Abstract
Objectives
Community-living older Medicare and Medicaid enrollees (“dual-enrollees”) have high care needs and commonly receive paid and unpaid long-term services and supports (LTSS) to help with routine activities. Little is known about whether receiving paid help or individuals’ state and neighborhood environmental context (“LTSS environment”) relates to dual-enrollees’ care experiences.
Methods
We examine a sample of n = 979 community-dwelling dual-enrollees with disabilities from 2011 to 2015 National Health and Aging Trends Study, linked to measures of neighborhood disadvantage and state Medicaid home and community-based services (HCBS) generosity. Logistic regression models stratified by dementia status assess associations between paid help and: (a) adverse consequences due to unmet care needs, and (b) participation restrictions in valued activities, among dual-enrollees with and without dementia, adjusting for individual and LTSS environmental characteristics.
Results
Use of paid help was greater for those with (versus without) dementia (46.9% vs. 37.8%). Neighborhood disadvantage was associated with greater use of paid help among dual-enrollees living with dementia. High state Medicaid HCBS generosity was associated with the use of paid help, regardless of dementia status. Dual-enrollees with dementia receiving paid help had higher odds of experiencing adverse consequences due to unmet need (adjusted odds ratio = 2.05; 95% confidence interval 1.16–3.61; p = .02)―no significant associations were observed for participation restrictions. Use of paid help and LTSS environment were not significantly associated with care experiences for dual-enrollees without dementia.
Discussion
Findings highlight the complexities of caring for dual-enrollees, particularly those with dementia, and emphasize the need to strengthen the delivery of paid care with considerations for the LTSS environment.
Keywords: Cognitive impairment, Home care, Social determinants of health
Approximately 7.4 million low-income older adults with disabilities were dually-enrolled in Medicare and Medicaid in 2018 (CMS Medicare-Medicaid Coordination Office, 2019), of whom nearly 75% lived in community settings (Zhanlian Feng, 2018). Compared to Medicare beneficiaries who are not enrolled in Medicaid (nonduals), dual-enrollees have more limited social and economic resources (Musumeci, 2017) and are more often living with dementia (McGarry et al., 2020). Despite higher annual costs of care compared to nonduals, (Johnston & Joynt Maddox, 2019) dual-enrollees are more likely to report negative care experiences, including adverse consequences due to unmet needs and participation restrictions in valued activities (Allen et al., 2013).
Dual-enrollees with physical and cognitive disability may receive long-term services and supports (LTSS) from paid helpers through Medicaid home and community-based services (HCBS) benefits (Garfield et al., 2015), as well as family and unpaid caregivers. Medicaid state and waiver programs vary significantly by state (Meucci et al., 2018), although the trend has been toward rebalancing from an institutionally-biased system toward HCBS to better align with older adult preferences to remain independent in the community (Ng et al., 2015). As a result, community-living dual-enrollees, including those with significant disability and dementia, are better able to access paid services than in the past (Kasper et al., 2015; Reckrey, Morrison et al., 2020). Nationally, per-enrollee Medicaid HCBS spending is higher for those with dementia than those without (Gorges et al., 2019).
The policy, economic, and legal determinants of population health are well-established (Montez et al., 2021). Although few studies have specifically examined the extent to which social and policy attributes of the environment contribute to the care experiences of older adults with disabilities, this is a potentially important area of investigation. For example, neighborhood disadvantage is associated with dementia diagnosis and greater care needs (Ryan Powell et al., 2020). Individuals living in more socioeconomically disadvantaged neighborhoods often have more limited access to health care services (Butler et al., 2013; Kind et al., 2014), and worse quality of life (Mather & Scommegna, 2017). The environmental context of dual-enrollees is especially relevant due to the socioeconomically disadvantaged status of this population which predisposes them to live in neighborhoods with more limited resources and amenities (Sapra et al., 2020). State policy may also play a role in dual-enrollees’ care experiences. The share of a state’s Medicaid LTSS expenditures that finance HCBS (Burr et al., 2005), known as Medicaid HCBS generosity, is associated with the use of paid services, for example (Fabius et al., 2019). Additionally, high Medicaid HCBS generosity is inversely associated with caregiving stress regardless of HCBS use (Hong & Casado, 2015).
Conceptual Framework
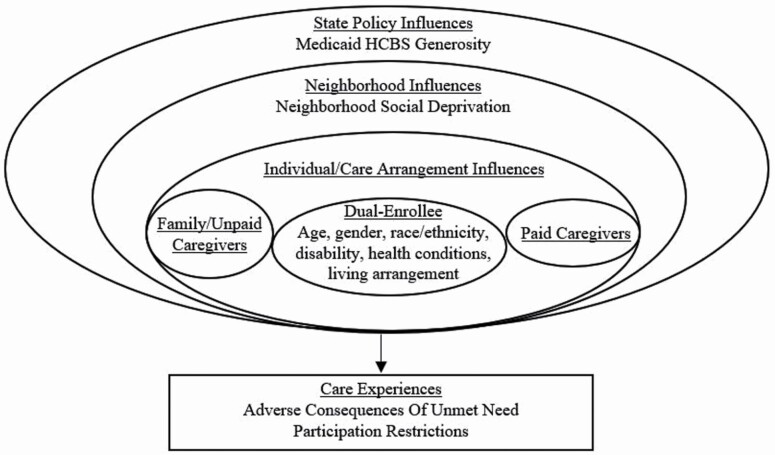
The present exploratory study draws on the Convoys of Care model (Kemp et al., 2012), which was developed to understand the care experiences of older adults and family and paid caregivers in assisted living, and defines convoys as “dynamic networks of close personal relationships through which social support is distributed or exchanged” (Kemp et al., 2012). The model proposes that convoys are nested in and affected by individual, social, economic, and political factors (Kemp et al., 2012). Our adapted model posits that care experiences (i.e., adverse consequences due to unmet need, participation restrictions) are a result of multiple influences, including individual (e.g., age) characteristics, available support (e.g., living arrangements), and the LTSS environment (e.g., neighborhood disadvantage; Figure 1).
Figure 1.
Adapted conceptual model based on the Convoys of Care model.
