Abstract
Baihuasheshecao (Hedyotis diffusa), a Chinese herb for cancer treatment, is frequently adulterated by a related species Hedyotis corymbosa. DNA sequencing of the complete internal transcribed spacer region was applied to differentiate H. diffusa from H. corymbosa and other closely related species. The molecular data showed that four out of seven herb samples of Baihuasheshecao were adulterants. Chemical analyses by TLC and HPLC were used to authenticate H. diffusa and H. corymbosa. Two marker compounds were identified exclusively in H. diffusa: 6-O-(E)-p-coumaroyl scandoside methyl ester (compound 1) and 10(S)-hydroxypheophytin a (compound 2). Both compounds showed moderate anti-proliferation effect on PC3 human androgen-independent prostate cancer cells, while compound 2 also showed strong anti-proliferation effect on LNCaP human androgen-sensitive prostate cancer cells. Accordingly, these bioactive marker compounds could be applied to verify the authenticity and assess the quality of Baihuasheshecao.
Keywords: Hedyotis diffusa, Hedyotis corymbosa, Baihuasheshecao, Internal transcribed spacer, 6-O-(E)-p-coumaroyl scandoside methyl ester, 10(S)-hydroxypheophytin a
1. Introduction
Baihuasheshecao is an herb derived from the whole plant of Hedyotis diffusa Willd. in the family Rubiaceae. The Pharmacopoeia of the People’s Republic of China (2005) chose to use the name Oldenlandia diffusa (Willd.) Roxb., which is regarded as a synonym by Flora Republicae Popularis Sinicae (Ko, 1999). It is commonly used in the Orient and tropical Asia (Ahmad et al., 2005; Perry, 1980), for making teas and botanicals for the relief of ‘heat’, removal of ‘toxins’ and promotion of diuresis to eliminate ‘wetness-evil’. It is used also in the West (Kane, Kane, & Jain, 1995). A more direct application is for the treatment of prostate cancer and other tumors (Liang, 2004). The anti-cancer properties of Baihuasheshecao have generated not a few studies (Gupta, Zhang, Yi, & Shao, 2004; Willimott, Barker, Jones, & Opara, 2007). Its anti-cancer activities are related to immuno-stimulating activity on the immune system (Shan, Zhang, Du, & Li, 2001), superoxide burst and caspase activation (Yadav & Lee, 2006).
In the market, Baihuasheshecao is frequently adulterated by a related species Hedyotis corymbosa (L.) Lam (Zhao & Li, 2005). They share similar morphological characters but the functions and efficacies of these two herbs are not quite the same (Lin, Ng, & Yang, 2004). In order to ensure effective and correct use of Baihuasheshecao, it is necessary to develop efficient methods and reliable markers for the authentication and quality control of Baihuasheshecao. Various approaches have been applied to differentiate the genuine Baihuasheshecao derived from H. diffusa from the adulterant derived from H. corymbosa and related species. The two species look very much alike even when fresh, differing only by the variable number of flowers, length of pedicels and shape of stems (Ko, 1999). These characters become unapparent when dried and dispensed in short fragments. Various attempts were made using chemical methods to authenticate Baihuasheshecao by thin layer chromatography (TLC) (Xie, Zhang, & Lu, 1997) and high performance liquid chromatography (HPLC) (Liang, He, Fong, Jiang, & Zhao, 2008; Liang, Jiang, Leung, & Zhao, 2006b). However, these studies relied on unidentified spots or markers with no reference to biological functions. DNA sequences of the internal transcribed spacer 1 (ITS-1) and internal transcribed spacer 2 (ITS-2) regions were separately proposed to distinguish Baihuasheshecao derived from H. diffusa from H. corymbosa (Hao, Liu, & Wang, 2004; Liu & Hao, 2005; Liu, Hao, & Wang, 2004). The accuracy of these sequences, however, has not been confirmed. Therefore, it is necessary to overhaul the molecular and chemical analyses for authentication of Baihuasheshecao.
In this study, we applied molecular authentication of Baihuasheshecao using the whole region of internal transcribed spacer (ITS) to distinguish H. diffusa from H. corymbosa and other Hedyotis species. Bioactive chemical markers with anti-proliferation effects on prostate cancer cells were identified from H. diffusa. These markers could be used for identification and quality assessment of Baihuasheshecao.
2. Materials and methods
2.1. Samples studied
A total of 21 voucher specimens, consisting of 14 Hedyotis species, and seven herb samples of Baihuasheshecao retailed in the market were included in this study. Leaves were picked from 14 voucher specimens deposited in Hong Kong Herbarium, Agriculture, Fisheries and Conservation Department, for molecular study. The names of these specimens are listed here together with the collectors’ number and the corresponding NCBI Genbank accession number of the ITS region in a bracket: Hedyotis auricularia L. (Y.W. Lam 1545, EF570980; Y.W. Lam 1018, EF570979), Hedyotis biflora (L.) Lam. (Y.W. Lam 1478, EF570993), Hedyotis bracteosa Hance (Y.S. Lau 3195, EF570998; K.L. Yip 4084, EF570997), Hedyotis consanguinea Hance (B. Walden, EF570995), Hedyotis costata (Roxb.) Kurz (Y.W. Lam 1248, EF570982), Hedyotis effusa Hance (Y.W. Lam 1241, EF570994), Hedyotis pinifolia Wall. ex G. Don (F. Yip, EF570989), Hedyotis shiuyingiae T. Chen (Y.W. Lam 245, EF570999; F.W. Xing 6937, EF571000), Hedyotis tenelliflora Blume (Y.W. Lam 898, EF570990), Hedyotis verticillata (L.) Lam (Y.W. Lam 878, EF570991; Y.W. Lam 909, EF570992). Seven fresh plant samples were collected in the field in Hong Kong and their voucher specimens were deposited in the Herbarium of the Department of Biology, The Chinese University of Hong Kong (CUHK): Hedyotis acutangula Champ. (S.Y. Hu & P. But 24053, EF570996), Hedyotis corymbosa (L.) Lam. (S.Y. Hu & P. But 24052, EF570974; W.L. Chu 005, EF570975), Hedyotis diffusa Willd. (M. Li 041, EF570985; M. Li 035, EF570986; M. Li 042, EF570988), and Hedyotis hedyotidea (DC.) Merr. (M. Li 044, EF570981). The identities of the samples of H. diffusa and H. corymbosa were double confirmed by Hong Kong Herbarium. Seven dried herb samples purchased from Guangzhou (China), Hong Kong (China) and Boston (USA) were deposited in the Museum of Chinese Medicine, Institute of Chinese Medicine, The Chinese University of Hong Kong. They are listed here together with their accession number in the Museum and source placed in a bracket: Baihuasheshecao 1 (2005–2686; Guangzhou), Baihuasheshecao 2 (2005–2687; Guangzhou), Baihuasheshecao 3 (2005–2688; Guangzhou), Baihuasheshecao 4 (2005–2683; Hong Kong), Baihuasheshecao 5 (2005–2684; Hong Kong), Baihuasheshecao 6 (2005–2685; Hong Kong) and Baihuasheshecao 7 (2005–2686; Boston).
