Abstract
Clostridium septicum is responsible for several diseases in humans and animals. The bacterium is capable of a simple kind of multicellular behavior known as swarming. In this investigation, environmental and physiologic factors affecting growth and swarm cell formation in C. septicum were studied over a range of dilution rates (D = 0.02 to 0.65 h−1) in glucose-limited, glucose-excess, and mucin-limited chemostats. Cellular differentiation was observed at low specific growth rates, irrespective of the carbon and energy source, showing that swarming occurred in response to nutrient depletion. Differential expression of virulence determinants was detected in swarm cells. Hemolysin was secreted by short motile rods but not swarm cells, whereas in cultures grown with glucose, only swarm cells formed DNase, hyaluronidase, and neuraminidase. However, neuraminidase and, to a lesser degree, hyaluronidase were induced in short motile rods in mucin-limited cultures. Both swarm cells and short rods were cytotoxic to Vero cells. Mucin was chemotaxic to C. septicum, and large amounts of mucin-degrading enzymes (β-galactosidase, N-acetyl β-glucosaminidase, glycosulfatase, and neuraminidase) were produced. Synthesis of these enzymes was catabolite regulated. In chemostat experiments, glycosulfatase secretion occurred only in swarm cells at low dilution rates in mucin-limited cultures. Determinations of oligosaccharide utilization demonstrated that N-acetylglucosamine, galactose, and N-acetylgalactosamine were the main carbon sources for C. septicum in mucin. Neuraminic acid was not assimilated, showing that neuraminidase does not have a direct nutritional function in this pathogen.
Clostridium septicum is the etiologic agent of several diseases in humans and animals (7, 20, 33). Unlike many clostridia, which are indigenous to the large intestine, C. septicum is not part of the normal microbiota in humans (16). Despite this, the main route of nontraumatic infection is the large bowel (11). Infection is associated primarily with mucosal damage, resulting from a number of causes including neutropenia, radiation therapy, cancer, and chemotherapy (7, 29, 41). C. septicum infections of the bowel are rapidly fulminating, and mortality is high (29).
Like many other bacterial pathogens (17), virulence in C. septicum is multifactorial, and the organism secretes several toxins and spreading factors that take part in destruction of the bowel wall (38, 51). Four major toxins are recognized, including alpha-toxin and delta-toxin, which are hemolysins, beta-toxin (DNase), and gamma-toxin (hyaluronidase) (38, 46). C. septicum also produces a variety of other secretory products, including neuraminidase (22, 45).
Alpha-toxin is hemolytic and has delayed action on red blood cells, whereas delta-toxin is oxygen labile and rapidly acting (45). Beta-toxin is one of the main extracellular proteins formed by C. septicum (46). Few studies have been performed on this enzyme, but DNases secreted by other bacteria are often highly toxic, and beta-toxin is thought to act as a spreading agent in C. septicum infections (50). More is known about the role of hyaluronidase in this bacterium, which is involved in tissue breakdown (22). The enzyme is secreted by C. septicum after intramuscular injection in experimental animals (22). A similar role has been linked to hyaluronidase in Treponema pallidum, where it is involved in cell adhesion as well as dissemination of the organism through the body (18).
An interesting characteristic of C. septicum is its ability to differentiate into giant hyperflagellated swarm cells, which can participate in rapid and concerted population migrations across surfaces (51). Swarm cell formation also occurs in other clostridia, such as C. tetani, C. sporogenes, and C. bifermentans, as well as in species belonging to the genera Vibrio, Proteus, Serratia, Bacillus, Chromobacterium, and Salmonella (3). Swarm cell formation in Proteus mirabilis has been directly linked to virulence (6), and swarming forms of C. septicum have been observed in necrotic tissue in the large bowel (28, 52), suggesting that pathogenicity may also be associated with cellular differentiation in this bacterium.
The aims of this study were to use the chemostat to investigate multicellular behavior in C. septicum, in relation to the synthesis and secretion of toxins and other virulence factors, and the mucinolytic capabilities of the organism, since in the large intestine, penetration of the mucus layer is a prelude to invasion of the underlying mucosa.
MATERIALS AND METHODS
Bacterium.
Clostridium septicum NCTC 282 was supplied by the National Collection of Type Cultures, Public Health Laboratory Service, London, United Kingdom.
Microscopy.
Swarming of C. septicum over surfaces was studied by removing a 15-mm section of agar containing the swarm zone edge from a culture of C. septicum grown anaerobically on Wilkins-Chalgren agar. This was stained with 200 μl of Baclight Live/Dead viability stain, comprising 1.5 μl of SYTO 9 and 1.5 μl of propidium iodide in 1 ml of anaerobic distilled H2O (Molecular Probes Europe BV, Leiden, The Netherlands), and placed in an anaerobic jar in the dark for 10 min. A Nikon Eclipse E800 microscope attached to a Nikon PCM 2000 confocal system with a 488-nm argon laser (green fluorescence, live) and a 543-nm helium-neon laser (red fluorescence, dead) was used to visualize the section. Images were captured using C.Imaging software (Compix Inc., Cranberry Township, Pa.). Cell morphology of bacteria grown in chemostats was studied by phase-contrast microscopy using a Zeiss Axiophot photomicroscope. For transmission electron microscopy of negatively stained preparations, the cells were centrifuged for 30 s (13,000 × g) in Eppendorf tubes. The bacterial pellets were subsequently resuspended in ammonium acetate (1% [wt/vol]) solution, placed onto Formvar (0.3% [wt/vol] in ethylene chloride)-coated copper grids (Agar Scientific Ltd., Stansted, United Kingdom), and stained with uranyl acetate (0.5% [wt/vol]).
