Keywords: cancer, COVID-19, extracellular vesicles, proteoglycans, SARS-CoV-2
Abstract
As structural components of the glycocalyx, heparan sulfate proteoglycans (HSPGs) are involved in multiple pathophysiological processes at the apex of cell signaling cascades, and as endocytosis receptors for particle structures, such as lipoproteins, extracellular vesicles, and enveloped viruses, including SARS-CoV-2. Given their diversity and complex biogenesis regulation, HSPGs remain understudied. Here we compile some of the latest studies focusing on HSPGs as internalizing receptors of extracellular vesicles (“endogenous virus”) and SARS-CoV-2 lipid-enclosed particles and highlight similarities in their biophysical and structural characteristics. Specifically, the similarities in their biogenesis, size, and lipid composition may explain a common dependence on HSPGs for efficient cell-surface attachment and uptake. We further discuss the relative complexity of extracellular vesicle composition and the viral mechanisms that evolve towards increased infectivity that complicate therapeutic strategies addressing blockade of their uptake.
PROTEOGLYCAN STRUCTURE AND FUNCTIONAL DIVERSITY
Proteoglycans (PGs) are highly complex macromolecules constituted by a core protein covalently decorated with sulfated glycosaminoglycan (GAG) chains of diverse length and composition. PGs are key components of the extracellular matrix (ECM) in virtually all mammalian tissues (1) and have important functions in numerous physiological processes, including embryonic development (2, 3) as well as in pathophysiological contexts, such as atherosclerosis, infectious disease, and cancer (4–7). Even if the PG core proteins can participate in protein-protein and protein-glycan interactions (8), the highly sulfated, polyanionic GAG chains have an overarching role as binding partners for a plethora of bioactive compounds, spanning from morphogens and growth factors to pathogens and lipid particles (9). There are four different subgroups of GAGs in mammals based on their disaccharide composition, including 1) heparin/heparan sulfate (Hep/HS), 2) chondroitin/dermatan sulfate (CS/DS), 3) keratan sulfate (KS), and 4) hyaluronic acid or hyaluronan (HA). The GAGs consist of repeating disaccharide units of a hexosamine (glucosamine or galactosamine) and either an uronic acid (glucoronic acid or iduronic acid) or galactose. Their synthesis is fine-tuned by more than 20 glucosyltransferases, O- and N-sulfotransferases, and epimerases in the ER and Golgi (10), as well as intra- and extracellular sulfatases (11). PG complexity also comes from the fact that the central dogma of molecular biology does not apply to their biosynthesis; from one protein coding gene, different PGs can arise depending on their GAG substitution and sulfation pattern.
Plasma membrane associated PGs include transmembrane PGs [members of the syndecan (SDC) family, and other transmembrane proteins such as CSPG4, CD44, carbonic anhydrase 9 (CA9), among others] and glycosylphosphatidylinositol (GPI)-anchored PGs (glypican family), which together with other glycoconjugates such as glycolipids and glycoproteins form the pericellular matrix or glycocalyx. Extracellular GAGs and PGs, including HA and versican, are major components of the ECM. Overall, pericellular and extracellular PGs largely influence the cell’s availability to stimuli and nutrients from the extracellular milieu and regulate cell processes such as cell adhesion and migration. Furthermore, PGs cannot be described in solitude since their end function is greatly impacted by the environment, i.e., the extracellular electrostatic charge distribution, which may influence ligand binding kinetics; or the availability of partner receptors for signal transduction. One example of this is the well-established HSPG-FGFR symbiosis (12–14), where FGF is presented to its receptor via interaction with GAG chains of cell-surface PGs (15). Moreover, GAG modifications may occur in other proteins outside of bona fide PG core proteins giving rise to so-called part-time PGs, influencing functional and biological properties. As an example of this, previous studies from our laboratory have described the occurrence of GAG substitution of CA9 in glioma cells, which influences its submembrane localization and endocytic profile with implications for antibody-drug conjugate delivery (16). Another recent example is HS sulfatase-2 (Sulf-2) that was shown to carry a CS/DS chain at the catalytic domain, thereby fine-tuning Sulf-2 activity in vitro and in vivo (17).
PGs AS SCAVENGING RECEPTORS
Beside their role as structural components of the ECM and as coreceptors of signal transduction, PGs take a central role as facilitators of endocytic uptake, i.e., by ligand binding and presentation to relevant uptake receptors, but also as sole mediators of internalization (18). Our laboratory found the first solid evidence for HSPGs as sole uptake receptors using an array of single-chain-fragment-variable (scFv) antibodies directed to different HS epitopes, which colocalized with endosomal markers (19). Anti-HS antibody uptake was somewhat epitope specific, with a preference for 2-O-sulfation, whereas members of both glypican and SDC families were involved (20).
Functionally relevant polybasic ligands that enter cells via PGs include polyamines (21), cell-penetrating peptides (22), and tau and α-synuclein protein aggregates (23, 24). Another well-studied example is the clearance of lipoproteins (specifically low-density lipoproteins, LDL) from circulating blood by the liver, which is mediated by LDL apolipoprotein binding to SDC-1 HS chains and presentation to LDL receptor. This was shown in vivo as mice deficient in hepatocyte HSPG accumulated remnant LDL in the circulation (25–27).
In addition, intracellular pathogens depend on traversing the glycocalyx and plasma membrane for infection. Various pathogens, including bacteria (28) and viruses such as the herpes virus simplex, HIV (29, 30), and coronavirus, such as the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) (31) depend on HSPG for efficient cell entry.
Interestingly, PGs may conversely serve inhibitory functions on phagocytosis of big particles (32). The negatively charged glycocalyx can inhibit phagocytosis in synovial macrophages by mechanisms including steric hindrance and repulsion from constitutive receptors at the surface of the phagocyte (33). Polyanionic GAG chains and other membrane O- and N-glycans may represent obstructions to the engagement of other phagocytic receptors at the cell surface, which may explain why macrophages mostly internalize positively charged particles (34).
PGs IN EV UPTAKE BY CANCER CELLS
Together with cell-to-cell signaling via soluble factors, extracellular vesicle (EV)-mediated communication has been established in the last decades as a key process in different pathological conditions, with special relevance in cancer biology. The secretion of EVs is a common mechanism for all cell types and EVs comprise different classes of lipid bilayer-enclosed vesicles, with varying biogenesis pathways, cargo enrichment, and biophysical properties (35). Major EV classes include microvesicles, which arise after plasma membrane budding, and exosomes that are derived from endolysosomal compartments and hence are enriched in endosomal markers (35); and apoptotic bodies that are released by cells dying through apoptosis. EVs were initially described to mediate cellular waste product clearance of potentially harmful intracellular metabolites or misfolded proteins (36, 37). Importantly, further development of EV research has established EVs as key players in cell homeostasis due to their extraordinary signaling transduction potential, mediated by EV luminal and membrane-associated molecules. EV-mediated signal transduction occurs via EV protein-cell surface interaction or via EV internalization, leading to the transfer of luminal and membrane EV contents, i.e., nucleic acids, proteins, and lipids. Alterations in the EV pathway have been reported in numerous pathologies, including in cancer. Tumor cells exhibit augmented EV secretion (38, 39) and internalization (40), conferring promalignant responses in recipient cells (malignant and nonmalignant stromal cells) by the transfer of oncoproteins, enzymes, and metabolites (40, 41). In addition, EVs may remodel the tumor microenvironment by enzymatic processing of the surrounding matrix for enhanced cell invasion (42, 43). Owing to the extensive contribution of EVs in different steps of cancer disease progression, it appears imperative to further investigate the mechanisms and specific molecules participating in EV-cell interactions, with the intention of finding novel therapeutic targets.