2.2. Molecular authentication by DNA sequencing
Fresh materials, herbarium specimens and herb samples were subjected to total DNA extraction using a modified DNA extraction method as previously described (Poon, Shaw, Simmons, & But, 2007). Complete ITS regions were amplified in a 50 μl reaction containing 27.5 μl distilled water, 5 μl 10× buffer solution (750 mM Tris–HCl, pH 8.8, 200 mM (NH4)2SO4, 0.1% Tween 20), 4 μl 25 mM MgCl2, 4 μl 2.5 mM dNTPs, 2 μl 10 mM forward primer Sol-18d (5ʹ -GAG GAA GGA GAA GTC GTA ACA AG-3ʹ), 2 μl 10 mM reverse primer Sol-28 cc (5ʹ -GGT AGT CCC GCC TGA CCT GG-3ʹ), 1 unit Taq polymerase and 3 μl total DNA extract. Polymerase chain reaction (PCR) was performed in a thermocycler through 35 cycles of 95 °C for 30 s; 60 °C for 20 s; 72 °C for 1 min. The PCR products were resolved in a 1.7% agarose gel and then purified with Gel-M™ Gel Extraction System (Viogene). Purified PCR products were cloned using pGEM®–T Easy Vector System I (Promega) and transformed into competent Escherichia coli cells. Plasmids were extracted using the spin method of Mini-M™ Plasmid DNA Extraction System (Viogene) and stored at −20 °C. Purified plasmids were sent to Macrogen Inc. for the process of DNA sequencing using sequencing primer T7-promoter (5ʹ -TAA TAC GAC TCA CTA TAG GG-3ʹ) and primer SP6 (5ʹ -ATT TAG GTG ACA CTA TAG AAT-3ʹ). The forward and reverse sequences were aligned using software ClustalX version 1.83 (Thompson, Gibson, Plewniak, Jeanmougin, & Higgins, 1997). Manual amendments were performed and percentage similarities were calculated using computer program BioEdit Sequence Alignment Editor Version 5.0.9 (Hall, 1999). Dendrogram was constructed by unweighted pair group method with arithmetic mean (UPGMA) tree construction method with bootstrap test of 1000 replicates using software MEGA version 2.1 (Kumar, Tamura, Jakobsen, & Nei, 1999).
2.3. Chemical authentication by TLC and HPLC
The test solutions were prepared by extracting 2.0 g of herbal materials with 4 ml of methanol under ultrasonic condition for 2 h followed by filtration. Precoated silica gel plates were used for analytical TLC using mobile phase methylene dichloride: acetone: methanol 6:4:1. Spots were detected by spraying with vanillin in 10% sulfuric acid and then heated on a hot plate. Analytical HPLC was performed on a Beckman System Gold instrument equipped with a 125 solvent module, a 168 photo diode array detector and a 508 autosampler. Chromatographic separation was carried out on a C18 column (250 × 4.6 mm, 5 μm; Beckman) using a gradient solvent system comprised of water and acetonitrile begin with 10% acetonitrile to 70% in 60 min. On-line UV spectra were recorded with a diode array detector from 200 to 400 nm and detection at 235 nm.
2.4. Chemical profile guided isolation of marker compounds
Dried herb (600 g) of H. diffusa was refluxed with 95% ethanol (8 L) for 2.5 h twice. After filtration, the filtrate was evaporated to dryness under reduced pressure at 45 °C. The ethanol extract was suspended in distilled water (400 ml) and partitioned by hexane (100 ml × 4), methylene dichloride (100 ml × 4) and ethyl acetate (100 ml × 4) successively to afford the hexane, methylene dichloride and ethyl acetate fractions, respectively. Through TLC and HPLC comparison of the chemical profiles of H. diffusa from different sources and all three fractions, marker compounds 1 and 2 were found to be present in the ethyl acetate and hexane fractions, respectively.
To obtain the marker compound 1, the ethyl acetate fraction was concentrated to give a syrup (1.1 g) that was chromatographed on a silica gel column with 600 ml methylene dichloride: acetone: methanol 6:4:1 as eluent. The eluates were monitored by TLC, combined and then concentrated to give the marker compound 1 (150 mg) for structural determination by nuclear magnetic resonance (NMR) and mass spectrometry (MS).
To obtain marker compound 2, the hexane fraction was concentrated under reduced pressure to give a syrup (6.0 g). The hexane extract was chromatographed on a silica gel column with methylene dichloride as eluent. The eluates were monitored by TLC, combined and grouped into nine fractions. After examination of all fractions by HPLC, fraction 8 containing the marker compound 2 was rechromatographied on silica gel and eluted with hexane: ethyl acetate 6:4 monitored by HPLC to give the marker compound 2 (22 mg) for NMR and MS analyses.
2.5. Anti-proliferation assays on prostate cancer cells
LNCaP androgen-sensitive and PC3 androgen-independent human prostate cancer cells were cultured in cell culture medium (Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 unit/mL penicillin, and 100 ng/mL streptomycin) and maintained at 37 °C in 5% CO2 humidified atmosphere. The anti-proliferation effects of marker compound 1 or 2 were then determined by CellTiter 96® AQueous Cell Proliferation Assay (MTS) (Promega) as previous described (Jiang et al., 2007). In brief, cells were cultured overnight in cell culture medium in 96-well plates at a density of 3 × 103 and 5 × 103 cell/well for PC3 and LNCaP, respectively. The cells were then treated with marker compound 1 or 2 at concentrations 5, 10, 20 and 50 μM or the vehicle (DMSO) for 48 h. CellTiter 96® AQueous reagent MTS (20 μl/well) was added and incubated for 2–4 h at 37 °C in 5% CO2 humidified atmosphere. The absorbance of formazan formed was measured at 490 nm using microplate reader (VersaMax). The experiments were performed thrice.
2.6. Statistical analysis
The results of anti-proliferation assays were expressed as mean ± SEM (standard error of mean). Differences among groups were analysed by one-way ANOVA using software GraphPad Prism 5. P-value < 0.05 was considered statistically significant.
3. Results
3.1. Molecular authentication
Complete ITS regions were amplified by PCR, cloned and sequenced successfully. The size of the ITS regions were 570 bp for H. diffusa and ranged from 565 bp to 566 bp for H. corymbosa (data not shown). After sequence alignment of H. diffusa and H. corymbosa, 102 bp were found intra-specifically conserved but inter-specifically polymorphic between the two species (Fig. 1), including 84 base substitutions and 18 base insertions/deletions. The intraspecific similarities of the complete ITS regions of H. diffusa and H. corymbosa were 99.5% and 99.8%, respectively, and the interspecific similarity between H. diffusa and H. corymbosa was 84.0%. The dendrogram constructed by UPGMA method using the complete ITS region clearly showed that H. diffusa and H. corymbosa formed separate clusters, supported by bootstrap frequencies of 100% (Fig. 2). Among the seven herb samples of Baihuasheshecao, the samples from Guangzhou (Baihuasheshecao 1–3) clustered with H. diffusa and their ITS regions were 99.9% similar to that of the ITS region of H. diffusa. The three herb samples from Hong Kong (Baihuasheshecao 4–6) and the sample from Boston (Baihuasheshecao 7) fell into the clade of H. corymbosa and their ITS regions were 99.6% similar to that of H. corymbosa (Fig. 2).
