Abstract
Lung dendritic cells were identified by immunohistochemistry in lung tissue sections from C57BL/6 mice. Following isolation from the lungs using CD11c magnetic beads, the flow cytometric analysis of I-Ab+ and CD11c+ cells indicated a mixed population of dendritic cells at different stages of maturation, with most expressing an immature phenotype. When cultured for 7 days with recombinant murine granulocyte-macrophage colony-stimulating factor, 99% of cells were CD11c+ and had a morphology typical of immature dendritic cells. These cells were negative for CD34, CD14, and CD8α antigens but expressed low levels of the myeloid marker F4/80 and moderate levels of MAC3. All expressed high levels of CD11a (LFA-1), CD11b (Mac1), and CD54 antigens, with low levels of class II major histocompatibility complex. Most cells expressed CD80 but only a small percentage of cells were positive for CD40 and CD86. Both overnight and 7-day cultures of lung dendritic cells were able to phagocytose Mycobacterium tuberculosis, and this was associated with the production of interleukin-12 and stimulation of both naïve and immune T cells to produce gamma interferon.
Disease caused by Mycobacterium tuberculosis continues to be the major cause of mortality from infectious disease worldwide (2). While development of new chemotherapy as well as effective administration of current therapies may provide some reduction in the incidence of tuberculosis in the near term, in the longer term an effective vaccine against the disease would be preferred. However, while current innovation in new vaccine development is encouraging (16, 18, 19), the field is still limited by a lack of precise knowledge of the host response, especially that regarding early events soon after exposure to the bacillus.
It is generally believed that the first cell to encounter M. tuberculosis in the lungs is the alveolar macrophage. If the macrophage is unable to kill the bacillus, it is thought that M. tuberculosis then somehow erodes into the interstitium, where it encounters other macrophages (how this happens is more a case of speculation [17] than of hard evidence) and thus establishes a site of infection. An influx of macrophages, probably of both local and blood-borne origin, then ensues, thickening the septa and giving rise to a local interstitial pneumonitis.
Acquired immunity is expressed relatively slowly in the lungs (5, 7). The reason for this is unclear, as is the site where the protective T cells become sensitized. The lymphoid tissues surrounding the bronchi are potential sites, but for these to be the sites, antigen has to be physically carried to these tissues due to the lack of lymphatic drainage to the mouse alveolus.
One type of myeloid cell capable of such an action is the dendritic cell. In the current study it is shown that CD11c-positive dendritic cells are well distributed throughout the alveolar region, with most exhibiting an immature phenotype when isolated and analyzed by flow cytometry. The study demonstrates that these cells are capable of phagocytosing live M. tuberculosis bacteria, leading to the secretion of interleukin-12 (IL-12) and stimulation of CD4 T cells to produce gamma interferon (IFN-γ). Given the knowledge (1, 25, 28) that dendritic cells are motile and capable of homing from the peripheral tissues to lymphoid organs, the data support the hypothesis that dendritic cells that engulf M. tuberculosis play an important role in the transition from the initial innate response in the lungs to a state of acquired specific immunity.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free C57BL/6 female mice (Jackson Laboratories, Bar Harbor, Maine) of 6 to 8 weeks of age were used.
Bacterium.
M. tuberculosis strain H37Rv was grown from low-passage seed lots in Proskauer-Beck liquid medium containing 0.02% Tween 80 to mid-log phase then aliquoted and frozen at −70°C until use. The viability of the frozen stock was 8.3 × 108 CFU/ml.
Culture media.
Dendritic cells were cultured in RPMI medium (cRPMI) consisting of RPMI 1640 medium (Sigma-Aldrich, Ltd., St. Louis, Mo.) supplemented with 1% glutamine, 0.1 mM nonessential amino acids (Life Technology, Grand Island, N.Y.), 50 μM 2-mercaptoethanol (Sigma-Aldrich), 1% penicillin-streptomycin (Sigma-Aldrich), 10% fetal bovine serum (FBS), and 20 ng of recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) (Pepro Tech, Rocky Hill, N.J.) per ml. In some cultures tumor necrosis factor alpha (TNF-α) (Pepro Tech) was added during the last 48 h of culture at a concentration of 10 ng/ml.
RPMI medium lacking biotin and phenol red (dRPMI) (Irvine Scientific, Santa Ana, Calif.) was supplemented with 1% l-glutamine, 1% HEPES, 0.1% N3Na, and 2% FBS and was used to wash and stain cells during flow cytometric studies.
Phosphate-buffered saline (PBS) without calcium chloride and magnesium chloride (Life Technology) supplemented with 0.5% FBS was used to isolate and purify lung dendritic cells.
Identification of dendritic cells in lung sections from C57BL/6 mice.
Lungs from C57BL/6 mice were inflated with 30% OCT (Tissue-Tek, Inc., Torrance, Calif.) in PBS through the trachea. After the lungs were removed from the pulmonary cavity, they were embedded in OCT and frozen in a bath of liquid nitrogen for a few seconds. Sections, 7 μm thick, were cut on a cryostat (Leica, product no. CM 1850), fixed in cold acetone for 10 min, and air dried. These sections were incubated overnight at 4°C with monoclonal antibody (MAb) CD11c (N418) (Serotec, Oxford, United Kingdom), which specifically recognizes dendritic cells.
