Abstract
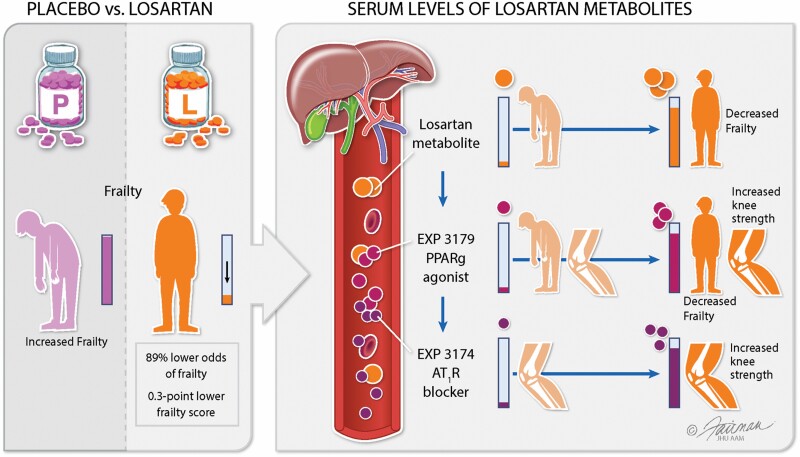
Losartan is an oral antihypertensive agent that is rapidly metabolized to EXP3174 (angiotensin-subtype-1-receptor blocker) and EXP3179 (peroxisome proliferator-activated receptor gamma [PPARγ] agonist), which was shown in animal studies to reduce inflammation, enhance mitochondrial energetics, and improve muscle repair and physical performance. We conducted an exploratory pilot study evaluating losartan treatment in prefrail older adults (age 70–90 years, N = 25). Participants were randomized to control (placebo) or treatment (daily oral losartan beginning at 25 mg per day and increasing every 8 weeks) for a total of 6 months. Fatigue, hyperkalemia, and hypotension were the most observed side effects of losartan treatment. Participants in the losartan group had an estimated 89% lower odds of frailty (95% confidence interval [CI]: 18% to 99% lower odds, p = .03), with a 0.3-point lower frailty score than the placebo group (95% CI: 0.01–0.5 lower odds, p = .04). Frailty score was also negatively associated with serum losartan and EXP3179 concentrations. For every one standard deviation increase in EXP3179 (ie, 0.0011 ng/μL, based on sample values above detection limit) and EXP3174 (ie, 0.27 ng/μL, based on sample values above detection limit), there was a 0.0035 N (95% CI: 0.0019–0.0051, p < .001) and a 0.0027 N (95% CI: 0.00054–0.0043, p = .007) increase in average knee strength, respectively.
Keywords: Citric acid cycle; EXP3174; EXP3179; Frailty, Losartan
Graphical Abstract
Losartan treatment was associated with lower concentrations of tumor necrosis factor α receptor 1; improvements in mitochondrial health biomarkers, such as higher serum concentrations of arginine and spermidine, with lower concentrations of nitrotyrosine, and lower extracellular concentrations of several intermediates of energy metabolism. Our findings suggest that losartan treatment improves molecular and clinical measures of frailty and may show a role for losartan’s nonangiotensin PPARγ pathway.
Frail older adults are particularly vulnerable to a myriad of adverse health outcomes, including early mortality, functional decline, disability, and falls (1,2). Physical frailty is determined by an aggregate score that includes weight loss, low activity levels, muscle weakness, exhaustion, and slow walking speed and categorizes patients as frail, prefrail, or robust (1). The etiopathogenesis of physical frailty and the adverse outcomes associated with it are not well defined, but chronic inflammation, mitochondrial damage with bioenergetic failure, cellular senescence, and impaired autophagy are associated with frailty in older adults (3–6).
The renin–angiotensin system (RAS), a central regulator of blood pressure (BP) and sodium balance, is involved (via activation of the angiotensin type 1 receptor [AT1R]) in several molecular mechanisms that are linked to frailty, including chronic inflammation, oxidative stress damage, mitochondrial decline, and impaired autophagy (7–9). Angiotensin receptor blockers (ARBs) have established renal, cardiovascular, and BP benefits and are in widespread clinical use. In contrast, the impact of angiotensin receptor blockade on physical function and life span in humans remains unclear (10). RAS blockade in animal studies reduced inflammation, enhanced mitochondrial energetics, improved muscle repair and physical performance (11) and led to a 25% increase in life span (12). However, in human studies, the impact of RAS blockade on muscle function and physical performance has varied widely (13–17).
Losartan is the first nonpeptide orally active AT1R blocker to be approved for use as an antihypertensive agent. It is a prodrug that is metabolized by the liver into 2 distinct metabolites (18). Approximately 14% of a losartan dose is converted to the EXP3174 metabolite (19), which is 10- to 40-fold more potent than losartan and mediates most of its AT1R-blocking effects. The second metabolite, EXP3179 (20), has no AT1R-blocking activity but possesses strong peroxisome proliferator-activated receptor gamma (PPARγ) agonistic activity and exerts antiinflammatory effects by inhibiting cyclooxygenase (COX)-2. Furthermore, EXP3179 stimulates the release of nitric oxide (NO), and it has been suggested that NO mediates some of the antidiabetic activity of losartan (21). The use of losartan in studies involving older animal models showed promise in improving the regeneration of injured muscle and protecting against muscle wasting due to inactivity (22,23).
In this study, we postulated that in prefrail older adults, the use of losartan would be associated with reductions in the concentrations of markers of inflammation and oxidative stress, improved physical function, and improved frailty status. We focused on prefrail older adults because they have an intermediate risk of poor health outcomes. Hence, their risk of morbidity and mortality is lower than those who are frail, and hypothetically, may have better chances of experiencing improvements in molecular and physical outcomes due to targeted treatment. We conducted a randomized, placebo-controlled pilot clinical trial of losartan in prefrail older adults aged 70 and older that aimed to assess the safety, tolerability, and dosing range of losartan treatment as well as assess the overall efficacy and dose–response of losartan on muscle strength. We also analyzed the effects of 6 months of losartan treatment on physical function and the molecular pathways that are associated with frailty.
Method
The following sections contain a brief overview of the methods used for results presented in this article. For further details of these methodologies, see Supplementary Materials online.
Participant Recruitment
Participants were included if they were aged 70 years and older and classified as prefrail based on the physical frailty phenotype (PFP) (1). All participants were from Baltimore and were recruited from a Johns Hopkins University (JHU) Institutional Review Board (IRB)-approved registry of older adults willing to participate in research studies as well as the general community through newspaper advertisements. The study was approved by the JHU IRB with approval #NA_00078435 and registered at ClinicalTrials.gov #NCT01989793. This pilot study was conducted under Investigational New Drug Exemption from the Food and Drug Administration for the off-label indication use of Losartan. A formal Data Safety Monitoring Board was established before the trial commenced and met regularly throughout the trial to review all recruitment efforts, participant’s safety, and any reported side effects. All potential participants underwent initial phone screening, and those who qualified were provided with a consent form to review before their in-person screening visit. Potential participants were excluded if they had unstable cardiovascular disease, current use of ARBs or angiotensin-converting enzyme (ACE) inhibitors, a prior allergic reaction to or hyperkalemia while taking any ARB, chronic renal failure with a glomerular filtration rate of <30 mL/min/1.73 m2, daily use of nonsteroidal antiinflammatory agents, use of steroids, lower-extremity disability that would prevent muscle strength testing, echocardiogram-diagnosed cardiac failure as evidenced by a left ventricular ejection fraction less than 50%, cognitive impairment based on a Mini-Mental State Examination score <24, or a BP consistently less than 110/70 mmHg.
