Introduction:
The American Academy of Pediatrics recommends premedication for all nonemergent neonatal intubations, yet there remains significant variation in this practice nationally. We aimed to standardize our unit’s premedication practices for improved intubation success and reduced adverse events.
Methods:
The study workgroup developed educational material and protocol content. Process measures included premedication use, education, and audit form completion. Primary (success on first intubation attempt and adverse event rates) and secondary (trainee success) study outcomes are displayed using statistical process control charts and pre-post cohort comparisons.
Results:
Forty-seven percent (97/206) of nurses completed educational intervention before protocol release, with an additional 20% (42/206) following a staff reminder. Two hundred sixteen (216) patients were intubated per protocol with 81% (174/216) audit completion. Compared with baseline (n = 158), intubation attempts decreased from 2 (IQR, 1–2) to 1 (IQR, 1–2) (P = 0.03), and success on the first attempt increased from 40% (63/158) to 57% (124/216) (P < 0.01), with a notable improvement in trainee success from less than 1% (1/40) to 43% (31/72) (P < 0.01). The rate of severe and rare adverse events remained stable; however, there was a rise in nonsevere events from 30% (48/158) to 45% (98/216). The tachycardia rate increased with atropine use. There was no change in chest wall rigidity, number of infants unable to extubate following surfactant, or decompensation awaiting medications.
Conclusions:
Standardizing procedural care delivery reduced intubation attempts and increased the attempt success rate. However, this was accompanied by an increase in the rate of nonsevere adverse events.
INTRODUCTION
Although a life-saving intervention, the physiologic response to endotracheal intubation potentiates various adverse events (AEs) that impact neonatal morbidity and mortality.1–4 Preterm infants are at higher risk of severe intraventricular hemorrhage and adverse neurodevelopmental outcomes in those requiring multiple intubation attempts.5–7 Single-site studies have reported intubation-related AEs in 22%−39% of intubations.5,8,9 A registry study of 10 academic centers reported ≥1 AE in 18% of neonatal intensive care unit (NICU) and 17% of delivery room intubations, including severe oxygen desaturation in 48% of NICU and 31% of delivery room intubations.1 Notably, there were significant differences in site-specific AEs, suggesting facility practice variation contributed to disparities in procedure safety and outcomes.1
Factors associated with improved intubation success are also protective against procedural AEs.5,8,10 Proceduralist experience directly correlates with an increased success rate.10,11 One single-center study reported overall first attempt success at 50% ± 8%, with the lowest success for novice providers (pediatric residents 42% ± 9%) compared with experienced clinicians (nonphysician clinicians 52% ± 9%, NICU fellows 63% ± 14%, and NICU attendings 64% ± 16%).10
Although provider skill level is important, performing a successful intubation can be impacted by physiologic stability, airway anatomy, and the safety culture. Physiologic instability (eg, bradycardia and oxygen desaturation) is the most cited reason for unsuccessful attempts and a target for QI efforts.12 Therefore, creating optimal intubation conditions characterized by jaw relaxation, open and immobile vocal cords, and suppression of laryngeal reflexes is essential.13 Premedication with a vagolytic to prevent bradycardia, an analgesic for pain control, and a neuromuscular blocking agent (NMBA) for paralysis improves intubating conditions, decreases the number of attempts, and minimizes AEs.14,15 In 2010, the American Academy of Pediatrics recommended premedication for all nonemergent intubations. However, this practice has been inconsistently adopted in the US and international NICUs.1,5,16,17
Proposed barriers to premedication use include a lack of consensus regarding the optimal drug regimen based on gestational age.18 Additionally, long-term benefits and adverse effects are not well studied in premature neonates. Surveys of providers have reported a perceived lack of benefit in improving intubation conditions and concern for medication errors or adverse drug events.16 Providers in our unit echoed these responses and raised concerns regarding the ability to extubate following surfactant administration.
QI methodology is increasingly used to address barriers to premedication use.3–5,19 These studies demonstrated that written policies could aid staff in medication administration and reduce AEs without significant threat to clinical deterioration while awaiting medication.3,4,8,19,20 However, despite the extensive body of literature supporting premedication, a 2020 survey of clinicians in 70 countries reported that 12% of practitioners do not use premedication, 32% of units do not have a standardized protocol, and 60% of providers chose premedication according to personal preference.17 This report suggests that targeted facility-level education and protocol standardization is needed to improve compliance.
Our unit aimed to improve procedural outcomes by implementing a standardized premedication protocol with accompanying staff education. Our primary aim was to increase intubation success on the first attempt from 40% to 50% and decrease overall AEs from 50% to 40% for all nonemergent intubations in our level IV NICU. Secondarily, we aimed to improve trainee intubation success on the first attempt from <1% to 40%.
METHODS
Study Context
This QI project was conducted at the Medical University of South Carolina (MUSC) Shawn Jenkins Children’s Hospital NICU, an 85-bed level IV regional perinatal center. Approximately 2,700 newborns are delivered annually, and an additional 12,000 neonates are born within our referral area, with approximately 1,000 annual admissions. Intubations are performed by residents (categorical pediatrics and internal medicine/pediatrics), neonatal nurse practitioners (NNPs), physician assistants (PAs), fellows, attending neonatologists, and rarely, respiratory therapists (RTs) or subspecialty physicians (otorhinolaryngology and anesthesia).
