To the Editor:
High-flow nasal cannula (HFNC) is increasingly used in patients with acute hypoxemic respiratory failure (AHRF) and has been shown to improve outcome in specific patient categories, including community acquired pneumonia (1) and after extubation (2). Because HFNC failure and delayed intubation is associated with adverse clinical outcome (3), predicting HFNC failure is of clinical importance.
In patients with pneumonia and hypoxemic failure treated with HFNC, the ROX index (oxygen saturation as measured by pulse oximetry [SpO2]/fraction of inspired oxygen [FiO2] over respiratory rate), has been validated to predict the risk for endotracheal intubation (4, 5). Increased respiratory rate, an important component of ROX, is used as an estimate for high respiratory drive, although it is well known that respiratory rate is insensitive to early changes in respiratory drive. Indeed, it has been shown that ROX worked best only after 12 hours after HFNC initiation. Earlier and more sensitive predictors of HFNC failure would be of clinical importance. Initially, elevated respiratory drive increases tidal volume (VT), but not respiratory rate (6). In addition, high VT has been linked to patient self-inflicted lung injury (P-SILI) and such may increase intubation rate in patients with AHRF (7). Taken together, from a physiological perspective, elevated VT may be a better predictor for HFNC failure compared with respiratory rate. Hence, we report an approach to measure VT generated by patients supported with HFNC and establish a novel index named VOX (Volume-OXygenation) based on VT to predict HFNC failure in patients with AHRF.
Methods
This single-center prospective observational study was performed from May 2021 to January 2022 in a 60-bed ICU at Zhongda Hospital, China. The research premise was granted approval by the local Ethics Committee (2020ZDSYLL303-P01) and registered in the Chinese Clinical Trial Registry (ChiCTR2100046461). All patients admitted to ICU for AHRF and eligible for treatment with HFNC (Optiflow™, Fisher and Paykel) according to clinical protocol were screened for eligibility.
The VOX index was defined as the ratio of SpO2/FiO2 over VT. We briefly interrupted HFNC (3 min) to measure VT using a mechanical ventilator (SV800, Mindray) in noninvasive ventilation (NIV) mode, as an “NIV test”. Inspiratory support was set at 5 cmH2O and 5 cmH2O positive end-expiratory pressure level for all patients, and initial oxygen concentration was set as in HFNC. NIV was delivered through a face mask (ZS-MZ-A, Zhongshan Medical) and a double-pipe system, while minimizing leaks. In consideration of variations in VT, we recorded mean VT and respiratory rate for 1 minute under stable conditions.
HFNC therapy was started within 15 minutes after recruitment. We adjusted FiO2, targeting SpO2 of 92% or more, and the rate of flow was set based on the physician’s judgement. HFNC discontinuation and invasive mechanical ventilation (IMV) initiation were based on the intubation criteria defined in our clinical protocol, finial decisions were made by the physicians in charge, who were blinded to the VT during NIV test. HFNC failure was defined as a need for IMV, on account of NIV is not employed as the second line of ventilatory support in the event of HFNC failure, in the participating units. The time of HFNC onset was defined as 0 hours. Vital signs; HFNC settings including FiO2, flow rate, and temperature; clinical respiratory variables including respiratory rate, VT, and SpO2 were recorded at 0, 2, 6, 12, 18, and 24 hours following initiation of HFNC treatment. Multivariable logistic regression was performed to explore the association between VOX index and HFNC failure. Receiver-operating characteristic curves (ROC) were generated to show clinical respiratory variables, ROX index and VOX index in predicting the failure of HFNC. Differences between area under the receiver operating curve (AUROC) were estimated using a nonparametric approach, by using the theory on generalized U statistics to generate an estimated covariance matrix (MedCalc software). The maximum value of the Youden's J statistic was utilized as the selection criteria of the optimum cut-off point of the ROC curves. P < 0.05 signifies statistical significance.
Results
Sixty-two patients were enrolled (age 65 ± 12 years, 39 males) and 29 patients (46.8%) failed HFNC. Pneumonia (36/62, 58%) was the primary causes of AHRF; none of the patients were diagnosed with coronavirus disease (COVID-19). Initial flow rate was 54 ± 6 L/min and FiO2 was 0.47 ± 0.08. Patients failing HFNC had significantly higher APACHE II score (21.0 ± 5.8 versus 15.2 ± 5.0, P < 0.001), number of quadrants affected on chest-X-ray (2.3 ± 0.9 versus 1.5 ± 0.7, P = 0.001) and initial FiO2 (0.51 ± 0.09 versus 0.44 ± 0.06, P < 0.001) when compared with patients successfully treated with HFNC. However, age, etiology for AHRF, and comorbidities were not significantly different between patient failing or not failing HFNC. The median duration of HFNC treatment in the success and failure groups was 78 (52–96) hours and 7 (6–23) hours, respectively. Among HFNC failure group, 18 patients (62.1%) were initiated on MV within 12 hours. Patients failing HFNC had higher ICU mortality (34.5% versus 0, P < 0.001), hospital mortality (37.9% versus 3.0%, P < 0.001), and longer ICU length of stay (11 versus 6 days, P = 0.012), compared HFNC success patients.
