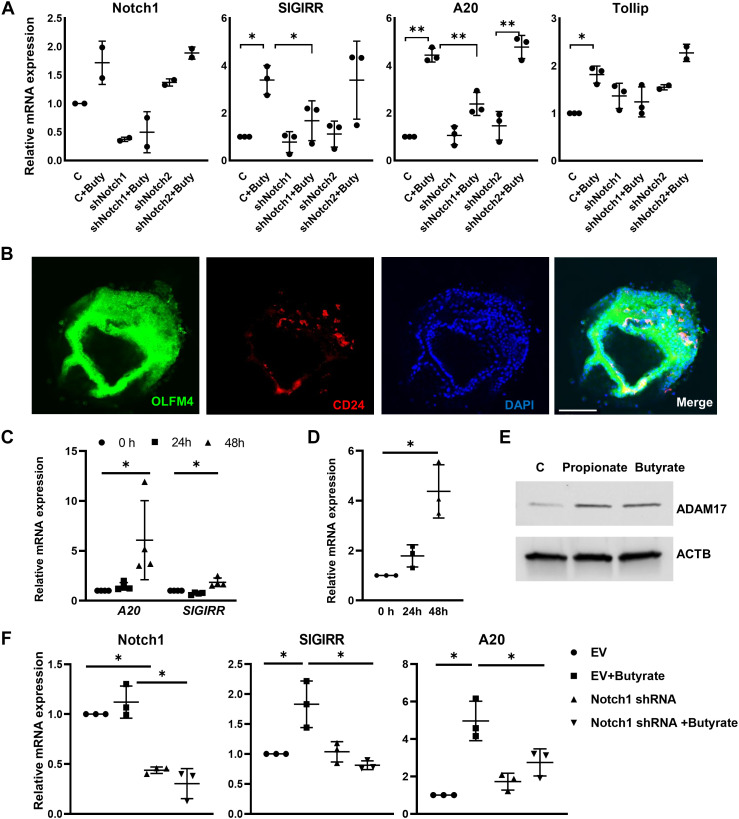
To investigate the role of Notch1 in promoting the expression of TLRs negative regulator after SCFA treatment, we used a lentiviral shRNA knockdown strategy to generate HIEC (HIECNotch1-) with >60% stable knockdown of NOTCH1 (Fig. 3A). Butyrate was able to induce A20, SIGIRR, and TOLLIP expression in control cells that were transduced with scrambled shRNA but not in NOTCH1-deficient cells (Fig. 3A). Interestingly, in HIEC when Notch2 was silenced (Supplemental Fig. S2), butyrate still induced SIGIRR and A20 expression (Fig. 3A), indicating that Notch1 is involved in butyrate inducing TLRs negative regulator expression in HIEC but not Notch2.
Figure 3.
Notch1 is required for butyrate-induced TLR-negative regulator expression in mouse enteroids and HIECs. A: HIECs stably expressing lentiviral Notch1 shRNA, Notch2 shRNA, or scramble control shRNA were treated with 5 mM butyrate for 16 h. TLR inhibitors and Notch1 expression were quantified by real-time PCR. n = 3. B: Immunofluorescent confocal images of mouse enteroids stained with antibodies against indicated proteins. n = 3. Scale bar = 50 µm. C: SIGIRR and A20 expression in mouse enteroids treated with 5 mM butyrate for 48 h were quantified by real-time PCR. *P < 0.05. n = 4. D: ADAM17 expression in enteroids with 48 h butyrate treatment was quantified by real-time PCR. *P < 0.05. n = 3. E: whole cell lysate of HIECs with 5 mM butyrate was immunoblotted for AMAM17 and β-actin. n = 3. F: enteroids transduced by or scramble shRNA or shRNA specificaly targeting Notch1 mRNA were treated with 5 mM butyrate for 48 h, then lysed for mRNA exrepssion by real-time PCR. *P < 0.05. n = 3. Data shown as means ± SD, *P < 0.05, **P < 0.01. EV, empty vector, HIEC, human intestinal epithelial cell; SIGIRR, single immunoglobulin interleukin-1-related receptor; TOLLIP, toll interacting protein.
To further prove the role of Notch1 in butyrate-induced A20 and SIGIRR expression, we deployed intestinal organoids (enteroids). We pursued this strategy as Notch-/- mice show embryonic lethality. Enteroids generated from day of life (DOL)10–11 wild-type C57BL/6 pups were immunopositive for olfactomedin 4 (OLFM4) (Fig. 3B), an intestinal stem cell marker, and showed enriched expression of the stem cell markers, such as Cd133, Lrig, Musashi, Dclk1, and Lgr5 (Supplemental Fig. S3). The enteroids were surrounded by CD24-positive Paneth cells (Fig. 3B), which may mimic the intestinal stem cell and its niche. A20 and SIGIRR expression in the enteroids were greatly induced after 48 h of butyrate treatment (Fig. 3C). In rat enteroids culture, butyrate has been reported to induce expression of a disintegrin and metalloproteinase 17 (ADAM17), a protease that initiates Notch1 proteolytic activation under ligand-independent conditions (41). In mouse enteroids, butyrate was able to induce Adam17 expression (Fig. 3D). Enhanced ADAM17 expression induced by SCFAs was confirmed in HIECs as well (Fig. 3E). Next, the requirement of Notch1 for butyrate-induced SIGIRR and A20 expression in mouse enteroids was examined. Enteroids were transduced with lentiviral Notch1 shRNA for 5 days before butyrate treatment. Similarly, butyrate failed to induce A20 SIGIRR expression in mouse enteroids with less Notch1 expression (Fig. 3F). Taken together, Notch1 regulates butyrate-mediated induction of TLRs negative regulators in human intestinal epithelial cells and neonatal mouse enteroids.
