Abstract
We present evidence that Bacillus anthracis lethal toxin (LT) suppresses rather than induces proinflammatory cytokine production in macrophages. Suppression is observed with extremely low levels of LT and involves inhibition of transcription of cytokine messenger RNA. Thus, LT may contribute to anthrax pathogenesis by suppressing the inflammatory response.
Lethal toxin (LT), produced by Bacillus anthracis, the etiologic agent for anthrax, is composed of two subunits, protective antigen (PA) and lethal factor (LF). PA is a pore-forming protein that mediates the entry of LF into the cell (3, 4). LF is a metalloprotease that exhibits an exclusive cytotoxicity for monocytes and macrophages. LF has been shown to cleave short N-terminal fragments from mitogen or extracellular signal-regulated kinase kinase 1 (MEK1) MEK2, and MEK3, the upstream activators of extracellular signal-regulated kinase 1 (ERK1), ERK2, and p38, respectively (1, 5, 7).
Effect of LT on macrophage cytokine expression.
The response of LT-sensitive (RAW 264.7, J774A.1) and LT-resistant (IC-21) murine macrophage cell lines to low levels of anthrax LT was investigated. A saturating level of PA (0.1 μg/ml; S. F. Little, unpublished observations) and various concentrations of LF were added to the cultures (Table 1). Each of the three cell lines expressed significant levels of tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) upon exposure to lipopolysaccharide (LPS) (Eschericha coli 026:B6; Sigma-Aldrich, St. Louis, Mo.) (data not shown). Expression of TNF-α was apparent within 30 min of exposure, well within the time during which the cells were still viable upon exposure to a lytic concentration of LT. Parallel cultures of macrophages were exposed to LT for 6 h and assessed for viability [by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2-H-tetrazolium, bromide (MTT) reduction assay (Roche Molecular Biochemicals, Indianapolis, Ind.)] or cytokine expression by a capture enzyme-linked immunosorbent assay (ELISA) (BioSource International, Camarillo, Calif, and R&D Systems, Minneapolis, Minn.). None of the cell lines expressed either TNF-α or IL-1β at any level of toxin administered to the cultures (Table 1).
TABLE 1.
Cytokine expression by macrophage cell lines
| LTa (μg/ml) | RAW 264.7 macrophages
|
J774A.1 macrophages
|
IC-21 macrophages
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| % viability | TNF-α (pg/ml) | II-1β (pg/ml) | % viability | TNF-α (pg/ml) | II-1β (pg/ml) | % viability | TNF-α (pg/ml) | II-1β (pg/ml) | |
| 102 | 41.2 ± 2.9 | 0.7 ± 2.7 | 0 ± 5.3 | 0.8 ± 0.5 | 10.4 ± 14.8 | 4.2 ± 13.7 | 88.6 ± 2.0 | 7.2 ± 4.7 | 20.2 ± 6.7 |
| 10−3 | 100 ± 3.4 | 0 ± 0.9 | 0 ± 29.1 | 58.5 ± 4.4 | 1.8 ± 5.8 | 0 ± 6.6 | 90.2 ± 3.1 | 0 ± 5.9 | 7.6 ± 33.2 |
| 10−6 | 100 ± 5.9 | 0 ± 2.8 | 16.5 ± 13.9 | 100 ± 8.5 | 13.8 ± 0.0 | 22.5 ± 16.8 | 99.4 ± 0.9 | 24.0 ± 25.6 | 18.5 ± 0.0 |
A concentration of 0.1 μg of PA/ml plus the indicated concentration of LF were used. Data are means ± standard errors of the mean.
Inhibition of cytokine expression.
Expression of TNF-α by macrophages in response to LPS involves at least three MAP kinase pathways (6). Thus, LPS-induced cytokine expression was a logical choice for examining the effect of LT upon ERK-dependent processes. Since the LF protease attacks the upstream activators of ERK isoforms (MEKs), it seemed likely that LT would have an inhibitory effect upon cytokine expression. Because some degree of killing in J774A.1 cultures was observed with as little as 10−4 μg of LT/ml, but not with lesser concentrations (Table 1 and data not shown), 10−6 μg of LT/ml was used as a sublytic amount (Table 1) to determine if low concentrations of toxin had any effect on cytokine expression by the cells. Preincubating macrophages with LT progressively inhibited the ability of the cells to express TNF-α upon subsequent exposure to LPS (Fig. 1). For J774A.1 cells, inhibition of TNF-α was detectable within 1 h of exposure to LT, reaching a maximum by 4 h after exposure. Inhibition of TNF-α in RAW 264.7 cells became apparent by 4 h posttreatment and was almost complete after overnight treatment. IC-21 cells displayed pronounced inhibition of TNF-α within 1 h of LT treatment, and in some experiments, coincubating the cells with LT and LPS was sufficient to inhibit cytokine expression. The cultures were viable by MTT assay (data not shown); thus, lack of response to LPS could not be accounted for by cell death. No inhibition of TNF-α was detected with 0.01 pg of LT/ml, and higher levels of LT failed to accelerate the time course of inhibition. Inhibition was specific to LT, as neither LF nor PA added alone had any effects on TNF-α expression (data not shown).
FIG. 1.
Inhibition of LPS-induced TNF-α expression. RAW 264.7 (open bars), J774A.1 (hatched bars), and IC-21 (cross-hatched bars) macrophages were cultured with media alone, LPS alone, or LT alone, were cocultured with LT and LPS, or were pretreated with LT (1 pg/ml) for 1, 4, or 16 h before LPS stimulation (10 ng/ml) for an additional 6 h. TNF-α expression was determined by cytokine capture ELISA. Data are the means ± standard errors of the results of two experiments with triplicate samples.
