OBJECTIVES:
Our objective was to compare norepinephrine plus dobutamine versus epinephrine as the first-line agent in children with fluid refractory cold septic shock.
DESIGN:
Open-label randomized controlled study.
SETTING:
A single-center PICU from North India.
PATIENTS:
Children 2 months to less than 18 years old with fluid refractory cold septic shock.
INTERVENTIONS:
In the intervention group, norepinephrine and dobutamine were started and in the control group, epinephrine was started as the first-line vasoactive agent. The primary outcome was the proportion attaining shock resolution (attaining all the therapeutic endpoints) at 1 hour of therapy.
MEASUREMENTS AND MAIN RESULTS:
We enrolled 67 children: 34 in the norepinephrine plus dobutamine group (intervention) and 33 in the epinephrine group (control). There was no difference in shock resolution at 1 hour (17.6% vs 9%; risk ratio [RR], 2.0; 95% CI, 0.54–7.35; p = 0.25), 6 hours (76.4% vs 54.5%; RR, 1.69; 95% CI, 0.92–3.13; p = 0.06), and 24 hours between the intervention and control groups, respectively. Children in the norepinephrine plus dobutamine group attained shock resolution earlier (measured from starting of vasoactive agents to attaining all the therapeutic endpoints) (hazard ratio, 1.84 [1.1–3.08]). The difference in 28-day mortality was not significant (23.5% vs 39.3% in the intervention and control groups, respectively [RR, 0.59; 95% CI, 0.28–1.25]).
CONCLUSIONS:
In children with fluid refractory cold septic shock, with use of norepinephrine plus dobutamine as first-line agents, the difference in the proportion of children attaining shock resolution at 1 hour between the groups was inconclusive. However, the time to shock resolution was earlier in the norepinephrine plus dobutamine group. Also, fewer children in the intervention group were refractory to treatment. Further studies powered to detect (or exclude) an important difference would be required to test this intervention.
Keywords: cold, septic shock, critically ill children, epinephrine, fluid refractory, norepinephrine plus dobutamine, vasoconstricted shock
KEY POINTS
Question: To compare a combination of norepinephrine plus dobutamine with epinephrine as a first-line agent in children with fluid refractory cold septic shock.
Findings: In this open-label, randomized controlled trial enrolling 67 children, there was no difference in shock resolution at 1 hour of initiation of therapy. However, children in the norepinephrine plus dobutamine group attained earlier shock resolution.
Meaning: A combination of norepinephrine plus dobutamine may be beneficial as first-line agents in children with fluid refractory cold septic shock.
In children with fluid refractory septic shock, vasoactive agents are used to maintain organ perfusion after fluid resuscitation. The American College of Critical Care Medicine (ACCM) 2017 guidelines classified children based on clinical presentation into cold shock (cool extremities, feeble pulses, and prolonged capillary refill) and warm shock (warm or flushed extremities, bounding pulses, and instant capillary refill) and recommended epinephrine in cold shock and norepinephrine in warm shock (1). The rationale for recommending epinephrine was—in children, low cardiac output with high systemic vascular resistance (SVR) (cold shock) was commonly observed compared with adults who presented with low SVR (2, 3).
However, it is increasingly being recognized that there is discordance between clinical assessment and hemodynamic variables measured invasively and therefore classifying children into warm shock or cold shock solely based on clinical parameters may not be desirable (4–6). The recently published surviving sepsis guidelines (SSG) have suggested to not use clinical signs alone to classify shock into warm or cold shock and have recommended the use of advanced hemodynamic monitoring (invasive blood pressure, echocardiography, SVR, and mixed venous oxygen saturation) to better classify the pathophysiology of shock (7). However, this may not be feasible in all circumstances as advanced hemodynamic monitoring is not widely available, especially in developing nations. Also, SSG have recommended the use of either epinephrine or norepinephrine in children with septic shock, highlighting the need for further studies to find the best regime to treat pediatric septic shock.
Owing to the high prevalence of septic myocardial dysfunction (8), the dynamic pathophysiology of shock and the poor ability of clinical features to identify the true pathophysiology, it is difficult for a single vasoactive agent to address all these issues. A combination of vasoactive agents, with different physiologic effects, may be preferred for the reason that lower doses of the individual drugs would be required compared with the use of a single agent resulting in lesser side effects. Transient increase in lactate, tachyarrhythmias, myocardial oxygen demand, and decreased splanchnic circulation have been reported with the use of epinephrine (9). The use of norepinephrine and dobutamine have also been reported to have adverse hemodynamic consequences such as tachycardia, tachyarrhythmias, and vasoconstriction (norepinephrine). However, in adults, limited studies evaluating norepinephrine plus dobutamine found the combination to be associated with lower heart rates, lactate levels, improved splanchnic perfusion, and decreased tachyarrhythmias compared with epinephrine (10–13).
In children, there is no data comparing the combination therapy with epinephrine alone. We planned to compare the combination of norepinephrine and dobutamine with epinephrine in children presenting with a clinical phenotype of cold shock. We chose only the cold shock phenotype with a narrow pulse pressure (vasoconstricted shock) instead of both cold and warm shock (vasodilated) as norepinephrine was already recommended for warm shock in line with the ACCM 2017 guidelines prevailing at the time of initiation of the study.
