Abstract
Temporomandibular disorders (TMD) symptoms develop into chronic pain for some patients, but the reasons for this are unclear. Psychosocial factors and chronic overlapping pain conditions are believed to contribute to the development of pain-related disability. We examined the role of jaw function, negative and positive psychological factors, and chronic overlapping pain conditions (COPCs) on pain-related disability while controlling for demographic variables. We collected demographics, medical and psychosocial history, and the Graded Chronic Pain Scale, a measure of pain intensity and pain interference from 400 participants with chronic TMD. Structural equation modeling was used to assess a model of COPCs and the latent variables of psychological unease (pain catastrophizing, somatic symptoms, and negative affect), positive valence factors (optimism and positive affect), jaw function (chewing, opening, and expression limitation), and pain-related disability (pain intensity and pain interference) while controlling for demographic variables. We achieved good fit of a parsimonious model (root-mean-square error of approximation = .063 (90% CI) [.051-.075]), comparative fit index =.942, standard root-mean-square residual = .067). Jaw function was the strongest latent variable predictor, followed by psychological unease and COPCs, suggesting resources focused on improving joint function, psychosocial support, and management of COPCs will improve pain-related disability in TMDs. These findings not only increase the body of knowledge related to TMD clinical phenotypes but also have translational impact in further supporting the potential value of targeting physical therapy such as jaw exercise along with psychological interventions as multidisciplinary nonpharmacological therapeutic solutions.
Keywords: Temporomandibular disorders, positive valence factors, psychological unease, chronic overlapping pain conditions
1. INTRODUCTION
Temporomandibular disorders (TMD) are common in the United States, affecting up to 12% of the population with considerable associated costs (National Institute of Dental and Craniofacial Research 2018). TMD sufferers experience excruciating pain in the temporomandibular joints, significantly impairing daily functioning and reducing the quality of life (de Leeuw and Klasser 2013; National Academies of Sciences and Medicine 2020; Schiffman et al., 2014). TMD symptoms can be acute, self-limiting, or vary over time, and for unclear reasons, 15% of TMD sufferers will have persistent pain five years after the complex symptoms appear (National Institute of and Craniofacial 2018; Schiffman et al., 2017; Yadav et al., 2018). A greater understanding of the multidimensional nature of TMD pain-related disability will enhance our ability to isolate which patient characteristics exacerbate pain disability and, equally important, which patient characteristics protect against poor pain outcomes.
Previous studies suggest the complex relationship between clinical characteristics, psychosocial factors, orofacial function, and underlying pain sensitivity impacts TMD pain symptoms and outcomes (Fillingim et al., 2018; List and Jensen 2017). TMD are often associated with chronic overlapping pain conditions (COPCs) like fibromyalgia, irritable bowel syndrome, headaches, migraines, and chronic low back pain due to an underlying central nervous system hypersensitivity (Aaron et al., 2000; Maixner et al., 2016; Plesh et al., 1996; Robinson et al., 2016; Sanders et al., 2013). Mainly, painful jaw characteristics common to TMD exist independently in other COPCs, and the presence of greater than 3 COPCs is associated with greater TMD symptomology (Sharma et al., 2020). The contribution of comorbidities to pain outcomes in TMD warrants further study as poor treatment outcomes may be linked to associated COPCs (Krogstad et al., 1996), and incorporating COPCs into assessment, management and intervention may be beneficial to pain outcomes (Chen et al., 2012).
Other factors that can influence disability are depression and pain catastrophizing, a heightened focus on the potential signs and dangers of pain (Ankawi et al., 2017; de Moraes Vieira et al., 2014; Turk et al., 2016). Significant positive associations were found between psychological distress, social stressors, arousal, and pain symptoms in patients with TMD (Davis et al., 2010), and between pain disability, psychological difficulties, and jaw function (Miller et al., 2020). While the role of psychological distress in chronic pain behavior is well recognized, much less is known about the contribution of positive valence factors (Wang et al., 2022) like optimism and positive affect which may counter the adverse effects of psychological distress in chronic pain outcomes. (Sturgeon and Zautra 2013).
This cross-sectional study aims to determine how positive and negative psychological constructs, jaw function, and COPCs contribute to pain-related disability in TMD using a structural equation modeling (SEM) approach. We hypothesize that: 1) positive psychological constructs will be inversely associated with pain-related disability; 2) negative psychological constructs will have a direct and positive relationship to pain-related disability; 3) jaw function limitation will have a direct and positive relationship to pain-related disability, and 4) COPCs will have a direct and positive impact on pain-related disability.
2. METHODS
This cross-sectional study comprised participants who were suffering from TMD for at least three months between the years 2017 and 2020. The sample was nested in a parent study investigating the genetic predictors of orofacial pain. The University of Maryland Institutional Review Board approved this study (HP-00068315), and all willing participants signed informed consent before data collection procedures. The risks, benefits, and alternatives were explained before obtaining written consent from all the participants. We excluded vulnerable groups such as children and prisoners from the study, and we took every precaution to protect the privacy of all participants. The participants received compensation for their time ($100).
2.1. Participants
We enrolled 409 participants and excluded nine as they did not meet eligibility criteria or withdrew from the study (see Figure 1). The final sample consisted of 400 participants. Recruitment occurred via advertisements, community events, health fairs, and letters to patients at the Brotman Facial Pain Clinic at the University of Maryland. Demographic data (age, sex, race, and education) were collected, participants completed a series of questionnaires, and a trained examiner confirmed the TMD diagnosis. A nurse or physician conducted telephone and in-person screenings to verify eligibility to participate in this study.
Figure 1.

Flow Diagram. This flow diagram represents sample selection and missing data among the TMD cohort.
Diagnostic Criteria for Temporomandibular Disorders
A trained examiner with expertise in orofacial pain confirmed the presence of TMD with an in-person clinical exam at the Brotman Facial Pain Clinic at the University of Maryland School of Dentistry. The DC/TMD symptom questionnaire was used to assess the reported pain history and arrive at a research diagnosis based on the presence of myalgia, arthralgia, and myofascial pain with referral (Schiffman et al., 2014). A trained examiner assessed the location of pain across the joint and muscle mass, incisal relationships, and mandibular movements. The exam included evaluating the presence of myalgia, arthralgia, or self-reported headache of any type in the temporal area in the previous 30 days and any headache modified by jaw movement, function, or parafunction. The grading of instruments was per the DC/TMD Scoring Manual for Self-Report Instruments (Ohrbach and Knibbe 2016).