Chemotaxis of C. septicum to mucin.
Chemotaxis studies were done using 25-μl glass capillary tubes (Camlab Ltd., Cambridge, United Kingdom). A 5-μl volume of a solution of purified porcine gastric mucin (1% [wt/vol]) in anaerobic 50 mM potassium phosphate buffer (pH 6.8) was added to the tubes, which were then attached to universal bottles (BDH Ltd., Poole, United Kingdom) containing either C. septicum swarm cells (9.4 × 108 ± 4.5 × 108 cells ml−1) or short rod forms (2.1 × 109 ± 1.9 × 109 ml−1). Control tubes contained phosphate buffer. The capillaries were incubated anaerobically at 37°C for 60 min. Following incubation, the contents were ejected and serial dilutions (10−2 to 10−7) were made in half-strength peptone water (pH 6.8). Samples (0.1 ml) were spread onto Wilkins-Chalgren agar plates containing 4% (wt/vol) purified agar to inhibit swarming (51). The chemotaxis ratio (Rche) was determined by dividing the number of C. septicum cells in mucin capillaries by the number of cells in the controls (1).
Synthesis of mucinolytic enzymes in batch culture.
Universal bottles (30 ml) were prepared containing the following anaerobic basal culture medium (in grams per liter): peptone water, 5.0; tryptone, 5.0; yeast extract, 1.0; cysteine, 0.8; NaCl, 4.5; KH2PO4, 1.5; KCl, 0.45; NH4Cl, 1.0; MgCl2 · 6H2O, 0.20; CaCl2 · 2H2O, 0.10; FeCl3, 0.005. The pH was 6.8. The basal medium was supplemented with either 7.5 g of glucose liter−1, 7.5 g of mucin liter−1, or a mixture of glucose and mucin (each 7.5 g liter−1). The bottles were inoculated with 1 ml of an overnight culture of C. septicum and incubated at 37°C for 24 h. A 20-ml volume of culture was subsequently centrifuged at 14,000 × g (20 min), and the cell-free culture supernatants were retained for enzyme assays.
Substrate specificities of extracellular glycosulfatases.
C. septicum was grown for 12 h on the essentially sulfate-free basal culture medium described above, containing partially purified porcine gastric mucin (0.5 g liter−1) as the carbon source. Bacteria were removed by centrifugation at 12,000 × g (30 min), and portions of cell-free supernatant (5 ml) were filter sterilized through 0.2-μm-pore-size filters into universal bottles containing 100 mM sodium phosphate buffer (pH 6.8), with either partially purified mucin (10 g liter−1), N-acetylglucosamine-6-sulfate, N-acetylglucosamine-3-sulfate, glucose-6-sulfate, or galactose-6-sulfate (each 5.0 g liter−1). Samples (1 ml) were taken periodically for up to 6 h for measurements of sulfate release. The controls were substrate solutions incubated without C. septicum culture supernatant and cell-free supernatants incubated without added substrate.
Sulfate measurements.
Samples were diluted 10-fold in purified water. Propan-2-ol (0.5 ml) was added to 0.5 ml of diluted sample, which was centrifuged at 13,000 × g (5 min). The supernatants (0.5 ml) were then added to 4.5 ml of 1.8 M Na2CO3. Sodium sulfate standards (0.1, 0.5, 1.0, 2.5, and 5.0 mM) were prepared identically to the test samples. Free sulfate was determined by anion-exchange chromatography with anion conductivity detection (19). An AS4 guard column and anion-exchange column (Dionex Ltd., Camberley, United Kingdom) were used, with sodium carbonate (1.8 mM) eluant and cationic suppression with dilute H2SO4 (25 mM), to improve the signal-noise ratio.
Growth of C. septicum in chemostats.
Chemostats are open systems that work efficiently at high cell population densities, and bacterial growth is strictly controlled by the concentrations of limiting nutrients in the feed medium. A useful advantage of continuous culture is that it enables studies to be performed under a multitude of externally imposed steady-state conditions that are not possible with closed batch-type cultures. C. septicum was grown in 0.28-liter (working volume) glass fermentation vessels containing anaerobic culture medium which comprised (in grams per liter) peptone water, 5.0; tryptone, 5.0; yeast extract, 2.5; cysteine, 0.8; NaCl, 4.5; KH2PO4, 1.5; KCl, 0.45; NH4Cl, 0.5; MgCl2 · 6H2O, 0.20; CaCl2 · 2H2O, 0.10; and FeCl3, 0.005. Carbohydrate sources in the chemostat feed media were either 4.0 g of porcine gastric mucin (Sigma grade III) liter−1, 15.0 g of glucose liter−1 (glucose-excess culture), or 1.5 g of glucose liter−1 (glucose-limited culture). Anaerobic growth conditions were maintained by passing O2-free N2 gas (2.5 liters h−1) though the fermentors. The temperature (37°C) and pH (6.5) were maintained as described previously (32). Samples for bacteriologic measurements and determinations of toxin and enzyme formation were taken during steady-state growth, after a minimum of nine culture turnovers at each dilution rate. Steady-state conditions were assessed by measuring viable counts of the organism and concentrations of short-chain fatty acids in spent culture media (14). Culture effluents from the glucose chemostats were analyzed by high-pressure liquid chromatography (see below) to ensure that the carbon source was either limiting or in excess.
Measurements of toxins and mucinolytic enzymes.
