Abstract
Auxotrophic mutants of Mycobacterium tuberculosis have been proposed as new vaccine candidates. We have analyzed the virulence and vaccine potential of M. tuberculosis strains containing defined mutations in genes involved in methionine (metB), proline (proC), or tryptophan (trpD) amino acid biosynthesis. The metB mutant was a prototrophic strain, whereas the proC and trpD mutants were auxotrophic for proline and tryptophan, respectively. Following infection of murine bone marrow-derived macrophages, H37Rv and the metB mutant strain survived intracellularly for over 10 days, whereas over 90% of proC and trpD mutants were killed during this time. In SCID mice, both H37Rv and the metB mutant were highly virulent, with mouse median survival times (MST) of 28.5 and 42 days, respectively. The proC mutant was significantly attenuated (MST, 130 days), whereas the trpD mutant was essentially avirulent in an immunocompromised host. Following infection of immunocompetent DBA mice with H37Rv, mice survived for a median of 83.5 days and the metB mutant now showed a clear reduction in virulence, with two of five infected mice surviving for 360 days. Both proC and trpD mutants were avirulent (MST of >360 days). In vaccination studies, prior infection with either the proC or trpD mutant gave protection equivalent (proC mutant) to or better (trpD mutant) than BCG against challenge with M. tuberculosis H37Rv. In summary, proC and trpD genes are essential for the virulence of M. tuberculosis, and mutants with disruptions in either of these genes show strong potential as vaccine candidates.
Mycobacterium tuberculosis continues to be a major cause of morbidity and mortality throughout the world, resulting in 2 million deaths and over 8 million cases of tuberculosis each year (6). Given the scale of the tuberculosis problem, vaccination is a priority and remains the only realistic public health intervention that is likely to affect both the incidence and prevalence of disease (32). The currently available drug treatment involves a minimum of 6 months of chemotherapy, with a cocktail of drugs to which resistance is increasing (24). The current vaccine, Mycobacterium bovis BCG, provides inconsistent efficacy, varying between 0 and 80% in randomized control trials (9). It confers protection against childhood forms of tuberculosis but has only limited efficacy against adult disease in selected geographical locations (4). Therefore, second-generation antituberculosis vaccines urgently need to be developed.
The development of new genetic techniques has facilitated the identification and construction of targeted mutants of M. tuberculosis (26). Thus, whereas avirulent BCG was generated as a result of serial passage, a new live, attenuated vaccine can now be generated in a rational manner (10). The complete genome sequence of M. tuberculosis reveals numerous potential new drug and vaccine targets for investigation (3). In addition, it demonstrates that the tubercle bacillus has the potential to make all the essential amino acids, vitamins, and enzyme cofactors, although some of the biosynthetic pathways involved may differ from those of other bacteria. We have recently described the construction of defined mutants in amino acid biosynthesis genes of M. tuberculosis H37Rv (25), on the basis that such mutants may be attenuated in vivo. This has been particularly well documented with Salmonella enterica serovar Typhimurium (23), where the aro series of mutants can effectively be used to vaccinate against Salmonella and to act as delivery vehicles for heterologous antigens. Furthermore, in the context of mycobacterial infection, auxotrophic mutants of BCG deficient in biosynthetic pathways for methionine, leucine, or isoleucine-leucine-valine were attenuated in mice (11). In addition, a leucine auxotroph and purine auxotroph of M. tuberculosis have been shown to confer some protection against challenge infection with wild-type bacteria (14, 15).
In this paper we describe the analysis of virulence in vitro and in vivo of three defined mutant strains of M. tuberculosis H37Rv (24): a prototrophic methionine (metB) mutant, a proline auxotroph, and a tryptophan auxotroph. In vitro studies were performed to test their phenotype in a macrophage model. We describe studies designed to test their safety for use in immunocompromised hosts using SCID mice and investigate their characteristics in immunocompetent mice. Finally, the auxotrophic mutants were tested as vaccine candidates in comparison with BCG and showed equal or better protection on challenge with virulent M. tuberculosis in a murine model.
MATERIALS AND METHODS
Mice.
DBA/2 mice were obtained from OLAC, Bicester, United Kingdom. CB-17/Icr SCID mice were initially obtained from C. M. Hetherington (National Institute for Medical Research, Mill Hill, London, United Kingdom) and were bred under aseptic conditions at the London School of Hygiene and Tropical Medicine. Female mice between 8 and 10 weeks of age were used in all experiments. Experimental animals were maintained in microisolator cages (Techniplast, Kettering, United Kingdom) until use, when they were transferred to negative-pressure flexible-film isolators following infection with M. tuberculosis in a Class I/III biohazard safety cabinet.
Bacterial strains and media.
Mycobacteria were grown in Middlebrook 7H9 broth (Difco) containing 0.05% (wt/vol) Tween 80 and 10% (vol/vol) OADC supplement (Becton Dickinson, Oxford, United Kingdom), or on Middlebrook 7H10 agar (Difco) plus 10% (vol/vol) OADC supplement. The wild-type M. tuberculosis strain used was H37Rv (ATCC 25618). Defined mutant strains containing stable disruptions of amino acid biosynthesis genes were constructed by allelic replacement. The construction of these strains from the parental strain M. tuberculosis H37Rv is described by Parish et al. (25). The defined mutant strains were TAME1 (metB::hyg), TAME2 (proC::hyg), and TAME3 (trpD::hyg). Strains TAME2 and TAME3 have deletions in the proC and trpD genes, respectively (together with an insertion of the hygromycin resistance cassette), and strain TAME1 has an insertion of the hygromycin resistance gene into metB. TAME1 (prototrophic strain) was grown without any additional supplements, while TAME2 (proline auxotroph) and TAME3 (tryptophan auxotroph) were grown with 50-μg/ml l-proline and l-tryptophan, respectively. Hygromycin B was used at 100 μg/ml where required. Freeze-dried live BCG Danish Strain 1331 (Staten Serum Institute) was reconstituted in normal saline prior to use.