2.2. Inclusion and Exclusion Criteria
Participants were English speakers between 18 and 65 years of age. Inclusion criteria of the study were facial pain for at least the immediate three months prior and symptoms that met the DC/TMD (Schiffman et al., 2014). Facial pain characteristics included pain occurring a minimum of three months in the jaw, temple, ear, in front of the ear, or on either side immediately before the examination. The inclusion criteria of the study were facial pain that was always present or intermittent for at least the immediate three months prior. Participants provided their medical history, including current medications, via a checklist of common medical conditions. We excluded participants with a history of severe psychiatric disease, degenerative neuromuscular disease, cardiovascular disease, pulmonary, kidney disease, liver disease, and cancers, all within three years. Participants who were pregnant, breastfeeding, color blind, had uncorrected hearing, facial trauma, cervical stenosis, and alcohol or drug dependence were also excluded.
2.3. Measurements
Pain-Related Disability was measured using the Graded Chronic Pain Scale version 2.0 (Von Korff 2011). The GCPS v2.0 is a reliable and valid tool for measuring the impact of TMD pain on the patients’ ability to function (Von Korff 2011; Von Korff et al., 1992). The GCPS consists of 8 items; the first inquiring about pain over the last six months is not included in the scoring. Three subscale scores, characteristic pain intensity (CPI), pain interference score, and disability days, are derived from the remaining seven items. The pain intensity items are ranked on a scale of 0 (no pain) to 10 (pain as bad as possible). The pain interference score is derived from questions 6 through 8 regarding the inability to do daily work or social activities. Interference questions are ranked on a scale of 0 (no interference) to 10 (unable to carry out activities). Pain intensity and interference scores were averaged and multiplied by 10. Higher scores indicate higher pain intensity and disability (Bartley et al., 2019). Pain intensity and pain interference scores were used in this analysis.
Jaw Limitation was measured using the jaw function limitation scale (JFLS-20) (Ohrbach et al., 2008), a 20-item scale measuring jaw mastication, jaw mobility, and emotional and verbal expression. The scale has excellent reliability for temporomandibular disorder patients.
Psychological unease a latent variable previously created by Miller et al, (Miller et al., 2020), was created from subscales: 1) negative affect, from the positive and negative affect schedule (PANAS) (Watson et al., 1988), 2) the pain catastrophizing scale (PCS) (Osman et al., 2000; Sullivan et al., 1995), and 3) the patient health questionnaire-15 (PHQ-15) (Kroenke et al., 2002) the somatic symptoms scale. Negative affect, the personal feeling of distress and unpleasant emotions accompanying negative mood states, was assessed by the negative affect subscale from the PANAS (Watson et al., 1988). Participants ranked on a 5-point Likert scale of 1 (very slightly) to 5 (extremely), the extent to which ten negative mood descriptors represented their mood during the past week. Lower scores ranging from 10–50 mean less negative affect. Pain catastrophizing was measured using the 13-item instrument PCS. Participants rate their feelings and thoughts regarding pain from 0 (not at all) to 4 (all the time). The total PCS score ranges from 0-to 52 (Osman et al., 2000). The PHQ-15 (Kroenke et al., 2002) assessed somatic symptom severity. It includes 15 medically unexplained physical symptoms. Participants rate the level they are bothered by a medical complaint. The total score is 30, with 5, 10, and 15 representing the cutoff for mild, moderate, and severe somatic symptom severity (Kroenke et al., 2002). The TMD model proposed by the Orofacial Pain Prospective Evaluation and Risk Assessment (OPPERA) study demonstrated that somatic symptoms, have a strong association with psychological distress and are among the psychological variables which predict the risk of new onset TMD (Bair et al., 2013; Fillingim et al., 2013; Fillingim et al., 2011; Slade et al., 2013a; Slade et al., 2013c).
Positive Valence Factors latent variable was adapted from the National Institute of Mental Health Research Domain Criteria framework in which specific psychological traits and characteristics indicate a phenotype (Insel, 2014). Specifically, positive affect and optimism are part of a broader construct in which motivation and reward seeking influence behavior (Colloca and Benedetti 2007; Sanislow et al., 2010; Wang et al., 2022). The latent variable was created by using the positive affect score from the 1) PANAS (Watson et al., 1988) and the 2) the optimism subscale from the life orientation test-revised (LOT-R) scale (Scheier et al., 1994). Positive Affect is a measure of enthusiasm, attentiveness, and drive and is linked to optimism and task persistence in patients with chronic musculoskeletal pain (Esteve et al., 2018; Watson et al., 1988; Zautra et al., 2005). Positive affect scores are derived from 10 items from the PANAS (Watson et al., 1988), ranging from 10–50. Participants ranked on a 5-point Likert scale of 1 (very slightly) to 5 (extremely), the extent to which 10 positive mood descriptors represented their mood during the past week. Higher scores indicate higher levels of positive affect. Optimism, a key personality trait that increases the likelihood of resilient outcomes, was measured using the life orientation test-revised (LOT-R) scale (Scheier et al., 1994). The 10-item scale consists of 3 optimism subscale questions, three pessimism subscale questions, and four filler items (Scheier et al., 1994). Each item is scored on a 5-point Likert scale of 0 (strongly disagree) to 4 strongly agree). The total score is comprised of direct score optimism items and reverse scored pessimism items. High total scores represent a higher level of dispositional optimism.
Our population’s demographic and clinical characteristics are presented in Table 1, including demographic variables such as sex, race, educational level, and the number of chronic overlapping pain conditions (COPCs). Some studies have found that TMD incidence and prevalence are greater among females and African Americans (Slade 2014; Slade et al., 2013b); however, the impact of socioeconomic status is somewhat mixed (Slade et al., 2011; Slade et al., 2013b). Likewise, the number of COPCs, namely irritable bowel syndrome, fibromyalgia, migraine headache, and low back pain, were included as they are believed to share common pathophysiology and risk factors that contribute to the onset and continuance of chronic pain conditions (Fillingim et al., 2020; Maixner et al., 2016; Ohrbach et al., 2020).
Table 1:
Demographic representation of the sample
| Variables N=400 | Mean (range) or Frequency n (%) |
|---|---|
| Age (years) | 41.3(18–65) |
| Sex | |
| Male | 94 (23.5) |
| Female | 306 (76.5) |
| Race | |
| American Indian | 1(0.2) |
| Asian | 29 (7.2) |
| African American | 141(35.2) |
| White | 202(50.4) |
| Mixed Race | 28 (7.0) |
| Education | |
| Did not complete High School | 0 |
| Completed High School | 50(12.5) |
| Some College | 104(26) |
| College Graduate | 138(34.5) |
| Professional or Postgraduate | 108(27) |
| Chronic Overlapping Pain Conditions | 178/222 |
| None | 222(55.5) |
| Migraine headache | 58(14.5) |
| Fibromyalgia | 20(5.0) |
| Irritable Bowel Syndrome | 15(3.8) |
| Low Back Pain | 136(34) |
| TMD symptoms onset in months | 142.5(3–480) |
2.4. Statistical Analyses
All variables were screened for normality. The proportion of missing data was small (1–6.5%, among the variables), and missing value analysis revealed that the data were missing completely at random (Little’s MCAR test: Chi-Square = 49, df = 47, p =.36; see also Supplementary Table A).
