Abstract
Background
Malnutrition causes diverse alterations in the immune system, and COVID-19 is an infection affecting the immune system, consequently leading to malnutrition.
Aims
This study aimed to investigate the use of prognostic nutritional index (PNI) and selected inflammatory indices for malnutrition screening among COVID-19 hospitalized patients.
Material and methodology
This is a single-center retrospective study that enrolled 289 hospitalized COVID-19 patients between 1st January to 30th April 2021, their median age was 59 years. Demographic and biochemical data were collected from patients’ records. The PNI, platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), and an early warning score to predict mortality risk (ANDC) were calculated. Univariate and multivariate logistic regression analyses were performed. A P-value of < 0.05 was considered statistically significant.
Results
about 30 % of patients were admitted to the intensive care unit (ICU), and ICU patients had significantly higher levels of white blood cell (WBCs) count, neutrophils, C-reactive protein (C-RP), and D-dimer (P < 0.05). On the other hand, they had significantly lower levels of lymphocytes and serum albumin (P < 0.001; for both). Those with high ANDC scores were more likely to develop severe conditions affecting nutritional status compared to non-ICU (OR = 1.04, 95 % CI:1.014–1.057; P < 0.001). ANDC showed good discrimination ability with an AUC of 0.784 (cut-off value > 68.19 score).
Conclusion
It is suggested that ANDC could be used as a predictor for nutritional status and severity in COVID-19 hospitalized patients.
Keywords: Inflammation, Malnutrition, Inflammatory indices, PNI, COVID-19
Abbreviations: PNI, prognostic nutritional index; ANDC, an early warning score to predict mortality risk; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ICU, The intensive care unit; C-RP, C-reactive protein
Introduction
Coronavirus disease 2019 (COVID-19) is a life-threatening respiratory disease, and it was recognized by the WHO as a pandemic in 2020 [1]. Patients with COVID-19 have a wide range of clinical manifestations, ranging from asymptomatic disease and mild, self-limiting respiratory illness with nonspecific symptoms to severe pneumonia with life-threatening complications, including complex and mixed pulmonary conditions and multiorgan failure that can lead to death [1], [2], [3]. The physiological and immunological mechanisms that cause COVID-19 clinical traits have not yet been fully elucidated to allow for adequate therapeutic identification and planning of successful therapies [4]. Also, 10–20 % of patients diagnosed with COVID-19 require critical care, which places a huge burden upon healthcare facilities [5]. A post-COVID syndrome, also known as Long COVID-19, is one of the dreaded COVID-19 sequelae. It is defined as the persistence of signs and symptoms in COVID-19 survivors, and it usually manifests three months after the initial infection with symptoms that last at least two months and cannot be accounted for by any other condition. According to research on the prevalence of post-COVID-19 symptoms, between 30 % and 50 % of people who recover from a COVID-19 infection experience persistent symptoms that can last up to a year. Up to 42 % of infected patients also experienced long COVID-19 symptoms, which are linked to a worse health-related quality of life [6], [7].
COVID-19 triggers an inflammatory response, thus, controlling the inflammatory response seems to be an essential strategy in combating the virus [8]. The practical prognostication of critically ill patients with COVID-19 may result in optimizing the allocation of healthcare resources [9], [10]. In this regard, Mehta, and colleagues (2020) reported that all patients with severe COVID-19 should be screened for hyper-inflammation [11].
Curiously, malnutrition causes the most diverse alterations in the immune system, suppressing the immune response and increasing the susceptibility to infections such as COVID-19 [8], [12], [13]. Many studies underlined the fact that elderly patients and those with comorbidities are the most vulnerable to death and at risk of malnutrition [14]. Moreover, recent evidence has shown that malnutrition is a critical prognostic factor in many different diseases, including autoimmune diseases [15]. Interestingly, high degrees of malnutrition correlates with high levels of inflammation [16], highlighting the role of malnutrition risk screening and its prognostic value for COVID-19 patients. Numerous studies found that hospitalized patients had a high prevalence of symptoms related to nutritional status such as loss of appetite, nausea, vomiting, diarrhea, dysphagia, fatigue, loss of smell and taste), malnutrition, micronutrient deficiencies, and obesity, all of which were linked to an increased risk of mortality and morbidity [17]. According to a systematic review [18], hospitalized COVID-19 patients had a pooled prevalence of malnutrition of 49.1 %, and their mortality risk was 10 times higher than that of well-nourished patients. Additionally, many systemic reviews and observational studies revealed that malnutrition among critically sick patients can reach 70 % and that it is substantially correlated with death, length of stay in the hospital, and time spent on mechanical ventilation [18].