In vitro infection of macrophages.
Bone marrow-derived macrophages were generated by culturing bone marrow cells harvested from the femurs of adult BALB/c mice in the presence of L-cell-conditioned medium for 8 days as described previously (1). Adherent cells were harvested and plated at a density of 106/ml in 24-well plates (Nunc) in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum, 4,500 mg of glucose/liter, 110 mg of sodium pyruvate/l-pyrodoxin · HCl, NaHCO2/liter, and 4 mM glutamine, in the absence of penicillin and streptomycin. Cells were infected at a multiplicity of infection of 1 bacterium per cell for 4 h and were then washed six times in warm tissue culture medium. The infection dose was assayed independently by plating the inoculum. At this time (taken as time zero) or at various intervals over a 14-day period, the number of viable mycobacteria was assessed on 7H10 plates, after lysis of the macrophage monolayer with 1 ml of sterile distilled water containing 0.1% Triton X-100 per well. The relevant amino acid was used to supplement plates where necessary. Results were expressed as the means and standard errors of duplicate wells.
Infection of mice and tissue analysis.
Viable stocks of wild-type M. tuberculosis and mutants were grown in 10 ml of liquid medium until an optical density at 600 nm between 0.5 and 1.0 was reached. These were washed once in phosphate-buffered saline (PBS), resuspended in 5 ml of sterile PBS, and stored at −70°C in aliquots until use. Mice were infected with 106 viable mycobacteria in 200 μl of normal saline via a lateral tail vein. Where appropriate, infected mice were killed by cervical dislocation in accordance with humane end point protocols under the Animals Scientific Procedures Act, 1986 (United Kingdom). Median survival times were calculated for each group, and statistical analysis was performed using the Log Rank tests of survival. For tissue analysis, lungs, livers, and spleens were collected aseptically into 10 ml of media and were passed through a 100-micron-pore-size sieve (Falcon) in 7H9 medium containing 0.05% Tween 80. Serial 10-fold dilutions were plated in 100-μl volumes, and CFU were counted after 4 weeks and checked at 6 weeks to allow for any change in growth rate for the mutants ex vivo. A reliable level of detection by plating was therefore defined as ≥100 bacteria per organ.
For histological analysis, tissues were fixed in formol-buffered saline and were embedded in paraffin. One- to 3-μm-thickness sections were cut using a Reichert-Jung 2030 microtome. Sections were stained with Ziehl-Neelsen and were photographed using a Reichert-Jung Polyvar microscope.
Analysis of protective efficacy of auxotrophic mutants against challenge with virulent M. tuberculosis.
DBA/2 mice were immunized intravenously (i.v.) with 106 BCG or auxotrophic mutants. Six weeks after infection, mice were challenged i.v. with 106 CFU of wild-type M. tuberculosis H37Rv. Mice were also harvested prior to challenge infection to quantitate the residual tissue burden of the auxotrophs or BCG. After 4 and 8 weeks, bacterial loads from lungs, livers, and spleens were determined (n = 6 mice per time point). To distinguish between wild-type or vaccinating organisms, tissues were plated either on 7H10 plates (on which both M. tuberculosis and BCG grow) or on 7H10 plates containing 5-μg/ml thiophen-2-carboxylic acid hydrazide (Sigma), which is selective for the growth of BCG (30). Wild-type bacteria were distinguished from auxotrophs by plating on 7H10 or on hygromycin and the appropriate amino acid.
Statistical analysis.
Statistical analysis was performed for bacterial CFU data using Student's t test and for survival curves using Kaplan-Meier plots and Log Rank tests.
RESULTS
Effect of mutations on the intracellular multiplication of M. tuberculosis.
To determine the effect of mutations in genes for amino acid biosynthesis on intracellular survival, murine bone marrow-derived macrophages were infected with either parental wild-type or mutant strains. At intervals over 12 days, cells were lysed and viable bacteria were counted. The wild-type or metB bacterial counts remained between 1 × 106 and 3 × 106 CFU/ml over the 12 days of assay (Fig. 1). In contrast, a 25-fold reduction for the proC mutant and a 10-fold reduction for the trpD mutant were seen. Culture medium alone (Dulbecco's modified Eagle medium) did not support the extracellular growth of mycobacteria. In order to check whether strains had different intrinsic growth rates, we compared the growth rates in bacterial culture. All three mutants grew with the same kinetics as the wild-type strain (data not shown). Thus, these results demonstrate that disruption of the trpD or proC gene alters the ability of M. tuberculosis to multiply in murine macrophages.
FIG. 1.
Effect of auxotrophy on the intracellular multiplication of M. tuberculosis. Murine macrophages were infected at a multiplicity of infection of 1:1 with either H37Rv (●) or mutant strain metB (○), proC (▾), or trpD (▿). The numbers of bacteria were quantified and were expressed as means and standard errors for each strain per time point. All standard errors were less than 0.2%. Results are representative of three independent experiments.