Butyrate Inhibits TLR-Mediated Inflammation in Cell Culture
The functional relevance of butyrate induction of TLR-negative regulators was first examined using HEK293/TLR4 line, which is responsive to the TLR4 ligand, lipopolysaccharide (LPS), a key component of Gram-negative bacteria implicated in NEC. Similar to HIEC, HEK293/TLR4 cells treated with butyrate or propionate expressed higher levels of TLR negative regulators in association with activated Notch1 signaling (Fig. 4, A and B, and Supplemental Fig. S1). Proinflammatory cytokines such as interleukin 8 (IL8), iNOS, and nuclear factor κ B subunit 1(NFKB1), an inflammatory response central mediator, were induced by LPS stimulation but were strongly suppressed by SCFAs pretreatment, in companion with elevated SIGIRR expression (Fig. 4C). Next, the butyrate-mediated inflammation suppression was examined in HIECs. HIECs show anergy to TLR4 stimulation due to low TLR4 expression, so we used flagellin to stimulate TLR5. Butyrate pretreatment effectively attenuated flagellin-stimulated TLR5-mediated inflammation response, evident by decreased expression of proinflammatory cytokines C-X-C motif chemokine ligand 2 (CXCL2), TNFα, and proinflammatory cellular adhesion molecule ICAM1 (Fig. 4D). Besides TLR signaling, A20 and SIGIRR are important negative regulators for IL1R signaling pathway (19, 42). Subsequently, we examined the beneficial effect of butyrate on inflammation induced by IL-1β, an agonist of IL1R signaling. Butyrate pretreatment suppressed IL-1β-induced inflammation in HIECs (Fig. 4E) (43, 44), indicating a broad benefit of SCFAs for inflammatory conditions, including those implicated in NEC. To evaluate whether induction of TLR negative regulators, specifically SIGIRR, mediated the anti-inflammatory effects of butyrate in HIEC, we used a lentiviral shRNA strategy to generate HIECs with stable knockdown of SIGIRR expression (Fig. 4F). In HIECs with reduced SIGIRR, butyrate did not suppress flagellin-induced IL1β and CXCL2 expression to the extent observed in HIEC transduced with scrambled shRNA lentivirus (Fig. 4F), indicating that induction of SIGIRR is important to butyrate-mediated inflammation suppression.
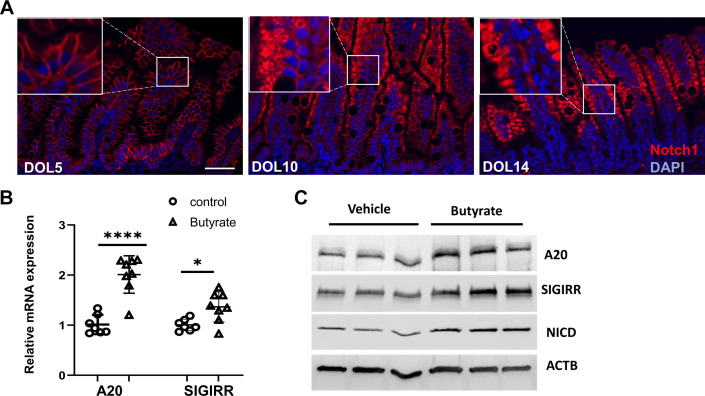
SCFAs Promotes A20 and SIGIRR Expression in the Neonatal Intestine
SCFAs enhanced Notch1-mediated expression of TLR-negative regulators in human intestinal epithelial cells and HEK239 cells. We next verified this finding in vivo using wild-type neonatal C57BL/6 (B6) mice. Our previous work had shown that SIGIRR expression gradually increased in the developing intestines (22). This induction correlated temporally with enhanced expression of NICD, as evidenced by the translocation of NOTCH1 from cell membrane to cytoplasm at DOL10 and 14 compared with DOL5 (Fig. 5A), indicating Notch1 is temporally activated in the neonatal intestine. To further prove SCFAs could induce TLR-negative regulators expression in developing intestines, mouse pups were fed with 100 µL of 400 mM butyrate twice a day via oral gavage from DOL5 for 3 days before IEC isolation for mRNA and protein expression analysis. A20 and SIGIRR expression were significantly induced in mouse IEC isolated from butyrate-treated terminal ileum compared with the littermate vehicle-treated control, in association with increased NICD expression (Fig. 5C). Taken together, our data show that butyrate enhances A20 and SIGIRR expression in the neonatal intestine.
Figure 5.