We used a structural equation modeling (SEM) approach that allowed us to create latent variables representing our research constructs. The latent variables are created by factor analysis using the overlapping variance of correlated measured variables. Latent variables are not directly measured but better represent our constructs because they contain less measurement error than the directly measured variables (Beran and Violato 2010). Using confirmatory factor analysis (CFA), we examined the strength of relationships between the latent variables jaw function, psychological constructs, and pain-related disability while controlling for the presence of chronic overlapping pain conditions (irritable bowel syndrome, fibromyalgia, migraines, and low back pain) and demographic covariates (sex, race, and education). All demographic covariates were made dichotomous in the analysis as follows: sex (M/F) with male coded as 1; race (white/non-white) recoded with white coded as 1; and education (college graduate/non-college graduate) recoded with college graduate coded as 1.
COPCs (fibromyalgia, irritable bowel syndrome, low back pain, and migraines) (Slade 2020) were captured from self-reported medical history and confirmed by a trained examiner during the in-person medical history review. A continuous variable was created as a count variable (0–4), capturing the number COPCs reported by each participant. Categorical variables may be analyzed as continuous variables if the data approximate a normal distribution and has four or more categories (Atkinson 1988; Bentler and Chou 1987); therefore, the number of COPCs was entered as a predictor into the model.
Four latent variables were constructed in the hypothesized measurement model (see Figure 2). Two observed variables, pain intensity and pain interference were the factors for the latent variable of pain-related disability. Jaw function limitation latent variable consisted of the JFLS subscales chewing, opening, and expression limitation measurement scores. Negative affect, pain catastrophizing, and somatic symptoms were the factors of the latent variable of psychological unease. The latent variable positive valence factors comprised positive affect and optimism scores.
Figure 2.

Hypothesized Model of Pain-Related Disability showing the associations between the latent variables and covariates. Structural equation modeling evaluated the relationships between observed variables, latent variables, the number of chronic overlapping pain conditions and demographic variables, sex, race, education. The relationships between latent and observed variables were tested using confirmatory factor analysis and are represented by arrows towards the corresponding observed variables. Circles represent latent variables, and rectangles represent observed variables. Solid arrows between the observed variables, latent variables, and covariates represent the relationships tested.
Confirmatory factor analysis of the latent variables in the measurement model was performed using maximum likelihood estimator, and goodness of fit indices and factor loadings were evaluated (Table 2). The extent to which the observed data fit the proposed model is reflected in the goodness of fit indices. Model fit was assessed using the chi-square test, comparative fit index (CFI), Tucker-Lewis index (TLI), root mean square error approximation (RMSEA), and standard root mean square residual (SRMR). The criteria for a well-fitting model were model, a p-value of chi-square > 0.05, CFI ≥.95, TLI ≥ .95, RMSEA <.08, and SRMR <.08 (Hu and Bentler 1999).
Table 2.
Results of confirmatory factor analysis of measurement model with factor loadings of psychological unease, positive valence factors, jaw limitation, and pain-related disability latent variables
| Variables | Model 1a | Model 2b |
|---|---|---|
| Pain disability | ||
| Pain Intensity | 0.779 | 0.747 |
| Pain Interference | 0.793 | 0.827 |
| Jaw function | ||
| Chewing Limitation | 0.857 | 0.874 |
| Opening Limitation | 0.902 | 0.817 |
| Expression Limitation | 0.822 | 0.915 |
| Psychological Unease | ||
| Negative Affect | 0.619 | 0.616 |
| Pain Catastrophizing | 0.616 | 0.613 |
| Somatic Symptoms | 0.612 | 0.617 |
| Positive Valence Factors | ||
| Positive Affect | 0.527 | 0.524 |
| Optimism | 0.847 | 0.853 |
| Model Fit Statistics | ||
| Chi-square (df) | 119.8(29) | 89.81(26) |
| P value | <.001 | <.001 |
| RMSEA (95% CI) | 0.088 (.072-.105) | 0.076 (.059-.094) |
| CFI | 0.941 | 0.960 |
| TLI | 0.92 | 0.933 |
| SRMR | 0.054 | 0.052 |
All variables in the proposed model.
Added error covariances between chewing limitation and opening limitation & chewing limitation and expression limitation.
Next, we tested the SEM model, which examined the relationships between the latent variables jaw limitation, psychological unease, positive valence factors, pain-related disability, and the number of COPCs, with the covariates, sex, race, and education, and evaluated global and local model fit and parameter estimates. Maximum likelihood estimator was used to assess the model fit, and global and local model fit were based on previously described indices. Data screening, exploration, and missing data analysis were conducted in SPSS version 27 (IBM 2020). All CFA and SEM analyses were performed in Mplus (Muthén and Muthén 2017). A p<0.05 was set as the level of significance.
In regards to sample size, a golden criteria is to have approximately 5–10 observations per parameter (Bentler and Chou 1987). For this study, there were 48 freely estimated parameters in the model. Based on these criteria, the sample size of 400 provided sufficient sample size for the SEM analysis.
3. RESULTS
3.1. Descriptive statistics
Four hundred participants were included in the analysis. Seventy-six percent of the sample was female, with African Americans comprising 35% of the sample. The pain intensity and pain interference scores were normally distributed with mean scores of 47.64(1.11 SE) and 27.47(1.35 SE) using a scale of 1–100, respectively, with a strong correlation between scores (r = .62, p <.001). Within our sample, TMD pain was characterized by 95% myalgias, 85% arthralgia 52%TMD headache.
Pain catastrophizing, negative affect, and somatic symptom scores were all normally distributed with medium-sized correlations (r=.33 to .43, p<.01) between these variables. Similarly, optimism and positive affect scores were normally distributed with a medium correlation between optimism and positive affect r =.45, p<.001. Jaw function limitation scores had an acceptable distribution, with jaw expression score having a skew/kurtosis value of 1.8 and 2.9, respectively. These were deemed acceptable as kurtosis was less than 3 (Finney and DiStefano 2013). The correlations between the jaw function variables were strong (r =.67 to .79) as expected, with the largest correlation (r =.79, p < .001) occurring between jaw chewing and jaw opening and the smallest occurring between expression limitation and chewing limitation (r = .67, p < .001). Correlations among the variables are reported in Supplemental table A.