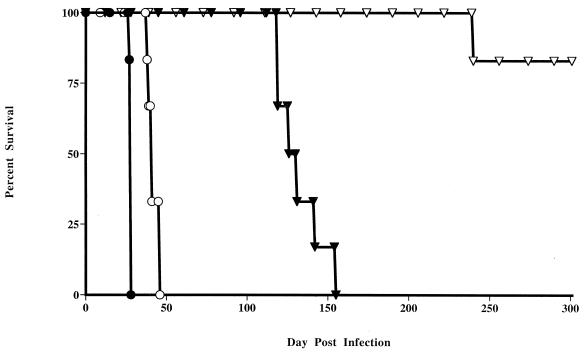
Virulence of M. tuberculosis mutants in SCID mice.
A requirement of live attenuated vaccines is that they should be safe even when used in immunocompromised hosts. To test for bacterial virulence in the absence of specific immunity, SCID mice, lacking both T and B cells and previously shown to be highly susceptible to M. tuberculosis infection (22), were inoculated with H37Rv or mutant strains (Fig. 2). All mice infected with H37Rv became moribund and died on day 28 or 29. The mice infected with the metB mutant showed a slight but consistent increase in length of survival (median survival time [MST], 42 days). In contrast, mice infected with the proC mutant survived significantly longer with a median survival time of 130 days (P < 0.001). The trpD mutant was even more attenuated, with only one death occurring, at 241 days, in an experiment which was terminated at 301 days. At this time the remaining trpD mutant-infected mice appeared healthy, although on autopsy they showed histological evidence of granuloma formation in the liver associated with the presence of mycobacteria. Importantly, no lesions or mycobacteria were detected in the lung tissue of these mice. Thus a significant reduction in virulence for the proline and tryptophan auxotrophs was seen compared to that for H37Rv in immunodeficient mice.
FIG. 2.
Virulence of mutant M. tuberculosis strains in SCID mice. Mice were infected i.v. with 106 H37Rv (●) or mutant strain metB (○), proC (▾), or trpD (▿), and survival was monitored over 11 months. Each group contained six mice, and the results are representative of two separate experiments.
Growth kinetics of H37Rv versus mutant M. tuberculosis strains and tissue inflammatory responses in SCID mice.
To monitor the effects of defects in amino acid biosynthesis on the replication of M. tuberculosis in vivo, SCID mice were infected with either H37Rv or the metB, proC, or trpD mutant strain, and bacterial burdens were assayed in the liver, lungs, and spleens. Bacterial numbers recovered from mice infected with H37Rv rose rapidly in the liver and spleen and especially in the lung by day 22 (Fig. 3). Bacterial growth was significantly delayed in the group infected with the metB mutant (P < 0.01) but thereafter followed an in vivo growth rate similar to that for H37Rv in all three organs.
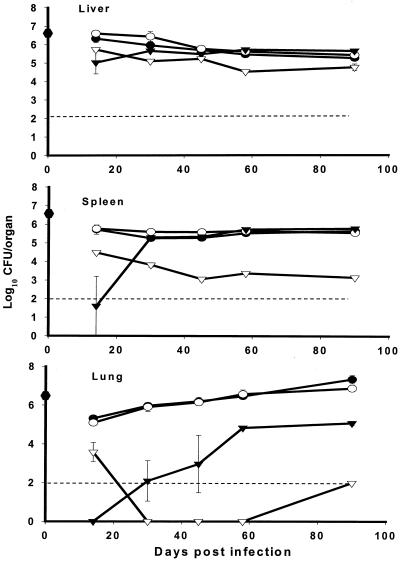
FIG. 3.
Effect of auxotrophy on the survival and multiplication of M. tuberculosis in the tissues of SCID mice. Mice were infected i.v. with 106 H37Rv (●) or mutant strain metB (○), proC (▾), or trpD (▿). CFU were assayed on 7H10 plates with amino acid supplementation where required. The results represent means ± standard errors for three mice per group with significance measured using Student's t test. The dotted line represents the reliable limit of detection. Results are representative of two separate experiments.
The rise in bacterial burden was significantly delayed in mice infected with the proC mutant, compared to mice infected with H37Rv, consistent with the former's increased survival times. Thus at day 20, the proC mutant was attenuated in vivo both in the liver and spleen (P < 0.0001) and also in the lungs, compared to H37Rv (P < 0.01). The mice infected with the proC mutant continued to demonstrate a degree of control over bacterial replication in the liver and spleen, but importantly they showed a gradual loss of control in the lung after day 20 (Fig. 3).
Mice infected with the trpD mutant had consistently lower bacterial burdens than did either H37Rv- or proC mutant-infected mice at all time points examined, which correlated with prolonged survival of trpD mutant-infected mice. Although bacterial numbers in the livers of SCID mice infected with the trpD mutant were maintained at approximately log10 5, in both the spleen and lung there was a rapid decrease in organ load postinfection to around or below the limits of detection by plating. This was maintained until the termination of the experiment at day 90. In summary, the loss of virulence of the trpD mutant in SCID mice correlated with its control in all target organs, whereas the eventual death of SCID mice infected with the proC mutant was particularly associated with outgrowth in the lungs.