Fig. 1.
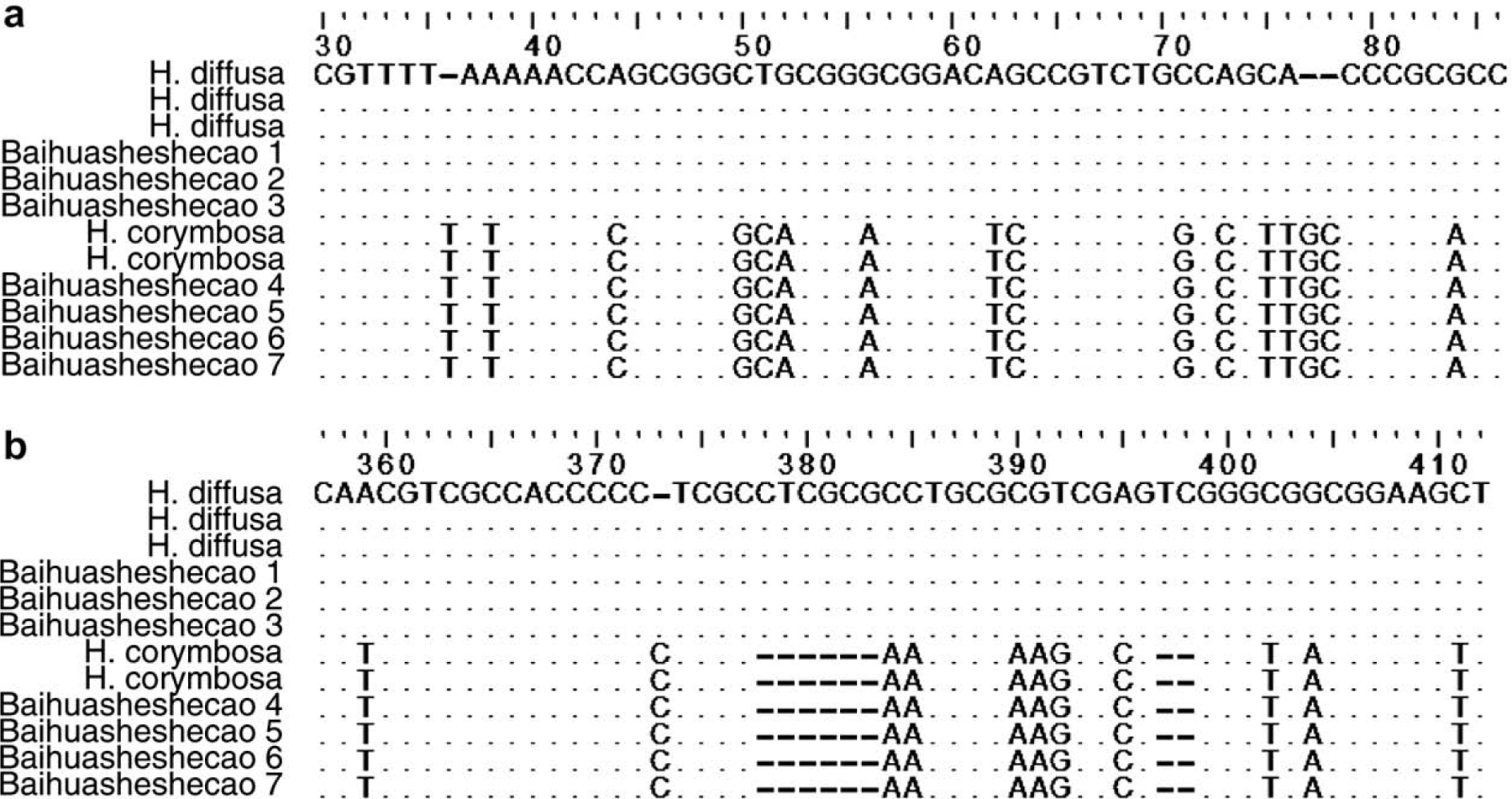
Molecular identification of H. diffusa, H. corymbosa and other Hedyotis species using complete ITS region. Partial alignment of the complete ITS regions showing notable base polymorphisms between H. diffusa and H. corymbosa in (a) ITS-1 and (b) ITS-2 regions. The numbers on the top line represent the base numbers in sequence alignment. ‘.’ represents the base being identical to the first sequence. ‘–’ represents gap.
Fig. 2.
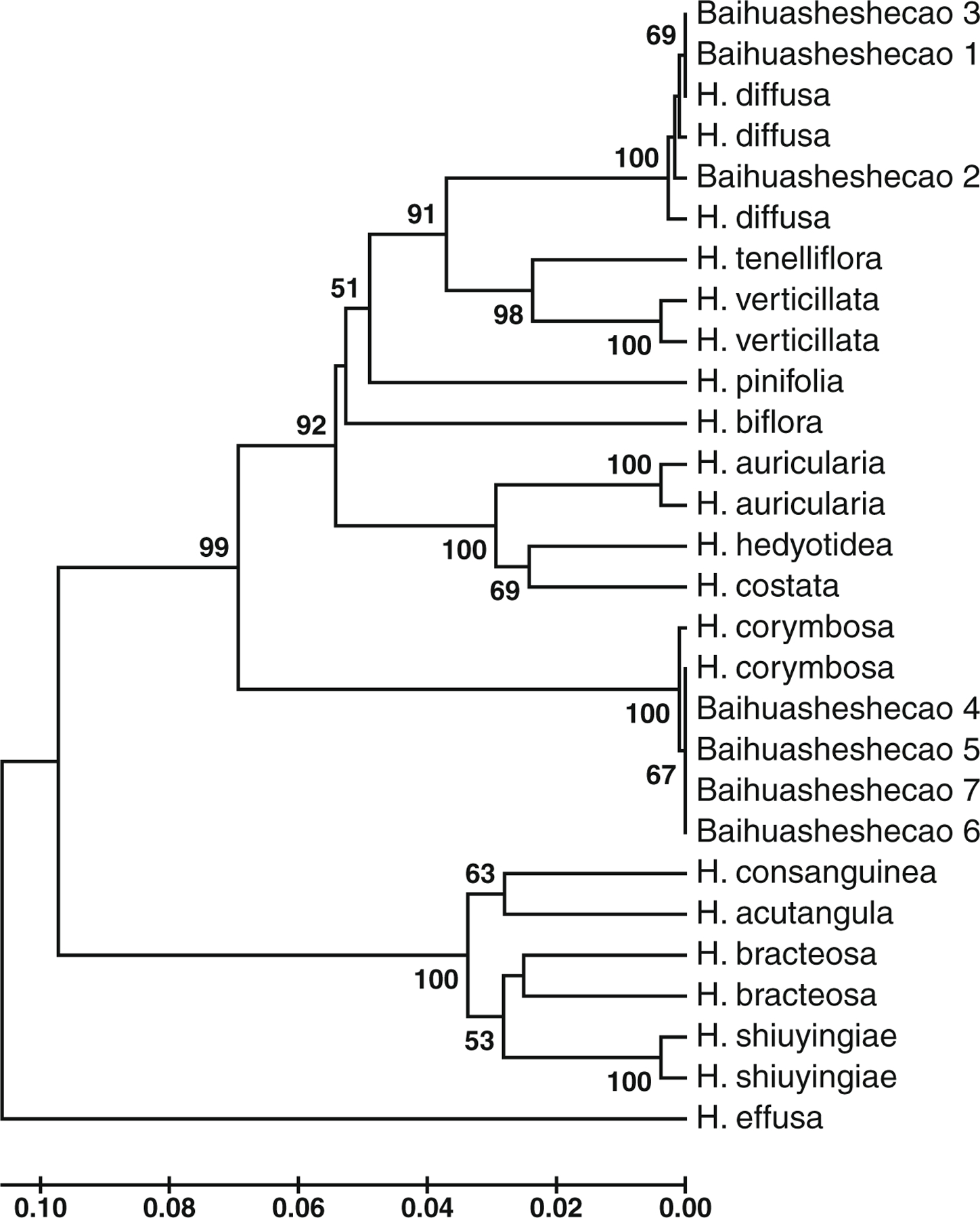
Clustering analysis based on complete ITS region of H. diffusa, H. corymbosa and other Hedyotis species constructed using the UPGMA method. Retailed herb samples of Baihuasheshecao 1–7 are also included in the dendrogram. Bootstrap frequencies (1000 replicates) with cutoff value of 50% are shown next to the branches. The scale bar represents the branch lengths of the dendrogram.