There is no standard nutritional screening tool or specific method for the assessment of nutritional status in COVID-19 patients [19]. The dysregulation of immune response and inflammation is a significant component of COVID-19 pathogenesis. The association between several inflammatory indices such as C-RP, hypoalbuminemia, neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) have been associated with disease severity and increased mortality rate among COVID-19 patients [20], [21].
Recently, the prognostic nutrition index (PNI) has been proposed as a method for quantifying the immune status, as it consists of easily accessible biochemical parameters, including serum albumin level and total lymphocyte count [22], [23], [24]. It was used as an indicator of the severity of COVID-19 [25], and also, it was used as a predictor of mortality in hospitalized COVID-19 patients [26]. Moreover, the PNI could reflect the nutritional and inflammatory status more broadly in COVID-19 patients [27]. Another indicator was proposed by Weng and his colleagues depending on four predictors including age, NLR, D-dimer, and CRP; the summation of these predictors is named ANDC (an early warning score to predict mortality risk). Patients with low ANDC scores (< 59) were classified as having a low death probability risk [27]. Additionally, ANDC was used as a death predictor in hospitalized COVID-19 malignancy cases [28].
Although studies documented the relationship between inflammation and malnutrition in COVID-19 patients [29], the use of inflammatory indices as malnutrition predictors is still uncommon. Therefore, the present study aimed to investigate the role of PNI and selected inflammatory indices as malnutrition screening indices in predicting malnutrition in hospitalized COVID-19 patients, which is reflected by their condition severity.
Materials and methods
Study design and settings
This study is a retrospective observational study in an accredited hospital in Jordan. COVID-19 was confirmed by nasal polymerase chain reaction (PCR). Confirmed COVID-19 adult patients were included after being admitted to the hospital. All patients had a clear clinical outcome of either hospital discharge or death. COVID-19 severity upon hospital admission was defined based on the fifth version of the National Health Commission Guideline on the Management of Novel Coronavirus Pneumonia. A total of 289 patients who were admitted to the hospital with COVID-19 were enrolled in this study between 1st January to 30th April 2021. Patients younger than 18 years old, pregnant, and lactating women were excluded.
Data collection
Demographic, symptoms, past medical history, laboratory results, duration of hospital stay, ICU admission, and in-hospital mortality were obtained retrospectively from patients’ electronic medical records. The electronic medical records of all enrolled patients were reviewed and needed data were extracted. Laboratory test results of WBCs, neutrophils, lymphocytes, and platelet counts, as well as serum albumin, C-RP, and D-dimer, were obtained from electronic medical records on admission. The selected inflammatory indices which are considered a potential malnutrition predictors included: PNI, ANDC, NLR, and PLR. PNI was calculated as serum albumin (g/L) + 5 × lymphocyte count (109 /L) [25]. ANDC was calculated as (1.14 × age – 20) (years) + 1.63 ×NLR + 5.00 × D-dimer (mg/L) + 0.14 × C-RP (mg/L) [27]. Also, NLR and PLR were calculated.
Ethical approval of the study protocol
The study protocol was reviewed and approved by the institutional review board of The Hashemite University (No.1/14/2020/2021) and by the Helsinki Declaration. The requirement for written consent was canceled due to the retrospective design of the study.
Statistical analysis
Data were analyzed using IBM SPSS Statistics (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp). Shapiro–Wilk test was used to verify the normality of variables. Normally distributed continuous variables were expressed as means ± standard deviations, while non-normally distributed variables were shown as medians with interquartile ranges. Chi-square (χ2) test was performed for categorical variables and presented as frequencies and percentages ( %). Independent-samples t-test and Mann-Whitney U test were performed. Significant independent variables which not included in the malnutrition screening indices calculations were tested for multicollinearity. The variance inflation factor (VIF) was performed, and no problem was detected with an overall VIF < 2 [30]. Univariate and multivariate logistic regression analyses were performed to examine the determinants of transferring COVID-19 patients from hospital wards to the ICU. Then, we performed receiver-operating characteristic (ROC) curve analyses to determine the discriminatory power of the nutritional screening tool ANDC as a classifier for malnutrition presence. Next, we calculated the area under the curve (AUC) to detect the discriminatory power of the index and the optimal cut-off point was recognized. Findings with a P-value of < 0.05 were considered statistically significant.