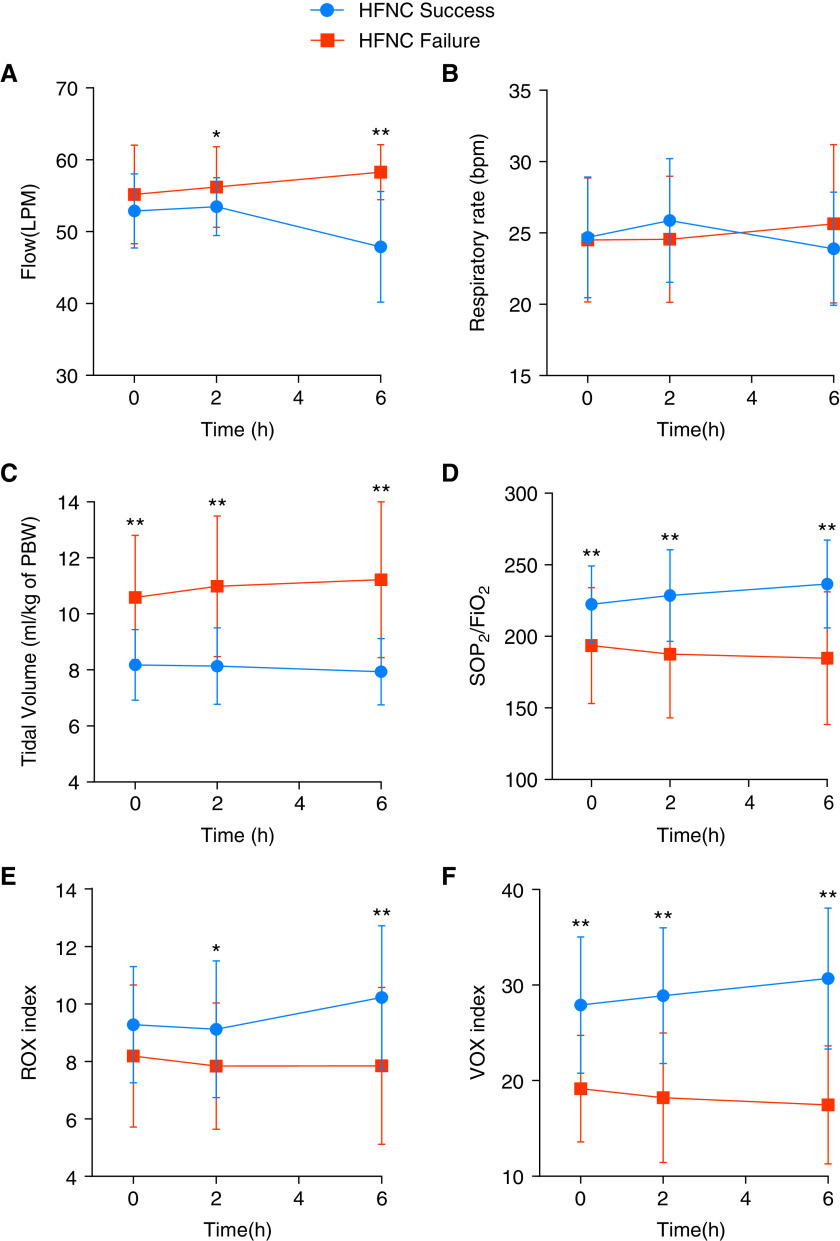
Between HFNC success and failure group, no significant differences in the respiratory rate were found at 0, 2, and 6 hours (Figure 1B). Whereas, VT per predicted body weight (PBW) at 0 h (8.2 ± 1.3 vs. 10.6 ± 2.2 ml/kg; P < 0.001), 2 h (8.1 ± 1.4 vs. 11 ± 2.5 ml/kg; P < 0.001), and 6 h (7.9 ± 1.2 vs. 11.2 ± 2.8 ml/kg; P < 0.001) were all significantly higher in the HFNC failure group (Figure 1C). Although no difference was found in the ROX index at 0 h between success and failure patients, the HFNC success group had significantly higher VOX index compared with failure group at 0 h (27.91 ± 7.13 vs. 19.16 ± 5.58; P < 0.001), 2 h (28.89 ± 7.10 vs. 18.21 ± 6.78; P < 0.001), and 6 h (30.68 ± 7.38 vs. 17.46 ± 6.18; P < 0.001) (Figures 1E and 1F).