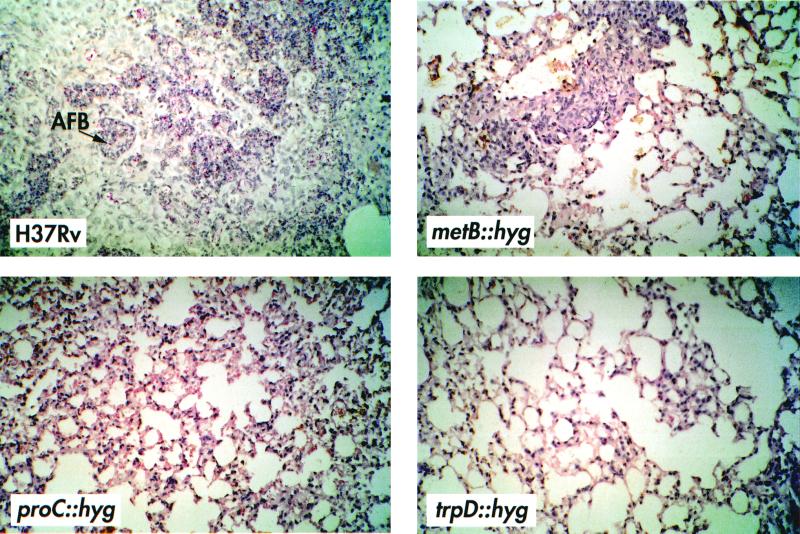
It has been previously shown that SCID mice form granulomas in response to mycobacterial infection in the absence of acquired immunity (12, 21, 22, 29). Therefore, we asked whether the difference in progression of disease and bacterial burdens between H37Rv and auxotrophic mutants was accompanied by visible changes in the tissues over the course of the infection. Figure 4 shows sections from the lungs of SCID mice infected with either H37Rv or mutant strains on day 22 that were stained for acid-fast bacteria and were counterstained to visualize inflammatory responses. The inflammatory response to H37Rv was characterized by massive cellular infiltration, with thickening of the airway epithelia and occupation of 20 to 30% of the tissue by well-demarcated granulomas by day 22 (Fig. 4). This response was delayed in the metB mutant infections, and the lesions were smaller and fewer (Fig. 4). Whilst the lung granulomas were less extensive in the metB mutant-infected mice, they were also accompanied by a thickening of the lung epithelium and the presence of visible mycobacteria in the lesions, again reflective of large numbers of mycobacteria in these organs. In stark contrast to the H37Rv- or metB mutant-infected mice, no visible mycobacteria or lesions were seen in either the proC (despite evidence of thickening of the lung epithelium) or trpD mutant-infected mice at 22 days (Fig. 4). However, by 90 days of infection in proC mutant-infected mice, a few small discrete granulomas were seen with visible mycobacteria. In trpD mutant-infected mice, there was no evidence of infection, airway thickening, or granuloma formation at any time over the 90 days (data not shown).
FIG. 4.
Cellular infiltration and granuloma formation in the lungs of SCID mice infected with wild-type or mutant strains of M. tuberculosis. Mice were infected with 106 bacteria from H37Rv or mutant strain metB, proC, or trpD, and lungs were harvested on day 22 of infection. Sections were stained with Ziehl-Neelsen stain, and photographs were taken at a magnification of ×25. AFB, acid-fast bacteria.
Virulence of M. tuberculosis mutants in immunocompetent mice.
To determine whether the disruption of genes involved in amino acid biosynthesis affected the growth rate of M. tuberculosis in the immunocompetent host, DBA/2 mice were infected with either H37Rv or mutant strains. Mice infected with H37Rv died between 78 and 100 days postinfection (MST, 83.5 days). In contrast two of five mice infected with the metB mutant survived for over 350 days (Fig. 5). All proC or trpD mutant-infected mice survived for the duration of the experiment, which was terminated at 350 days. Thus, although H37Rv was virulent in DBA/2 mice, the metB mutant was somewhat attenuated, whilst proC and trpD mutants were essentially avirulent.
FIG. 5.
Virulence of mutant M. tuberculosis in immunocompetent mice. DBA/2 mice were infected intravenously with 106 H37Rv (●) or mutant strain metB (○), proC (▾), or trpD (▿), and survival was monitored over 11 months. The results are representative of two separate experiments with 6 mice per group.
Growth kinetics of wild-type versus mutant M. tuberculosis and tissue inflammatory responses in immunocompetent mice.
We then asked whether the prolonged survival times in mice infected with auxotrophs were associated with clearance of bacteria from the tissues. The course of infection in either H37Rv- or metB mutant-infected mice was remarkably similar in all organs, and statistical analysis revealed no differences in tissue burdens at any time. Thus bacterial numbers were stable or controlled in the liver and spleen but increased notably in the lungs from log10 5.0 to log10 7.4 for H37Rv and log10 6.9 for the metB mutant over 90 days (Fig. 6).
FIG. 6.
Effect of auxotrophy on survival and multiplication in the tissues of DBA/2 mice. Mice were infected with 106 H37Rv (●) or mutant strain metB (○), proC (▾), or trpD (▿). CFU were assayed at various intervals on 7H10 agar with or without appropriate amino acid supplementation. The results represent means ± standard errors for three mice per group. Significance was measured using Student's t test. The dotted line represents the reliable limit of detection. The results are representative of two separate experiments.
In contrast, the numbers of bacteria recovered from the livers of proC mutant-infected mice were 100-fold lower on day 14 (P < 0.0001). Over the course of the experiment, these rose by approximately 10-fold to equal the number recovered from mice infected with H37Rv (Fig. 6). Unusually for a liver response to infection with M. tuberculosis, the numbers of bacteria recovered from trpD mutant-infected mice fell 10-fold over the 90 days, providing further evidence of attenuation in this strain.
The numbers of bacteria recovered from spleens of mice infected with either H37Rv or the metB mutant were stable. After an initially low recovery of the proC mutant at day 14, the number rose to the levels recovered from H37Rv-infected mice by day 30. In contrast the trpD mutant was progressively reduced to a stable level of approximately 1,000 bacteria per spleen by day 45.