Bound antibody was detected using biotinylated goat anti-hamster immunoglobulin G (IgG) (BD PharMingen, San Diego, Calif.). Finally, the reaction was developed using alkaline phosphatase linked to avidin and new fuchsin (Biogenex, San Ramon, Calif.) as substrate. Sections were counterstained with Meyer's hematoxylin.
MAbs.
MAbs specific for CD11c (N418) (Serotec) and CD11c (clone HL3, hamster IgG), CD11a (LFA-1, clone 2D7, rat IgG2a), CD11b (Mac-1, M1/70, rat IgG2a), MAC3 (clone M3/84, rat IgG1), I-Ab (clone 9AFG-120, mouse IgG2a), CD54 (ICAM-1, clone 3E2, hamster IgG), CD40 (clone 3/23, rat IgG2a), CD86 (B7-2, clone GL1, rat IgG2a), CD80 (B7-1, clone 16-10A1, hamster IgG), rat IgG2a, rat IgG2b, rat IgG1, and hamster IgG were purchased from BD PharMingen as direct conjugates to fluorescein isothiocyanate (FITC), phycoerythrin, or biotin. In addition, MAb F4/80 (clone CI:A3-1, rat IgG2a) as direct conjugate to either FITC or phycoerythrin was purchased from Serotec together with its isotype control. Goat F(ab)2 IgG anti-hamster IgG-FITC (Caltag, Burlingame, Calif.) and streptavidin conjugated to peridinin chlorophyll a protein (PerCP; Becton Dickinson, San Jose, Calif.) were used as secondary antibody where necessary.
Lung dendritic cell isolation.
Isolation of lung dendritic cells was performed by modification of protocols already described by others (22). Briefly, mice were euthanatized and the pulmonary cavities were opened. The blood circulatory system in the lungs was cleared by perfusion through the pulmonary artery with 3 ml of saline containing 50 U of heparin (Sigma-Aldrich) per ml. Lungs were aseptically removed and cut into small pieces in cold RPMI medium. The dissected tissue was then incubated in RPMI medium containing collagenase XI (0.7 mg/ml; Sigma-Aldrich) and type IV bovine pancreatic DNase (30 μg/ml; Sigma-Aldrich) for 30 to 45 min at 37°C. The action of the enzymes was stopped by adding 10 ml of cRPMI, and digested lungs were further disrupted by gently pushing the tissue through a nylon screen. The single-cell suspension was then washed and centrifuged at 200 × g. To lyse contaminating red blood cells, the cell pellet was incubated for 5 min at room temperature with 5 ml of Gey's solution (NH4Cl and KHCO3). Cells were then washed with PBS containing 0.5% FBS, counted, and incubated at the appropriate ratio with MACS CD11c microbeads (Miltenyi Biotec, Auburn, Calif.) for 15 min at 6 to 12°C. After being washed again with 15 ml of PBS, cells were diluted in 5 ml of PBS containing 0.5% FBS. Finally, CD11c+ cells were separated by passing the antibody-coated cell suspension over a VS+ column on a SuperMACS magnetic cell separator. Positive cells were collected by removing the column from the magnetic field and then flushing it with PBS–0.5% FBS. Macrophages were removed from the CD11c+ cell population by plastic adherence incubation overnight at 37°C in a 5% CO2 atmosphere. Thereafter, cells were washed with RPMI medium and used for further studies or cultured again for 7 days in cRPMI. Cultures were fed every 48 h.
Bone marrow-derived dendritic cells.
Bone marrow-derived dendritic cells were prepared using a procedure similar to one described previously (11). Briefly, mice were euthanatized by exposure to CO2 atmosphere and their femurs were dissected out. After trimming the bones at both ends, the marrow was flushed out with cold cRPMI supplemented with 10% FBS. Cells were washed once in cRPMI and incubated at a concentration of 2 × 105 cells/ml in cRPMI. The medium was changed at 48 h and replaced with cRPMI without antibiotics. After 7 days of incubation, dendritic cells were infected with M. tuberculosis as described below for lung dendritic cells.
Cell staining.
Lung dendritic cells were isolated as discussed above and cultured either overnight or for 7 days at 37°C and 5% CO2. Cell suspensions of 5 × 105 cells/ml were prepared and 100 μl was centrifuged onto glass slides. To confirm adherent dendritic cell morphology, cells were cultured using Thermovox coverslips (Nalge Nunc International, Naperville, Ill.). Either cells centrifuged onto glass or cells adhered to plastic were stained using the Hema 3 stain set (Biochemical Science, Swedesboro, N.J.).
In vitro infection of lung dendritic cells.
Purified lung dendritic cells from overnight or 7-day culture were cultured in 24-well plates at 2 × 105 cells per ml in cRPMI without antibiotics and infected with M. tuberculosis (H37Rv strain) at a ratio of 5 or 10 bacilli per cell. At different times postinfection cells were washed, centrifuged onto glass slides as indicated above, and stained for acid-fast bacilli using Ziehl-Neelsen stain (Becton Dickinson Microbiology Systems, Sparks, Md.) or stained for cell surface markers and analyzed on a flow cytometer.
