Abstract
Recently published studies have found an impaired immune response after SARS-CoV-2 vaccination in solid organ recipients. However, most of these studies have not assessed immune cellular responses in liver and heart transplant recipients. We prospectively studied heart and liver transplant recipients eligible for SARS-CoV-2 vaccination. Patients with past history of SARS-CoV-2 infection or SARS-CoV-2 detectable antibodies (IgM or IgG) were excluded. We assessed IgM/IgG antibodies and ELISpot against the S protein 4 weeks after receiving the second dose of the mRNA-1273 (Moderna) vaccine. Side effects, troponin I, liver tests and anti-HLA donor-specific antibodies (DSA) were also assessed. A total of 58 liver and 46 heart recipients received two doses of mRNA-1273 vaccine. Median time from transplantation to vaccination was 5.4 years (IQR 0.3–27). Sixty-four percent of the patients developed SARS-CoV-2 IgM/IgG antibodies and 79% S-ELISpot positivity. Ninety percent of recipients developed either humoral or cellular response (87% in heart recipients and 93% in liver recipients). Factors associated with vaccine unresponsiveness were hypogammaglobulinemia and vaccination during the first year after transplantation. Local and systemic side effects were mild or moderate, and none presented DSA or graft dysfunction after vaccination. Ninety percent of our patients did develop humoral or cellular responses to mRNA-1273 vaccine. Factors associated with vaccine unresponsiveness were hypogammaglobulinemia and vaccination during the first year after transplantation, highlighting the need to further protect these patients.
KEYWORDS: basic (laboratory) research/science, clinical research/practice, heart transplantation/cardiology, immune regulation, infection and infectious agents - viral, infectious disease, liver transplantation/hepatology, monitoring: immune, T cell biology
Abbreviations: COVID-19, Coronavirus Disease 2019; DSA, donor-specific antibodies; ELISpot, Enzyme-linked Immunosorbent Spot; GFR, glomerular filtrate; HLA, human leukocyte antigen; HTR, heart transplant recipient; LTR, liver transplant recipient; mRNA, messenger RNA; SOT, solid organ transplant
1. INTRODUCTION
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic has rapidly spread around the world with more than 160 million reported cases and more than 3 million deaths.1
The approved mRNA vaccines, BNT162b2 (Comirnaty) (Pfizer/BioNTech)2 and mRNA-1273 (Moderna)3 have shown 94.1%–95% efficacy preventing Coronavirus Disease 2019 (COVID-19) in immunocompetent population.
Solid organ transplant (SOT) recipients are among the most vulnerable patients to develop severe COVID-19 with higher reported morbidity and mortality compared with the general population.4 , 5
Neither SOT patients nor immunocompromised patients were included in the phase 3 clinical trials of the mRNA vaccines, but despite the lack of information on their safety and immunogenicity, the European Society for Organ Transplantation and the American Society for Transplantation recommend vaccination of SOT recipients, considering the potential benefits of the vaccine likely outweigh the risks.
Recently, data on the serologic response to mRNA SARS-Cov-2 vaccine in SOT has been reported. A study including SOT recipients receiving mRNA vaccines (48% received the mRNA-1273 vaccine) showed that only 17% of the patients developed humoral response (anti-S1 or anti–receptor-binding domain) at a median of 20 days after the first dose of the vaccine,6 and 54% after the second dose.7 Consistent with this finding, previous studies on influenza vaccination have shown decreased effectiveness in immunocompromised individuals,8 so additional or higher doses are recommended to increase its immunogenicity.8
Our hypothesis is that the elicited humoral and cellular immune responses to mRNA-1273 SARS-CoV-2 vaccination in liver transplant recipient (LTR) and heart transplant recipient (HTR) could be lower than that reported in the general population because of both the immunosuppressive therapy and primary underlying co-morbid conditions.
Therefore, the primary objectives of the study were to determine (a) the humoral and cellular immune response to the mRNA-1273 vaccine in SARS-CoV-2 naïve LTR and HTR by assessing the detection of de novo antibodies (IgM/IgG) targeting the receptor-binding domain (RBD) of the S1 spike protein (IgM or IgG) and the cellular response to the S protein of SARS-CoV-2 by the ELISpot technique. The secondary objective was to assess the safety of the mRNA-1273 vaccine in SARS-CoV-2 naïve LTRs and HTRs.
2. MATERIALS AND METHODS
All HTRs and LTRs followed up at our institution received two doses of the mRNA-1273 SARS-CoV-2 vaccine (100 mcg administered in the deltoid region, 4 weeks apart from the first dose) as part of the national vaccination program. All consecutive patients receiving the vaccine were screened for this study.