Examination of bacterial growth in the lungs highlighted the greatest differences between the mutants and H37Rv. Again, H37Rv and the metB mutant showed identical patterns of sustained growth in this organ. In marked contrast the proC mutant was cleared to levels below detection (i.e., less than 100 bacteria per organ) in the lungs at day 14. However, by day 45 some of the mice had recoverable bacteria in the lungs, and significant numbers were present in all mice in the group. Although in trpD mutant-infected mice significant numbers were recovered at 14 days, after this time clearance occurred in this organ. Thus, loss of the ability to synthesize proline or tryptophan rendered these auxotrophs less virulent than H37Rv, and the trpD mutant was most severely attenuated. Granuloma formation is a key component of the adaptive response to mycobacterial infection and is influenced by the tissue burden and virulence of the infecting organism. Therefore, we examined whether the persistence of mycobacteria affected the growth and development of granulomas in the lung tissues. Analysis of the lung tissue of mice infected with H37Rv showed that by day 90, granulomatous areas spread over 20% of the tissue. This was classified as stage 3 (27), and mycobacteria could clearly be seen associated with tissue necrosis in some areas. Histological changes in the lungs of the metB mutant-infected mice were similar but delayed. However, no inflammatory responses or mycobacteria were visible in the lungs of mice infected with the proC or trpD mutant (data not shown).
Protective efficacy of auxotrophic mutants against challenge with virulent M. tuberculosis.
To determine whether auxotrophic mutants were able to protect mice against subsequent challenge with virulent M. tuberculosis, DBA/2 mice were infected with either 106 proC or trpD mutant or BCG bacteria i.v. and were challenged with wild-type M. tuberculosis 6 weeks later. The residual tissue burdens of vaccinating organisms were determined in samples taken at the time of challenge (see Fig. 7 legend). The number of wild-type versus auxotroph M. tuberculosis at each time point postchallenge also revealed that the course of infection with vaccinating organisms was unchanged from that described for Fig. 6.
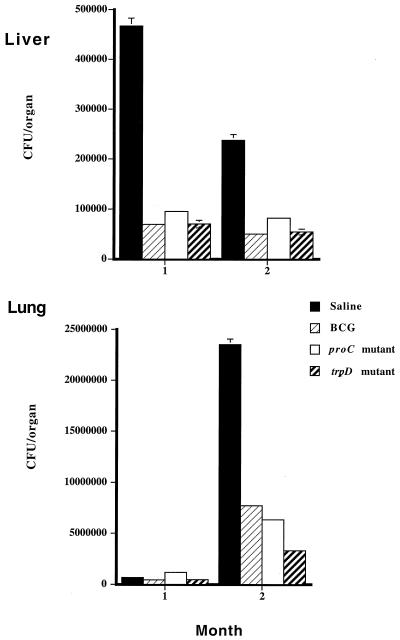
FIG. 7.
Protective efficacy of auxotrophic mutants against challenge with virulent M. tuberculosis in DBA/2 mice. Mice were inoculated i.v. with 106 M. tuberculosis auxotrophs (proC or trpD mutants) or BCG or given saline alone and were challenged i.v. 6 weeks later with 106 H37Rv. Mice were killed 4 or 8 weeks postchallenge, and homogenates of lungs and livers were serially diluted on differential plates to distinguish between inoculum and challenge (see Materials and Methods). Results represent mean ± standard error for six mice per group. Statistical significance was measured using Student's t test. Residual burden of vaccinating inoculum at time of challenge with H37Rv in CFU per organ for BCG: liver, log10 3.1; lung, log10 0.8; spleen, log10 3.2; for proC mutant: liver, log10 5.8; lung, log10 4.4; spleen, log10 5.6; for trpD mutant: liver, log10 4.5; lung, none detected; spleen, log10 2.9. Residual bacteria at 1 month for proC mutant: lung, log10 4.4; liver, log10 5.2; spleen, log10 4.6; for trpD mutant: lung, log10 1.5; liver, log10 4.2; spleen, none detected. Residual bacteria at 2 months for proC mutant: lung, log10 4.2; liver, log10 5.2; spleen, log10 4.8; for trpD mutant: lung, none detected; liver, log10 3.1; spleen, log10 1.7.
By 1 month postchallenge, no significant reduction in bacterial burden was seen in the lungs of mice previously infected with either BCG or auxotrophic M. tuberculosis. However, at this time, very low numbers of bacteria were recovered from this organ. In contrast, in the spleen the numbers of wild-type bacteria recovered were significantly reduced in vaccinated mice, by 67% for BCG (P < 0.0013), 60% for the trpD mutant (P < 0.02), and 70% for the proC mutant (P < 0.0039). In the liver, all three vaccinations significantly reduced the numbers of M. tuberculosis H37Rv bacteria recovered compared to challenge controls (Fig. 7). This represented an 85% reduction in H37Rv bacteria recovered from mice vaccinated with either BCG (P < 0.0008) or the trpD mutant (P < 0.0001) and an 80% reduction for the proC mutant vaccination (P < 0.0024). At 2 months postchallenge, levels of protection were found in the lung for both mutants in excess of the 67% for BCG vaccination (P < 0.01), compared with challenge controls. A 73% reduction in tissue burden for the proC mutant (P < 0.009) and an 86% reduction for the trpD mutant prior to challenge were seen, compared with challenge controls (P < 0.004). In addition, the levels of protection (log10 0.9) seen in the lungs of mice vaccinated with the trpD mutant were significantly higher than those seen with BCG (log10 0.5) (P < 0.0001). Evidence of protection was also seen in the liver at this time but not in the spleen. Thus, both proC and trpD mutants were able to protect against challenge with virulent M. tuberculosis at levels equivalent to or greater than those for BCG vaccination in mice.