Period 1. Baseline Data Collection (May 1, 2019–January 27, 2020)
Baseline data extraction occurred by retrospective electronic medical record (EMR) review for the 9 months (period 1) preceding all study activities. These data included patient demographics, proceduralist characteristics, intubation indication, the number of attempts, and AEs. In addition, the medication administration record provided data on premedication use.
Period 2. Education and Protocol Development (January 28, 2020–August 5, 2020)
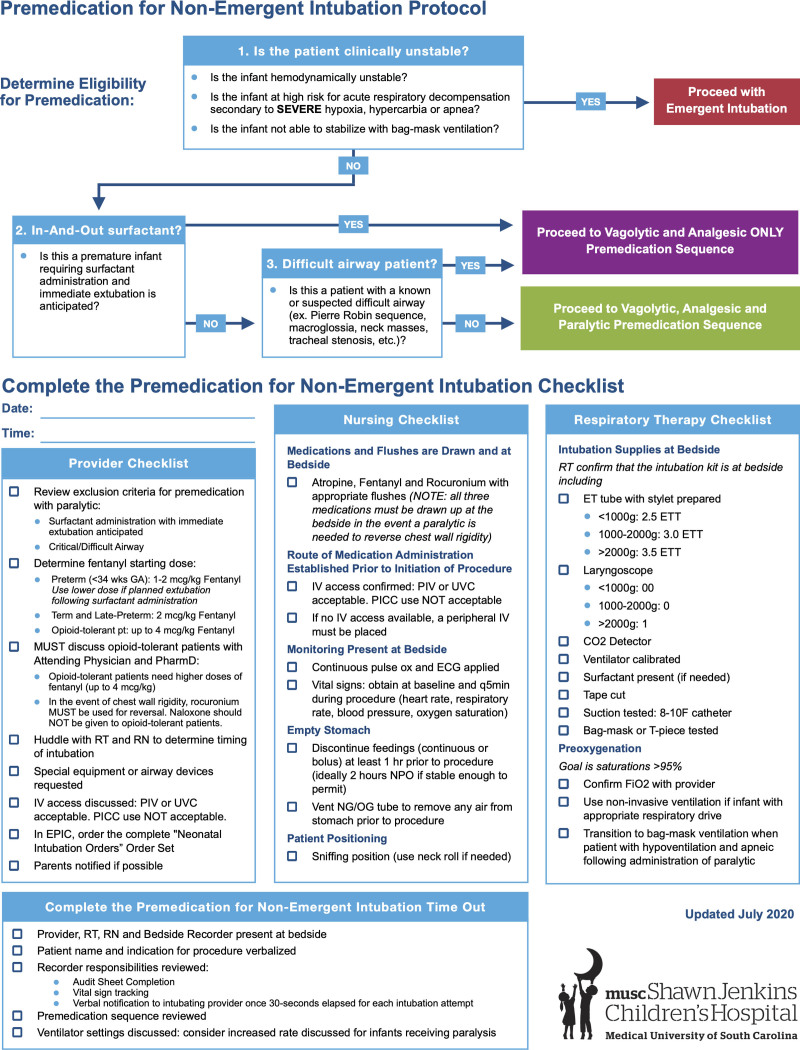
A multidisciplinary workgroup with representation from bedside nursing staff, nurse management, RT, pharmacy, and providers (NNPs, PAs, and MD/DOs) conducted an evidence-based literature review to adapt national guidelines to the local clinical context. As a result, the MUSC Pre-Medication for Nonemergent Intubation Protocol (Fig. 1) received consensus-based clinical guideline approval on July 1, 2020. The protocol instituted educational, process, and systems-based changes to target interdisciplinary communication, clear role assignment, medication utilization, and AE tracking.
Fig. 1.
Medical University of South Carolina premedication for nonemergent intubation protocol.
Educational Intervention
Provider education was through lecture-based instruction. Education addressed gaps in knowledge related to indications for premedication use, fentanyl dosing considerations to ensure the smallest effective dose, and inappropriate use of an NMBA for agitation. Also, an educational video series for RNs reviewed optimal intubation conditions, outlined drug classes, demonstrated medication administration, and introduced the novel role of the bedside recorder. The video release was through a secure streaming service on July 31, 2020.
Premedication Computerized Order Set Intervention
Revisions to the existing EMR premedication order set (created in 2011) streamlined the ordering, procurement, and administration processes. All 3 medications (atropine, fentanyl, and rocuronium) were preset to be automatically ordered to ensure that all medications were at the bedside, including rocuronium, in cases of chest wall rigidity. Fentanyl defaulted to 2 mcg/kg, so providers were required to change the order if requiring an alternative dose (eg, opioid-tolerant infants or preterm infants intubated for surfactant delivery). Atropine would need to be deselected if contraindicated (eg, history of arrhythmia). All medications were available in automated dispensing cabinets in the patient care unit. If a patient required a rocuronium dilution, the pharmacy prepared a patient-specific dose within 30 minutes per the institution’s “STAT” medication policy. Nurses completed a double check of all high-alert medications removed from automated dispensing cabinets as required by the institution’s policy.
Determine Eligibility for Premedication Intervention
Any condition necessitating emergent intubation excluded a patient from the protocol. Infants with suspected or known critical/difficult airway or planned extubation following surfactant did not receive NMBAs. Infants with a history of arrhythmia did not receive atropine.