Study Design
Study visits
Four study visits, 8 weeks apart, took place in the Institute for Clinical and Translational Research (ICTR) clinical research unit (CRU) on the Johns Hopkins Bayview Medical Center campus. Additional phone calls were made every 2 weeks to monitor safety and compliance. Participants who gave their informed consent attended a screening visit, during which their prefrail status was confirmed based on grip strength and walking speed assessments and selfreported physical activity/fatigue/weight loss. Once they were deemed eligible to enroll, participants were randomized to either the control group or the treatment group with a computerized randomization program. Participants were started on 25 mg tablets (or the placebo) and were instructed to take the pills every morning. Every 8 weeks, those in the losartan treatment arm were given an increased dose of losartan, from 25 mg to 50 mg to 100 mg. Matching placebo pills were also given out at these intervals to those in the control group. Validation of compliance was achieved by asking participants about their pill (placebo or losartan) intake. Patients were evaluable if they stated that they took 100% of the planned treatment dose. Participants were monitored for potential side effects or symptoms listed in the exclusion criteria, such as hypotension, falls with fracture, hyperkalemia, and elevated serum creatinine concentration. All exams for primary and secondary parameters were done by trained CRU staff who were blinded to both groups.
Physical measures
The primary outcome, isokinetic strength, was measured as the bilateral knee concentric strength using a Biodex System 3 dynamometer as previously described (24).
Frailty, which was the secondary outcome, was measured using the PFP criteria of unintentional weight loss, weakness, poor endurance, slowness, and low physical activity (1,25). Those meeting none of the 5 criteria were categorized as robust, those with 1 or 2 criteria were categorized as prefrail, and those with three or more criteria were considered frail. In this study, only older individuals who were prefrail at baseline were included.
Phlebotomy and blood processing
Nonfasting blood collection was also performed in the morning on the day of each visit before the losartan dose was increased or the placebo was continued. Blood was processed, and serum aliquots were then transferred to cryovials and stored at −80°C until the lab assays were performed. The number of freeze-thaw cycles was limited to ≤3.
Laboratory Analyses
Blood chemistry and serum cytokines
Comprehensive metabolic panels were analyzed by the JHU Bayview Hospital Clinical Laboratory. Serum cytokines (interleukin-6 [IL6], tumor necrosis factor α [TNFα], tumor necrosis factor α receptor 1 [TNFαR1], and interleukin-1β [IL1β]) were assayed using a quantitative sandwich ELISA (Mesoscale Diagnostics, Rockville, MD) following the manufacturer’s protocol. The performance characteristics of the cytokine immunoassay included a lower limit of detection of 0.09 pg/mL and a lower limit of quantitation of 0.30 pg/mL. These measures were performed in the JHU ICTR Core Laboratory.
Losartan, EXP3174, and EXP3179
Samples from the losartan treatment group were analyzed for losartan, EXP3174, and EXP3179 concentrations by ultra-performance liquid chromatography (LC/MS/MS) in 1 bioanalytical run. The analytes were extracted from the serum by protein precipitation using acetonitrile containing an internal standard (thioridazine). The mass spectrometer was programmed to monitor the following MRM transitions: 437.1 > 207.2 for EXP3174; 421.1 > 207.1 for EXP3179; 423.1 > 207.2 for losartan; and 371.0 > 126.0 for thioridazine. The calibration curve was computed using the area ratio peak of the analysis to the internal standard based on a quadratic equation with a 1/x2 weighting function over the concentration range of 5–2 000 ng/mL for losartan, 25–2 000 ng/mL for EXP3174, and 0.1–200 ng/mL for EXP3179. Accuracy for losartan, EXP3174, and EXP3179 was >91.6%, >91.7%, and >97.8%, respectively. This accuracy met the acceptance criteria for bioanalytical assays (26)
Metabolomics
Metabolites were extracted, and their concentrations in the losartan treatment group were obtained using the AbsoluteIDQ kit p180 (Biocrates Life Science AG, Austria), following the manufacturer’s protocol for the API5500 LC/MS/MS System (AB SCIEX, Framingham, MA, USA) running with Analyst 1.5.2 software and equipped with an electrospray ionization source, a Shimadzu CBM-20A command module, LC-20AB pump, Shimadzu SIL-20AC-HT autosampler and a CTO-10Ac column oven heater as previously described (27). Of the 186 preconfigured metabolites and ratios in the kit, 121 metabolites were quantifiable in our serum samples.
Tricarboxylic acid (TCA) cycle and glycolysis metabolites were extracted from the serum samples using 80% (vol/vol) mass spectrometry grade methanol. Data acquisition from the samples was performed using a Thermo Scientific Q Exactive Plus Orbitrap Mass Spectrometer and a Vanquish UPLC system (Waltham, MA). Acquired data were analyzed using Thermo Scientific Compound Discoverer and TraceFinder software. The intensities of each metabolite were obtained by integrating chromatographic peaks. Fold change was calculated by dividing the intensities by the average intensity of the baseline group for each metabolite.
Statistical Analysis
Participant characteristics, baseline chemistry lab values, physical measurements, and inflammatory lab values were summarized stratified by study arm using means and standard deviations (SD) or medians and interquartile ranges (IQR) for continuous variables and frequencies for categorical variables. Thirty-seven patients were recruited for the study. Twenty-five participants completed all study visits and included 10 in the treatment group and 15 who received placebo.
Study arm comparisons
Population-average marginal generalized linear models were fit to assess differences between the treatment arms for the primary (isokinetic strength) and secondary (frailty) outcomes as well as walking speed, the 6-minute walk test (m), and dominant-hand grip strength (kg). The change from baseline at each visit was used as the dependent variable. The independent variables were baseline outcome level, study arm, visit and visit by arm interaction to evaluate whether the change in outcome by arm differed by visit. If the interaction term was not statistically significant at the 0.05 level, the final model included only the study arm, baseline outcome, and visit. Additional adjustments were performed for age and sex. The models were estimated using generalized estimating equations (GEEs) with an exchangeable working correlation structure.
Frailty status was dichotomized as frail, represented in these analyses by a score of 3 or more, or not frail/prefrail, which were defined as a score of 0 or 1–2, respectively. To compare frailty status by arm, the GEE model described earlier was extended to include binomial family and logit links to estimate the odds ratios for frailty in the losartan and placebo groups, with adjustment for visit.
Losartan group analyses
We assessed the changes in the outcomes according to losartan dose in the treatment arm and the correlations of losartan and its metabolite concentrations with the outcomes. These analyses should be considered exploratory; therefore, no multiplicity adjustments were used.
To assess the changes in the outcomes with an increase in the losartan dose, population-average marginal generalized linear models with logarithmic links and robust variance were used with visit as the predictor to estimate the percent changes in outcomes. In the results section, we report the changes observed at the end of the study (Visit 4: 100 mg). These models for the active arm were refit to include losartan or its serum metabolite concentrations and visit to assess the associations of physical measures, serum cytokines, oxidative stress markers, targeted metabolomics outcomes with the concentrations of losartan and its metabolites while controlling for the dose/visit. The level of statistical significance was at the 0.05 level.
The analysis was performed using SAS (Statistical Analysis Software 9.4, SAS Institute Inc, Cary, NC) and Stata 15 Statistical Software (StataCorp, College Station, TX).