SCFAs promote A20 and SIGIRR expression in neonatal intestine. A: immunofluorescent confocal images of DOL5, 10, and 14 mouse terminal ilea stained with Notch1 antibody. n = 3 mice per time point. Scale bar = 50 µm. B: A20 and SIGIRR expression in IECs isolated from terminal ileum of DOL8 mice with 3 days butyrate oral gavage were quantified by real-time PCR. n = 6 or 7 mice/group. C: mouse IEC cell lysates were analyzed by immunoblotting with indicated antibodies. n = 3 mice/group. Data shown as means ± SD, *P < 0.05, ****P < 0.005. DOL, day of life; IEC, intestinal epithelial cell; NICD, Notch intracellular domain; SCFA, short-chain fatty acid; SIGIRR, single immunoglobulin interleukin-1-related receptor.
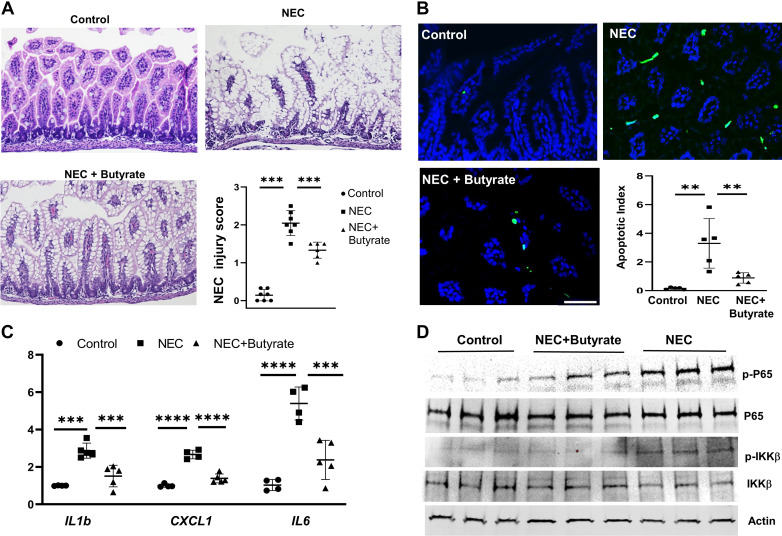
Butyrate Attenuates Experimental NEC Injury
Pretreatment with SCFAs ameliorated LPS or flagellin-stimulated inflammation in cell culture, and butyrate did induce SIGIRR and A20 expression in the neonatal intestine. We therefore examined the protective effect of butyrate-induced TLR-negative regulators in the neonatal intestine using an experimental mouse NEC model where pups were fed a combination of formula milk, enteral LPS, and timed hypoxia exposure. Histologic evaluation of the terminal ileum was performed based on pathological damage on a scale of 0 (normal) to 4 (bowel necrosis)(45). Histological score of 2 and above was considered indicative of significant injury. No morphological changes were observed in breast-fed control pups (NEC grade: 0) (Fig. 6A). In NEC pups, the epithelial layer displayed typical NEC damages, such as swelling of the epithelial cells, disrupted crypt morphology, villus sloughing and separation of submucosa/lamina propria (Fig. 6A). Cellular edema was noted prominently in NEC group (46) (Fig. 6A). After 3 days of experimental NEC procedure, five of seven neonates developed NEC for a mean NEC grade of 2.0 (Fig. 6A). Butyrate treatment significantly reduced the NEC-induced damage, such as a re-emergence of normal villi morphology and decreased epithelial disruption. The butyrate-treated NEC group has an improved average NEC injury score of 1.33. Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) assay results demonstrated that NEC-induced apoptosis in terminal ileum was improved by enteral butyrate treatment (Fig. 6B). Inflammatory cytokines, such as IL1b, CXCL1, and IL-6, were greatly repressed in enteral butyrate-treated NEC group compared with the NEC-only group (Fig. 6C). Consistent with the above results, markers of proinflammatory TLR signaling, such as phosphorylated p65 (RELA, NFκB subunit) and phosphorylated inhibitor of κ B kinase β, were elevated in NEC group but were repressed in enteral butyrate treatment group (Fig. 6D). Taken together, oral butyrate administration alleviated NEC-like intestinal injury through enhancing SIGIRR, TOLLIP, and A20 expression, and consequently suppressing TLR4-mediated inflammation in the neonatal intestine.
Figure 6.
Butyrate alleviated NEC-like intestinal injury in neonatal mice. A: hematoxylin and eosin staining of terminal ileum from littermate control (control), experimental NEC (NEC) and NEC with butyrate administration (NEC + butyrate). Quantification of intestinal injury by a validated scoring tool shows decrease in injury score following butyrate administration. n = 5–7 mice/group. B: TUNEL staining of terminal ileum of indicated group, with quantification shown graphically. n = 5 mice/group. Scale bar = 50 µm. C: real-time PCR analysis of proinflammatory cytokines expression in terminal ileum from indicated mouse group. n = 4 or 5 mice/group. D: mouse terminal ileum lysates from control group, NEC group, and NEC plus enteral butyrate administration group were analyzed by immunoblotting with indicated antibodies. n = 3 mice/group. Data shown as means ± SD, **P < 0.01, ***P < 0.005. ****P < 0.001. NEC, necrotizing enterocolitis.