Anthrax LT and expression of cytokine RNAs.
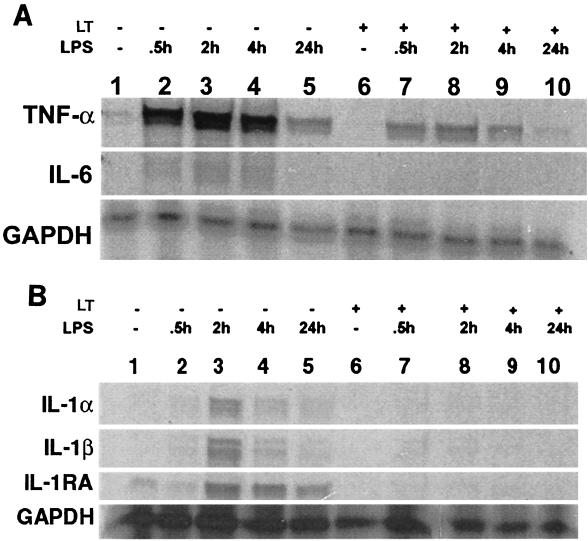
Because ERK pathways are involved in LPS-induced transcription of TNF-α mRNA (2), it is reasonable to expect that LT might inhibit LPS-induced transcription. Levels of RNA encoding IL-1β and TNF-α, as well as IL-1α, IL-1 receptor antagonist (IL-1RA), and IL-6, were examined in murine macrophage cell lines. The cell lines were cultured overnight in the presence or absence of 10−6 μg of LF/ml plus 0.1 μg of PA/ml. After incubation, LPS was added to the cells, which were cultured for 30 min or 2, 4, or 24 h and lysed. A control culture with no treatment and a culture that received only LT were included in the experiments. RNAs were harvested and probed with multi-probe templates (BD Pharmingen, San Diego, Calif.) that included probes for TNF-α and IL-6 (Fig. 2A) or IL-1α, IL-1β, and IL-1RA (Fig. 2B). Target RNA (2 μg) was reacted with mouse cytokine primer sets mCK-3b and mCK-2b (2.9 × 105 cpm/μl) labeled with [33P]UTP, after which free probe and single-stranded RNAs were digested with RNases. The protected RNA was resolved on a 5% polyacrylamide gel electrophoresis gel. The gel was then transferred onto Whatman paper, dried, and exposed to an X-ray film (X-AR; Eastman Kodak Co., Rochester, N.Y.). Culturing the cells with LT alone had no effect on expression of cytokine RNAs while LPS induced increases in the levels of mRNA for TNF-α, IL-1β, IL-1α, IL-1RA, and IL-6. Preincubation with LT inhibited the observed LPS-induced increase in the levels of these RNAs. Inhibition persisted over the 24-h time course of the experiments. As is demonstrated in Fig. 2B, IL-1β mRNA was induced by LPS in RAW 264.7 cells and was completely inhibited by LT pretreatment. Similar results were observed for J774A.1 and IC-21 macrophages; however, the level of mRNA expression induced by LPS alone was not as high as it was for RAW 264.7 macrophages (data not shown).
FIG. 2.
Analysis of cytokine expression by RNase protection assay. RNase protection assays were performed to measure RNA expression for TNF-α and IL-6 (A) or IL-1α, IL-1β, and IL-1RA (B). Lanes 1 to 5, cells cultured with medium for 16 h before addition of 10 ng of LPS/ml; lanes 6 to 10, cells cultured with 1 pg of LT/ml for 16 h before addition of 10 ng of LPS/ml. Lanes 1 and 6, cells cultured without LPS; lanes 2 and 7, cells cultured with LPS for 30 min; lanes 3 and 8, cells cultured with LPS for 2 h; lanes 4 and 9, cells cultured with LPS for 4 h; lanes 5 and 10, cells cultured with LPS for 24 h.
In summary, we have shown that LT does not induce proinflammatory cytokine expression. Moreover, LPS-induced cytokine expression is inhibited by LT. Our findings suggest that LT may act to impair host response in reducing the inflammatory response and thus enhance bacterial virulence.
REFERENCES
- 1.Duesbery N S, Webb C P, Leppla S H, Gordon V M, Klimpel K R, Copeland T D, Ahn N G, Oskarsson M K, Fukasawa K, Paull K D, Vande Woude G F. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 2.English J, Pearson G, Wilsbacher J, Swantek J, Karandikar M, Xu S, Cobb M H. New insights into the control of MAP kinase pathways. Exp Cell Res. 1999;253:255–270. doi: 10.1006/excr.1999.4687. [DOI] [PubMed] [Google Scholar]
- 3.Friedlander A M. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986;261:7123–7126. [PubMed] [Google Scholar]
- 4.Klimpel K R, Molloy S S, Thomas G, Leppla S H. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc Natl Acad Sci USA. 1992;89:10277–10281. doi: 10.1073/pnas.89.21.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellizzari R, Guidi-Rontani C, Vitale G, Mock M, Montecucco C. Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFN gamma-induced release of NO and TNFalpha. FEBS Lett. 1999;462:199–204. doi: 10.1016/s0014-5793(99)01502-1. [DOI] [PubMed] [Google Scholar]
- 6.Swantek J L, Cobb M H, Geppert T D. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol Cell Biol. 1997;17:6274–6282. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]