Results
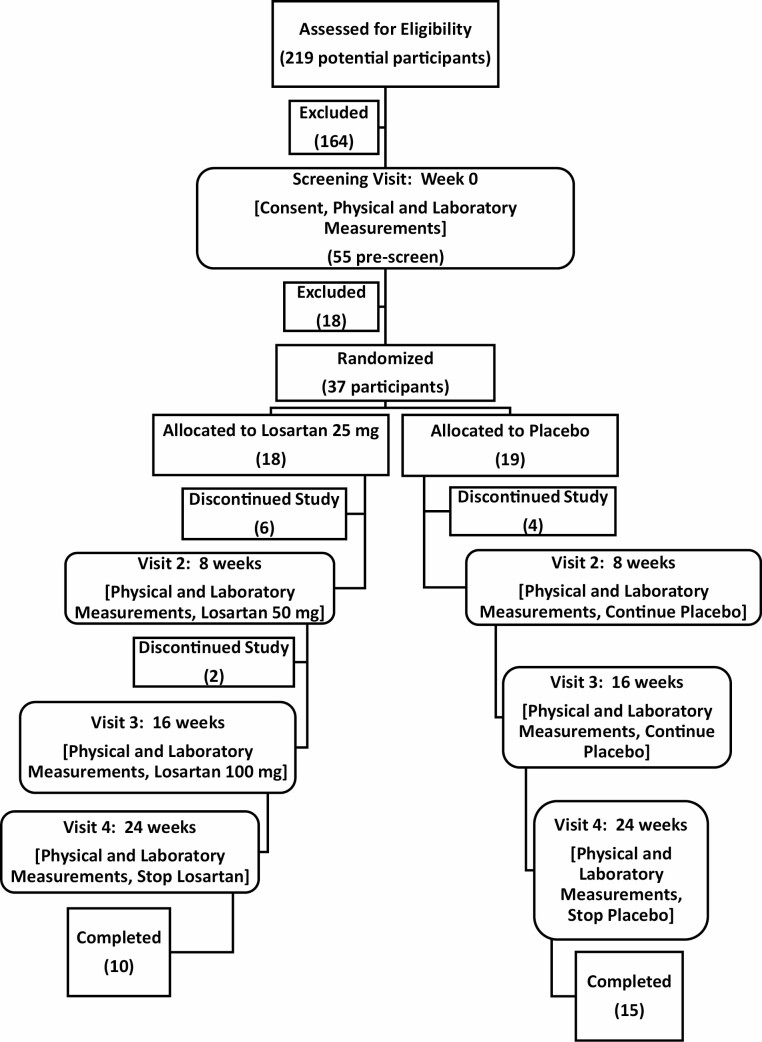
We enrolled a total of 37 participants who were 70 years or older (Figure 1). Eighteen participants were randomized to the losartan treatment group, while 19 were randomized to the placebo control group. During the study, 4 participants withdrew from the placebo group, leaving 15 participants who completed the study. Six participants withdrew from the losartan group, with an additional 2 participants who were withdrawn by study investigators, leaving 10 participants who completed the study. Supplementary Table 1 shows the detailed reasons participants withdrew or were excluded. In the treatment group, there were 4 participants who experienced side effects that were more likely related to losartan, raising some questions about the tolerability. An independent Data Safety Monitoring Board evaluated the withdrawal reasons and determined that the trial could continue safely. The baseline demographic and clinical variables of the participants who completed the study are presented in Table 1.
Figure 1.
Study design with participant enrollment, withdrawal, and completion.
Table 1.
Baseline Measurements in Participants Who Completed the Study
| Placebo | Losartan | p Value | |
|---|---|---|---|
| N = 15 | N = 10 | ||
| Physical measurements* | |||
| Age (years) | 78.1 (6.3) | 75.1 (5.0) | .22 |
| Sex | .44 | ||
| Male Female |
10 (66.7) 5 (33.3) |
5 (50.0) 5 (50.0) |
|
| Systolic blood pressure (mmHg) | 138.2 (16.9) | 139.4 (21.1) | .88 |
| Diastolic blood pressure (mmHg) | 73.6 (12.5) | 72.1 (12.5) | .77 |
| Heart rate (beats per minute) | 70.6 (11.4) | 63.2 (5.8) | .062 |
| Body mass index (kg/m2) | 27.3 (5.0) | 30.2 (4.4) | .14 |
| Dominant-hand grip strength (kg) | 21.9 (7.1) | 24.1 (9.9) | .55 |
| Walking speed (m/sec) | 1.1 (0.2) | 1.1 (0.2) | .73 |
| Six minute walk (m) | 435.9 (132.9) | 423.0 (80.6) | .78 |
| Biodex both knee max strength (N) | 77.7 (27.6) | 85.9 (39.1) | .54 |
| Chemistry lab values* | |||
| Potassium (mEq/L) | 4.1 (0.4) | 4.2(0.4) | .53 |
| Blood urea nitrogen (mg/dL) | 16.4 (4.2) | 19.4 (6.9) | .20 |
| Creatinine (mg/dL) | 1.0 (0.2) | 1.0 (0.3) | .66 |
| Chloride (mEq/L) | 104.4 (2.8) | 105.8 (1.6) | .14 |
| Carbon dioxide(mmol/L) | 28.1 (2.5) | 27.7 (2.1) | .67 |
| Aspartate aminotransferase (IU/L) | 18.8 (3.6) | 22.2 (10.6) | .27 |
| Alanine aminotransferase (IU/L) | 22.9 (6.8) | 32.0 (19.3) | .11 |
| Albumin (g/dL) | 3.8 (0.9) | 3.9 (0.3) | .46 |
| Total bilirubin (mg/dL) | 0.7 (0.6) | 0.7 (0.3) | .78 |
| Inflammatory lab values** | |||
| Interleukin-1β (pg/mL) | 24.1 (11.6–84.1) | 20.4 (14.0–88.8) | .61 |
| Tumor necrosis factor-α (pg/mL) | 3.8 (2.1–10.2) | 2.9 (2.2–6.5) | .25 |
| Tumor necrosis factor-α receptor 1 (pg/mL) | 1 313.7 (713.4–2344.8) | 1 189.1 (633.0–1785.0) | .57 |
Notes:
*Data are presented as the means (standard deviation [SD]) for continuous measures.
**Data are presented as the medians (interquartile ranges [IQRs]) for continuous measures.
Losartan Serum Concentrations Increased With Increasing Doses With High Variability Among Individuals
Losartan is a unique ARB because it is a prodrug that is absorbed after oral administration, reaches a plasma peak in 2 hours and is metabolized by CYP3A4 and CYP2C9 into 2 active metabolites, EXP3174 (a potent, selective AT1R blocker) and EXP3179 (a PPAR-γ agonist). Given potential variability in drug exposure with aging due to differences in drug metabolism and drug interactions from polypharmacy, we measured the serum concentrations of losartan and its active metabolites (EXP3174 and EXP3179) at each visit. Our data show an overall increase in the concentrations of losartan and its active metabolites that correlated with the increase in dose (Supplementary Figure 1). Thus, on average, we observed increases in the serum concentrations of losartan and its metabolites with each increase in the dose of oral losartan. However, we observed significant differences in interpatient serum drug concentrations, even though all those in the losartan treatment arm were given the same dose schedule. After being administered a 25 mg (Visit 2) oral dose, 1 patient had a losartan serum concentration of 46.8 ng/mL, which was approximately twofold higher (24.4 ng/mL) than that in a patient who was administered a 100 mg (Visit 4) oral dose.