Preintubation Procedure Checklist Intervention
Before timeout, each team member completed their portion of the premedication procedure checklist (Fig. 1). Peripheral intravenous or umbilical venous catheters were the only acceptable form of vascular access, given the need for the rapid push of vagolytic and NMBAs. Peripherally inserted central catheters were unacceptable as the 1.9 French lumen diameter required slow bolus infusion. Infants without IV access required peripheral intravenous placement. Recommended items included discontinuation of feeds (1−2 hours prior if possible for clinically stable infants), requests for special equipment, and an attempt to contact a parent/guardian.
Designated Bedside Recorder Role Intervention
A bedside recorder was designated from available nursing staff to complete the clinical audit form (Fig. 2) and instruct providers to terminate an attempt for vital sign instability or if exceeding 30 seconds. An intubation attempt is any visual evaluation of the airway with a laryngoscope, even without an endotracheal tube (ETT) passage (traditionally referred to as an intubation view).
Fig. 2.
Clinical audit form. Employed by the designated bedside recorder for tracking protocol process, outcome, and balancing measures during the intubation procedure.
Procedure Timeout Intervention
The protocol mandated that all team members be present to initiate a timeout and must remain at the bedside for premedication administration.
Chest Wall Rigidity Prevention Intervention
To prevent chest wall rigidity, the intubation team delivered fentanyl over 5 minutes, followed by a sodium chloride (NaCl) flush over 5 minutes. Fentanyl delivery could be shortened to 1−3 minutes if administered with rocuronium. In the event of chest wall rigidity, rocuronium was pushed by IV. Naloxone for opioid reversal in opioid-naive patients corrected prolonged sedation effects preventing extubation.
Period 3. Study Intervention (August 6, 2020–July 30, 2021)
On August 6, 2020, the protocol (Fig. 1) was announced to NICU staff by email notification. Access to an electronic version was available on the unit’s online clinical protocol repository. In addition, printed protocols and audit forms were placed on all intubation carts and in provider workrooms. Twelve months of data tracking occurred between August 6, 2020, and July 30, 2021.
Patient and Proceduralist Characteristics
Data tracking included patient demographics, proceduralist discipline, and level of experience.
Study Measures
The clinical audit form (Fig. 2) tracked study measures. Process measures included educational video completion, audit form completion, and premedication use. Outcome measures included the number of intubation attempts, rate of AEs (nonsevere, severe, and rare), procedural events, and clinician response to pain/agitation. Nonsevere AEs included bradycardia [<100 beats per minute (BPM) and ≥60 BPM], tachycardia >180 BPM, desaturation <88% SpO2, esophageal intubation (immediate recognition), oral or airway bleeding, difficult bag-mask ventilation, and emesis. Severe AEs included severe bradycardia <60 BPM, severe desaturation >20% from SpO2 baseline, esophageal intubation (delayed identification), chest wall rigidity, direct airway trauma, and transition to emergent intubation. Rare AEs included hypotension requiring intervention, chest compressions, code medications, pneumothorax, and death. Balancing measures included decompensation awaiting medications, medication errors and side effects, and inability to extubate following surfactant administration if planned. Medication errors were tracked using the hospital’s standardized patient safety reporting system. Medication side effects were tracked using the clinical audit form.
PDSA Cycles
The workgroup met every 6−10 weeks during period 3 through a virtual platform to discuss unit staff feedback and review statistical process control (SPC) charts. Change ideas were generated and selected for PDSA cycles to address staff feedback.
Statistical Analysis
Pre-Post Cohort Design
Comparisons of selected measures between the pre- (period 1) and post- (period 3) cohorts occurred to detect differences. All variables were assessed for normalcy. Demographic variables, intubation indication, and premedication use used a χ2 test for nominal data and an independent t test for continuous, unmatched data. Outcome, process, and balancing measures utilized a χ2 test for discrete data and an independent t test for continuous data. All continuous outcomes required Levene’s test for equality of variances. If significant variance occurred between groups, degrees of freedom were adjusted using the Welch-Satterthwaite method. χ2 analyses or Fisher’s exact tests assessed categorical variables as appropriate. Data analyses were performed using IBM SPSS Statistical Analysis Software (version 28.0.1.0) and compared using a P value α < 0.05.
Time Series Analysis
SPC charts identified special cause variation for primary and secondary outcome measures using QI Macros for Excel version 2020 (KnowWare International, Inc., Denver, Colo.). Rules for identification of special cause variation follow Western Electric rules.21,22 Centerline shifts and upper and lower control limits were adjusted when meeting the criteria for special cause variation.
Ethical Consideration
The MUSC Office of Research Integrity did not require IRB review as the project did not constitute research defined under the Common Rule 45 CFR 46.102(d).
RESULTS
Period 1
In period 1, 38% (158/418) of infants intubated in the NICU received some form of premedication. The most commonly used analgesic was fentanyl at 99% (156/158). Rarely were vagolytics and NMBAs used: 1% (2/158) vagolytic and 11% (17/158) NMBAs (Table 1). The indication for NMBAs was a rescue therapy for agitation in 24% (4/17), for chest wall rigidity in 12% (2/17), for vocal cord relaxation on a second or third intubation attempt in 18% (3/17), and prophylactically in 47% (8/17).