Results
Baseline characteristics among COVID-19 in-hospital patients
A total of 289 patients who were admitted to the hospital with COVID-19 were enrolled in this study. With an ICU admission rate of roughly 30 % of the study population, 88 of these patients were classified as the "ICU" group at any point during their hospital stay, while the remaining 201 patients were categorized as the "non-ICU" group. Their demographic and clinical characteristics are listed in Table 1. The median age of the study population was 59 (48.5–71 years) and about 61 % of the enrolled patients were males. The patients requiring ICU care were noted to be significantly older (69 vs. 54 years, P < 0.001) and to require a longer duration of stay (12 vs. 7 days, P < 0.001) than the patients that did not require an intensive level of care. Total in-hospital death was 42 (14.5 %) patients in the study population, 41 patients of them were admitted to the ICU (P < 0.001). Having diarrhea and/or vomiting as one of the initial symptoms was found to be significant in non-ICU patients (P = 0.007). The proportions of diabetes, hypertension, and cardiovascular diseases were significantly different between the two groups (P < 0.05) with more frequencies among the non-ICU group. On the other hand, patients with renal diseases were found to be significantly more frequent in the ICU group (57.1 % vs. 42.9 %, P = 0.001).
Table 1.
Baseline demographics and clinical characteristics of the study population stratified by COVID-19 severity.
| Variable | All (n = 289) |
COVID-19 Severity |
p-value | ||
|---|---|---|---|---|---|
| ICU (n = 88) |
Non-ICU (n = 201) |
||||
| Age, median (IQR), years | 59 (48.5–71) | 69 (59–77) | 54 (44.5–65) | < 0.001 | |
| Hospital admission, median (IQR), days | 8 (5.5–12) | 12 (8–19) | 7 (5–10) | < 0.001 | |
| Gender, n (%) | |||||
| Male | 175 (60.6) | 53 (30.3) | 122 (69.7) | 0.94 | |
| Female | 114 (39.4) | 35 (30.7) | 79 (69.3) | ||
| In-hospital Mortality, n (%) | |||||
| Yes | 42 (14.5) | 41 (97.6) | 1 (2.4) | < 0.001 | |
| No | 247 (85.5) | 47 (19) | 200 (81) | ||
| Symptoms in admission, n (%) | |||||
| Fever | 214 (74) | 67 (31.3) | 147 (68.7) | 0.592 | |
| Cough | 229 (79.2) | 66 (28.8) | 163 (71.2) | 0.24 | |
| Diarrhea and/or vomiting | 61 (21.1) | 10 (16.4) | 51 (83.6) | 0.007 | |
| Anorexia | 133 (46) | 38 (28.6) | 95 (71.4) | 0.522 | |
| Dyspnea | 245 (84.8) | 80 (32.7) | 165 (67.3) | 0.055 | |
| Fatigue | 266 (92) | 81 (30.5) | 185 (69.5) | 0.999 | |
| Comorbidities, n (%) | |||||
| Diabetes | 102 (35.3) | 39 (38.2) | 63 (61.8) | 0.034 | |
| Hypertension | 146 (50.5) | 62 (42.5) | 84 (57.5) | < 0.001 | |
| Cardiovascular diseases | 89 (30.8) | 41 (46.1) | 48 (53.9) | < 0.001 | |
| Renal diseases | 28 (9.7) | 16 (57.1) | 12 (42.9) | 0.001 | |
| Cancer | 10 (3.5) | 4 (40) | 6 (60) | 0.504 | |
| Multi-comorbidities | 125 (43.3) | 56 (44.8) | 69 (55.2) | 0.061 | |
ICU-admitted patients showed significantly higher: WBCs (8.45 vs. 6.80 ×109 /L, P = 0.001), neutrophils count (7.41 vs. 5.36 ×109 /L, P < 0.001), C-RP (11.34 vs. 8.61 mg/L, P = 0.021), and D-dimer (526.0 vs. 280.5 ng/L, P = 0.005) at admission. While lymphocyte count and serum albumin were significantly lower among ICU patients (0.64 vs. 0.96 ×109 /L, P < 0.001; and 34.00 vs. 37.00 g/L, P < 0.001, respectively) as shown in Table 2.
Table 2.