MATERIALS AND METHODS
Study Design
This open-label randomized controlled trial was conducted between November 2018 and June 2020 in the Pediatric Emergency and ICU of All India Institute of Medical Sciences (AIIMS), New Delhi. The trial protocol, available at Clinical Trials Registry of India (CTRI number: CTRI/2018/09/015844), was approved by the institute ethics committee of AIIMS, New Delhi (IECPG-299/28.6.2018, RT 11/30/08/2018). We obtained written informed consent from the parent/guardian and followed procedures as per the ethical standards of the Institute ethics committee on human experimentation and with the Declaration of Helsinki of 1975.
Patients
Children 2 months to 18 years old with fluid refractory cold septic shock were screened for inclusion. Those with fulminant myocarditis, congenital heart disease, severe acute malnutrition, chronic kidney disease, those already on vasoactive agents, and whose parent/guardian did not give consent were excluded.
Study Definitions
Septic shock was defined as children who had a suspected infection and at least two of the following clinical signs of decreased perfusion with or without hypotension (defined as systolic blood pressure less than fifth centile as per Pediatric Advanced Life Support guidelines, 2010) (14), including altered mental status, prolonged capillary refill of greater than 2 seconds, cool mottled extremities, diminished pulses, or history of decreased urine output (15, 16). Fluid refractory cold shock (vasoconstricted shock) was defined as the presence of cool peripheries, capillary refill time greater than 2 seconds, diminished pulses, and narrow pulse pressure (pulse pressure < 40 mm Hg) despite 40 mL/kg of fluid bolus OR if there was worsening after the fluid bolus in the form of new onset rales or hepatomegaly (1, 17).
Outcomes
The primary outcome was the proportion of children attaining shock resolution at 1 hour of vasoactive therapy. Shock resolution was defined as normal mean arterial pressure for age and any four of the following criteria: 1) Normal pulses with no difference between peripheral and central pulses, 2) Warm extremities, 3) Capillary refill time less than 2 seconds, 4) Improving mental status, and 5) Urine output greater than 1 mL/kg/hr without need for fluid bolus or vasoactive dose escalation for 4 hours. The secondary outcomes were: the proportion attaining shock resolution at 6 and 24 hours of therapy; the duration of vasoactive support; 28-day mortality; Vasoactive-Inotropic Score (VIS) at 24 hours; proportion requiring other vasoactive agents; time to attain shock resolution; occurrence of tachyarrhythmias; pediatric Sequential Organ Failure Assessment (pSOFA); and Pediatric Logistic Organ Dysfunction-2 (PELOD-2) scores at 24, 48, and 72 hours of randomization (Please see Supplemental Digital Content, Table 1, http://links.lww.com/CCX/B101 for specific definitions of secondary outcomes) (18–20).
Study Protocol
As soon as a child presented to the emergency in shock or a child in emergency or PICU developed shock, they were screened for inclusion and exclusion criteria by the resident on call. Those who were eligible with no exclusion criteria were considered for possible inclusion in the study after fluid boluses. The resident on call informed the primary author, coauthor, or corresponding author (K.K.B., U.V.K., J.S.). Three investigators and one support staff were available for the process of enrollment during the study period. The children were given fluid boluses as per the study protocol. If the patient was fluid refractory and vasoconstricted, the authors approached the parents/guardian for informed consent. Verbal and written informed consents were serially obtained from parents/guardian before enrollment. Central venous catheters were recommended to be inserted in all children once they were fluid refractory.
Randomization
Participants were randomized to receive either norepinephrine plus dobutamine or epinephrine. Block randomization was carried out by a statistician blinded to patient identity or status with the use of varying block sizes of two to eight generated using a computer-based random number table generator (using Stata 13 [Stata Corp, College Station, TX]). Sealed envelopes with random numbers were kept in the PICU. When the decision to start vasoactive agents was taken, sealed envelopes were opened, and child was randomized to the respective treatment arm as per the random number sequence.
Interventions
1) Norepinephrine plus dobutamine group: Norepinephrine and dobutamine were started simultaneously at a dose of 0.1 and 10 μg/kg/min, respectively. The children were monitored continuously. Those children who failed to attain therapeutic endpoints at 15 minutes of intervention, the vasoactive agents were titrated as per the physiologic status. In children with low blood pressure, norepinephrine dose was increased and in children with normal blood pressure and signs of poor perfusion, dobutamine was increased. We assessed shock resolution at 1 hour. Children who did not attain shock resolution on norepinephrine at 0.3 μg/kg/min or dobutamine at 20 μg/kg/min were labeled as treatment refractory. Subsequent management of these children was as per the physiologic status and in accordance with the ACCM 2017 guidelines (Supplemental Digital Content, Fig. 1, http://links.lww.com/CCX/B101) (1).
2) Epinephrine group: Epinephrine was started at a dose of 0.1 μg/kg/min. In children who failed to attain therapeutic endpoints, epinephrine dose was titrated every 15 minutes. We assessed shock resolution at 1 hour. Children who did not attain shock resolution on epinephrine at 0.3 μg/kg/min were labeled as treatment refractory. Subsequent management was as per the ACCM 2017 guidelines (Supplemental Digital Content, Fig. 1, http://links.lww.com/CCX/B101) (1).