Toxin and mucinolytic enzyme levels were measured using C. septicum cell-free culture supernatants and bacterial cell extracts. Bacterial pellets were obtained by centrifugation (14,000 g for 30 min), resuspended in 5 ml of sterile anaerobic buffer (0.01 M Tris [pH 6.5]), and passed twice through a French pressure cell (1.1 × 105 kPa). Hemolysin assays were carried out in Eppendorf tubes by using sheep red blood cells (Oxoid Ltd., Basingstoke, United Kingdom) as described by Allison et al. (6). One unit of hemolytic activity resulted in an increase in absorbance at 540 nm of 0.10 h−1 (sensitivity, 0.05 U ml−1). Sterile saline replaced sample material in the controls. Cell-free culture supernatants from the chemostats were incubated for up to 18 h, to detect both hemolysins. Hyaluronidase and neuraminidase were determined using colorimetric methods described by Linker (31) and Warren (49), respectively. One unit of neuraminidase activity is defined as 1 μg of N-acetylneuraminic acid released h−1 (sensitivity, 5 U ml−1), and 1 unit of hyaluronidase is defined as 1 μmol of N-acetylglucosamine liberated h−1 (sensitivity, 3 U ml−1). N-Acetyl-β-glucosaminidase and β-galactosidase levels were measured by monitoring the release of chromogen from p-nitrophenyl-N-acetyl-β-d-glucosaminide and p-nitrophenyl-β-d-galactopyranoside (32). One unit of glycosidase activity corresponds to 1 μmol of p-nitrophenol released h−1 (sensitivity, 1 U ml−1). DNase measurements in cell-free culture supernatants were made using the method of Bergmeyer et al. (10). One unit of DNase activity was taken as a reduction in the absorbance at 260 nm of 0.001 min−1 (sensitivity, 5 U ml−1). Glycosulfatase activities were detected by monitoring the liberation of free sulfate from partially purified porcine gastric mucin, as described above. One unit of sulfatase activity defined as the release of 1 μmol of SO42−h−1.
Cytotoxin assay.
The cytopathic effect of culture supernatants was demonstrated using African Green Monkey (Vero) cells (Public Health Laboratory Service, Cambridge, United Kingdom). Cell-free culture supernatants (0.1 ml) were added in duplicate to 96-well microtiter plates containing confluent Vero cells and incubated at 37°C for 12 h before being subjected to microscopic examination for cytopathic effects. Cytotoxin-positive samples were serially diluted in phosphate-buffered saline (pH 7.0) and the toxin titer was determined as the dilution at which less than 50% of the monolayer showed evidence of cytopathic effect. Uninoculated culture medium was used as a control.
Carbohydrate measurements.
The chemical compositions of the oligosaccharides in the mucin preparations were determined by hydrolyzing uninoculated medium and culture samples in 2 M H2SO4 for 2 h at 100°C. Sugars were then separated by high-pressure anion-exchange chromatography with pulsed amperometric detection, on a Dionex CarboPac PA 10 column, using 15 mM NaOH as the eluant (42, 43). The N-Acetylneuraminic acid level was determined by hydrolyzing samples in 0.05 M H2SO4 for 1 h at 80°C. The neuraminic acid released was then detected colorimetrically using the periodate method (49). Residual glucose in the glucose-excess chemostats was also measured using the Dionex system. Glucose was not detected in effluent from the glucose-limited cultures.
Culture dry weights.
Culture dry weights were measured as described by Degnan and Macfarlane (14).
Chemicals.
Bacteriologic culture media were obtained from Oxoid. All fine chemicals were purchased from the Sigma Chemical Co. (Poole, United Kingdom).
RESULTS
Swarming in C. septicum.
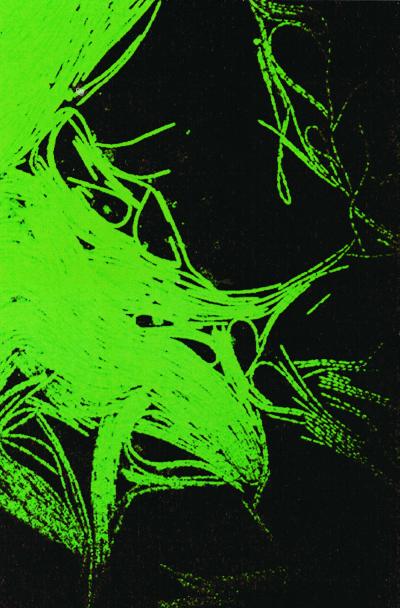
After inoculation onto plates containing 1% agar, C. septicum spread rapidly over the surface, covering the entire plate within hours. Figure 1 shows a fluorescent light micrograph of an advancing raft of giant multinucleate swarm cells at the edge of a swarm zone. Figure 2A shows a hyperflagellated C. septicum swarmer taken from the edge of the swarm zone on a 1% agar plate. These cell forms varied considerably in size, ranging from 10 to 40 μm long. Figure 2B shows C. septicum short motile rods, taken from a colony on a 4% agar plate, where swarming was inhibited.
FIG. 1.
Fluorescent light micrograph showing a raft of C. septicum swarm cells at the edge of the swarm zone on an agar plate. The bacteria were stained with Baclight Live/Dead viability stain. Magnification, ×522.
FIG. 2.
Transmission electron micrographs of a C. septicum swarm cell (A) and C. septicum short motile rods (B). Magnification, ×5000.
Mucin as a chemotactic substance.
Measurements of mucin as a chemotactic substance were made using capillary tubes attached to liquid suspensions of either short motile rods or C. septicum swarm cells. The results demonstrated that mucin was strongly chemotactic to C. septicum swarm cells (Rche, 5.9 ± 1.4 [standard deviation]; n = 5), compared to short motile rod forms (Rche, 1.7 ± 0.6).
Induction and repression of mucin-degrading enzymes.