Higher Losartan Doses and Serum Concentrations Were Associated With Decreased BP and Increased Blood Urea Nitrogen
To determine the effects of losartan on the body systems traditionally impacted by ARBs, we examined changes in BP, heart rate (HR), and comprehensive metabolic panel (CMP) parameters with increasing losartan doses in participants in the treatment group (Table 2).
Table 2.
Clinical and Laboratory Measures in the Losartan Treatment Group
| Physical measurements (losartan treatment group) | % Change, baseline to Visit 4 | 95% CI p-value |
Losartan | 95% CI p-value |
EXP3174 | 95% CI p-value |
EXP3179 | 95% CI p-value |
|---|---|---|---|---|---|---|---|---|
| Systolic blood pressure (mmHg) | ↓ 10% | 0.84, 0.96 .002 |
― | ― | ― | ― | ― | ― |
| Diastolic blood pressure (mmHg) | ↓ 7% | 0.87, 1.00 .046 |
0.04 | 0.02, 0.06 <.001 |
2.3 | 0.6, 4.1 .008 |
||
| Heart rate (beats per minute) | ↑ 11% | 1.02, 1.20 .01 |
― | ― | ― | ― | ― | ― |
| Blood urea nitrogen (mg/dL) | ↑ 15% | 1.09, 1.22 ≤.001 |
― | ― | ― | ― | ― | ― |
| Chloride (mEq/L) | ↑ 2% | 1.00, 1.03 .02 |
0.01 | 0.003, 0.02 .003 |
― | ― | ― | ― |
| CO2 (mmol/L) | ↓ 5% | 0.91, 0.99 .015 |
― | ― | ― | ― | ― | ― |
| Knee srength (N) | ― | ― | ― | ― | 0.01 | 0.002, 0.02 .007 |
3.2 | 1.7, 4.6 <.001 |
| Frailty score | ― | ― | −0.001 | −0.001, −0.003 .005 |
― | ― | −0.09 | −0.16, −0.01 .02 |
Note: Upward arrows indicate positive correlations, downward arrows indicate negative correlations, and dashes indicate no correlation.
As expected, the BP decreased with losartan treatment. The seemingly paradoxical increase in HR after the drop in BP in our treated participants was expected, as tachycardia is a baroreceptor functional response to a decrease in BP. This provided additional functional validation of the measured serum concentrations of losartan and its metabolites. Similarly, the CMP showed several renal function and acid–base balance changes commonly seen with the use of losartan (28–30). Given the potential associations among metabolic markers, liver function, and creatinine clearance, we conducted an analysis comparing serum losartan, EXP3174, and EXP3179 concentrations using Child-Pugh scores to represent the liver function and the estimated glomerular filtration rate (eGFR) calculated with the CKD-EPI equation to represent the renal function. There were no associations between drug concentrations and liver function or eGFR.
Losartan Treatment Led to Improvements in Knee Strength and Frailty
The primary goal of this study was to determine whether oral losartan treatment positively impacts muscle strength in prefrail older adults (graphical abstract).
The change in average knee strength over 6 months was 0.09 N (95% confidence interval [CI]: −5.62–5.81) higher in the losartan group than in the placebo group after adjustment for losartan dose and baseline strength, though the changes were not significant. A comparison of physical performance between the losartan-treated cohort and the placebo group showed no significant differences in the 6-minute walk distance, walking speed, or grip strength.
Within the losartan group itself, we noted an estimated 10% (95% CI: 4% to 15% decline, p = .001) reduction in knee strength at Visit 4 compared to baseline. Similarly, we observed a 7% (p = .08) reduction in knee strength within the placebo group when comparing their final visit to baseline. While it appears that knee strength declined faster in the losartan arm, the 10% versus 7% progression of decline was not statistically significant between the 2 arms (Supplementary Table 2).
When examining the serum concentrations of losartan and its metabolites as potential contributors to physical function in the treatment group, there were no statistically significant improvements in grip strength, 6-minute walk distance, or walking speed. However, both EXP3174 and EXP3179 were positively correlated with knee strength after adjustment for losartan dose (Table 2). Specifically, for every one SD increase in EXP3179 (ie, 0.0011 ng/μL, based on sample values above detection limit) and EXP3174 (ie, 0.27 ng/μL, based on sample values above detection limit), there was a 0.0035 N (95% CI: 0.0019–0.0051, p < .001) and a 0.0027 N (95% CI: 0.00054–0.0043, p = .007) increase in average knee strength, respectively.
Because knee strength is specifically a measure of muscle strength, we also wanted to explore overall function by examining changes in frailty status with every losartan dose increase and between baseline and the end of the study. Our data showed that at Visit 4, 27% (4/15) of the placebo group participants were classified as frail, while none of the losartan group participants were classified as frail. The treatment group had an estimated 89% lower odds of frailty than the control group after adjustment for the losartan dose (95% CI: 18% to 99% lower odds, p = .03). After controlling for the losartan dose, patients in the losartan group also had a 0.3-point lower frailty score (95% CI: 0.01–0.5 lower odds, p = .04) than those in the placebo group. The frailty score was also negatively associated with the serum losartan (p = .005) and EXP3179 (p = .02) concentrations but not the EXP3174 concentration after controlling for the losartan dose in the treatment group.
Losartan Treatment Increased IL1β and Decreased TNFαR1
Substantial evidence supports strong links between chronic inflammation and an increased risk of frailty and overall poor health outcomes; thus, we explored changes in serum cytokines concentrations in the losartan treatment group.
No changes were identified in IL6. However, IL1β increased by 41% from baseline to Visit 4 (95% CI: 34–48 increase, p < .001). While the functional consequences of this increase are unknown, some studies indicate that IL1β may have beneficial and protective effects with regard to neurological amyloid plaque clearance and atherosclerosis (31–35).
Our data did show a significant decrease in TNFαR1 with increasing serum concentrations of losartan. Overall, for each nanogram increase in the losartan serum concentration, there was a 1.3 pg/mL decrease in TNFαR1 (p = .009) after adjustment for the oral losartan dose. Paradoxically, we also observed a smaller but significant increase in TNFα of 0.004 pg/mL (p = .001) for every nanogram increase in the serum losartan concentration. The reason for and significance of this modest increase in serum TNFα in the context of a more marked decrease in TNFαR1 remains unclear, but lack of availability of TNFαR1 to bind circulating TNFα may have played a role (31).
Losartan Changes Metabolism, Energy Metabolism, and Biomarkers of Mitochondrial Health
Prior research suggests that angiotensin system dysregulation plays a role in age-related mitochondrial decline (7,23,36–38). We employed a targeted metabolomic platform to pinpoint specific biological pathways connecting losartan metabolites to metabolic mechanisms and energy utilization. We quantified an array of metabolites from 2 substance classes (amino acids and biogenic amines) and serum metabolites of the TCA cycle. We compared these metabolites across visits and with serum losartan, EXP3174, and EXP3179 concentrations in the treatment group. Supplementary Table 3 lists the major metabolites from each substance class that changed significantly during losartan treatment.