Regarding COPCs, 15% of the sample reported migraines, 5% reported fibromyalgia, and 4% reported irritable bowel syndrome. Mean, minimum and maximum scores of observed variables are displayed in Supplementary Table B.
3.2. Structural Equation Model
The first step of SEM was the specification of the latent constructs of psychological unease, positive valence factors, jaw limitation, and pain-related disability with observed variables (see Figure 2). Confirmatory factor analysis was used to check that the concepts in the latent variables were valid as a single latent variable, and the factor loadings are shown in Table 2. Three exogenous latent variables, jaw function, psychological unease, and positive valence factors, and one endogenous latent variable, pain-related disability, were constructed. Results from the final model revealed factor loading values of 0.52 to 0.92, and the overall model fit was enhanced by adding error correlations. The final CFA can be found in Table 2 Model 2.
A structural model was constructed to examine the relationships between psychological unease, positive valence factors, and jaw limitation on pain-related disability with the covariates of chronic overlapping pain conditions (irritable bowel syndrome, fibromyalgia, and migraines) and demographic covariates (sex, race, and education). All variables and covariates were entered into the model, with significant relationships observed between the predictors and the outcome variable. Still, a sub-optimal model fit was noted (Model 1 (χ2 (234.16) = 63, RMSEA = 0.082 CFI = 0.899). Overall, model fit was greatly improved by allowing an additional error correlation between the number of COPCs and somatic symptoms. Studies have shown a strong association between somatic symptom burden and the number of COPCs (Fillingim et al., 2020). The final model revealed a good fit of the data with χ2 (160.01) = 62, RMSEA of 0.063, and CFI 0.942, indicating that the data was a good fit for the proposed model. The latent variables, jaw limitation, psychological unease, positive valence factors, and the number of COPCs were significant predictors of pain-related disability latent variable. The R-squared for the final model accounted for 68% of the variance in the latent variable pain-related disability. The final path estimates are reported in Table 3 and Supplemental Table C.
Table 3.
Structural equation model with path coefficients demonstrating the relationship between latent variables, covariates, and the latent variable pain-related disability
| Structural Model (β) | a Model 1 | b Model 2 |
|---|---|---|
| c UNEASE→ d PRD | 0.272 ** | 0.265 ** |
| e POSVAL→PRD | 0.159 * | 0.158 * |
| f JAWL→PRD | 0.671 *** | 0.670 *** |
| g COPC→PRD | 0.153 * | 0.165 *** |
| h Sex→PRD | −0.056 | −0.055 |
| i Race → PRD | −0.019 | −0.010 |
| j Education →PRD | − 0.088 * | −0.088 * |
| Model Fit Statistics | ||
| Chi-square (df) | 234.16 (63) | 160.01 (62) |
| P value | <.001 | <.001 |
| RMSEA (90% CI) | 0.082 (0.071–0.094) | 0.063 (0.051–0.075 |
| CFI | 0.899 | 0.942 |
| TLI | 0.864 | 0.921 |
| SRMR | 0.084 | 0.067 |
Full model with latent variables from confirmatory factor analysis, chronic overlapping pain conditions, and demographic variables. We allowed error correlations between the following pairs: chewing limitation with opening limitation, and expression limitation with chewing limitation.
We allowed error correlations between the following pairs: chewing limitation with opening limitation, expression limitation with chewing limitation, and COPCs and Somatic Symptoms.
UNEASE = Psychological Unease,
PRD = Pain-related disability,
POSVAL= Positive Valence Factors,
JAWL = Jaw function limitation,
COPC = chronic overlapping pain conditions,
female is referent,
non-white is referent,
educational level - non college graduate is referent.
p < .05,
p <.01,
p < .001
While all latent variables had significant relationships with a pain-related disability, the Jaw function limitation latent construct had the most substantial impact (β = .66, p <.001) on the outcome variable when compared with psychological unease (β = .31, p <.01) and positive valence factors (β=.16, p<.05). The number of COPCs also predicted (β = .16, p <.001) increased pain-related disability. When compared to non-college-educated, being a college graduate predicted less pain-related disability (β = −.088, p = .048). The demographic covariates sex, and race, were non-significant predictors of pain-related disability in this model.
4. DISCUSSION
The current cross-sectional study evaluated the role of psychological unease (pain catastrophizing, somatic symptoms, and negative affect), positive valence factors (optimism, positive affect), and jaw function limitation (chewing, opening, and expression), the number of COPCs on pain-related disability. SEM is a well-organized and parsimonious way of avoiding the repetitive testing of multiple regression equations while controlling for random error and the systematic error characteristic of common method variance (Beran and Violato 2010). We found significant associations between the positive and negative psychological constructs, COPC, and pain-related disability in chronic TMD participants. Jaw function limitation had the most substantial positive impact on pain-related disability, followed by psychological unease and positive valence factors among TMD patients. We also found that the number of COPC was positively associated with pain-related disability.
4.1. Jaw Function and Pain-Related Disability
Jaw dysfunction directly affects activities such as chewing, smiling, and laughing. It can profoundly impact an individual’s quality of life and psychosocial functioning (Ohrbach et al., 2008). Few studies have examined jaw dysfunction’s impact on TMD pain-related disability. In our study, the impact of jaw function was stronger than psychological unease on pain-related disability. This effect size is significantly larger than a similar study in which the effect of jaw function limitation associated with pain-related disability was less than that of the negative psychological construct (Miller et al., 2020). This difference may be due to Miller et al.’s (2020) latent variable of pain-related disability, which included the concept of work restriction and a decrease in work efficiency (Miller et al., 2020). While an earlier study found no significant relationship between oral parafunction and pain intensity and unpleasantness symptoms, pain interference was excluded (Davis et al., 2010). The substantial, strong impact of jaw function demonstrated in our study is further confirmed as similar strong correlations were seen in a construct combining TMD jaw function and GCPS disability points (Chantaracherd et al., 2015). The strength of the relationship between jaw function and disability indicates that resources should be focused on finding and providing practical strategies such as physical therapy, manual therapy applied to the craniomandibular structures, and exercise which can benefit jaw function (Asquini et al., 2021; Gil-Martinez et al., 2018; Paço et al., 2016). Furthermore, patient education and support in this area might be beneficial (Xu et al., 2021).
4.2. Psychological Unease and Pain-Related Disability
We found that the positive association between psychological unease (i.e. pain catastrophizing, negative affect, and somatic symptoms) and pain-related disability was less strong than that of jaw dysfunction and pain-related disability. On the contrary, a recent study reported that the impact of the negative psychological construct was larger than that of jaw function limitation (Miller et al., 2020). Several factors may have driven this difference including the indicator variables being used to create the psychological unease construct, demographic (i.e. younger participants for the Miller’s cohort) and clinical phenotypes (i.e. TMD duration). In our model, we did not include work absenteeism as a component of the pain-related disability variable. Yet, our findings confirm that psychological vulnerability remains an important contributor to pain disability and that all indicator variables contributed almost equally to the latent construct of psychological unease.