3.2. Comparison of TLC and HPLC profiles between H. diffusa and H. corymbosa
The TLC profiles of H. diffusa and H. corymbosa showed that marker compound 1 was found only in H. diffusa (Fig. 3). Marker compound 1 was a white powder, having a molecular formula C26H30O13 as indicated by MS, 1H and 13C NMR spectra (data not shown). It was identified as 6-O-(E)-p-coumaroyl scandoside methyl ester (Fig. 5) by comparison of its spectral data with the reported values (Nishihama, Masuda, Yamaki, Takagi, & Sakina, 1981). The HPLC profiles of H. diffusa and H corymbosa indicated that marker compound 2 was found only in H. diffusa with retention time at 32 min (Fig. 4). Marker compound 2 was an amorphous black powder, having a molecular formula C55H74N4O6 as indicated by MS, 1H and 13C NMR spectra (data not shown). It was identified as 10(S)-hydroxypheophytin a (Fig. 5) by comparison of its spectral data with the reported values (Nakatani, Ourisson, & Beck, 1981).
Fig. 3.
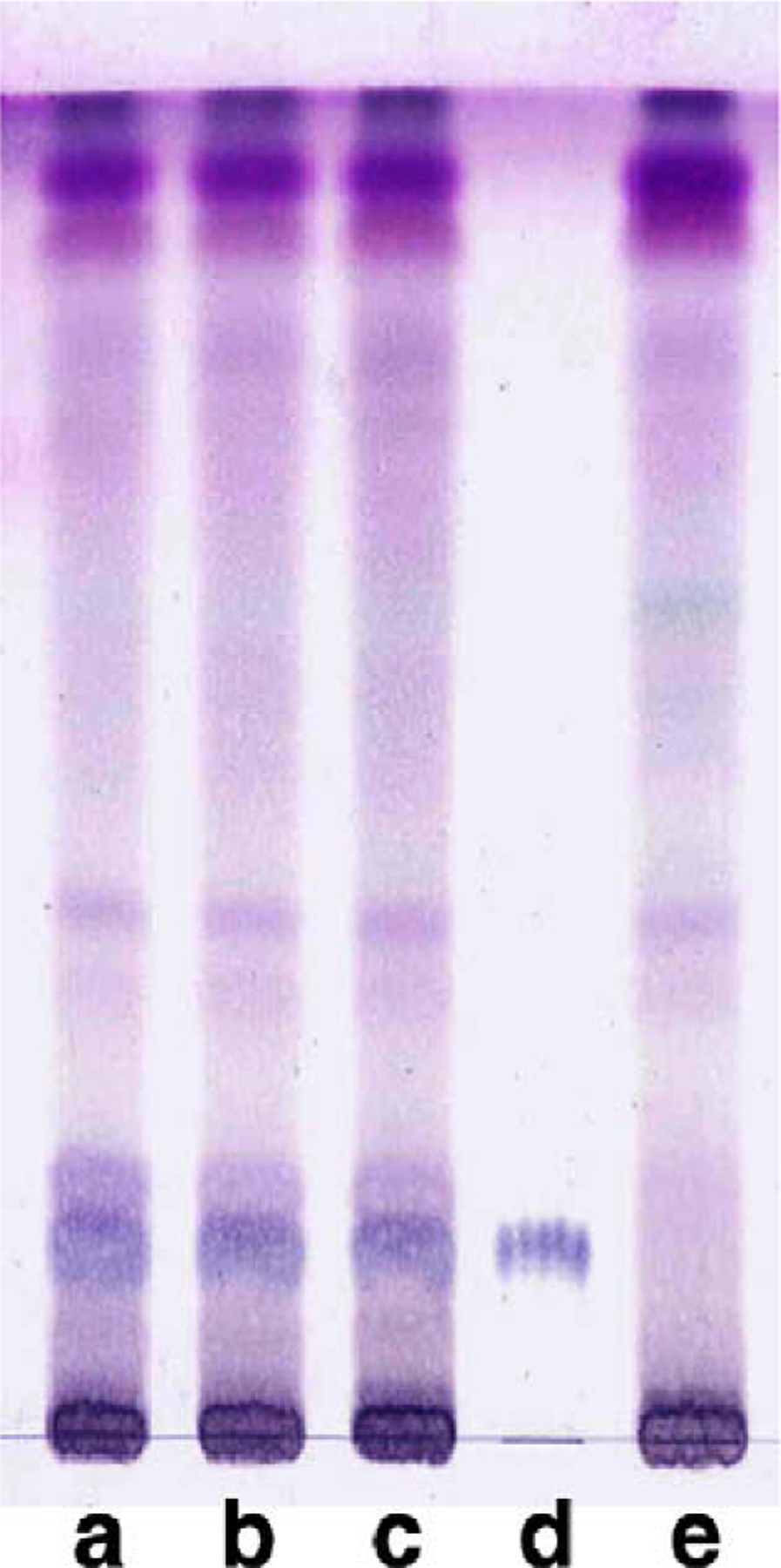
TLC profiles of H. diffusa (lanes a–c), H. corymbosa (lane e) and marker compound 1 (lane d). Marker compound 1 was found in H. diffusa but undetectable in H. corymbosa.
Fig. 5.