Table 1.
Demographic Data, Indication for Intubation, Premedication Practice Characteristics, Process Measures, Outcome Measures, Balancing Measures, and Adverse Event Rates for the 9-Month Baseline (Period 1) and 12-Month Study Intervention Period (Period 3)
| Patient Demographic Data | |||
|---|---|---|---|
| Period 1 (n = 158) | Period 3 (n = 216) | P | |
| Postnatal age, median day (IQR) | 2 (1– 22) | 2 (1– 19) | 0.42 |
| Postmenstrual age, median week (IQR) | 30 (27– 36) | 31 (28– 35) | 0.34 |
| Weight, median gram (IQR) | 1,288 (836– 2,418) | 1,405 (991– 2,168) | 0.87 |
| Sex (female), n (%) | 56 (35) | 97 (45) | 0.07 |
| Critical airway, n (%) | 3 (2) | 0 (0) | 0.04 |
| Indication for Intubation | |||
| Period 1 (n = 158) | Period 3 (n = 216) | P | |
| Need for mechanical ventilation (apnea, hypercarbia, hypoxemia, increased work of breathing, or pneumothorax), n (%) | 91/158 (58) | 112/216 (52) | 0.27 |
| Surfactant administration, n (%) | 53/158 (34) | 83/216 (38) | 0.29 |
| Unplanned extubation with ETT replacement required, n (%) | 1/158 (1) | 3/216 (1) | 0.48 |
| Upsize ETT, n (%) | 11/158 (7) | 15/216 (7) | 1.00 |
| Exchange ETT, n (%) | 2/158 (1) | 2/216 (1) | 0.75 |
| Need for stable airway ahead of surgical procedure, n (%) | 0/158 (0) | 1/216 (1) | 0.39 |
| Premedication Use | |||
| Period 1 (n = 158) | Period 3 (n = 216) | P | |
| Any premedcation use, n (%) | 158/418 (38) | 216/498 (43) | 0.09 |
| Opiate only | 141/158 (89) | 2/216 (1) | <0.01 |
| Opiate + muscle relaxant use | 15/158 (10) | 0/216 (0) | <0.01 |
| Opiate + vagolytic use | 0/158 (0) | 76/216 (35) | <0.01 |
| Opiate + muscle relaxant + vagolytic use | 2/158 (1) | 138/216 (64) | <0.01 |
| Process, Outcome, and Balancing Measures | |||
| Period 1 (n = 158) | Period 3 (n = 216) | P | |
| Process measures | |||
| Education intervention completion, n (%) | |||
| Before protocol initiation | N/a | 97/206 (47) | — |
| After protocol initiation | N/a | 42/206 (20) | — |
| Protocol completion, n (%) | N/a | 216 | — |
| Clinical audit form completion rate, n (%) | N/a | 174/216 (81) | — |
| Outcome measures | |||
| Intubation success on first attempt (all providers), n (%) | 63/158 (40) | 124/216 (57) | <0.01 |
| Proceduralist on successful first attempt, n (%) | |||
| Trainee (pediatric resident, PA student, and NNP student) | 1/40 (0) | 31/72 (43) | <0.01 |
| NNP/PA | 44/88 (50) | 72/110 (66) | 0.03 |
| Fellow | 17/28 (61) | 18/29 (62) | 0.92 |
| RT | 1/2 (50) | 4/5 (80) | 0.43 |
| Attending physician | 0 (0) | 0 (0) | — |
| Intubation attempts, median (IQR) | 2 (2–3) | 1 (1–2) | 0.03 |
| Adverse events, n (%) | |||
| Nonsevere | 48/158 (30) | 98/216 (45) | <0.01 |
| Severe | 30/158 (19) | 40/216 (19) | 0.91 |
| Rare | 1/158 (0) | 1/216 (0) | 0.82 |
| Procedural Events, n (%) | 19/158 (12) | 30/216 (14) | 0.60 |
| Response to inadequate analgesia | |||
| Second dose of analgesia | 2/6 (33) | 8/9 (89) | 0.03 |
| Rocuronium infused | 4/6 (67) | 0/9 (0) | <0.01 |
| No corrective action | 0/6 (0) | 1/9 (11) | 0.40 |
| Balancing measures | |||
| Tachycardia | 0/158 (0) | 10/216 (5) | <0.01 |
| Decompensation awaiting medications | N/a | 0/216 (0) | — |
| Chest wall rigidity | 7/158 (4) | 6/216 (3) | 0.39 |
| Inability to extubate after surfactant | 5/158 (3) | 10/216 (5) | 0.48 |
Period 2
Period 2 data were excluded from the pre-post cohort comparison as educational interventions were underway. Forty-seven percent (97/206) of nursing staff viewed educational video content during period 2.
Period 3
During period 3, 43% (216/498) of all intubated infants completed the study protocol, and 81% (174/216) had a completed audit form.