Pain catastrophizing increases the risk of pain persistence by 6-fold and is a significant limitation to treatment response in a patient with TMD (Litt and Porto 2013; Reiter et al., 2018), while negative affect and somatic symptoms are risk factors for TMD incidence (Fillingim et al., 2013). While the overall effect is less in this study, it does not negate the need for psychological treatment targets such as cognitive-behavioral therapies to address this issue (Litt and Porto 2013).
4.3. Positive Valence Factors and Pain-Related Disability
While this manuscript did not use the direct resilience measurement tool, we used the resilience traits of optimism and positive affect which are recognized as resilience traits and are believed to lead to improvement and perhaps protection against poor pain outcomes (Hassett and Finan 2016). We identified therefore a higher order construct that represents a phenotype in which motivation (such as escaping pain) and reward seeking (pain relief or pain suppression) produce behavior outcomes such as activity/pain avoidance or approach behaviors (Navratilova and Porreca 2014; Wiech and Tracey 2013).
Unexpectedly, there was a positive association between positive valence factors (optimism and positive affect) and pain-related disability. We hypothesized that the positive psychological factors would have an inverse and protective relationship with pain-related disability. The duration of TMD symptoms among our participants may have obscured positive valence factor’s effect on disability in this cross-sectional study. However, in sub-analyses, we controlled for the duration of symptoms, and the impact of positive valence factors on the disability construct remained unchanged. The protective effect of this construct on disability may be limited to the acute or subacute phases of pain rather than chronicity (Jegan et al., 2017). In our sample, the mean pain intensity scores were less than 50% of the maximum possible score and pain interference scores less than 30% of the maximum possible score on the GCPS suggesting ceiling effects on the role of positive affect on pain disability.
4.4. Chronic Overlapping conditions and Pain-Related Disability
An increasing number of COPCs (migraine headache, fibromyalgia, irritable bowel syndrome, or low back pain) was associated with increased pain disability. COPCs represent a cluster of pain disorders with no identifiable cause for pain persistence, but they share an underlying hypersensitivity of the central nervous system (Slade 2020). COPCs and TMD are reported to have similar and overlapping pain characteristics (Maixner et al., 2016). For example, patients with fibromyalgia reported widespread body pain in greater percentages than patients with TMD alone (Ohrbach et al., 2020). Psychological variables such as somatic symptoms, negative affect, and pain catastrophizing are strongly linked with COPCs (Fillingim et al., 2020). In addition, TMD characteristics such as masticatory function and nonspecific jaw symptoms are shared with other COPCs, with the association being most prominent for fibromyalgia (Sharma et al., 2020). Our finding confirms that independent of latent and demographic variables, the impact of COPCs on pain disability is significant. Control and management of both TMD and COPCs symptomology remain viable targets for intervention.
4.5. Sociodemographic variables and Pain-Related Disability
Sex and race had a minimal impact on this model. However, higher education trended with lower pain-related disability. Socioeconomic status variables such as race and ethnicity have had varying associations within chronic pain; however, our education findings are consistent with other data national data confirming that a higher educational level is associated with lower pain severity ratings and lower odds of high-impact pain (Anderson et al., 2009; Dahlhamer et al., 2018; Grol-Prokopczyk 2017; National Academies of Sciences and Medicine 2020). Higher education is associated with higher income, thus increasing access to health care and medications, health insurance, and treatment information (Magalhaes et al., 2014; Poleshuck and Green 2008). In addition, higher education has been linked to a higher cognitive reserve and more effective coping resources resulting in less pain disability (Gale et al., 2012; Gomez-Beldarrain et al., 2015; Stern 2009). Lower education increased risk of depressive symptoms in a sample of older adults with physical disability (McGiffin et al., 2019). Education level through improving access to financial and cognitive resources may lessen chronic pain’s impact on daily life and protect against disability.
4.6. Limitations and Strengths
The cross-sectional design of this study limits the ability to conclude the direction of the relationships between the variables or the effects of the predictor latent variables over time. In addition, all self-report survey tools carry a threat of social-desirability bias (Grimm 2010). Thus, the questionnaire data on constructs such as positive affect and optimism may not reflect actual states but desired states of the participants as they have a certain amount of wishful thinking, which may have influenced their optimism scores (Rauch et al., 2007).
In addition, longitudinal mediational analyses approach would be advantageous in determining whether the relationships between the relationships between latent constructs and the outcome causal and vary over time (Baron and Kenny 1986; O’Laughlin et al., 2018; Resnick and Inguito 2011). Further research is needed to address other positive valence factors which may modify chronic pain outcomes for TMD patients.
On the other hand, this study adds several tangible and innovative aspects. First, it quantifies the comparative effects of jaw dysfunction, COPCs, and positive and negative psychological constructs in pain outcomes. Second, it demonstrates that COPCs have a significant role in pain outcomes and should be evaluated and managed alongside TMD symptomology. Finally, positive valence factors such as optimism and positive affect in the pain disability model may be bidirectional and warrant further study.
5. CONCLUSION
This study provides helpful insights leading to the development of nonpharmacological chronic pain therapeutics for the prevention and clinical management of pain-related disability. We shed light on which critical factors clinicians should target in orofacial pain management. The use of strategies such as exercise, physical therapy, and psychological therapy, as well as the management of comorbid conditions, should be part of the overall therapeutic plan.
Supplementary Material
Figure 3.