Biochemical characteristics and Malnutrition screening indices of the study population stratified by COVID-19 severity.
| Variable | Total | COVID-19 Severity |
p-value |
||
|---|---|---|---|---|---|
| ICU | Non-ICU | ||||
| White blood cell count, median (IQR), × 109 /L | 7.60(5.4–10.75) | 8.45 (6.25–12.2) | 6.80 (5.2–10.25) | 0.001 | |
| Absolute Neutrophils Count, median (IQR), × 109 /L | 6.09 (4–9.32) | 7.41 (5.23–10.79) | 5.36 (3.81–8.4) | < 0.001 | |
| Absolute Lymphocyte Count, median (IQR), × 109 /L | 0.83 (0.56–1.23) | 0.64 (0.44–0.97) | 0.96 (0.64–1.31) | < 0.001 | |
| Platelet count, mean ± SD, × 109 /L | 241.32 ± 92.27 | 236.44 ± 88.84 | 243.45 ± 93.86 | 0.553 | |
| Serum Albumin, median (IQR), g/L | 36.00 (32–39) | 34.00 (31–36) | 37.00 (33–40) | < 0.001 | |
| C-reactive protein, median (IQR), mg/L | 9.45 (4.18–15.99) | 11.34 (5.61–21.1) | 8.61 (3.74–15.07) | 0.021 | |
| D-Dimer, median (IQR), ng/mL | 336.00 (154–925) | 526.00 (195–1600) | 280.50 (142–777.5) | 0.005 | |
| Malnutrition screening indices, median (IQR) | |||||
| PNI | 40.47 (36.12–44.47) | 37.26 (33.71–41.52) | 41.29 (38.11–45.59) | < 0.001 | |
| PLR | 266.67 (177.25–423.67) | 339.68 (224.25–519.66) | 243.06 (161.37–384.01) | < 0.001 | |
| NLR | 7.64 (4.05–13) | 11.63 (7.75–18.35) | 5.93 (3.24–10.69) | < 0.001 | |
| ANDC | 62.01 (46.74–81.13) | 80.33 (68.46–96.7) | 55.14 (43.04–70.54) | < 0.001 | |
ICU: intensive care unit; PNI: the prognostic nutrition index; NLR: neutrophil-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; ANDC: an early warning score to predict mortality risk.
Table 2 shows the malnutrition screening indices and their relation to COVID-19 severity, it was found that PNI was significantly lower in the ICU patients as compared to non-ICU patients (37.26 vs. 41.29, P < 0.001), the other three indices (PLR, NLR, and ANDC) were significantly higher among the ICU patients (339.68 vs. 243.06, P < 0.001; 11.63 vs. 5.93, P < 0.001; and 80.33 vs. 55.14, P < 0.001, respectively).
Association between malnutrition screening indices and COVID-19 severity
Table 3 shows a univariate and multivariate logistic regression analysis for malnutrition screening indices. In univariate analysis, PNI, PLR, NLR, and ANDC were strongly associated with COVID-19 severity (PNI = 0.91, P < 0.001; PLR = 1, P = 0.008; NLR = 1.06, P < 0.001; ANDC = 1.04, P < 0.001).
Table 3.
Univariate and multivariate logistic regression analysis of risk factors for malnutrition among in-hospital COVID-19 patients expressed by ICU admission.
| Variable | Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|---|
| OR | 95 CI % | p-value | OR | 95 CI % | p-value | ||
| Diarrhea and/or vomiting at admission | 0.38 | 0.18–0.78 | 0.009 | 0.47 | 0.20–1.07 | 0.073 | |
| Comorbidities | |||||||
| Diabetes | 1.74 | 1.04–2.92 | 0.035 | 0.96 | 0.49–1.88 | 0.903 | |
| Hypertension | 3.32 | 1.94–5.68 | < 0.001 | 1.69 | 0.834–3.41 | 0.146 | |
| Cardiovascular diseases | 2.78 | 1.64–4.72 | < 0.001 | 1.51 | 0.80–2.86 | 0.203 | |
| Renal diseases | 3.50 | 1.58–7.76 | 0.002 | 2.29 | 0.904–5.78 | 0.081 | |
| Malnutrition screening Indices | |||||||
| PNI | 0.91 | 0.869–0.949 | < 0.001 | 0.98 | 0.978–1.015 | 0.237 | |
| PLR | 1.00 | 1.000–1.003 | 0.008 | 1.00 | 0.998–1.002 | 0.990 | |
| NLR | 1.06 | 1.033–1.096 | < 0.001 | 0.96 | 0.900–1.023 | 0.203 | |
| ANDC | 1.04 | 1.026–1.051 | < 0.001 | 1.04 | 1.014–1.057 | 0.001 | |
The multivariate logistic regression analysis showed that ANDC was an independent predictor for COVID-19 severity and patients with higher ANDC scores were more likely to get worse conditions (OR = 1.04, CI:1.014–1.057; P < 0.001).