Patients with multiorgan transplant, with past history of SARS-CoV-2 infection or positive SARS-CoV-2 serology at baseline, were excluded from the study.
Patients who agreed to participate signed the informed consent, and blood samples were drawn at baseline and 4 weeks after each vaccine dose. The choice of the time-points was based according to the previous experience of the phase-1 trial.3 In all the time-points, we studied the antibodies response against the S protein (IgM/IgG) and the cellular response to the S protein of SARS-CoV-2 virus by means of the ELISpot technique. The Institutional Ethics Committee approved the study (HCB/2021/0222).
During the study period, the 14-day accumulated incidence of COVID-19 in Barcelona was between 176 and 248 cases/100.000.9
2.1. Patient information
The following variables were collected for analysis: age, gender, body mass index, comorbidities, date of transplant, immunosuppressive regimen, and use of induction or rejection treatment in the last year. Lymphopenia (defined as less than 1000 lymphocytes/mm3), hypogammaglobulinemia (defined as less than 6.8 g/L of total IgG at the time of inclusion), ultrasensitive troponin levels, liver function tests, chronic kidney disease (CKD) (defined as maintained GFR <60 ml/min/1.73 m2 in the last 3 months) were also collected.
Secondary outcomes included the analysis of all the baseline factors associated with no-response to the vaccine for either cellular or humoral response or both. Safety analysis included phone interview with patients 48–72 h after each dose to assess patient’s reported side effects, using a predefined questionnaire on local and systemic symptoms with a semiquantitative scale (none/mild/moderate/severe). Troponin I levels and liver tests were also performed at baseline, before the second dose and 4 weeks after the second dose. We additionally screened anti-HLA donor-specific HLA antibodies at baseline and 4 weeks after the second dose by a Luminex-based bead assay technique. Samples were screened using the Lifecodes LifeScreen Deluxe kit (Lifecodes, division of Immucor, Stamford, CT). If positive, antibody HLA specificities were determined using the Lifecodes Single Antigen bead assay (Lifecodes, division of Immucor, Stamford, CT). All beads showing a MFI >3000 were considered positive.
2.2. Quantification of antibodies to SARS-CoV-2
A serological assay based on the chemiluminescent immunoassay by Atellica analyzer was used to determine the presence of total (IgG and IgM) antibodies against SARS-CoV-2, Siemens SARS-CoV-2 Total (COV2T). When COV2T showed to be positive, Siemens SARS-CoV-2 IgG (COV2G) antibody test was realized, following manufacturer instructions. Both COV2T and COV2G antibody tests are directed against the spike 1 protein receptor binding domain (S1-RBD). Sensitivity and specificity of COV2T of 93.2%–97.5% and 99.5%–100%, respectively, at >21 days after PCR positivity has been reported,10 , 11 whereas a COV2G sensitivity of 92.2% and specificity of 100% between days 7 and 13 after PCR positivity was published.11
2.3. IFN-γ ELISpot
Stimulation was conducted with 2 × 105 PBMCs in X-VIVOTM 15 medium (Lonza) supplemented with 10% heat-inactivated AB serum and PepTivator® SARS-CoV-2 Prot_S peptide pools1 (1 µg/ml, Miltenyi Biotec). Negative control wells lacked peptides, and positive control wells included mAb CD3-2 of Kit. Cells were incubated overnight (16–20 h) at 37°C 5% CO2 in precoated anti-IFN-γ MSIP white plates (mAb 1-D1K, Mabtech). Plates were then washed five times with PBS (Sigma-Aldrich) and incubated for 2 h at room temperature with horseradish peroxidase–conjugated anti-IFN-γ detection antibody (1 μg/ml; clone mAb-7B6-1; Mabtech). After five further washes with PBS, tetramethylbenzidine substrate was added and spots were counted using an automated ELISpot Reader System (Autoimmun Diagnostika GmbH).
To quantify positive peptide-specific responses, spots of the unstimulated wells were subtracted from the peptide-stimulated wells, and the results expressed as spot-forming units (SFU)/2 × 105 peripheral blood mononuclear-stem cells (PBMCs). We determined SARS-CoV-2–specific spots by spot increment, defined as stimulated spot numbers ≥6 SFU/2 × 105 PBMCs. This cutoff was defined by calculating the mean ± 2 standard deviations of SFU/2 × 105 PBMCs in a group of healthy donors obtained prior to the start of the pandemic of SARS-CoV-2.