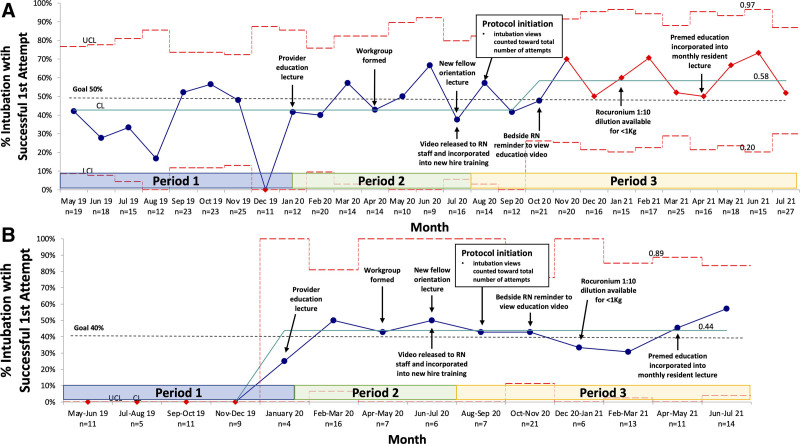
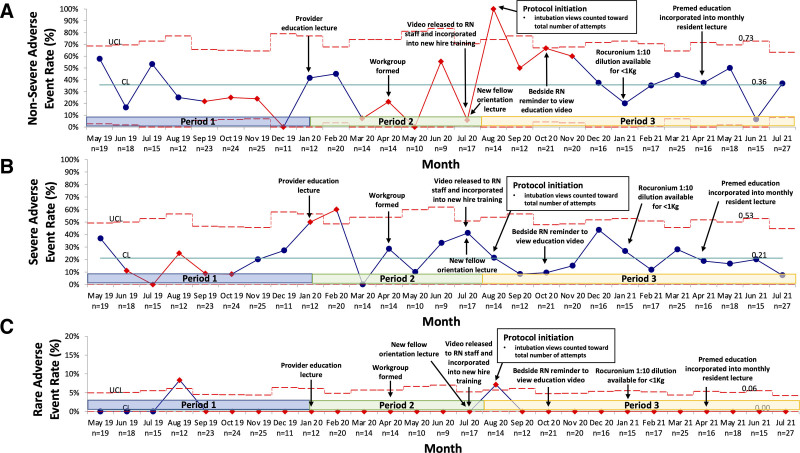
SPC charts for periods 1–3 display key outcome measures (Figs. 3–5). A centerline shift from 43% to 58% occurred for the success rate on the first intubation attempt in October 2020 (Fig. 3A) and from 0% to 44% for trainee success in January 2020 (Fig. 3B). AE rates did not meet process change criteria (Figs. 4, 5). Arrows indicate corresponding PDSA cycle interventions and are also summarized in Table 1, Supplemental Digital Content, http://links.lww.com/PQ9/A443. Interventions included video view promotion through email and staff meeting alerts [increasing video views to 68% (139/206)]. In addition, in January 2021, a 1:10 rocuronium dilution was made available for infants <1 kg due to nursing concern that doses were nearing the minimum volume required to ensure dose delivery. This concern was a theoretical risk as no audits reported the need for additional NMBA doses before this PDSA cycle, and reassuringly, there continued to be no reports of inadequate paralysis following this intervention.
Fig. 3.
First intubation attempt success rate (%) P-chart. A, Aggregate first attempt success rate for all providers (trainee, NNP/PA, fellow, and RT) p-chart. No attending physicians made a first intubation attempt during any of the 3 periods, and thus, are not included in the “all provider” designation. B, First attempt success rate for trainees (resident physician, NNP student, and PA student) p-chart.
Fig. 5.
Nonsevere, severe, and rare adverse event rate (%) p-chart. A, Nonsevere adverse event rate p-chart. Nonsevere adverse events include bradycardia (<100 BPM and ≥60 BPM), tachycardia >180 BPM, desaturation <88% SpO2, esophageal intubation with immediate recognition, oral or airway bleeding, difficult bag-mask ventilation, and emesis. B, Severe adverse event rate p-chart. Severe adverse events include severe bradycardia <60 BPM, severe desaturation >20% from SpO2 baseline, esophageal intubation with delayed identification, chest wall rigidity, direct airway trauma, and transition to emergent intubation. C, Rare adverse event rate p-chart. Rare adverse events include hypotension requiring intervention, chest compressions, code medications, pneumothorax, and death.
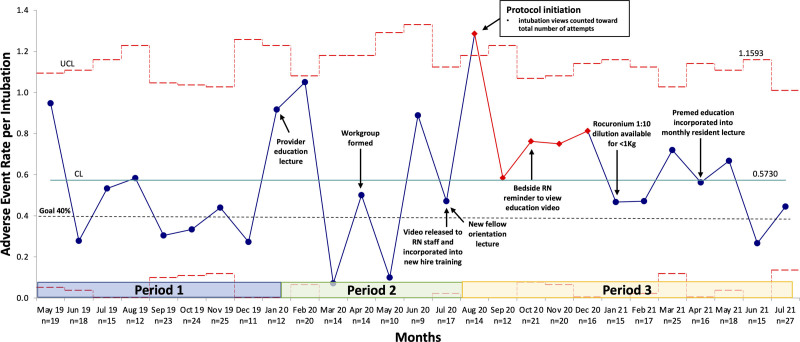
Fig. 4.
Overall adverse event rate per intubation event U-chart. This figure displays the cumulative number of overall AEs (nonsevere, severe, and rare) as the numerator over a denominator of total intubation events per month. Each discrete intubation event could have up to 18 overall AEs (7 nonsevere AEs, 6 severe AEs, and 5 rare AEs). Therefore, the cumulative number of reported AEs might exceed the total number of intubation events per month.