In the amino acid class, we observed a significant increase in arginine by an estimated 28% (95% CI: from 7% to 52% increase, p = .006) from baseline to Visit 4. Our data also showed that each ng/mL increase in the serum concentration of EXP3179 was associated with a 10.8 μmol/L estimated increase in arginine (95% CI: 3.1–18.6 μmol/L, p = .006) after adjustment for the oral losartan dose. Arginine is the biological precursor of NO, which serves as an important signaling and effector molecule. The Global Arginine Bioavailability index, which is a more accurate measure of the availability of arginine to form NO (39), was also positively correlated with the serum concentrations of EXP3174 (0.00035, 95% CI: 0.00012–0.00058, p < .01) and EXP3179 (0.12, 95% CI: 0.073–0.17, p < .001). An improvement in NO bioavailability is traditionally seen as an indicator of an effective AT1R blockade (40,41).
Other important polyamines, such as the autophagy marker spermidine, increased by an estimated 70% (p = .01) from baseline to Visit 4. Our data also showed a significantly negative association between the serum EXP3179 concentration and the oxidative stress marker nitrotyrosine (p = .026) after adjustment for the losartan dose. Nitrotyrosine is generally higher in frail adults (42,43).
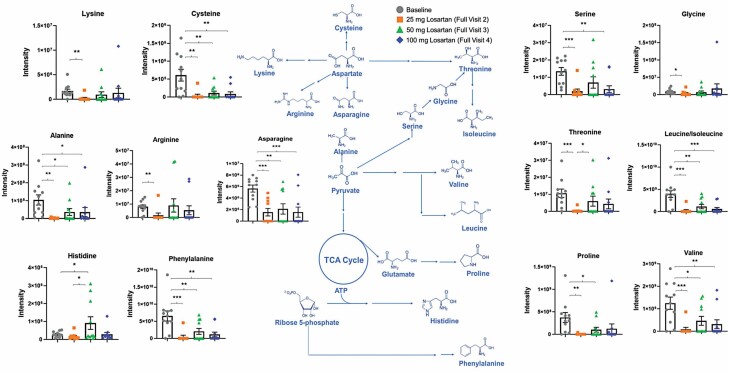
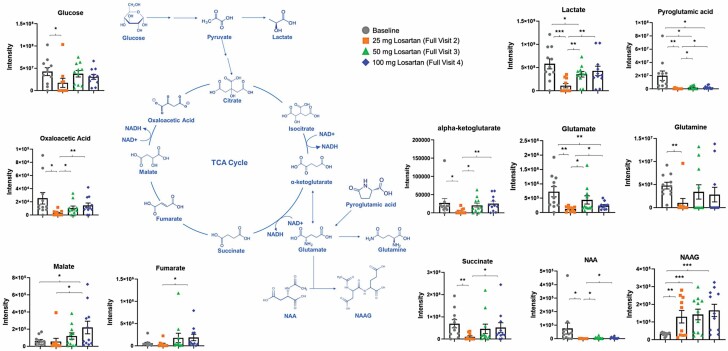
There were nominally significant differences in TCA metabolites. The accumulation of serum (extramitochondrial) metabolites are linked to poor outcomes and may indicate poor utilization (44–49). Our data showed significantly lower concentrations of key TCA metabolites following the initiation of treatment with losartan in the treatment group (Figures 2 and 3; Supplementary Figure 2).
Figure 2.
Effects of losartan doses on the amino acid precursor metabolites that feed into the intermediate metabolites of the TCA cycle. Comparisons of the distributions of metabolites by losartan dose in the treatment group were conducted using Kruskal–Wallis one-way ANOVA with Dunn’s multiple comparisons test. *p < .05, **p < .01, and ***p < .005. TCA = tricarboxylic acid.
Figure 3.
Effects of losartan doses on TCA cycle metabolites. Comparisons of the distributions of metabolites by losartan dose in the treatment group were conducted using Kruskal–Wallis one-way ANOVA with Dunn’s multiple comparisons test. *p < .05, **p < .01, and ***p < .005. TCA = tricarboxylic acid.
Most of the energy metabolites decreased in concentration from baseline to Visit 4 in the losartan treatment group and showed linear decreases per visit, regardless of the concentrations of serum losartan and its metabolites. N-acetyl aspartate decreased by an estimated 93% overall (p = .003) from baseline to Visit 4. Cysteine decreased by an estimated 82% (p = .03), and with each increase in the dose of losartan, cysteine showed linear decreases at any given concentration of serum losartan (p = .01), EXP3174 (p = .04), and EXP3179 (p = .02). Leucine/isoleucine decreased by an estimated 78% (p = .001) from baseline to Visit 4, and additional decreases were seen with losartan dose increases at any given concentration of serum losartan (p < .001), EXP3174 (p = .001), and EXP3179 (p < .001). Hippuric acid decreased by an estimated 85% (p = .001) from baseline to Visit 4, and decreases were seen with losartan dose increases at any given concentration of losartan (p = .04). Indole-3-lactic acid decreased by an estimated 83% (p < .001) from baseline to Visit 4, and a linear decrease was observed by losartan dose at any given concentration of losartan (p = .006), EXP3174 (p = .01), and EXP3179 (p = .004). L-Phenylalanine decreased by an estimated 74% (p = .001), and decreases were seen with subsequent losartan dose increases at any given concentration of losartan (p = .002), EXP3174 (p = .007), and EXP3179 (p = .005). L-Pyroglutamate decreased by an estimated 86% (p = .001), and decreases were seen with subsequent losartan dose increases at any given concentration of losartan (p = .04). L-Glutamic acid decreased by an estimated 60% (p < .001), and a linear decrease was seen with subsequent losartan dose increases at any given concentration of EXP3179 (p = .04). Interestingly, our untargeted metabolomics analysis revealed that losartan treatment led to an upregulation in the serum concentration of γ-aminobutyric acid (GABA), which is a neurotransmitter linked to neuromuscular function (Supplementary Figure 3). Prior research suggested that the enzyme responsible for GABA synthesis is downregulated in frail, aging individuals (50).
A few of the energy metabolites increased from baseline to Visit 4 during losartan treatment and showed linear increases per visit, regardless of the concentrations of serum losartan and its metabolites. N-acetyl-aspartyl-glutamate increased by 7.5-fold (p < .001) from baseline to Visit 4, and there was a linear increase with each losartan dose increase at any given concentration of losartan (p < .001), EXP3174 (p = .001), and EXP3179 (p < .001). Malate increased by 4.4-fold (p = .01), and linear decreases were seen with subsequent losartan dose increases at any given concentration of EXP3179 (p = .04). Fumarate increased by 7.5-fold (p = .04) from baseline to Visit 4. Taken together, these metabolomic results suggest that losartan treatment increases the concentrations of amino acids involved in mitigating oxidative stress and recycling damaged tissue and improves the utilization of key TCA metabolites.
Discussion
Losartan is an oral antihypertensive agent that is rapidly metabolized to EXP3174 (AT1R blocker) and EXP3179 (PPARγ agonist). Our data showed that the use of losartan in prefrail older adults resulted in an estimated 89% lower odds of frailty with an average 0.3-point lower frailty score as compared to the placebo group. Frailty score was negatively associated with serum losartan and EXP3179 concentrations, while higher EXP3179 and EXP3174 concentrations were associated with increased knee strength.
Losartan was approved for clinical use in the United States in 1995. In the last 25 years, there have been numerous studies examining the beneficial effects of ARBs on cellular and molecular mechanisms and physical and cognitive performance phenotypes that extend beyond their effects on BP. While the results are often variable and inconsistent, the availability of corroborating human phenotypic data is limited, blocking AT1R in animals has been reported to improve muscle repair ability, aerobic capacity, and longevity (22,23,51). In this study, we have only analyzed through samples. The full pharmacokinetic profile of the EXP3179 metabolite has not been described in the literature. Therefore, no current population pharmacokinetic model exists to assess losartan, EXP3174, and EXP3179.