The present study specifically focuses on the relationship between the use of paid help and care experiences of dual-enrollees with and without dementia given the greater availability of paid help in this population. Because older adults with dementia more often experience unmet needs and low social engagement compared to those without dementia (Beach et al., 2020; Hackett et al., 2019), we examine care experiences for each group separately. We also examine the role of contextual factors in care experiences, as these are relevant to the availability and accessibility of care and highly variable across state and local geographies. As a result of having greater needs that drive the use of paid help (Beach et al., 2020), we hypothesize that dual-enrollees receiving paid help will be more likely to report negative care experiences, regardless of dementia status. Because living in disadvantaged neighborhoods increases risks for poor health and limited access to resources (Shavers, 2007), we also hypothesize that dual-enrollees living in neighborhoods with high social disadvantage will more often report negative care experiences, and those living in a state with high Medicaid HCBS generosity will less often report negative care experiences.
Methods
Data and Sample
We use data from the National Health and Aging Trends Study (NHATS), a nationally representative study of Medicare beneficiaries ages 65 and older, as well as linked census tract data from the American Community Survey (ACS) and state-level data related to the Medicaid program HCBS generosity (Eiken et al., 2014, 2018) based on NHATS participants’ geocoded place of residence. NHATS was originally fielded in 2011, with annual follow-up interviews, and the sample replenished in 2015 (Freedman & Kasper, 2019). In person interviews are conducted with study participants or proxy respondents if the participant is unable to respond.
We pooled data from NHATS rounds 1–5 (2011–2015). The study cohort includes 979 (weighted N = 3,647,143) community-dwelling older adults who were receiving help with self-care, mobility, or household activities, and reported being enrolled in Medicaid in the 2011 NHATS or a subsequent follow-up interview (2012–2015). We relied on survey responses to identify each participant’s first year of Medicaid eligibility, so each participant appears in the data set once (Supplemental Table 1). We excluded NHATS participants who were not enrolled in Medicaid at any time during the observation period, as well as those living in nursing homes or residential care settings, or not receiving help with self-care, mobility, or household activities for a health and function reason.
Measures
Care experiences
Adverse consequences due to unmet need.—
Older adults with high needs more often experience adverse consequences due to unmet self-care and mobility-related needs, rather than household needs (e.g., shopping; Beach et al., 2020), and self-care and mobility needs drive decisions regarding Medicaid eligibility due to disability. For these reasons, we focus on adverse consequences due to unmet self-care and mobility-related needs. We first identified older adults with self-care and mobility limitations. NHATS asks older adults about how they perform self-care (eating, bathing, dressing, and toileting) and mobility (indoor and outdoor, transferring in and out of bed). For each activity, older adults are asked whether they receive help, and the level of difficulty if they performed the activity with or without assistance. If participants reported performing the activity without assistance, they indicated how difficult it was to do the activity alone. Respondents were considered to have a need for assistance if they reported that they had assistance with a self-care or mobility activity, or that they performed an activity themselves with difficulty. We then constructed binary measures indicating limitations by individual activity and activity domain (e.g., self-care). Participants who reported receiving help or having difficulty completing an activity were asked whether they experienced an adverse consequence due to no one being there to provide help or the activity being too difficult for them to complete on their own. Adverse consequences included the following: going without eating, being unable to shower, taking a bath, or wash up, wetting or soiling yourself, going without getting dressed, having to stay in the house, being unable to get around inside the home, and having to stay in bed. We created a summary measure that indicated whether a participant experienced at least one adverse consequence due to an unmet need, as in prior work (Allen et al., 2013; Wolff et al., 2019).
Participation restrictions.—
Interdisciplinary Aging Research to Address Health Disparities in Alzheimer’s Disease and Related Dementias: Participation restrictions refer to activities reported as being very or somewhat important to the respondent that were limited in the prior month due to health or functioning reasons. Care recipients were asked to report whether the following activities were important to them: visiting friends and family, attending religious services, attending club meetings or group activities, and going out for enjoyment. Care recipients were characterized as having a participation restriction if the activity was somewhat or very important, and they were unable to participate due to a health reason, an approach employed in previous literature (Fabius, Wolff et al., 2020; Wolff et al., 2016).
Older adult characteristics
Dementia refers to probable dementia, identified via self-reported dementia diagnosis, a score indicating dementia on the AD8 Dementia Screening Interview, or performance on cognitive tests of memory, orientation, and executive function (Kasper et al., 2013). This methodology more accurately estimates the population of older adults living with dementia, which is often underdiagnosed and underreported (Amjad et al., 2018; Connolly et al., 2011; Lang et al., 2017; Savva & Arthur Antony, 2015). To identify paid help, we created a dichotomous variable indicating that a person helping with self-care, mobility, or household activities (for a health reason) was paid.
We include several individual characteristics: age (65–74; 75–84; and >85), sex, and race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Hispanic and other race/ethnicity). Multimorbidity refers to a number of self-reported diagnosed conditions, from the following: heart attack, heart disease, high blood pressure, arthritis, osteoporosis, diabetes, lung disease, stroke, or cancer (Beach et al., 2020). We include two measures that reflect support characteristics. First, to assess an older adult’s level of disability, which is associated with the use of paid help and care experiences (Fabius et al., 2021; Thomas & Applebaum, 2015), we include the number of daily activities (self-care and mobility) older adults received help with. Living arrangement was included as a proxy measure that may reflect level of available support (Beach et al., 2020). Respondents indicated whether they lived alone, with a spouse or with a spouse and others, or with others only.
LTSS environment characteristics include area social and economic disadvantage and Medicaid HCBS generosity. We used a census tract-level measure of neighborhood social disadvantage: the social deprivation index (Butler et al., 2013), which is a composite of seven demographic characteristics from the ACS and reflects 5-year estimates from 2011 to 2015. We examined a dichotomous variable representing the most (top 15%) socially disadvantaged neighborhoods in the United States. Medicaid HCBS generosity was assessed for each study year, drawing on state-specific Medicaid HCBS expenditures from publicly available sources (Eiken et al., 2014, 2018), and measured categorically based on quartiles for the analytic sample.
Analyses
Because older adults with dementia are more likely to use paid help (Kasper et al., 2015), and have worse care experiences than those without dementia (Beach et al., 2020; Hackett et al., 2019), all analyses are stratified by dementia status. We first compare characteristics of dual-enrollees with and without dementia, by use of paid help. We present frequencies for categorical measures and means for continuous measures (as well as 95% confidence intervals [CI]). Second, we assess differences among dual-enrollees with and without dementia who reported adverse consequences due to unmet needs and participation restrictions, by receipt of paid help. Finally, we present multivariate logistic regression models to assess associations between the LTSS environment and our two measures of care experiences after adjusting for individual factors. Analyses were conducted with Stata, version 15 (StataCorp, 2017) using weighted data and variables that account for the complex survey design (Montaquila et al., 2012).