Pre-Post Cohort Comparison
Table 1 summarizes patient demographics, intubation indication, premedication use, study measures, and AEs for periods 1 and 3. Key differences to highlight include decreased intubation attempts from 2 [interquartile range (IQR), 1–2] to 1 (IQR, 1–2) (P = 0.03). In addition, there was a significant increase in success on the first intubation attempt for all providers (trainees, NNP/PAs, fellows, and RTs) from 40% (63/158) to 57% (124/216) (P < 0.01) with a notable improvement in trainee success on the first attempt from <1% (1/40) to 43% (31/72) (P < 0.01). Also, trainees performed an increased number of first intubation attempts: 25% (40/158) in period 1 versus 33% (72/216) in period 3 (P < 0.01).
Patients experiencing nonsevere AEs increased from 30% (48/158) to 45% (98/216) (P < 0.01), with a significant rise in documented desaturation events from 6% (10/158) to 30% (65/216) (P < 0.01). The overall rate of severe and rare AEs and procedural events was not different between periods 1 and 3 (Table 1). Table 2, Supplemental Digital Content, http://links.lww.com/PQ9/A444 further delineates the types and rates of AE and procedural event. Notable findings include improvement in difficulty with bag-mask ventilation, decreased direct airway trauma, and fewer cases of equipment deficiency. Severe desaturations accounted for a proportionally larger number of severe AEs [2% (3/158) versus 13% (28/216); P < 0.01].
While there was no difference in rates of inadequate analgesia, there was a practice change in response to this procedural event. Patients inappropriately received rocuronium in 67% (4/6) cases, and only 33% (2/6) received additional analgesia in period 1, whereas 89% (8/9) of patients appropriately received a second dose of fentanyl to address inadequate analgesia in period 3.
Regarding balancing measures, no infants decompensated awaiting medication from the pharmacy, and there were no reported medication errors. The rate of tachycardia with atropine use increased from 0% (0/158) to 5% (10/216) (P < 0.01). Seventy percent (7/10) of tachycardia was observed in preterm (<34 weeks) infants, with 10% (1/10) in late preterm (34 weeks and 0 days—36 weeks and 6 days) infants and 20% (2/10) in term (37 weeks and older) infants. One infant did not receive atropine due to a history of wide-complex tachyarrhythmia, and 2 infants did not receive atropine as there was no acceptable IV access. There was no change in the rate of chest wall rigidity [4% (7/158) versus 3% (6/216); P = 0.39] or the number of infants unable to extubate following surfactant (3% (5/158) versus 5% (10/216); P = 0.48). No infants required naloxone for opioid reversal.
DISCUSSION
Premedication for nonemergent intubation is not the standard of care in many NICUs.3,4,10,13,15,17 This observation was true in our unit as we observed significant practice disparity in the baseline period. This finding is likely due to the absence of a formalized protocol and premedication utilization being at the provider’s discretion. Interestingly, a premedication EMR order set was available nearly a decade ahead of our quality initiative. Unfortunately, this order set may have failed to effectually change clinical practice given its implementation without corresponding staff education. Thus, our unit elected to employ an education-focused QI approach with representation from key stakeholders to ensure change ideas would lead to sustained practice change and improved patient outcomes. As a result, our unit has decreased the number of intubation attempts and increased success on the first attempt, with a notable increase in trainee success.
Opportunities for pediatric trainees to intubate have declined with stricter duty hours, practice changes in meconium tracheal aspiration, and increased use of noninvasive ventilation for preterm infants.23–25 The increased risk of AEs also lends to hesitancy in allowing inexperienced trainees to intubate this medically fragile population.1,25 Furthermore, published studies show the failure of residents to achieve intubation competency ahead of program completion.24,26,27 Our QI initiative shows the potential to mitigate this trend by demonstrating increased trainee intubation opportunities and a significant improvement in success on the first attempt.
Previous studies have shown that interdisciplinary communication and clear role assignment improve team function.13 Furthermore, high team stress levels increase rates of AEs, and premedication has a protective effect.28 The preintubation checklist distinctly defined procedural responsibilities for improved coordination and staff accountability. This intervention standardized procedural preparedness and communication, likely contributing to the observed outcomes.
Before this quality initiative, the intubating provider was responsible for AE reporting, preventing accurate tracking. Furthermore, EMR procedure documentation only included 1 free text box in the note template to document “complications” without clearly defining a procedure complication. We highlight these significant gaps in our unit’s AE reporting system to emphasize the inaccuracy of AE tracking in period 1. The study workgroup targeted this reporting gap by implementing the role of a bedside recorder. Thus, the study group anticipated the observed rise in nonsevere AEs with the introduction of a bedside recorder and routine atropine use. Additional considerations that may account for the rise in hypoxemia include masking hypoxemic bradycardia with atropine, and hypoventilation-induced hypoxemia with paralytic use.29,30 Our protocol attempted to minimize these causes by limiting views to 30 seconds.29,31 Of note, a recent randomized control trial using a high-flow nasal cannula offers a potential intervention to reduce hypoxemia.32
Our unit improved selective safety measures (eg, reduced airway trauma, improved equipment presence, and effective bag-mask ventilation) and appropriate provider response to inadequate analgesia. In addition, balancing measures remained stable, except for tachycardia with atropine use. Although tachycardia is a well-tolerated side effect in infants with normal cardiovascular function, there is a lack of published data on atropine safety in the neonatal population.33–35 Forgoing atropine in term infants, who might tolerate a vagal response with less profound bradycardia, could be considered. However, there are no published data to support this approach.