Flow cytometric analysis.
Lung dendritic cells from either overnight or 7-day culture were washed in dRPMI. After blocking Fc receptors by using 1 μg of anti-mouse CD16/CD32 MAb (Caltag) per 106 cells for 10 min on ice, cells were stained for 30 min on ice with purified or directly conjugated antibodies. Where necessary, cells were washed twice in dRPMI and incubated with an immunoglobulin-specific secondary antibody. Cell acquisition was performed on a FACScalibur (Becton Dickinson, Mountain View, Calif.) and data were analyzed using CellQuest software (Becton Dickinson).
Antigen stimulation of dendritic cells.
Lung dendritic cells, either from overnight or 7-day culture, were incubated with 100 μl of cRPMI (without GM-CSF) per well in 96-well plates. Cell number per well varied depending on the study: 2 × 104 cells per well were used for stimulation of immune T cells and 5 × 104 cells per well were used for stimulation of naïve T cells. In naïve T-cell stimulation, cells were stimulated with 0, 1, 5, or 10 bacilli per cell or with 0, 10, 25, or 50 μg of culture filtrate protein (CFP) of M. tuberculosis (received from J. Belisle, Mycobacteria Research Laboratories, Department of Microbiology, Colorado State University, under NIH contract no. AI 75320) per ml. For immune T-cell stimulation, cells were stimulated with 0 or 5 bacilli per cell and 25 μg of CFP per ml. Cells and antigens or bacteria were incubated overnight at 37°C in 5% CO2 atmosphere prior to lymphocyte addition.
M. tuberculosis-immune T cells.
Twelve mice per experiment were used. Each received 2 × 105 M. tuberculosis bacilli (H37Rv strain) in saline by intravenous injection via a lateral tail vein.
Stimulation of IFN-γ production.
The stimulatory activity of the lung dendritic cells was assessed by coculturing these cells with either naïve or immune CD4 T cells. Briefly, splenocytes were prepared as previously described (7) and resuspended in PBS–0.5% FBS. Cells were incubated with CD4+ microbeads for 15 min at 6°C and then passed over a VS+ column on a SuperMACS magnetic cell separator. The column was removed from the separator and the cells were collected in PBS–0.5% FBS and counted. CD4+ T cells at a ratio of 10:1 were overlaid onto antigen-presenting cells that were previously stimulated with antigens (as indicated above). The mixed cell cultures were then incubated for 72 h at 37°C and 5% CO2. In addition, IL-2 (Pepro Tech) at 5 U per well was added to support CD4+ T-cell proliferation. In some experiments, cultures received 0.1 ng of murine recombinant IL-12 (Pepro Tech) per ml.
ELISA.
Supernatants were assayed for IFN-γ by a sandwich enzyme-linked immunosorbent assay (ELISA) using MAbs R4.6A2 and biotinylated XMG1.2 (BD PharMingen) as previously described (20). IL-12 “total” (p40 and p70) or IL-12 p70 were detected using the Endogen mouse IL-12 total or IL-12 p70 ELISA. Sensitivity of the assay for IL-12 total was 12 pg/ml and for IL-12 p70 was <5 pg/ml.
RESULTS
Identification of dendritic cells in lung tissue from C57BL/6 mice.
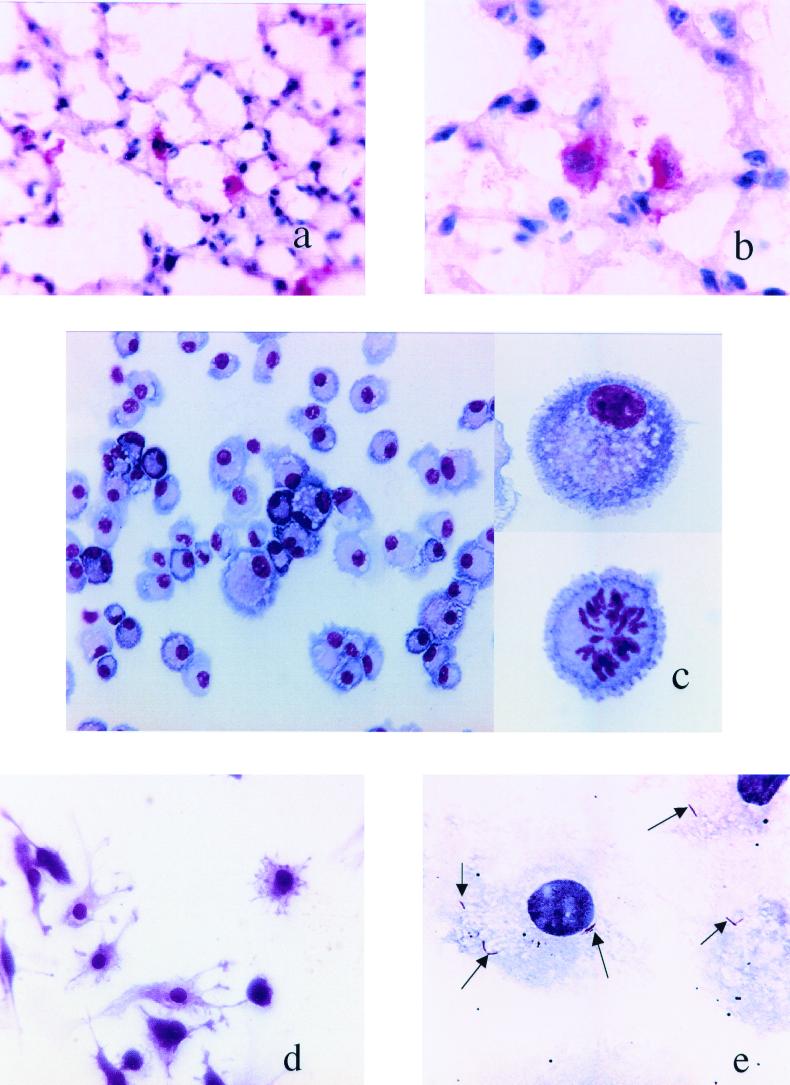
Lung tissue sections were stained with monoclonal anti-CD11c (Fig. 1a and b). Dendritic cells were present in the airway epithelium, close to the alveolar walls, interstitial spaces, and alveoli of lungs. At higher magnifications, lung dendritic cells showed a typical morphology, including a lobulated nucleus with abundant cytoplasm and small cytoplasmic projections.
