Abstract
Jejunal biopsies from volunteers challenged with Cryptosporidium parvum were examined for tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1β mRNA. Postchallenge biopsies from 15 of 28 (54%) volunteers expressed TNF-α; 14% expressed IL-1β. Cytokine expression did not correlate with enteric symptoms, suggesting that TNF-α and IL-1β are not key mediators of diarrhea in human cryptosporidiosis.
Cryptosporidiosis is characterized by watery diarrhea. The mechanisms by which Cryptosporidium parvum causes diarrhea have not been clearly defined. Some studies suggested that disease was mediated by an enterotoxin (8, 9). However, no parasite enterotoxin has been identified. Others have suggested that host molecules might account for enterotoxin-like activity. In a porcine model, C. parvum infection was associated with increased chloride secretion as well as with sodium and glucose malabsorption (1–3). The altered chloride secretion and solute malabsorption were inhibited by prostaglandin synthesis inhibitors and were reproduced by the addition of prostaglandins (1). Prostaglandins are produced by the intestinal wall in response to proinflammatory cytokines or C. parvum infection (1, 12).
The proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1β are key stimulators of prostaglandin synthesis. In cryptosporidiosis, TNF-α has been detected in the gut in porcine and human xenograft models (11, 20). Treatment of porcine mucosa with TNF-α mimicked the effects of C. parvum infection (11). This response was reversible with prostaglandin synthesis inhibitors. Similarly, IL-1β can induce intestinal chloride secretion via prostaglandins (10).
These same proinflammatory cytokines could also directly alter the mucosal epithelial integrity, leading to diminished barrier function (14, 22). The increased permeability may result in back diffusion of ions and water into the gut lumen, thereby contributing to a greater loss in gastrointestinal fluids. Altered barrier function has been documented in several studies of human cryptosporidiosis (7, 13, 24). Some studies have noted proinflammatory cytokines in biopsies from AIDS patients with chronic cryptosporidiosis (17). However, enzyme-linked immunosorbent assays (ELISAs) did not identify TNF-α protein in stools of patients with cryptosporidiosis (21).
To clarify the role of TNF-α and IL-1β in the pathogenesis of Cryptosporidium-induced diarrhea, we studied the expression of these cytokines in jejunal intestinal biopsy specimens from normal volunteers experimentally challenged with C. parvum. Cytokine expression was then analyzed in relation to the development and timing of symptoms and of oocyst shedding.
Thirty immunocompetent volunteers were challenged with defined doses of C. parvum oocysts as described previously (4, 5, 15, 16). Ten prechallenge biopsies were available (from 10 volunteers, including 2 with only prechallenge biopsies). Thirty-eight postchallenge biopsies from 28 volunteers (obtained from 1 to 31 days postchallenge) were examined for TNF-α, and 21 of these biopsies from 21 volunteers were also examined for IL-1β. Prechallenge anti-C. parvum immunoglobulin G and immunoglobulin M antibody status was screened by ELISA using oocyst antigen (5). Oocyst excretion was measured and quantitated by direct immunofluorescence (6). The volunteers recorded all enteric symptoms for 6 weeks postchallenge. Jejunal biopsies were obtained as previously described (19, 23), fixed in diethyl pyrocarbonate-treated paraformaldehyde, and stored in 70% alcohol until sectioning. Bluescript SK(−) plasmids containing cDNA for human TNF-α and IL-1β (American Type Culture Collection, Manassas, Va.) were prepared using ion-exchange chromatography (Qiagen Inc., Chatsworth, Calif.) (18, 23). The plasmid cDNA was linearized with the appropriate restriction enzymes. Sense and antisense RNA probes were synthesized by in vitro transcription using the appropriate polymerases in the presence of [35S]-UTP (TNF-α [sense, HindIII, SP6 polymerase; antisense, HincII, T7 polymerase] and IL-1β [sense, KpnI, T3 polymerase; antisense, EcoRI, T7 polymerase]). In situ hybridization was performed on paraffin-embedded jejunal biopsy tissue sections (19, 23). The concentration of probe providing an optimal positive signal with minimal background was assessed for each probe using peripheral blood mononuclear cells stimulated with lipopolysaccharide and phytohemagglutinin as the positive control. The sense strand of each probe was used as the negative control. The slides were examined by bright-field microscopy, and the number of cells overlaid with numerous silver granules was counted. The number of positive cells was quantitated as previously described (19, 23).