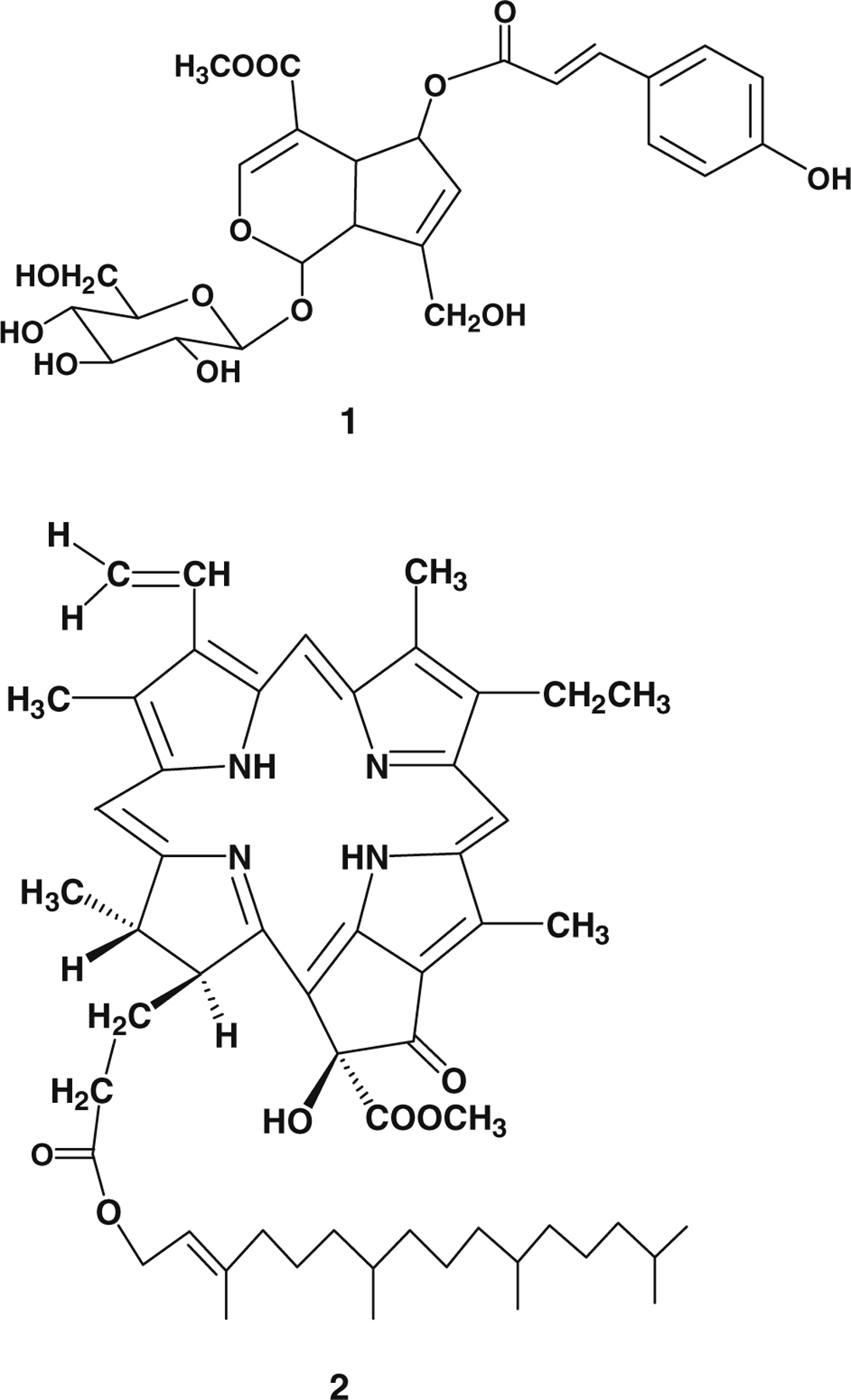
Chemical structures of marker compound 1: 6-O-(E)-p-coumaroyl scandoside methyl ester and marker compound 2: 10(S)-hydroxypheophytin a.
Fig. 4.
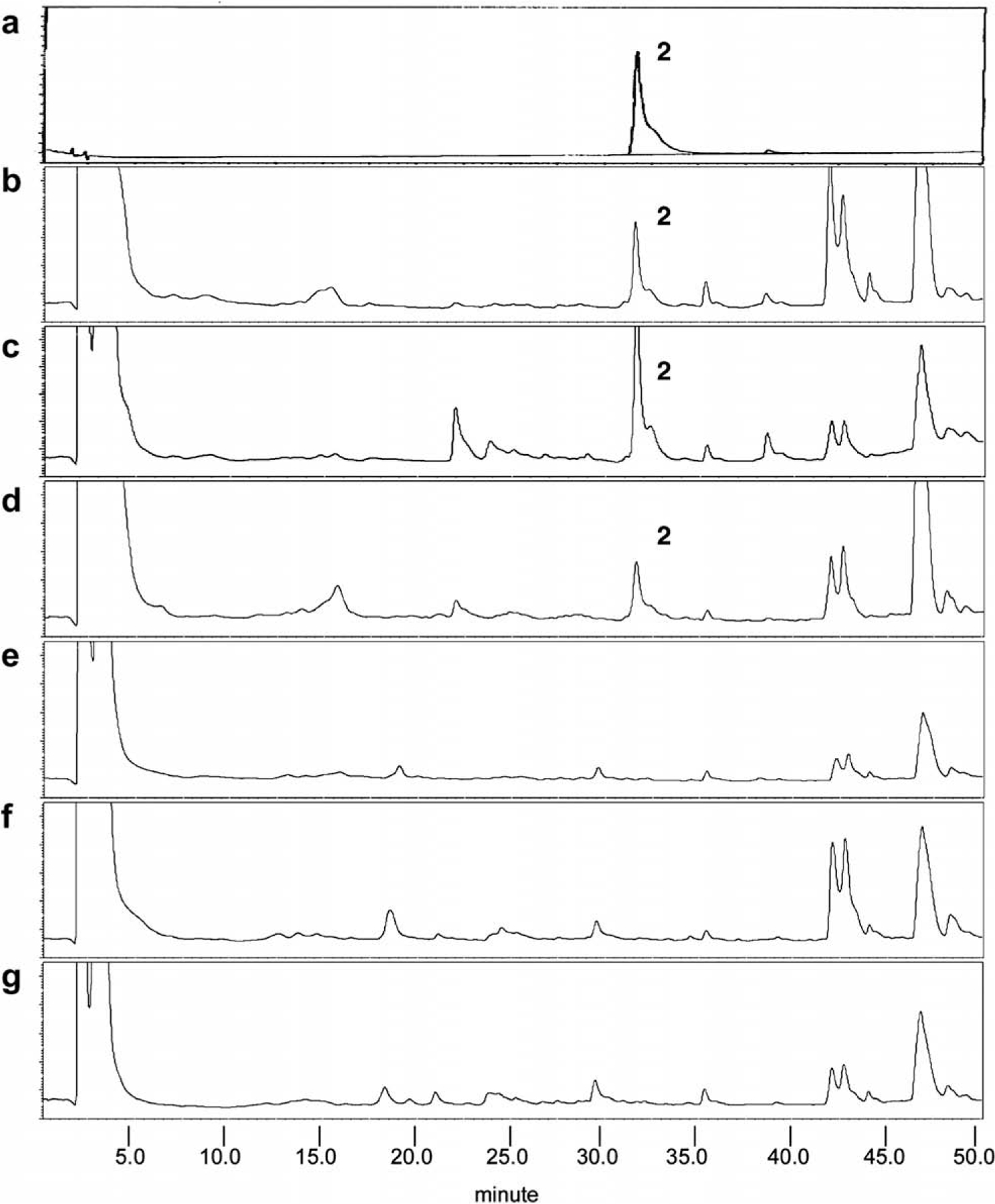
HPLC analysis of H. diffusa and H. corymbosa showing the presence of marker compound 2 (a) in H. diffusa (b–d) at 32 min but undetectable in H. corymbosa (e–g).
