Abstract
Objectives
The objective of this study was to understand disparities in cognitive impairment between middle-aged formerly incarcerated (FI) and nonincarcerated individuals.
Methods
The 1979 National Longitudinal Survey of Youth is a nationally representative longitudinal data set containing information on incarceration, cognitive functioning, and other health conditions. Using a modified version of the Telephone Interview for Cognitive Status (TICS-m), adapted from the Health and Retirement Study, we analyzed the association between incarceration and cognitive impairment, cognitive impairment—not dementia and dementia. Multivariable regression models were estimated, including prior incarceration status and covariates associated with incarceration and cognitive functioning.
Results
FI individuals had lower unadjusted scores on TICS-m (−2.5, p < .001) and had significantly greater unadjusted odds ratios (OR) for scoring in the cognitive impairment (OR = 2.4, p < .001) and dementia (OR = 2.7, p < .001) range. Differences were largely explained by a combination of risk factors associated with incarceration and cognition. Education and premorbid cognition (measured by Armed Forces Qualification Test) separately and completely explained differences in the odds of dementia. Regardless of incarceration status, Blacks and Hispanics had significantly greater odds of cognitive impairment and dementia relative to Whites, holding other factors constant.
Discussion
The association between prior incarceration and cognitive impairment in middle age was largely explained by differences in educational attainment and premorbid cognitive functioning, supporting the cognitive reserve hypothesis. Greater prevalence of cognitive impairment and dementia among the FI could create challenges and should be considered in reentry planning. Structural and institutional factors should be considered when addressing health disparities in Alzheimer’s Disease and Related Dementias.
Keywords: Cognitive impairment, Cognitive reserve, Formerly incarcerated, Health disparities, Reentry
The Role of Incarceration as a Risk Factor for Cognitive Impairment
For more than 40 years, the United States conducted a political experiment in mass incarceration (Raphael & Stoll, 2013), such that it has the highest incarceration rate in the world (Fair & Walmsley, 2021). Although incarceration rates began declining in 2009 (Carson, 2015), over 600,000 individuals are released from prison each year. Mass incarceration has many documented costs (Cox, 2016, 2018); however, an understudied area is the consequences related to healthy aging and the ability of people in communities disproportionately affected by incarceration to successfully age (Cox, 2018). Specifically, there is a paucity of research focusing on aging and prisoner reentry even though people in prison aged 55 or older have increased by 400% since 1993, 95% will be released into society, and 48% of those released are 35 or older (Carson & Sabol, 2016). At the same time, the baby boomer generation has begun to pass the age of 65, and the number of people living with cognitive impairment is expected to increase, with greater increases among Hispanics and non-Hispanic Blacks (Rajan et al., 2021). Cognitive impairment is costly to society: Alzheimer’s and other dementias are the most expensive diseases to treat in the United States, with an estimated cost of $355 billion (Alzheimer’s Association, 2021). If incarceration affects cognitive functioning, these costs could grow by even more than expected, with the potential cost burden varying not only by state, but also by communities experiencing differential rates of incarceration, potentially causing increased disparities in Alzheimer’s Disease and Related Dementias (ADRD).
This study addresses an important gap in the literature. To the best of our knowledge, it is the first to investigate the relationship between cognitive functioning, cognitive impairment, and incarceration postrelease (within a reentry context) using a nationally representative sample of a cohort of formerly incarcerated (FI) and nonincarcerated (NI) middle-aged people. Early declines in cognitive functioning not only have direct medical costs, but also affect the quality of life and ability to function day-to-day. Transitioning from prison or jail into society is challenging with numerous barriers to success; lower cognitive functioning and increased cognitive impairment could create additional challenges to successfully reintegrate individuals from incarceration to society.
Racial Disparities in Mass Incarceration and Cognition
Due to mass incarceration, the imprisonment rate of African Americans increased to the point that a Black male born in 2001 had a 1 in 3 chance of going to prison in his lifetime (Bonczar, 2003). African American men are imprisoned at rates six times that of their White counterparts, whereas the corresponding rates for Hispanic men are 2.4 times that of their White counterparts. At the same time, the risk of cognitive impairment is greater for African Americans than Whites (Lines et al., 2014; Mayeda et al., 2016; Rajan et al., 2021; Schwartz et al., 2004; Sloan & Wang, 2005; Zhang et al., 2016). Racial disparities in the prevalence of cognitive impairment between African Americans and Whites are larger in midlife age groups. Specifically, the prevalence of cognitive impairment among African Americans who are 85 or older is roughly two times that of their White counterparts, whereas the prevalence is four times that of Whites for African Americans aged 55–64 (Lines et al., 2014). A significant relationship between incarceration and cognitive functioning could help to shed light on racial disparities in early-onset cognitive impairment.
Mass Incarceration, Health, and Aging
Conceptually, the era of mass incarceration could be viewed as a historical event that has changed the epidemiological environment of those exposed to it, with lasting affects on the health of surviving members later in life and possible cohort and generational effects (Finch & Crimmins, 2004). Contact with this environment could affect health for the duration of life after exposure, and whether this effect is positive or negative could depend on the strength of the “protective” factors of incarceration (e.g., access to social services and other human capital investments inaccessible prior to incarceration) relative to the risk factors and how the mark of or exposure to an incarceration might affect someone’s ability to function in everyday life (Cox, 2018).
Prior research found incarceration to be associated with prolonged levels of stress, the transmission of infectious disease, vascular disease, and head trauma (Anderson et al., 2016; Bick, 2007; Maruschak et al., 2015; Massoglia, 2008; Shiroma et al., 2012). Secondarily, incarceration may cause heightened stress and health problems postrelease due to stigma and barriers to reintegration (Cox, 2018;Cox et al., 2021; Massoglia, 2008; Schnittker et al., 2012).
Given these findings, mass incarceration should be modeled from a life course perspective that aims to comprehend its effects on the cohort in question and successive birth cohorts, as well as its expression in disease trends over time in the population (Lynch & Smith, 2005).
Incarceration and Cognition
Incarceration may have direct and indirect effects on cognitive functioning. Although decline in cognitive fluid abilities is a normal part of aging (Murman, 2015), the effect of incarceration on cognitive functioning is conceptually ambiguous. On the one hand, incarceration can cause decreases in human capital (e.g., education, skill development, and health). Specifically, exposure to incarceration during adolescence could disrupt the development of protective factors such as educational attainment (Aizer & Doyle, 2015), and the experience during (Ezenwa et al., 2020; Umbach et al., 2018) and after incarceration may increase factors that collectively lead to brain injury and cognitive decline. Examples of these factors are head trauma, vascular disease, infectious diseases, substance use disorders (e.g., smoking and alcohol), and chronic stress, which are also risk factors for cognitive impairment (Barnes et al., 2006; Black, 2002; Case & Paxson, 2009; Forton et al., 2002; Gardner & Yaffe, 2014; Gorelick et al., 2011; Katan et al., 2013; Li et al., 2016; Lopez et al., 2003; McEwen & Sapolsky, 1995; Ozen et al., 2015; Sandi, 2004). The direct and indirect effects of incarceration through stress (Wilson et al., 2007) could negatively affect cognitive functioning, increase cognitive impairment, and ultimately cause a greater risk of ADRD. On the other hand, access to social services and opportunities for human capital investments (see Author Note 1) during confinement (e.g., mental health, education) could improve human capital and, therefore, cognitive functioning.
To make matters more complicated, incarcerated individuals are a highly selected population. They are more likely to experience other factors that influence the relationship between cognitive functioning and incarceration, such as adverse childhood experiences (ACEs), early onset of psychiatric disorders, and early problems with substance abuse (Schnittker et al., 2012). This suggests early life experiences should be controlled in models measuring the relationship between incarceration and cognitive functioning. Schnittker et al. (2012) found that early life experiences explain the relationship between incarceration and most psychiatric disorders. Nonetheless, after controlling for early life experiences related to childhood and substance abuse, incarceration was still strongly associated with mood disorders. When discussing the selection of the incarcerated population, it is important to note that structural factors (e.g., racism) can lead to environmental conditions that not only create barriers to develop and access protective factors for cognitive functioning (such as educational attainment) but may also increase exposure to stressful events that are potential risk factors for cognitive impairment (e.g., incarceration), especially for non-Whites (see Derenoncourt, 2022).
Taken together, the conceptualization of the relationship between incarceration and cognition should be modeled from a life course framework that incorporates how “early- and later-life biological, behavioral, social, and psychological exposures affect adult health” in general (Lynch & Smith, 2005, p. 2), and adult cognition in particular. Indeed, Richards and Deary (2005) propose a life course framework for cognitive reserve (a functional concept that emphasizes how the efficiency of neural networks can serve as protective factors against neuropathology), by placing a central focus on premorbid cognitive ability, which can be strengthened or, presumably, weakened over the life course and is influenced by the social and material environment, occupation, physical health, lifestyle, and health behaviors. Likewise, within the National Institute on Aging Health Disparities Framework (Hill et al., 2015), mass incarceration would be considered a structural factor that affects population health across the life course, and its impact should be analyzed at the environmental, sociocultural, behavioral, and biological levels.