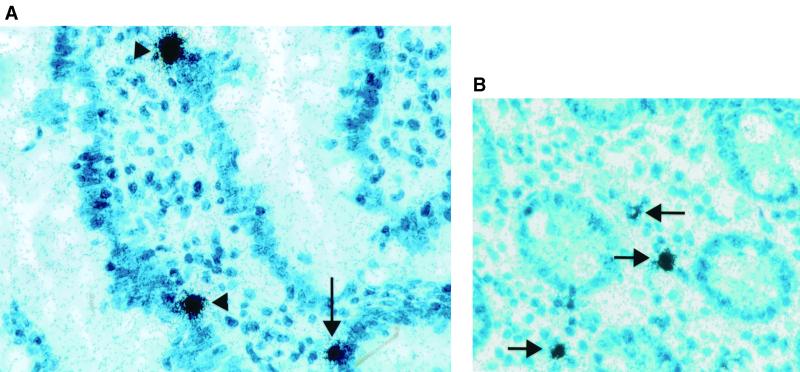
TNF-α mRNA was detected on 16 of 38 (42%) postchallenge biopsies, compared to only 1 of 10 prechallenge biopsies. Fifteen of 28 (54%) volunteers had at least 1 biopsy demonstrating TNF-α mRNA. TNF-α was primarily observed overlying cells in the lamina propria of both crypts and villi (Fig. 1). IL-1β mRNA was detected in only 3 of 21 (14%) postchallenge biopsies, which was not significantly different from prechallenge.
FIG. 1.
Jejunal biopsies from normal volunteers with experimental human cryptosporidiosis were probed by in situ hybridization using [35S]-labeled riboprobes for cytokine mRNA. Cytokine mRNA is detected as numerous black-silver grains overlying cells primarily in the lamina propria of the villi (arrowhead) and crypts (arrows). The original magnification of both photographs was 500×. (A) TNF-α. (B) IL-1β.
While expression increased after exposure to C. parvum, TNF-α was not associated with illness. TNF-α was detected in biopsies from 12 of 23 (52%) symptomatic and from 3 of 5 (60%) asymptomatic volunteers (P = 1.0, Fisher's exact test). Among the symptomatic volunteers, TNF-α expression was not significantly different for biopsies obtained before, during, or after the period of symptoms (3 of 12 [25%], 4 of 8 [50%], or 5 of 11 [45%], respectively). Overall, 4 of 11 biopsies obtained from days 6 to 13 postchallenge (near the time of symptoms) expressed TNF-α compared to 4 of 11 obtained earlier and 8 of 16 obtained 14 or more days postchallenge. There was also no relationship between the quantity of cells expressing TNF-α mRNA and either the presence or timing of symptoms. Thus, TNF-α expression was not associated with either the presence or timing of symptoms. Likewise, postchallenge biopsies from 1 of 3 asymptomatic and 2 of 18 symptomatic volunteers expressed IL-1β (P = 0.39, Fisher's test). Thus, IL-1β was also not associated with symptoms. The number of positive biopsies was too small to comment on the timing of expression.
Both seropositive and seronegative volunteers expressed TNF-α and IL-1β. TNF-α was found in 7 of 11 (64%) seropositive and 8 of 17 (47%) seronegative volunteers (P = 0.39, chi-square test). IL-1β was found in 1 of 10 (10%) seropositive and 3 of 14 (21%) seronegative volunteers (P = 0.61, Fisher's test). Neither TNF-α nor IL-1β significantly correlated with the presence or absence of oocyst shedding (Table 1). There was also no association with the challenge dose.
TABLE 1.
Biopsies from immunocompetent volunteers challenged with C. parvum were probed for expression of TNF-α and IL-1β mRNA
| Category of volunteers | TNF-α positive/total (%) | IL-1β positive/total (%) |
|---|---|---|
| Volunteers with prechallenge biopsies | 1/10 (10) | 1/7 (14) |
| Volunteers with postchallenge biopsies | 15/28 (54) | 3/21 (14) |
| Volunteers who shed oocysts | 7/13 (54) | 1/9 (11) |
| Volunteers not shedding oocysts | 8/15 (53) | 2/12 (16) |
| Asymptomatic volunteers | 3/5 (60) | 1/3 (33) |
| Symptomatic volunteers | 12/23 (52) | 2/18 (11) |
| Biopsies before symptoms | 3/12 (25) | 1/6 (16) |
| Biopsies during symptoms | 4/8 (50) | 1/6 (16) |
| Biopsies after symptoms | 5/11 (45) | 0/9 (0) |
In summary, we noted increased expression of TNF-α but not of IL-1β in response to the C. parvum challenge. This is consistent with prior data from model infections of pigs and of human intestinal xenographs (11, 20). Others have not found TNF-α protein in stool by ELISA (21). This may be a reflection of greater sensitivity of our assay. Alternatively, since TNF-α was primarily found in the lamina propria, it may not be transported into the gut lumen or may be degraded during the passage from the site of infection in the small bowel to the stool.