Batch culture experiments showed that the activities of a number of enzymes involved in mucin breakdown (N-acetyl-β-glucosaminidase, β-galactosidase, glycosulfatase, and neuraminidase) were increased during growth on mucin and partially or completely suppressed by a readily fermentable carbon source such as glucose. This was particularly evident with neuraminidase and glycosulfatase (Table 1).
TABLE 1.
Induction and repression of extracellular mucin-degrading enzymes in C. septicuma
| Carbon sourceb | Activity (U/mg [dry wt] of cells) of:
|
|||
|---|---|---|---|---|
| Neuraminidase | Glycosulfatase | β-Galactosidase | N-Acetyl β-glucosaminidase | |
| Glucose | NDc | ND | 0.03 ± 0.01 | 0.18 ± 0.02 |
| Mucin | 620 ± 22.1 | 5.1 ± 0.3 | 0.41 ± 0.02 | 0.48 ± 0.02 |
| Glucose and mucin | ND | 0.8 ± 0.3 | 0.38 ± 0.01 | 0.25 ± 0.02 |
Results are means and standard deviations of data obtained in four separate experiments. Samples were taken for analysis during the mid-exponential phase of growth in batch culture.
Cultures contained either 7.5 g of glucose or mucin per liter or 7.5 g each of glucose and mucin per liter.
ND, not detected.
Desulfation of carbohydrates.
Cultures of C. septicum grown on low concentrations of mucin secreted glycosulfatase. The specificities associated with this enzyme activity (in micromoles of SO42− released per hour per milligram [dry weight] of cells ± standard deviation; n = 4) against different sugar sulfates were as follows: mucin, 0.201 ± 0.026; glucose-6-sulfate, 0.125 ± 0.002; N-acetylglucosamine-6-sulfate, 0.120 ± 0.009; galactose-6-sulfate, 0.055 ± 0.004; and N-acetylglucosamine-3-sulfate, 0.013 ± 0.001. Thus, free sulfate was released most rapidly from mucin, while the location of sulfate on the C-3 position of the sugar molecule markedly reduced the rate of hydrolysis.
Expression of virulence determinants in glucose-excess and glucose-limited continuous cultures.
Changes in the dilution rate did not markedly affect bacterial viability in these chemostats (Table 2). However, cell counts did increase with specific growth rate under carbon limitation, while the reverse occurred in glucose-excess fermentors. Cell morphology was strongly affected by dilution rate, irrespective of glucose availability. At low dilution rates, essentially all of the bacteria observed in the chemostats were found to be swarm cells, whereas at D = 0.36 and 0.65 h−1, C. septicum reverted to short rods (results not shown). Table 2 also shows the effects of nutrient limitation and dilution rate on the synthesis of toxins and mucinolytic enzymes. Hemolysin was detected only in cultures containing short motile rods, especially during glucose-excess growth. Conversely, DNase, hyaluronidase, and neuraminidase were formed only by swarm cells at low specific growth rates. Furthermore, DNase secretion occurred principally under glucose limitation, while cell-associated and extracellular neuraminidase and hyaluronidase were formed mainly in glucose-excess culture vessels.
TABLE 2.
Effect of glucose availability on the production of toxins and spreading factors in continuous cultures of C. septicum
| Db (h−1) | Tdc (h) | Culture conditions | Cell count (ml−1) | Activity (U/mg [dry wt] of cells) ofa:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Hemolysin | DNase | Hyaluronidase
|
Neuraminidase
|
||||||
| Cell-bound | Extracellular | Cell-bound | Extracellular | ||||||
| 0.02 | 34.7 | Glucose excess | (4.0 ± 1.2) × 108 | NDd | 4.5 ± 2.7 | 14.2 ± 3.8 | 55.8 ± 3.4 | 360 ± 20.3 | 270 ± 24.3 |
| Glucose limited | (2.3 ± 1.2) × 108 | ND | 25.2 ± 3.6 | ND | 14.5 ± 2.1 | 89.6 ± 6.6 | 55.8 ± 4.8 | ||
| 0.10 | 6.9 | Glucose excess | (3.6 ± 0.5) × 108 | ND | 1.1 ± 0.4 | 2.1 ± 1.1 | 22.7 ± 2.9 | 200 ± 18.2 | 150 ± 12.1 |
| Glucose limited | (5.9 ± 0.8) × 108 | ND | 8.3 ± 1.9 | ND | ND | ND | ND | ||
| 0.36 | 1.9 | Glucose excess | (9.2 ± 1.1) × 107 | 34.5 ± 5.6 | ND | ND | ND | ND | ND |
| Glucose limited | (6.7 ± 2.1) × 108 | 15.2 ± 3.4 | ND | ND | ND | ND | ND | ||
| 0.65 | 1.1 | Glucose excess | (8.8 ± 1.4) × 107 | 108 ± 12.7 | ND | ND | ND | ND | ND |
| Glucose limited | (6.8 ± 0.7) × 108 | 82.6 ± 8.5 | ND | ND | ND | ND | ND | ||
Results are means ± standard deviations of data obtained in three separate experiments.
Dilution rate.
Cell doubling time, calculated as 0.693/D.
ND, not detected.
Synthesis of toxins and mucinolytic enzymes in mucin-limited chemostats.
The effect of growth rate on the formation of toxins and spreading factors was investigated in mucin-limited continuous cultures. Table 3 shows that as before, hemolysin formation was strongly growth rate associated whereas glycosulfatase was secreted only at low dilution rates. However, very high levels of cell-bound hyaluronidase and neuraminidase, as well as other mucinolytic enzymes (β-galactosidase and N-acetyl-β-glucosaminidase), were detected at all growth rates. Low levels of DNase were detected in the mucin chemostats at low dilution rates (results not shown). Although viable-cell counts did not significantly change in the cultures, swarm cells again predominated at low growth rates, changing to short rods at D = 0.23 h−1 and above. Measurements of cytotoxicity to Vero cells demonstrated that cell-free culture supernatants were highly toxigenic under all growth conditions. This included nonhemolytic supernatants obtained at D = 0.04 h−1, which completely destroyed Vero cell monolayers in tissue culture after 12 h of incubation.