Predictive value of ANDC
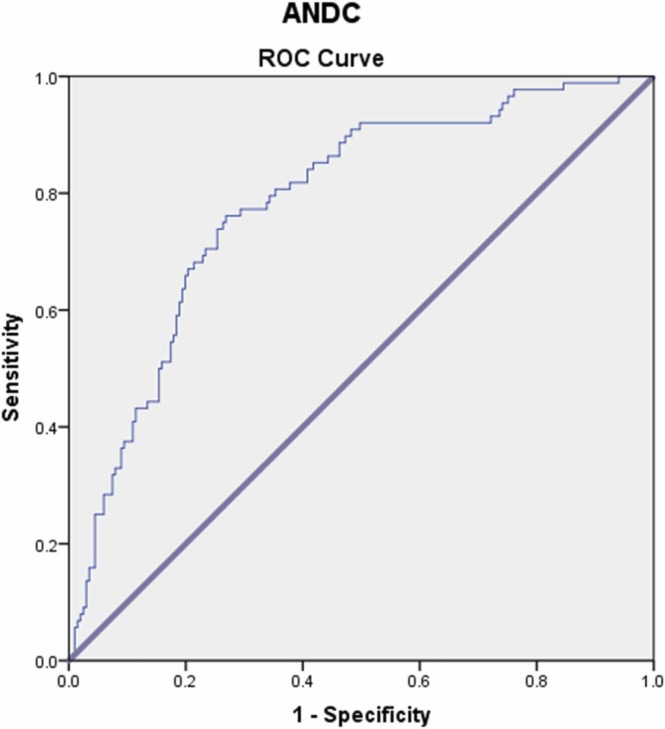
The values of ANDC in the prediction of the disease course and disease severity were analyzed with the ROC curve and found to be statistically significant (p < 0.001). The ROC curves and the AUC in detecting the predictive abilities of ANDC for COVID-19 severity among our study population are presented in Table 4 and Fig. 1.
Table 4.
Area under ROC curve (AUC), optimal cut-off value, sensitivity, and specificity of ANDC as malnutrition screening index in predicting COVID-19 severity.
| Variable | AUC (95 % CIs) | Cut-off | Sensitivity | Specificity |
|---|---|---|---|---|
| ANDC | 0.784 (0.732–0.830) | > 68.19 | 76.14 | 73.13 |
Fig. 1.
The ROC curves for detecting the discrimination power of ANDC as a malnutrition screening index in predicting COVID-19 severity.
In general, the ability to predict the severity of COVID-19 is considered to be well done by ANDC which showed good discrimination ability with an AUC of 0.784. In addition, we found that, when the cut-off value was taken as > 68.19 ANDC score, it was found to have 76.14 % sensitivity and 73.13 % specificity for prediction of the disease severity and ICU admission (Table 4), and the probability of being admitted to the ICU found to be 8.5 times higher.
Discussion
Several studies have confirmed the effect of inflammation on nutritional status, as well as the effect of nutritional status on the development of inflammation. The past findings concluded that inflammatory indices (NLR, PLR, PNI, and ANDC) were a predictor of disease severity in COVID-19 patients [22], [31], [32], but in this study, we combined them all to find out the best malnutrition screening predictor. Many studies underlined the fact that malnutrition is a critical prognostic factor in many diseases, including autoimmune diseases and infectious diseases [15], [25]. Curiously, high levels of malnutrition had been correlated with high levels of inflammation [33], thus highlighting the role of malnutrition risk screening and its prognostic value for COVID-19 patients.
In line with our results, Yang and colleagues (2020) studied 69 non-severe and 24 severe patients with COVID‐19 and found that the ratio of NLR and PLR was statistically significantly higher in severe patients [32]. Moreover, Erdogan and his colleagues concluded that NLR and PLR ratios can be used as more significant biomarkers in predicting the COVID-19 prognosis of patients [34]. Also, Qu et al. found that PLR might provide a new indicator in monitoring patients with COVID‐19 [35]. This study indicates the usefulness of a similar ratio in predicting the prognosis of patients with COVID‐19. We found a significantly higher ratio among the ICU group when compared to the other group.
Since albumin is frequently used to gauge patients' nutritional status, systemic inflammation lowers albumin synthesis and increases albumin breakdown [36]. However, the PNI, which is calculated from albumin and lymphocyte levels, is an objective reflection of inflammatory and nutritional status in COVID-19 patients [25]. Previous results have shown that albumin levels were inversely correlated with unfavorable progression and outcomes in COVID-19 patients [22], [37]. Furthermore, low albumin levels during infectious diseases and inflammation could be an indicator of liver function, as inflammatory cytokines could decrease hepatocytes’ synthesis of albumin [25]. The present results also observed a significantly lower PNI score for ICU-admitted patients when compared to non-ICU patients. These results are consistent with many other studies’ findings. Hence, Wang and colleagues concluded that the PNI is inversely associated with outcomes in COVID-19 patients, and PNI scores are also beneficial for clinicians to evaluate progression and strengthen monitoring for COVID-19 patients [25]. In 2021, in a study to explore the predictive role of the PNI on COVID-19 severity, the authors reported that the PNI is a valuable biomarker that could be used to discriminate COVID-19 severity [38]. Additionally, Nalbant and colleagues studied 118 hospitalized patients 40 of them admitted to the ICU, they found that the PNI scores are useful for the prediction of the ICU need and the disease severity in COVID-19 [39]. Still, using albumin level to calculate the PNI might weaken its inflammation prediction ability; as researchers mistrust the patient’s liver’s function.