Although, theoretically, the association between incarceration and cognition is ambiguous (see Appendix Figure 1 for a visualization of the conceptual framework), we hypothesize that the overwhelming negative direct and indirect effects of incarceration on the individual during and after an incarceration will compound the early life disadvantages disproportionately experienced by this population, resulting in lower cognitive reserve and a higher prevalence of early-onset cognitive impairment (no dementia and dementia) relative to individuals with no reported incarceration. Moreover, differences in cognitive impairment will largely be explained by the aforementioned factors that influence cognitive reserve.
Method
Data
The association between incarceration and cognitive functioning was estimated in a cohort of middle-aged men and women using the 1979 National Longitudinal Survey of Youth (NLSY79, U.S. Bureau of Labor Statistics, 2019). The NLSY79 is a unique panel data set that contains information on variables measuring criminal history, health, employment, education, parental information, cognitive ability, and childhood experiences. It offers substantial data to study the association between incarceration and cognitive functioning among middle-aged individuals because the cohort consists of respondents followed from 1979 to the present who were between 14 and 22 years old at baseline. At the time of the cognition module, respondents ranged in age from 46 to 60 years. Two health surveys were administered at age 40 or older and again at age 50 or older, starting in 1998 and 2008, respectively. Beginning in 2006, a modified version of the Telephone Interview for Cognitive Status (TICS-m; Crimmins et al., 2011; Gure et al., 2012) adapted from the Health and Retirement Study was administered as part of the 48+ cognition module (CM). By 2016, 8,021 men and women took the cognition survey. Multiple imputation by chained equations (MICE) was used to impute missing values due to item nonresponse. Although the survey is longitudinal, the TICS-m was only measured at one point. However, the longitudinal structure and richness of the data allow for analyzing a broad set of control variables suggested by the conceptual framework.
Cognitive Functioning Scale and Cognitive Impairment
The TICS-m measures fluid intelligence and was adapted from the Health and Retirement Study (Ofstedal et al., 2005). The scale measures episodic memory through immediate and delayed 10-word recall tests, working memory through a serial 7s subtraction test, and mental status using the backward count from 20 test (Ofstedal et al., 2005). The TICS-m score was calculated by summing the scores from each component of the test, with a range of 0–27 (Crimmins et al., 2011; Fisher et al., 2014; Langa et al., 2017; Ofstedal et al., 2005). Lower scores represent worse cognitive functioning. To assess the association between incarceration and early cognitive impairment, a categorical variable was created using cutpoints established in the literature (Crimmins et al., 2011; Langa et al., 2017). Specifically, scores between 7 and 11 were categorized as cognitive impairment—not dementia (CIND), whereas scores less than or equal to 6 were coded as dementia. Finally, we assessed overall cognitive impairment by combining respondents who were categorized as scoring in dementia or CIND range. That is, respondents with scores ranging from 0 to 11 on the 27-point scale were coded as cognitive impairment, and those with scores greater than or equal to 12 were coded as no cognitive impairment.
Incarceration
The NLSY79 directly asked questions regarding criminal participation and incarceration history until 1980. After 1980, incarceration status is captured, using the household variable “Type Of Residence R Is Living In” (e.g., own dwelling unit, jail, and parent household), which is asked in every survey wave (see Appendix Figure 2 for the distribution of proportion incarcerated by survey wave). Respondents whose residence is listed as “jail” are coded as incarcerated for the corresponding survey wave, while those whose residence is not reported as “jail” are coded as not incarcerated. In this way, incarceration status is documented in every survey year, which allows us to create our key independent variable of interest: incarcerated prior to the 48+ cognition survey (N = 567). Given the conceptual framework and research suggests that there could be direct effects of incarceration on cognition during incarceration, as well as indirect effects after release, we also created a dichotomous variable to control for those incarcerated at the time of the 48+ cognition survey year for which the TICS-m was administered (N = 60, of which 6 were not incarcerated prior to the 48+ cognition survey). One benefit of the data is that the NLSY project, when possible, obtained permission to interview respondents during incarceration spells (U.S. Bureau of Labor Statistics, 2011). Our measure of incarceration likely undercounts incarceration status because those with short periods of incarceration between waves are less likely to be captured. Moreover, this variable does not distinguish between the type of incarceration (i.e., jail or prison).
Control Variables
Following the conceptual framework and literature, sociodemographic covariates correlated with both incarceration and cognitive functioning were included in the regression models. The “standard” regression model included typical covariates accounted for in the literature (Langa et al., 2017). These covariates were age at the time of interview, race and ethnicity (i.e., non-Hispanic Black, Hispanic, and non-Hispanic and non-Black; see Author Note 2), highest grade completed (<12 years, 12 years, 13–15 years, and ≥16 years), and net-worth quartiles. In addition, reported diagnoses of chronic vascular conditions (i.e., ever being diagnosed with heart problems, high blood pressure, diabetes, and stroke) and body mass index (BMI), which was calculated from self-reported weight and height, were also controlled for (Langa et al., 2017). In addition, the standard regression model was augmented with early life variables associated with incarceration and cognitive functioning. These variables included premorbid cognition (i.e., 1980 Armed Forces Qualification Test [AFQT] score) and ACEs, such as if they had poor health as a child; if they were confined to home or bed for 4 or more weeks; if they were ever hospitalized for at least 2 weeks; whether they lived with anyone with depression, mental illness, or suicidal behavior; whether they lived with a problem drinker or alcoholic; how often a parent or adult physically harmed them; and how much parental love and affection they received. An indicator variable for having smoked more than 100 cigarettes during their lifetime was also included (Rodriguez et al., 2018).
Empirical Analysis
We estimated the association between TICS-m scores and incarceration using multiple regression analysis. The difference in odds of cognitive impairment between FI and NI were estimated using logistic regression. The generalized ordered logit estimator was used to model the odds of scoring in the CIND range relative to unimpaired and the odds of scoring in the dementia range relative to CIND and unimpaired (Williams, 2016).
Ten models were estimated for each of the three dependent variables by sequentially adding categories of the earlier mentioned covariates. Model 1 regresses the outcome variables on incarceration status (prior and current) and does not adjust for covariates. Models 2–5 sequentially add in the standard covariates and Models 6–9 individually add in the augmented regressors (described earlier) to the fully specified standard regression model (Model 5). Model 10 is the fully specified augmented model, which includes all standard covariates and all augmented regressors. For clarity and brevity, only Models 1, 5, 6 (Model 5 plus ACEs), and 10 are displayed; all other models can be found in the online Appendix. Missing values were imputed using MICE. All regressions, except Model 1, included urban and region fixed effects and were run using Stata/MP 16 (StataCorp, 2019).
Results
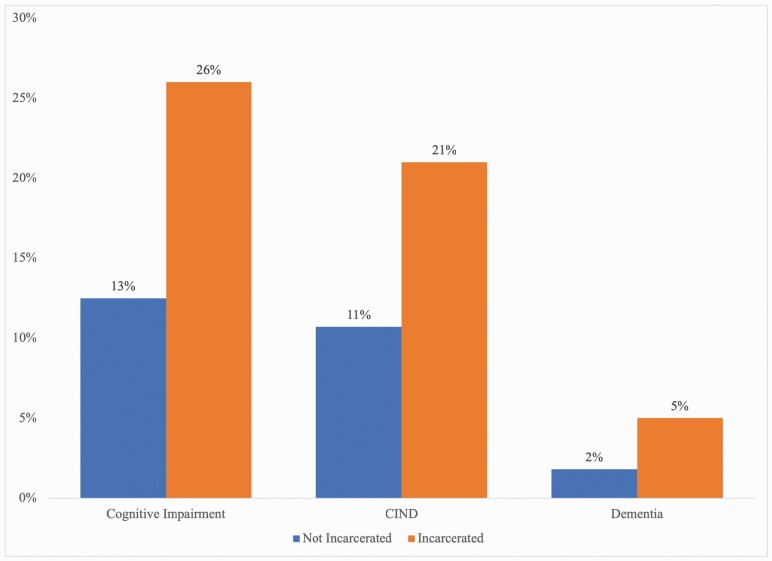
The population means for each independent variable are displayed in Table 1 for formerly and currently incarcerated (FI/I) and nonincarcerated (NI) individuals (see Appendix Table 4 for overall means of each variable). They show that FI/I was significantly different than NI in many characteristics. FI/I was significantly older, was more likely to be non-Hispanic Blacks or Hispanics, and was less likely to be women. In terms of health factors, a higher proportion of FI/I was underweight and overweight, but a lower proportion was obese. FI/I was also more likely to have been diagnosed with a heart problem, high blood pressure, and emotional problems or depression. Moreover, roughly 87% had smoked at least 100 cigarettes in their lifetime, compared to 56% among NI. In terms of ACEs, FI/I was significantly more likely to have reported hospitalization, being bedridden for at least 4 weeks, being physically harmed more than once by an adult, and receiving little to no parental love before age 18. A much greater proportion of FI/I was at the lower end of the income distribution relative to their NI counterparts, and a greater proportion had less than high school education. Finally, FI/I scored almost 21 points lower on the 1980 AFQT than NI. Table 1 also shows that FI/I score significantly lower on the TICS-m. Figure 1 shows that a greater proportion of the TICs-m scores for FI/I were in the range of cognitive impairment, CIND, and dementia. Based on the results in Table 1, FI/I was more disadvantaged than the NI group, and this disadvantage may have driven the large differences in cognitive impairment.