Previously, Kandil and colleagues suggested that TNF-α is a major mediator of diarrhea in porcine cryptosporidiosis (11). In contrast, our data demonstrate that neither TNF-α nor IL-1β was significantly associated with symptoms in human volunteers. We cannot exclude the possibility that TNF-α or IL-1β may synergize some unmeasured factor to cause diarrhea in cryptosporidiosis. However, our data suggest that it is unlikely that the diarrhea in human cryptosporidiosis is primarily mediated by these cytokines.
Interestingly, similar proportions of patients who were uninfected (asymptomatic and no oocysts shed), infected (oocyst positive), and presumably infected (symptomatic without oocysts) expressed TNF-α. This contrasts with prior data that demonstrated an association between expression of gamma interferon and prevention of oocyst shedding (23) and an association of IL-15 with control of oocyst shedding in seronegative volunteers (P. Robinson et al., submitted for publication). Indeed, expression of TNF-α did not correlate with expression of either gamma interferon or IL-15. While TNF-α expression correlated with C. parvum exposure, it is not clear whether it plays a role in clearing the parasite or is merely an incidental finding. For now we can conclude that expression of TNF-α is not sufficient to explain the pathogenesis of diarrhea in cryptosporidiosis.
Acknowledgments
These studies were approved by the committee for the protection of human subjects at the University of Texas at Houston and the Institutional Review Board for Human Subjects at Baylor College of Medicine. We thank Stanley Cron for assistance with the statistical analysis.
Grant support was provided by the National Institutes of Health (Baylor Center for AIDS Research [AI36211], RO1 AI41735, and the General Clinical Research Centers [RR02558]) and the U.S. Environmental Protection Agency (CR819814).
REFERENCES
- 1.Argenzio R A, Lecce J, Powell D W. Prostanoids inhibit intestinal NaCl absorption in experimental porcine cryptosporidiosis. Gastroenterology. 1993;104:440–447. doi: 10.1016/0016-5085(93)90412-6. [DOI] [PubMed] [Google Scholar]
- 2.Argenzio R A, Liacos J A, Levy M L, Meuten D J, Lecce J G, Powell D W. Villous atrophy, crypt hyperplasia, cellular infiltration, and impaired glucose-Na absorption in enteric cryptosporidiosis of pigs. Gastroenterology. 1990;98:1129–1140. doi: 10.1016/0016-5085(90)90325-u. [DOI] [PubMed] [Google Scholar]
- 3.Argenzio R A, Rhoads J M, Armstrong M, Gomez G. Glutamine stimulates prostaglandin-sensitive Na(+)-H+ exchange in experimental porcine cryptosporidiosis. Gastroenterology. 1994;106:1418–1428. doi: 10.1016/0016-5085(94)90393-x. [DOI] [PubMed] [Google Scholar]
- 4.Chappell C L, Okhuysen P C, Sterling C R, Wang C, Jakubowski W, Dupont H L. Infectivity of Cryptosporidium parvum in healthy adults with pre-existing anti-C. parvum serum immunoglobulin G. Am J Trop Med Hyg. 1999;60:157–164. doi: 10.4269/ajtmh.1999.60.157. [DOI] [PubMed] [Google Scholar]
- 5.DuPont H L, Chappell C L, Sterling C R, Okhuysen P C, Rose J B, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 6.Goodgame R W, Genta R M, White A C, Chappell C L. Intensity of infection in AIDS-associated cryptosporidiosis. J Infect Dis. 1993;167:704–709. doi: 10.1093/infdis/167.3.704. [DOI] [PubMed] [Google Scholar]
- 7.Goodgame R W, Kimball K, Ou C N, White A C, Jr, Genta R M, Lifschitz C H, Chappell C L. Intestinal function and injury in acquired immunodeficiency syndrome-related cryptosporidiosis. Gastroenterology. 1995;108:1075–1082. doi: 10.1016/0016-5085(95)90205-8. [DOI] [PubMed] [Google Scholar]
- 8.Guarino A, Canani R B, Casola A, Pozio E, Russo R, Bruzzese E, Fontana M, Rubino A. Human intestinal cryptosporidiosis: secretory diarrhea and enterotoxic activity in Caco-2 cells. J Infect Dis. 1995;171:976–983. doi: 10.1093/infdis/171.4.976. [DOI] [PubMed] [Google Scholar]