Limitations of this study include the cohort design, which is hypothesis-generating and cannot prove causality. The study measures were obtained primarily by bedside recorder reports, which may lead to observer reporting bias. Furthermore, a majority consensus by the workgroup determined that paper audit forms were preferred to electronic ones given the ease of use but lent to gaps in data form tracking when not placed in appropriate collection receptacles. Although the generalizability of single-center initiatives may be limited, similarities in the infrastructure (trainee representation, patient volume, and frequency of intubation) of level III and IV academic perinatal centers lend to adaptation. Yet, there remains a great need for multicenter studies to evaluate the long-term neurodevelopmental outcomes of premedication standardization for benefit-risk assessment. Counseling for patient families regarding long-term neurodevelopmental outcomes was not standardized as part of the preprocedure checklist. Our improvement team would encourage institutions to include a more robust plan for benefit-risk discussion and disclosure in their protocol development. Additionally, the educational video was not a mandated requirement before the protocol launch, which likely contributed to the low completion rate (47%). This limitation was addressed with targeted staff promotion with an email alert and a verbal announcement at the monthly nursing staff meeting (increasing view rate to 68%) and incorporation into required new hire nursing orientation.
CONCLUSIONS
The QI strategies developed in this study can serve as a model to standardize premedication use for nonemergent intubation; however, this will require appropriate integration with local safety and pharmacologic practices. Commissioning interdisciplinary input from procedural stakeholders for improved feasibility and protocol usability is recommended for facility-specific applications.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article. Supported, in part, by the National Center for Advancing Translational Sciences of the National Institutes of Health under Grant Number UL1 TR001450.
Supplementary Material
Footnotes
Published online December 27, 2022
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
Drs. Wagner and Ross contributed equally as co-senior authors.
Presented at the Pediatric Academic Society (PAS) Meeting, Denver, Colo., April 2022, Oral Presentation; at the Southern Society for Pediatric Research (SSPR) Meeting, New Orleans, La., February 2022, Oral Presentation; at the American Academy of Pediatrics (AAP) National Conference, Section on Neonatal-Perinatal Medicine, Philadelphia, Pa., October 2021, Virtual Conference Poster Presentation; at the 13th Pediatrics Darby Children’s Research Institute (DCRI) Research Symposium, April 2021, Charleston, SC., Virtual Conference Poster Presentation; at the Association of Pediatric Program Directors (APPD) Annual Spring Meeting, March 2021, Virtual Conference Poster Presentation.
However, the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
To Cite: Diego EK, Malloy K, Cox T, Broomall A, Orr L, Baxter C, Meany S, Baker N, Fraser J, Corbin KS, Gregoski MJ, Wagner CL, Ross JR. Implementation of a Standardized Premedication Bundle to Improve Procedure Success Rate and Reduce Adverse Events for Nonemergent Neonatal Intubations. Pediatr Qual Saf 2022;8:e622.
REFERENCES
- 1.Foglia EE, Ades A, Sawyer T, et al. Neonatal intubation practice and outcomes: an International Registry Study. Pediatrics. 2019;143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawyer T, Johnson K. Neonatal intubation: past, present, and future. Neoreviews. 2020;21:e335–e341. [DOI] [PubMed] [Google Scholar]
- 3.Herrick HM, Pouppirt N, Zedalis J, et al. Reducing severe tracheal intubation events through an individualized airway bundle. Pediatrics. 2021;148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shay R, Weikel BW, Grover T, et al. Standardizing premedication for nonemergent neonatal tracheal intubations improves compliance and patient outcomes. J Perinatol. 2022;42:132–138. [DOI] [PubMed] [Google Scholar]
- 5.Hatch LD, Grubb PH, Lea AS, et al. Interventions to improve patient safety during intubation in the neonatal intensive care unit. Pediatrics. 2016;138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sauer CW, Kong JY, Vaucher YE, et al. Intubation attempts increase the risk for severe intraventricular hemorrhage in preterm infants-a retrospective cohort study. J Pediatr. 2016;177:108–113. [DOI] [PubMed] [Google Scholar]
- 7.Wallenstein MB, Birnie KL, Arain YH, et al. Failed endotracheal intubation and adverse outcomes among extremely low birth weight infants. J Perinatol. 2016;36:112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foglia EE, Ades A, Napolitano N, et al. Factors associated with adverse events during tracheal intubation in the NICU. Neonatology. 2015;108:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatesh V, Ponnusamy V, Anandaraj J, et al. Endotracheal intubation in a neonatal population remains associated with a high risk of adverse events. Eur J Pediatr. 2011;170:223–227. [DOI] [PubMed] [Google Scholar]