However, the present study identified the ANDC as an independent predictor of COVID-19 severity, our results suggested that evaluation of ANDC is important for COVID-19 risk stratification. This finding agrees with Weng and his colleagues who reported that the application of ANDC would help clinicians make a prompt and reasonable decision to optimize patient stratification management and potentially reduce fatality [30]. On the other hand, Bilge et al. found no difference in PNI and ANDC between malignancy and without malignancy groups in COVID-19 patients, and they concluded that this can be partially accounted for the lack of existence of an adequate inflammatory response and a poor nutritional status for malignancy group [28].
The ANDC was first developed by Weng and his colleagues as a nomogram for predicting the prognosis of patients with COVID-19 as multiple risk factors (age, NLR, D-dimer, and C-RP), it showed good discrimination and calibration [30]. Weng and colleagues analyzed these four factors specifically and identified that they were associated with inflammation, immunity, and coagulation function, which might contribute to the pathogenesis of COVID-19. So, they speculated that the inflammatory response to SARS-CoV-2 infection may be the core of the pathogenesis of COVID-19, and the dysregulation of the immune and/or coagulation system will result in worse disease outcomes [27]. In our study, ICU-admitted patients had significantly low lymphocyte levels and higher levels of neutrophils, D-dimer, and C-RP than non-ICU. These results were the same as those documented by Weng and colleagues [27]. Also, we found that the median ages of ICU patients (69 years) were significantly higher than non-ICU patients (54 years), and these findings are consistent with Pijls et al., who conducted a meta-analysis of 59 studies comprising 36.470 patients showed that infection, severe disease, ICU admission, and death are more likely to occur among patients aged 70 and above [40]. In our study, the aforementioned four predictors (age, NLR, D-dimer, and CRP) obtained on admission that was used to calculate the ANDC might contribute to making a reasonable and individualized therapeutic strategy for COVID-19 patients with severe conditions as well as for patients with high mortality risk without worrying about the physiological functions of patient’s organs.
Our study recognized an ANDC score of > 68.19 to be the optimal cut-off point for the risk of malnutrition, and a COVID-19 patient of this score or more is at higher risk of being admitted to the ICU 8.5 times more than those who have a lower score. Malnutrition is usually diagnosed by either percentage of weight loss within the known duration of time or by calculating the body mass index. However, in this study, we didn’t use any of the common methods in malnutrition diagnosis because of a lack of information about the patient’s weight or height in his/her medical file due to the urgency of the epidemic disease. So, we tried to identify a method to diagnose malnutrition by using the available information. Any of our chosen malnutrition screening indices can be measured from the patient’s medical file. By identifying malnourished COVID-19 patients, we can use this information to identify potentially critically ill COVID-19 patients at an early stage and design an intervention plan to minimize the number of patients requiring ICU admission or/and who will develop severe consequences.
This study had several limitations. Firstly, the selection bias is due to the nature of a single-center study. Secondly, the absence of anthropometric measurements is due to the disease’s nature and the strict health protocols in dealing with patients. Also being a single-center study and having a retrospective design is another limitation. Hence, more extensive prospective studies are required. Moreover, the biochemical data for the participants and malnutrition status were not documented, and there was a wide range of age groups.
Nevertheless, as this topic’s data and findings are scarce, it is a good starting point for future clinical trials validated in a larger sample size with a longer proper follow-up period.
Conclusion
The findings of this study showed that the ANDC had an independent prognostic value in predicting COVID-19 severity among hospitalized patients. The ANDC components are collected routinely in hospitals and can be easily and rapidly calculated. So, it might be the best choice for prediction of the malnutrition, as well as it may be useful for early detection of the ICU need in COVID-19 patients. Moreover, ANDC is of vital importance for providing medical care and improving the prognosis.
Funding
There were no external funding sources for this study.
Conflicts of interest/Competing interests
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
Not applicable.