Results
Drawing on weighted estimates from the 2015 NHATS, among community-living older adults dually-enrolled in both Medicare and Medicaid, use of paid help was greater for those with (versus without) dementia (46.9% vs. 37.8%; Table 1). Use of paid help was greater for those 85 and older, with more health conditions, receiving help with more daily activities, and those living alone, regardless of dementia status. Paid help use did not differ by sex, race, or ethnicity. Paid help use was more common among those living in the most (versus all others) disadvantaged neighborhoods among those with dementia only (39.0% vs. 27.3% and 25.9% vs. 28.9% for those without dementia). Medicaid HCBS generosity was associated with the use of paid help for dual-enrollees, regardless of dementia status.
Table 1.
Individual Characteristics of Community-Dwelling Dual-Enrollees With and Without Dementia Receiving Assistance, by Use of Paid Help
| No dementia | Dementia | |||
|---|---|---|---|---|
| No paid help | Paid help | No paid help | Paid help | |
| 62.2 (56.9, 67.2) | 37.8 (32.8, 43.1) | 53.1 (47.2, 58.9) | 46.9% (41.0, 52.8) | |
| n = 264 | n = 191 | n = 252 | n = 272 | |
| Weighted estimate (in thousands) | 1,164 | 707 | 943 | 832 |
| Individual characteristics | ||||
| Age | ||||
| 65–74 | 41.8 (34.0, 49.9) | 27.0 (18.9, 37.1) | 26.1 (18.5, 35.5) | 17.1 (10.7, 26.2) |
| 75–84 | 42.2 (34.2, 50.7) | 48.3 (40.5, 56.2) | 46.5 (37.5, 32.0) | 38.7 (32.0, 45.8) |
| >85 | 16.0 (11.6, 21.8) | 24.7 (18.6, 31.9) | 27.5 (22.2, 33.5) | 44.2 (37.0, 51.7) |
| Female | 65.7 (59.4, 71.5) | 71.8 (65.2, 77.5) | 64.9 (54.4, 74.1) | 66.6 (59.2, 73.3) |
| Race | ||||
| White | 53.9 (46.5, 61.1) | 53.2 (41.6, 64.3) | 39.5 (30.8, 49.0) | 40.7 (31.8, 50.2) |
| Black | 19.8 (16.0, 24.2) | 19.5 (16.0, 24.2) | 21.0 (15.7, 27.6) | 20.9 (16.4, 26.3) |
| Hispanic | 20.0 (15.1, 26.0) | 20.1 (15.1, 26.0) | 22.9 (16.2, 31.6) | 26.1 (19.5, 34.1) |
| Other | 6.3 (3.2, 12.1) | 7.3 (2.7, 18.1) | 16.6 (9.8, 26.7) | 12.2 (6.5, 21.8) |
| Number of health conditions, M (95% CI) | 3.2 (3.0, 3.4) | 3.5 (3.3, 3.8) | 2.9 (2.7, 3.2) | 3.3 (3.1, 3.6) |
| Support characteristics | ||||
| Number of daily activities receiving help with, M (95% CI) | 2.1 (1.7, 2.4) | 3.2 (2.7, 3.6) | 3.3 (2.9, 3.7) | 4.7 (4.4, 5.1) |
| Living arrangement | ||||
| Alone | 26.3 (20.1, 33.6) | 44.8 (36.1, 53.9) | 16.5 (10.8, 24.4) | 26.2 (19.2, 34.7) |
| Spouse | 38.1 (30.9, 45.9) | 14.9 (10.1, 21.5) | 37.6 (29.2, 46.8) | 16.0 (11.4, 22.0) |
| Other | 35.6 (29.5, 42.2) | 40.2 (32.2, 48.9) | 45.9 (38.5, 53.5) | 57.8 (48.3, 66.6) |
| LTSS environment characteristics | ||||
| Neighborhood social | ||||
| Disadvantage | ||||
| Least disadvantaged (<85) | 71.5 (63.8, 78.2) | 74.1 (66.1, 80.8) | 72.7 (64.8, 79.4) | 61.0 (50.7, 70.4) |
| Most disadvantaged (≥85) | 28.5 (21.9, 36.2) | 25.9 (19.2, 33.9) | 27.3 (20.6, 35.2) | 39.0 (29.6, 49.3) |
| Medicaid generosity | ||||
| Q1 (8.6%–27.4%) | 26.4 (17.8, 37.3) | 15.4 (9.2, 24.6) | 31.0 (21.1, 43.1) | 14.1 (8.3, 22.9) |
| Q2 (27.4%–36.4%) | 21.6 (13.4, 32.8) | 22.5 (14.4, 33.3) | 20.2 (13.0, 30.0) | 19.8 (12.7, 29.4) |
| Q3 (36.8%–55.4%) | 26.5 (18.5, 14.4) | 23.1 (14.4, 35.0 | 22.7 (14.6, 33.5) | 30.1 (20.4, 41.9) |
| Q4 (55.6%–77.7%) | 25.5 (19.6, 32.6) | 39.0 (28.4, 50.9) | 26.1 (17.0, 37.9) | 36.0 (26.5, 46.9) |
Notes: CI = confidence interval; LTSS = long-term services and supports. All estimates are survey weight adjusted; estimates presented are weighted percentages and 95% confidence intervals unless otherwise noted; National Health and Aging Trends Study 2011–2015; 979 Medicaid-enrolled persons aged 65 and older living in community settings (excluding those in nursing homes and residential care facilities) and reporting receiving assistance with self-care, mobility, or household tasks (for a health reason).
Dual-enrollees receiving paid help were more likely to report adverse consequences due to unmet needs and participation restrictions, regardless of dementia status (Table 2). For example, compared to those who did not use paid help, those with and without dementia receiving paid help were more likely to experience adverse consequences due to unmet needs relating to self-care (82.1% vs. 62.7% and 70.9% vs. 53.3%, respectively). Dual-enrollees with dementia using paid help were more likely to experience mobility-related adverse consequences due to unmet need (69.7 vs. 54.1%). Similarly, dual-enrollees with and without dementia using paid help were more likely to report participation restrictions (78.0% vs. 63.4% and 71.0% vs. 58.1%, respectively), with both groups experiencing participation restrictions related to going out for enjoyment (42.6% vs. 30.3% and 44.6% vs. 22.2%, respectively). Dual-enrollees with dementia using paid help also more often experienced restrictions in attending religious services (61.0% vs. 43.6%) and attending club meetings or group activities (31.3% vs. 19.0%).
Table 2.