DISCUSSION
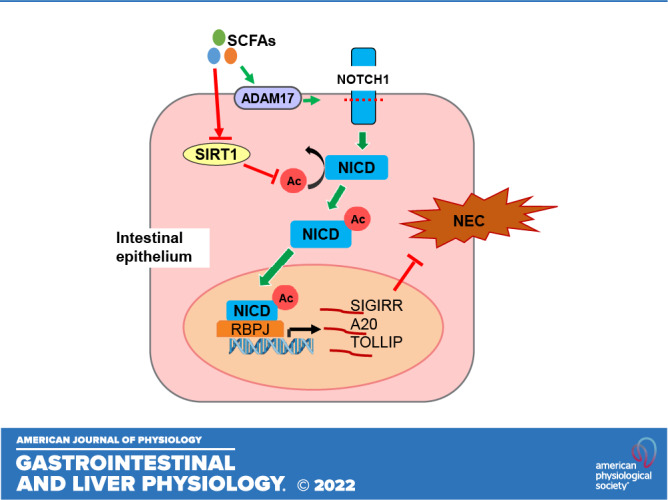
NEC is a severe intestinal inflammatory disease developed in 5% to 14% of preterm infants with a mortality of 20%–35% (47). Aberrant intestinal TLR4 activation plays a key role in NEC pathogenesis, and direct inhibition of TLR4 function has emerged as a promising method of prevention and cure for NEC (7, 22). TLR4 antagonists, C34 and C35 have shown protective effects in mouse models of NEC (8, 48). TLR-negative regulators, such as A20 and SIGIRR, are developmentally regulated with decreased expression during the NEC vulnerability window, and are also downregulated in NEC (28). Transgenic mice expressing human IL-37, a ligand of SIGIRR, are protected against NEC-like intestinal injury (49). All of the evidence indicates that the enhancement of genes that repress TLR signaling may be an efficient strategy for NEC prevention. Herein, we found that SCFAs, natural metabolites of symbiotic gut bacteria, activate SIGIRR and A20 expression through Notch, thereby repressing TLR-mediated inflammation and alleviating NEC-like intestinal injury.
In term infants, intestinal concentrations of SCFAs gradually increase with age postnatally(27). Importantly, preterm infants, who are more vulnerable to NEC, have lower SCFA concentrations when compared with term infants (30). Emerging evidence demonstrates that SCFAs play an important role in intestinal homeostasis and inflammation regulation through the inhibition of histone deacetylase (HDAC) or activating G protein-coupled receptors (GPCRs) (15, 17). In this work, we show that NOTCH1-dependent activation of SIGIRR and A20, major inhibitors of TLR signaling is important for the anti-inflammatory effects of SCFA. This mechanism appears conserved as we were able to demonstrate in human neonatal intestinal epithelial cells, HEK293 cells, neonatal mouse IEC, and mouse ex vivo enteroids. Epithelial SIGIRR and A20 are critical for intestinal postnatal immune tolerance, and we have shown that lack of SIGIRR function predisposes to NEC in humans and mouse models (2, 21, 22, 50). Similar to our results, butyrate was shown to directly suppress NFκB activity in intestinal epithelial cells and isolated lamina propria cells (51, 52). Weng et al. (53) in their study further reported that butyrate induces A20 expression and suppressed pathogen-stimulated IL8 expression in Caco-2 cells, but the in vivo relevance and molecular mechanisms were not explored. Our data expand the existing knowledge about the anti-inflammatory and intestinal homeostasis-promoting effect of SCFAs by showing that it regulates the expression of the TLR inhibitors SIGIRR, A20, and TOLLIP, important for promoting postnatal intestinal homeostasis.
Using ChIP and Notch1 RNA interference, we identified Notch1-dependent SIGIRR and A20 upregulation as a novel pathway through which butyrate promotes the anti-inflammatory response in mouse and human IEC and mouse enteroids. Notch2 silencing did not alter SCFA responsiveness. In pheochromocytoma (PC-12) cells, butyrate was shown to activate Notch1 signaling through enhancing NOTCH1 expression after 2 days of treatment (40). However, in HIECs, rapid increase of HEY1 and HEY2, Notch1 downstream targets was observed after 6-h butyrate treatment, but significant changes in NOTCH1 expression was not observed, suggesting activation of NOTCH function, rather than expression. Based on this observation, we inferred that butyrate may directly activate Notch1 transcriptional activity. We determined whether SCFAs modulate NOTCH activation through acetylation-dependent NICD stabilization. Focusing on SIRT1, a NICD deacetylase, we found that SIRT1 expression was downregulated in propionate and butyrate-treated HIECs, consistent with previous reports in colorectal cancer cells and cholangiocarcinoma cells (54, 55). Accumulation of acetylated NICD was seen in SCFA-treated HIECs indicating that SCFAs enhance NICD transcriptional activity through acetylation. Consistent with this, acetylation-deficient NICD 14KR (32) could not activate SIGIRR/A20 expression as efficiently as wild-type NICD. More importantly, forced expression of SIRT1 inhibited butyrate-mediated SIGIRR/A20 upregulation. The aforementioned results imply the regulation of SIGIRR and A20 through the SCFA-SIRT1-NICD transcriptional program. The other mechanism we examined is whether SCFAs may function through ADAM17, which can proteolytically activate Notch receptors in a ligand-independent way (41). Our data show that butyrate enhances ADAM17 expression, implying a potential role in NOTCH1 activation.