Several, different cell-surface receptors have been proposed as mediators of EV internalization, likely owing to the vast array of proteins exposed at the EV surface. These include integrins (44) and phosphatidylserine receptors such as Tim4 (45). Work by our group showed that EVs depend on docking to HSPGs at the cell surface for efficient binding and uptake, whereas CSPGs seemed not to trigger EV internalization. Furthermore, PG members of both glypican and SDC families could mediate EV uptake (46). Therefore, the relative expression of PG core proteins or different EV receptors on target cells may dictate EV tropism and uptake efficiency in various cell types. Importantly, despite the identification of receptors involved in EV uptake, the ligand partners on the surface of EVs remain ill defined. This is partly due to the complexity of EV composition, which depends on the signaling, mutational, and metabolic status of the secreting cancer cell. Hypoxic glioma tumor cells were shown to secrete EV populations carrying a hypoxia signature that elicited proangiogenic signaling in recipient endothelial cells. Similarly, EV tropism and uptake magnitude are likely dictated by changes in EV surface proteome composition, for example, integrins (44), tetraspanins (47), or heparin-binding proteins as well as differential biogenesis mechanisms, i.e., small EVs (from the endolysosomal system) versus large EVs (from the plasma membrane). Conceivably, multiple membrane-associated EV components sequentially contribute to cell-surface docking and uptake in a cooperative manner, where facilitating receptors (HSPGs) could present EVs to cell-type-specific endocytic receptors. Adding on to this complexity, several groups have suggested a “sandwich” model for EV uptake. According to this model, EV-membrane-associated glycoconjugates, such as PGs and glycoproteins bind intermediate proteins during dispersion or circulation, which in turn trigger the binding to cell receptors for uptake (48) (Fig. 1).
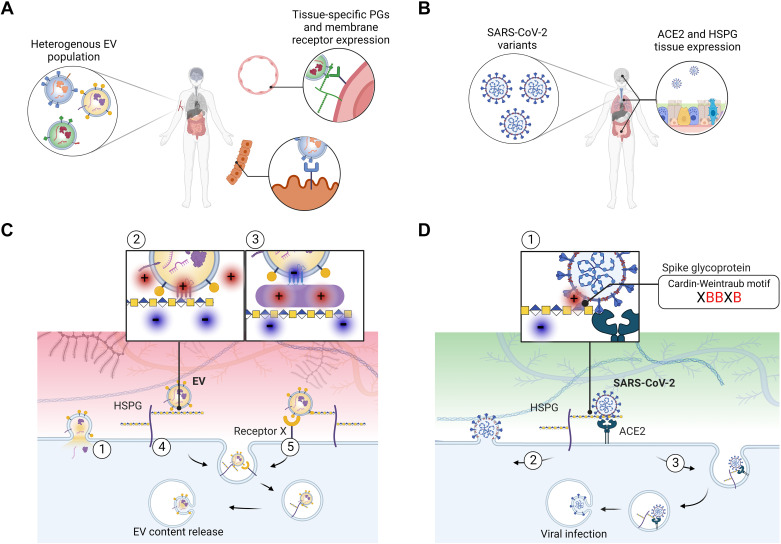
Figure 1.
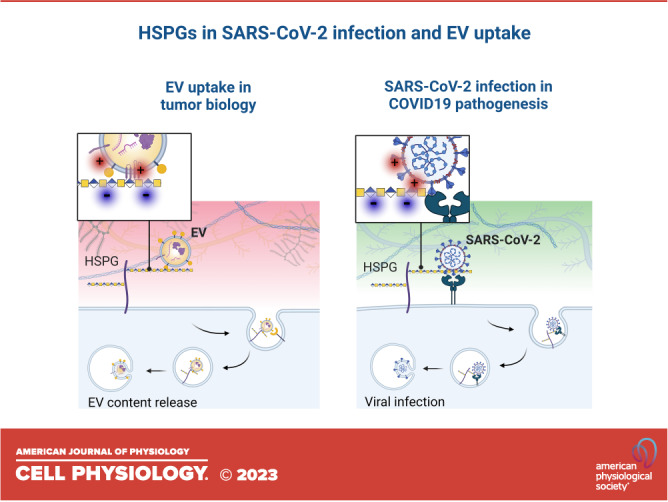
Heparan sulfate (HS) as a portal of entry for EVs and SARS-CoV-2 viral particles. A: EVs carry membrane-associated proteins whose relative abundance highly depends on the status of the secreting cell, leading to heterogeneous populations of EVs in tissues and in circulation. The expression levels of proteoglycans and other cell membrane receptors at the target tissue, together with specific EV-ligand proteins likely dictate EV tropism. B: SARS-CoV-2 particles are relatively less heterogeneous than EVs in their molecular composition; however SARS-CoV-2 variants differ in the efficiency of infection. ACE2 and HSPG tissue expression influence SARS-CoV-2 infection. C: mechanistically, EV transfer to recipient cells may occur via 1) direct fusion of lipid membranes or via an endocytic route following attachment to membrane receptors. Heparan sulfate proteoglycans (HSPGs) can 2) bind to polybasic domains of membrane EV proteins or 3) proteins associated to the EV surface by electrostatic interactions. After initial attachment, 4) HSPGs may trigger internalization or 5) facilitate EV uptake by other membrane receptors. After internalization, EV contents may be recycled or released in the recipient cell. D: similarly, SARS-CoV-2 uptake depends on initial binding of the spike protein to HSPGs through Cardin-Weintraub motifs within the S1-S2 region (where X is a hydrophobic amino acid and B denotes a positively charged (red) basic residue). Further, 1) binding to ACE2 facilitates 2) viral fusion with the cell membrane or 3) receptor-mediated endocytosis, leading to viral infection. EVs, extracellular vesicles.
The resemblance of EV and enveloped virus characteristics, their common dependence on HSPGs for efficient binding and uptake by target cells, and the possibility of an evolutionary conserved system of EV-virus codependence was previously discussed (49). The relatively well-defined repertoire of proteins exposed to enveloped viruses has enabled the research community to identify their GAG-binding receptors. However, as indicated above, EVs display a much more abundant and diverse surface proteome, harboring several putative, HS-binding domains that are likely to interact during HSPG-mediated uptake (50) (Fig. 1). Below, we will discuss what could be learned from recent SARS-CoV-2-HSPG studies in the context of EV uptake mechanisms.