3.3. Anti-cancer activity of the marker compounds
Marker compound 1 suppressed the proliferation of PC3 cells in a dose-dependent manner with significant effect at 50 μM (Fig. 6a). In contrast, marker compound 1 did not exhibit any anti-proliferation effect on LNCaP cells within the tested concentration range. On the other hand, marker compound 2 reduced the proliferation rate of PC3 cells in a dose-dependent manner with significant effects at 50 μM (Fig. 6b). It also showed dose-dependent anti-proliferation effects on LNCaP cells with significant effect at 5 μM and IC50 at 20 μM. It was shown that the proliferation of LNCaP cells could be significantly suppressed by marker compound 2 but not by marker compound 1, while the proliferation of PC3 cells could be suppressed by both marker compounds.
Fig. 6.
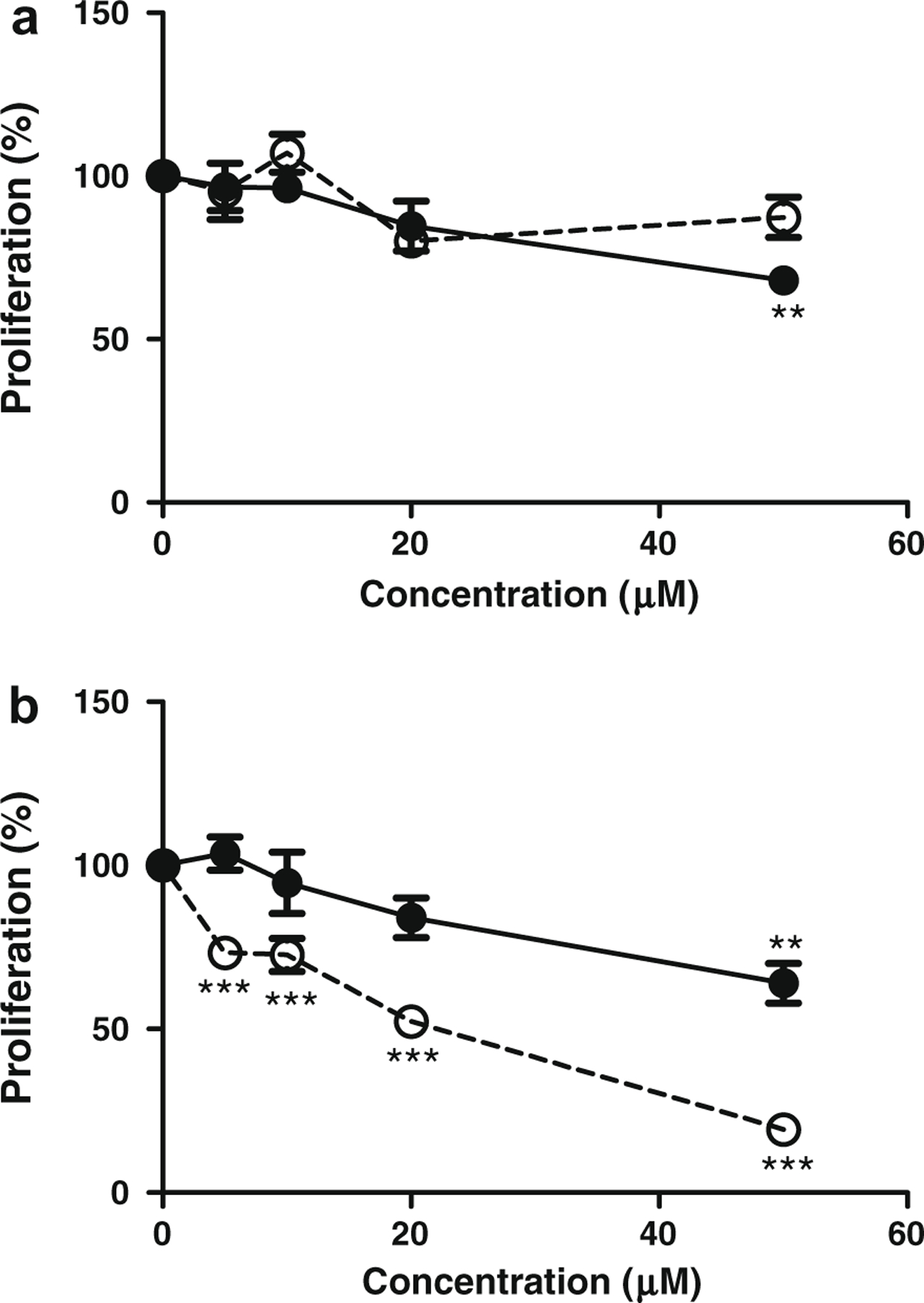
Anti-proliferation effects of (a) marker compound 1 and (b) marker compound 2 on LNCaP androgen-sensitive and PC3 androgen-independent human prostate cancer cells. DMSO was applied as the vehicle control. (–○– LNCaP, –●– PC3; mean ± SEM from three independent experiments; one-way ANOVA, **p < 0.01, ***p < 0.001).
4. Discussion
According to the Flora Republicae Poluparis Sinicae, Baihuasheshecao derived from H. diffusa has solitary flowers on thick and short pedicels (2–4 mm in length) growing from cylindrical stems. The adulterant derived from H. corymbosa differs in having corymbose inflorescences on slender peduncles (8–15 mm in length) growing from tetragonal stems (Ko, 1999). However, these characters become unapparent in dried and cut materials. To ensure the correct use of herbal materials, molecular sequencing was applied to differentiate H. diffusa from H. corymbosa and other closely related species, by taking advantage of the fact that molecular technique is independent of phenotypic and environmental variations and can help pinpointing plant materials to specific taxon. In this study, a total of 14 Hedyotis species were used in the molecular study using complete ITS region. This region was found to provide the highest number of informative characters and resolving power in the phylogenetic analysis of Hedyotis and related genera in Europe and Americas (Karehed, Groeninckx, Dessein, Motley, & Bremer, 2008). Our molecular data showed that the complete ITS region of H. diffusa and H. corymbosa were intra-specifically conserved but inter-specifically variable enough to differentiate these two species (Fig. 1). Clustering analysis among Hedyotis species also showed that H. diffusa and H. corymbosa formed distantly separate clusters (Fig. 2). Therefore, the ITS region was a suitable region for the authentication of Baihuasheshecao. Molecular data also revealed that 50% herb samples of Baihuasheshecao retailed in southeast China and the sample purchased from Boston, USA clustered to the species of adulterant H. corymbosa (Fig. 2) suggesting the problem of adulteration of Baihuasheshecao in the market is not a local phenomenon. To ensure proper use of herbal materials for efficient treatment, more attention should be put on identification and quality assessment of Baihuasheshecao.