TABLE 3.
Formation of toxins and mucinolytic enzymes in continuous cultures of C. septicum grown in mucin-limited chemostatsa
| Db (h−1) | Cell count (ml−1) | Cytotoxicity titer | Activity (U/mg [dry wt] of cells) of:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemolysin | Glycosulfatase | Neuraminidase
|
N-Acetyl β-glucosaminidase
|
β-Galactosidase
|
Hyaluronidase
|
|||||||
| Cell bound | Extracellular | Cell bound | Extracellular | Cell bound | Extracellular | Cell bound | Extracellular | |||||
| 0.04 | (6.6 ± 0.8) × 108 | 16 | NDc | 5.2 ± 0.1 | 1118 ± 56 | 73 ± 8.1 | 3.3 ± 0.13 | 0.6 ± 0.20 | 2.6 ± 0.20 | 0.2 ± 0.02 | 58.3 ± 6.0 | 198 ± 12.3 |
| 0.16 | (6.7 ± 0.2) × 108 | 32 | 2.5 ± 0.4 | 5.0 ± 0.3 | 921 ± 43 | 13 ± 1.9 | 4.0 ± 0.10 | 2.1 ± 0.11 | 3.7 ± 0.20 | 0.3 ± 0.03 | 163 ± 13.2 | 143 ± 11.7 |
| 0.23 | (6.8 ± 0.3) × 108 | 16 | 16.6 ± 0.2 | 0.4 ± 0.1 | 1462 ± 71 | ND | 5.0 ± 0.24 | 0.5 ± 0.02 | 1.5 ± 0.06 | 0.2 ± 0.02 | 99.5 ± 7.0 | 127 ± 15.1 |
| 0.36 | (7.1 ± 0.1) × 108 | 16 | 109 ± 6.0 | ND | 956 ± 35 | ND | 4.4 ± 0.16 | 0.5 ± 0.03 | 0.8 ± 0.02 | 0.6 ± 0.04 | ND | 121 ± 11.0 |
| 0.63 | (8.2 ± 0.4) × 108 | 16 | 256 ± 10.6 | ND | 1186 ± 92 | ND | 5.0 ± 0.24 | 0.9 ± 0.05 | 0.9 ± 0.06 | 0.5 ± 0.01 | ND | ND |
Results are means ± standard deviations of data obtained in three separate experiments.
Dilution rate.
ND, not detected.
Utilization of mucin oligosaccharides by C. septicum.
Measurements of mucin oligosaccharide carbohydrate in the chemostat feed medium and residual glycoprotein in spent culture fluid showed that quantitatively, N-acetylglucosamine served as the principal carbon source for C. septicum, with utilization ranging from 53 to 64%. Mannose was also a preferred carbon source (55 to 84% utilization) while N-acetylgalactosamine (20 to 34%) and galactose (24 to 42%), together with small amounts of glucose in the preparation, were also fermented. Fucose and neuraminic acid were not assimilated to a significant degree under any growth conditions.
DISCUSSION
Bacterial swarm cells are capable of swift concordant movement over surfaces, but individual organisms are incapable of swarming by themselves. This form of multicellular behavior proceeds only when groups of bacteria aggregate and act in concert (9, 25). In Proteus, swarming is a cyclical phenomenon during growth on agar plates, because differentiated cells periodically revert to short rods and concentric swarm zones form (3); however, this type of consolidation process does not take place in C. septicum. For most bacteria, little information is available concerning the environmental factors and intercellular signaling processes involved in controlling swarming, but studies with P. mirabilis show that glutamine both induces cellular differentiation and is a chemoattractant for swarm cells (5) while studies with Vibrio parahaemolyticus show that swarming is induced by iron limitation (35).
The chemostat experiments undertaken in this investigation showed that cellular differentiation was induced at low dilution rates, irrespective of whether glucose or mucin served as the carbon source. This was not an effect of growth rate per se, because swarming bacteria on agar plates grow very rapidly. Instead, the results suggest that swarming in C. septicum is associated, to some degree, with nutrient (energy) depletion, which has also been linked to swarming in Proteus (12).
Swarm cells often differ biochemically and metabolically from short motile forms (8), and studies described in this paper demonstrated that major changes occur in virulence characteristics during swarming in C. septicum. Hemolysin formation was principally an attribute of short rods, either on blood agar plates or during growth in chemostats (Tables 2 and 3). In contrast, the production of DNase and hyaluronidase, as well as that of putative spreading factors such as neuraminidase, was associated primarily with swarm cells in the absence of mucin (Tables 2 and 3). This mucin glycoprotein can therefore modulate the virulence characteristics of C. septicum.
Marked differences have been reported in the expression of several virulence attributes in P. mirabilis swarm cells, including increased urease, hemolysin, and flagellin synthesis, while swarm cells were found to be more invasive to epithelial cells than the short rod forms (4). However, the reverse is true in C. septicum, where short motile rods are more hemolytic and are more cytotoxic and invasive to Caco-2 and HEp-2 cells than are swarming bacteria (51).