Table 1.
Weighted Summary Statistics of Covariates and Outcomes by Incarceration Status
| Variables | Incarceration status | Mean | 95% CI |
|---|---|---|---|
| Demographics | |||
| Age at interview | |||
| Not incarcerated | 48.312 | (48.271, 48.353) | |
| Incarcerated | 48.536** | (48.377, 48.694) | |
| Black | |||
| Not incarcerated | 0.296 | (0.283, 0.310) | |
| Incarcerated | 0.513*** | (0.469, 0.557) | |
| Hispanic | |||
| Not incarcerated | 0.192 | (0.181, 0.203) | |
| Incarcerated | 0.218*** | (0.183, 0.253) | |
| Female | |||
| Not incarcerated | 0.539 | (0.524, 0.555) | |
| Incarcerated | 0.122*** | (0.095, 0.149) | |
| Body mass index | |||
| Underweight | |||
| Not incarcerated | 0.008 | (0.006, 0.010) | |
| Incarcerated | 0.014+ | (0.004, 0.024) | |
| Overweight | |||
| Not incarcerated | 0.378 | (0.367, 0.389) | |
| Incarcerated | 0.425+ | (0.384, 0.465) | |
| Obese | |||
| Not incarcerated | 0.367 | (0.356, 0.378) | |
| Incarcerated | 0.341+ | (0.303, 0.380) | |
| Reported diagnosis by health 50+ survey | |||
| Heart problem | |||
| Not incarcerated | 0.080 | (0.073, 0.086) | |
| Incarcerated | 0.121** | (0.092, 0.149) | |
| High blood pressure | |||
| Not incarcerated | 0.377 | (0.366, 0.389) | |
| Incarcerated | 0.453*** | (0.411, 0.494) | |
| Diabetes | |||
| Not incarcerated | 0.147 | (0.139, 0.156) | |
| Incarcerated | 0.138 | (0.108, 0.168) | |
| Stroke | |||
| Not incarcerated | 0.030 | (0.026, 0.034) | |
| Incarcerated | 0.044 | (0.026, 0.061) | |
| Emotional problem or depression | |||
| Not incarcerated | 0.192 | (0.183, 0.201) | |
| Incarcerated | 0.264*** | (0.227, 0.301) | |
| Substance use | |||
| Smoked 100 cigarettes during lifetime | |||
| Not incarcerated | 0.559 | (0.548, 0.571) | |
| Incarcerated | 0.871*** | (0.842, 0.899) | |
| Adverse childhood experiences | |||
| Poor health | |||
| Not incarcerated | 0.049 | (0.044, 0.055) | |
| Incarcerated | 0.067 | (0.045, 0.088) | |
| Hospitalized for at least 2 weeks | |||
| Not incarcerated | 0.084 | (0.077, 0.090) | |
| Incarcerated | 0.120** | (0.092, 0.148) | |
| Confined to home or bed for at least 4 weeks | |||
| Not incarcerated | 0.046 | (0.041, 0.051) | |
| Incarcerated | 0.071* | (0.049, 0.092) | |
| Lived with someone with depression, mental illness, or suicidal behavior | |||
| Not incarcerated | 0.072 | (0.066, 0.078) | |
| Incarcerated | 0.059 | (0.039, 0.079) | |
| Lived with a problem drinker or alcoholic | |||
| Not incarcerated | 0.182 | (0.173, 0.191) | |
| Incarcerated | 0.207 | (0.173, 0.240) | |
| Physically harmed by an adult at least once | |||
| Not incarcerated | 0.106 | (0.099, 0.114) | |
| Incarcerated | 0.165*** | (0.134, 0.196) | |
| Received little to no parental love | |||
| Not incarcerated | 0.174 | (0.165, 0.183) | |
| Incarcerated | 0.252*** | (0.213, 0.291) | |
| Net worth | |||
| First quartile | |||
| Not incarcerated | 0.202 | (0.191, 0.212) | |
| Incarcerated | 0.489*** | (0.445, 0.532) | |
| Second quartile | |||
| Not incarcerated | 0.336 | (0.325, 0.347) | |
| Incarcerated | 0.352*** | (0.312, 0.392) | |
| Third quartile | |||
| Not incarcerated | 0.269 | (0.257, 0.280) | |
| Incarcerated | 0.097*** | (0.069, 0.125) | |
| Fourth quartile | |||
| Not incarcerated | 0.193 | (0.182, 0.205) | |
| Incarcerated | 0.063*** | (0.040, 0.086) | |
| Education | |||
| <High school | |||
| Not incarcerated | 0.136 | (0.128, 0.144) | |
| Incarcerated | 0.452*** | (0.411, 0.493) | |
| High school | |||
| Not incarcerated | 0.377 | (0.366, 0.388) | |
| Incarcerated | 0.366*** | (0.328, 0.405) | |
| Some college | |||
| Not incarcerated | 0.249 | (0.239, 0.259) | |
| Incarcerated | 0.144*** | (0.114, 0.174) | |
| ≥College | |||
| Not incarcerated | 0.238 | (0.228, 0.248) | |
| Incarcerated | 0.037*** | (0.021, 0.052) | |
| 1980 Armed Forces Qualification Test | |||
| Not incarcerated | 42.269 | (41.533, 43.005) | |
| Incarcerated | 21.537*** | (19.838, 23.235) | |
| Cognition outcomes | |||
| TICS-m score | |||
| Not incarcerated | 16.563 | (16.457, 16.669) | |
| Incarcerated | 14.098*** | (13.710, 14.487) | |
| Cognitive impairment | |||
| Not incarcerated | 0.125 | (0.117, 0.134) | |
| Incarcerated | 0.260*** | (0.221, 0.299) | |
| No cognitive impairment, CIND, dementia | |||
| Normal | |||
| Not incarcerated | 0.875 | (0.866, 0.883) | |
| Incarcerated | 0.740*** | (0.701, 0.779) | |
| CIND | |||
| Not incarcerated | 0.107 | (0.099, 0.115) | |
| Incarcerated | 0.210*** | (0.174, 0.247) | |
| Dementia | |||
| Not incarcerated | 0.018 | (0.015, 0.022) | |
| Incarcerated | 0.050*** | (0.030, 0.069) | |
| N | 8,021 | ||
| N (FI/I) | 573 | ||
| N (NI) | 7,448 |
Notes: Missing data imputed using multiple imputation by chained equations. Weighted means by incarceration status. Standard errors calculated using Taylor-linearized variance estimation. F test from a multinomial regression was used to calculate significance levels for BMI, net worth quartiles, race and ethnicity (Black, Hispanic, and White), and cognitive impairment (i.e., normal, CIND, and dementia). All other variables were calculated using t test from linear regression with incarceration as the independent variable and the variable of interest as the dependent variable. N is the total number of observations, N (FI/I) is the total number of individuals with any report of incarceration at the time of or prior to the 48+ cognition module, N (NI) is the total number with no reported incarceration. CI = confidence interval; TICS-m = modified version of the Telephone Interview for Cognitive Status; CIND = cognitive impairment—not dementia; FI/I = formerly and currently incarcerated; NI = nonincarcerated; BMI = body mass index.
+p < .10; *p < .05; **p < .01; ***p < .001.
Figure 1.
Prevalence of cognitive impairment. Notes: Weighted means by incarceration status for cognitive impairment. Missing data were imputed using multiple imputation by chained equations. N = 8,021, N (FI/I) = 573, and N (NI) = 7,448. Following Langa et al. (2017), cognitively impaired (either with or without dementia) are those with TICS-m scores ≤ 11. Those with scores in the range 6 < TICS-m ≤ 11 were classified as CIND, and those with TICS-m scores ≤ 6 were categorized as dementia. All differences are significant at the 0.01 significance level or lower. FI/I = formerly and currently incarcerated; NI = nonincarcerated; TICS-m = modified version of the Telephone Interview for Cognitive Status; CIND = cognitive impairment—not dementia.