After initiation of vasoactive agents, continuous monitoring of the vital signs was done and the blood pressures were measured using indwelling arterial catheters. In children who arterial line could not be established, noninvasive blood pressure measurement was done by oscillometric method using Mindray-BeneView T5 system using age-appropriate cuffs. The need for further fluid boluses was assessed by the treating team. Inferior vena cava distensibility index/collapsibility index was assessed to predict fluid responsiveness. Further fluids were considered in children who had shock and did not manifest overt features of fluid overload and whose echocardiographic parameters suggested fluid responsiveness. Stress dose steroids (hydrocortisone at 100 mg/m2/d) were given in children with catecholamine refractory shock. During the study period, the framework provided for management of septic shock in children in both groups were as per the ACCM guidelines 2017 (1).
Data Collection
Data collection included demographics, clinical course, investigations, treatment received, and outcome variables. To establish infection as the etiology of shock, cultures were sent from various organs or body sites, depending upon specific localizing signs and symptoms.
For assessing primary outcome (shock resolution), hemodynamic parameters were recorded at baseline, before initiation of vasoactive agents, and at every step while escalating therapy till 1 hour. Subsequently, the data were recorded every 60 minutes till attainment of shock resolution and then every 4 hours till the vasoactive agents were stopped.
The secondary outcomes assessed were 28-day mortality, adverse events, proportion attaining shock resolution at 6 and 24 hours, time to shock resolution, duration of vasoactive therapy, and use of other vasoactive agents. Other outcomes compared were the pSOFA scores, PELOD scores at 24, 48, and 72 hours, fluids received, transfusion of blood products, duration and requirement of mechanical ventilation, renal replacement therapy, and length of PICU and hospital stay between the groups.
Enrolled children were followed till day 28 of randomization and outcomes were recorded. In those children who reported for follow-up, a physical follow-up was done and in the remaining telephonic consultation was performed.
Functional echocardiography was performed within the first 6 hours of enrollment using Philips Ultrasound CX-50 machine (Philips Ultrasound, Bothell, WA), using S5-1 probe. Myocardial contractility was assessed using fractional shortening method in the parasternal long axis (PLAX) view. Systolic dysfunction was defined by an ejection fraction of less than 55% (21). Cardiac output was measured at 1, 6, and 24 hours where feasible using the formula: heart rate × stroke volume. Stroke volume was calculated using the formula: velocity time integral (VTI) × aortic area; VTI was measured in the apical five-chamber view and aortic area was measured in PLAX view at the aortic annulus (22).
Children were monitored for the occurrence of any adverse events including rhythm abnormalities and peripheral ischemic changes. The adverse events were reported to the institute ethics committee. There was no separate data safety monitoring board for this study.
Sample Size Calculation
Our primary objective was to evaluate shock resolution at 1 hour of therapy. With shock resolution with epinephrine of 40% (23) and assuming a shock resolution of 60% with intervention group (no previous data), alpha error of 0.05% and power of 80%, we had calculated that 95 children would be required in each group (total 190). However, as a result of the COVID-19 pandemic, we could enroll only 67 patients.
Statistical Analysis
Data were entered into Microsoft Excel and analyzed using Stata 13 (Stata Corp). Categorical variables are presented as n (%), while continuous variables are presented as mean (sd), if normally distributed and median (interquartile range [IQR]), if non-normally distributed. Tests of statistical significance were applied based on the type of variables. Continuous variables, if normally distributed, were analyzed using “t” test and if not normally distributed, were compared using Wilcoxon rank-sum test and Quasi-Poisson regression. Categorical variables were compared using Fisher exact test/chi-square test as applicable. A p value of less than 0.05 was taken as significant. Kaplan-Meier curves were used for the time-to-event outcomes and the effect of interventions on these outcomes was assessed using Cox proportional hazard model (post hoc analysis).