2.4. Statistical analysis
Continuous variables have been described as mean with standard deviation or median and interquartile range, according to data distribution. Categorical variables have been described as either absolute frequencies or percentages. The relationship between baseline variables and vaccine no-response was assessed with univariable logistic regression analysis. Age, sex and variables that were associated with the primary outcome with a p < .1 were finally entered into the multivariable logistic analysis. Changes in the ELISpot and antibodies titles through time points were assessed by Wilcoxon test for related samples. Difference in the ELISpot forming units and antibodies titles between groups was analyzed by Mann–Whitney test for independent samples. All statistical tests have been conducted with a 95% confidence interval and a p-value <.05 has been considered significant. To perform all the analysis, the software SPSS v.25 (SPSS inc) has been used. Figures were designed with GraphPad v.5 (GraphPad Software).
3. RESULTS
A total of 116 liver and HTRs were screened for the study, 11 patients had baseline positive IgG SARS-CoV-2 antibodies and one presented SARS-CoV-2 mild infection 2 weeks after the first dose and therefore were excluded from the study. A total of 104 patients (58 liver and 46 heart recipients) received two doses of the mRNA-1273 SARS-CoV-2 vaccine and were prospectively followed.
3.1. Baseline characteristics
Baseline characteristics according to type of organ transplanted are described in Table 1. Median age in both groups was 60 years and predominantly male. None of the patients had more than one transplant. HTRs presented more frequently dyslipidemia and CKD. Vaccination during the first year after transplant was significantly more common in LTRs, 10 (17.2%), compared with 2 (4.3%) in HTRs and the majority of them, 10 (83%), received the vaccine between 3 and 6 months posttransplant. Patients who required induction immunosuppression received basiliximab. None of the patients experienced rejection within 12 months of vaccination needing steroids boluses or lymphocyte depletion agents. Use of mycophenolate acid and prednisone was significantly more frequent in HTRs. Most of the HTRs had an immunosuppressive regimen containing three drugs, whereas most of the LTRs were of monotherapy or dual therapy. There were no major differences in other baseline characteristics.