Beneficial effects of butyrate on NEC-like intestinal injury have been reported recently, such as repression of inflammatory gene expression and enhancement of intestinal barrier function (56, 57). Our results expand these findings by demonstrating that SCFAs enhance Notch1-mediated A20 and SIGRR expression to repress the inflammatory response in normal human intestinal epithelial cells and experimental NEC. We confirmed that Notch1, and not Notch2, is required for butyrate-induced SIGIRR and A20 expression. Although Notch signaling is a major modulator of intestinal innate and adaptive immune responses, it has been reported to have conflicting pro- and anti-inflammatory effects in disease models (58, 59). Sodhi et al. (60) in their study reported that TLR4-mediated Notch signaling in intestinal epithelium contributes to NEC as inhibition of Notch by DBZ, a γ-secretase inhibitor reduced severity of NEC. These results contrast our findings. However, γ-secretase inhibition has several effects, independent of Notch including IL1R1 and E-cadherin (61). Inhibition of γ-secretase could also abolish IL1β-induced damage to epithelial cell junction (62, 63), attenuating intestinal injury independent of NOTCH. Notably, IL-1β is elevated in human and experimental NEC, so inhibiting IL1R1, its receptor, is likely to suppress NEC-induced intestinal injury. Loss of function of presenilin, catalytic components of γ-secretase in the brain, results in neurodegeneration and inflammation (64). Nascimento et al. (65) in their study recently reported that JLK6, a γ-secretase inhibitor that does not interfere with Notch signaling decreases inflammatory response in patients with cutaneous leishmaniasis, which also confirms the anti-inflammatory role of γ-secretase inhibitor. In human induced pluripotent stem cells derived endothelial cell, Notch haploinsufficiency suppressed anti-inflammatory gene networks activated by shear stress, suggesting that Notch represses inflammation. Given that neonatal milk diet also generates shear stress in the intestinal lumen, Notch1 may exert a similar anti-inflammatory effect under the regulation of SCFAs, which is indicated by temporal expression of Notch1, and SIGIRR in mouse neonatal intestinal epithelium. The embryonic lethality of Notch1−/− mice precluded us from more definitive studies. The relevance of Notch1 signaling and SCFAs in neonatal intestine immune tolerance to postnatal bacterial colonization needs to be studied using newer transgenic mouse models.
Maintenance of neonatal tolerance to gut bacteria through blockage of TLR4 signaling in intestinal epithelium is critical for postnatal gut adaptation, and prevention of NEC. SIGIRR and A20 play an important role in maintaining intestinal immune tolerance and preventing neonatal intestinal inflammation (2, 21, 22, 50, 66). In this study, we identify a new mechanism by which SCFAs regulate intestinal inflammation by inducing the key TLR and IL1R inhibitors, SIGIRR and A20, through transcriptional activation of the pluripotent transcriptional factor NOTCH1. These studies are translationally relevant as supplementation of the preterm human infant diet with oral SCFAs such as butyrate may offer a promising bacteria-free therapy to prevent NEC in neonates.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.21365664.
GRANTS
This study was supported by a research grant from the National Institutes of Health (NIH) (1R01 DK117296-A3; to V.S.), and Children’s Mercy Research Institute funds (to V.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.Y. and V.S. conceived and designed research; W.Y., A.V., H.L.M., and M.M. performed experiments; W.Y., A.V., H.L.M., and V.S. analyzed data; W.Y. and V.S. interpreted results of experiments; W.Y. prepared figures; W.Y. and V.S. drafted manuscript; W.Y., A.V., S.U., and V.S. edited and revised manuscript; W.Y., A.V., S.U., and V.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Michael Potente at the Max Planck Institute for Heart and Lung Research for providing NICD plasmid.