RESULTS
Baseline Characteristics
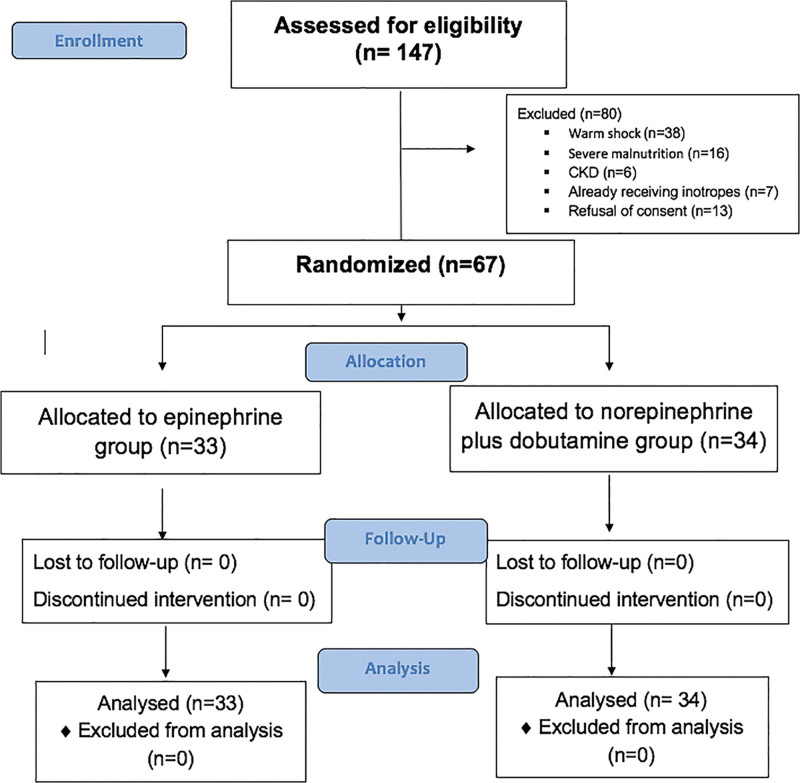
Of the 147 children with fluid refractory septic shock during the study period, 67 were randomized: 34 in the norepinephrine plus dobutamine group and 33 in the epinephrine group (Fig. 1). Thirty-eight (56.7%) were boys and the median age was 7 years (1.5–10 yr). Median (IQR) Pediatric Index of Mortality-3 (PIM-3) predicted mortality was 26.9% (17.6–40.1%). There were no differences in the baseline parameters including gender, PIM-3 score, underlying illness, hemodynamic, and laboratory parameters between the groups (Table 1). Most common focus of infection was respiratory (37.3%) followed by CNS (23.8%) and gastrointestinal (19.4%) infection. The time to initiation of antibiotic therapy, first fluid bolus or inotropes was similar between the groups and is provided in Supplemental Digital Content, Table 2 (http://links.lww.com/CCX/B101).
Figure 1.
Study flow chart.
TABLE 1.
Baseline Characteristics of the Study Population
| Characteristics | Norepinephrine Plus Dobutamine Group (n = 34) | Epinephrine Group (n = 33) |
|---|---|---|
| Age (yr) | 7.5 (3–10) | 5 (1.5–10) |
| Gender (boys), n (%) | 20 (58.8) | 18 (54.5) |
| Pediatric Index of Mortality-3 predicted mortality (%) | 25 (16.3–35.5) | 27.2 (18.8–40.5) |
| Underlying medical condition, n (%) | 17 (50) | 20 (60.6) |
| Neurologic | 5 (29.4) | 10 (50) |
| Hematological malignancies | 1 (5.8) | 2 (10) |
| Respiratory | 3 (17.6) | 5 (25) |
| Immunodeficiency | 0 | 1 (5) |
| Renal disorder | 5 (29.4) | 1 (5) |
| Connective tissue disorders | 3 (17.6) | 0 |
| Liver disorder | 0 | 1 (5) |
| Focus of infection, n (%) | ||
| Respiratory | 9 (26.4) | 16 (48.4) |
| Gastrointestinal | 8 (23.5) | 5 (15.1) |
| CNS | 10 (29.4) | 6 (18.1) |
| Skin and soft tissue | 3 (8.8) | 1 (3) |
| Others | 4 (11.7) | 5 (15.1) |
| Hemodynamic variables | ||
| Heart rate (beats/min) | 155 (136–172) | 156 (136–168) |
| Mean blood pressure (mm Hg) | 62 (58–73) | 61.5 (57–70.5) |
| Hypotensive shock (blood pressure < fifth centile), n (%) | 11 (32.3) | 13 (39.3) |
| Capillary refill time (s) | 4 (3–4) | 3 (3–4) |
| Laboratory parameters | ||
| Hemoglobin (g/dL) | 9.1 (7.4–11.1) | 8.9 (7.9–11.5) |
| Total leukocyte count (/mm3) | 9,505 (6,900–16,450) | 12,610 (5,980–22,470) |
| Platelet count (×105/mm3) | 1.3 (0.8–2.4) | 1.9 (1–4.3) |
| Bicarbonate (mEq/L) | 18.4 (15.4–21.7) | 17.2 (13.6–21.1) |
| Base deficit (mEq/L) | 7.7 (2.2–10.4) | 9 (4.5–13.6) |
| Lactate (mmol/L) | 2.6 (2.4–3.6) | 3.2 (2.5–4.5) |
| Amount of fluids given before starting vasoactive agent (mL/kg) | 40 (20–40) | 40 (20–40) |
| Median IVC distensibility index | 22.6 (10.5–36.5) (n = 13) | 23 (20.2–30.9) (n = 13) |
| Median IVC collapsibility index | 43.4 (26–53.6) (n = 6) | 37.5 (26.6–52) (n = 3) |
IVC = inferior vena cava.
Data are presented as median (interquartile range), unless otherwise specified.
Primary Outcome
In the norepinephrine plus dobutamine group, six children (17.6%) attained shock resolution and in the epinephrine group, three children (9%) attained shock resolution at 1 hour of therapy; the difference was not statistically significant (risk ratio [RR], 2; 95% CI, 0.54–7.35; p = 0.25) (Table 2).
TABLE 2.