Figure 1.
Respiratory variables during HFNC treatment: At time 0 hours, no significant difference was found in ROX index between HFNC failure and HFNC success, whereas VOX index (and VT) was already significantly different at 0 hours, and remained the most powerful predictor for failure during the first 6 hours of HFNC treatment. FiO2 = fraction of inspired oxygen; HFNC = high-flow nasal cannula; LPM = liters per minute; PBW = predicted body weight; ROX = respiratory rate-OXygenation; SpO2 = pulse oximetry; VOX = Volume-OXygenation. *P < 0.05; **P < 0.01.
After adjusting for age, gender, APACHE II, and oxygenation, VOX > 24.82 at 0 hours (odds ratio [OR] 0.81 [95% confidence interval {CI}, 0.69–0.94]; P = 0.007), VOX > 20.91 at 2h (OR 0.78 [95% CI 0.67–0.91]; P = 0.002) and VOX > 22.67 at 6 hours (OR 0.66 [95% CI, 0.51–0.86]; P =0.002) were associated with lower risk for HFNC failure. AUROC values of different variables at 0, 2, and 6 hours, which were used to predict the failure of HFNC treatment, are reported in Table 1. Among all the variables, the prediction accuracy of the VOX increased over time (AUROC 0 h 0.84; 2 h 0.88; 6 h 0.93). At 2 hours, a VOX index cutoff of 20.91 produced the highest sum of sensitivity (72.41%) and specificity (93.94%), positive predictive value (91.30%) and negative predictive value (76.92%). At 6 hours, a VOX index cutoff of 22.67 produced the highest sum of sensitivity (86.21%) and specificity (90.91%), positive predictive value (89.29%) and negative predictive value (88.28%). The overall discriminatory power computed via AUROC of VOX index was greater relative to the ROX index at 0 hours (P = 0.009), 2 hours (P = 0.014), and 6 hours (P = 0.008) for identification of HFNC failure. The AUROC of VOX index was slightly higher than for SpO2/FiO2 or VT alone at each time point, although this did not reach statistical significance (Table 1).
Table 1.
Diagnostic Accuracy of Different Respiratory Variables at Different Time Points of Need for IMV in Patients Treated With HFNC
| Time (h) | AUROC | 95% CI | P Value | |
|---|---|---|---|---|
| SpO2/FiO2 | 0 | 0.76 | 0.64–0.89 | <0.001 |
| 2 | 0.82 | 0.71–0.93 | <0.001 | |
| 6 | 0.84 | 0.73–0.95 | <0.001 | |
| Respiratory rate | 0 | 0.50** | 0.35–0.64 | 0.944 |
| 2 | 0.42** | 0.28–0.56 | 0.271 | |
| 6 | 0.51** | 0.36–0.66 | 0.893 | |
| ROX index | 0 | 0.66** | 0.52–0.80 | 0.034 |
| 2 | 0.70* | 0.56–0.85 | 0.006 | |
| 6 | 0.79** | 0.68–0.91 | 0.001 | |
| VT (ml/kg of PBW) | 0 | 0.83 | 0.71–0.94 | <0.001 |
| 2 | 0.87 | 0.77–0.97 | <0.001 | |
| 6 | 0.87 | 0.76–0.98 | <0.001 | |
| VOX index | 0 | 0.84 | 0.75–0.94 | <0.001 |
| 2 | 0.88 | 0.79–0.97 | <0.001 | |
| 6 | 0.93 | 0.86–0.99 | <0.001 |
Definition of abbreviations: AUROC = area under the receiver operating curve; CI = confidence interval; FiO2 = fraction of inspired oxygen; HFNC = high-flow nasal cannula; IMV = invasive mechanical ventilation; PBW = predicted body weight; ROX = respiratory rate-OXygenation; SpO2 = oxygen saturation as measured by pulse oximetry; VOX = Volume-OXygenation.
P < 0.05 compared with VOX index at the same time point.
P < 0.01 compared with VOX index at the same time point.
Discussion
We present the VOX index (SpO2/FiO2 to VT), as a novel early predictor for HFNC failure in patients with AHRF. This index is based on the premise that VT is a better estimate early increase in respiratory drive compared with respiratory rate, a key component of the ROX index. After adjusting for potential confounding, higher VOX index remained independently linked to a lower risk for HFNC failure. VOX index had a discriminatory potential of 0.88 (0.79–0.97) and 0.93 (95% CI, 0.86–0.99) in estimating HFNC failure within the first 2 and 6 hours of HFNC onset.