Cognitive Functioning
Table 2 presents regression coefficients measuring the effect of a prior incarceration on the TICS-m scores for Models 1, 5, 6, and 10 (see Appendix Figure 3 and Appendix Table 1 for the full models). Model 1 shows that FI scored roughly 2.4 (p < .001) points lower on the TICS-m than NI, there is no significant effect for current (at the time of interview) incarceration status. After including demographic variables (Appendix Table 1, Model 2), BMI, diagnoses of cardiovascular risks (i.e., heart disease, high blood pressure, diabetes, and stroke; Appendix Table 1, Model 3), and location fixed effects, the difference between FI and NI participants dropped to roughly −1.6 points (p < .001) but remained significant. After controlling for family net worth ( Appendix Table 2, Model 4), the difference in scores decreased further to roughly −1.1 but remained highly significant (p < .001). The gap between FI and NI respondents diminished after controlling for education (Model 5). Although the difference decreased in magnitude and significance, it remained marginally significant (−0.374, p < .10).
Table 2.
TICS-m Regressions
| Simple regression | Standard regression | Standard + ACEs | Fully augmented | |
|---|---|---|---|---|
| (1) | (5) | (6) | (10) | |
| Incarcerated prior to the 48+ cognition module | −2.457*** | −0.363+ | −0.355+ | −0.118 |
| (0.211) | (0.214) | (0.213) | (0.208) | |
| Incarcerated in the year of the 48+ cognition module | 0.116 | 0.759 | 0.615 | 0.653 |
| (0.719) | (0.659) | (0.664) | (0.649) | |
| Age at interview | −0.076** | −0.081** | −0.067* | |
| (0.029) | (0.029) | (0.028) | ||
| Non-Hispanic Black | −1.594*** | −1.552*** | −0.345** | |
| (0.125) | (0.126) | (0.132) | ||
| Hispanic | −1.406*** | −1.365*** | −0.539*** | |
| (0.139) | (0.139) | (0.137) | ||
| Female | 0.623*** | 0.623*** | 0.894*** | |
| (0.099) | (0.100) | (0.097) | ||
| BMI | ||||
| Underweight: BMI <18.5 | −0.748 | −0.696 | −0.755 | |
| (0.559) | (0.553) | (0.535) | ||
| Overweight: 25 ≤ BMI < 30 | 0.433*** | 0.433*** | 0.447*** | |
| (0.124) | (0.124) | (0.120) | ||
| Obese: BMI ≥30 | 0.596*** | 0.600*** | 0.610*** | |
| (0.136) | (0.136) | (0.129) | ||
| Reported diagnoses by health 50+ survey | ||||
| High blood pressure | −0.455*** | −0.452*** | −0.432*** | |
| (0.107) | (0.107) | (0.104) | ||
| Diabetes | −0.246+ | −0.226 | −0.183 | |
| (0.143) | (0.143) | (0.137) | ||
| Net worth | ||||
| First quartile | −1.439*** | −1.409*** | −0.885*** | |
| (0.214) | (0.213) | (0.209) | ||
| Second quartile | −0.687*** | −0.691*** | −0.302+ | |
| (0.171) | (0.170) | (0.166) | ||
| Third quartile | −0.104 | −0.119 | 0.067 | |
| (0.156) | (0.156) | (0.153) | ||
| Education | ||||
| HGC <12 years | −3.687*** | −3.599*** | −1.303*** | |
| (0.182) | (0.183) | (0.199) | ||
| HGC = 12 years | −2.259*** | −2.204*** | −0.585*** | |
| (0.131) | (0.131) | (0.140) | ||
| 12 < HGC < 16 years | −0.995*** | −0.977*** | −0.049 | |
| (0.133) | (0.133) | (0.135) | ||
| Augmented variables | ||||
| ACEs | ||||
| Fair or poor health | −1.129*** | −0.946*** | ||
| (0.229) | (0.219) | |||
| Lived with someone depressed, mentally ill, or suicidal | 0.609** | 0.533** | ||
| (0.189) | (0.186) | |||
| Physically harmed by parent or adult more than once | 0.246 | 0.279+ | ||
| (0.169) | (0.161) | |||
| Little to no love from parent | −0.309* | −0.159 | ||
| (0.136) | (0.132) | |||
| Emotional disorder or depression | −0.542*** | |||
| (0.127) | ||||
| 1980 Armed Forces Qualification Test score | 0.055*** | |||
| (0.002) | ||||
| Observations | 8,021 | 8,021 | 8,021 | 8,021 |
| N (FI/I) | 573 | 573 | 573 | 573 |
| N (NI) | 7,448 | 7,448 | 7,448 | 7,448 |
Notes: Robust standard errors in parentheses. All models except the simple regression include fixed effects for type of city at baseline, region at baseline, type of city at year of survey, and region at year of survey. Missing data imputed using multiple imputation by chained equations. Results for heart problems, stroke, hospitalized for greater than or equal to 2 weeks, confined to home or bed for greater than or equal to 4 weeks, lived with a problem drinker, and smoked at least 100 cigarettes in one’s lifetime omitted from the table due to lack of significance and space but can be found in Appendix Table 1. N is the total number of observations, N (FI/I) is the total number of individuals with any report of incarceration at the time of or prior to the cognition survey, N (NI) is the total number with no reported incarceration. ACE = adverse childhood experiences; BMI = body mass index; FI/I = formerly and currently incarcerated; HGC = highest grade completed; NI = nonincarcerated.
+p < .10; *p < .05; **p < .01; ***p < .001.
We then augmented the standard models with variables for ACEs, smoking behavior, diagnoses of emotional problems or depression, and a premorbid measure of cognitive functioning, AFQT. Models 6–10 added these variables to the standard regression model, first one-by-one (Models 6–9) and then all together (Model 10), which we refer to as the fully specified regression. Models 6–8 in Appendix Table 1 show that the inclusion of ACEs, smoking behavior, and prior diagnoses of an emotional disorder had a small impact on the difference in TICS-m scores relative to the standard regression model (Model 5). Although smoking behavior was not significant in the model, some ACEs and being diagnosed with an emotional or depressive disorder significantly affected TICS-m scores. After including AFQT (see Model 9), the remaining difference between FI and NI decreased to −0.17 and was no longer statistically significant. Additional analyses regressing TICS-m scores on prior incarceration status adding in each control variable separately to the model reveal that no control variable completely explained the gap in TICS-m scores between FI and NI. However, differences in cognitive functioning between these groups seem to be associated with disparities in human capital (health and education) and early childhood experiences.
Focusing on the full TICS-m model in Table 2, regardless of incarceration status, TICS-m scores were significantly affected by demographic factors, health factors, education, ACEs, diagnosis of an emotional disorder or depression, and premorbid cognitive functioning (i.e., AFQT scores). Specifically, each additional year of age was associated with a decrease in TICS-m scores (−0.067, p < .05). Moreover, Blacks (−0.345, p < .01) and Hispanics (−0.539, p < .001) scored significantly lower on the TICS-m, even after controlling for the rich set of covariates in the model. However, women scored significantly higher than men (0.894, p < .001) on the TICS-m, holding other factors constant. Overweight (0.447, p < .001) and obese (0.610, p < .001) participants also scored higher on the TICS-m, whereas underweight individuals scored lower (−0.755, p = .159) than individuals with normal weight. Regarding cardiovascular risks, although diagnosed heart problems, high blood pressure, diabetes, or stroke significantly affected TICS-m scores in Model 3, only high blood pressure (−0.432, p < .001) remained statistically significant in the full model. Lower levels of net worth also significantly affected TICS-m scores. Individuals in the bottom quartile (−0.885, p < .001) or second quartile (−0.302, p < .10) of net worth scored significantly lower on the TICS-m than those in the top quartile. Likewise, those with less than (−1.303, p < .001) or equal to (−0.585, p < .001) 12 years of education scored lower on the TICS-m relative to individuals with at least 16 years of education. Regarding ACEs, individuals who reported having fair or poor health (−0.946, p < .001); having lived with someone with depression, other mental illness, or suicidal behavior (−0.533, p < .01); or being physically harmed by a parent or adult more than once (−0.279, p < .10) during childhood had significantly lower TICS-m scores relative to individuals who did not report these ACEs, holding other factors constant. In addition, those diagnosed with an emotional disorder or depression (−0.542, p < .001) scored lower than those who had not been diagnosed. Finally, each additional point on the AFQT was associated with a higher TICS-m score (0.055, p < .001).
Cognitive Impairment, CIND, and Dementia
Cognitive impairment
Overall cognitive impairment
Table 3 displays odds ratios (OR) measuring the effect of a prior incarceration on cognitive impairment (see Appendix Table 2 and Appendix Table 2 for the full regressions). Cognitive impairment is first estimated (instead of dementia) because of the relatively young age of the study population. As in the TICS-m score analysis, Models 1–5 represent the standard regression models, and Models 6–10 represent the augmented regression models. Table 3 displays Models 1, 5, 6, and 10 for clarity and brevity. Models 1–4 show that prior incarceration significantly increased the odds of cognitive impairment. Although demographic factors, cardiovascular risk, and net worth explained some of the positive association between prior incarceration and cognitive impairment, FI still had significantly increased odds of cognitive impairment (OR = 1.351, p < .05). However, after controlling for education (Model 5), the effect is removed both in significance and magnitude. Additional regressions not displayed here show that, as in the TICS-m analysis, one factor alone did not explain the difference in the odds of cognitive impairment between FI and NI groups. Rather, a combination of factors explained the between-group difference in cognition.