REFERENCES
- 1. Chassin C, Kocur M, Pott J, Duerr CU, Gütle D, Lotz M, Hornef MW. MiR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe 8: 358–368, 2010. doi: 10.1016/j.chom.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 2. Yu W, Haque I, Venkatraman A, Menden HL, Mabry SM, Roy BC, Xia S, Prokop JW, Umar S, Geurts AM, Sampath V. SIGIRR mutation in human necrotizing enterocolitis (NEC) disrupts STAT3-dependent microRNA expression in neonatal gut. Cell Mol Gastroenterol Hepatol 13: 425–440, 2022. doi: 10.1016/J.JCMGH.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dominguez-Bello MG, Godoy-Vitorino F, Knight R, Blaser MJ. Role of the microbiome in human development. Gut 68: 1108–1114, 2019. doi: 10.1136/GUTJNL-2018-317503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cuna A, Morowitz MJ, Ahmed I, Umar S, Sampath V. Dynamics of the preterm gut microbiome in health and disease. Am J Physiol Gastrointest Liver Physiol 320: G411–G419, 2021. doi: 10.1152/ajpgi.00399.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10: 131–144 , 2010. [Erratum in Nat Rev Immunol 10: 215, 2010]. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 6. Caballero S, Pamer EG. Microbiota-mediated inflammation and antimicrobial defense in the intestine. Annu Rev Immunol 33: 227–256, 2015. doi: 10.1146/annurev-immunol-032713-120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 179: 4808–4820, 2007. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 8. Hackam DJ, Sodhi CP. Toll-like receptor-mediated intestinal inflammatory imbalance in the pathogenesis of necrotizing enterocolitis. Cell Mol Gastroenterol Hepatol 6: 229–238.e1, 2018. doi: 10.1016/j.jcmgh.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neal MD, Sodhi CP, Dyer M, Craig BT, Good M, Jia H, Yazji I, Afrazi A, Richardson WM, Beer-Stolz D, Ma C, Prindle T, Grant Z, Branca MF, Ozolek J, Hackam DJ. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J Immunol 190: 3541–3551, 2013. doi: 10.4049/jimmunol.1202264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453: 620–625, 2008. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 11. Tahara Y, Yamazaki M, Sukigara H, Motohashi H, Sasaki H, Miyakawa H, Haraguchi A, Ikeda Y, Fukuda S, Shibata S. Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci Rep 8: 1–12, 2018. doi: 10.1038/s41598-018-19836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singer JR, Blosser EG, Zindl CL, Silberger DJ, Conlan S, Laufer VA, DiToro D, Deming C, Kumar R, Morrow CD, Segre JA, Gray MJ, Randolph DA, Weaver CT. Preventing dysbiosis of the neonatal mouse intestinal microbiome protects against late-onset sepsis. Nat Med 25: 1772–1782, 2019. doi: 10.1038/s41591-019-0640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuna A, Yu W, Menden HL, Feng L, Srinivasan P, Chavez-Bueno S, Ahmed I, Umar S, Sampath V. NEC-like intestinal injury is ameliorated by Lactobacillus rhamnosus GG in parallel with SIGIRR and A20 induction in neonatal mice. Pediatr Res 88: 546–555, 2020. doi: 10.1038/s41390-020-0797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients 3: 858–876, 2011. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parada Venegas D, De La Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Corrigendum: short chain fatty acids (SCFAs) mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10: 1486, 2019. doi: 10.3389/fimmu.2019.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, De los Reyes-Gavilán CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 7: 185, 2016. doi: 10.3389/FMICB.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gasaly N, de Vos P, Hermoso MA. Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front Immunol 12: 658354, 2021. doi: 10.3389/FIMMU.2021.658354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165: 1708–1720, 2016. [Erratum in Cell 167: 1137, 2016]. doi: 10.1016/j.cell.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, a negative regulator of toll-like receptor - interleukin 1 receptor signaling. Nat Immunol 4: 920–927, 2003. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 20. Garlanda C, Anders HJ, Mantovani A. TIR8/SIGIRR: an IL-1R/TLR family member with regulatory functions in inflammation and T cell polarization. Trends Immunol 30: 439–446, 2009. doi: 10.1016/j.it.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 21. Sampath V, Menden H, Helbling D, Li K, Gastonguay A, Ramchandran R, Dimmock DP. SIGIRR genetic variants in premature infants with necrotizing enterocolitis. Pediatrics 135: e1530–e1534, 2015. doi: 10.1542/peds.2014-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fawley J, Cuna A, Menden HL, McElroy S, Umar S, Welak SR, Gourlay DM, Li X, Sampath V. Single-immunoglobulin interleukin-1-related receptor regulates vulnerability to TLR4-mediated necrotizing enterocolitis in a mouse model. Pediatr Res 83: 164–174, 2018. doi: 10.1038/pr.2017.211. [DOI] [PubMed] [Google Scholar]