Care Experiences of Community-Dwelling Older Adults Receiving Assistance, by Use of Paid Help
| No dementia | Dementia | |||
|---|---|---|---|---|
| No paid help | Paid help | No paid help | Paid help | |
| 62.2 (56.9, 67.2) | 37.8 (32.8, 43.1) | 53.1 (47.2, 58.9) | 46.9% (41.0, 52.8) | |
| n = 264 | n = 191 | n = 252 | n = 272 | |
| Weighted estimate (in thousands) | 1,164 | 707 | 943 | 832 |
| Adverse consequences due to unmet needs | ||||
| Any adverse consequences due to unmet need | 53.3 (45.1, 61.3) | 70.9 (62.1, 78.5) | 62.7 (75.9, 86.9) | 82.1 (75.9, 86.9) |
| Self-care disability (weighted estimate) | N = 1,493,100 | N = 1,561,362 | ||
| Self-care related adverse consequences | 36.2 (26.9, 46.6) | 52.2 (41.8, 62.4) | 48.4 (40.4, 56.4) | 68.7 (61.1, 75.3) |
| Mobility disability (weighted estimate) | N = 1,655,825 | N = 1,652,992 | ||
| Mobility related adverse consequences | 58.9 (51.2, 66.2) | 60.5 (52.3, 68.2) | 54.1 (46.3, 61.8) | 69.7 (62.1, 76.4) |
| Participation restrictions | ||||
| Any participation restrictions | 58.1 (49.3, 66.5) | 71.0 (61.2, 79.2) | 63.4 (54.3, 71.6) | 78.0 (69.9, 84.4) |
| Attending religious services | 38.3 (30.4, 46.9) | 46.2 (38.1, 54.5) | 43.6 (35.2, 52.3) | 61.0 (52.7, 68.7) |
| Visiting family and friends | 33.4 (26.2, 41.4) | 39.1 (32.2, 46.5) | 40.0 (32.9, 47.5) | 45.7 (37.0, 55.7) |
| Going out for enjoyment | 22.2 (16.8, 28.7) | 44.6 (35.7, 53.9) | 30.3 (23.1, 38.5) | 42.6 (35.3, 50.3) |
| Attending club meetings or group activities | 20.4 (14.7, 27.6) | 28.0 (21.1, 36.1) | 19.0 (13.8, 25.5) | 31.3 (23.8, 39.8) |
Notes: All estimates are survey weight adjusted; estimates presented are weighted percentages and 95% confidence intervals; National Health and Aging Trends Study 2011–2015; 979 Medicaid-enrolled persons aged 65 and older living in community settings (excluding those in nursing homes and residential care facilities) and reporting receiving assistance with self-care, mobility, or household tasks (for a health reason).
In fully adjusted logistic regression models, use of paid help was associated with adverse consequences due to unmet need for dual-enrollees with dementia only (Table 3). Those living with dementia and receiving paid help were nearly twice as likely to experience adverse consequences due to unmet need compared to those who did not use paid help (adjusted odds ratio = 2.05; 95% CI 1.16–3.61). Using paid help was not associated with adverse consequences due to unmet need or participation restrictions for dual-enrollees with or without dementia (Table 4). LTSS environment characteristics were not associated with care experiences.
Table 3.
Associations Between Paid Help and Self-Care and Mobility Related Adverse Consequences Due to Unmet Need Among Community-Dwelling Dually-Enrolled Older Adults With and Without Dementia
| Any adverse consequence due to unmet need | ||||
|---|---|---|---|---|
| No dementia adjusted odds ratio (95% CI) | p | Dementia adjusted odds ratio (95% CI) | p | |
| Uses paid help | 1.47 (0.67, 3.2) | .33 | 2.05 (1.16, 3.61) | .02 |
| Individual characteristics | ||||
| Age | ||||
| 65–74 | Reference | Reference | ||
| 75–84 | 0.97 (0.52, 1.82) | .93 | 1.48 (0.60, 3.65) | .39 |
| >85 | 0.73 (0.34, 1.81) | .42 | 0.63 (0.23, 1.73) | .37 |
| Female | 1.45 (0.66, 3.21) | .35 | 2.00 (1.05, 3.91) | .04 |
| Race | ||||
| Non-Hispanic White | Reference | Reference | ||
| Non-Hispanic Black | 0.76 (0.46, 1.25) | .35 | 0.84 (0.41, 1.70) | .62 |
| Hispanic and other | 1.01 (0.41, 2.49) | .99 | 4.26 (1.83, 9.94) | <.01 |
| Number of health conditions | 1.22 (1.04, 1.43) | .01 | 1.52 (1.26, 1.84) | <.001 |
| Support characteristics | ||||
| Number of daily activities receiving help with | 1.94 (1.61, 2.34) | <.001 | 1.68 (1.49, 1.91) | <.001 |
| Living arrangement | ||||
| Alone | Reference | Reference | ||
| Spouse | 1.53 (0.67, 3.49) | .30 | 0.49 (0.19, 1.27) | .14 |
| Others | 0.81 (0.33, 1.99) | .65 | 0.50 (0.21, 1.16) | .11 |
| LTSS environment characteristics | ||||
| Most socially disadvantaged neighborhoods | 1.57 (0.82, 3.00) | .16 | 1.01 (0.56, 1.79) | .99 |
| Medicaid generosity | ||||
| Q1 (8.6%–27.4%) | Reference | Reference | ||
| Q2 (27.4%–36.4%) | 0.47 (0.21, 1.05) | .06 | 1.86 (0.90, 3.83) | .09 |
| Q3 (36.8%–55.4%) | 0.68 (0.25, 1.83) | .44 | 1.41 (0.58, 3.47) | .44 |
| Q4 (55.6%–77.7%) | 0.66 (0.30, 1.41) | .27 | 0.67 (0.28, 1.59) | .36 |
Notes: CI = confidence interval; LTSS = long-term services and supports. All estimates are survey weight adjusted; National Health and Aging Trends Study 2011–2015; 979 Medicaid-enrolled persons aged 65 and older living in community settings (excluding those in nursing homes and residential care facilities) and reporting receiving assistance with self-care, mobility, or household tasks (for a health reason).
Table 4.