HSPG-MEDIATED EV AND SARS-COV-2 UPTAKE
The COVID-19 pandemic has caused a severe impact on global health and the world economy. Due to the high mutational rate of the virus, new variants arise with increased virulence, and its prevention and treatment remain a challenge worldwide. SARS-CoV-2 binding to cell receptors and subsequent infection depend on the viral spike protein, a glycosylated trimer protruding from the viral lipid envelope (51). Hoffman et al. (52) were among the first to identify angiotensin-converting enzyme (ACE2) as a key cellular receptor for SARS-CoV-2, as well as the importance of the serine protease TMPRSS2 for spike protein priming. It was then demonstrated that the spike protein first binds to negatively charged GAGs on the cell surface, which is followed by docking to ACE2, providing the virus an entry point into the cell (31, 51, 53). According to our current understanding, HS interaction with the receptor binding domain (RBD) favors the shift to an “open” spike conformation, i.e., the receptor-accessible state, and establishes the cooperation between HS and ACE2 receptor for efficient virus entry (31). The ACE2 dependence for efficient viral entry has, however, been questioned. It is important to note that the expression level of ACE2 may be rather low in human airway epithelial cells and lung tissue (54, 55). Furthermore, the correlation between SARS-Cov-2 organ/cell tropism and ACE2 distribution remains unclear, opening the possibility of additional receptors for viral entry (56). Puray-Chavez et al. showed that the H522 lung adenocarcinoma cell line, which does not express detectable levels of ACE2 or TMPRSS2, exhibited efficient SARS-CoV-2 replication. The authors underline the dependence on HSPGs for SARS-CoV-2 infection, possibly alone or in combination with alternative receptors (57). Following cellular attachment, the virus can enter either via an endosomal pathway or by direct fusion with the cellular membrane (58). To what extent HSPG docking directs the virus for endocytic versus direct entry remains to be investigated.
The importance of HSPGs in both lung physiology and pathophysiology is well established (59). However, the detailed characterization of HSPG expression in the lung is technically challenging and there is still conflicting data concerning the expression patterns as these alter with different sample preparations and staining methods (51). Could the differential expression of HSPGs across specific cell types, inside as well as outside the respiratory tract, explain the virus tissue tropism? Several HSPG core proteins, including SDCs, are expressed on lung epithelium, and specifically SDC-1 and 4 have been pointed out as important attachment receptors for SARS-CoV-2 (53). Furthermore, SDC4, which shows the highest expression in the lung among the SDC family members, was shown to mediate SARS-CoV-2 uptake more efficiently than other SDCs (60). Most cells, however, express more than one type of HSPG. To better understand the tissue tropism of SARS-CoV-2, the question of whether the spike protein has a preference for specific HS epitopes or sulfation motifs should be further explored.
SARS-CoV-2 surface spike protein sequence analysis revealed well-known GAG-binding domains, resembling the so-called Cardin-Weintraub motifs, residing within the spike protein S1-S2 region (61). These consensus amino acid sequences, such as “XBBXB” and “XBBBXXBX,” where X is a hydrophobic amino acid and B denotes a basic residue (including arginine, lysine, and histidine) are well known to be of key importance in protein-GAG interactions (61) (Fig. 1). Interestingly, recent work underscored the importance of GAG-protein electrostatic interactions for viral binding, increasing infectivity of SARS-CoV-2 mutants. The increased transmission of virus variants of concern, including delta and omicron, has been associated with mutations of the spike protein leading to the gain of basic amino acids and increased positive surface charge in the receptor binding domain (51, 62). Furthermore, kinetic and structural analysis of the spike protein and heparin interaction, using surface plasmin resonance analysis, showed a much higher association rate of the omicron variant compared with the wild type and delta variants. The interactions were shown to be pH sensitive, highlighting the importance of the gain of charged amino acid residues at the omicron spike protein-HS interface (63).
Some of the insights and gained knowledge from studies on SARS-CoV-2 cell entry mechanisms may have more general relevance in our understanding of EV-HSPG interactions. Oncogenetic events initially drive malignant development, and great efforts have been made in the collection of tumor samples for genetic sequencing and analysis. However, the importance of the changes conferred by these mutations at the protein level during tumor evolution deserves more attention. One base pair change may lead to several possible amino acid substitutions, and although different types of cancers have unique properties, they may share some substitution patterns. Several groups have recently analyzed amino acid signatures across a variety of cancers. Interestingly, the basic amino acid arginine was pointed out to be the most mutated amino acid in cancer cells (64). A comprehensive, systematic analysis of the missense, silent insertion and deletion mutations at the protein level in cancer genes of 41 different cancer types showed that arginine is highly mutated, enriched both in driver and passenger substitutions. However, also arginine to histidine substitutions (Arg > His) were enriched in driver mutations compared with passenger mutations (65). Another study using genome-wide mutational spectra analysis identified arginine as the most favorable amino acid alteration in 17 out of the 23 analyzed cancer types (66). In a tumor-normal tissue paired mutation database, consisting of almost 7,000 samples across 29 cancer types, a subset of mutation signatures was shown to be dominated by either Arg > His substitutions or glutamate to lysine (Glu > Lys) (67). Furthermore, analyses of amino acid substitutions caused by mutations in around 2,000 protein-coding genes showed a gain of cysteine, histidine, and tryptophan by single nucleotide substitutions at the expense of a net loss of arginine (68).
Taken together, in analogy with evolutionary selected SARS-CoV-2 spike protein variants, there seems to be a preference for charge-shifting mutations in a broad range of tumors. Regardless of whether these amino acid changes are surface-exposed or intraluminal, they have the potential to alter protein conformation and hence HSPG interactions and biological functions.
Although the functional effects conferred by these mutations are still to be explored, the Arg > His substitution is of special interest in the context of the tumor microenvironment. The complex and adaptive system of the tumor microenvironment, including hypoxia and acidosis, fosters the selection of prometastatic subpopulations in highly heterogeneous tumors (69). Under conditions of tumor acidosis, or low extracellular pH (pHe), which occurs concomitantly with increased intracellular pH (pHi) (70), the Arg > His alteration has been shown to enhance pH sensing capacity of cancer cells. In other words, swapping a positively charged amino acid (Arg) for a titratable amino acid (His) may enhance adaptive, oncogenic signaling and limit tumor suppression in a pH-dependent manner (71).
In the context of EV-HSPG interplay in tumor paracrine signaling, a gain of basic amino acids and increased positive surface charge of GAG-binding EV surface protein domains could be of special relevance. Such alterations could generate cancer cell-derived EVs with relatively higher affinity for HSPGs on target cells, resulting in more efficient paracrine transfer of the EV message, especially in the acidic tumor microenvironment (Fig. 2). Thus, whether heparin-binding motifs of membrane protein extracellular domains preferentially harbor amino acid mutations associated with net charge changes is worth further investigations. One potential avenue for such studies is a recently developed method for unbiased mapping of the tumor surfaceome, i.e., proteins integrated with the plasma membrane (72). Using similar methods for the exploration of the EV surfaceome combined with tumor cell whole genome sequencing should bring new insights into the enrichment of mutant surface proteins in EVs. If so, this would point at the possibility to profile the mutant surfaceome landscape noninvasively by an EV liquid biopsy.
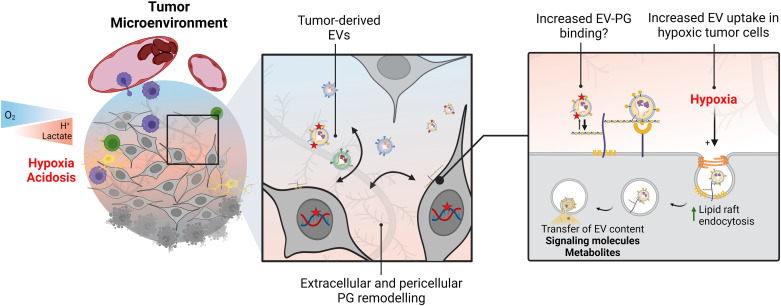
Figure 2.