Ethics approval
The study protocol was carried out following the principles of the Declaration of Helsinki as revised in 2000. Ethical approval for this research was obtained from the Ethical Review Board at Hashemite University (No.1/14/2020/2021).
Consent for publication
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
References
- 1.World Health Organization. Virtual press conference on COVID-19 – 11 March 2020; 2020. 〈https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audioemergencies-coronaviruspress-conference-full-and-final-11 mar2020.pdf?sfvrsn = cb432bb3_2〉 (Accessed March 2020).
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2019;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Morales A., Cardona-Ospina J., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tay M., Poh C., Rénia L., MacAry P., Ng L. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khinda J., Janjua N.Z., Cheng S., van den Heuvel E.R., Bhatti P., Darvishian M. Association between indices of immune response at hospital admission and COVID-19 disease severity and mortality: a meta-analysis and meta-regression. J Med Virol. 2021;93:1078–1098. doi: 10.1002/jmv.26411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández-de-las-Peñas C., Notarte K.I., Peligro P.J., Velasco J.V., Ocampo M.J., Henry B.M., et al. Long-COVID symptoms in individuals infected with different SARS-CoV-2 variants of concern: a systematic review of the literature. Viruses. 2022;14(12):2629. doi: 10.3390/v14122629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Notarte K.I., Catahay J.A., Velasco J.V., Pastrana A., Ver A.T., Pangilinan F.C., et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. EClinicalMedicine. 2022 1;53 doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morais A., Aquino J., da Silva-Maia J., Vale S., Maciel B., Passos T. Nutritional status, diet and viral respiratory infections: perspectives for severe acute respiratory syndrome coronavirus 2. Br J Nutr. 2020;125(8):851–862. doi: 10.1017/S0007114520003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight S., Ho A., Pius R., Buchan I., Carson G., Drake T., et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterization Protocol: development and validation of the 4C Mortality Score. BMJ. 2020:m3339. doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang W., Liang H., Ou L., Chen B., Chen A., Li C., et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Int Med. 2020;180(8):1081. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta P., McAuley D., Brown M., Sanchez E., Tattersall R., Manson J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moser J., Galindo-Fraga A., Ortiz-Hernández A., Gu W., Hunsberger S., Galán-Herrera J., et al. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Resp Virus. 2018;13(1):3–9. doi: 10.1111/irv.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrakis D., Margină D., Tsarouhas K., Tekos F., Stan M., Nikitovic D., et al. Obesity - a risk factor for increased COVID-19 prevalence, severity and lethality (Review) Mol Med Rep. 2020;22(1):9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont R., Longué M., Galinier A., Cinq Frais C., Ingueneau C., Astudillo L., et al. Impact of micronutrient deficiency & malnutrition in systemic sclerosis: cohort study and literature review. Autoim Rev. 2018;17(11):1081–1089. doi: 10.1016/j.autrev.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Sueta D., Hokimoto S., Sakamoto K., Akasaka T., Tabata N., Kaikita K., et al. Validation of the high mortality rate of Malnutrition-Inflammation-Atherosclerosis syndrome. Inter J Cardiol. 2017;230:97–102. doi: 10.1016/j.ijcard.2016.12.072. [DOI] [PubMed] [Google Scholar]
- 17.van der Meij B.S., Ligthart-Melis G.C., de van der Schueren M.A.E. Malnutrition in patients with COVID-19: assessment and consequences. Curr Opin Clin Nutr Metab Care. 2021 1;24(6):543–554. doi: 10.1097/MCO.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 18.Abate S.M., Chekole Y.A., Estifanos M.B., Abate K.H., Kabthymer R.H. Prevalence and outcomes of malnutrition among hospitalized COVID-19 patients: a systematic review and meta-analysis. Clin Nutr ESPEN. 2021;43 doi: 10.1016/j.clnesp.2021.03.002. 174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck M.A., Levander O.A. Host nutritional status and its effect on a viral pathogen. J Infect Dis. 2000;182(1):93–96. doi: 10.1086/315918. [DOI] [PubMed] [Google Scholar]
