Highlights
-
•
Alien and indigenous springtail species differ in basal desiccation resistance.
-
•
That difference is more pronounced at lower than at higher temperatures.
-
•
Strong phylogenetic signal suggests that differences lie with certain taxa.
-
•
Plasticity of desiccation resistance to temperature does not differ between the groups.
-
•
Trait-based approaches provide insights into soil faunal invasions.
Keywords: Alien species, Laboratory evolution, Soil fauna, Springtails, Traits, Water balance
Abstract
Biological invasions have significant ecological and economic impacts. Much attention is therefore focussed on predicting establishment and invasion success. Trait-based approaches are showing much promise, but are mostly restricted to investigations of plants. Although the application of these approaches to animals is growing rapidly, it is rare for arthropods and restricted mostly to investigations of thermal tolerance. Here we study the extent to which desiccation tolerance and its phenotypic plasticity differ between introduced (nine species) and indigenous (seven species) Collembola, specifically testing predictions of the ‘ideal weed’ and ‘phenotypic plasticity’ hypotheses of invasion biology. We do so on the F2 generation of adults in a full factorial design across two temperatures, to elicit desiccation responses, for the phenotypic plasticity trials. We also determine whether basal desiccation resistance responds to thermal laboratory natural selection. We first show experimentally that acclimation to different temperatures elicits changes to cuticular structure and function that are typically associated with water balance, justifying our experimental approach. Our main findings reveal that basal desiccation resistance differs, on average, between the indigenous and introduced species, but that this difference is weaker at higher temperatures, and is driven by particular taxa, as revealed by phylogenetic generalised least squares approaches. By contrast, the extent or form of phenotypic plasticity does not differ between the two groups, with a ‘hotter is better’ response being most common. Beneficial acclimation is characteristic of only a single species. Laboratory natural selection had little influence on desiccation resistance over 8–12 generations, suggesting that environmental filtering rather than adaptation to new environments may be an important factor influencing Collembola invasions.
1. Introduction
Invasive alien species are one of the leading drivers of environmental change. They can cause significant economic and environmental damage (Simberloff et al., 2013; Diagne et al., 2021), and are increasing in number and global extent (Seebens et al., 2017). Much effort has focussed on understanding the intrinsic (e.g., life history characteristics) and extrinsic (e.g., receiving environment, propagule pressure) factors which contribute to the successful movement of species along the invasion pathway (Richardson and Pyšek, 2006; Blackburn et al., 2011; Enders et al., 2020). Increasing appreciation of traits-based approaches to understanding ecological communities (Kearney et al. 2006; Gallagher et al., 2020) has seen growth in their application to biological invasions (van Kleunen et al., 2010a; Hulme and Bernard-Verdier, 2018; Renault et al., 2018), building on early work demonstrating their utility (Kolar and Lodge, 2001; Jeschke and Strayer, 2008).
Until recently, plants have been the main focus of such trait-based approaches (van Kleunen et al., 2010b; Davidson et al., 2011) and they continue to dominate empirical research in this area (Gallien et al., 2019; Milanović et al., 2020). A suite of traits is thought to underlie the success of plant invasions, related in part to the typical form of such traits (e.g., fast growth) (Van Kleunen et al., 2015) and in part to the extent of their phenotypic plasticity (Enders et al., 2020). Yet comparative information is now accumulating about animals in this context, with similar, consistent variation in traits often being found between indigenous and introduced species (Kelly, 2014; Capellini et al., 2015; Allen et al., 2017; Redding et al., 2019). Surprisingly, only a few studies have sought to adopt a traits-based approach to understanding arthropod biological invasions, despite the diversity and importance of terrestrial arthropods as invasive species (Hill et al., 2016; Cicconardi et al., 2017), and their extraordinary economic impacts (Bradshaw et al., 2016). Typically, these studies have concerned thermal traits of the groups under investigation (Janion et al., 2010; Weiner et al., 2010; Yu et al., 2012; Jarošík et al., 2015; Janion-Scheepers et al., 2018).