Not all bacterial pathogens are motile, but motility frequently favors survival and enhances colonization in the host (17). A number of motile intestinal bacteria exhibit chemotaxis (5, 30) or possibly viscotaxis in response to mucin. Motility also promotes virulence in many organisms, including Pseudomonas (26) and Salmonella (44), and is often controlled by chemotaxis. The present study shows that this occurs in C. septicum, where mucin is a chemoattractant, as well as in Campylobacter jejuni, which is chemotactic toward serine and fucose residues in mucus (27). Nonmotile C. jejuni mutants are unable to invade the gut because they cannot pass through the mucus layer (37), while nonchemotactic but motile C. jejuni mutants are similarly deficient in invasive qualities (48). Virulence is also related to chemotaxis in Brachyspira (Serpulina) hyodysenteriae, where pathogenic strains are considerably more chemotactic toward mucin than are nonvirulent isolates (36).
Mucus plays a protective role against enteric pathogens such as Yersinia enterocolitica by reducing binding of the organisms to brush border membranes (34), while sulfomucins prevent colonization of the gastric mucosa by Helicobacter pylori (40). Conversely, the abilities of some gram-negative pathogens to colonize the mouse gut are specifically related to their abilities to adhere to mucus (13). For example, campylobacters do not degrade mucus, but they bind to the glycoprotein before gaining access to cell membrane receptors (47).
N-Acetylglucosamine, N-acetylgalactosamine, galactose, fucose, and neuraminic acid are the principal constituents of mucin oligosaccharide side chains; however, small amounts of glucose and mannose are also present in mucin preparations (2). Although α-fucosidase and N-acetyl-α-glucosaminidase production was never detected in C. septicum (results not shown), the bacterium synthesized several mucinolytic enzymes that, in principle, would allow extensive digestion of this chemically complex, highly sulfated glycoprotein. Their formation was catabolite regulated, being induced by mucin and repressed by glucose (Table 1). The glucose effect was reduced at low dilution rates in the chemostats (Table 2), where the carbon source was strongly limiting, whereas the cell growth rate had little effect on glycoprotein-degrading enzymes in the mucin-limited chemostats, due to the continuous presence of the inducer (Table 3). These results show that carbon availability will affect the expression of virulence determinants in C. septicum invading the large bowel while the occurrence of high levels of cell-associated enzymes, especially in the presence of mucin glycoprotein (Table 3), may confer a significant competitive advantage in the mucus layer lining the bowel.
Although the major cytotoxin produced by C. septicum is believed to be alpha-toxin, which is hemolytic, necrotizing, and lethal (45), it has also been observed that neuraminidase is important in cell damage and spread of the bacterium through body tissues when cytolytic activities are low or undetectable (22). This was also shown in the present study, where nonhemolytic cell-free culture supernatants were found to be highly toxic to Vero cells. C. septicum neuraminidase seems to be particularly active against glycoproteins (45, 53). Unlike in other bacteria such as C. perfringens (39) or Bacteroides fragilis (24), the release of neuraminic acid residues by C. septicum neuraminidase does not serve a direct nutritional function. However, it is likely to provide access to the mucin molecule for other digestive enzymes. Moreover, through affecting its gel-forming properties, neuraminidase may enhance invasiveness in a similar way to that in Vibrio cholerae (23). Invasive clostridia, such as C. septicum and C. perfringens, form large amounts of neuraminidase, whereas the noninvasive C. tetani and C. botulinum do not (21). Secretion of neuraminidase by C. septicum was demonstrated by Gadalla and Collee (22) in animal experiments, as well as in studies of gas gangrene in humans (21). In contrast to neuraminic acid and fucose, C. septicum was able to utilize other mucin oligosaccharide constituents, particularly N-acetylglucosamine, showing that the high levels of N-acetyl-β-glucosaminidase and other mucin-degrading glycosidases formed were nutritionally important. Catabolite regulation undoubtedly plays a role in virulence in the bowel, and substrate induction of mucinolytic enzymes is likely to facilitate the movement of C. septicum through the viscous mucus layer and underlying gut mucosa.
Relatively few studies have been performed on bacterial glycosulfatases, their substrates, or their modes of activity. It was therefore of interest to find that C. septicum cell-free culture supernatants desulfated partially purified porcine gastric mucin, together with a number of sulfated monosaccharides. Sulfate residues on the mucin molecule are believed to have a protective effect, in that through steric hindrance they assist in preventing its destruction by bacterial glycosidases (43). Galactose and N-acetylglucosamine are the principal sulfated moities in mucin, with the hydrophilic sulfate group usually occurring in the 6′ position (15). Extracellular sulfatase activity in C. septicum was active against glucose-6-sulfate, N-acetylglucosamine-6-sulfate, and galactose-6-sulfate but not N-acetylglucosamine-3-sulfate, showing a high degree of steriospecificity in function.
In conclusion, pathogenicity in C. septicum is multifactorial, and physiologic studies in the chemostat show that the organism secretes several toxins and spreading factors under a variety of growth conditions, while evidence has been obtained for the differential expression of virulence determinants in swarming bacteria. Further studies are now needed to elucidate the environmental and molecular mechanisms that regulate these processes.