DISCUSSION
In this paper we describe the phenotypic characteristics of mutants in defined amino acid biosynthesis genes. Targeted disruption of the proC and trpD genes resulted in auxotrophic mutants that had reduced intracellular survival in macrophages in vitro. In immunocompromised SCID mice, the proC mutant showed significant attenuation and the trpD mutant was rendered avirulent. In contrast, both proC and trpD mutants were avirulent in immunocompetent mice. In vaccination experiments for mice, both proC and trpD mutants showed protection equivalent to or greater than that of BCG, the current vaccine.
In axenic culture both the tryptophan and proline auxotrophs, with appropriate amino acid supplementation, had intrinsic growth rates similar to that of H37Rv. In contrast, both these auxotrophs were attenuated in a macrophage model of in vitro infection, whereas the metB mutant (which was prototrophic) showed the same kinetics of growth as H37Rv. It has been shown previously that leucine auxotrophs of both BCG and M. tuberculosis were restricted in their growth in macrophages (2). In this case, leucine was provided as a supplementation to the media employed for macrophage culture, suggesting that after phagocytosis the organisms were sequestered in an intracellular compartment from which they could not obtain this amino acid. In our experiments the macrophage culture media contained both methionine and tryptophan but not proline. However, the relevance of this to the in vivo situation is unclear, since we do not know what concentrations of amino acids are available in the phagosomal environment.
In considering auxotrophic mutants as vaccine candidates, it is essential that they are sufficiently attenuated such that they do not cause disease even in immunodeficient individuals who are particularly at risk from tuberculosis. As a primary screen to identify changes in virulence, M. tuberculosis mutants were injected into SCID mice, which lack both T and B cells. Wild-type M. tuberculosis and the metB mutant were extremely virulent in these mice. Both the proC and trpD mutants were significantly attenuated in SCID mice, with the trpD mutant most attenuated (survival times exceeded 300 days for the majority of mice). Thus the trpD auxotroph had a degree of attenuation equivalent to that for the recently described leucine auxotroph, which was not only unable to replicate in macrophages but also caused no deaths in SCID mice (14). In addition, these prolonged survival times for SCID mice infected with the tryptophan auxotroph far exceed those described for the course of infection with BCG in SCID mice, which generally succumb by 8 to 10 weeks (11, 21, 22). Analysis of tissue burdens showed that the growth of the trpD mutant was controlled or stabilized in all organs and was essentially cleared from the lung and spleen by day 20. In contrast, proC mutant-infected mice controlled the infection in the liver but importantly lost control of infection in the lung after day 20. Loss of control in the lung also contributed significantly to progression to death in mice infected with H37Rv or the metB mutant. Thus, even in the SCID mouse, the lung is clearly the key target organ in determining bacterial virulence in the murine model, as it is for immunocompetent mice (5).
Researchers have previously shown that SCID mice are able to form granulomas even in the absence of T cells and that this ability operates as a key part of the early response to infection (21, 29). The increased bacterial numbers found in SCID mice infected with H37Rv or the metB mutant were accompanied by rapid cellular infiltration and granuloma formation. In contrast, minimal histological changes were seen in the lung tissue of mice infected with the proC mutant and none in mice infected with the trpD mutant. These results suggest that the trpD mutant may be safe to use in immunodeficient hosts. In addition, from these studies we may conclude that the SCID mouse is a useful and sensitive model for distinguishing between strains of different virulence.
Using immunocompetent mice, we were able to demonstrate the prolonged survival, over 300 days, of mice infected with either the proC or trpD mutant. We found that infection with H37Rv led to death in 50% of immunocompetent DBA/2 mice within 100 days, similar to what was found for previous reports (19). It was noted that mice infected with the metB mutant survived longer than H37Rv-infected mice, suggesting some degree of attenuation, although the kinetics of in vivo bacterial growth for the metB mutant were very similar. This attenuation in vivo was not predicted from in vitro studies either in axenic culture or in macrophages. A methionine auxotroph of BCG (18) had previously been shown to grow in vivo in BALB/c mice to a similar level and with kinetics similar to that of wild-type BCG (11).
In parallel to the results just described in SCID mice, differences in the growth of M. tuberculosis and the mutants in immunocompetent mice were most striking in the lung. While the metB mutant remained at numbers similar to those for H37Rv over the 90 days, the proC mutant was originally detected at very low levels; those levels were maintained for 30 to 40 days and then increased over the course of infection. In contrast, the trpD mutant was controlled at an early time point and then throughout the 90 days. No overt changes were seen in the lungs of mice infected with either the proC or trpD mutant compared to infection with H37Rv, where granulomas were clearly visible. These granulomas were discretely spread over the lung area and seemed to coalesce in later stages of infection. In mice, as in humans, tuberculosis is a chronic disease of the lungs, the only organ in which the organism causes excessive pathology, regardless of whether the infection is initiated via the respiratory or intravenous route.
The results of the protection studies with the proC or trpD mutants demonstrated significant protection in all organs, with liver burdens reduced at both 4 and 8 weeks after challenge similar to BCG-vaccinated controls. The numbers of virulent M. tuberculosis organisms in the spleen were significantly reduced 1 month after challenge. Importantly, in the lung they were significantly reduced 2 months after challenge with greater levels of protection in this organ than seen with BCG vaccination. This compares very favorably with other auxotrophs of M. tuberculosis, the leucine auxotroph which conferred less protection than BCG (14) and the purine auxotroph which produced equal protection in the lung but not in the spleen (15).