Disentangling the PPARγ effects of losartan from its angiotensin effects is difficult given that both EXP3179 and EXP3174 are products of losartan administered orally to the participants. However, our data sheds some light on the nonangiotensin system effects of losartan, which are not dependent on angiotensin II binding to AT1R. Exploratory analysis of the data suggested that EXP3179, a PPARγ agonist with no AT1R effects, may have a positive effect on physical function, frailty markers, cytokine concentrations, oxidative stress marker concentrations, and metabolomics. Our data are consistent with the reported antiinflammatory and platelet antiaggregatory properties of EXP3179 (52).
Our data showed that it is challenging to balance intra and interpatient and intra and interindication variability with regard to pharmacokinetics and the observed effects. There were signs that several of the participants who were in the losartan intervention group were not taking losartan, as they had undetectable serum concentrations of losartan and its metabolites. Among those who did take losartan, there was still significant variability regarding their blood concentrations of losartan and its metabolites, even though they all received the same oral dose schedule of losartan. This has implications for patients taking losartan to treat hypertension or cardiovascular diseases because there may be a dose–response relationship. Altogether, these data also highlight an opportunity to practice precision medicine when prescribing losartan, tailoring the treatment doses to the specific patient and indication.
Our cytokine data highlighted some of this variability. Evaluation of the effects of losartan on cytokine concentrations showed a significant decrease in the concentration of TNFαR1 with increasing serum concentrations of losartan. Previous studies have indicated that increased TNFαR1 concentrations are associated with worse outcomes (53) and frailty (54,55). However, we also observed a significant increase in the concentration of IL1β. Although this is not consistent with prior reports from cell cultures or animal models (56), to the best of our knowledge, there are no previous reports of the impact of angiotensin system blockade on IL1β in human clinical trials. Because individual cytokines often play roles that are both helpful and harmful, our results may reflect these dual roles of cytokines in the inflammatory process.
The limitations of this study include the small number of prefrail older adults. Despite the finding of significant differences in outcomes, the small sample size impeded the ability to perform robust adjustments in the molecular analyses. Perhaps more importantly, the study was designed to ascertain associations and not causality. While we observed beneficial effects of losartan on frailty and muscle condition in older adults, that effect may have been mediated by higher muscle blood flow or other molecular factors, such as increased concentrations of growth factors. Larger studies are needed to fine-tune the utilization of serum concentrations of losartan and its metabolites to guide treatment adjustments and evaluate the impact on skeletal muscle injury, frailty, disuse atrophy, and other aging-related skeletal muscle problems. These studies should also involve pharmacogenomic analyses because CYP2C9 polymorphisms appear to be linked to the pharmacokinetics of losartan and EXP3174 (57).
In summary, our study showed that the use of losartan at doses used for general BP management was associated with improvements in the molecular markers of frailty, knee strength, and frailty scores. These improvements were achieved, at least in part, through a nonangiotensin II-dependent pathway. Moreover, we gained a better understanding of the way in which intermediate endpoints and biomarkers could be employed to efficaciously test therapies for frailty and aging-related skeletal muscle injuries and function. This furthers the ultimate goal of identifying tailored treatments to help older adults preserve their strength and independence as they age.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Thomas Finucane for his intellectual contribution to the manuscript, including guidance with the clinical implications of the data.
Clinical Trials Registration Number: NCT01989793
Contributor Information
Jessica L Lee, Department of Medicine, Division of Geriatric Medicine and Gerontology, The Johns Hopkins University School of Medicine, , Baltimore, Maryland, USA; Department of Internal Medicine, Division of Geriatric and Palliative Medicine, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, Texas, USA.
Cissy Zhang, Department of Oncology, Division of Cancer Chemical and Structural Biology, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Reyhan Westbrook, Department of Medicine, Division of Geriatric Medicine and Gerontology, The Johns Hopkins University School of Medicine , Baltimore, Maryland, USA.
Mariann M Gabrawy, Department of Medicine, Division of Geriatric Medicine and Gerontology, The Johns Hopkins University School of Medicine , Baltimore, Maryland, USA.
Lolita Nidadavolu, Department of Medicine, Division of Geriatric Medicine and Gerontology, The Johns Hopkins University School of Medicine , Baltimore, Maryland, USA.
Huanle Yang, Department of Medicine, Division of Geriatric Medicine and Gerontology, The Johns Hopkins University School of Medicine , Baltimore, Maryland, USA.
Ruth Marx, Department of Medicine, Division of Geriatric Medicine and Gerontology, The Johns Hopkins University School of Medicine , Baltimore, Maryland, USA.
Yuqiong Wu, Department of Medicine, Division of Geriatric Medicine and Gerontology, The Johns Hopkins University School of Medicine , Baltimore, Maryland, USA.
Nicole M Anders, Department of Oncology, Division of Cancer Chemical and Structural Biology, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; The Johns Hopkins Analytical Pharmacology Core Laboratory, Clinical Pharmacology, Baltimore, MD, USA.
Lina Ma, Department of Geriatrics, Xuanwu Hospital, Capital Medical University, China National Clinical Research Center for Geriatric Disorders, Beijing, China.
Marcela-Dávalos Bichara, Department of Medicine, Division of Geriatric Medicine and Gerontology, The Johns Hopkins University School of Medicine , Baltimore, Maryland, USA.
Min-Ji Kwak, Department of Internal Medicine, Division of Geriatric and Palliative Medicine, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, Texas, USA.
Brian Buta, Department of Medicine, Division of Geriatric Medicine and Gerontology, The Johns Hopkins University School of Medicine , Baltimore, Maryland, USA.
Mohammed Khadeer, Translational Gerontology Branch, National Institute on Aging, National Institutes of Health, Baltimore, Maryland, USA.
Gayane Yenokyan, Johns Hopkins Biostatistics Center, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Jing Tian, Department of Medicine, Division of Geriatric Medicine and Gerontology, The Johns Hopkins University School of Medicine , Baltimore, Maryland, USA.
Qian-Li Xue, Department of Medicine, Division of Geriatric Medicine and Gerontology, The Johns Hopkins University School of Medicine , Baltimore, Maryland, USA.
Helmy M Siragy, Department of Medicine, Division of Endocrine and Metabolism, University of Virginia, Charlottesville, Virginia, USA.
Robert M Carey, Department of Medicine, Division of Endocrine and Metabolism, University of Virginia, Charlottesville, Virginia, USA.
Rafael de Cabo, Translational Gerontology Branch, National Institute on Aging, National Institutes of Health, Baltimore, Maryland, USA.
Luigi Ferrucci, Translational Gerontology Branch, National Institute on Aging, National Institutes of Health, Baltimore, Maryland, USA.
Ruin Moaddel, Translational Gerontology Branch, National Institute on Aging, National Institutes of Health, Baltimore, Maryland, USA.
Michelle A Rudek, The Johns Hopkins Analytical Pharmacology Core Laboratory, Clinical Pharmacology, Baltimore, MD, USA; Department of Medicine, Division of Clinical Pharmacology, The Johns Hopkins University School of Medicine , Baltimore, Maryland, USA.
Anne Le, The Johns Hopkins Analytical Pharmacology Core Laboratory, Clinical Pharmacology, Baltimore, MD, USA; Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Jeremy D Walston, Department of Medicine, Division of Geriatric Medicine and Gerontology, The Johns Hopkins University School of Medicine , Baltimore, Maryland, USA.