Hao et al. (2004), Liu and Hao (2005) and Liu et al. (2004) applied ITS-1 and ITS-2 regions to differentiate Baihuasheshecao derived from H. diffusa and other related species. Interestingly, their sequences were different from our data. For the ITS-1 region, their sequences of H. diffusa (Genbank accession No. AY438319) and H. tenelliflora (Genbank accession No. AY438317) matched our sequences of H. corymbosa and H. tenelliflora (both 99.4%), respectively. Both of their sequences of H. corymbosa (Genbank accession No. AY438318) and H. pinifolia (Genbank accession No. AY438325) matched our sequence of H. diffusa (both 98.4%) (Hao et al., 2004; Liu & Hao, 2005). For the ITS-2 region, their sequences of H. diffusa (Genbank accession No. AY438319) and H. pinifolia (Genbank accession No. AY438316) matched our sequence of H. corymbosa (99.5%.) and H. diffusa (94.5%), respectively (Liu et al., 2004). Their sequences of H. corymbosa (Genbank accession No. AY438323) and H. tenelliflora (Genbank accession No. AY438324) did not match any of our sequences. Blasting of these two sequences to NCBI Genbank showed that these two sequences were most similar to plants from the families Caryophyllaceae and Cyperaceae, respectively, indicating a possibility of using improperly identified starting materials adulterated by plants from other families. Those authors also designed a H. diffusa-specific primer based on the ITS-2 region which amplified an additional DNA fragment of size 439 bp in H. diffusa (Liu et al., 2004). Similarly, even when using the primers specified by Liu et al. (2004), we were unable to reproduce this additional DNA fragment in our samples of H. diffusa, but an additional DNA fragment of correct size was amplified in H. corymbosa. The inconsistency between these molecular results of those reported and our data further confirmed the importance of correct identities of starting materials. Also, a single method may not be sufficient for proper identification of the genuineness of herbal materials. Additional markers and methods, such as TLC and HPLC, for authentication should also be applied to cross check the identities of materials.
Chemical markers for the authentication of Baihuasheshecao were identified by TLC and HPLC. Marker compound 1, identified as 6-O-(E)-p-coumaroyl scandoside methyl ester, was found specifically present in H. diffusa but undetectable in H. corymbosa (Fig. 3). Our results were consistent with previous findings that marker compound 1 was found exclusively in the ethyl acetate and n-butanol fractions of methanol extract of H. diffusa, but not in H. corymbosa (Liang et al., 2008). Marker compound 1 was also reported to be absent in H. tenelliflora, which was sometimes found adulterating H. diffusa, making this compound more applicable to differentiate H. diffusa from other common adulterants (Liang, Jiang, Leung, Peng, & Zhao, 2006a). Marker compound 2, identified as 10(S)-hydroxypheophytin a, was reported to be found in algae and silkworm excreta (Dai et al., 1992; Huang et al., 2007; Jiang et al., 2008). However, this is the first time to find compound 2 in Hedyotis species. Our results showed that it can be used for identification of genuine Baihuasheshecao derived from H. diffusa.
Compound 1 was studied on anti-proliferative effects on human hepatoma HepG2 and colon carcinoma CaCo-2 cells as well as anti-angiogenesis effects in the zebrafish model, but negative results were obtained (Liang et al., 2008). In this paper, compound 1 was found to have anti-proliferation effects against prostate cancer cells. It inhibited the growth of PC3 androgen-independent human prostate cancer cells but showed no inhibiting effects on LNCaP androgen-sensitive human prostate cancer cells (Fig. 6a). On the other hand, compound 2 showed significant anti-proliferation effects on both LNCaP androgen-sensitive and PC3 androgen-independent prostate cancer cells in a dose-dependent manner (Fig. 6b). It was reported to be a strong photodynamic therapeutic sensitizer to reduce the survival rate of human and mouse tumor cells (Dai et al., 1992). Its anti-tumor effects may be related to the inhibition of nuclear factor-κB activation induced by tumor necrosis factor alpha (Huang et al., 2007). Our study found that compound 1 was more effective in killing LNCaP androgen-sensitive prostate cancer cells while compound 2 was more effective in killing PC3 androgen-independent prostate cancer cells. The bioactive compounds were present only in H. diffusa but undetectable in H. corymbosa. This finding suggested H. diffusa should not be substituted by H. corymbosa for the treatment of prostate cancer.
Identity and quality are two of the most important elements for food quality control and drug development. We have demonstrated the DNA sequence of complete ITS region and the marker compounds 1 and 2 could be used to verify the identity of Baihuasheshecao independently. Combined use of these molecular and chemical markers could increase the confidence of analysis by cross-checking each other. The bioactive maker compounds 1 and 2 could also be applied for the quality control of Baihuasheshecao. Usually, the quality of Baihuasheshecao was assessed by the contents of ursolic acid and oleanolic acid. However, these compounds were commonly found in other herbs, as well as in the adulterant H. corymbosa (Liang et al., 2008), making them inapplicable for differentiation among these species. Therefore, we suggest that bioactive marker compounds 1 and 2 should be included as additional markers for identity determination and quality assessment of Baihuasheshecao, especially in the field of prostate cancer treatment.
In summary, the identity of the materials of Baihuasheshecao was confirmed by molecular authentication using DNA sequencing of the ITS regions of H. diffusa, H. corymbosa and related species. Base pair polymorphisms between H. diffusa and H. corymbosa could be used as molecular markers for authentication. Clustering analysis revealed the relationship among the closely related Hedyotis species, including H. diffusa and H. corymbosa. Using the molecular authentication technique, four out of seven retailed Baihuasheshecao samples were found to be adulterated by H. corymbosa. Chemical profiles of H. diffusa and H. corymbosa were compared by TLC and HPLC. Marker compound 1, identified as 6-O-(E)-p-coumaroyl scandoside methyl ester and marker compound 2, identified as 10(S)-hydroxypheophytin a, were specifically found in H. diffusa. Marker compound 1 showed moderate anti-proliferation effect on PC3 human androgen-independent prostate cancer cells while marker compound 2 showed moderate anti-proliferation effect on PC3 cells but strong anti-proliferation effect on LNCaP human androgen-sensitive prostate cancer cells.
Acknowledgements
We thank the Hong Kong Herbarium and Prof. Tao Chen, an Editor of Rubiaceae in Flora of China, for identifying Hedyotis samples and providing specimens for molecular authentication. This project was partially supported by Hong Kong Jockey Club Charities Trust. The support from Program for New Century Excellent Talent in University (NCET-08–0612) to R.W.J. is also deeply appreciated.