FIG. 1.
(a and b) Identification of dendritic cells on frozen lung tissue sections from normal lung tissue of C57BL/6 mice. Shown are tissue sections stained with anti-CD11c+ (N418) at magnifications of ×400 (a) and ×1,000 (b). (c) Hema 3 staining of nonadherent CD11c+ lung cells after overnight culture and centrifugation onto glass slides. Cells in the left panel show the homogeneity of the culture, with most cells demonstrating the typical morphology of the immature dendritic cells. All the cells presented large nuclei, abundant cytoplasm, and small cytoplasmic projections (top right panel). In these cultures, there were also cells apparently in the proliferation stage (bottom right panel). The sizes of the cells varied (see Fig. 2, FSC and SSC in the flow cytometric analysis). (d) Adherent CD11c+ lung cells were stained using Hema 3 stain after 7 days of culture and adherence on Thermovox coverslips. These cells had elongated morphology with very long cytoplasmic processes and large central nuclei (magnification, ×200). (e) In vitro infection of lung dendritic cells. Lung dendritic cells were infected in vitro at a ratio of 10 bacilli per cell. After overnight infection and centrifugation onto glass slides, cells were stained for acid-fast bacilli (arrows) using Ziehl-Neelsen stain (magnification, ×400).
Figure 1c shows the morphology of lung dendritic cells after overnight culture. All the cells possessed large lateral nuclei, abundant cytoplasm, and small cytoplasmic projections and were of various sizes. The side panels show a higher magnification of dendritic cells with lateral nuclei and veiled morphology. Some were clearly in a state of mitosis.
When the cells were cultured in Thermovox coverslips, their dendritic nature could be more clearly seen, as demonstrated by long cytoplasmic processes and large central nuclei (Fig. 1d).
To determine the ability of the harvested cells to engulf M. tuberculosis, lung dendritic cells were infected in vitro with 5 to 10 bacilli per cell. After overnight infection, the cells were centrifuged onto glass slides and stained for acid-fast bacilli. Intracellular bacilli were clearly evident (Fig. 1e).
Cell surface marker expression by purified dendritic cells.
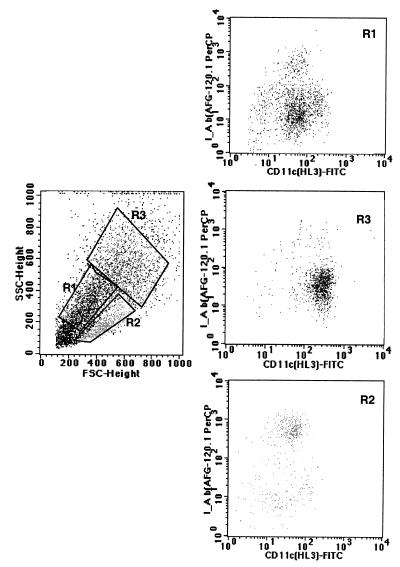
Purified lung dendritic cells were analyzed by flow cytometry. After purification with CD11c microbeads, 80 to 90% of cells were CD11c+ (Fig. 2). Based upon cell surface expression of CD11c and I-Ab antigens, purified lung dendritic cells after overnight culture could be classified into three populations, as shown in Fig. 2: (i) I-Ab low, CD11c+ low, FSClow, SSClow (R1, top right panel); (ii) I-Ab high, CD11+ low, FSCmid, SSClow (R2, bottom right panel); and (iii) I-Ab low, CD11c+ high, FSChigh, SSChigh (R3, central right panel).
FIG. 2.