REFERENCES
- 1.Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973;74:77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- 2.Allen A. Structure of gastrointestinal mucus glycoproteins and the gel-forming properties of mucus. Br Med Bull. 1978;34:28–33. [PubMed] [Google Scholar]
- 3.Allison A, Hughes C. Bacterial swarming: an example of prokaryotic differentiation and multicellular behaviour. Sci Prog Edin. 1991;75:403–422. [PubMed] [Google Scholar]
- 4.Allison C, Coleman N, Jones P L, Hughes C. Ability of P. mirabilis to invade human urothelial cells is coupled to motility and swarming differentiation. Infect Immun. 1992;60:4740–4746. doi: 10.1128/iai.60.11.4740-4746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allison C, Lai H-C, Gygi D, Hughes C. Cell differentiation of Proteus mirabilis is initiated by glutamine, a specific chemoattractant for swarming cells. Mol Microbiol. 1993;8:53–60. doi: 10.1111/j.1365-2958.1993.tb01202.x. [DOI] [PubMed] [Google Scholar]
- 6.Allison C, Lai H-C, Hughes C. Co-ordinate expression of virulence genes during swarm cell differentiation and population migration of Proteus mirabilis. Mol Microbiol. 1992;6:1583–1591. doi: 10.1111/j.1365-2958.1992.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous. Clostridium septicum and neutropenic enterocolitis. Lancet. 1987;ii:608. [PubMed] [Google Scholar]
- 8.Armitage J P. Changes in metabolic activity of Proteus mirabilis during swarming. J Gen Microbiol. 1981;125:445–450. doi: 10.1099/00221287-125-2-445. [DOI] [PubMed] [Google Scholar]
- 9.Belas R. The swarming phenomenon of Proteus mirabilis. ASM News. 1992;58:15–22. [Google Scholar]
- 10.Bergmeyer H U, Gawelin K, Grassl M. Deoxyribonuclease I. In: Bergmeyer H U, editor. Methods of enzymatic analysis. Vol. 1. London, United Kingdom: Academic Press, Ltd.; 1975. p. 447. [Google Scholar]
- 11.Bratton S L, Krane E J, Park J R, Burchette S. Clostridium septicum infections in children. Pediatr Infect Dis J. 1992;11:569–575. doi: 10.1097/00006454-199207000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Brogan T D, Nettleton J, Reid C. The swarming of Proteus on semisynthetic media. J Med Microbiol. 1971;4:2–11. doi: 10.1099/00222615-4-1-1. [DOI] [PubMed] [Google Scholar]
- 13.Cohen P S, Wadolkowski E A, Laux D C. Adhesion of a human fecal Escherichia coli strain to a 50.5 KDal glycoprotein receptor present in mouse colonic mucus. Microecol Ther. 1986;16:231–241. [Google Scholar]
- 14.Degnan B A, Macfarlane G T. Arabinogalactan utilization in continuous cultures of Bifidobacterium longum. Effect of co-culture with Bacteroides thetaiotaomicron. Anaerobe. 1995;1:103–112. doi: 10.1006/anae.1995.1005. [DOI] [PubMed] [Google Scholar]
- 15.Dodgson K S, White G F, Fitzgerald J W. Sulfatases of microbial origin. Boca Raton, Fla: CRC Press, Inc.; 1982. [Google Scholar]
- 16.Drasar B S, Goddard P, Heaton S, Peach S, West B. Clostridia isolated from faeces. J Med Microbiol. 1976;9:63–71. doi: 10.1099/00222615-9-1-63. [DOI] [PubMed] [Google Scholar]
- 17.Finlay B B, Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989;53:210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerald T J, Repesh L A. The hyaluronidase associated with Treponema pallidum facilitates treponemal dissemination. Infect Immun. 1987;55:1023–1028. doi: 10.1128/iai.55.5.1023-1028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Florin T H J, Neale G, Gibson G R, Christl S U, Cummings J H. Metabolism of dietary sulphate-absorption and excretion in humans. Gut. 1991;32:766–773. doi: 10.1136/gut.32.7.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fowler N G, Hussaini F N. Clostridium septicum infection and antibiotic treatment in broiler chickens. Vet Rec. 1975;96:14–15. doi: 10.1136/vr.96.1.14. [DOI] [PubMed] [Google Scholar]
- 21.Frazer A G. Neuraminidase production by clostridia. J Med Microbiol. 1978;11:269–280. doi: 10.1099/00222615-11-3-269. [DOI] [PubMed] [Google Scholar]
- 22.Gadalla M S A, Collee J G. The relationship of the neuraminidase of Clostridium septicum to the haemagglutinin and other soluble products of the organism. J Pathol Bacteriol. 1968;96:169–185. doi: 10.1002/path.1700960118. [DOI] [PubMed] [Google Scholar]
- 23.Galen J E, Ketley J M, Fasano A, Richardson S H, Wassermann S S, Kaper J B. Role of Vibrio cholerae neuraminidase in the function of cholera toxin. Infect Immun. 1992;60:406–415. doi: 10.1128/iai.60.2.406-415.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godoy V G, Dallas M M, Russo T A, Malamy M H. A role for Bacteroides fragilis neuraminidase in bacterial growth in two model systems. Infect Immun. 1993;61:4415–4426. doi: 10.1128/iai.61.10.4415-4426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harshey R M, Matsuyama T. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc Natl Acad Sci USA. 1994;91:8631–8635. doi: 10.1073/pnas.91.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holder I A, Wheeler R, Montie T C. Flagellar preparations from Pseudomonas aeruginosa: animal protection studies. Infect Immun. 1982;35:276–280. doi: 10.1128/iai.35.1.276-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hugdahl M B, Doyle M P. Chemotactic behaviour of Campylobacter jejuni. In: Pearson A D, Skirrow M B, Lior H, Rowe B, editors. Campylobacter III. London, United Kingdom: Public Health Laboratory Service; 1985. p. 143. [Google Scholar]