Table 3.
Logistic Regressions for Cognitive Impairment
| Simple regression | Standard regression | Standard + ACEs | Fully augmented | |
|---|---|---|---|---|
| (1) | (5) | (6) | (10) | |
| Incarcerated prior to the 48+ cognition module | 2.402*** | 1.001 | 1.003 | 0.887 |
| (0.275) | (0.131) | (0.131) | (0.119) | |
| Incarcerated in the year of the 48+ cognition module | 1.127 | 0.887 | 0.955 | 0.950 |
| (0.375) | (0.310) | (0.334) | (0.334) | |
| Age at interview | 1.041* | 1.045* | 1.040+ | |
| (0.020) | (0.020) | (0.021) | ||
| Non-Hispanic Black | 2.562*** | 2.495*** | 1.288* | |
| (0.247) | (0.242) | (0.134) | ||
| Hispanic | 2.202*** | 2.135*** | 1.323* | |
| (0.241) | (0.235) | (0.153) | ||
| Female | 0.781** | 0.785** | 0.683*** | |
| (0.061) | (0.063) | (0.057) | ||
| BMI | ||||
| Underweight: BMI <18.5 | 0.943 | 0.905 | 1.000 | |
| (0.377) | (0.363) | (0.422) | ||
| Overweight: 25 ≤ BMI< 30 | 0.662*** | 0.658*** | 0.655*** | |
| (0.065) | (0.065) | (0.067) | ||
| Obese: BMI ≥30 | 0.622*** | 0.620*** | 0.619*** | |
| (0.067) | (0.067) | (0.068) | ||
| Reported diagnoses by health 50+ survey | ||||
| High blood pressure | 1.284** | 1.287** | 1.278** | |
| (0.110) | (0.110) | (0.112) | ||
| Diabetes | 1.251* | 1.237* | 1.202+ | |
| (0.131) | (0.131) | (0.129) | ||
| Stroke | 1.411+ | 1.412+ | 1.289 | |
| (0.267) | (0.268) | (0.253) | ||
| Net worth | ||||
| First quartile | 1.963*** | 1.949*** | 1.386+ | |
| (0.358) | (0.357) | (0.260) | ||
| Second quartile | 1.388+ | 1.394* | 1.067 | |
| (0.230) | (0.231) | (0.179) | ||
| Third quartile | 0.876 | 0.887 | 0.772 | |
| (0.155) | (0.158) | (0.141) | ||
| Education | ||||
| HGC <12 years | 6.083*** | 5.863*** | 1.420* | |
| (0.932) | (0.898) | (0.241) | ||
| HGC = 12 years | 3.331*** | 3.243*** | 1.154 | |
| (0.474) | (0.462) | (0.174) | ||
| 12 < HGC < 16 years | 1.645** | 1.637** | 0.868 | |
| (0.262) | (0.261) | (0.145) | ||
| Augmented variables | ||||
| ACEs | ||||
| Fair or poor health | 1.430* | 1.322+ | ||
| (0.216) | (0.201) | |||
| Lived with problem drinker or alcoholic | 0.821+ | 0.896 | ||
| (0.089) | (0.101) | |||
| Little to no love from parent | 1.226+ | 1.119 | ||
| (0.131) | (0.124) | |||
| Emotional disorder or depression | 1.296* | |||
| (0.131) | ||||
| 1980 Armed Forces Qualification Test score | 0.960*** | |||
| (0.003) | ||||
| N | 8,021 | 8,021 | 8,021 | 8,021 |
| N (FI/I) | 573 | 573 | 573 | 573 |
| N (NI) | 7,448 | 7,448 | 7,448 | 7,448 |
Notes: Odds ratios with robust standard errors in parentheses. All models except the simple regression include fixed effects for type of city at baseline, region at baseline, type of city at year of survey, and region at year of survey. Missing data imputed using multiple imputation by chained equations. Results for diagnosis with a heart problem, hospitalized for greater than or equal to 2 weeks, confined to home or bed for greater than or equal to 4 weeks, lived with someone depressed, mentally ill, or suicidal, physically harmed by parent or adult more than once, and smoked at least 100 cigarettes in one’s lifetime omitted from the table due to lack of significance and space but can be found inAppendix Table 2. N is the total number of observations, N (FI/I) is the total number of individuals with any report of incarceration at the time of or prior to the cognition survey, N (NI) is the total number with no reported incarceration. ACE = adverse childhood experiences; BMI = body mass index; FI/I = formerly and currently incarcerated; HGC = highest grade completed; NI = nonincarcerated.
+p < .10; *p < .05; **p < .01; ***p < .001.
Irrespective of incarceration status, the augmented regressions in Table 3 and Appendix Table 2, show certain ACEs (Model 6), having been diagnosed with an emotional disorder or depression (Model 8), and AFQT scores (Model 9) were significantly associated with increased odds of cognitive impairment when individually added to the standard regression model (Model 5). However, in the fully specified regression in column 4 of Table 3 only age at interview, race and ethnicity, female sex, BMI, diagnoses of certain chronic illnesses, net worth, education, reporting fair or poor health during childhood, being diagnosed with an emotional disorder or depression, and AFQT scores remained significant. Age at interview, a diabetes diagnosis, being in the lowest quartile of net worth relative to the top quartile, and reporting fair or poor health as a child was associated with increased odds of cognitive impairment but were only marginally statistically significant, holding other factors constant. Blacks or Hispanics relative to Whites, those with high blood pressure, those with less than 12 years of education relative to those with 16 or more years of education, and those diagnosed with an emotional disorder or depression had significantly higher odds of cognitive impairment. Finally, women, overweight or obese relative to normal weight participants, and each additional point scored on the 1980 AFQT were associated with significantly lower odds of cognitive impairment, holding other factors constant.
CIND versus normal
The generalized ordered logit regressions comparing those scoring in the CIND range to those with no cognitive impairment can be found in Appendix Figure 5 and Appendix Table 3, Panel A. The results are almost identical to those presented in Table 3 for the overall cognitive impairment analysis; therefore, we omit the presentation and discussion of the results here due to space constraints.
Dementia versus CIND and no cognitive impairment
Table 4 presents the results for regression models estimating the likelihood of scoring in the dementia range on the TICS-m relative to scoring in the unimpaired or CIND range (see Appendix Figure 5 and Appendix Table 3 for the full regressions). Prior incarceration was significantly and positively associated with increased odds of dementia; however, the difference disappeared after including net worth to the model containing demographic and health covariates (Model 4, Appendix Table 3, Panel B). Separate regressions assessing the effect of including each variable separately, show that most variables, including net worth, did not individually explain differences in the likelihood of dementia between FI and NI groups. However, education and AFQT scores separately explained these differences. Once these variables were individually added to the simple regression model, the effect significantly decreased in size and was no longer significant at traditional levels. The remaining discussion focuses on the fully specified regression presented in column 4 of Table 4 to understand risk factors that explain the likelihood of scoring in the dementia range (irrespective of incarceration status) on the TICS-m.
Table 4.