Flow cytometric analysis of lung dendritic cells after overnight culture of nonadherent CD11c+ lung cells. Cells were washed and stained with CD11c+ -FITC and I-Ab–biotin plus avidin PerCP. The left panel shows the variable size of the CD11c+ cells. Cells could be gated into three populations as follows: region 1 (R1) with FSClow and SSClow corresponding to small cells; region 2 (R2) with FSCmid and SSClow corresponding to large cells with low granularity; and region 3 (R3) with FSChigh and SSChigh corresponding to larger and more granular cells. The panels on the right side represent the expression of CD11c+-FITC (x axis) and I-Ab–PerCP (y axis). The expression of these antigens on each of the different populations is shown in region 1 (upper dot plot was gated in R1), region 2 (bottom dot plot was gated in R2), and region 3 (middle dot plot was gated in R3). According to their cell surface expression of CD11c and I-Ab antigens, purified lung dendritic cells after overnight culture constitute a mixed population of cells and can be classified in three populations: (R1 population) I-Ab low CD11c+ low expression, FSClow and SSClow; (R2 population) I-Ab high and CD11+ low, FSCmid SSClow; and (R3 population) I-Ab low CD11c+ high, FSChigh and SSChigh.
Lung dendritic cells constituted less than 1% of the total number of cells in the lung. When cells were cultured in the presence of recombinant murine GM-CSF, they proliferated (Fig. 1c, bottom right photograph), all cells remained CD11c+, and the total numbers of cells after 7 days in vitro increased two- to threefold compared to the number after the initial purification.
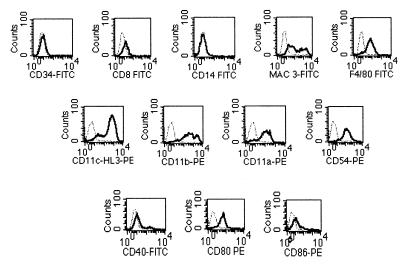
The flow cytometric analysis, described for the overnight culture, was also performed on the expanded 7-day lung dendritic cell culture. All cells were I-Ab low and CD11c+ low to high and were similar to the R1 population from the overnight culture in Fig. 2 (data not shown). These cells were negative for the hemopoietic marker CD34, the peripheral blood marker CD14, and the lymphoid-related CD8α but expressed low levels of the myeloid marker F4/80 and moderate to high levels of MAC3 antigens (Fig. 3). All cells expressed high levels of CD11a, CD11b, CD11c, and antigen CD54. In addition, most cells expressed CD80, but only a small percentage of cells were positive for CD40 and CD86.
FIG. 3.
Flow cytometric analysis of lung dendritic cells cultured for 7 days. When CD11c+ lung cells were cultured for 7 days in cRPMI containing recombinant murine GM-CSF, all cells remained CD11c+ and were similar to the R1 population in Fig. 2. Levels of cell surface expression for different markers on 7-day culture of lung dendritic cells were analyzed. Lung dendritic cells were incubated with MAbs against CD34, CD8, CD14, MAC3, F4/80, CD11c (N418), CD11c (HL3), CD11b CD11a CD54, CD40, CD80, and CD86 antigens. The histogram for each specific MAb was overlaid against its corresponding isotype control (dashed line). Data are shown for differentiation markers (top row), integrins and adhesion molecules (middle row), and costimulatory molecules (bottom row). PE, phycoerythrin.
Flow cytometric analysis of infected lung and bone marrow-derived dendritic cells.
It has been reported previously that priming of naïve T cells by dendritic cells requires the upregulation of accessory and costimulatory cell surface antigens. To investigate if this occurred following in vitro infection with M. tuberculosis, the cell surface expression of CD11a, CD11b, CD11c, CD54, CD40, CD80, CD86, and I-Ab antigens on uninfected versus expression on M. tuberculosis-infected lung dendritic cells were compared.
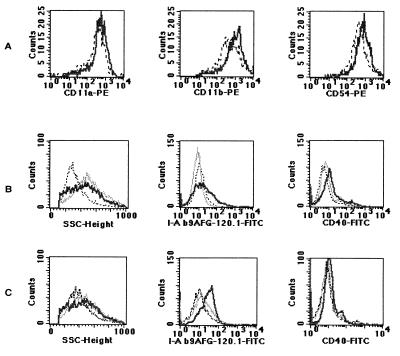
The results of this study are shown in Fig. 4. Upregulation of the CD54 molecule and CD11a and CD11b for lung dendritic cells was seen after overnight infection (Fig. 4A). In addition, lung dendritic cells infected for 48 h or cultured in the presence of TNF-α had an upward shift of the side scatter parameter (SSC) for both populations (Fig. 4B). These cells also upregulated CD40 and I-Ab antigens (Fig. 4B). CD80 and CD86 antigens appeared to be upregulated only very late in infection (96 h) (data not shown). Bone-marrow-derived dendritic cells infected for 48 h presented the same pattern of upregulation of antigens as lung dendritic cells, but I-Ab antigens were consistently upregulated to a higher level in the bone marrow-derived dendritic cells than in lung dendritic cells (Fig. 4C).
FIG. 4.