- 23. Zhao J, Bulek K, Gulen MF, Zepp JA, Karagkounis G, Martin BN, Zhou H, Yu M, Liu X, Huang E, Fox PL, Kalady MF, Markowitz SD, Li X. Human colon tumors express a dominant-negative form of SIGIRR that promotes inflammation and colitis-associated colon cancer in mice. Gastroenterology 149: 1860–1871.e8, 2015. doi: 10.1053/J.GASTRO.2015.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song HY, Rothe M, Goeddel DV. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-κB activation. Proc Natl Acad Sci USA 93: 6721–6725, 1996. doi: 10.1073/PNAS.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol 5: 1052–1060, 2004. [Erratum in Nat Immunol 6: 114, 2005]. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 26. Nagpal R, Tsuji H, Takahashi T, Nomoto K, Kawashima K, Nagata S, Yamashiro Y. Ontogenesis of the gut microbiota composition in healthy, full-term, vaginally born and breast-fed infants over the first 3 years of life: a quantitative bird’s-eye view. Front Microbiol 8: 1388, 2017. doi: 10.3389/fmicb.2017.01388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsukuda N, Yahagi K, Hara T, Watanabe Y, Matsumoto H, Mori H, Higashi K, Tsuji H, Matsumoto S, Kurokawa K, Matsuki T. Key bacterial taxa and metabolic pathways affecting gut short-chain fatty acid profiles in early life. ISME J 15: 2574–2590, 2021. doi: 10.1038/s41396-021-00937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, Llanos A, Claud EC, Walker WA. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One 6: e17776, 2011. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact 16: 79, 2017. doi: 10.1186/S12934-017-0691-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arboleya S, Binetti A, Salazar N, Fernández N, Solís G, Hernández-Barranco A, Margolles A, de los Reyes-Gavilán CG, Gueimonde M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol Ecol 79: 763–772, 2012. doi: 10.1111/J.1574-6941.2011.01261.X. [DOI] [PubMed] [Google Scholar]
- 31. Perreault N, Beaulieu JF. Use of the dissociating enzyme thermolysin to generate viable human normal intestinal epithelial cell cultures. Exp Cell Res 224: 354–364, 1996. doi: 10.1006/excr.1996.0145. [DOI] [PubMed] [Google Scholar]
- 32. Guarani V, Deflorian G, Franco CA, Krüger M, Phng LK, Bentley K, Toussaint L, Dequiedt F, Mostoslavsky R, Schmidt MHH, Zimmermann B, Brandes RP, Mione M, Westphal CH, Braun T, Zeiher AM, Gerhardt H, Dimmeler S, Potente M. Acetylation-dependent regulation of endothelial Notch signalling by the SIRT1 deacetylase. Nature 473: 234–238, 2011. doi: 10.1038/nature09917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc 8: 2471–2482, 2013. doi: 10.1038/NPROT.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Braverman J, Yilmaz ÖH. From 3D organoids back to 2D enteroids. Dev Cell 44: 533–534, 2018. doi: 10.1016/J.DEVCEL.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 35. Lanik WE, Xu L, Luke CJ, Hu EZ, Agrawal P, Liu VS, Kumar R, Bolock AM, Ma C, Good M. Breast milk enhances growth of enteroids: an ex vivo model of cell proliferation. J Vis Exp 56921, 2018. doi: 10.3791/56921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xia S, Yu W, Menden H, Younger ST, Sampath V. FOXC2 autoregulates its expression in the pulmonary endothelium after endotoxin stimulation in a histone acetylation-dependent manner. Front Cell Dev Biol 9: 657662, 2021. doi: 10.3389/fcell.2021.657662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cummings JH, Pomare EW, Branch HWJ, Naylor CPE, MacFarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28: 1221–1227, 1987. doi: 10.1136/GUT.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noah TK, Shroyer NF. Notch in the intestine: regulation of homeostasis and pathogenesis. Annu Rev Physiol 75: 263–288, 2013. doi: 10.1146/annurev-physiol-030212-183741. [DOI] [PubMed] [Google Scholar]
- 39. Shang Y, Smith S, Hu X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell 7: 159–174, 2016. doi: 10.1007/S13238-016-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cayo MA, Cayo AK, Jarjour SM, Chen H. Sodium butyrate activates Notch1 signaling, reduces tumor markers, and induces cell cycle arrest and apoptosis in pheochromocytoma. Am J Transl Res 1: 178–183, 2009. [PMC free article] [PubMed] [Google Scholar]
- 41. Bozkulak EC, Weinmaster G. Selective use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol Cell Biol 29: 5679–5695, 2009. doi: 10.1128/MCB.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Catrysse L, Vereecke L, Beyaert R, van Loo G. A20 in inflammation and autoimmunity. Trends Immunol 35: 22–31, 2014. doi: 10.1016/J.IT.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 43. Zheng N, Gao Y, Zhu W, Meng D, Walker WA. Short chain fatty acids produced by colonizing intestinal commensal bacterial interaction with expressed breast milk are anti-inflammatory in human immature enterocytes. PLoS One 15: e0229283, 2020. doi: 10.1371/journal.pone.0229283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gao Y, Davis B, Zhu W, Zheng N, Meng D, Walker WA. Short-chain fatty acid butyrate, a breast milk metabolite, enhances immature intestinal barrier function genes in response to inflammation in vitro and in vivo. Am J Physiol Gastrointest Liver Physiol 320: G521–G530, 2021. doi: 10.1152/AJPGI.00279.2020/ASSET/IMAGES/MEDIUM/GI-00279-2020R01.PNG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res 92: 71–77, 2000. doi: 10.1006/jsre.2000.5877. [DOI] [PubMed] [Google Scholar]