TABLE 1.
Baseline characteristics according to type of transplantation
| Heart recipients n = 46 | Liver recipients n = 58 | p-value | |
|---|---|---|---|
| Age (years), median | 60 (20–80) | 61.5 (18–88) | .6 |
| Sex (female) | 13 (28%) | 18 (31%) | .7 |
| Hypertension | 28 (61%) | 33 (57%) | .7 |
| Diabetes | 14 (30%) | 21 (36.2%) | .5 |
| BMI, median (IQR) | 24.8 (18–36.5) | 26.3 (17–42) | .8 |
| Dyslipidemia | 36 (78%) | 22 (38%) | <.001 |
| HIV infection | 0 | 2 (3.4%) | 1 |
| Median time from transplantation, years (IQR) | 6.3 (0.4–21) | 4.6 (0.3–26.8) | .4 |
| First-year posttransplant | 2 (4.3%) | 10 (17.2%) | .04 |
| Prior transplantation | 0 | 0 | — |
| Acute allograft rejection (last year) | 0 | 0 | — |
| Induction (past year) | .6 | ||
| ATG | — | — | |
| Basiliximab | 2 (4.3%) | 4 (7%) | |
| Rituximab | — | — | |
| Immunosuppressive regimen | <.001 | ||
| Quadruple therapy | 3 (6.5%) | — | |
| Triple therapy | 35 (76%) | 7 (12%) | |
| Bitherapy | 8 (17%) | 23 (40%) | |
| Monotherapy | — | 27 (47%) | |
| No therapy | — | 1 (2%) | |
| Type of immunosuppressive drug | <.001 | ||
| Calcineurin inhibitors | 45 (98%) | 53 (91%) | |
| Mycophenolate | 33 (72%) | 15 (26%) | |
| Prednisone | 38 (83%) | 13 (22%) | |
| mTOR inhibitors | 15 (33%) | 13 (22%) | |
| Lymphopenia (<1000/mm3) (%yes) | 4 (8.7%) | 12 (20.7%) | .09 |
| Hypogammaglobulinemia (<6.8 g/L IgG) | 9 (19.6%) | 8 (14%) | .4 |
| Chronic kidney disease (GFR <60 ml/min/1.73 m2) | 21(45.6%) | 15 (26%) | .004 |
3.2. Humoral and cellular response to the mRNA-1273 vaccine
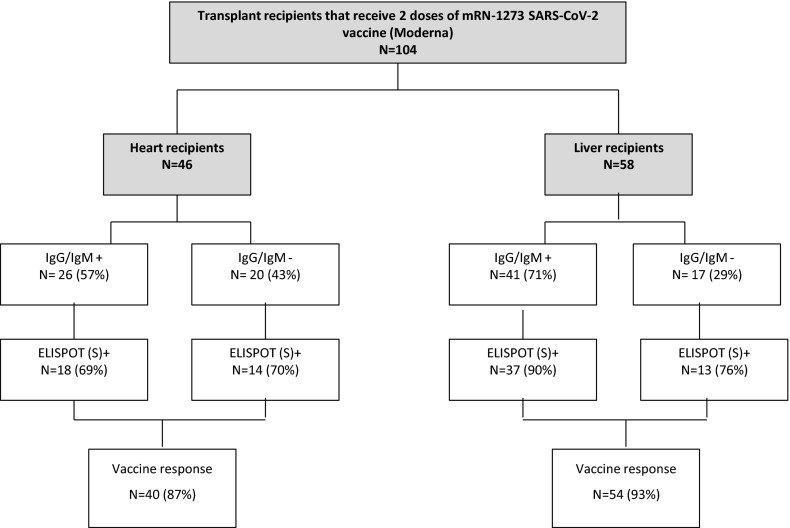
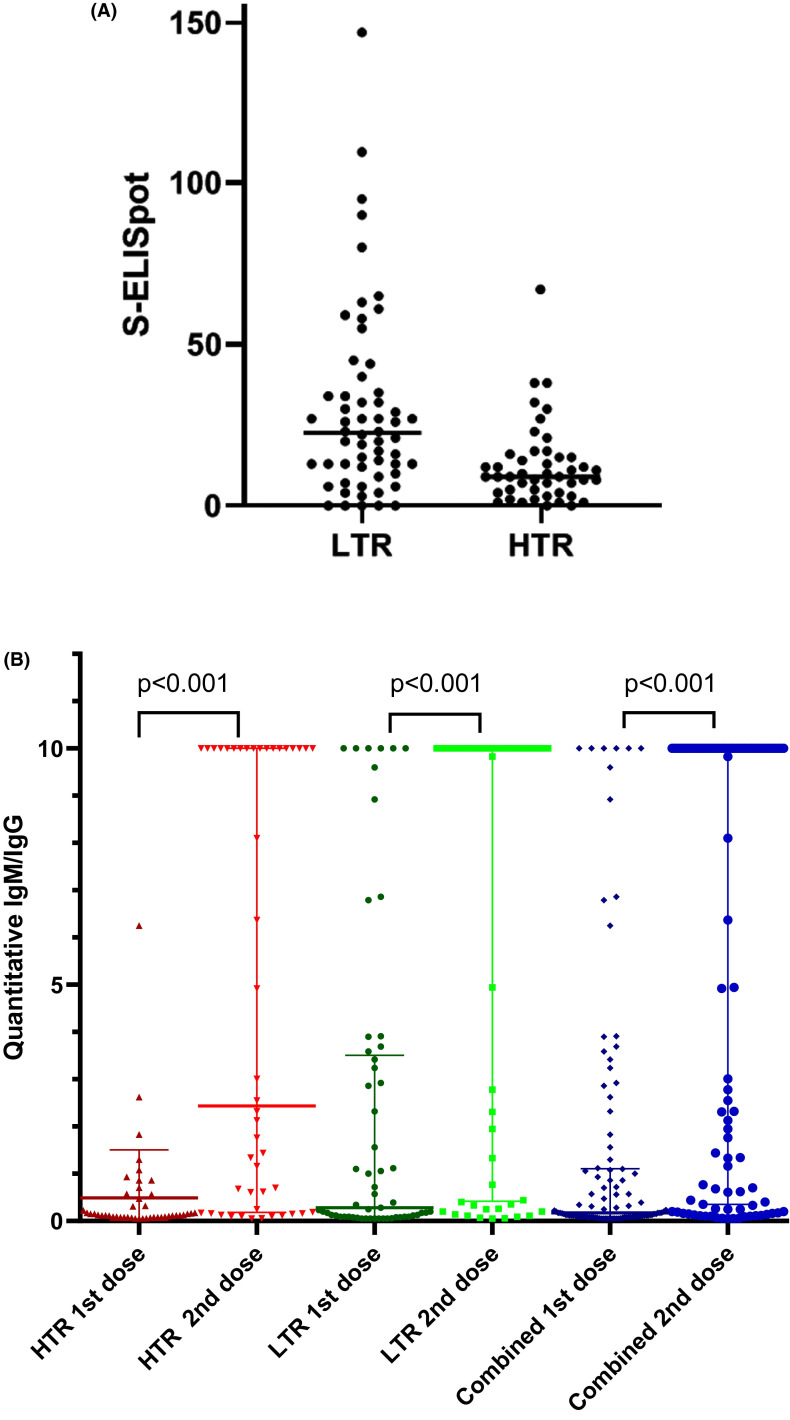
Figure 1 shows the development of humoral and/or cellular response in SARS-CoV-2-naïve patients after administration of two doses the mRNA-1273 vaccine. Out of 58 LTR, 22 (37.9%) had SARS-CoV-2 antibodies after the first dose of the vaccine, and 41 (71%) after the second dose. Out of 46 HTRs, 5 (11%) had SARS-CoV-2 antibodies after the first dose of the vaccine and 26 (57%) after the second dose. After the second dose of the vaccine, 50 (86%) LTR showed S-ELISpot positivity, and 32 (70%) HTRs showed S-ELISpot positivity. Six (13%) HTRs and four (7%) LTRs did not develop any kind of response to the vaccine. Figure 2A shows ELISpot 4 weeks after the second dose of vaccination. Median S-ELISpot response was significantly higher in LTRs compared with HTRs (p < .001).