Analysis of either lung-derived or bone-marrow-derived dendritic cell responses to TNF-α and bacteria. Lung- or bone-marrow-derived dendritic cells were cultured for 7 days and then exposed to bacteria (thick line) or TNF-α (thin line) or were left untreated (dotted line). Treated cells were incubated either overnight (A) or for 48 h (B and C). Row A contains histograms demonstrating rapid upregulation of adhesion markers and integrins within 24 h of exposure to bacteria. Rows B and C show the different responses of lung-derived dendritic cells (B) and bone-marrow-derived dendritic cells (C) following exposure to either bacteria or TNF-α. Histograms correspond to 3,000 (A) or 10,000 (B and C) events per sample. Results are from one representative experiment of six experiments. PE, phycoerythrin.
IFN-γ production by stimulated T cells.
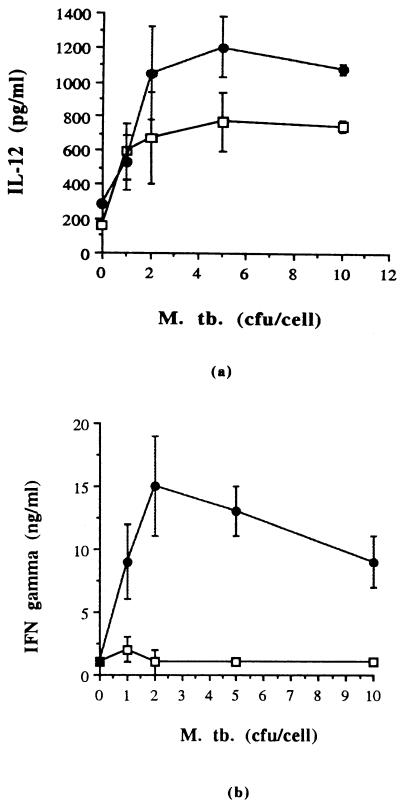
Lung dendritic cells were infected with M. tuberculosis at a ratio of 0, 1, 2, 5, or 10 CFU per cell and overlaid with purified naïve CD4+ T cells. After 72 h of culture, supernatants were assayed for the presence of IL-12 and IFN-γ. Figure 5b shows that lung dendritic cells were capable of stimulating naïve CD4+ T cells to produce IFN-γ in a dose-dependent manner. IL-12 total was also detected in these cultures in a dose-dependent manner (Fig. 5a), but IL-12 p70 was not detected by ELISA, indicating either that it was not present or that the amount present in these cultures was below the sensitivity levels of the assay.
FIG. 5.
Stimulation of naïve CD4 T cells by lung dendritic cells infected with M. tuberculosis (M. tb.). Lung dendritic cells were infected overnight with M. tuberculosis at a ratio of 0, 1, 2, 5, or 10 CFU per cell and were overlaid with purified naïve CD4+ T cells. After 72 h of culture, supernatants were assayed by ELISA for murine IFN-γ and IL-12 total and IL-12 p70 bioactive form. IL-12 p70 was not detected by ELISA ( , lung dendritic cells plus M. tuberculosis; and
, lung dendritic cells plus M. tuberculosis; and  , lung dendritic cells plus M. tuberculosis overlaid with naïve CD4+ T cells). Results are from one representative experiment of three experiments.
, lung dendritic cells plus M. tuberculosis overlaid with naïve CD4+ T cells). Results are from one representative experiment of three experiments.
It has been reported before (9) that exogenous addition of murine IL-12 to cultures increases priming of naïve T cells by dendritic cells. We wanted to analyze if this was also true for M. tuberculosis-infected lung dendritic cells and naïve CD4+ T cells, and we added IL-12 (0.1 ng/ml) to some cultures. Our results confirmed that exogenous addition of IL-12 increases by two- to threefold the production of IFN-γ by naïve CD4+ T cells when cultured with M. tuberculosis-infected lung dendritic cells (data not shown).
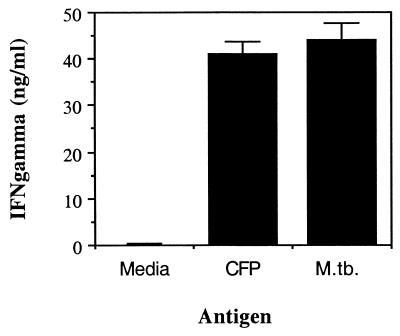
We then analyzed whether immune CD4+ T cells obtained by intravenous inoculation of mice with M. tuberculosis were also stimulated by in vitro M. tuberculosis-infected lung dendritic cells. Figure 6 shows that immune CD4+ T cells produced IFN-γ when cultured in the presence of M. tuberculosis-infected lung dendritic cells or when cultured with CFP-stimulated lung dendritic cells.
FIG. 6.