Roca and colleagues introduced the ROX index to predict HFNC failure in patients with hypoxemic pneumonia. Consistent with the original study (4, 5), in the current study, ROX index was associated with HFNC failure. However, the VOX index appears to have a better predictive performance. In fact, VOX could already reliably predict HFNC failure at initiation of HFNC (ROC 0.84). Moreover, the overall discriminatory ability of VOX index was superior to that of ROX index for identification of HFNC failure at all the time points studied. There are some possible explanations for the superior performance of VOX compared with ROX. Even though respiratory drive constitutes a frequency component, respiratory rate alone is a rather insensitive marker for the quantification of respiratory drive and effort. In fact, respiratory rate increased only when respiratory drive was 3 to 4 times higher than normal (8). In our study, patients requiring MV did not exhibit higher respiratory rate than patients successfully treated with HFNC. Our data may further underline the inability of respiratory rate alone to identify patients with harmful respiratory drive. Nonintubated patients with AHRF may exhibit a high respiratory drive resulting in intense inspiratory effort, thus generating the inflation of high VT (9). We observed that patients requiring MV were more likely to generate high VT (>10 ml/kg) at HFNC initiation compared with patients successfully treated with HFNC. Interestingly, Carteaux (10) reported high tidal volume is independently associated with NIV failure in patients with acute hypoxemic failure. Also, Tonelli (7) reported that higher tidal volume, but not respiratory rate, are independently associated with NIV failure in hypoxemic patients. These studies support the important role of TV in failure of noninvasive respiratory support in acute hypoxemic failure patients. This is a likely explanation for the better performance of VOX (incorporating VT), compared with ROX.
This study has limitations. First, measurement of VT required interruption of HFNC and a short period of NIV for diagnostic purposes. This is cumbersome, and more feasible methods for assessment of VT should be used in future validation studies. Second, we used low levels of support during NIV, to guarantee patient comfort and arbitrarily mimic level of support during HFNC. Finally, given the fact that this was a single center study, with a relatively small number of patients, VOX requires further validation. Nevertheless, our findings are important for the design of larger prospective multicenter clinical trials aimed at validating the VOX index and determining the optimal intubation time.
Footnotes
Supported, in part, by Jiangsu Provincial Key Research and Development Program (BE2020786 and BE2019749).
Author Contributions: D.C., H.Q, Y.Y., and L.L. contributed to the conception and design. C.P. and D.C. collected the data. J.X. and D.C. contributed to data analysis and interpretation. D.C., L.H, Y.Y., and L.L. contributed to drafting manuscript for important intellectual content. All authors helped to revise the draft of the manuscript. All authors read and approved the final manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202203-0561LE on June 7, 2022
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. FLORALI Study Group REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med . 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 2. Hernández G, Vaquero C, González P, Subira C, Frutos-Vivar F, Rialp G, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA . 2016;315:1354–1361. doi: 10.1001/jama.2016.2711. [DOI] [PubMed] [Google Scholar]
- 3. Kang BJ, Koh Y, Lim CM, Huh JW, Baek S, Han M, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med . 2015;41:623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 4. Roca O, Messika J, Caralt B, García-de-Acilu M, Sztrymf B, Ricard JD, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J Crit Care . 2016;35:200–205. doi: 10.1016/j.jcrc.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 5. Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med . 2019;199:1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 6. Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Pathophysiology and clinical implications. Respiratory drive in critically ill patients. Am J Respir Crit Care Med . 2020;201:20–32. doi: 10.1164/rccm.201903-0596SO. [DOI] [PubMed] [Google Scholar]
- 7. Tonelli R, Fantini R, Tabbì L, Castaniere I, Pisani L, Pellegrino MR, et al. Early inspiratory effort assessment by esophageal manometry predicts noninvasive ventilation outcome in de novo respiratory failure. A pilot study. Am J Respir Crit Care Med . 2020;202:558–567. doi: 10.1164/rccm.201912-2512OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med . 2020;46:606–618. doi: 10.1007/s00134-020-05942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grieco DL, Menga LS, Eleuteri D, Antonelli M. Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol . 2019;85:1014–1023. doi: 10.23736/S0375-9393.19.13418-9. [DOI] [PubMed] [Google Scholar]
- 10. Carteaux G, Millán-Guilarte T, De Prost N, Razazi K, Abid S, Thille AW, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med . 2016;44:282–290. doi: 10.1097/CCM.0000000000001379. [DOI] [PubMed] [Google Scholar]