FIGURE 1.
Development of humoral and/or cellular responses in SARS-CoV-2-naïve patients after administration of the mRNA-1273 vaccine. Patients with previous SARS-CoV-2 humoral response were excluded from the analysis
FIGURE 2.
(A) S-ELISpot 4 weeks after 2 doses of mRNA-1273 vaccine. (B) Humoral responses 4 weeks after first and second dose of mRNA-1273 vaccine. HTR, heart transplant recipients; LTR, liver transplant recipients [Color figure can be viewed at wileyonlinelibrary.com]
Table 2 and Figure 2B show quantitative data on humoral and cellular responses. Median IgG/IgM antibodies were significantly higher after the second dose of the vaccine in HTRs, LTRs, and all patients combined (p < .001).
TABLE 2.
Humoral and cellular responses to the mRNA-1273 vaccine
| HTR Median (IQR) |
LTR Median (IQR) |
Combined Median (IQR) |
|
|---|---|---|---|
| IgG/IgM antibodies 4 weeks after first dose of mRNA–1273 vaccine | 0.13 (0.44) | 0.28 (3.42) | 0.17 (1.01) |
| IgG/IgM antibodies 4 weeks after second dose of vaccine | 2.44 (9.81) | 10 (9.58) | 10 (9.65) |
| No of spots per 200 000 4 weeks after second dose of mRNA–1273 vaccine | 10 (12) | 24.5 (27.5) | 14 (22.5) |
Abbreviations: HTR, heart transplant recipient, LTR, liver transplant recipient.
Overall, 94 (90%) patients had humoral or cellular response to the mRNA-1273 SARS-CoV-2 vaccine.
3.3. Factors associated with lack of response to the vaccine
Table 3 describes the logistic regression analysis of factors associated with vaccine unresponsiveness, absence of SARS-CoV-2 IgG/IgM antibodies, 4 weeks after the second dose of mRNA-1273 SARS-CoV-2 vaccine by type of transplantation. Risk factors associated with lack of antibody response in both groups were hypogammaglobulinemia (OR 61 [95% CI 3.7–1000.4]), vaccination in the first posttransplant year (OR 30.7 [95% CI 3.1–307.2]), and high-dose mycophenolate acid use (OR 10.1 [95% CI 2.3–44.3]). Age was the only significant factor associated with lack of humoral response in HTRs. In contrast, hypogammaglobulinemia, vaccination within 1 year after LT and use of mycophenolate were significantly associated with lack of humoral response in LTR.
TABLE 3.
Multivariable analysis on factors associated with vaccine unresponsiveness (absence of SARS-CoV-2 IgG/IgM antibodies) 4 weeks after the second dose of mRNA-1273 SARS-CoV-2 vaccine
| Variable | Combined (OR; 95% CI) | Heart |
Liver |
|||
|---|---|---|---|---|---|---|
| p-value | (OR; 95% CI) | p-value | (OR; 95% CI) | p-value | ||
| Age | 1.04 (1–1.08) | .077 | 1.06 (1.004–1.12) | .035 | ||
| Sex (female) | 0.32 (0.1–1) | .05 | 0.09 (0.01–0.8) | .032 | ||
| Hypogammaglobulinemia | 5.7 (1.6–21.1) | .008 | 61 (3.7–1004.3) | .004 | ||
| Vaccination first year after transplant | 11.9 (2.4–58.42) | .002 | 30.7 (3.1–307.2) | .004 | ||
| High dose of mycophenolic acid (≥1500 mg/day) | 3.34 (1.4–7.7) | .005 | 10.1 (2.3–44.3) | .002 | ||
Table 4 describes the logistic regression analysis of factors associated with the absence of S-ELIspot positivity 4 weeks after the second dose of mRNA-1273 SARS-CoV-2 vaccine. Hypogammaglobulinemia was the only variable associated with the absence of cellular response in the entire cohort (OR 5.54 [95% CI 1.73–17.7]) and also in the HTR cohort, whereas this association was not observed in LTRs.