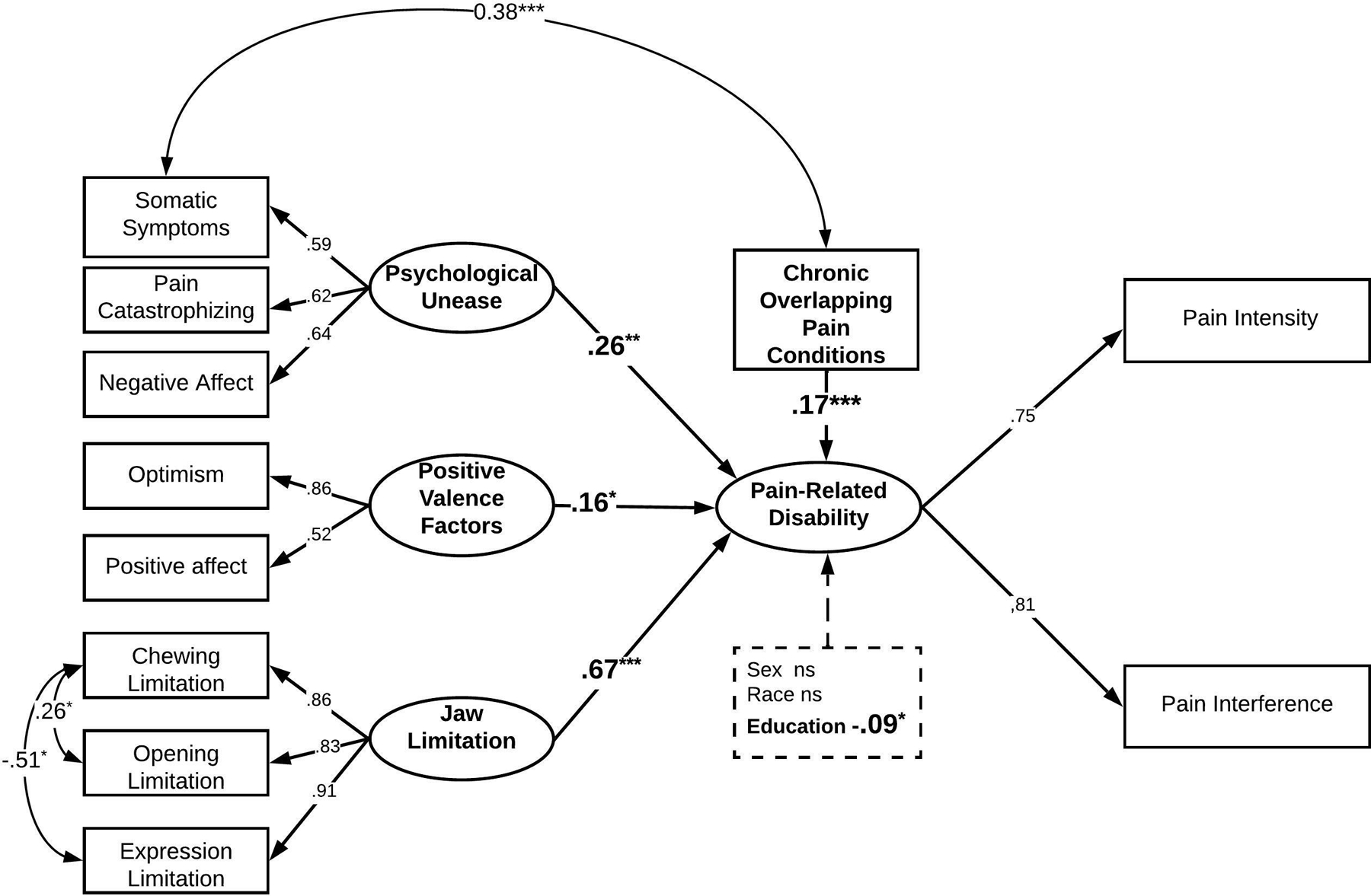
Final model with standardized parameter estimates demonstrating the significant relationships between latent variables and pain-related disability. A dashed line with significant parameter estimates bolded represents the relationship between demographic variables and pain-related disability. *p<.05, **p<.01, ***p<.001. Curved arrows represent error covariances between observed variables.
Acknowledgements.
We acknowledge our TMD patients for their time and wiliness to participate in our study, Dr Shijun Zhu PhD, DrE and Dr Paul Sacco PhD, MSW their help with the statistical parts of this project and Jane Phillips for conducting the screening to confirm the diagnosis of TMD.
Funding Sources
This research is supported by National Institute Dental Craniofacial Research, NIDCR (R01 DE025946, LC) and Biology and Behavior Across the Lifespan (BBAL) Facilitation award (ST). The funding agencies have no roles in the study. The views expressed here are the authors own and do not reflect the position or policy of the National Institutes of Health or any other part of the federal government.
This study was funded by NIDCR grant number 1R01DE025946–01 (PI Colloca) and by Biology and Behavior Across the Lifespan Facilitation award (PI: Thomas)
Footnotes
Conflicts of Interest: LC received in the past 5 years research grants from the US National Institutes of Health, and honoraria for lecturing, serving as an expert witness and/or panelist or consulting for methodological aspects from Chiesi, Averitas, Shionogi and Patient-Centered Outcomes Research Institute. ST has received Biology and Behavior Across the Lifespan Facilitation award, University of Maryland School of Nursing. The rest of the authors have no conflicts of interest to declare.
References
- Aaron LA, Burke MM, Buchwald D. Overlapping Conditions Among Patients With Chronic Fatigue Syndrome, Fibromyalgia, and Temporomandibular Disorder 2000;160. [DOI] [PubMed] [Google Scholar]
- Anderson KO, Green CR, Payne R. Racial and Ethnic Disparities in Pain: Causes and Consequences of Unequal Care. Journal of Pain 2009;10: 1187–1204. [DOI] [PubMed] [Google Scholar]
- Ankawi B, Slepian PM, Himawan LK, France CR. Validation of the Pain Resilience Scale in a Chronic Pain Sample. Journal of Pain 2017. [DOI] [PubMed] [Google Scholar]
- Asquini G, Rushton A, Pitance L, Heneghan N, Falla D. The effectiveness of manual therapy applied to craniomandibular structures in the treatment of temporomandibular disorders: protocol for a systematic review. Syst Rev 2021;10: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson L. The measurement-statistics controversy: Factor analysis and subinterval data. Bulletin of the Psychonomic Society 1988;26: 361–364. [Google Scholar]
- Bair E, Brownstein NC, Ohrbach R, Greenspan JD, Dubner R, Fillingim RB, Maixner W, Smith SB, Diatchenko L, Gonzalez Y, Gordon SM, Lim PF, Ribeiro-Dasilva M, Dampier D, Knott C, Slade GD. Study protocol, sample characteristics, and loss to follow-up: the OPPERA prospective cohort study. J Pain 2013;14: T2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM and Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology 1986. [DOI] [PubMed] [Google Scholar]
- Bartley EJ, LaGattuta NR, Robinson ME, Fillingim RB. Optimizing resilience in orofacial pain: a randomized controlled pilot study on hope. Pain Rep 2019;4: e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler PM and Chou C-P. Practical issues in structural modeling. Sociological methods & research 1987;16: 78–117. [Google Scholar]
- Beran TN and Violato C. Structural equation modeling in medical research: a primer. BMC Res Notes 2010;3: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantaracherd P, John MT, Hodges JS, Schiffman EL. Temporomandibular joint disorders’ impact on pain, function, and disability. Journal of Dental Research 2015;94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Slade G, Lim PF, Miller V, Maixner W, Diatchenko L. Relationship between temporomandibular disorders, widespread palpation tenderness, and multiple pain conditions: a case-control study. J Pain 2012;13: 1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L and Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol 2007;20: 435–439. [DOI] [PubMed] [Google Scholar]
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults — United States, 2016. MMWR Morbidity and Mortality Weekly Report 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CE, Carlson CR, Studts JL, Curran SL, Hoyle RH, Sherman JJ, Okeson JP. Use of a structural equation model for prediction of pain symptoms in patients with orofacial pain and temporomandibular disorders. Journal of orofacial pain 2010;24. [PubMed] [Google Scholar]
- de Leeuw R and Klasser GD. Diagnosis and Management of TMDs In: Fifth Edit. Chicago, Illinois: Quintessence Publishing Co Inc; 2013; 127–186. [Google Scholar]
- de Moraes Vieira EB, de Goes Salvetti M, Damiani LP, de Mattos Pimenta CA. Self-efficacy and fear avoidance beliefs in chronic low back pain patients: coexistence and associated factors. Pain Manag Nurs 2014;15: 593–602. [DOI] [PubMed] [Google Scholar]