In contrast to other studies, we also determined the residual tissue burden of the vaccinating mutants, both at the time of challenge and 1 and 2 months later. The results obtained paralleled the data for bacterial growth in mice without the overlay of challenge infection. To induce optimal protection, attenuated strains of M. tuberculosis need to persist for some time, to home to an appropriate organ to engender immunity and to be sufficiently metabolically active to synthesize relevant antigens. This suggests that organ-specific responses to the vaccinating strain may be of key importance in protective mechanisms. It is known that immune response to infection can vary markedly in different organs of the same animal. As shown here, in some organs the infection can resolve with subsequent immunity to reinfection, whereas in other organs pathogens can persist (7). Of real interest was that the trpD mutant derived from the immunization was reduced below detectable levels in the lung, and in this organ the highest degree of protection against M. tuberculosis was obtained, exceeding that seen for BCG vaccination. Our data for proline and tryptophan auxotrophs of M. tuberculosis contrast with that for a purine auxotroph of M. tuberculosis which was eliminated from the liver (15). In our study, the proC mutant multiplied in this organ to levels found in the H37Rv-infected mice by the termination of the experiment, and while the trpD mutant did fall significantly, it never fell below detectable limits in this organ. In contrast we saw trpD mutant clearance from the lung and the spleen even during challenge with H37Rv. The differential organ clearance of the various auxotrophic strains is intriguing. However, it is unclear whether this is due to a simple requirement for amino acid lacking in the organs in question or is related to differences in immunological competence at these sites. These mutants will give us a unique opportunity to investigate almost identical strains of M. tuberculosis, which are differentially variable in their growth characteristics in different organs.
The development of attenuated, auxotrophic mutants as potential vaccine candidates has been clearly demonstrated in many pathogen systems. These include S. enterica serovar Typhimurium, Corynebacterium paratuberculosis, and Legionella pneumophila and show attenuation for growth in macrophages or in vivo (8, 13, 16, 20, 28). Indeed, Salmonella typhi Ty21, an auxotrophic vaccine against typhoid fever, is already widely used in humans (31) and is safe, although it may not be optimally effective. The description of auxotrophs originally developed from BCG as vaccine candidates against tuberculosis has given hope that safe auxotrophic strains could be produced from virulent M. tuberculosis itself (11, 14, 15). In addition, the genome sequence has revealed information for targeting specific mutations and has highlighted the areas of homology between M. tuberculosis and BCG vaccine strains (17). This is important, since it is likely that there are unique protective and immunogenic antigens and epitopes in M. tuberculosis that are not present in BCG or are inappropriately expressed. Since BCG in humans provides inconsistent efficacy and protection in adults (4, 9), we believe that further studies with these auxotrophs will both extend our understanding of mycobacterial virulence and provide an opportunity for further genetic manipulation to produce an optimally attenuated vaccine candidate. One advantage of using attenuated M. tuberculosis is the greater antigenic homology with the infecting organism than that for BCG. Additionally, our data suggest that vaccination targeted to different organs may engender stronger and more appropriate protection. In the future we will test the differential organ protection seen here against a more physiological challenge by the aerosol route.
In conclusion, we have analyzed auxotrophic mutants of M. tuberculosis which clearly demonstrate the essential role of the proC and trpD genes in virulence. The promising levels of protection achieved in comparison with BCG suggest that further investigation using different mouse strains, routes, doses, and times of administration will help us to optimize and evaluate the protective effects of these mutants. In addition, these auxotrophs may serve as a basis for further genetic manipulation to enhance induction of protective immunity.
ACKNOWLEDGMENTS
We are grateful to Heidi Alderton and also to Helen Counihan for her excellent technical assistance with histology as well as to the Staff of the Biological Services Facility at the London School of Hygiene and Tropical Medicine.
Debbie A. Smith and Tanya Parish were supported by the Glaxo Wellcome Action TB initiative.
REFERENCES
- 1.Bancroft G J, Collins H L, Sigola L B I, Cross C E. Modulation of murine macrophage behaviour in vivo and in vitro. Methods Cell Biol. 1994;45:129–146. doi: 10.1016/s0091-679x(08)61849-x. [DOI] [PubMed] [Google Scholar]
- 2.Bange F C, Brown A M, Jacobs W R. Leucine auxotrophy restricts growth of Mycobacterium bovis BCG in macrophages. Infect Immun. 1996;64:1794–1799. doi: 10.1128/iai.64.5.1794-1799.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 4.Davis, P. D. O. Clinical tuberculosis, 2nd ed. Chapman & Hall Medical, London, United Kingdom.