Peter M Abadir, Department of Medicine, Division of Geriatric Medicine and Gerontology, The Johns Hopkins University School of Medicine , Baltimore, Maryland, USA.
Funding
This work was supported by the Johns Hopkins University Claude D. Pepper Older Americans Independence Center, which is funded by the National Institute on Aging of the National Institutes of Health under award number the Bright Focus Foundation Research Award (P.M.A.); and the Nathan W. and Margaret T. Shock Aging Research Foundation, Nathan Shock Scholar in Aging (P.M.A. and R.W.). The project was also supported by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30CA006973 and UL1TR001079 and the Shared Instrument Grant [S10RR026824]). The project was also supported by grant number UL1TR001079 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. This research was supported in part by the Intramural Research Program at the National Institute on Aging. Its contents are solely the authors’ responsibility and do not necessarily represent the official view of the NCATS or NIH.
Conflict of Interest
None declared.
Author Contributions
J.L.L., P.M.A., L.F., and J.D.W. participated in the study conception and design. J.D.W. acted as the PI for the study and J.L.L. and P.M.A. recruited the participants, monitored participant safety, and obtained the functional, demographic, and serum data. M.A.R. and N.M.A were responsible for developing the methods and quantifying the serum levels of losartan, EXP3174, and EXP 3179. J.L.L. and C.Z. were responsible for developing the targeted metabolomics measurements of TCA and glycolysis and quantification in our patient cohort. R.M. and M.K. were responsible for the targeted metabolomics measurement of changes in metabolites in 5 substance classes. R.W. and M.M.G. were responsible for processing the samples for the metabolomic analysis and facilitated the development of the TCA cycle metabolomic measurements. L.N., L.M., M.-D.B., H.Y., R.M., and Y.W. participated in processing the samples for the molecular measurements. G.Y., Q.-L.X., M.-J.K., J.L.L., J.D.W., P.M.A., J.T., and B.B. participated in analyzing and interpreting the data. All authors participated in drafting the manuscript and approved the final version. G.Y., Q.-L.X, J.L.L., J.D.W., R.M.C., H.M.S., R.d.C., and P.M.A. were responsible for the critical revision of the manuscript for important intellectual content and approval of the final version. G.Y., Q.-L.X., J.L.L., J.D.W., and P.M.A. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 2. Varadhan R, Seplaki CL, Xue QL, Bandeen-Roche K, Fried LP. Stimulus-response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mech Ageing Dev. 2008;129(11):666–670. doi: 10.1016/j.mad.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ko F, Abadir P, Marx R, et al. Impaired mitochondrial degradation by autophagy in the skeletal muscle of the aged female interleukin 10 null mouse. Exp Gerontol. 2016;73:23–27. doi: 10.1016/j.exger.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aas SN, Hamarsland H, Cumming KT, et al. The impact of age and frailty on skeletal muscle autophagy markers and specific strength: a cross-sectional comparison. Exp Gerontol. 2019;125:110687. doi: 10.1016/j.exger.2019.110687 [DOI] [PubMed] [Google Scholar]
- 6. Walston JD. Connecting age-related biological decline to frailty and late-life vulnerability. Nestle Nutr Inst Workshop Ser. 2015;83:1–10. doi: 10.1159/000382052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vajapey R, Rini D, Walston J, Abadir P. The impact of age-related dysregulation of the angiotensin system on mitochondrial redox balance. Front Physiol. 2014;5:439. doi: 10.3389/fphys.2014.00439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abadir PM. The frail renin-angiotensin system. Clin Geriatr Med. 2011;27(1):53–65. doi: 10.1016/j.cger.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cosarderelioglu C, Nidadavolu LS, George CJ, et al. Brain renin-angiotensin system at the intersect of physical and cognitive frailty. Front Neurosci. 2020;14:586314. doi: 10.3389/fnins.2020.586314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eckel SP, Bandeen-Roche K, Chaves PH, Fried LP, Louis TA. Surrogate screening models for the low physical activity criterion of frailty. Aging Clin Exp Res. 2011;23(3):209–216. doi: 10.1007/BF03324962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calvani R, Picca, A, Marini F, et al. A distinct pattern of circulating amino acids characterizes older persons with physical frailty and sarcopenia: results from the BIOSPHERE study. Nutrients. 2018;10:1691. doi: 10.3390/nu10111691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huffman KM, Koves TR, Hubal MJ, et al. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia. 2014;57(11):2282–2295. doi: 10.1007/s00125-014-3343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown JD, Smith SM, Strotmeyer ES, et al. Comparative effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on response to a physical activity intervention in older adults: results from the Lifestyle Interventions and Independence for Elders Study. J Gerontol A Biol Sci Med Sci. 2020;75(5):1010–1016. doi: 10.1093/gerona/glz120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heisterberg MF, Andersen JL, Schjerling P, et al. Losartan has no additive effect on the response to heavy-resistance exercise in human elderly skeletal muscle. J Appl Physiol (1985). 2018;125(5):1536–1554. doi: 10.1152/japplphysiol.00106.2018 [DOI] [PubMed] [Google Scholar]
- 15. Buford TW, Manini TM, Hsu FC, et al. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J Am Geriatr Soc. 2012;60(7):1244–1252. doi: 10.1111/j.1532-5415.2012.04045.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simon CB, Lee-McMullen B, Phelan D, Gilkes J, Carter CS, Buford TW. The renin-angiotensin system and prevention of age-related functional decline: where are we now? Age (Dordr). 2015;37(1):9753. doi: 10.1007/s11357-015-9753-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harper SA, Baptista LC, Roberts LM, et al. Angiotensin converting enzyme inhibitors combined with exercise for hypertensive seniors (The ACES trial): study protocol of a randomized controlled trial. Front Med (Lausanne). 2019;6:327. doi: 10.3389/fmed.2019.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lo MW, Goldberg MR, McCrea JB, Lu H, Furtek CI, Bjornsson TD. Pharmacokinetics of losartan, an angiotensin II receptor antagonist, and its active metabolite EXP3174 in humans. Clin Pharmacol Ther. 1995;58(6):641–649. doi: 10.1016/0009-9236(95)90020-9 [DOI] [PubMed] [Google Scholar]
- 19. Sachinidis A, Ko Y, Weisser P, et al. EXP3174, a metabolite of losartan (MK 954, DuP 753) is more potent than losartan in blocking the angiotensin II-induced responses in vascular smooth muscle cells. J Hypertens. 1993;11(2):155–162. doi: 10.1097/00004872-199302000-00007 [DOI] [PubMed] [Google Scholar]
- 20. Krämer C, Sunkomat J, Witte J, et al. Angiotensin II receptor-independent antiinflammatory and antiaggregatory properties of losartan: role of the active metabolite EXP3179. Circ Res. 2002;90(7):770–776. doi: 10.1161/01.res.0000014434.48463.35 [DOI] [PubMed] [Google Scholar]
- 21. Rossi GP. Losartan metabolite EXP3179: an AT1-receptor-independent treatment strategy for patients with the metabolic syndrome? Hypertension. 2009;54(4):710–712. doi: 10.1161/HYPERTENSIONAHA.109.138883 [DOI] [PubMed] [Google Scholar]
- 22. Burks TN, Andres-Mateos E, Marx R, et al. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med. 2011;3(82):82ra37. doi: 10.1126/scitranslmed.3002227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin CH, Yang H, Xue QL, et al. Losartan improves measures of activity, inflammation, and oxidative stress in older mice. Exp Gerontol. 2014;58:174–178. doi: 10.1016/j.exger.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang X, Miller GD, Messier SP, Nicklas BJ. Knee strength maintained despite loss of lean body mass during weight loss in older obese adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62(8):866–871. doi: 10.1093/gerona/62.8.866 [DOI] [PubMed] [Google Scholar]
- 25. Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–266. doi: 10.1093/gerona/61.3.262 [DOI] [PubMed] [Google Scholar]
- 26. Food and Drug Administration Center for Drug Evaluation and Research, U.S.D.o.H.a.H.S. Docket # FDA-2013-D-1020. 2018. Bioanalytical Method Validation Guidance for Industry. 1–37. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry [Google Scholar]
- 27. Westbrook R, Chung T, Lovett J, et al. Kynurenines link chronic inflammation to functional decline and physical frailty. JCI Insight. 2020;5:e136091. doi: 10.1172/jci.insight.136091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ripley E, Hirsch A. Fifteen years of losartan: what have we learned about losartan that can benefit chronic kidney disease patients? Int J Nephrol Renovasc Dis. 2010;3:93–98. doi: 10.2147/ijnrd.s7038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Holtkamp FA, de Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80(3):282–287. doi: 10.1038/ki.2011.79 [DOI] [PubMed] [Google Scholar]
- 30. Cozaar (Losartan Potassium Tablets). 2021:1–28. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020386s058lbl.pdf. Accessed 2021.