Associations Between Paid Help and Participation Restrictions Among Community-Dwelling Dually-Enrolled Older Adults With and Without Dementia
| Any participation restrictions | ||||
|---|---|---|---|---|
| No dementia adjusted odds ratio (95% CI) |
p | Dementia adjusted odds ratio (95% CI) |
p | |
| Uses paid help | 1.50 (0.82, 2.73) | .18 | 1.42 (0.74, 2.72) | .30 |
| Individual characteristics | ||||
| Age | ||||
| 65–74 | Reference | Reference | ||
| 75–84 | 1.04 (0.52, 2.06) | .98 | 1.06 (0.46, 2.47) | .89 |
| >85 | 1.48 (0.72, 3.02) | .28 | 1.16 (0.53, 2.51) | .71 |
| Female | 1.15 (0.60, 2.20) | .67 | 1.20 (0.59, 2.46) | .61 |
| Race | ||||
| Non-Hispanic White | Reference | Reference | ||
| Non-Hispanic Black | 1.08 (0.61, 1.91) | .79 | 1.95 (0.98, 3.87) | .06 |
| Hispanic and other | 0.54 (0.23, 1.28) | .16 | 1.85 (0.99, 3.46) | .06 |
| Number of health conditions | 1.14 (0.96, 1.35) | .14 | 1.31 (1.11, 1.55) | <.01 |
| Support characteristics | ||||
| Number of daily activities receiving help with | 1.20 (1.02, 1.41) | .03 | 1.25 (1.14, 1.36) | <.001 |
| Living arrangement | ||||
| Alone | Reference | Reference | ||
| Spouse | 1.30 (0.67, 2.52) | .43 | 0.35 (0.15, 0.84) | .02 |
| Others | 1.36 (0.75, 2.46) | .31 | 0.28 (0.14, 0.57) | <.001 |
| LTSS environment characteristics | ||||
| Most socially disadvantaged neighborhoods | 1.03 (0.53, 1.99) | .93 | 0.72 (0.40, 1.30) | .27 |
| Medicaid generosity | ||||
| Q1 (8.6%–27.4%) | Reference | Reference | ||
| Q2 (27.4%–36.4%) | 1.34 (0.64, 2.82) | .43 | 0.80 (0.34, 1.87) | .60 |
| Q3 (36.8%–55.4%) | 1.72 (0.69, 4.27) | .24 | 1.03 (0.47, 2.28) | .94 |
| Q4 (55.6%–77.7%) | 1.00 (0.48, 2.05) | .99 | 0.97 (0.48, 1.98) | .94 |
Notes: CI = confidence interval; LTSS = long-term services and supports. All estimates are survey weight adjusted; National Health and Aging Trends Study 2011–2015; 979 Medicaid-enrolled persons aged 65 and older living in community settings (excluding those in nursing homes and residential care facilities) and reporting receiving assistance with self-care, mobility, or household tasks (for a health reason).
Discussion
In the coming years, the demand for paid help among the growing numbers of persons living to very old ages, including those with dementia will increase (Matthews et al., 2019). Due to the long and costly course of the disease (Hurd et al., 2015), older adults with dementia are often ultimately enrolled in Medicaid. The present study provides important insight into the role of paid help in care experiences of community-dwelling dual-enrollees with and without dementia. We find that more than four in 10 dual-enrollees use paid services, regardless of dementia status. Our first hypothesis was partially supported―in fully adjusted models, use of paid help was associated with experiencing adverse consequences due to unmet need for dual-enrollees with dementia only. Our second hypothesis was not supported―we found no association between using paid help and participation restrictions, or LTSS environment characteristics and care experiences among dual-enrollees with or without dementia.
Findings call attention to the complexity of supporting dual-enrollees with dementia in the community. Prior research supports our finding that people with dementia using paid help more often experience adverse consequences due to unmet need (Fabius et al., 2021). However, to understand why this might be the case, the structure of paid help must be considered. For example, despite strides in rebalancing Medicaid LTSS spending toward HCBS, dual-enrollees may still go without needed services due to barriers like Medicaid HCBS waiver waitlists―in 2017, there were over 700,000 people on waitlists in 40 states (Musumeci et al., 2019). As a result, dual-enrollees may be unable to afford all the help they need. The cost of services for older adults (e.g., home health and homemaking) amounted to an annual cost of $55,000 in 2020 (Genworth, 2020). Additionally, even for those receiving extensive help, paid helpers provide care on schedules that may not address the intermittent needs of people with dementia. For example, care recipients may only receive services to help with bathing a few times a week, restricting them from completing these tasks as often as they would prefer (Fabius, Shugrue et al., 2020), which, if not addressed, result in costly adverse consequences due to unmet need (Wolff et al., 2019). States should consider expanding the ways in which they support social services (e.g., cash assistance and transportation) that are associated with better health outcomes and cost savings (Bradley et al., 2016). Such efforts have also proved efficacious for addressing the needs of racial/ethnic minority groups and might address the disparities observed in the present study (Adler et al., 2016; National Hispanic Council on Aging, 2015)―dual-enrollees with dementia who identified as Hispanic or other racial/ethnic group were more than four times as likely to experience adverse consequences due to unmet needs compared to White dual-enrollees.
Our finding that older adults with dementia using paid help more often experience adverse consequences due to unmet need also highlight the potential for better coordination across paid, unpaid, and medical care. When there are gaps in care, family and unpaid caregivers are often left to help, especially those assisting an older adult living with dementia (Torres et al., 2015). Additionally, recent findings demonstrate that dementia family caregivers often must manage paid caregivers in the home (Reckrey et al., 2022). Caregivers vary in feelings of preparedness for this role, and commonly also must reconcile the demands of caregiving with other responsibilities such as child care, or work, which may impact their availability as well as subsequent older adult care experiences (Mahoney et al., 2019; Pot et al., 2005). More broadly, our work and that of others indicate that caregiving is often a collaborative effort between paid helpers and family and unpaid caregivers, suggesting the need for interventions to improve communication and role negotiation between members of care networks (Reckrey et al., 2021). This includes medical providers, who less often interact with paid caregivers, but stand to gain important information about changes in care recipients’ health or behavior from them (Reckrey, Geduldig et al., 2020). New strategies should include support for paid caregivers through training, wage increases, and addressing nurse delegation, which have yet to be widely implemented in community settings (Stone & Bryant, 2019). Additionally, dual-enrollees with dementia could benefit from the scaling of efficacious community-based programs (Fortinsky et al., 2020; Hirth et al., 2009) that consider both family and unpaid caregivers and paid helpers in dementia-specific care delivery.
While no association between LTSS environment characteristics and care experiences was observed, our results substantiate the relevance of individual characteristics and supports. Moreover, bivariate analyses demonstrate the importance of LTSS environment characteristics in the use of paid help, which is likely a result of availability, affordability, accessibility, and acceptability of services. Conversely, adverse consequences due to unmet need and participation restrictions are more subjective in nature and may be more affected by individual factors (i.e., number of chronic conditions and daily activities receiving help with), especially for dual-enrollees who already have a high need for LTSS (Allen et al., 2013; Willink et al., 2019).