- Esteve R, López-Martínez AE, Peters ML, Serrano-Ibáñez ER, Ruiz-Párraga GT, Ramírez-Maestre C. Optimism, Positive and Negative Affect, and Goal Adjustment Strategies: Their Relationship to Activity Patterns in Patients with Chronic Musculoskeletal Pain. Pain Research and Management 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain 2013;14: T75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, Baraian C, Slade GD, Maixner W. Potential psychosocial risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case-control study. Journal of Pain 2011;12: T27–T45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Ohrbach R, Greenspan JD, Sanders AE, Rathnayaka N, Maixner W, Slade GD. Associations of Psychologic Factors with Multiple Chronic Overlapping Pain Conditions. J Oral Facial Pain Headache 2020;34: s85–s100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Slade GD, Greenspan JD, Dubner R, Maixner W, Bair E, Ohrbach R. Long-term changes in biopsychosocial characteristics related to temporomandibular disorder: Findings from the OPPERA study. Pain 2018;159: 2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney SJ and DiStefano C. Non-normal and categorical data in structural equation modeling. Structural equation modeling: A second course 2013. [Google Scholar]
- Gale CR, Deary IJ, Cooper C, Batty DG. Intelligence in childhood and chronic widespread pain in middle age: the National Child Development Survey. Pain 2012;153: 2339–2344. [DOI] [PubMed] [Google Scholar]
- Gil-Martinez A, Paris-Alemany A, Lopez-de-Uralde-Villanueva I, La Touche R. Management of pain in patients with temporomandibular disorder (TMD): challenges and solutions. J Pain Res 2018;11: 571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Beldarrain M, Anton-Ladislao A, Aguirre-Larracoechea U, Oroz I, Garcia-Monco JC. Low cognitive reserve is associated with chronic migraine with medication overuse and poor quality of life. Cephalalgia 2015;35: 683–691. [DOI] [PubMed] [Google Scholar]
- Grimm P Social Desirability Bias, Part 2 Marketing Research 2010. [Google Scholar]
- Grol-Prokopczyk H. Sociodemographic disparities in chronic pain, based on 12-year longitudinal data. Pain 2017;158: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett AL and Finan PH.The role of resilience in the clinical management of chronic pain 2016. [DOI] [PubMed] [Google Scholar]
- Hu LT and Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling 1999;6. [Google Scholar]
- IBM.SPSS Statistics for Macintosh, Version 27.0; 2020. [Google Scholar]
- Insel TR (2014). The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. American Journal of Psychiatry, 171(4), 395–397. [DOI] [PubMed] [Google Scholar]
- Jegan NR, Brugger M, Viniol A, Strauch K, Barth J, Baum E, Leonhardt C, Becker A. Psychological risk and protective factors for disability in chronic low back pain - a longitudinal analysis in primary care. BMC Musculoskelet Disord 2017;18: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosomatic Medicine 2002;64. [DOI] [PubMed] [Google Scholar]
- Krogstad BS, Jokstad A, Dahl BL, Vassend O. Relationships between risk factors and treatment outcome in a group of patients with temporomandibular disorders. J Orofac Pain 1996;10: 48–53. [PubMed] [Google Scholar]
- List T and Jensen RH. Temporomandibular disorders: Old ideas and new concepts. Cephalalgia 2017;37: 692–704. [DOI] [PubMed] [Google Scholar]
- Litt MD and Porto FB. Determinants of pain treatment response and nonresponse: identification of TMD patient subgroups. J Pain 2013;14: 1502–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes BG, de-Sousa ST, de Mello VV, da-Silva-Barbosa AC, de-Assis-Morais MP, Barbosa-Vasconcelos MM, Caldas-Junior AD. Risk factors for temporomandibular disorder: binary logistic regression analysis. Med Oral Patol Oral Cir Bucal 2014;19: e232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD.Overlapping Chronic Pain Conditions: Implications for Diagnosis and Classification 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGiffin JN, Galatzer-Levy IR, Bonanno GA. Socioeconomic resources predict trajectories of depression and resilience following disability. Rehabil Psychol 2019;64: 98–103. [DOI] [PubMed] [Google Scholar]
- Miller VE, Chen D-G, Barrett D, Poole C, Golightly YM, Sanders AE, Ohrbach R, Greenspang JD, Fillingim RB, Slade GD. Understanding the relationship between features associated with pain-related disability in people with painful temporomandibular disorder: an exploratory structural equation modeling approach. Pain 2020;March: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK and Muthén BO. Mplus User’s Guide Eighth Edition. Los Angeles, CA: Muthén & Muthén; 2017. [Google Scholar]
- National Academies of Sciences E and Medicine. Temporomandibular Disorders: Priorities for Research and Care (2020) Washington DC: The National Academies Press. 2020. [PubMed] [Google Scholar]
- National Institute of D and Craniofacial R.Prevalence of TMJD and its Signs and Symptoms 2018. [Google Scholar]
- National Institute of Dental and Craniofacial Research. Facial Pain 2018; Available from: https://www.nidcr.nih.gov/research/data-statistics/facial-pain.