Generalized Ordered Logit Regressions: Dementia Versus CIND and Normal
| Simple regression | Standard regression | Standard + ACEs | Fully augmented | |
|---|---|---|---|---|
| (1) | (5) | (6) | (10) | |
| Incarcerated prior to the 48+ cognition module | 2.683*** | 0.955 | 0.957 | 0.881 |
| (0.662) | (0.270) | (0.276) | (0.258) | |
| Incarcerated in the year of the 48+ cognition module | 0.966 | 0.733 | 0.822 | 0.835 |
| (0.720) | (0.564) | (0.641) | (0.645) | |
| Age at interview | 1.146*** | 1.150*** | 1.148*** | |
| (0.045) | (0.045) | (0.046) | ||
| Non-Hispanic Black | 3.865*** | 3.832*** | 2.103* | |
| (0.999) | (1.027) | (0.610) | ||
| Hispanic | 3.634*** | 3.575*** | 2.268** | |
| (1.097) | (1.104) | (0.716) | ||
| Female | 0.787 | 0.793 | 0.716+ | |
| (0.143) | (0.146) | (0.133) | ||
| BMI | ||||
| Underweight: BMI <18.5 | 1.241 | 1.158 | 1.299 | |
| (1.005) | (0.946) | (1.067) | ||
| Overweight: 25 ≤ BMI < 30 | 0.575* | 0.565* | 0.573* | |
| (0.131) | (0.129) | (0.131) | ||
| Obese: BMI ≥30 | 0.478** | 0.468** | 0.478** | |
| (0.117) | (0.116) | (0.121) | ||
| Net worth | ||||
| First quartile | 2.541+ | 2.484+ | 1.762 | |
| (1.304) | (1.280) | (0.918) | ||
| Second quartile | 1.622 | 1.625 | 1.254 | |
| (0.800) | (0.800) | (0.627) | ||
| Third quartile | 0.696 | 0.705 | 0.622 | |
| (0.449) | (0.452) | (0.405) | ||
| Education | ||||
| HGC <12 years | 7.810*** | 7.622*** | 1.967 | |
| (3.812) | (3.762) | (1.014) | ||
| HGC = 12 years | 4.113** | 4.010** | 1.446 | |
| (1.986) | (1.948) | (0.735) | ||
| 12 < HGC < 16 years | 1.437 | 1.442 | 0.783 | |
| (0.794) | (0.806) | (0.438) | ||
| Augmented variables | ||||
| 1980 Armed Forces Qualification Test score | 0.961*** | |||
| (0.007) | ||||
| Observations | 8,021 | 8,021 | 8,021 | 8,021 |
| N (FI/I) | 573 | 573 | 573 | 573 |
| N (NI) | 7,448 | 7,448 | 7,448 | 7,448 |
Notes: Reported odds ratios with robust standard errors in parentheses. All models except the simple regression include fixed effects for type of city at baseline, region at baseline, type of city at year of survey, and region at year of survey. Please see the Appendix Table 3A for the CIND versus normal analysis. Missing data imputed using multiple imputation by chained equations. Results for all reported health diagnoses by the health 50+ survey, all ACEs variables, smoked at least 100 cigarettes in one’s lifetime, and having an emotional disorder or depression were omitted from the table due to lack of significance and space but can be found in Appendix Table 3. N is the total number of observations, N (FI/I) is the total number of individuals with any report of incarceration at the time of or prior to the cognition survey, and N (NI) is the total number with no reported incarceration. CIND = cognitive impairment—not dementia; HGC = highest grade completed; ACE = adverse childhood experiences; BMI = body mass index; FI/I = formerly and currently incarcerated; NI = nonincarcerated.
+p < .10; *p < .05; **p < .01; ***p < .001.
Column 4 of Table 4 demonstrates that age at interview, Black, Hispanic, female, BMI, and AFQT were significantly associated with dementia. Specifically, women, overweight or obese individuals (relative to those of normal weight), and each additional point on the AFQT were associated with lower odds of dementia relative to unimpaired or CIND, holding other factors constant. On the other hand, each additional year of age, being Black, and being Hispanic was associated with greater odds of dementia relative to unimpaired or CIND, holding all other factors constant.
Discussion
Recent research found declines in cognitive functioning during incarceration (Ezenwa et al., 2020; Umbach et al., 2018) and high prevalence rates of cognitive functioning among older incarcerated people (Ahalt et al., 2018; Perez et al., 2021). Nonetheless, these studies used specific samples of currently incarcerated people, and, as a result, exclude a sizable portion of individuals who have had contact with the correctional system. Moreover, there is scant evidence of risk factors for the higher prevalence rates found in this population (Lloyd, 2019). In a relatively sizable nationally representative survey of middle-aged men and women between the ages of 46 and 60 years, we found the unadjusted prevalence of cognitive impairment and early-onset dementia among FI men and women was at least two times the prevalence of those with no reported incarceration. Nonetheless, these differences were explained by a combination of factors associated with incarceration and cognitive functioning. The results suggest that incarceration could be affecting cognitive impairment through its effects on human capital (educational attainment, physical and mental health) and net worth. However, premorbid cognitive functioning was also significant. Incarceration could affect premorbid cognition and educational attainment through its timing (see Aizer & Doyle, 2015, for the link between juvenile detention, educational attainment, and later incarceration). The results provide further evidence for considering ADRD from a life course perspective in general, and a cumulative disadvantage framework in particular (Dannefer, 2003).
For cognitive impairment, it seems that incarceration may compound disadvantages over the life course leading to early cognitive decline. While differences between FI and NI for cognitive impairment were explained by a combination of factors, differences for dementia were independently explained by premorbid cognitive functioning and education. Nonetheless, while education becomes insignificant after controlling for AFQT scores, AFQT scores continue to significantly influence early-onset dementia above and beyond its effect through education (i.e., holding constant education levels; see Author Note 3). Finally, it is important to note the relatively resilient association between a prior diagnosis of an emotional disorder or depression and cognitive impairment could be a proxy for chronic psychological distress (Wilson et al., 2007) resulting from an incarceration (Schnittker et al., 2012).
The findings also align with research investigating the cognitive reserve hypothesis (Greenfield et al., 2020; Meng & D’Arcy, 2012; Schmand et al., 1997; Stern, 2012). This hypothesis purports that individuals with high levels of brain reserve can better endure age-related changes to the brain, which allows them to have a greater tolerance for disease, leading to a delay in the onset of dementia (larger brain reserve slows disease progression). Previous research have tested this hypothesis using education levels, IQ tests, reading tests, etc. This study included both education level and a premorbid test of cognition (i.e., 1980 AFQT). Consistent with prior studies (e.g., Schmand et al., 1997; Valenzuela & Sachdev, 2006), we found that premorbid measures of cognitive functioning captured by the AFQT were consistently important in explaining cognitive impairment. In fact, on its own, the AFQT significantly diminished differences in cognitive impairment, and both education and the AFQT separately absorbed the difference in odds of early-onset dementia between FI and NI groups. Given that AFQT scores are a reflection of environmental and socioeconomic factors (Cordero-Guzmán, 2001; Rodgers & Spriggs, 1996), our findings highlight the importance of modeling ADRD within a life course framework as adverse experiences throughout one’s life will affect cognitive reserve and, therefore, cognition later in life.
Irrespective of incarceration status, the findings are consistent with previous research that found significant associations between cognitive functioning and race (Lopez et al., 2003; Sloan & Wang, 2005), ethnicity (Garcia et al., 2018; Sloan & Wang, 2005), gender (Sloan & Wang, 2005), BMI (Langa et al., 2017), vascular diseases (Blazer & Wallace, 2016; Gorelick et al., 2011), some ACEs (Blazer & Wallace, 2016; Case & Paxson, 2009; Ritchie et al., 2011), emotional disorders and depression (Barnes et al., 2006; Blazer & Wallace, 2016; Rodriguez et al., 2018), education (Langa et al., 2017; Rodriguez et al., 2018), and premorbid measures of cognitive functioning (e.g., IQ test, reading test, and in our case, AFQT scores; Greenfield et al., 2020; Meng & D’Arcy, 2012; Schmand et al., 1997; Stern, 2012). It is worth noting that differences in cognitive impairment between Hispanics and non-Hispanic Blacks relative to Whites were persistent, holding other factors constant (including premorbid cognition), suggesting that discrimination and other barriers to social opportunities play a role in disparities in early-onset of ADRD.
Conclusion and Limitations
This study focused on understanding disparities in cognitive functioning among FI and NI people within a nationally representative sample of middle-aged women and men. We found large differences in cognitive functioning, cognitive impairment, and dementia between FI and NI (postrelease) that were explained by sociodemographic and human capital factors associated with incarceration as well as the selectivity of the FI population (e.g., higher rates of ACEs). Greater prevalence of cognitive impairment and dementia could create additional barriers for FI and should be incorporated into reentry planning. Moreover, given the resilience of the effect of race and ethnicity in this study after the inclusion of a rich set of covariates, the findings suggest that to address health disparities in ADRD, structural factors, such as racism and its direct and indirect effects on premorbid cognition (e.g., effect of discrimination on health, inequities in accessing high-quality educational opportunities), must be taken into consideration across the life course.
This study had several limitations. First, incarceration history was constructed from the variable measuring residence at the time of the interview. As a result, individuals who experienced short prison or jail stays were less likely to be categorized as incarcerated. This might have caused differences to be biased toward zero. Another limitation is that we do not observe the exact date of diagnosis of various health conditions. In addition, the results should be interpreted as conditional on having survived to take the survey. To the extent that incarcerated individuals are more likely to die before age 50, our results might be biased downwards. Of major concern is that the TICS-m and other similar tests capture differences in education (both quality and quantity) and not true differences in cognitive decline, especially among low-income populations like incarcerated people. If this is true, then we may not be capturing differences in cognitive decline but rather differences in education levels. Although we control for education, this could elucidate why education individually explained the difference in odds of dementia between FI and NI. Finally, our findings highlight the need to collect data that will support causal inference to better disentangle the mechanisms through which incarceration affects cognition.
Supplementary Material
Acknowledgments
We would like to thank the USC Edward R. Roybal Institute on Aging and the Interdisciplinary Aging Research to Address Health Disparities in Alzheimer’s Disease and Related Dementias: A Scientific Training Program for their support.
Contributor Information
Robynn J A Cox, School of Public Policy, University of California, Riverside, California, USA.