Some states use targeted initiatives to support dual-enrollees with dementia in Medicaid HCBS programs. Our bivariate analysis findings provide more context―dual-enrollees with dementia were more often receiving paid help if they were living in the most disadvantaged neighborhoods, and older adults with dementia are more likely than those without dementia to be receiving paid help within states with the highest quartiles of Medicaid generosity. One example of this support can be found in Massachusetts, where the waiver program includes dementia coaching and other home care services. Despite targeted supports, regardless of LTSS environment characteristics, fewer than half of dual-enrollees in our study received paid help. This may be because many dementia-tailored waivers are limited to providing services in residential care settings, such as assisted living (Garfield et al., 2015). Future HCBS strategies should target people with dementia living in the community, including through managed LTSS programs, which are also delivered through Medicaid HCBS waivers (Lewis et al., 2018).
We acknowledge several limitations. Our study is cross-sectional and relies on a relatively small sample, and results cannot be interpreted as causal. We also acknowledge the limitations associated with conducting multiple comparisons. Still, this descriptive and exploratory study yields important information about the care experiences of duals with and without dementia. To address the challenges associated with multiple comparisons, we do not present p-values in our descriptive analyses. We cannot draw conclusions about the availability of services (e.g., service area) of dual-enrollees that might impact care experiences and access to services or to identify all types of paid services that participants were receiving. The type of services people use is often associated with quality of life and health service utilization outcomes (Fabius, Shugrue et al., 2020). We are unable to determine the share of a state’s level of Medicaid HCBS expenditures allocated to services in residential care settings (e.g., assisted living). Future work should disentangle the distribution of Medicaid HCSB expenditures across settings. Despite these limitations, the present study yields important findings for better understanding the care experiences of dual-enrollees with and without dementia.
Findings from this study have implications for LTSS policy and delivery for dual-enrollees with dementia. Given the projected growth in both dementia prevalence and the costs associated with LTSS, innovative strategies are needed to meet the increasing demands for paid care. Results emphasize the complex nature of caring for dual-enrollees with dementia and indicate a need for greater efforts to strengthen the availability and generosity of HCBS that include support for dual-enrollees with dementia, family and unpaid caregivers, and paid helpers.
Supplementary Material
Contributor Information
Chanee D Fabius, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Safiyyah M Okoye, Johns Hopkins School of Nursing, Baltimore, Maryland, USA.
John Mulcahy, University of Minnesota School of Public Health, Minneapolis, Minnesota, USA.
Julia G Burgdorf, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Visting Nurse Service of New York, New York, New York, USA.
Jennifer L Wolff, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Funding
Funds to support this pilot study were provided by the National Institute on Aging (NIA) through the Hopkins’ Economics of Alzheimer’s Disease & Services (HEADS) Center under award number P30AG066587 (C. D. Fabius and J. L. Wolff), funding from the Johns Hopkins University Alzheimer’s Disease Resource Center for Minority Aging Research (P30AG059298; C. D. Fabius), Health Services and Outcomes Research for Aging Populations (T32AG066576; J. G. Burgdorf and S. M. Okoye), the Epidemiology and Biostatistics of Aging Training Grant (T32AG000247; S. M. Okoye), the NHATS/NSOC Dementia Care Conference at the Michigan Center on the Demography of Aging (P30AG012846), and the National Study of Caregiving (R01AG054004), as well as by the National Institute for Minority Health Disparities (NIMHD) through the Johns Hopkins Center for Health Disparities Solutions of the under award U54MD000214 (C. D. Fabius).
Conflict of Interest
None declared.
References
- Adler, N. E., Cutler, D. M., Fielding, J. E., Galea, S., Glymour, M. M., Koh, H. K., & Satcher, D. (2016). Addressing social determinants of health and health disparities: A vital direction for health and health care. NAM Perspectives, 6, 9. doi: 10.31478/201609t [DOI] [Google Scholar]
- Allen, S. M., Piette, E. R., & Mor, V. (2013). The adverse consequences of unmet need among older persons living in the community: Dual-Eligible versus medicare-only beneficiaries. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 69, S51–S58. doi: 10.1093/geronb/gbu124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amjad, H., Roth, D. L., Sheehan, O. C., Lyketsos, C. G., Wolff, J. L., & Samus, Q. M. (2018). Underdiagnosis of demenia: An observational study of patterns in diagnosis and awareness in US older adults. Journal of General Internal Medicine, 33(7), 1131–1138. doi: 10.1007/s11606-018-4377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach, S. R., Schulz, R., Friedman, E. M., Rodakowski, J., Martsolf, R. G., & James, A. E. (2020). Adverse consequences of unmet needs for care in high-need/high-cost older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(2), 459–470. doi: 10.1093/geronb/gby021 [DOI] [PubMed] [Google Scholar]
- Bradley, E. H., Canavan, M., Rogan, E., Talbert-Slagle, K., Ndumele, C., Taylor, L., & Curry, L. A. (2016). Variation in health outcomes: The role of spending on social services, public health, and health care, 2000–2009. Health Affairs, 35(5), 760–768. doi: 10.1377/hlthaff.2015.0814 [DOI] [PubMed] [Google Scholar]
- Burr, J. A., Mutchler, J. E., & Warren, J. P. (2005). State commitment to home and community-based services. Journal of Aging and Social Policy, 17(1), 1–18. doi: 10.1300/J031v17n01_01 [DOI] [PubMed] [Google Scholar]
- Butler, D. C., Petterson, S., Phillips, R. L., & Bazemore, A. W. (2013). Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Services Research, 48(2pt1), 539–559. doi: 10.1111/j.1475-6773.2012.01449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- CMS Medicare-Medicaid Coordination Office. (2019). Data analysis brief: Medicare-Medicaid enrollment 2006 through 2018.https://www.cms.gov/Medicare-Medicaid-Coordination/Medicare-and-Medicaid-Coordination/Medicare-Medicaid-Coordination-Office/DataStatisticalResources/Downloads/MedicareMedicaidDualEnrollmentEverEnrolledTrendsDataBrief2006-2018.pdf
- Connolly, A., Gaehl, E., Martin, H., Morris, J., & Purandare, N. (2011). Underdiagnosis of dementia in primary care: variations in the observed prevalence and comparisons to the expected prevalence. Aging & Mental Health, 15(8), 978–984. [DOI] [PubMed] [Google Scholar]
- Eiken, S., Sredl, K., Gold, L., Kasten, J., Burwell, B., & Saucier, P. (2014). Medicaid expenditures for long-term services and supports ion FFY 2012.