- 5.Dunn P L, North R J. Virulence ranking of some Mycobacterium tuberculosis and Mycobacterium bovis strains according to their ability to multiply in the lungs, induce lung pathology, and cause mortality in mice. Infect Immun. 1995;63:3428–3437. doi: 10.1128/iai.63.9.3428-3437.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dye C, Scheele S, Dolin P, Pathania V, Raviglione M C. Global burden of tuberculosis. Estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 7.Engwerda C R, Kaye P M. Organ-specific responses associated with infectious disease. Viewpoint Immunol Today. 2000;21:73–78. doi: 10.1016/s0167-5699(99)01549-2. [DOI] [PubMed] [Google Scholar]
- 8.Fields P L, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5191. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine P E M. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 10.Grange J M, Gibson J, Osborn T W, Collins C H, Yates M D. What is BCG? Tubercle. 1983;64:129–139. doi: 10.1016/0041-3879(83)90038-7. [DOI] [PubMed] [Google Scholar]
- 11.Guleria I, Teitelbaum R, McAdam R A, Kalpana G, Jacobs W R, Jr, Bloom B R. Auxotrophic vaccines for tuberculosis. Nat Med. 1996;2:334–337. doi: 10.1038/nm0396-334. [DOI] [PubMed] [Google Scholar]
- 12.Hansch H C, Smith D A, Mielke M E, Hanh H, Bancroft G J, Ehlers S. Mechanisms of granuloma formation in murine Mycobacterium avium infection: the contribution of CD4+ T cells. Int Immunol. 1996;8:1299–1310. doi: 10.1093/intimm/8.8.1299. [DOI] [PubMed] [Google Scholar]
- 13.Hoiseth S K, Stocker B A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 14.Hondalus M K, Bardarov S, Russell R, Chan J, Jacobs W R, Bloom B R. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect Immun. 2000;68:2888–2898. doi: 10.1128/iai.68.5.2888-2898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson M, Phalen S W, Lagranderie M, Ensergueix D, Chavarot P, Marchal G, McMurray D N, Gicquel B, Guilhot C. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect Immun. 1999;67:2867–2873. doi: 10.1128/iai.67.6.2867-2873.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung K Y, Findley B B. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc Natl Acad Sci USA. 1991;88:11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahairas G G, Sabo P J, Hickey M J, Singh D C, Stover C K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAdam R A, Weisbrod T R, Martin J, Scuderi J D, Brown A M, Cirillo J D, Bloom B R, Jacobs W R., Jr In vivo growth characteristics of leucine and methionine auxotrophic mutants of Mycobacterium bovis BCG generated by transposon mutagenesis. Infect Immun. 1995;63:1004–1012. doi: 10.1128/iai.63.3.1004-1012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina E, North R J. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology. 1998;93:270–274. doi: 10.1046/j.1365-2567.1998.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mintz C S, Chen J, Shuman H A. Isolation and characterization of auxotrophic mutants of Legionella pneumophila that fail to multiply in human monocytes. Infect Immun. 1988;56:1449–1455. doi: 10.1128/iai.56.6.1449-1455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.North R J, Izzo A A. Granuloma formation in severe combined immunodeficient (SCID) mice in response to progressive BCG infection. Tendency not to form granulomas in the lung is associated with faster bacterial growth in this organ. Am J Pathol. 1993;142:1959–1966. [PMC free article] [PubMed] [Google Scholar]
- 22.North R J, Izzo A A. Mycobacterial virulence. Virulent strains of Mycobacterium tuberculosis have faster in vivo doubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J Exp Med. 1993;177:1723–1733. doi: 10.1084/jem.177.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Callaghan D, Maskell D, Liew F Y, Easmon C S F, Dougan G. Characterization of aromatic and purine-dependent Salmonella typhimurium: attenuation, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun. 1988;56:419–423. doi: 10.1128/iai.56.2.419-423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pablos-Mendez A, Raviglione M C, Laszlo A, Binkin N, Rieder H L, Bustreo F, Cohn D L, Lambregts-van Weezenbeek C S B, Kim S J, Chaulet P, Nunn P. Global surveillance for antituberculosis-drug resistance, 1994–1997. N Engl J Med. 1998;338:1641–1649. doi: 10.1056/NEJM199806043382301. [DOI] [PubMed] [Google Scholar]
- 25.Parish T, Gordhan B G, McAdam R A, Duncan K, Mizrahi V, Stoker N G. Production of mutants in amino acid biosynthesis genes of Mycobacterium tuberculosis by homologous recombination. Microbiology. 1999;145:3497–3503. doi: 10.1099/00221287-145-12-3497. [DOI] [PubMed] [Google Scholar]
- 26.Parish T, Stoker N G. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology. 2000;146:1969–1975. doi: 10.1099/00221287-146-8-1969. [DOI] [PubMed] [Google Scholar]
- 27.Rhoades E R, Frank A A, Orme I M. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 28.Simmons C P, Hodgson A L M, Strugnell R A. Attenuation and vaccine potential of aroQ mutants of Corynebacterium pseudotuberculosis. Infect Immun. 1997;65:3048–3056. doi: 10.1128/iai.65.8.3048-3056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith D, Hansch H, Bancroft G, Ehlers S. T-cell-independent granuloma formation in response to Mycobacterium avium: role of tumour necrosis factor-alpha and interferon-gamma. Immunology. 1997;92:413–421. doi: 10.1046/j.1365-2567.1997.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vestal A L, Kubica G P. Differential identification of mycobacteria. Use of thiacetazone, thiophen-2-carboxylic acid hydrazide and tripheyltetrazolium chloride. Scand J Respir Dis. 1967;48:142–148. [PubMed] [Google Scholar]
- 31.Wahdan M, Serie C, Cerisier Y, Sallam S, Germanier R. A controlled field trial of live Salmonella typhi strain Ty21a oral vaccine against typhoid: three-year results. J Infect Dis. 1982;145:292–295. doi: 10.1093/infdis/145.3.292. [DOI] [PubMed] [Google Scholar]
- 32.Young D B, Fruth U. New vaccines against tuberculosis. In: Levine M, Woodrow G, Kaper J, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker; 1997. pp. 631–645. [Google Scholar]