Robert B Wallace, College of Public Health, The University of Iowa, Iowa City, IA, USA.
Author Notes
1. Note, the quality of services varies across location and type of carceral institution (e.g., prison or jail).
2. For brevity, we refer to non-Hispanic Black as Black and non-Hispanic and non-Black as White.
3. Results available upon request.
Funding
This work was supported by the National Institute on Aging of the National Institutes of Health (grant numbers P30AG043073 and R13AG063477-01).
Conflict of Interest
None declared.
Author Contributions
R. J. A. Cox had full access to all data in the study. R. J. A. Cox developed the concept, designed the study, acquired, analyzed and interpreted the data, and drafted the manuscript. R. B. Wallace and R. J. A. Cox contributed substantially to the interpretation of data, provided critical revisions of the manuscript that were important for intellectual content and accuracy, as well as provided administrative, technical, or material support. The authors have approved the final version of the manuscript and take responsibility for all aspects of the work.
References
- Ahalt, C., Stijacic‐Cenzer, I., Miller, B. L., Rosen, H. J., Barnes, D. E., & Williams, B. A. (2018). Cognition and incarceration: Cognitive impairment and its associated outcomes in older adults in jail. Journal of the American Geriatrics Society, 66(11), 2065–2071. doi: 10.1111/jgs.15521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizer, A., & Doyle, J. J. (2015). Juvenile incarceration, human capital, and future crime: Evidence from randomly assigned judges. Quarterly Journal of Economics, 130(2), 759–803. doi: 10.1093/qje/qjv003 [DOI] [Google Scholar]
- Alzheimer’s Association. (2021). 2021 Alzheimer’s disease facts and figures. Alzheimer’s and Dementia, 17(3), 327–406. doi: 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- Anderson, R. E., Geier, T. J., & Cahill, S. P. (2016). Epidemiological associations between posttraumatic stress disorder and incarceration in the National Survey of American Life. Criminal Behaviour and Mental Health, 26(2), 110–123. doi: 10.1002/cbm.1951 [DOI] [PubMed] [Google Scholar]
- Barnes, D. E., Alexopoulos, G. S., Lopez, O. L., Williamson, J. D., & Yaffe, K. (2006). Depressive symptoms, vascular disease, and mild cognitive impairment: Findings from the Cardiovascular Health Study. Archives of General Psychiatry, 63(3), 273–279. doi: 10.1001/archpsyc.63.3.273 [DOI] [PubMed] [Google Scholar]
- Black, P. H. (2002). Stress and the inflammatory response: A review of neurogenic inflammation. Brain, Behavior, and Immunity, 16(6), 622–653. doi: 10.1016/S0889-1591(02)00021-1 [DOI] [PubMed] [Google Scholar]
- Blazer, D. G., & Wallace, R. B. (2016). Cognitive aging: What every geriatric psychiatrist should know. The American Journal of Geriatric Psychiatry, 24(9), 776–781. doi: 10.1016/j.jagp.2016.06.013 [DOI] [PubMed] [Google Scholar]
- Bick, J. A. (2007). Infection control in jails and prisons. Clinical Infectious Diseases, 45(8), 1047–1055. doi: 10.1086/521910 [DOI] [PubMed] [Google Scholar]
- Bonczar, T. P. (2003). Prevalence of imprisonment in the US population, 1974–2001. http://www.bjs.gov/content/pub/pdf/piusp01.pdf
- Carson, E. A. (2015). Prisoners in 2014. US Department of Justice. https://bjs.ojp.gov/library/publications/prisoners-2014 [Google Scholar]
- Carson, E. A., & Sabol, W. J. (2016). Aging of the state prison population, 1993–2013. Bureau of Justice Statistics. https://www.bjs.gov/content/pub/pdf/aspp9313.pdf [Google Scholar]
- Case, A., & Paxson, C. (2009). Early life health and cognitive function in old age. The American Economic Review, 99(2), 104–109. doi: 10.1257/aer.99.2.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Guzmán, H. R. (2001). Cognitive skills, test scores, and social stratification: The role of family and school-level resources on racial/ethnic differences in scores on standardized tests (AFQT). Review of Black Political Economy, 28(4), 31–71. doi: 10.1007/s12114-001-1008-2 [DOI] [Google Scholar]
- Cox, R. (2016). The effect of private sector work opportunities in prison on labor market outcomes of the formerly incarcerated. Journal of Labor Research, 37(4), 412–440. doi: 10.1007/s12122-016-9229-0 [DOI] [Google Scholar]
- Cox, R. (2018). Mass incarceration, racial disparities in health, and successful aging. Generations, 42(2), 48–55. https://www.jstor.org/stable/26556360 [Google Scholar]
- Cox, R., Lahey, J., Rhoades, H., Henwood, B., & Wenzel, S. (2021). Does the timing of incarceration impact the timing and duration of homelessness? Evidence from “The Transitions to Housing” study. Justice Quarterly, 38(6), 1070–1094. doi: 10.1080/07418825.2019.1709883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins, E. M., Kim, J. K., Langa, K. M., & Weir, D. R. (2011). Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(Suppl. 1), i162–i171. doi: 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannefer, D. (2003). Cumulative advantage/disadvantage and the life course: Cross-fertilizing age and social science theory. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 58(6), S327–S337. doi: 10.1093/geronb/58.6.s327 [DOI] [PubMed] [Google Scholar]
- Derenoncourt, E. (2022). Can you move to opportunity? Evidence from the Great Migration. American Economic Review, 112(2), 369–408. doi: 10.1257/aer.20200002 [DOI] [Google Scholar]
- Ezenwa, M. O., Orjiakor, C. T., & Onu, D. U. (2020). Incarceration impacts cognitive performance, and prisoner status matters. Journal of Forensic Psychiatry and Psychology, 31(4), 613–622. doi: 10.1080/14789949.2020.1784249 [DOI] [Google Scholar]
- Fair, H., & Walmsley, R. (2021, December). World prison population list. World Prison Brief. Retrieved March 16, 2022, from https://www.prisonstudies.org/sites/default/files/resources/downloads/world_prison_population_list_13th_edition.pdf [Google Scholar]
- Finch, C. E., & Crimmins, E. M. (2004). Inflammatory exposure and historical changes in human life-spans. Science, 305(5691), 1736–1739. doi: 10.1126/science.1092556 [DOI] [PubMed] [Google Scholar]
- Fisher, G. G., Stachowski, A., Infurna, F. J., Faul, J. D., Grosch, J., & Tetrick, L. E. (2014). Mental work demands, retirement, and longitudinal trajectories of cognitive functioning. Journal of Occupational Health Psychology, 19(2), 231. doi: 10.1037/a0035724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forton, D. M., Thomas, H. C., Murphy, C. A., Allsop, J. M., Foster, G. R., Main, J., Wesnes, K. A., & Taylor‐Robinson, S. D. (2002). Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology, 35(2), 433–439. doi: 10.1053/jhep.2002.30688 [DOI] [PubMed] [Google Scholar]
- Garcia, M. A., Saenz, J., Downer, B., & Wong, R. (2018). The role of education in the association between race/ethnicity/nativity, cognitive impairment, and dementia among older adults in the United States. Demographic Research, 38, 155–168. doi: 10.4054/DemRes.2018.38.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, R. C., & Yaffe, K. (2014). Traumatic brain injury may increase risk of young onset dementia. Annals of Neurology, 75, 339–341. doi: 10.1002/ana.24121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick, P. B., Scuteri, A., Black, S. E., DeCarli, C., Greenberg, S. M., Iadecola, C., & Seshadri, S. (2011). Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 42(9), 2672–2713. doi: 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield, E. A., Akincigil, A., & Moorman, S. M. (2020). Is college completion associated with better cognition in later life for people who are the least, or most, likely to obtain a bachelor’s degree? The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(6), 1286–1291. doi: 10.1093/geronb/gbz132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gure, T. R., Blaum, C. S., Giordani, B., Koelling, T. M., Galecki, A., Pressler, S. J., & Langa, K. M. (2012). Prevalence of cognitive impairment in older adults with heart failure. Journal of the American Geriatrics Society, 60(9), 1724–1729. doi: 10.1111/j.1532-5415.2012.04097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, C. V., Pérez-Stable, E. J., Anderson, N. A., & Bernard, M. A. (2015). The National Institute on Aging health disparities research framework. Ethnicity and Disease, 25(3), 245. doi: 10.18865/ed.25.3.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan, M., Moon, Y. P., Paik, M. C., Sacco, R. L., Wright, C. B., & Elkind, M. S. (2013). Infectious burden and cognitive function: The Northern Manhattan Study. Neurology, 80(13), 1209–1215. doi: 10.1212/WNL.0b013e3182896e79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa, K. M., Larson, E. B., Crimmins, E. M., Faul, J. D., Levine, D. A., Kabeto, M. U., & Weir, D. R. (2017). A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Internal Medicine, 177(1), 51–58. doi: 10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Risacher, S. L., McAllister, T. W., & Saykin, A. J. (2016). Traumatic brain injury and age at onset of cognitive impairment in older adults. Journal of Neurology, 263, 1280–1285. doi: 10.1007/s00415-016-8093-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines, L. M., Sherif, N. A., & Wiener, J. M. (2014). Racial and ethnic disparities among individuals with Alzheimer’s disease in the United States: A literature review. RTI Press. doi: 10.3768/rtipress.2014.rr.0024.1412 [DOI] [Google Scholar]