- Eiken, S., Sredl, K., Brian, B., & Amos A. (2018). Medicaid expenditures for long-term services and supports (LTSS) in FY 2015.
- Fabius, C. D., Ogarek, J., & Shireman, T. I. (2019). Racial disparities in medicaid home and community-based service utilization among white, black, and hispanic adults with multiple sclerosis: Implications of state policy. Journal of Racial and Ethnic Health Disparities, 6(6), 1200–1207. doi: 10.1007/s40615-019-00621-9 [DOI] [PubMed] [Google Scholar]
- Fabius, C., Shugrue, N., & Robison, J. T. (2020). Outcomes associated with home and community-based service use among older adults following a nursing home transition. Journal of Gerontological Social Work, 63(8), 807–821. doi: 10.1080/01634372.2020.1830328 [DOI] [PubMed] [Google Scholar]
- Fabius, C. D., Wolff, J. L., & Kasper, J. D. (2020). Race differences in characteristics and experiences of black and white caregivers of older Americans. The Gerontologist, 60(7), 1244–1253. doi: 10.1093/geront/gnaa042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabius, C. D., Wolff, J. L., Willink, A., Skehan, M. E., Mulcahy, J., Kasper, J. (2021). Community-based long-term services and supports: Are the needs of older adults and their caregivers being met?
- Freedman, V. A., Kasper, J. D. (2019). Cohort profile: The National Health and Aging Trends Study (NHATS). International Journal of Epidemiology, 48(4), 1044–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortinsky, R. H., Gitlin, L. N., Pizzi, L. T., Piersol, C. V., Grady, J., Robison, J. T., Molony, S., & Wakefield, D. (2020). Effectiveness of the care of persons with dementia in their environments intervention when embedded in a publicly funded home- and community-based service program. Innovation in Aging, 4(6), igaa053. doi: 10.1093/geroni/igaa053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield, R., Musumeci, M., Reaves, E. L., & Damico, A. (2015). Serving low-income seniors where they live: Medicaid’s role in providing community-based long-term services and supports. Henry J. Kaiser Family Foundation. [Google Scholar]
- Genworth. (2020). Cost of care survey. https://www.genworth.com/aging-and-you/finances/cost-of-care.html [Google Scholar]
- Gorges, R. J., Sanghavi, P., & Konetzka, R. T. (2019). A national examination of long-term care setting, outcomes, and disparities among elderly dual eligibles. Health Affairs, 38(7), 1110–1118. doi: 10.1377/hlthaff.2018.05409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett, R. A., Steptoe, A., Cadar, D., & Fancourt, D. (2019). Social engagement before and after dementia diagnosis in the English Longitudinal Study of Ageing. PLoS One, 14(8), e0220195. doi: 10.1371/journal.pone.0220195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth, V., Baskins, J., & Dever-Bumba, M. (2009). Program of all-inclusive care (PACE): Past, present, and future. Journal of the American Medical Directors Association, 10(3), 155–160. doi: 10.1016/j.jamda.2008.12.002 [DOI] [PubMed] [Google Scholar]
- Hong, M., & Casado, B. L. (2015). Caregiver stress: Does states’ expenditure on home- and community-based services matter? Home Health Care Services Quarterly, 34(2), 85–100. doi: 10.1080/01621424.2015.1029186 [DOI] [PubMed] [Google Scholar]
- Hurd, M., Martorell, P., & Langa, K. (2015). Future monetary costs of dementia in the United States under alternative dementia prevalence scenarios. Journal of Population Ageing, 8(1), 101–112. doi: 10.1007/s12062-015-9112-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, K. J., & Joynt Maddox, K. E. (2019). The role of social, cognitive, and functional risk factors in medicare spending for dual and nondual enrollees. Health Affairs, 38(4), 569–576. doi: 10.1377/hlthaff.2018.05032 [DOI] [PubMed] [Google Scholar]
- Kasper, J. D., Freedman, V. A., & Spillman, B. (2013). Classification of persons by dementia status in the national health and aging trends study. Technical Paper #5. Baltimore: Johns Hopkins University School of Public Health.www.NHATS.org [Google Scholar]
- Kasper, J. D., Freedman, V. A., Spillman, B. C., & Wolff, J. L. (2015). The disproportionate impact of dementia on family and unpaid caregiving to older adults. Health Affairs, 34(10), 1642–1649. doi: 10.1377/hlthaff.2015.0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp, C. L., Ball, M. M., & Perkins, M. M. (2012). Convoys of care: Theorizing intersections of formal and informal care. Journal of Aging Studies, 27(1), 15–29. doi: 10.1016/j.jaging.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind, A. J. H., Jencks, S., Brock, J., et al. (2014). Neighborhood socioeconomic disadvantage and 30-day rehospitalization: A retrospective cohort study. 161, 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, L., Clifford, A., Wei, L., Zhang, D., Leung, D., Augustine, G., & Chen, R. (2017). Prevalence and determinants of undetected dementia in the community: A systematic literature review and a meta-analysis. BMJ open, 7(2), e011146. doi: 10.1136/bmjopen-2016-011146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, E., Eiken, S., Amos, A., & Saucier, P. (2018). The growth of managed long term services and supports programs: 2017 update. Mena Report. https://www.medicaid.gov/medicaid/managed-care/managed-long-term-services-and-supports/index.html [Google Scholar]
- Mahoney, E. K., Simon-Rusinowitz, L., Loughlin, D. M., Ruben, K., & Mahoney, K. J. (2019). Preparedness of representatives for people with dementia in a self-directed program. Journal of Gerontological Social Work, 62(2), 172–194. doi: 10.1080/01634372.2018.1500965 [DOI] [PubMed] [Google Scholar]
- Mather, M., & Scommegna, P. (2017). How neighborhoods affect the health and well-being of older Americans. Today’s Research on Aging, 35, 1–11. https://www.prb.org/resources/how-neighborhoods-affect-the-health-and-well-being-of-older-americans/ [Google Scholar]
- Matthews, K. A., Xu, W., Gaglioti, A. H., Holt, J. B., Croft, J. B., Mack, D., & McGuire, L. C. (2019). Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer’s and Dementia, 15(1), 17–24. doi: 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry, K., Bollens-Lund, E., Rahman, O., Ferreira, K., & Ferreira, K. B. (2020). Residential setting and the cumulative financial burden of dementia in the 7 years before death. Journal of the American Geriatrics Society, 68(6), 1319. doi: 10.1111/jgs.16414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci, M. R., Kurth, N. K., Shireman, T. I., & Hall, J. P. (2018). Availability of Medicaid home- and community-based services for older Americans and people with physical disabilities. Home Health Care Services Quarterly, 37(1), 41–59. doi: 10.1080/01621424.2018.1425175 [DOI] [PubMed] [Google Scholar]