Outcomes and Organ Dysfunction in the Study Population
| Variable | Norepinephrine Plus Dobutamine Group (n = 34) | Epinephrine Group (n = 33) | Risk Ratio/Mean Difference (95% CI) | p |
|---|---|---|---|---|
| Primary outcome | ||||
| Proportion achieving shock resolution at 1 hr, n (%) | 6 (17.6) | 3 (9) | 2 (0.54–7.35) | 0.25 |
| Secondary outcomes | ||||
| Proportion achieving shock resolution at 6 hr, n (%) | 26 (76.4) | 18 (54.5) | 1.69 (0.92–3.13) | 0.06 |
| Proportion achieving shock resolution at 24 hr, n (%) | 33 (97.1) | 28 (84.8) | 1.14 (0.97–1.33) | 0.08 |
| 28-d mortality, n (%) | 8 (23.5) | 13 (39.3) | 0.59 (0.28–1.25) | 0.16 |
| Time to attain shock resolution (hr), median (IQR) | 3 (2–6) | 6 (3–10) | 1.84 (1.1–3.08)a | 0.02 |
| Duration of vasoactive therapy (hr), median (IQR) | 52 (25–146) | 65 (40–124) | 1.21 (0.7–2.08)a | 0.5 |
| Other vasoactive agents added, n (%) | 10 (29.4) | 27 (82) | 2.78 (1.61–4.80) | < 0.001 |
| Dopamine | 3 (9) | 9 (27) | 1.15 (0.39–3.41) | 0.82 |
| Dobutamine | NA | 21 (64) | NA | NA |
| Milrinone | 6 (18) | 11 (33) | 0.71 (0.36–1.39) | 0.34 |
| Vasopressin | 2 (6) | 5 (26) | 0.96 (0.22–4.18) | 0.96 |
| Levosimendan | 1 (3) | 2 (6) | 0.77 (0.08–7.57) | 0.82 |
| Norepinephrine in the epinephrine group | NA | 13 (39) | NA | |
| Epinephrine in the norepinephrine group | 7 (21) | NA | NA | NA |
| Fluids administered | ||||
| Volume of fluid boluses received before starting inotropes (mL/kg) | 40 (20–40) | 40 (20–40) | NA | 0.97 |
| Volume of fluid boluses received after starting inotropes (mL/kg) | 20 (20–30) | 20 (10–30) | NA | 0.74 |
| Volume of fluids received in 24 hr (mL/kg) | 75 (60–91) | 84 (60–96) | NA | 0.58 |
| Cumulative fluid balance till shock resolution (%) | 3.3 (1.2–4.3) | 4.1 (0.3–6.4) | NA | 0.34 |
| Adverse events | ||||
| Rhythm abnormalities, n (%) | 2 (5.8) | 4 (12.1) | NA | 0.32 |
| Arterial gangrene, n (%) | 0 (0) | 1 (3) | NA | 0.49 |
| Duration of PICU stay (d), median (IQR) | 10 (6–14) | 6 (5–13) | 1.19 (0.77–1.85)b | 0.43 |
| Duration of hospital stay (d), median (IQR) | 19 (10–29) | 15 (9–28) | 1.03 (0.70–1.52)b | 0.88 |
| Organ dysfunction and support | ||||
| Ventilation, n (%) | 23 (68) | 28 (85) | NA | 0.18 |
| Duration of mechanical ventilation, median (IQR) | 8 (6–12) | 6.5 (3.2–19) | 1.34 (0.79–2.35)b | 0.28 |
| Proportion with septic myocardial dysfunction, n (%) | 15 (44.1) | 14 (42.4) | 1.03 (0.67–1.56) | 0.88 |
| Proportion receiving hydrocortisone for catecholamine refractory shock, n (%) | 15 (45) | 15 (44) | 1.03 (0.60–1.75) | 0.91 |
| Packed RBC transfusion, n (%) | 22 (64.7) | 21 (63.6) | NA | 0.92 |
| Renal replacement therapy, n (%) | 7 (20.5) | 9 (27.2) | NA | 0.52 |
| Pediatric Sequential Organ Failure Assessment, median (IQR) | ||||
| Day 1 | 8.5 (5–11) | 9 (8–12) | NA | 0.59 |
| Day 2 | 8 (5–10) | 9 (8–11) | 0.04 | |
| Day 3 | 6 (3–10) | 8 (5–10) | 0.1 | |
| Pediatric Logistic Organ Dysfunction-2, median (IQR) | ||||
| Day 1 | 6 (2–9) | 8 (5–10) | NA | 0.09 |
| Day 2 | 4 (2–7) | 6 (4–7.5) | 0.05 | |
| Day 3 | 4.5 (2–7) | 5 (4–8) | 0.13 | |
IQR = interquartile range, NA = not applicable.
Hazard ratio.
Quasi-Poisson regression coefficient.
Secondary Outcomes
At 6 hours of therapy, the proportion attaining shock resolution was more in the norepinephrine plus dobutamine group, the difference being insignificant (76.4% vs 54.5%; RR, 1.69; 95% CI, 0.92–3.13; p = 0.06). At 24 hours of therapy also, the difference in results was insignificant (97% vs 84.8%; RR, 1.14; 95% CI, 0.97–1.33; p = 0.08).
Mortality at day 28 of randomization was 23.5% versus 39.3% in the norepinephrine plus dobutamine group and epinephrine groups, respectively (RR, 0.59; 95% CI, 0.28–1.25; p = 0.16) (Table 2).