- Lloyd, S. L. (2019). Cognitive health and incarceration among older adults. Journal of the American Geriatrics Society, 67(3), 622–623. doi: 10.1111/jgs.15699 [DOI] [PubMed] [Google Scholar]
- Lopez, O. L., Jagust, W. J., Dulberg, C., Becker, J. T., DeKosky, S. T., Fitzpatrick, A., Breitner, J., Lyketsos, C., Jones, B., Kawas, C., Carlson, M., & Kuller, L. H. (2003). Risk factors for mild cognitive impairment in the cardiovascular health study cognition study. Archives of Neurology, 60(10), 1394. doi: 10.1001/archneur.60.10.1394 [DOI] [PubMed] [Google Scholar]
- Lynch, J., & Smith, G. D. (2005). A life course approach to chronic disease epidemiology. Annual Review of Public Health, 26(1), 1–35. doi:10.1146/annurev.publhealth.26.021304.144505 [DOI] [PubMed] [Google Scholar]
- Maruschak, L. M., Berzofsky, M., & Unangst, J. (2015). Medical problems of state and federal prisoners and jail inmates, 2011–12. Bureau of Justice Statistics. https://bjs.ojp.gov/content/pub/pdf/mpsfpji1112.pdf [Google Scholar]
- Massoglia, M. (2008). Incarceration as exposure: The prison, infectious disease, and other stress-related illnesses. Journal of Health and Social Behavior, 49(1), 56–71. doi: 10.1177/002214650804900105 [DOI] [PubMed] [Google Scholar]
- Mayeda, E. R., Glymour, M. M., Quesenberry, C. P., & Whitmer, R. A. (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s and Dementia, 12(3), 216–224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, B. S., & Sapolsky, R. M. (1995). Stress and cognitive function. Current Opinion in Neurobiology, 5(2), 205–216. doi: 10.1016/0959-4388(95)80028-x [DOI] [PubMed] [Google Scholar]
- Meng, X., & D’arcy, C. (2012). Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One, 7(6), e38268. doi: 10.1371/journal.pone.0038268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murman, D. L. (2015). The impact of age on cognition. In Seminars in hearing (Vol. 36, No. 03, pp. 111–121). Thieme Medical Publishers. doi: 10.1055/s-0035-1555115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofstedal, M. B., Fisher, G. G., & Herzog, A. R. (2005). Documentation of cognitive functioning measures in the Health and Retirement Study. University of Michigan, 10. http://hrsonline.isr.umich.edu/sitedocs/userg/dr-006.pdf?_ga=2.75282898.1114577250.1662476251-381895763.1662476251 [Google Scholar]
- Ozen, L., Fernandes, M., Clark, A., & Roy, E. (2015). Evidence of cognitive decline in older adults after remote traumatic brain injury: An exploratory study. Aging, Neuropsychology, and Cognition, 22(5), 517–533. doi: 10.1080/13825585.2014.993584 [DOI] [PubMed] [Google Scholar]
- Perez, A., Manning, K. J., Powell, W., & Barry, L. C. (2021). Cognitive impairment in older incarcerated males: Education and race considerations. The American Journal of Geriatric Psychiatry, 29(10), 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan, K. B., Weuve, J., Barnes, L. L., McAninch, E. A., Wilson, R. S., & Evans, D. A. (2021). Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s & dementia, 17(12), 1966–1975. doi: 10.1002/alz.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael, S., & Stoll, M. A. (2013). Why are so many Americans in prison? Russell Sage Foundation. [Google Scholar]
- Richards, M., & Deary, I. J. (2005). A life course approach to cognitive reserve: a model for cognitive aging and development? Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 58(4), 617–622. doi: 10.1002/ana.20637 [DOI] [PubMed] [Google Scholar]
- Ritchie, K., Jaussent, I., Stewart, R., Dupuy, A. M., Courtet, P., Malafosse, A., & Ancelin, M. L. (2011). Adverse childhood environment and late‐life cognitive functioning. International Journal of Geriatric Psychiatry, 26(5), 503–510. doi: 10.1002/gps.2553 [DOI] [PubMed] [Google Scholar]
- Rodriguez, F. T., Aranda, M. P., Lloyd, D. A., & Vega, W. A. (2018). Racial and ethnic disparities in dementia risk among individuals with low education. American Journal of Geriatric Psychiatry, 26(9), 966–976. doi: 10.1016/j.jagp.2018.05.011 [DOI] [PubMed] [Google Scholar]
- Rodgers, W. M., & Spriggs, W. E. (1996). What does the AFQT really measure: Race, wages, schooling and the AFQT score. Review of Black Political Economy, 24(4), 13–46. doi: 10.1007/bf02690041 [DOI] [Google Scholar]
- Sandi, C. (2004). Stress, cognitive impairment and cell adhesion molecules. Nature Reviews Neuroscience, 5(12), 917–930. doi: 10.1038/nrn1555 [DOI] [PubMed] [Google Scholar]
- Schwartz, B. S., Glass, T. A., Bolla, K. I., Stewart, W. F., Glass, G., Rasmussen, M., Bressler, J., Shi, W., & Bandeen-Roche, K. (2004). Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environmental Health Perspectives, 112(3), 314–320. doi: 10.1289/ehp.6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmand, B., Smit, J. H., Geerlings, M. I., & Lindeboom, J. (1997). The effects of intelligence and education on the development of dementia. A test of the brain reserve hypothesis. Psychological Medicine, 27(6), 1337–1344. doi: 10.1017/s0033291797005461 [DOI] [PubMed] [Google Scholar]
- Schnittker, J., Massoglia, M., & Uggen, C. (2012). Out and down: Incarceration and psychiatric disorders. Journal of Health and Social Behavior, 53(4), 448–464. doi: 10.1177/0022146512453928 [DOI] [PubMed] [Google Scholar]
- Shiroma, E. J., Ferguson, P. L., & Pickelsimer, E. E. (2012). Prevalence of traumatic brain injury in an offender population: A meta-analysis. Journal of Head Trauma Rehabilitation, 27(3), E1–E10. doi: 10.1097/htr.0b013e3182571c14 [DOI] [PubMed] [Google Scholar]
- Sloan, F. A., & Wang, J. (2005). Disparities among older adults in measures of cognitive function by race or ethnicity. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60(5), P242–P250. doi: 10.1093/geronb/60.5.p242 [DOI] [PubMed] [Google Scholar]
- StataCorp (2019). Stata statistical software: Release 16. StataCorp LLC. [Google Scholar]
- Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurology, 11(11), 1006–1012. doi: 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach, R., Raine, A., & Leonard, N. R. (2018). Cognitive decline as a result of incarceration and the effects of a CBT/MT intervention: A cluster-randomized controlled trial. Criminal Justice and Behavior, 45(1), 31–55. doi: 10.1177/0093854817736345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Bureau of Labor Statistics, U.S. Department of Labor (2019) National Longitudinal Survey of Youth 1979 cohort, 1979–2016 (rounds 1–27). Produced and distributed by the Center for Human Resource Research (CHRR). The Ohio State University. [Google Scholar]
- U.S. Bureau of Labor Statistics (2011). NLS News (Publication No. 11-146). U.S. Department of Labor, U.S. Bureau of Labor Statistics. https://www.bls.gov/nls/additional-publications/news-letter-discontinued/release-146.pdf [Google Scholar]
- Valenzuela, M. J., & Sachdev, P. (2006). Brain reserve and dementia: A systematic review. Psychological Medicine, 36(4), 441–454. doi: 10.1017/S0033291705006264 [DOI] [PubMed] [Google Scholar]
- Williams, R. (2016). Understanding and interpreting generalized ordered logit models. Journal of Mathematical Sociology, 40(1), 7–20. doi: 10.1080/0022250x.2015.1112384 [DOI] [Google Scholar]
- Wilson, R. S., Schneider, J. A., Boyle, P. A., Arnold, S. E., Tang, Y., & Bennett, D. A. (2007). Chronic distress and incidence of mild cognitive impairment. Neurology, 68(24), 2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82 [DOI] [PubMed] [Google Scholar]
- Zhang, Z., Hayward, M. D., & Yu, Y. L. (2016). Life course pathways to racial disparities in cognitive impairment among older Americans. Journal of Health and Social Behavior, 57(2), 184–199. doi: 10.1177/0022146516645925 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.