- Montaquila, J., Freedman, V. A., Spillman, B., Kasper, J. D. (2012). National Health and Aging Trends Study Development of Round 1 Survey Weights. NHATS Technical Paper #2. Baltimore: Johns Hopkins University School of Public Health. www.NHATS.org [Google Scholar]
- Montez, J. K., Hayward, M. D., & Zajacova, A. (2021). Trends in U.S. population health: The central role of policies, politics, and profits. Journal of Health and Social Behavior, 62(3), 286–311. doi: 10.1177/00221465211015411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci, M. (2017). Medicaid’s role for medicare beneficiaries.https://statistical.proquest.com/statisticalinsight/result/pqpresultpage.previewtitle?docType=PQSI&titleUri=/content/2017/R5710-216.xml
- Musumeci, M., Chidambaram, P., & Watts, M. O. (2019). Key questions about medicaid home and community-based services waiver waiting lists.https://www.kff.org/medicaid/issue-brief/key-questions-about-medicaid-home-and-community-based-services-waiver-waiting-lists/
- National Hispanic Council on Aging. (2015). Status of Hispanic Older Adults: Insights from the Field – Reframing Aging. https://www.diverseelders.org/wp-content/uploads/2018/10/2018-Status-of-Hispanic-Older-Adults.pdf
- Ng, T., Stone, J., & Harrington, C. (2015). Medicaid home and community-based services: How consumer access is restricted by state policies. Journal of Aging and Social Policy, 27(1), 21–46. doi: 10.1080/08959420.2015.969078 [DOI] [PubMed] [Google Scholar]
- Pot, A. M., Zarit, H., Twisk, J. W. R., & Townsend, A. L. (2005). Transitions in caregivers’ use of paid home help: Associations with stress appraisals and well-being. Psychology and Aging, 20(2), 211–219. doi: 10.1037/0882-7974.20.2.211 [DOI] [PubMed] [Google Scholar]
- Reckrey, J. M., Boerner, K., Franzosa, E., Bollens-Lund, E., & Ornstein, K. A. (2021). Paid caregivers in the community-based dementia care team: Do family caregivers benefit? Clinical Therapeutics, 43(6), 1–12. doi: 10.1016/j.clinthera.2021.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckrey, J. M., Geduldig, E. T., Lindquist, L. A., Morrison, R. S., Boerner, K., Federman, A. D., & Brody, A. A. (2020). Paid caregiver communication with homebound older adults, their families, and the health care team. The Gerontologist, 60(4), 745–753. doi: 10.1093/geront/gnz067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckrey, J. M., Morrison, R. S., Boerner, K., Szanton, S. L., Bollens-Lund, E., Leff, B., & Ornstein, K. A. (2020). Living in the community with dementia: Who receives paid care? Journal of the American Geriatrics Society, 68(1), 186–191. doi: 10.1111/jgs.16215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckrey, J. M., Watman, D., Tsui, E. K., Franzosa, E., Perez, S., Fabius, C. D., & Ornstein, K. A. (2022). “I Am the Home Care Agency”: The dementia family caregiver experience managing paid care in the home. International Journal of Environmental Research and Public Health, 19(3), 1311. doi: 10.3390/ijerph19031311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan Powell, W., Buckingham, W. R., Larson, J. L., Veila, L., Yu, M., Shahriar Salamat, M., Bendlin, B. B., Rissman, R. A., & Kind, A. J. H. (2020). Association of neighborhood-level disadvantage with Alzheimer Disease neuropathology. JAMA Network Open, 3(6), e207559. doi: 10.1001/jamanetworkopen.2020.7559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapra, K. J., Yang, W., Walczak, N. B., & Cha, S. S. (2020). Identifying high-cost Medicare beneficiaries: impact of neighborhood socioeconomic disadvantage. Population Health Management, 23(1), 12–19. [DOI] [PubMed] [Google Scholar]
- Savva, G. M., & Arthur, A. (2015). Who has undiagnosed dementia? A cross-sectional analysis of participants of the Aging, Demographics and Memory Study. Age and Ageing, 44(4), 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. (2017). Stata statistical software: Release 15. College Station, TX: StataCorp LLC. [Google Scholar]
- Shavers, V. L. (2007). Measurement of socioeconomic status in health disparities research. Journal of the National Medical Association, 99(9), 1013–1023. https://www.ncbi.nlm.nih.gov/pubmed/17913111 [PMC free article] [PubMed] [Google Scholar]
- Stone, R. I., & Bryant, N. S. (2019). The future of the home care workforce: Training and supporting aides as members of home-based care teams. Journal of the American Geriatrics Society, 67(S2), S444–S448. doi: 10.1111/jgs.15846 [DOI] [PubMed] [Google Scholar]
- Thomas, K. S., & Applebaum, R. (2015). Long-term services and supports (LTSS): A growing challenge for an aging America. Public Policy and Aging Report, 25(2), 56–62. doi: 10.1093/ppar/prv003 [DOI] [Google Scholar]
- Torres, J. M., Kietzman, K. G., & Wallace, S. P. (2015). Walking the line: navigating market and gift economies of care in a consumer‐directed home‐based care program for older adults. The Milbank Quarterly, 93(4), 732–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willink, A., Davis, K., Mulcahy, J., Wolff, J. L., & Kasper, J. (2019). The financial hardship faced by older Americans needing long-term services and supports. Issue Brief Commonw Fund, 2019, 1–12. [PubMed] [Google Scholar]
- Wolff, J. L., Nicholas, L. H., Willink, A., Mulcahy, J., Davis, K., & Kasper, J. D. (2019). Medicare spending and the adequacy of support with daily activities in community-living older adults with disability: An observational study. Annals of Internal Medicine, 170(12), 837–844. doi: 10.7326/M18-2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff, J. L., Spillman, B. C., Freedman, V. A., & Kasper, J. D. (2016). A national profile of family and unpaid caregivers who assist older adults with health care activities. JAMA Internal Medicine, 176(3), 372–379. doi: 10.1001/jamainternmed.2015.7664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhanlian, F. (2018). Dual eligibles: Who are they and why are they important? Public Policy and Aging Report 28, 56–63. doi: 10.1093/ppar/pry013 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.