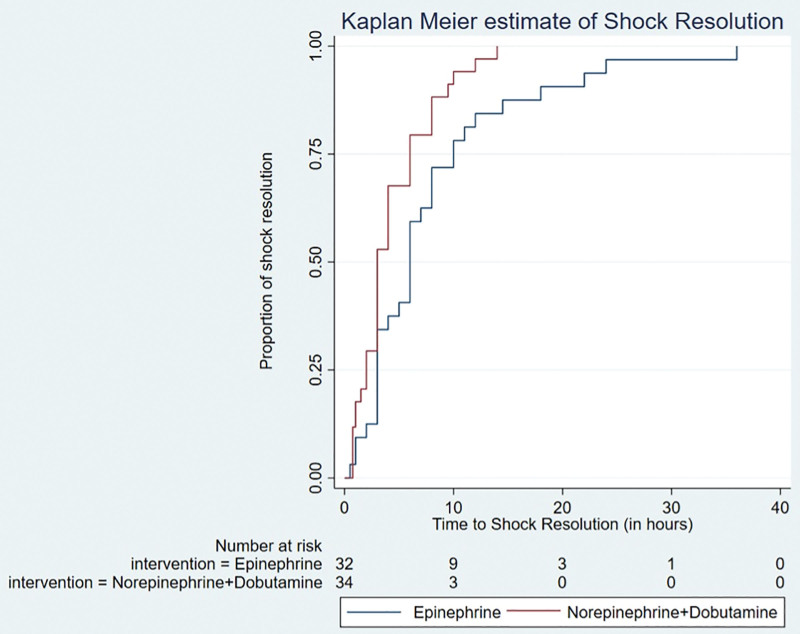
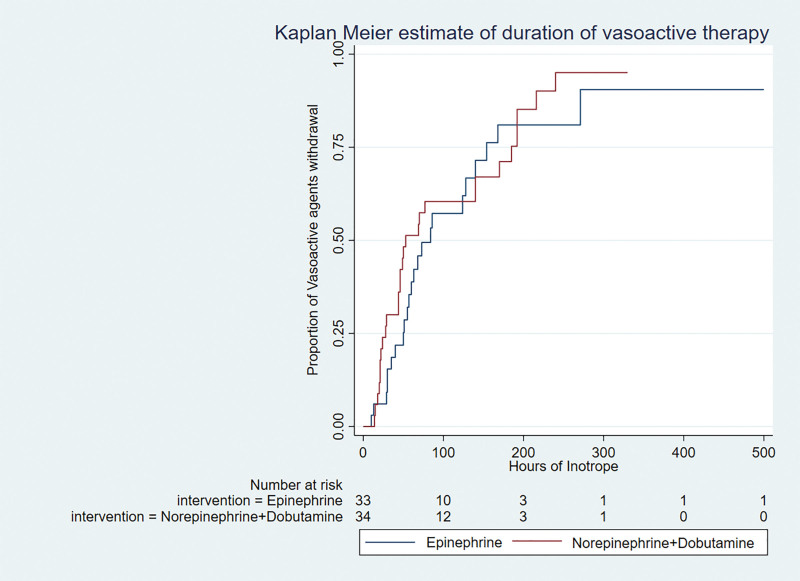
The time to shock resolution was shorter in the norepinephrine plus dobutamine group (hazard ratio [HR], 1.84 [1.10–3.08]; p = 0.02) and remained significant after adjustment for PIM-3 score (HR, 1.88 [1.12–3.14]; p = 0.02) (Fig. 2). The duration of vasoactive therapy was similar between the groups even after adjustment for PIM-3 score (HR, 1.21 [0.7–2.08]; p = 0.5) (Fig. 3). A total of 13 children died (nine in the norepinephrine plus dobutamine group and four in the epinephrine group) before the inotropes could be stopped. The average duration of vasoactive agent therapy in survivors in the norepinephrine plus dobutamine was 47.5 hours (22–140 hr) and in the epinephrine group was 56 hours (32.5–78.5 hr) (p = 0.84). There was no difference in the VIS score at 6 and 24 hours between the groups (30 [21–55] vs 30 [22.5–42.5] and 21.7 [10–40] vs 31.2 [20–60], respectively).
Figure 2.
Kaplan-Meier curve for time to shock resolution adjusted for Pediatric Index of Mortality-3.
Figure 3.
Kaplan-Meier curve for duration of vasoactive therapy adjusted for Pediatric Index of Mortality-3.
A total of 18 children required additional fluid boluses in the study (nine in each group). In the intervention group, 10 children required addition of other vasoactive agents. In nine out of 10 children, these were added within 24 hours (milrinone in six and epinephrine in five). In the control group, 27 children required addition of other vasoactive agents. In 25 out of 27 children, these were added within 24 hours (dobutamine in 21, norepinephrine in 13, and milrinone in nine) (Table 2).
Organ Dysfunction and Organ Support
The pSOFA scores and PELOD-2 scores on day 2 of randomization were lower in the norepinephrine plus dobutamine group, the difference being statistically significant (Table 2). The mean ejection fraction in the norepinephrine plus dobutamine group was 48.5 ± 14.1 and in the epinephrine group, it was 48.4 ± 13.9 (p = 0.96). There was no difference between the groups with regard to the proportion with systolic dysfunction (44.1% vs 42.4%; p = 0.88). At 6 hours of therapy, the mean cardiac index (L/min/m2) was higher in the norepinephrine plus dobutamine group (4 ± 1 vs 3.1 ± 1.2; p = 0.01) and the difference persisted at 24 hours of therapy (p = 0.05) (Supplemental Digital Content, Table 3, http://links.lww.com/CCX/B101).