References
- Ahmad R, Ali AM, Israf DA, Ismail NH, Shaari K, & Lajis NH (2005). Antioxidant, radical-scavenging, anti-inflammatory, cytotoxic and antibacterial activities of methanolic extracts of some Hedyotis species. Life Sciences, 76(17), 1953–1964. [DOI] [PubMed] [Google Scholar]
- Dai R, Shoemaker R, Farrens D, Han MJ, Kim CS, & Song PS (1992). Characterization of silkworm chlorophyll metabolites as an active photosensitizer for photodynamic therapy. Journal of Natural Products, 55(9), 1241–1251. [DOI] [PubMed] [Google Scholar]
- Gupta S, Zhang D, Yi J, & Shao J (2004). Anticancer activities of Oldenlandia diffusa. Journal of Herbal Pharmacotherapy, 4(1), 21–33. [PubMed] [Google Scholar]
- Hall TA (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98. [Google Scholar]
- Hao MG, Liu ZQ, & Wang JL (2004). Application of the sequences of rDNA ITS to identify Chinese crude drug Hedyotis diffusa. Journal of Anhui Normal University (Natural Sciecne), 27(2), 188–191. [Google Scholar]
- Huang X, Li M, Xu B, Zhu X, Deng Z, & Lin W (2007). Proteasome and NF-kappaB inhibiting phaeophytins from the green alga Cladophora fascicularis. Molecules, 12(3), 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang RW, Hay ME, Fairchild CR, Prudhomme J, Roch KL, Aalbersberg W, et al. (2008). Antineoplastic unsaturated fatty acids from Fijian macroalgae. Phytochemistry, 69(13), 2495–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang RW, Zhou JR, Hon PM, Li SL, Zhou Y, Li LL, et al. (2007). Lignans from Dysosma versipellis with inhibitory effects on prostate cancer cell lines. Journal of Natural Products, 70(2), 283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JA, Kane SP, & Jain S (1995). Hepatitis induced by traditional Chinese herbs; possible toxic components. Gut, 36(1), 146–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karehed J, Groeninckx I, Dessein S, Motley TJ, & Bremer B (2008). The phylogenetic utility of chloroplast and nuclear DNA markers and the phylogeny of the Rubiaceae tribe Spermacoceae. Molecular Phylogenetics and Evolution, 49(1), 843–866. [DOI] [PubMed] [Google Scholar]
- Ko WC (1999). Hedyotis. In Luo XR (Ed.). Flora Republicae Popularis Sinicae (vol. 71, pp. 32–77). Beijing: Science Press. [Google Scholar]
- Kumar S, Tamura K, Jakobsen IB, & Nei M (1999). MEGA2: Molecular evolutionary genetics analysis software Tempe: Arizona State University. [DOI] [PubMed] [Google Scholar]
- Liang XY (2004). A case of prostate cancer treated with Chinese medicines. Academic Periodical of Changchun College of Traditional Chinese Medicine, 20(2), 29. [Google Scholar]
- Liang ZT, He MF, Fong WF, Jiang ZH, & Zhao ZZ (2008). A comparable, chemical and pharmacological analysis of the traditional Chinese medicinal herbs Oldenlandia diffusa and O. Corymbosa and a new valuation of their biological potential. Phytomedicine, 15(4), 259–267. [DOI] [PubMed] [Google Scholar]
- Liang ZT, Jiang ZH, Leung KS, Peng Y, & Zhao ZZ (2006a). Distinguishing the medicinal herb Oldenlandia diffusa from similar species of the same genus using fluorescence microscopy. Microscopy Research and Technique, 69(4), 277–282. [DOI] [PubMed] [Google Scholar]
- Liang ZT, Jiang ZH, Leung KS, & Zhao ZZ (2006b). Determination of iridoid glucosides for quality assessment of Herba Oldenlandiae by high-performance liquid chromatography. Chemical and Pharmaceutical Bulletin (Tokyo), 54(8), 1131–1137. [DOI] [PubMed] [Google Scholar]
- Lin CC, Ng LT, & Yang JJ (2004). Antioxidant activity of extracts of peh-huejuwa-chi-cao in a cell free system. American Journal of Chinese Medicine, 32(3), 339–349. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, & Hao MG (2005). Determination of rDNA ITS sequences in Hedyotis diffusa. Shaanxi Journal of Traditional Chinese Medicine, 26(2), 167–169. [Google Scholar]
- Liu ZQ, Hao MG, & Wang JL (2004). Application of allele-specific primer in the identification of Hedyotis diffusa. Journal of Chinese Medicinal Materials, 27(7), 484–487. [PubMed] [Google Scholar]
- Nakatani Y, Ourisson G, & Beck JP (1981). Chemistry and biochemistry of Chinese drugs VII. Cytostatic pheophytins from silkworm excreta, and derived photocytotoxic pheophorbides. Chemical & Pharmaceutical Bulletin (Tokyo), 29(8), 2261–2269. [DOI] [PubMed] [Google Scholar]
- Nishihama Y, Masuda K, Yamaki M, Takagi S, & Sakina K (1981). Three new iridoid glucosides from Hedyotis diffusa. Planta Medica, 43(9), 28–33. [DOI] [PubMed] [Google Scholar]
- Perry LM (1980). Medicinal plants of East and Southeast Asia: Attributed properties and uses Cambridge: MIT Press; (pp. 350–351). [Google Scholar]
- Poon WS, Shaw PC, Simmons MP, & But PPH (2007). Congruence of molecular, morphological, and biochemical profiles in Rutaceae: A cladistic analysis of the subfamilies Rutoideae and Toddalioideae. Systematic Botany, 32(4), 837–846. [Google Scholar]
- Shan BE, Zhang JR, Du XN, & Li KX (2001). Immunommodulatory activity and anti-tumor activity of Oldenlandia diffusa in vitro. Chinese Journal of Integrated Traditional and Western Medicine, 21(5), 370–374. [PubMed] [Google Scholar]
- State Pharmacopoeia Commission of People’s Republic China (2005). Appendix III. In State Pharmacopoeia Commission of People’s Republic China, Pharmacopoeia of the People’s Republic of China (p. 22). Beijing: Chemical Industry Press. [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, & Higgins DG (1997). The ClustalX windows interface. Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25(24), 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willimott S, Barker J, Jones LA, & Opara EI (2007). Apoptotic effect of Oldenlandia diffusa on the leukaemic cell line HL60 and human lymphocytes. Journal of Ethnopharmacology, 114(3), 290–299. [DOI] [PubMed] [Google Scholar]
- Xie Z, Zhang Y, & Lu R (1997). Identification of Herba Hedyotis Diffusae and its confused material Herba Hedyotis Pinifoliae. Journal of Chinese Medicinal Materials, 20(5), 287–290. [PubMed] [Google Scholar]
- Yadav SK, & Lee SC (2006). Evidence for Oldenlandia diffusa-evoked cancer cell apoptosis through superoxide burst and caspase activation. Journal of Chinese Integrative Medicine, 4(5), 485–489. [DOI] [PubMed] [Google Scholar]
- Zhao ZZ, & Li YS (2005). Easily confused chinese medicines in Hong Kong Hong Kong: Chinese Medicine Merchants Association. [Google Scholar]