Tumor stress adaptation involves changes in HSPG-mediated extracellular vesicle (EV) uptake. Aggressive solid tumors are characterized by areas of low oxygen levels, or hypoxia, which is often accompanied by metabolic rewiring leading to low extracellular pH (acidosis). Cell-to-cell communication in the tumor microenvironment includes exchange of extracellular vesicles (EVs), which may carry cancer-enriched, or cancer-specific proteins (red star). Certain amino acid modifications may favor binding to PGs through electrostatic interactions. Moreover, changes in proteoglycan (PG) expression in the stressed tumor niche will likely impact PG-dependent EV uptake by cancer cells. Importantly, under hypoxic conditions, increased PG-dependent EV uptake leads to metabolite transfer and lipid accumulation in recipient cells, following a lipid-raft dependent endocytic pathway. HSPG, heparan sulfate proteoglycans.
PG REMODELING IN STRESSED TISSUES
PG remodeling is well documented in various pathologies including tissue fibrosis (73, 74), lung inflammation (75), and cancer (76, 77), contributing to different aspects of disease pathogenesis such as growth factor signaling and ECM remodeling (78, 79). Importantly, a preferential GAG substitution by CS and alterations in CS sulfation patterns have been observed in numerous solid tumors including glioblastoma (GBM), breast, prostate, and renal cancer and were associated with increased tumor aggressiveness, cancer cell proliferation, and poor prognosis (76, 80–83). Furthermore, induction of DS epimerase (DSE), the enzyme that converts CS into DS, is common in several tumor types (84, 85). Although CS seems to play a fundamental role in cancer development in many tumor entities, the biological role is not yet determined. However, recently it was shown that CS is an essential component in androgen receptor-resistant prostate cancer, required for both growth and survival of aggressive castrate-resistant prostate cancer. The authors suggest a targeting strategy based on either synthetic GalNac sugar mimetics or enzymatic degradation of CS (86).
The exogenous cues and cellular mechanisms that mediate changes in PG expression are not characterized in detail. In cancer, tumor microenvironment conditions, including hypoxia and acidosis are of key importance in PG remodeling. For instance, hypoxia-induced HA associates with higher proliferation, migration, and immune evasion in GBM (87), and favors stem cell-like properties (88), possibly by the interaction with its receptor, the putative stem cell marker CD44 (89). In addition, changes in PG core protein expression have been studied as biomarkers for various tumor types, including in GBM, where SDC-4 was proposed as a marker of therapy response (90). Work from our group showed that the levels of another member of the SDC family, SDC-1, in circulating EVs could discriminate between GBM and low-grade gliomas (WHO grade II) (91). However, although PG remodeling has been well documented in cancer, the potential of PG-targeting therapeutic strategies is underexplored.
Others have shown that acute responses to environmental acidosis that largely overlaps with hypoxic areas facilitate EV transfer through direct membrane fusion (92). Similarly, LDL, i.e., lipid particles that depend on HPSGs for efficient uptake and whose physicochemical properties resemble those of EVs (93), are more efficiently internalized by cancer cells after short-term (2–24 h) exposure to hypoxia or acidosis (94). Moreover, work from our laboratory investigated the effects of acute hypoxia on EV uptake by glioma cells, finding increased EV uptake levels in hypoxic cells (40). Mechanistically, EV uptake in hypoxia was mediated by cell surface HSPGs, and HSPG inhibition resulted in decreased EV uptake and reversal of the hypoxic induction. Notably, basal anti-HS antibody uptake was also increased by hypoxia, indicating an overall upregulation of HSPG internalization in stressed glioma cells. Hypoxic cell EV uptake occurred mostly in a lipid-raft-dependent manner, and it was shown that internalized EVs colocalize with the major cell-surface HSPG SDC1 as well as the lipid raft endocytosis marker cholera toxin subunit B (CtxB). These data point at a specific involvement of a nonclassical endocytosis pathway in hypoxia-driven EV scavenging. Even though the mechanisms that regulate HSPG internalization are not fully understood, HSPG receptor clustering at the plasma membrane is necessary for endocytic vesicle formation in cholesterol rich lipid raft membrane microdomains. Therefore, it may be speculated that metabolic stress conferred by hypoxia modulates plasma membrane cholesterol recruitment and distribution, further leading to differential sorting and clustering of HSPGs at the cell surface (Fig. 2).
Notably, most studies on lipoprotein and EV particle uptake have focused on acute responses to hypoxia and acidosis, and long-term effects of tumor stress factors may differ, influenced by, for example, changes in expression levels of PG core proteins and GAG modifying enzymes. Thus, the dynamics of PG remodeling and PG-mediated scavenging during the evolution of the tumor microenvironment is an open field for further investigations that also should include stress-induced alterations of ECM-intercalated PGs involved in ligand sequestration.
OPEN QUESTIONS AND FUTURE DIRECTIONS
Several questions regarding PG remodeling and function in cancer and viral pathogenesis remain unresolved. What are the cell intrinsic (e.g., oncogenetic events, signaling, metabolic status) and extrinsic (e.g., environmental stress, matrix interactions) cues that regulate PG remodeling? PG core protein expression and cell surface HS/CS abundance are not altered by short-term hypoxia in glioma cells (40, 94). However, in the context of infectious inflammation, such as in COVID-19, studies have demonstrated vast remodeling of PGs, in particular pointing at the CSPG versican (95). How do changes in GAG composition and sulfation pattern affect ligand interaction, signaling, and uptake? Most importantly, how do these changes translate functionally? It is conceivable that changes in GAG composition and charge distribution can lead to altered ligand distribution and availability to cells. As a relevant example, enzymatic CS digestion improved temozolomide penetrance into GBM tumors and increased cancer cell killing in in vivo models (96). However, from these studies, it cannot be ruled out that cell-surface CS eradication contributes to enhanced cell toxicity through additional, sensitizing mechanisms.
The abnormal transfer, not only of EVs and lipoproteins, but also of other extracellular nutrients in the acidic tumor niche may result in the alteration of vital functions related to stress adaptation and enhanced metastatic potency. It is hypothesized that stress-induced recruitment of EVs through the HSPG uptake route represents a new mechanism of cancer cell adaptation. How similar stress conditions in the inflammatory context of SARS-COV-2 infection modulate HSPG expression, structure, and internalization, and the consequences for viral infectivity, deserve further attention. Other avenues for future investigations include the finding of ACE2 as well as HSPG expression on the surface of EVs (46, 97). This raises the possibility that EVs act as decoys for viral transmission. Another possibility is EV-mediated transfer and incorporation of ACE2 and HSPG in the plasma membrane of recipient cells, resulting in increased capacity for viral uptake and susceptibility for infection. Finally, the potential competitive effect of EVs engineered to overexpress ACE2 and HSPG may be further exploited for therapeutic development.
GRANTS
This work was supported by grants (to M.B.) from the Swedish Cancer Fund CAN 20 0745 PjF; the Swedish Research Council VR-MH 2018-02562; the Swedish Childhood Cancer Foundation PR2020-0129; the Fru Berta Kamprad Foundation; the Sjöberg Foundation; the Skåne University Hospital Donation Funds; the governmental funding of clinical research within the National Health Services, ALF; and a generous donation by Viveca Jeppsson.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.C.-M. prepared figures; M.C.-M., A.B.-R., and M.B. drafted manuscript; M.C.-M., A.B.-R., and M.B. edited and revised manuscript; M.C.-M., A.B.-R., and M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
All figures including the graphical abstract were created with BioRender and published with permission.
This article is part of the special collection “Deciphering the Role of Proteoglycans and Glycosaminoglycans in Health and Disease.” Liliana Schaefer, MD, served as Guest Editor of this collection.