- 28.King A T, Rampling A, Wight D G D, Warren R E. Neutropenic enterocolitis due to Clostridium septicum infection. J Clin Pathol. 1984;37:335–343. doi: 10.1136/jcp.37.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kornbluth A A, Danzig J B, Bernstein L H. Clostridium septicum infection and associated malignancy: report of two cases and review of the literature. Medicine. 1989;68:30–37. doi: 10.1097/00005792-198901000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Lee S G, Changsung K, Young C H. Successful cultivation of a potentially pathogenic coccoid organism with trophism for gastric mucin. Infect Immun. 1997;65:49–54. doi: 10.1128/iai.65.1.49-54.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linker A. Hyaluronidase. In: Bergmeyer H U, editor. Methods of enzymatic analysis. I. London, United Kingdom: Academic Press, Ltd.; 1974. pp. 944–948. [Google Scholar]
- 32.Macfarlane G T, Hay S, Macfarlane S, Gibson G R. Effect of different carbohydrates on growth, polysaccharidase and glycosidase production by Bacteroides ovatus, in batch and continuous cultures. J Appl Bacteriol. 1990;68:179–1879. doi: 10.1111/j.1365-2672.1990.tb02564.x. [DOI] [PubMed] [Google Scholar]
- 33.MacLennan J D. The histotoxic clostridial infections of man. Bacteriol Rev. 1962;26:177–276. [PMC free article] [PubMed] [Google Scholar]
- 34.Mantle M, Basaraba L, Peacock S C, Gall D G. Binding of Yersinia enterocolitica to rabbit brush border membranes, mucus, and mucin. Infect Immun. 1989;57:3292–3299. doi: 10.1128/iai.57.11.3292-3299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarter L, Silverman M. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol Microbiol. 1990;4:1057–1062. doi: 10.1111/j.1365-2958.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 36.Milner J A, Sellwood R. Chemotactic response to mucin by Serpulina hyodysenteriae and other porcine spirochetes: potential role in intestinal colonization. Infect Immun. 1994;62:4095–4099. doi: 10.1128/iai.62.9.4095-4099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morooka T, Umeda A, Amako K. Motility as an intestinal colonisation factor for Campylobacter jejuni. J Gen Microbiol. 1985;131:1973–1980. doi: 10.1099/00221287-131-8-1973. [DOI] [PubMed] [Google Scholar]
- 38.Moussa R S. Complexity of toxins from Clostridium septicum and Clostridium chauvoei. J Pathol Bacteriol. 1958;76:538–545. doi: 10.1128/jb.76.5.538-545.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nees S, Schauer R. Induction of neuraminidase from Clostridium perfringens and the co-operation of this enzyme with acylneuraminate pyruvate lyase. Behring Inst Mitt. 1974;55:68–78. [Google Scholar]
- 40.Piotrowski J, Slomiany A, Murty V L N, Fekete Z, Slomiany B L. Inhibition of Helicobacter pylori colonization of sulfated gastric mucin. Biochem Int. 1991;24:749–756. [PubMed] [Google Scholar]
- 41.Prolla J C, Kirsner J B. The gastrointestinal lesion and complication of the leukemias. Ann Intern Med. 1964;61:1084–1103. doi: 10.7326/0003-4819-61-6-1084. [DOI] [PubMed] [Google Scholar]
- 42.Quigley M E, Englyst H N. Determination of neutral sugars and hexosamines by high performance liquid chromatography with pulsed amperometric detection. Analyst. 1992;117:1715–1718. doi: 10.1039/an9941901511. [DOI] [PubMed] [Google Scholar]
- 43.Quigley M E, Kelly S M. Structure, function and metabolism of host mucus glycoproteins. In: Gibson G R, Macfarlane G T, editors. Human colonic bacteria: role in nutrition, physiology and pathology. Boca Raton, Fla: CRC Press, Inc.; 1995. pp. 175–199. [Google Scholar]
- 44.Siitonen A, Nurminen M. Bacterial motility is a colonization factor in experimental urinary tract infection. Infect Immun. 1992;60:3918–3920. doi: 10.1128/iai.60.9.3918-3920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith L O S, Williams B L. Clostridium septicum. In: Balows A, editor. The pathogenic anaerobic bacteria. 3rd ed. Springfield, Ill: Charles C. Thomas; 1984. pp. 180–190. [Google Scholar]
- 46.Swiatek P J, Allen S D, Siders J A, Lee C H. DNase production by Clostridium septicum. J Clin Microbiol. 1987;25:437–438. doi: 10.1128/jcm.25.2.437-438.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sylvester F A, Philpott D, Gold B, Lastovica A, Forstner J F. Adherence to lipids and intestinal mucin by a recently recognized human pathogen, Campylobacter upsaliensis. Infect Immun. 1996;64:4060–4066. doi: 10.1128/iai.64.10.4060-4066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takata T, Fujimoto S, Amako K. Isolation of nonchemotactic mutants of Campylobacter jejuni and their colonization of the mouse intestinal tract. Infect Immun. 1992;60:3596–3600. doi: 10.1128/iai.60.9.3596-3600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959;234:1971–1975. [PubMed] [Google Scholar]
- 50.Willis A T. Clostridia of wound infections. London, United Kingdom: Butterworths; 1969. [Google Scholar]
- 51.Wilson L M, Macfarlane G T. Cytotoxicity, adhesion and invasion of Clostridium septicum in cultured human epithelial cells (CACO-2, Hep-2): pathological significance of swarm cell differentiation. Anaerobe. 1996;2:71–79. [Google Scholar]
- 52.Yeong M L, Nicholson G I. Clostridium septicum infection in neutropenic enterocolitis. Pathology. 1988;20:194–197. doi: 10.3109/00313028809066634. [DOI] [PubMed] [Google Scholar]
- 53.Zenz K I, Roggentin P, Schauer R. Isolation and properties of the natural and the recombinant sialidase from Clostridium septicum NC 0054714. Glycoconj J. 1993;10:50–56. doi: 10.1007/BF00731187. [DOI] [PubMed] [Google Scholar]