Stimulation of immune CD4+ T cells by lung dendritic cells infected with M. tuberculosis (M. tb.). Lung dendritic cells were infected overnight with 5 CFU/cell or with 25 μg of CFP per ml and then overlaid with M. tuberculosis-immune CD4+ T cells. After culture for 72 h, supernatants were assayed by ELISA for IFN-γ. Results are from one representative experiment of two experiments
DISCUSSION
The results of this study show that specialized macrophages exhibiting a dendritic morphology and expression of the CD11c cell surface marker (dendritic cells) are distributed widely throughout the mouse lung tissues. Examination of tissue sections stained with an antibody to CD11c indicated that these cells were present in airway epithelia as well as within the interstitial spaces in the alveolar regions, suggesting that these cells are judiciously placed for any interaction with invading pathogens. This distribution has been reported to be similar in both mice and rats (14, 15, 22).
Isolation of these cells from the lung tissues revealed cells that were mostly circular. Compared to the total numbers of cells that could be harvested from the lungs, their numbers were low. Following flow cytometric analysis it was found that the great majority of these dendritic cells had a marker expression that is generally regarded by most studies (21, 27) as an immature phenotype, i.e., CD11cmid to high and major histocompatibility (MHC) class IIlow. A small number of cells, however, expressed high levels of I-A (Fig. 2), which is indicative of a more mature phenotype that is usually associated with dendritic cells that have encountered an antigen and have undergone interactions with lymphocytes (6, 21).
Previous reports (10, 12) have also described lung dendritic cells with high expression of cell surface MHC class II antigens. The bronchus-associated lymphoid tissue contains discrete T- and B-cell areas as well as interdigitating cells (3) and it may be that lung dendritic cells expressing high levels of MHC class II antigens could have interacted with T cells in the bronchus-associated lymphoid tissue and fully matured inside the lungs.
Harvested dendritic cells could be expanded by in vitro culture with GM-CSF, but most cells retained an immature phenotype. Expression of F4/80 and MAC3 was observed, but that of the myeloid progenitor marker CD34 or the lymphoid marker CD14 was not observed. It has also been suggested elsewhere (23) that a subset of mouse dendritic cells can be identified by the expression of the α chain of CD8, but we failed to observe this in the lung dendritic cells (Fig. 3). As anticipated, all dendritic cells expressed CD80, but only a few were seen to express CD40 or CD86. It has been demonstrated elsewhere (27) that this phenotype tends to identify large immature cells. Lung dendritic cells infected with M. tuberculosis and cultured overnight exhibited little change in the expression of CD40, CD80, CD86, and I-A antigens. By 48 h postinfection, however, CD40 and I-A antigens were upregulated. Despite the relative lack of costimulatory molecules on the overnight culture, these cells were still able to induce IFN-γ secretion by naïve T cells. Presumably, when in an in vivo environment this efficiency is even higher; in this regard it has been shown elsewhere (4, 13) that CD40/40L ligation upregulates the expression of CD80 and CD86 on dendritic cells, increasing the production of IL-12 and leading to increased IFN-γ secretion. Our own findings regarding the addition of exogenous IL-12 and stimulation of naïve CD4 T cells are in keeping with this.
Upregulation of MHC class II antigens after infection was consistently higher in bone-marrow-derived dendritic cells than in lung dendritic cells. Since both cells were cultured under the same conditions, it indicates that there are differences between both types of dendritic cells and that the origin of these cells may be an important factor to consider.
As stressed above, however, before dendritic cells can sensitize T cells they first have to reach them. We hypothesize that these cells take up bacilli from the interstitium and find their way to local lymphoid tissues in the bronchial region, eventually leading to the emergence of antigen-specific T cells. The fact that this will take a finite period of time may thus well explain why expression of acquired immunity in the lungs is such a slow event (5, 7).
In keeping with our hypothesis, we have demonstrated here that infected dendritic cells quickly upregulate molecules that will allow them to pass through the extracellular matrix (integrins CD11a and CD11b) as well as cross-blood-vessel endothelial surfaces (CD54), actions that would be fully consistent with these cells acquiring a motile phenotype. This change in phenotype has been seen by others in cultures of blood-derived dendritic cells (8) but not in immortalized dendritic cell lines infected with M. tuberculosis (26). In contrast, in the current study infection of dendritic cells with M. tuberculosis readily increased CD54 expression.
The concept that dendritic cells could be specifically targeted by new tuberculosis vaccine candidates is attractive. However, it is also clear that interactions between dendritic cells and T cells after their carriage of antigen to lymphoid tissues is a complex issue, in that not only are dendritic cells still relatively poorly understood, but the migratory routes of T-cell subsets, which appear to differ depending upon their selective expression of chemokine receptors such as CCR7 (24), also appear to be a very complicated matter.
ACKNOWLEDGMENTS
We thank J. Brinks for technical assistance and A. M. Cooper for critically reviewing the manuscript.
This work was supported by NIH grants HL55967 and AI-44072.