REFERENCES
- 1. Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol 42: 11–55, 2015. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Merry CL, Bullock SL, Swan DC, Backen AC, Lyon M, Beddington RS, Wilson VA, Gallagher JT. The molecular phenotype of heparan sulfate in the Hs2st-/- mutant mouse. J Biol Chem 276: 35429–35434, 2001. doi: 10.1074/jbc.M100379200. [DOI] [PubMed] [Google Scholar]
- 3. Johnson CE, Crawford BE, Stavridis M, Ten Dam G, Wat AL, Rushton G, Ward CM, Wilson V, van Kuppevelt TH, Esko JD, Smith A, Gallagher JT, Merry CLR. Essential alterations of heparan sulfate during the differentiation of embryonic stem cells to Sox1-enhanced green fluorescent protein-expressing neural progenitor cells. Stem Cells 25: 1913–1923, 2007. [Erratum in Stem Cells 25: 2389, 2007]. doi: 10.1634/stemcells.2006-0445. [DOI] [PubMed] [Google Scholar]
- 4. Thota LNR, Chignalia AZ. The role of the glypican and syndecan families of heparan sulfate proteoglycans in cardiovascular function and disease. Am J Physiol Cell Physiol 323: C1052–C1060, 2022. doi: 10.1152/ajpcell.00018.2022. [DOI] [PubMed] [Google Scholar]
- 5. Koo A, Dewey CFJ, García-Cardeña G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am J Physiol Cell Physiol 304: C137–C146, 2013. doi: 10.1152/ajpcell.00187.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aquino RS, Teng YH, Park PW. Glycobiology of syndecan-1 in bacterial infections. Biochem Soc Trans 46: 371–377, 2018. doi: 10.1042/BST20170395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deb G, Cicala A, Papadas A, Asimakopoulos F. Matrix proteoglycans in tumor inflammation and immunity. Am J Physiol Cell Physiol 323: C678–C693, 2022. doi: 10.1152/ajpcell.00023.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heremans A, De Cock B, Cassiman JJ, Van den Berghe H, David G. The core protein of the matrix-associated heparan sulfate proteoglycan binds to fibronectin. J Biol Chem 265: 8716–8724, 1990. doi: 10.1016/S0021-9258(19)38948-3. [DOI] [PubMed] [Google Scholar]
- 9. Xu D, Esko JD. Demystifying heparan sulfate-protein interactions. Annu Rev Biochem 83: 129–157, 2014. doi: 10.1146/annurev-biochem-060713-035314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prydz K, Dalen KT. Synthesis and sorting of proteoglycans. J Cell Sci 113: 193–205, 2000. doi: 10.1242/jcs.113.2.193. [DOI] [PubMed] [Google Scholar]
- 11. Vivès RR, Seffouh A, Lortat-Jacob H. Post-synthetic regulation of hs structure: the Yin and Yang of the Sulfs in cancer. Front Oncol 3: 331, 2014. doi: 10.3389/fonc.2013.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science 252: 1705–1708, 1991. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 13. Robinson CJ, Harmer NJ, Goodger SJ, Blundell TL, Gallagher JT. Cooperative dimerization of fibroblast growth factor 1 (FGF1) upon a single heparin saccharide may drive the formation of 2:2:1 FGF1.FGFR2c.heparin ternary complexes. J Biol Chem 280: 42274–42282, 2005. doi: 10.1074/jbc.M505720200. [DOI] [PubMed] [Google Scholar]
- 14. Brown A, Robinson CJ, Gallagher JT, Blundell TL. Cooperative heparin-mediated oligomerization of fibroblast growth factor-1 (FGF1) precedes recruitment of FGFR2 to ternary complexes. Biophys J 104: 1720–1730, 2013. doi: 10.1016/j.bpj.2013.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlessinger J, Lax I, Lemmon M. Regulation of growth factor activation by proteoglycans: What is the role of the low affinity receptors? Cell 83: 357–360, 1995. doi: 10.1016/0092-8674(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 16. Christianson HC, Menard JA, Chandran VI, Bourseau-Guilmain E, Shevela D, Lidfeldt J, Månsson A-S, Pastorekova S, Messinger J, Belting M. Tumor antigen glycosaminoglycan modification regulates antibody-drug conjugate delivery and cytotoxicity. Oncotarget 8: 66960–66974, 2017. doi: 10.18632/oncotarget.16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Masri RE, Seffouh A, Roelants C, Seffouh I, Gout E, Pérard J, Dalonneau F, Nishitsuji K, Noborn F, Nikpour M, Larson G, Crétinon Y, Friedel-Arboleas M, Uchimura K, Daniel R, Lortat-Jacob H, Filhol O, Vivès RR. Extracellular endosulfatase Sulf-2 harbors a chondroitin/dermatan sulfate chain that modulates its enzyme activity. Cell Rep 38: 110516, 2022. doi: 10.1016/j.celrep.2022.110516. [DOI] [PubMed] [Google Scholar]
- 18. Christianson HC, Belting M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol 35: 51–55, 2014. doi: 10.1016/j.matbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 19. Wittrup A, Zhang S-H, ten Dam GB, van Kuppevelt TH, Bengtson P, Johansson M, Welch J, Mörgelin M, Belting M. ScFv antibody-induced translocation of cell-surface heparan sulfate proteoglycan to endocytic vesicles: Evidence for heparan sulfate epitope specificity and role of both syndecan and glypican. J Biol Chem 284: 32959–32967, 2009. doi: 10.1074/jbc.M109.036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wittrup A, Zhang S-H, Svensson KJ, Kucharzewska P, Johansson MC, Mörgelin M, Belting M. Magnetic nanoparticle-based isolation of endocytic vesicles reveals a role of the heat shock protein GRP75 in macromolecular delivery. Proc Natl Acad Sci USA 107: 13342–13347, 2010. doi: 10.1073/pnas.1002622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belting M, Mani K, Jönsson M, Cheng F, Sandgren S, Jonsson S, Ding K, Delcros J-G, Fransson L-A. Glypican-1 is a vehicle for polyamine uptake in mammalian cells: a pivotal role for nitrosothiol-derived nitric oxide. J Biol Chem 278: 47181–47189, 2003. doi: 10.1074/jbc.M308325200. [DOI] [PubMed] [Google Scholar]
- 22. Silhol M, Tyagi M, Giacca M, Lebleu B, Vivès E. Different mechanisms for cellular internalization of the HIV-1 Tat-derived cell penetrating peptide and recombinant proteins fused to Tat. Eur J Biochem 269: 494–501, 2002. doi: 10.1046/j.0014-2956.2001.02671.x. [DOI] [PubMed] [Google Scholar]
- 23. Stopschinski BE, Holmes BB, Miller GM, Manon VA, Vaquer-Alicea J, Prueitt WL, Hsieh-Wilson LC, Diamond MI. Specific glycosaminoglycan chain length and sulfation patterns are required for cell uptake of tau versus α-synuclein and β-amyloid aggregates. J Biol Chem 293: 10826–10840, 2018. doi: 10.1074/jbc.RA117.000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stopschinski BE, Thomas TL, Nadji S, Darvish E, Fan L, Holmes BB, Modi AR, Finnell JG, Kashmer OM, Estill-Terpack S, Mirbaha H, Luu HS, Diamond MI. A synthetic heparinoid blocks Tau aggregate cell uptake and amplification. J Biol Chem 295: 2974–2983, 2020. doi: 10.1074/jbc.RA119.010353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stanford KI, Bishop JR, Foley EM, Gonzales JC, Niesman IR, Witztum JL, Esko JD. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J Clin Invest 119: 3236–3245, 2009. doi: 10.1172/JCI38251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsiantoulas D, Eslami M, Obermayer G, Clement M, Smeets D, Mayer FJ, Kiss MG, Enders L, Weißer J, Göderle L, Lambert J, Frommlet F, Mueller A, Hendrikx T, Ozsvar-Kozma M, Porsch F, Willen L, Afonyushkin T, Murphy JE, Fogelstrand P, Donzé O, Pasterkamp G, Hoke M, Kubicek S, Jørgensen HF, Danchin N, Simon T, Scharnagl H, März W, Borén J. APRIL limits atherosclerosis by binding to heparan sulfate proteoglycans. Nature 597: 92–96, 2021. doi: 10.1038/s41586-021-03818-3. [DOI] [PubMed] [Google Scholar]