TABLE 4.
Multivariable analysis on factors associated with vaccine unresponsiveness (absence of S-ELISpot positivity) 4 weeks after the second dose of mRNA-1273 SARS-CoV-2 vaccine
| Variable | Combined (OR; 95% CI) | Heart |
Liver |
|||
|---|---|---|---|---|---|---|
| p-value | (OR; 95% CI) | p-value | (OR; 95% CI) | p-value | ||
| Age | 0.99 (0.95–1.03) | .75 | ||||
| Sex (female) | 1.4 (0.4–5.1) | .6 | ||||
| Hypogammaglobulinemia | 5.5 (1.7–17.7) | .004 | 9.87 (1.65–59.15) | .012 | ||
| Vaccination first year after transplant | 4.6 (0.8–25.1) | .078 | ||||
Finally, we assessed the predictors of absence of both humoral and cellular response after two doses of the mRNA-1273 SARS-CoV-2 vaccine ( Table 5). In the entire cohort, hypogammaglobulinemia (OR 11.13 [95% CI 2.5–49.4]) and vaccination (OR 7 [95% CI 1.07–45.8]) within the first year of transplant were strongly correlated with lack of response.
TABLE 5.
Multivariable analysis on factors associated with vaccine unresponsiveness (absence of IgG antibodies AND S-ELISpot) 4 weeks after the second dose of mRNA-1273 SARS-CoV-2 vaccine
| Variable | Combined (OR; 95% CI) | Heart |
Liver |
|||
|---|---|---|---|---|---|---|
| p-value | (OR; 95% CI) | p-value | (OR; 95% CI) | p-value | ||
| Age | 1.04 (0.96–1.1) | .31 | ||||
| Sex (female) | 0.64 (0.1–4.02) | .64 | ||||
| Chronic kidney disease | 4.3 (0.8–22.7) | .08 | ||||
| Hypogammaglobulinemia | 11.13 (2.5–49.4) | .002 | 19.98 (1.99–200.16) | .011 | ||
| Vaccination first year after transplant | 7 (1.07–45.8) | .042 | 20.14 (1.8–221.7) | .014 | ||
3.4. Safety
Pain in the site of injection was the most common patient-reported side effect (80%), followed by fatigue (15%), swelling (12%), and low-grade fever (7%). There were no significant differences between the first and the second dose for all the reported side effects. Patients required analgesics in 33% and 40% of cases after the first and the second dose, respectively.
No evidence of organ damage was observed: In the HTR population, there were no significant changes in ultrasensitive troponine level thorough the study period. In the LTR population, there were no significant changes in transaminase levels thorough the study period.
No recipient showed an increase in HLA antibodies from baseline to 3 weeks after the second dose of the vaccine. No patient developed rejection after vaccination. There have been no cases of COVID-19 among the vaccinated recipients to date.
4. DISCUSSION
In our study, we found that mRNA SARS-CoV-2 vaccines were safe in LTRs and HTRs, with a seroconversion rate of 64% and immune cellular response of 79%. Overall, 90% of our study population showed a positive response to the vaccine. To our knowledge, this is the first study reporting both humoral and cellular responses to the mRNA-1273 vaccine in HTRs and LTRs.
SOT recipients have been reported to have worse outcomes after SARS-CoV-2 infection, with an overall 20% mortality rate.12 A lot of effort and hope has been put on vaccines, as a strategy to protect this group of patients; however, the large clinical trials did not include immunocompromised patients in their design. To date, only a few studies showing humoral responses in SOT have been published.