- 10.Sawyer T, Foglia E, Hatch LD, et al. Improving neonatal intubation safety: a journey of a thousand miles. J Neonatal Perinatal Med. 2017;10:125–131. [DOI] [PubMed] [Google Scholar]
- 11.Evans P, Shults J, Weinberg DD, et al. Intubation competence during neonatal fellowship training. Pediatrics. 2021;148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haubner LY, Barry JS, Johnston LC, et al. Neonatal intubation performance: room for improvement in tertiary neonatal intensive care units. Resuscitation. 2013;84:1359–1364. [DOI] [PubMed] [Google Scholar]
- 13.Kumar P, Denson SE, Mancuso TJ; Committee on Fetus and Newborn, Section on Anesthesiology and Pain Medicine. Premedication for nonemergency endotracheal intubation in the neonate. Pediatrics. 2010;125:608–615. [DOI] [PubMed] [Google Scholar]
- 14.Carbajal R, Eble B, Anand KJ. Premedication for tracheal intubation in neonates: confusion or controversy? Semin Perinatol. 2007;31:309–317. [DOI] [PubMed] [Google Scholar]
- 15.Ozawa Y, Ades A, Foglia EE, et al. ; National Emergency Airway Registry for Neonates (NEAR4NEOS) Investigators. Premedication with neuromuscular blockade and sedation during neonatal intubation is associated with fewer adverse events. J Perinatol. 2019;39:848–856. [DOI] [PubMed] [Google Scholar]
- 16.Shay R, Weikel BW, Grover T, et al. Standardizing premedication for nonemergent neonatal tracheal intubations improves compliance and patient outcomes. J Perinatol. 2021;42:132–138. [DOI] [PubMed] [Google Scholar]
- 17.Mari J, Franczia P, Margas W, et al. International consensus is needed on premedication for non-emergency neonatal intubation after survey found wide-ranging policies and practices in 70 countries. Acta Paediatr. 2020;109:1369–1375. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor TL. Premedication for nonemergent intubation in the NICU: a call for standardized practice. Neonatal Netw. 2021;40:8–13. [DOI] [PubMed] [Google Scholar]
- 19.Glenn TJ, Grathwol MM, McClary JD, et al. Decreasing time from decision to intubation in premedicated neonates: a quality improvement initiative. Pediatr Qual Saf. 2019;4:e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanLooy JW, Schumacher RE, Bhatt-Mehta V. Efficacy of a premedication algorithm for nonemergent intubation in a neonatal intensive care unit. Ann Pharmacother. 2008;42:947–955. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery DC. Introduction to Statistical Quality Control. 8th ed. Wiley; 2019. [Google Scholar]
- 22.Wheeler TA, Davis JT, Brilli RJ. The aggregate point rule for identifying shifts on P charts and U charts. Pediatr Qual Saf. 2018;3:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leone TA, Rich W, Finer NN. Neonatal intubation: success of pediatric trainees. J Pediatr. 2005;146:638–641. [DOI] [PubMed] [Google Scholar]
- 24.Downes KJ, Narendran V, Meinzen-Derr J, et al. The lost art of intubation: assessing opportunities for residents to perform neonatal intubation. J Perinatol. 2012;32:927–932. [DOI] [PubMed] [Google Scholar]
- 25.Johnston L, Sawyer T, Ades A, et al. ; NEAR4NEOS Investigators. Impact of physician training level on neonatal tracheal intubation success rates and adverse events: a report from National Emergency Airway Registry for Neonates (NEAR4NEOS). Neonatology. 2021;118:434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starr M, Sawyer T, Jones M, et al. A simulation-based quality improvement approach to improve pediatric resident competency with required procedures. Cureus. 2017;9:e1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Handley SC, Pouppirt N, Zucker E, et al. Improving the resident educational experience in a level IV neonatal/infant intensive care unit. Pediatr Qual Saf. 2020;5:e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umoren RA, Sawyer TL, Ades A, et al. ; National Emergency Airway Registry for Neonates (NEAR4NEOS) Investigators. Team stress and adverse events during neonatal tracheal intubations: a report from NEAR4NEOS. Am J Perinatol. 2020;37:1417–1424. [DOI] [PubMed] [Google Scholar]
- 29.Kothari R, Hodgson KA, Davis PG, et al. Time to desaturation in preterm infants undergoing endotracheal intubation. Arch Dis Child Fetal Neonatal Ed. 2021;106:603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones P, Dauger S, Peters MJ. Bradycardia during critical care intubation: mechanisms, significance and atropine. Arch Dis Child. 2012;97:139–144. [DOI] [PubMed] [Google Scholar]
- 31.Zaichkin J, Kamath-Rayne BD, Weiner G. The NRP 8th edition: innovation in education. Adv Neonatal Care. 2021;21:322–332. [DOI] [PubMed] [Google Scholar]
- 32.Hodgson KA, Owen LS, Kamlin COF, et al. Nasal high-flow therapy during neonatal endotracheal intubation. N Engl J Med. 2022;386:1627–1637. [DOI] [PubMed] [Google Scholar]
- 33.Barrington KJ. The myth of a minimum dose for atropine. Pediatrics. 2011;127:783–784. [DOI] [PubMed] [Google Scholar]
- 34.Barrington K. Premedication for endotracheal intubation in the newborn infant. Paediatr Child Health. 2011;16:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andriessen P, Janssen BJA, Berendsen RCM, et al. Cardiovascular autonomic regulation in preterm infants: the effect of atropine. Pediatr Res. 2004;56:939–946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.