- 27. Anower-E-Khuda F, Singh G, Deng Y, Gordts PLSM, Esko JD. Triglyceride-rich lipoprotein binding and uptake by heparan sulfate proteoglycan receptors in a CRISPR/Cas9 library of Hep3B mutants. Glycobiology 29: 582–592, 2019. [Erratum in Glycobiology 30: 760, 2020]. doi: 10.1093/glycob/cwz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Putten JP, Paul SM. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells. EMBO J 14: 2144–2154, 1995. doi: 10.1002/j.1460-2075.1995.tb07208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shieh M-T, WuDunn D, Montgomery RI, Esko JD, Spear PG. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol 116: 1273–1281, 1992. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koehler M, Delguste M, Sieben C, Gillet L, Alsteens D. Initial step of virus entry: virion binding to cell-surface glycans. Annu Rev Virol 7: 143–165, 2020. doi: 10.1146/annurev-virology-122019-070025. [DOI] [PubMed] [Google Scholar]
- 31. Clausen TM, Sandoval DR, Spliid CB, Pihl J, Perrett HR, Painter CD, et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell 183: 1043–1057.e15, 2020. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maschalidi S, Ravichandran KS. Phagocytosis: sweet repulsions via the glycocalyx. Curr Biol 31: R20–R22, 2021. doi: 10.1016/j.cub.2020.10.066. [DOI] [PubMed] [Google Scholar]
- 33. Imbert PRC, Saric A, Pedram K, Bertozzi CR, Grinstein S, Freeman SA. An acquired and endogenous glycocalyx forms a bidirectional “Don’t Eat” and “Don’t Eat Me” barrier to phagocytosis. Curr Biol 31: 77–89.e5, 2021. doi: 10.1016/j.cub.2020.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arias-Alpizar G, Kong L, Vlieg RC, Rabe A, Papadopoulou P, Meijer MS, Bonnet S, Vogel S, van Noort J, Kros A, Campbell F. Light-triggered switching of liposome surface charge directs delivery of membrane impermeable payloads in vivo. Nat Commun 11: 3638, 2020. doi: 10.1038/s41467-020-17360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21: 9–17, 2019. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 36. Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101: 942–948, 1985. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kavanagh EL, Lindsay S, Halasz M, Gubbins LC, Weiner-Gorzel K, Guang MHZ, McGoldrick A, Collins E, Henry M, Blanco-Fernández A, O’Gorman P, Fitzpatrick P, Higgins MJ, Dowling P, McCann A. Protein and chemotherapy profiling of extracellular vesicles harvested from therapeutic induced senescent triple negative breast cancer cells. Oncogenesis 6: e388, 2017. doi: 10.1038/oncsis.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boussadia Z, Lamberti J, Mattei F, Pizzi E, Puglisi R, Zanetti C, Pasquini L, Fratini F, Fantozzi L, Felicetti F, Fecchi K, Raggi C, Sanchez M, D'Atri S, Carè A, Sargiacomo M, Parolini I. Acidic microenvironment plays a key role in human melanoma progression through a sustained exosome mediated transfer of clinically relevant metastatic molecules. J Exp Clin Cancer Res 37: 245, 2018. doi: 10.1186/s13046-018-0915-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, Chaturvedi P, Green JJ, Semenza GL. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci USA 111: E3234–E3242, 2014. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cerezo-Magaña M, Christianson HC, van Kuppevelt TH, Forsberg-Nilsson K, Belting M. Hypoxic induction of exosome uptake through proteoglycan-dependent endocytosis fuels the lipid droplet phenotype in glioma. Mol Cancer Res 19: 528–540, 2021. doi: 10.1158/1541-7786.MCR-20-0560. [DOI] [PubMed] [Google Scholar]
- 41. Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, Alvarez H, Gupta S, Maiti SN, Cooper L, Peehl D, Ram PT, Maitra A, Nagrath D. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife 5: e10250, 2016. doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mu W, Rana S, Zöller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia 15: 875–887, 2013. doi: 10.1593/neo.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Jong OG, van Balkom BWM, Gremmels H, Verhaar MC. Exosomes from hypoxic endothelial cells have increased collagen crosslinking activity through up-regulation of lysyl oxidase-like 2. J Cell Mol Med 20: 342–350, 2016. doi: 10.1111/jcmm.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature 527: 329–335, 2015. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature 450: 435–439, 2007. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 46. Christianson HC, Svensson KJ, van Kuppevelt TH, Li J-P, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A 110: 17380–17385, 2013. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rana S, Yue S, Stadel D, Zöller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol 44: 1574–1584, 2012. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 48. Berenguer J, Lagerweij T, Zhao XW, Dusoswa S, van der Stoop P, Westerman B, de Gooijer MC, Zoetemelk M, Zomer A, Crommentuijn MHW, Wedekind LE, López-López À, Giovanazzi A, Bruch-Oms M, van der Meulen-Muileman IH, Reijmers RM, van Kuppevelt TH, García-Vallejo JJ, van Kooyk Y, Tannous BA, Wesseling P, Koppers-Lalic D, Vandertop WP, Noske DP, van Beusechem VW, van Rheenen J, Pegtel DM, van Tellingen O, Wurdinger T. Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. J Extracell Vesicles 7: 1446660, 2018. doi: 10.1080/20013078.2018.1446660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cerezo-Magaña M, Bång-Rudenstam A, Belting M. The pleiotropic role of proteoglycans in extracellular vesicle mediated communication in the tumor microenvironment. Semin Cancer Biol 62: 99–107, 2019. doi: 10.1016/j.semcancer.