Adverse Events
Six children developed rhythm abnormalities—two in the norepinephrine plus dobutamine group (heart block: 1, preterminal ventricular fibrillation: 1) and four in the epinephrine group (supraventricular tachycardia: 1, ectopic atrial rhythm: 1, preterminal ventricular tachycardia: 1, and cardiac arrest: 1). Only one child developed peripheral ischemic changes in the form of gangrenous changes over both hands and right foot in the epinephrine group. This child was on epinephrine, norepinephrine, and milrinone when the changes were noted. The most common cause of death was refractory shock (n = 14), refractory hypoxemia (n = 4), and refractory raised intracranial pressure (n = 3).
DISCUSSION
In this randomized controlled trial, we observed in vasoconstricted shock (cold shock), use of norepinephrine plus dobutamine as initial vasoactive agent did not result in greater proportion of children attaining shock resolution at 1, 6, and 24 hours of therapy. However, children in the norepinephrine plus dobutamine group attained shock resolution earlier and fewer were refractory to treatment compared with the epinephrine group. As the numbers enrolled were lower than the calculated sample size for any meaningful interpretation due to COVID-19, our results may be interpreted as pilot or preliminary data.
Septic shock is a dynamic condition. Hence, the choice of vasoactive agents may need to be optimized as per the physiologic status once advanced hemodynamic monitoring is available for favorable outcomes. Ceneviva et al (2), in 50 children with fluid refractory septic shock, observed that 22% responded to vasopressor and inotrope combination and five out of 10 children with hyperdynamic shock required addition of inotrope for evolving cardiac dysfunction. Greater number of children required additional agents in the epinephrine group compared with the combination therapy group, and although exploratory, this is an important observation in the present study.
We observed that the combination therapy was feasible and may benefit children with cold (vasoconstricted) shock in terms of earlier shock resolution. The hemodynamic variables including heart rate, capillary refill time, and mean blood pressure were better in the norepinephrine plus dobutamine group at varying time points from 1 hour to 72 hours of therapy. Lactates and mean cardiac index showed greater improvement at 6 hours of intervention in the norepinephrine plus dobutamine group. These findings highlight the possible beneficial effects of the use of norepinephrine plus dobutamine in children with cold (vasoconstricted) shock. However, it is pertinent to mention here that the addition of other vasoactive agents could have influenced this variable even though there was significant difference in the number of children who required additional agents as mentioned above.
Few adult studies have compared norepinephrine plus dobutamine with epinephrine (10–13). Annane et al (10), in 330 adults with septic shock, observed that there was no difference between epinephrine and norepinephrine plus dobutamine groups in terms of safety and efficacy. However, two recent systematic reviews in adults have found that the combination of vasoactive agents is associated with lower 28-day mortality (24, 25). The combination group has both cardiac and vascular effects and improved preload, myocardial contractility and perfusion with the added advantage of precise titration of individual effects when needed.
To our knowledge, this is the first study to evaluate this combination therapy in pediatric septic shock and provides preliminary data for the same. Our study has few limitations. First, we could not enroll the expected sample size due to COVID-19 and therefore may call it pilot or preliminary data that needs further evaluation. Second, we chose the phenotype of vasoconstricted shock only and not those with vasodilated shock. If we had included both clinical types, the results may have been more generalizable. However, this decision was based on the ACCM guidelines prevailing at the time of the initiation of the study. Third, the time to shock resolution could have been influenced by addition of other vasoactive agents within 24 hours. However, this would be an inherent difficulty in this type of trial design, as one would have to add other agents in case of nonresolution of shock. The observation that a greater proportion of children required additional vasoactive agents in the epinephrine group compared with the combination vasoactive therapy group (27/33 vs 10/34) indirectly supports the combination therapy group. Fourth, it was an open-label study, which may have introduced bias. Although we took utmost care and standardized the measurement of study outcomes, the possibility of bias still exists. Finally, our study was from a single center from a developing country with higher illness severity and therefore the generalizability of our results to other settings needs further exploration.
CONCLUSIONS
In children with fluid refractory cold septic shock, with the use of norepinephrine plus dobutamine as first-line agents, the difference in proportion of children attaining shock resolution at 1, 6, or 24 hours of therapy between the groups was inconclusive. However, the time to shock resolution was earlier in the norepinephrine plus dobutamine group. Also, fewer children in the intervention group were refractory to treatment. Further studies powered to detect (or exclude) an important difference would be required to test this intervention.
ACKNOWLEDGMENTS
We are thankful to the PICU nurses, residents, and support staff for their contribution to the conduct of the study.