In our study, the vaccine was found to be safe with only local adverse events reported, and no episodes of rejection were reported in the immediate follow-up. This is in concordance with the previous studies in solid organ transplantation.7 , 13
Regarding humoral response, our LTRs had 71% rate of seroconversion after the second dose of the vaccine. Rabinowich et al.13 found that 47.5% of their LTR seroconverted after vaccination with BNT162b2. In contrast, Boyarsky et al.7 found that 80% of their LTRs developed antibody response in their study with both mRNA-approved vaccines. Use of antimetabolites has been associated with worse seroconversion rates6 , 14; in our series, we also found an association with the use of mycophenolate, despite only having 26% of our LTRs with mycophenolate, compared with Boyarsky (72% of the patients with antimetabolites) and Rabinowich (50%). However, the factors that were more strongly correlated with the lack of response were hypogammaglobulinemia and vaccination during the first year after transplantation. Neither Rabinowich nor Boyarsky collected hypogammaglobulinemia as a variable of their study, and they did not find a correlation between time of transplant and vaccination. Hypogammaglobulinemia and vaccination during the first year after transplantation are strongly correlated, although more common in lung and HTR, some studies have found that up to 26% of LTRs have hypogammaglobulinemia in the first year after transplant.15
Our HTR had lower rates of seroconversion compared with the LTR, probably related to the use of more aggressive immunosuppressive regimens (76% on triple therapy vs. 12% in LTR). HTRs are known to have suboptimal vaccine responses, especially in the early posttransplant, mainly because of hypogammaglobulinemia16 and increased immunosuppressive regimens. In our study, 57% of the HTRs had positive serology after the second dose of the vaccine. This is concordant with the series reported by Boyarsky et al.7 (56%) and Itzhaki et al.17 (49%) but much higher than that reported by Peled et al.18 (18%) and much lower than that reported by Marinaki et al.19 (75%). It is hard to hypothesize which factors could explain this difference. The type of vaccine used in the studies differed: mRNA-1273 vaccine in our group versus BNT162b2 vaccine in Peled’s, Itzhaki’s, or Marinaki’s. Interestingly, compared with Peled, our recipients were more frequently on a triple immunosuppressive regimen (75% in our HTRs vs. 48% in Peled’s HTRs); nevertheless, the rate of mycophenolate use in both studies was similar (75% vs. 71%). The fact that we could not identify this association is because of the small size of our cohort because we were able to identify the association when we combined the whole study group. Small number of patients and heterogeneous populations included in all these reports probably account for the differences in seroconversion rates.
Reports of cellular immunity in SOTs are scarce. Cellular immunity probably plays an important role in the control of SARS-CoV-2 infection. Recent studies in lung and kidney transplant recipients found lower cellular responses compared with our results.20 , 21 In our study, we found very high rates of combined humoral and cellular responses of up to 90%. It is to note that more than 70% of patients who had negative antibodies after both doses of vaccines had S-ELISpot positivity. Cucchiari et al.20 found 35% S-ELISpot positivity in their cohort of kidney and kidney-pancreas transplant recipients receiving mRNA-1273 vaccine. Similarly, Havlin et al.21 were able to detect specific T cell in 33% of their lung transplant recipients, none of which had detectable humoral response after two doses of BNT162b2 vaccine. Overall, with the available data, LTRs and HTRs seem to have higher seroconversion rates to either of the mRNA vaccines,7 than kidney or lung transplant recipients, and therefore it is not surprising that the cellular responses are higher too.
Risk factors associated with lack of any type of response were again hypogammaglobulinemia and vaccination during the first year after transplantation, suggesting that these patients are probably the most vulnerable. Identifying these patients is crucial, advising them to maintain precautions until herd immunity is achieved and encouraging their close contacts to get vaccinated.
Our study has several limitations; first, we included a small number of patients, and we did not include a control group. However, there are extensive and consistent data in large clinical trials regarding efficacy of vaccine in healthy individuals.2 , 3 We did not evaluate baseline and in-between vaccine dose cellular responses; therefore, we could have missed patients with asymptomatic SARS-CoV-2 infection that did not produce antibodies. Our study analyzed immunity response to an specific mRNA SARS-CoV-2 vaccine, in line with recent published data,17 , 19, 20, 21 but immunity response to other vaccines should be evaluated to select the optimal vaccine in SOT recipients. Finally, whether cellular responses are associated with protection is yet to be elucidated.
We believe our study adds evidence to the scarce published data in SOTs. SOT recipients that receive SARS-CoV-2 vaccines should continue to maintain precautions and encourage vaccination of their household. Whether additional22 or delayed doses of vaccine might help to boost humoral and cellular responses to SARS-CoV-2 vaccines, similar to other respiratory viruses’ vaccines23 is yet to be elucidated. Similarly, other types of vaccines such as vector vaccines or protein subunit vaccines might have different immunogenic properties in this specific population.
In conclusion, mRNA-1273 SARS-CoV-2 vaccine showed a high rate of immunological response, with a combination of humoral and cellular responses close to 90% in our HTR and LTR cohort. Factors associated with vaccine unresponsiveness were hypogammaglobulinemia and vaccination during the first year after transplantation, highlighting the need to identify these patients.