REFERENCES
- 1.Austyn J M. New insights into the mobilization and phagocytic activity of dendritic cells. J Exp Med. 1996;183:1287. doi: 10.1084/jem.183.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom B R, Widdus R. Vaccine visions and their global impact. Nat Med. 1998;4(Suppl.):480–484. doi: 10.1038/nm0598supp-480. [DOI] [PubMed] [Google Scholar]
- 3.Breel M, de Ende M V, Sminia T, Kraal G. Subpopulations of lymphoid and non-lymphoid cells in bronchus-associated lymphoid (BALT) of the mouse. Immunology. 1988;63:657–662. [PMC free article] [PubMed] [Google Scholar]
- 4.Buka S, Thomas E K, Gong J, Barnes P F. Depressed CD40 ligand expression contributes to reduced gamma interferon production in human tuberculosis. Infect Immun. 2000;68:3002–3006. doi: 10.1128/iai.68.5.3002-3006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardona P J, Cooper A M, Luquin M, Ariza A, Filipo F, Orme I M, Ausina V. The intravenous model of murine tuberculosis is less pathogenic than the aerosol model owing to a more rapid induction of systemic immunity. Scand J Immunol. 1999;49:362–366. doi: 10.1046/j.1365-3083.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 6.Cella M, Salusto F, Lanzavechia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 7.Cooper A M, Callahan J E, Keen M, Belisle J T, Orme I M. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:67–73. doi: 10.1016/s0962-8479(97)90017-4. [DOI] [PubMed] [Google Scholar]
- 8.Henderson R A, Watkins S C, Flynn J L. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol. 1997;159:635–643. [PubMed] [Google Scholar]
- 9.Hilkens C M U, Lainski P, de Boer M, Kapsenberg M L. Human dendritic cells require exogenous interleukin-12 inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997;90:1920–1926. [PubMed] [Google Scholar]
- 10.Holt P G, Haininh S, Nelson D J, Sedgwick J D. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol. 1994;153:256–261. [PubMed] [Google Scholar]
- 11.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Maramatsu S, Steinman R M. Generation of large numbers of dendritic cells from bone marrow cultures supplemented with granulocyte/macrophage colony-stimulatory factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masten B J, Lipscomb M F. Comparison of lung dendritic cells and B cells in stimulating naive antigen-specific T cells. J Immunol. 1999;162:1310–1314. [PubMed] [Google Scholar]
- 13.McLellan A D, Sorg R V, Williams L A, Hart D N J. Human dendritic cells activate T lymphocytes via a CD40:CD40 ligand-dependent pathway. Eur J Immunol. 1996;26:1204–1210. doi: 10.1002/eji.1830260603. [DOI] [PubMed] [Google Scholar]
- 14.McWilliam A S, Napoli S, Marsh A M, Pemper F L, Nelson D J, Pimm C L, Stumbles P A, Wells T N C, Holt P G. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996;184:2429–2432. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McWilliam A S, Stumbles P A, Holt P G. Dendritic cells: biology and clinical applications. In: Lotze M T, Thomson A W, editors. Dendritic cells. San Diego, Calif: Academic Press; 1999. pp. 123–140. [Google Scholar]
- 16.Orme I M. Beyond BCG: the potential for a more effective TB vaccine. Mol Med Today. 1999;5:487–492. doi: 10.1016/s1357-4310(99)01594-4. [DOI] [PubMed] [Google Scholar]
- 17.Orme I M. The immunopathogenesis of tuberculosis: a new working hypothesis. Trends Microbiol. 1998;6:94–97. doi: 10.1016/s0966-842x(98)01209-8. [DOI] [PubMed] [Google Scholar]
- 18.Orme I M. New vaccines against tuberculosis. The status of current research. Infect Dis Clin N Am. 1999;13:169–185. doi: 10.1016/s0891-5520(05)70049-0. [DOI] [PubMed] [Google Scholar]
- 19.Orme I M. Prospects for new vaccines against tuberculosis. Trends Microbiol. 1995;3:401–404. doi: 10.1016/s0966-842x(00)88987-8. [DOI] [PubMed] [Google Scholar]
- 20.Orme I M, Roberts A D, Griffin J P, Abrams J S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–525. [PubMed] [Google Scholar]
- 21.Pierre P, Sahnnon T J, Gatti E, Hull M, Meltzer J, Mizra A, Inaba K, Steinman R M, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 22.Pollard A M, Lipscomb M F. Characterization of murine lung dendritic cells: similarities to langerhans cells and thymic dendritic cells. J Exp Med. 1990;172:159–167. doi: 10.1084/jem.172.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulendran B, Smith J L, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski C R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto F, Palermo B, Leing D, Miettinen M, Matikainen S, Julkunen I, Forster R, Burgstahler R, Lipp M, Lanzavecchia A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol. 1999;29:1617–1625. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 26.Tascon R E, Soares C S, Ragno S, Stavropoulos E, Hirst E M A, Colston M J. Mycobacterium tuberculosis-activated dendritic cells induce protective immunity in mice. Immunology. 2000;99:473–480. doi: 10.1046/j.1365-2567.2000.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson H L, Ni K, O'Neill H C. Identification of progenitor cells in long-term spleen stromal cultures that produce immature dendritic cells. Proc Natl Acad Sci USA. 2000;97:4784–4789. doi: 10.1073/pnas.080278897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia W, Pinto C E, Kradin R. The antigen-presenting activities of Ia+ dendritic cells shift dynamically from lung to lymph node after an airway challenge with soluble antigen. J Exp Med. 1995;181:1275–1283. doi: 10.1084/jem.181.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]