- 31. Parameswaran N, Patial S. Tumor necrosis factor-Î ± signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gomez D, Baylis RA, Durgin BG, et al. Interleukin-1β has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med. 2018;24:1418–1429. doi: 10.1038/s41591-018-0124-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rivera-Escalera F, Pinney JJ, Owlett L, et al. IL-1β-driven amyloid plaque clearance is associated with an expansion of transcriptionally reprogrammed microglia. J Neuroinflammation. 2019;16(1):261. doi: 10.1186/s12974-019-1645-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kühn K, Hashimoto S, Lotz M. IL-1 beta protects human chondrocytes from CD95-induced apoptosis. J Immunol. 2000;164(4):2233–2239. doi: 10.4049/jimmunol.164.4.2233 [DOI] [PubMed] [Google Scholar]
- 35. Collino S, Montoliu I, Martin FP, et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS One. 2013;8(3):e56564. doi: 10.1371/journal.pone.0056564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Cavanagh EM, Piotrkowski B, Fraga CG. Concerted action of the renin-angiotensin system, mitochondria, and antioxidant defenses in aging. Mol Aspects Med. 2004;25(1–2):27–36. doi: 10.1016/j.mam.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 37. Abadir PM, Foster DB, Crow M, et al. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA. 2011;108(36):14849–14854. doi: 10.1073/pnas.1101507108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burks TN, Marx R, Powell L, et al. Combined effects of aging and inflammation on renin-angiotensin system mediate mitochondrial dysfunction and phenotypic changes in cardiomyopathies. Oncotarget. 2015;6(14):11979–11993. doi: 10.18632/oncotarget.3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moaddel R, Shardell M, Khadeer M, et al. Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology (Berl). 2018;235(10):3017–3030. doi: 10.1007/s00213-018-4992-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42(4):600–604. doi: 10.1161/01.HYP.0000090323.58122.5C [DOI] [PubMed] [Google Scholar]
- 41. Mason RP, Jacob RF, Kubant R, et al. Effects of angiotensin receptor blockers on endothelial nitric oxide release: the role of eNOS variants. Br J Clin Pharmacol. 2012;74(1):141–146. doi: 10.1111/j.1365-2125.2012.04189.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kadoguchi T, Shimada K, Miyazaki T, et al. Promotion of oxidative stress is associated with mitochondrial dysfunction and muscle atrophy in aging mice. Geriatr Gerontol Int. 2020;20(1):78–84. doi: 10.1111/ggi.13818 [DOI] [PubMed] [Google Scholar]
- 43. Uchmanowicz I. Oxidative stress, frailty and cardiovascular diseases: current evidence. Adv Exp Med Biol. 2020;1216:65–77. doi: 10.1007/978-3-030-33330-0_8 [DOI] [PubMed] [Google Scholar]
- 44. Velasquez S, Prevedel L, Valdebenito S, et al. Circulating levels of ATP is a biomarker of HIV cognitive impairment. EBioMedicine. 2020;51:102503. doi: 10.1016/j.ebiom.2019.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cauwels A, Rogge E, Vandendriessche B, Shiva S, Brouckaert P. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis. 2014;5:e1102. doi: 10.1038/cddis.2014.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mills EL, Pierce KA, Jedrychowski MP, et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature. 2018;560(7716):102–106. doi: 10.1038/s41586-018-0353-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Littlewood-Evans A, Sarret S, Apfel V, et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 2016;213(9):1655–1662. doi: 10.1084/jem.20160061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martínez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun. 2020;11(1):102. doi: 10.1038/s41467-019-13668-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Volonté C, Amadio S, Cavaliere F, D’Ambrosi N, Vacca F, Bernardi G. Extracellular ATP and neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2(6):403–412. doi: 10.2174/1568007033482643 [DOI] [PubMed] [Google Scholar]
- 50. Brivio P, Paladini MS, Racagni G, Riva MA, Calabrese F, Molteni R. From healthy aging to frailty: in search of the underlying mechanisms. Curr Med Chem. 2019;26(20):3685–3701. doi: 10.2174/0929867326666190717152739 [DOI] [PubMed] [Google Scholar]
- 51. Benigni A, Corna D, Zoja C, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119(3):524–530. doi: 10.1172/JCI36703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sadoshima J. Novel AT(1) receptor-independent functions of losartan. Circ Res. 2002;90(7):754–756. doi: 10.1161/01.res.0000016843.82450.8c [DOI] [PubMed] [Google Scholar]
- 53. Naude PJ, Dobos N, van der Meer D, et al. Analysis of cognition, motor performance and anxiety in young and aged tumor necrosis factor alpha receptor 1 and 2 deficient mice. Behav Brain Res. 2014;258:43–51. doi: 10.1016/j.bbr.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 54. Piggott DA, Bandeen-Roche K, Mehta SH, et al. Frailty transitions, inflammation, and mortality among persons aging with HIV infection and injection drug use. AIDS. 2020;34:1217–1225. doi: 10.1097/QAD.0000000000002527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McAdams-DeMarco MA, Ying H, Thomas AG, et al. Frailty, inflammatory markers, and waitlist mortality among patients with end-stage renal disease in a prospective cohort study. Transplantation. 2018;102(10):1740–1746. doi: 10.1097/TP.0000000000002213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim E, Hwang SH, Kim HK, Abdi S, Kim HK. Losartan, an angiotensin II type 1 receptor antagonist, alleviates mechanical hyperalgesia in a rat model of chemotherapy-induced neuropathic pain by inhibiting inflammatory cytokines in the dorsal root ganglia. Mol Neurobiol. 2019;56(11):7408–7419. doi: 10.1007/s12035-019-1616-0 [DOI] [PubMed] [Google Scholar]
- 57. Park YA, Song YB, Yee, J, Yoon HY, Gwak HS. Influence of CYP2C9 genetic polymorphisms on the pharmacokinetics of losartan and its active metabolite E-3174: a systematic review and meta-analysis. J Pers Med. 2021;11:617. doi: 10.3390/jpm11070617 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.