ACKNOWLEDGMENTS
This study was funded by Marató TV3 grant (research projects on COVID-19). The authors thank Juan Carlos de la Fuente, Fina Casal, and Estefania Torrecilla for their support in logistic tasks.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
ENDNOTE
1 The PepTivator® SARS-CoV-2 Prot_S is a pool of peptides, consisting mainly of 15-mer sequences with 11 amino acids overlap, covering the immunodominant sequence domains of the spike glycoprotein (“S”) of SARS-Coronavirus 2 (GenBank MN908947.3, Protein QHD43416.1).
Funding information Fundació la Marató de TV3
Footnotes
Sabina Herrera and Jordi Colmenero contributed equally to this work.
REFERENCES
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report. [PubMed]
- 2.Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/nejmoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/nejmoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivinius R, Kaya Z, Schramm R, et al. COVID-19 among heart transplant recipients in Germany: a multicenter survey. Clin Res Cardiol. 2020;109(12):1531–1539. doi: 10.1007/s00392-020-01722-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumortier J, Duvoux C, Roux O, et al. Covid-19 in liver transplant recipients: the French SOT COVID registry. Clin Res Hepatol Gastroenterol. 2021;45(4) doi: 10.1016/j.clinre.2021.101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;22(12):7–9. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manuel O, Pascual M, Hoschler K, et al. Humoral response to the influenza a H1N1/09 monovalent AS03-adjuvanted vaccine in immunocompromised patients. Clin Infect Dis. 2011;52(2):248–256. doi: 10.1093/cid/ciq104. [DOI] [PubMed] [Google Scholar]
- 9.#COVID19aldiaBCN. https://aspb.shinyapps.io/COVID19_BCN/#Incidència_acumulada. Accessed July 1, 2021.
- 10.Harritshøj LH, Gybel-Brask M, Afzal S, et al. Comparison of 16 serological SARS-CoV-2 immunoassays in 16 clinical laboratories. J Clin Microbiol. 2021;59(5) doi: 10.1128/jcm.02596-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Florin L, Maelegheer K, Vandewal W, Bernard D, Robbrecht J. Performance evaluation of the siemens SARS-CoV-2 total antibody and IgG antibody test [published online ahead of print 2021]. Lab Med. 10.1093/labmed/lmab027 [DOI] [PMC free article] [PubMed]
- 12.Alfishawy M, Elbendary A, Mohamed MNM. COVID-19 mortality in transplant recipients. Int J organ Transplant Med. 2020;11(4):145–162. [PMC free article] [PubMed] [Google Scholar]
- 13.Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baluch A, Humar A, Eurich D, et al. Randomized controlled trial of high-dose intradermal versus standard-dose intramuscular influenza vaccine in organ transplant recipients. Am J Transplant. 2013;13(4):1026–1033. doi: 10.1111/ajt.12149. [DOI] [PubMed] [Google Scholar]
- 15.Doron S, Ruthazer R, Werner BG, Rabson A, Snydman DR. Hypogammaglobulinemia in liver transplant recipients: Incidence, timing, risk factors, and outcomes. Transplantation. 2006;81(5):697–703. doi: 10.1097/01.tp.0000180531.66518.9e. [DOI] [PubMed] [Google Scholar]
- 16.Sarmiento E, Jaramillo M, Calahorra L, et al. Evaluation of humoral immunity profiles to identify heart recipients at risk for development of severe infections: a multicenter prospective study. J Hear Lung Transplant. 2017;36(5):529–539. doi: 10.1016/j.healun.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Itzhaki Ben Zadok O, Shaul AA, Ben-Avraham B, et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients – a prospective cohort study [published online ahead of print 2021]. Eur J Heart Fail. 10.1002/ejhf.2199 [DOI] [PubMed]
- 18.Peled Y, Ram E, Lavee J, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Hear Lung Transplant. 2021;40(8):759–762. doi: 10.1016/j.healun.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients [published online ahead of print 2021]. Am J Transplant. 10.1111/ajt.16607 [DOI] [PMC free article] [PubMed]
- 20.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients [published online ahead of print May 26, 2021]. Am J Transplant. 2021. 10.1111/ajt.16701 [DOI] [PMC free article] [PubMed]
- 21.Havlin J, Svorcova M, Dvorackova E, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Hear Lung Transplant. 2021;40(8):754–758. doi: 10.1016/j.healun.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients [published online ahead of print 2021]. N Engl J Med. 10.1056/NEJMc2108861 [DOI] [PMC free article] [PubMed]
- 23.Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis. 2018;66(11):1698–1704. doi: 10.1093/cid/cix1082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.