- Navratilova E and Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci 2014;17: 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Laughlin KD, Martin MJ, Ferrer E. Cross-Sectional Analysis of Longitudinal Mediation Processes. Multivariate Behav Res 2018;53: 375–402. [DOI] [PubMed] [Google Scholar]
- Ohrbach E and Knibbe W.Diagnostic Criteria for Temporomandibular Disorders: Scoring Manual for Self-Report Instruments Version 29May2016. 2016. [Google Scholar]
- Ohrbach R, Larsson P, List T. The jaw functional limitation scale: Development, reliability, and validity of 8-item and 20-item versions. Journal of Orofacial Pain 2008;22. [PubMed] [Google Scholar]
- Ohrbach R, Sharma S, Fillingim RB, Greenspan JD, Rosen JD, Slade GD. Clinical Characteristics of Pain Among Five Chronic Overlapping Pain Conditions. J Oral Facial Pain Headache 2020;34: s29–s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The pain catastrophizing scale: Further psychometric evaluation with adult samples. Journal of Behavioral Medicine 2000. [DOI] [PubMed] [Google Scholar]
- Paço M, Peleteiro B, Duarte J, Pinho T. The Effectiveness of Physiotherapy in the Management of Temporomandibular Disorders: A Systematic Review and Meta-analysis. J Oral Facial Pain Headache 2016;30: 210–220. [DOI] [PubMed] [Google Scholar]
- Plesh O, Wolfe F, Lane N. The relationship between fibromyalgia and temporomandibular disorders: Prevalence and symptom severity. Journal of Rheumatology 1996;23: 1948–1952. [PubMed] [Google Scholar]
- Poleshuck EL and Green CR. Socioeconomic disadvantage and pain. Pain 2008;136: 235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch WA, Schweizer K, Moosbrugger H. Method effects due to social desirability as a parsimonious explanation of the deviation from unidimensionality in LOT-R scores. Personality and Individual Differences 2007;42: 1597–1607. [Google Scholar]
- Reiter S, Eli I, Mahameed M, Emodi-Perlman A, Friedman-Rubin P, Reiter MA, Winocur E. Pain Catastrophizing and Pain Persistence in Temporomandibular Disorder Patients. J Oral Facial Pain Headache 2018;32: 309–320. [DOI] [PubMed] [Google Scholar]
- Resnick BA and Inguito PL. The Resilience Scale: Psychometric Properties and Clinical Applicability in Older Adults. Archives of Psychiatric Nursing 2011. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Durham J, Newton JL. A systematic review of the comorbidity between Temporomandibular Disorders and Chronic Fatigue Syndrome. J Oral Rehabil 2016;43: 306–316. [DOI] [PubMed] [Google Scholar]
- Sanders AE, Slade GD, Bair E, Fillingim RB, Knott C, Dubner R, Greenspan JD, Maixner W, Ohrbach R. General health status and incidence of first-onset temporomandibular disorder: the OPPERA prospective cohort study. J Pain 2013;14: T51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Wang PS, Cuthbert BN. Developing constructs for psychopathology research: research domain criteria. J Abnorm Psychol 2010;119: 631–639. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing Optimism From Neuroticism (and Trait Anxiety, Self-Mastery, and Self-Esteem): A Reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology 1994. [DOI] [PubMed] [Google Scholar]
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet PJ, List T, Svensson P, Gonzalez Y, Lobbezoo F, Michelotti A, Brooks S, Ceusters W, Drangsholt M, Ettlin D, Gaul C, Goldberg L, Haythornthwaite J, Hollender L, Jensen R, John M, Delaat RA, Maixner W, Meulen M, Murray G, Nixdorf D, Palla S, Petersson A, Pionchon P, Smith, Visscher C, Zakrzewska J, Dworkin. Diagnostic Criteria for the Most Common Temporomandibular Disorders: Symptom Questionnaire and Clinical Examination Items. ournal of Oral & Facial Pain and Headache 2014;28: 6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman EL, Ahmad M, Hollender L, Kartha K, Ohrbach R, Truelove EL, Zhang L, Hodges JS, Sommers E, Anderson GC, Gonzalez YM, Guo X, Look JO. Longitudinal Stability of Common TMJ Structural Disorders. Journal of Dental Research 2017;96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Slade G, Fillingim R, Greenspan J, Rathnayaka N, Ohrbach R. Attributes Germane to Temporomandibular Disorders and Their Associations with Five Chronic Overlapping Pain Conditions. Journal of Oral & Facial Pain and Headache 2020;34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade G. Overlap of Five Chronic Pain Conditions: Temporomandibular Disorders, Headache, Back Pain, Irritable Bowel Syndrome, and Fibromyalgia. Journal of Oral & Facial Pain and Headache 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD. Epidemiology of temporomandibular joint disorders and related painful conditions. Molecular Pain 2014. [Google Scholar]
- Slade GD, Bair E, By K, Mulkey F, Baraian C, Rothwell R, Reynolds M, Miller V, Gonzalez Y, Gordon S, Ribeiro-Dasilva M, Lim PF, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Dampier D, Knott C, Ohrbach R. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J Pain 2011;12: T12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Knott C, Ohrbach R. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain 2013a;14: T20–32 e21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Knott C, Ohrbach R, Hill C, Hill C, Carolina N. Signs and Symptoms of First-Onset TMD and Sociodemographic Predictors of Its Development: The OPPERA Prospective Cohort Study. YJPAI 2013b;14: T20–T32.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD, Fillingim RB, Sanders AE, Bair E, Greenspan JD, Ohrbach R, Dubner R, Diatchenko L, Smith SB, Knott C, Maixner W. Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibular disorder: implications and future directions. J Pain 2013c;14: T116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia 2009;47: 2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgeon JA and Zautra AJ. Psychological resilience, pain catastrophizing, and positive emotions: Perspectives on comprehensive modeling of individual pain adaptation topical collection on psychiatric management of pain. Current Pain and Headache Reports 2013;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MJL, Bishop SR, Pivot J. The Pain Catastrophizing Scale: Development and Validation. Psychological Assessment 1995;7: 524–532. [Google Scholar]
- Turk DC, Fillingim RB, Ohrbach R, Patel KV. Assessment of Psychosocial and Functional Impact of Chronic Pain. Journal of Pain 2016. [DOI] [PubMed] [Google Scholar]
- Von Korff M Assessment of chronic pain in epidemiological and health services research: Empirical bases and new directions. In: Handbook of Pain Assessment New York: Guilford Press; 2011; 455–473. [Google Scholar]
- Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain 1992;50: 133–149. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan E, Dorsey SG, Campbell CM, Colloca L. Who are the placebo responders? A cross-sectional cohort study for psychological determinants. Pain 2022;163: 1078–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology 1988;54. [DOI] [PubMed] [Google Scholar]
- Wiech K and Tracey I. Pain, decisions, and actions: a motivational perspective. Front Neurosci 2013;7: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Cai B, Lu S, Fan S, Dai K. The Impact of Education and Physical Therapy on Oral Behaviour in Patients with Temporomandibular Disorder: A Preliminary Study. Biomed Res Int 2021;2021: 6666680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Yang Y, Dutra EH, Robinson JL, Wadhwa S, Yadav Bds S, Associate P. Temporomandibular Joint Disorders in the Elderly and Aging Population HHS Public Access. J Am Geriatr Soc 2018;66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. Journal of Consulting and Clinical Psychology 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