- 9.Guarino A, Canani R B, Pozio E, Terracciano L, Albano F, Mazzeo M. Enterotoxic effect of stool supernatant of Cryptosporidium-infected calves on human jejunum. Gastroenterology. 1994;106:28–34. doi: 10.1016/S0016-5085(94)94093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Homaidan F R, Zhao L, Chakroun I, Martin C A, Burakoff R. The mechanisms of action of interleukin-1 on rabbit intestinal epithelial cells. Mediat Inflamm. 1999;8:189–197. doi: 10.1080/09629359990342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandil H M, Berschneider H M, Argenzio R A. Tumour necrosis factor alpha changes porcine intestinal ion transport through a paracrine mechanism involving prostaglandins. Gut. 1994;35:934–940. doi: 10.1136/gut.35.7.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurent F, Kagnoff M F, Savidge T C, Naciri M, Eckmann L. Human intestinal epithelial cells respond to Cryptosporidium parvum infection with increased prostaglandin H synthase 2 expression and prostaglandin E2 and F2α production. Infect Immun. 1998;66:1787–1790. doi: 10.1128/iai.66.4.1787-1790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima A A, Silva T M, Gifoni A M, Barrett L J, McAuliffe I T, Bao Y, Fox J W, Fedorko D P, Guerrant R L. Mucosal injury and disruption of intestinal barrier function in HIV-infected individuals with and without diarrhea and cryptosporidiosis in northeast Brazil. Am J Gastroenterol. 1997;92:1861–1866. [PubMed] [Google Scholar]
- 14.Madara J L, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Investig. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okhuysen P C, Chappell C L, Crabb J, Valdez L M, Douglass E T, DuPont H L. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin Infect Dis. 1998;26:1324–1329. doi: 10.1086/516374. [DOI] [PubMed] [Google Scholar]
- 16.Okhuysen P C, Chappell C L, Crabb J H, Sterling C R, DuPont H L. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J Infect Dis. 1999;180:1275–1281. doi: 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- 17.Reka S, Garro M L, Kotler D P. Variation in the expression of human immunodeficiency virus RNA and cytokine mRNA in rectal mucosa during the progression of infection. Lymphokine Cytokine Res. 1994;13:391–398. [PubMed] [Google Scholar]
- 18.Robinson P, Atmar R L, Lewis D E, White A C., Jr Granuloma cytokines in murine cysticercosis. Infect Immun. 1997;65:2925–2931. doi: 10.1128/iai.65.7.2925-2931.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson P, Okhuysen P C, Chappell C L, Lewis D E, Shahab I, Lahoti S, White A C., Jr Transforming growth factor β1 is expressed in the jejunum after experimental Cryptosporidium parvum infection in humans. Infect Immun. 2000;68:5405–5407. doi: 10.1128/iai.68.9.5405-5407.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seydel K B, Zhang T, Champion G A, Fichtenbaum C, Swanson P E, Tzipori S, Griffiths J K, Stanley S L., Jr Cryptosporidium parvum infection of human intestinal xenografts in SCID mice induces production of human tumor necrosis factor alpha and interleukin-8. Infect Immun. 1998;66:2379–2382. doi: 10.1128/iai.66.5.2379-2382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharpstone D R, Rowbottom A W, Nelson M R, Lepper M W, Gazzard B G. Faecal tumour necrosis factor-alpha in individuals with HIV-related diarrhoea. AIDS. 1996;10:989–994. doi: 10.1097/00002030-199610090-00009. [DOI] [PubMed] [Google Scholar]
- 22.Stockmann M, Schmitz H, Fromm M, Schmidt W, Rokos K, Pauli G, Scholz P, Riecken E O, Schulzke J D. The mechanism of diarrhea in HIV is based on an impaired epithelial barrier function that could be induced by a specific cytokine pattern. Ann N Y Acad Sci. 1998;859:267–270. doi: 10.1111/j.1749-6632.1998.tb11143.x. [DOI] [PubMed] [Google Scholar]
- 23.White A C, Jr, Robinson P, Okhuysen P, Lewis D, Shahab I, Lahoti S, DuPont H L, Chappell C. Interferon gamma expression in jejunal biopsies in experimental human cryptosporidiosis correlates with prior sensitization and control of oocyst excretion. J Infect Dis. 2000;181:701–709. doi: 10.1086/315261. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Lee B, Thompson M, Glass R, Lee R C, Figueroa D, Gilman R, Taylor D, Stephenson C. Lactulose-mannitol intestinal permeability test in children with diarrhea caused by rotavirus and cryptosporidium. Diarrhea Working Group, Peru. J Pediatr Gastroenterol Nutr. 2000;31:16–21. doi: 10.1097/00005176-200007000-00006. [DOI] [PubMed] [Google Scholar]