- 20.Luo X., Zhou W., Yan X., Guo T., Wang B., Xia H., et al. Prognostic Value of C-Reactive Protein in Patients with Coronavirus 2019. Clin Infect Dis. 2020;71(16):2174–2179. doi: 10.1093/cid/ciaa641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang A.P., Liu J.P., Tao W.Q., Li H.M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H., Wang X., Fu Z., et al. Potential factors for prediction of disease severity of COVID-19 patients. Front Cell Infect Microbiol. 2020:10. doi: 10.3389/fcimb.2020.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du X., Liu Y., Chen J., Peng L., Jin Y., Cheng Z., et al. Comparison of the clinical implications among two different nutritional indices in hospitalized patients with COVID-19. 2020. medRxiv. 2020 Preprint posted online May 1, Preprint posted online May 1, [Google Scholar]
- 24.Zhou J., Ma Y., Liu Y., Xiang Y., Tao C., Yu H., et al. A correlation analysis between the nutritional status and prognosis of COVID-19 patients. J Nutr Health Aging. 2021;25(1):84–93. doi: 10.1007/s12603-020-1457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang R., He M., Yin W., Liao X., Wang B., Jin X., et al. The Prognostic Nutritional Index is associated with mortality of COVID‐19 patients in Wuhan, China. J Clin Lab Anal. 2020;34:10. doi: 10.1002/jcla.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Çınar T., Hayıroğlu M., Çiçek V., Kılıç Ş., Asal S., Yavuz S., et al. Is prognostic nutritional index a predictive marker for estimating all-cause in-hospital mortality in COVID-19 patients with cardiovascular risk factors? Heart Lung. 2021;50(2):307–312. doi: 10.1016/j.hrtlng.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng Z., Chen Q., Li S., Li H., Zhang Q., Lu S., et al. ANDC: an early warning score to predict mortality risk for patients with Coronavirus Disease 2019. J Trans Med. 2020;18:1. doi: 10.1186/s12967-020-02505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilge M., Akilli I., Karaayvaz E., Yesilova A., Kart Yasar K. Comparison of systemic immune-inflammation index (SII), early warning score (ANDC) and prognostic nutritional index (PNI) in hospitalized patients with malignancy, and their influence on mortality from COVID-19. Infect Agents Cancer. 2021;16:1. doi: 10.1186/s13027-021-00400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alkhatib B., Al Hourani H.M., Al-Shami I. Using inflammatory indices for assessing malnutrition among COVID-19 patients: a single-center retrospective study. J Infect Public Health. 2022 1;15(12):1472–1476. doi: 10.1016/j.jiph.2022.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston R., Jones K., Manley D. Confounding and collinearity in regression analysis: a cautionary tale and an alternative procedure, illustrated by studies of British voting behavior. Qual Quant. 2018;52(4):1957–1976. doi: 10.1007/s11135-017-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo X., Zhou W., Yan X., et al. Prognostic value of C-reactive protein in patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa641. https://doi. org/10.1093/cid/ciaa641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang A.P., Liu J.P., Tao W.Q., Li H.M. The diagnostic and predictive role of NLR, d-NLR, and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sueta D., Hokimoto S., Sakamoto K., Akasaka T., Tabata N., Kaikita K., et al. Validation of the High Mortality Rate of Malnutrition-inflammation-atherosclerosis syndrome. Inter J Cardiol. 2017;230:97–102. doi: 10.1016/j.ijcard.2016.12.072. [DOI] [PubMed] [Google Scholar]
- 34.Erdogan A., Ezgi Can F., Gönüllü H. Evaluation of the prognostic role of NLR, LMR, PLR, and LCR ratio in COVID‐19 patients. J Med Virol. 2021;93:5555–5559. doi: 10.1002/jmv.27097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu R., Ling Y., Zhang Y., Wei L., Chen X., Li X., et al. Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19. J Med Virol. 2020:1–9. doi: 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabrerizo S., Cuadras D., Gomez-Busto F., Artaza-Artabe I., Marín-Ciancas F., Malafarina V. Serum albumin and health in older people: review and meta-analysis. Maturitas. 2015;81(1):17–27. doi: 10.1016/j.maturitas.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Gong J., Ou J., Qiu X., Jie Y., Chen Y., Yuan L., et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk Nomogram in Wuhan and Guangdong. China Clin Infec Dis. 2020;71(15):833–840. doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z.H., Lin Y.-W., Wei X.-B., Li F., Liao X.L., Yuan H.-Q., et al. Predictive value of prognostic nutritional index on COVID-19 severity. Front Nutr. 2021;7 doi: 10.3389/fnut.2020.582736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nalbant A., Demirci T., Kaya T., Aydın A., Altındiş M., Güçlü E. Can prognostic nutritional index and systemic immune‐inflammatory index predict disease severity in COVID‐19? Inter Clin Pract. 2021;75:10. doi: 10.1111/ijcp.14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pijls B., Jolani S., Atherley A., Derckx R., Dijkstra J., Franssen G., et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open. 2021;11(1) doi: 10.1136/bmjopen-2020-044640. [DOI] [PMC free article] [PubMed] [Google Scholar]