- 46. Tanner SM, Berryhill TF, Ellenburg JL, Jilling T, Cleveland DS, Lorenz RG, Martin CA. Pathogenesis of necrotizing enterocolitis: modeling the innate immune response. Am J Pathol 185: 4–16, 2015. doi: 10.1016/J.AJPATH.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med . 364: 255–264, 2011. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Neal MD, Jia H, Eyer B, Good M, Guerriero CJ, Sodhi CP, Afrazi A, Prindle T Jr, Ma C, Branca M, Ozolek J, Brodsky JL, Wipf P, Hackam DJ. Discovery and validation of a new class of small molecule toll-like receptor 4 (TLR4) inhibitors. PLoS One 8: e65779, 2013. doi: 10.1371/JOURNAL.PONE.0065779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cho SX, Rudloff I, Lao JC, Pang MA, Goldberg R, Bui CB, McLean CA, Stock M, Klassert TE, Slevogt H, Mangan NE, Cheng W, Fischer D, Gfroerer S, Sandhu MK, Ngo D, Bujotzek A, Lariviere L, Schumacher F, Tiefenthaler G, Beker F, Collins C, Kamlin COF, König K, Malhotra A, Tan K, Theda C, Veldman A, Ellisdon AM, Whisstock JC, Berger PJ, Nold-Petry CA, Nold MF. Characterization of the pathoimmunology of necrotizing enterocolitis reveals novel therapeutic opportunities. Nat Commun 11: 1–19, 2020. doi: 10.1038/s41467-020-19400-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang J, Ouyang Y, Guner Y, Ford HR, Grishin AV. Ubiquitin-editing enzyme A20 promotes tolerance to lipopolysaccharide in enterocytes. J Immunol 183: 1384–1392, 2009. doi: 10.4049/jimmunol.0803987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Segain JP, Raingeard de la Blétière D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottière HM. Butyrate inhibits inflammatory responses through NFκB inhibition: implications for Crohn’s disease. Gut 47: 397–403, 2000. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology 118: 724–734, 2000. doi: 10.1016/S0016-5085(00)70142-9. [DOI] [PubMed] [Google Scholar]
- 53. Weng M, Walker WA, Sanderson IR. Butyrate regulates the expression of pathogen-triggered IL-8 in intestinal epithelia. Pediatr Res 62: 542–546, 2007. doi: 10.1203/PDR.0B013E318155A422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cao M, Zhang Z, Han S, Lu X. Butyrate inhibits the proliferation and induces the apoptosis of colorectal cancer HCT116 cells via the deactivation of mTOR/S6K1 signaling mediated partly by SIRT1 downregulation. Mol Med Rep 49: 3941–3947, 2019. doi: 10.3892/MMR.2019.10002/HTML. [DOI] [PubMed] [Google Scholar]
- 55. Pant K, Richard S, Gradilone SA. Short-chain fatty acid butyrate induces cilia formation and potentiates the effects of HDAC6 inhibitors in cholangiocarcinoma cells. Front Cell Dev Biol 9: 809382, 2021. doi: 10.3389/FCELL.2021.809382/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu J, Zhu H, Li B, Lee C, Alganabi M, Zheng S, Pierro A. Beneficial effects of butyrate in intestinal injury. J Pediatr Surg 55: 1088–1093, 2020. doi: 10.1016/J.JPEDSURG.2020.02.036. [DOI] [PubMed] [Google Scholar]
- 57. Sun Q, Ji Y-C, Wang Z-L, She X, He Y, Ai Q, Li L-Q. Sodium butyrate alleviates intestinal inflammation in mice with necrotizing enterocolitis. Mediators Inflamm 2021: 6259381, 2021. doi: 10.1155/2021/6259381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Radtke F, MacDonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol 13: 427–437, 2013. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- 59. Christopoulos PF, Gjølberg TT, Krüger S, Haraldsen G, Andersen JT, Sundlisæter E. Targeting the notch signaling pathway in chronic inflammatory diseases. Front Immunol 12: 668207, 2021. doi: 10.3389/FIMMU.2021.668207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, Prindle T Jr, Russo AM, Afrazi A, Good M, Brower-Sinning R, Firek B, Morowitz MJ, Ozolek JA, Gittes GK, Billiar TR, Hackam DJ. Intestinal epithelial toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143: 708–718.e5, 2012. doi: 10.1053/J.GASTRO.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haapasalo A, Kovacs DM. The many substrates of presenilin/γ-secretase. J Alzheimers Dis 25: 3–28, 2011. doi: 10.3233/JAD-2011-101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Elzinga BM, Twomey C, Powell JC, Harte F, McCarthy JV. Interleukin-1 receptor type 1 is a substrate for gamma-secretase-dependent regulated intramembrane proteolysis. J Biol Chem 284: 1394–1409, 2009. doi: 10.1074/JBC.M803108200. [DOI] [PubMed] [Google Scholar]
- 63. Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J 21: 1948–1956, 2002. doi: 10.1093/EMBOJ/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Saura CA. Presenilin/γ-secretase and inflammation. Front Aging Neurosci 2: 16, 2010. doi: 10.3389/FNAGI.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nascimento MT, Franca M, Carvalho AM, Amorim CF, Peixoto F, Beiting D, Scott P, Carvalho EM, Carvalho LP. Inhibition of gamma-secretase activity without interfering in Notch signalling decreases inflammatory response in patients with cutaneous leishmaniasis. 10: 1219–1226, 2021. doi: 10.1080/22221751.2021.1932608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vereecke L, Vieira-Silva S, Billiet T, van Es JH, Mc Guire C, Slowicka K, Sze M, van den Born M, De Hertogh G, Clevers H, Raes J, Rutgeerts P, Vermeire S, Beyaert R, van Loo G. A20 controls intestinal homeostasis through cell-specific activities. Nat Commun 5: 5103, 2014. doi: 10.1038/ncomms6103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.21365664.