Supplementary Material
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Davis AL, Carcillo JA, Aneja RK, et al. : American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med 2017; 45:1061–1093 [DOI] [PubMed] [Google Scholar]
- 2.Ceneviva G, Paschall JA, Maffei F, et al. : Hemodynamic support in fluid- refractory pediatric septic shock. Pediatrics 1998; 102:e19. [DOI] [PubMed] [Google Scholar]
- 3.Dalimonte MA, DeGrado JR, Anger KE: Vasoactive agents for adult septic shock: An update and review. J Pharm Pract 2020; 33:523–532 [DOI] [PubMed] [Google Scholar]
- 4.Ranjit S, Aram G, Kissoon N, et al. : Multimodal monitoring for hemodynamic categorization and management of pediatric septic shock: A pilot observational study. Pediatr Crit Care Med 2014; 15:e17–e26 [DOI] [PubMed] [Google Scholar]
- 5.Brierley J, Peters MJ: Distinct hemodynamic patterns of septic shock at presentation to pediatric intensive care. Pediatrics 2008; 122:752–759 [DOI] [PubMed] [Google Scholar]
- 6.Tibby SM, Hatherill M, Marsh MJ, et al. : Clinicians’ abilities to estimate cardiac index in ventilated children and infants. Arch Dis Child 1997; 77:516–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss SL, Peters MJ, Alhazzani W, et al. : Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med 2020; 21:e52–e106 [DOI] [PubMed] [Google Scholar]
- 8.Sankar J, Das RR, Jain A, et al. : Prevalence and outcome of diastolic dysfunction in children with fluid refractory septic shock--a prospective observational study. Pediatr Crit Care Med 2014; 15:e370–e378 [DOI] [PubMed] [Google Scholar]
- 9.Jentzer JC, Coons JC, Link CB, et al. : Pharmacotherapy update on the use of vasopressors and inotropes in the intensive care unit. J Cardiovasc Pharmacol Ther 2015; 20:249–260 [DOI] [PubMed] [Google Scholar]
- 10.Annane D, Vignon P, Renault A, et al. ; CATS Study Group: Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: A randomised trial. Lancet 2007; 370:676–684 [DOI] [PubMed] [Google Scholar]
- 11.Levy B, Bollaert P-E, Charpentier C, et al. : Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: A prospective, randomized study. Intensive Care Med 1997; 23:282–287 [DOI] [PubMed] [Google Scholar]
- 12.Levy B, Perez P, Perny J, et al. : Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Crit Care Med 2011; 39:450–455 [DOI] [PubMed] [Google Scholar]
- 13.Zhou S-X, Qiu H-B, Huang Y-Z, et al. : Effects of norepinephrine, epinephrine, and norepinephrine-dobutamine on systemic and gastric mucosal oxygenation in septic shock. Acta Pharmacol Sin 2002; 23:654–658 [PubMed] [Google Scholar]
- 14.Kleinman ME, Chameides L, Schexnayder SM, et al. : Part 14: Pediatric advanced life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122:S876–S908 [DOI] [PubMed] [Google Scholar]
- 15.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis: International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8 [DOI] [PubMed] [Google Scholar]
- 16.Carcillo JA, Fields A: Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. J Pediatr (Rio J) 2002; 78:449–466 [PubMed] [Google Scholar]
- 17.Choong K, Bohn D, Fraser DD, et al. ; Canadian Critical Care Trials Group: Vasopressin in pediatric vasodilatory shock: A multicenter randomized controlled trial. Am J Respir Crit Care Med 2009; 180:632–639 [DOI] [PubMed] [Google Scholar]
- 18.Gaies MG, Gurney JG, Yen AH, et al. : Vasoactive–inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010; 11:234–238 [DOI] [PubMed] [Google Scholar]
- 19.Matics TJ, Sanchez-Pinto LN: Adaptation and validation of a pediatric Sequential Organ Failure Assessment score and evaluation of the Sepsis-3 definitions in critically ill children. JAMA Pediatr 2017; 171:e172352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leteurtre S, Duhamel A, Salleron J, et al. ; Groupe Francophone de Réanimation et d’Urgences Pédiatriques (GFRUP): PELOD-2: An update of the PEdiatric logistic organ dysfunction score. Crit Care Med 2013; 41:1761–1773 [DOI] [PubMed] [Google Scholar]
- 21.Jain A, Sankar J, Anubhuti A, et al. : Prevalence and outcome of sepsis-induced myocardial dysfunction in children with “sepsis” “with” and “without shock”-a prospective observational study. J Trop Pediatr 2018; 64:501–509 [DOI] [PubMed] [Google Scholar]
- 22.García X, Mateu L, Maynar J, et al. : [Estimating cardiac output. Utility in the clinical practice. Available invasive and non-invasive monitoring]. Med Intensiva 2011; 35:552–561 [DOI] [PubMed] [Google Scholar]
- 23.Ramaswamy KN, Singhi S, Jayashree M, et al. : Double-blind randomized clinical trial comparing dopamine and epinephrine in pediatric fluid-refractory hypotensive septic shock. Pediatr Crit Care Med 2016; 17:e502–e512 [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Pang L, Wang Y, et al. : Combination era, using combined vasopressors showed benefits in treating septic shock patients: A network meta-analysis of randomized controlled trials. Ann Transl Med 2019; 7:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng L, Yan J, Han S, et al. : Comparative efficacy of vasoactive medications in patients with septic shock: A network meta-analysis of randomized controlled trials. Crit Care 2019; 23:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.