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 50. Indira Chandran V, Welinder C, Gonçalves de Oliveira K, Cerezo-Magaña M, Månsson A-S, Johansson MC, Marko-Varga G, Belting M. Global extracellular vesicle proteomic signature defines U87-MG glioma cell hypoxic status with potential implications for non-invasive diagnostics. J Neurooncol 144: 477–488, 2019. doi: 10.1007/s11060-019-03262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kearns FL, Sandoval DR, Casalino L, Clausen TM, Rosenfeld MA, Spliid CB, Amaro RE, Esko JD. Spike-heparan sulfate interactions in SARS-CoV-2 infection. Curr Opin Struct Biol 76: 102439, 2022. doi: 10.1016/j.sbi.2022.102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bermejo-Jambrina M, Eder J, Kaptein TM, van Hamme JL, Helgers LC, Vlaming KE, Brouwer PJM, van Nuenen AC, Spaargaren M, de Bree GJ, Nijmeijer BM, Kootstra NA, van Gils MJ, Sanders RW, Geijtenbeek TBH. Infection and transmission of SARS-CoV-2 depend on heparan sulfate proteoglycans. EMBO J 40: e106765, 2021. doi: 10.15252/embj.2020106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol 16: e9610, 2020. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aguiar JA, Tremblay BJ-M, Mansfield MJ, Woody O, Lobb B, Banerjee A, Chandiramohan A, Tiessen N, Cao Q, Dvorkin-Gheva A, Revill S, Miller MS, Carlsten C, Organ L, Joseph C, John A, Hanson P, Austin RC, McManus BM, Jenkins G, Mossman K, Ask K, Doxey AC, Hirota JA. Gene expression and in situ protein profiling of candidate SARS-CoV-2 receptors in human airway epithelial cells and lung tissue. Eur Respir J 56: 2001123, 2020. doi: 10.1183/13993003.01123-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu J, Li Y, Liu Q, Yao Q, Wang X, Zhang H, Chen R, Ren L, Min J, Deng F, Yan B, Liu L, Hu Z, Wang M, Zhou Y. Cell Discovery SARS-CoV-2 cell tropism and multiorgan infection. Cell Discov 7: 17, 2021. doi: 10.1038/s41421-021-00249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Puray-Chavez M, LaPak KM, Schrank TP, Elliott JL, Bhatt DP, Agajanian MJ, Jasuja R, Lawson DQ, Davis K, Rothlauf PW, Liu Z, Jo H, Lee N, Tenneti K, Eschbach JE, Shema Mugisha C, Cousins EM, Cloer EW, Vuong HR, VanBlargan LA, Bailey AL, Gilchuk P, Crowe JE, Diamond MS, Hayes DN, Whelan SPJ, Horani A, Brody SL, Goldfarb D, Major MB, Kutluay SB. Systematic analysis of SARS-CoV-2 infection of an ACE2-negative human airway cell. Cell Rep 36: 109364, 2021. doi: 10.1016/j.celrep.2021.109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 23: 3–20, 2022. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Haeger SM, Yang Y, Schmidt EP. Heparan sulfate in the developing, healthy, and injured lung. Am J Respir Cell Mol Biol 55: 5–11, 2016. doi: 10.1165/rcmb.2016-0043TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hudák A, Letoha A, Szilák L, Letoha T. Contribution of syndecans to the cellular entry of SARS-CoV-2. Int J Mol Sci 22: 5336, 2021. doi: 10.3390/ijms22105336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim SY, Jin W, Sood A, Montgomery DW, Grant OC, Fuster MM, Fu L, Dordick JS, Woods RJ, Zhang F, Linhardt RJ. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res 181: 104873, 2020. doi: 10.1016/j.antiviral.2020.104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang Z, Zhang J, Wang J. Surface charge changes in spike RBD mutations of SARS-CoV-2 and its variant strains alter the virus evasiveness via HSPGs: a review and mechanistic hypothesis. Front Public Health 10: 952916, 2022. doi: 10.3389/fpubh.2022.952916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gelbach AL, Zhang F, Kwon SJ, Bates JT, Farmer AP, Dordick JS, Wang C, Linhardt RJ. Interactions between heparin and SARS-CoV-2 spike glycoprotein RBD from omicron and other variants. Front Mol Biosci 9: 912887, 2022. doi: 10.3389/fmolb.2022.912887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Miller ML, Reznik E, Gauthier NP, Aksoy BA, Korkut A, Gao J, Ciriello G, Schultz N, Sander C. Pan-cancer analysis of mutation hotspots in protein domains. Cell Syst 1: 197–209, 2015. doi: 10.1016/j.cels.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Anoosha P, Sakthivel R, Gromiha MM. Exploring preferred amino acid mutations in cancer genes: applications to identify potential drug targets. Biochim Biophys Acta 1862: 155–165, 2016. doi: 10.1016/j.bbadis.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 66. Tan H, Bao J, Zhou X. Genome-wide mutational spectra analysis reveals significant cancer-specific heterogeneity. Sci Rep 5: 12566, 2015. doi: 10.1038/srep12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Szpiech ZA, Strauli NB, White KA, Ruiz DG, Jacobson MP, Barber DL, Hernandez RD. Prominent features of the amino acid mutation landscape in cancer. PLoS One 12: e0183273, 2017. doi: 10.1371/journal.pone.0183273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tsuber V, Kadamov Y, Brautigam L, Berglund UW, Helleday T. Mutations in cancer cause gain of cysteine, histidine, and tryptophan at the expense of a net loss of arginine on the proteome level. Biomolecules 7: 49, 2017. doi: 10.3390/biom7030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bång-Rudenstam A, Cerezo-Magaña M, Belting M. Pro-metastatic functions of lipoproteins and extracellular vesicles in the acidic tumor microenvironment. Cancer Metastasis Rev 38: 79–92, 2019. doi: 10.1007/s10555-019-09786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Voss NCS, Kold-Petersen H, Henningsen MB, Homilius C, Boedtkjer E. Upregulated Na(+)/H(+)-exchange protects human colon cancer tissue against intracellular acidification. Biomed Res Int 2019: 3702783, 2019. doi: 10.1155/2019/3702783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. White KA, Ruiz DG, Szpiech ZA, Strauli NB, Hernandez RD, Jacobson MP, Barber DL. Cancer-associated arginine-to-histidine mutations confer a gain in pH sensing to mutant proteins. Sci Signal 10: eaam9931, 2017. doi: 10.1126/scisignal.aam9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Governa V, Talbot H, Gonçalves de Oliveira K, Cerezo-Magaña M, Bång-Rudenstam A, Johansson MC, Månsson A-S, Forsberg-Nilsson K, Marko-Varga G, Enríquez Pérez J, Darabi A, Malmström J, Bengzon J, Welinder C, Belting M. Landscape of surfaceome and endocytome in human glioma is divergent and depends on cellular spatial organization. Proc Natl Acad Sci USA 119: e2114456119, 2022. doi: 10.1073/pnas.2114456119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schaefer L, Mihalik D, Babelova A, Krzyzankova M, Gröne H-J, Iozzo RV, Young MF, Seidler DG, Lin G, Reinhardt DP, Schaefer RM. Regulation of Fibrillin-1 by biglycan and decorin is important for tissue preservation in the kidney during pressure-induced injury. Am J Pathol 165: 383–396, 2004. doi: 10.1016/S0002-9440(10)63305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Baghy K, Iozzo RV, Kovalszky I. Decorin–TGFβ axis in hepatic fibrosis and cirrhosis. J Histochem Cytochem 60: 262–268, 2012. doi: 10.1369/0022155412438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tabary M, Gheware A, Peñaloza HF, Lee JS. The matricellular protein thrombospondin-1 in lung inflammation and injury. Am J Physiol Cell Physiol 323: C857–C865, 2022. doi: 10.1152/ajpcell.00182.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Svensson KJ, Christianson HC, Kucharzewska P, Fagerström V, Lundstedt L, Borgquist S, Jirström K, Belting M. Chondroitin sulfate expression predicts poor outcome in breast cancer. Int J Oncol 39: 1421–1428, 2011. doi: 10.3892/ijo.2011.1164. [DOI] [PubMed] [Google Scholar]
- 77. Salanti A, Clausen TM, Agerbæk MØ, Al Nakouzi N, Dahlbäck M, Oo HZ, Lee S, Gustavsson T, Rich JR, Hedberg BJ, Mao Y, Barington L, Pereira MA, LoBello J, Endo M, Fazli L, Soden J, Wang CK, Sander AF, Dagil R, Thrane S, Holst PJ, Meng L, Favero F, Weiss GJ, Nielsen MA, Freeth J, Nielsen TO, Zaia J, Tran NL. Targeting human cancer by a glycosaminoglycan binding malaria protein. Cancer Cell 28: 500–514, 2015. doi: 10.1016/j.ccell.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Belting M. Glycosaminoglycans in cancer treatment. Thromb Res 133: S95–S101, 2014. doi: 10.1016/S0049-3848(14)50016-3. [DOI] [PubMed] [Google Scholar]
- 79. Nikitovic D, Berdiaki A, Spyridaki I, Krasanakis T, Tsatsakis A, Tzanakakis GN. Proteoglycans-biomarkers and targets in cancer therapy. Front Endocrinol (Lausanne) 9: 69, 2018. doi: 10.3389/fendo.2018.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Theocharis AD, Tsolakis I, Tzanakakis GN, Karamanos NK, Overview IC. Chondroitin sulfate as a key molecule in the development of atherosclerosis and cancer progression. Adv Pharmacol 53: 281–295, 2006. doi: 10.1016/S1054-3589(05)53013-8. [DOI] [PubMed] [Google Scholar]
- 81. Ricciardelli C, Sakko AJ, Stahl J, Tilley WD, Marshall VR, Horsfall DJ. Prostatic chondroitin sulfate is increased in patients with metastatic disease but does not predict survival outcome. Prostate 69: 761–769, 2009. doi: 10.1002/pros.20926. [DOI] [PubMed] [Google Scholar]
- 82. Ucakturk E, Akman O, Sun X, Baydar DE, Dolgun A, Zhang F, Linhardt RJ. Changes in composition and sulfation patterns of glycoaminoglycans in renal cell carcinoma. Glycoconj J 33: 103–112, 2016. doi: 10.1007/s10719-015-9643-1. [DOI] [PubMed] [Google Scholar]
- 83. Tsidulko AY, Kazanskaya GM, Volkov AM, Suhovskih AV, Kiselev RS, Kobozev VV, Gaytan AS, Krivoshapkin AL, Aidagulova SV, Grigorieva EV. Chondroitin sulfate content and decorin expression in glioblastoma are associated with proliferative activity of glioma cells and disease prognosis. Cell Tissue Res 379: 147–155, 2020. doi: 10.1007/s00441-019-03127-2. [DOI] [PubMed] [Google Scholar]
- 84. Thelin MA, Svensson KJ, Shi X, Bagher M, Axelsson J, Isinger-Ekstrand A, van Kuppevelt TH, Johansson J, Nilbert M, Zaia J, Belting M, Maccarana M, Malmström A. Dermatan sulfate is involved in the tumorigenic properties of esophagus squamous cell carcinoma. Cancer Res 72: 1943–1952, 2012. doi: 10.1158/0008-5472.CAN-11-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liao W-C, Liao C-K, Tsai Y-H, Tseng T-J, Chuang L-C, Lan C-T, Chang H-M, Liu C-H. DSE promotes aggressive glioma cell phenotypes by enhancing HB-EGF/ErbB signaling. PLoS One 13: e0198364, 2018. doi: 10.1371/journal.pone.0198364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Al-Nakouzi N, Wang CK, Oo HZ, Nelepcu I, Lallous N, Spliid CB, Khazamipour N, Lo J, Truong S, Collins C, Hui D, Esfandnia S, Adomat H, Clausen TM, Gustavsson T, Choudhary S, Dagil R, Corey E, Wang Y, Chauchereau A, Fazli L, Esko JD, Salanti A, Nelson PS, Gleave ME, Daugaard M. Reformation of the chondroitin sulfate glycocalyx enables progression of AR-independent prostate cancer. Nat Commun 13: 4760, 2022. doi: 10.1038/s41467-022-32530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pibuel MA, Poodts D, Díaz M, Hajos SE, Lompardía SL. The scrambled story between hyaluronan and glioblastoma. J Biol Chem 296: 100549, 2021. doi: 10.1016/j.jbc.2021.100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Martínez-Ramos C, Lebourg M. Three-dimensional constructs using hyaluronan cell carrier as a tool for the study of cancer stem cells. J Biomed Mater Res B Appl Biomater 103: 1249–1257, 2015. doi: 10.1002/jbm.b.33304. [DOI] [PubMed] [Google Scholar]
- 89. Misra S, Hascall VC, Markwald RR, Ghatak S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front Immunol 6: 201, 2015. doi: 10.3389/fimmu.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Takashima S, Oka Y, Fujiki F, Morimoto S, Nakajima H, Nakae Y, Nakata J, Nishida S, Hosen N, Tatsumi N, Mizuguchi K, Hashimoto N, Oji Y, Tsuboi A, Kumanogoh A, Sugiyama H. Syndecan-4 as a biomarker to predict clinical outcome for glioblastoma multiforme treated with WT1 peptide vaccine. Future Sci OA 2: FSO96, 2016. doi: 10.4155/fsoa-2015-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Indira Chandran V, Welinder C, Månsson A-S, Offer S, Freyhult E, Pernemalm M, Lund SM, Pedersen S, Lehtiö J, Marko-Varga G, Johansson MC, Englund E, Sundgren PC, Belting M. Ultrasensitive immunoprofiling of plasma extracellular vesicles identifies syndecan-1 as a potential tool for minimally invasive diagnosis of glioma. Clin Cancer Res. 25: 3115–3127, 2019. doi: 10.1158/1078-0432.CCR-18-2946. [DOI] [PubMed] [Google Scholar]
- 92. Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem 284: 34211–34222, 2009. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Menard JA, Cerezo-Magaña M, Belting M. Functional role of extracellular vesicles and lipoproteins in the tumour microenvironment. Philos Trans R Soc Lond B Biol Sci 373: 20160480, 2018. doi: 10.1098/rstb.2016.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Menard JA, Christianson HC, Kucharzewska P, Bourseau-Guilmain E, Svensson KJ, Lindqvist E, Indira Chandran V, Kjellén L, Welinder C, Bengzon J, Johansson MC, Belting M. Metastasis stimulation by hypoxia and acidosis-induced extracellular lipid uptake is mediated by proteoglycan-dependent endocytosis. Cancer Res 76: 4828–4840, 2016. doi: 10.1158/0008-5472.CAN-15-2831. [DOI] [PubMed] [Google Scholar]
- 95. Jung S, Potapov I, Chillara S, Del Sol A. Leveraging systems biology for predicting modulators of inflammation in patients with COVID-19. Sci Adv 7: eabe5735, 2021. doi: 10.1126/sciadv.abe5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jaime-Ramirez AC, Dmitrieva N, Yoo JY, Banasavadi-Siddegowda Y, Zhang J, Relation T, Bolyard C, Wojton J, Kaur B. Humanized chondroitinase ABC sensitizes glioblastoma cells to temozolomide. J Gene Med 19: e2942, 2017. doi: 10.1002/jgm.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chuang H-C, Hsueh C-H, Hsu P-M, Huang R-H, Tsai C-Y, Chung N-H, Chow Y-H, Tan T-H. SARS-CoV-2 spike protein enhances MAP4K3/GLK-induced ACE2 stability in COVID-19. EMBO Mol Med 14: e15904, 2022. doi: 10.15252/emmm.202215904. [DOI] [PMC free article] [PubMed] [Google Scholar]