For terrestrial ectotherms, however, water balance – and desiccation resistance in particular (the ability to resist loss of body water through a variety of mechanisms (Hadley, 1994; Chown and Nicholson, 2004)) – have profound influences on the abundance and distribution of many species, including those that have become invasive (Holway et al., 2002; Parkash et al., 2008; Chown et al., 2011; Peguero et al., 2019). By contrast with thermal traits, much less attention has been given to the extent to which indigenous and invasive species might differ consistently in desiccation resistance. The few studies that have been undertaken on multiple species have either identified variation in basal desiccation resistance, or in plasticity of desiccation resistance, as important differences among indigenous and invasive species (Chown et al., 2007; Ajayi et al., 2020; Phillips et al., 2020; da Silva et al., 2021). But their outcomes have not been consistent, despite suggestions from environmental correlates of the success of invasive species (Staubus et al., 2019), and from macrophysiological investigations of species-level variation in desiccation resistance (Addo-Bediako et al., 2001; Kellermann et al., 2020), that expectations of reliable differences among indigenous and invasive species are plausible, and that these might largely be expected in basal resistance.
Therefore, whether desiccation resistance plays a role in the success of invasive animal ectotherm species remains unclear. Moreover, if it has a role, whether this role is through environmental filtering of species in the initial stages of invasion (Renault et al., 2018), or through evolutionary shifts in desiccation resistance following establishment in the introduced range, as is recognised for other traits (Van Kleunen et al., 2018; Reznick et al., 2019; Szűcs et al., 2019; but see also Bertelsmeier and Keller, 2018), remains unknown. Such differences lie at the heart of discussions about whether species distribution modeling is useful for understanding and forecasting the outcomes of invasions (Liu et al., 2020). For ectotherms this may be especially important because some factors associated with water balance or environmental precipitation are routinely identified by selection procedures used in such modeling (Bradie and Leung, 2017).
In this study, we therefore explicitly examine variation in desiccation resistance between indigenous and introduced species, focussing on basal resistance and on its plasticity in response to different thermal regimes (Chown et al., 2007). In doing so we test predictions from the two traits-based hypotheses central to invasion biology (Van Kleunen et al., 2018; Enders et al., 2020). The ‘ideal weed’ hypothesis predicts that invasive alien species have one or more traits (including physiological traits) which provide them with an advantage over their indigenous counterparts. The ‘plasticity’ hypothesis makes the prediction for which it is named. That is, phenotypic plasticity is key to the success of invasive species and is more pronounced in this group when compared with indigenous species (though these comparisons have some complexity (van Kleunen et al., 2010a, 2018)). As the exemplar group for testing these hypotheses, we use Collembola because of their significance in soil systems (Potapov et al., 2020), because invasive springtails displace indigenous species (Convey et al., 1999; Terauds et al., 2011; Chown et al., 2022), alter ecosystem structure (Greenslade, 2018; Treasure et al., 2019), and have the potential to affect ecosystem functioning (Leinaas et al., 2015; Potapov et al., 2020), and because tests of these hypotheses have so far been developed most extensively for this group, but only for species from the sub-Antarctic islands (Chown et al., 2007; Phillips et al., 2020).
In these previous investigations, temperature exposures in the form of acclimation treatments have been used to examine plasticity in desiccation resistance. Here we do so too. A response in desiccation resistance to temperature as a cue (and specifically temperature acclimation) alone has been demonstrated in hemiedaphic and other Collembola (Chown et al., 2007; Leinaas et al., 2009; Phillips et al., 2020) and in insects (Terblanche and Chown, 2006; Fischer and Kirste, 2018; Baumgart et al., 2022). Using temperature as a cue to elicit a response to dry conditions not only has these empirical precedents, but also has a firm basis in theory. From a theoretical perspective, at a constant humidity, lower temperatures necessarily mean a reduced vapor pressure (or saturation) deficit (Campbell and Norman, 1998). Moreover, hygrosensing in arthropods typically involves an integrated and combined sensory system, serving both thermo- and hygrosensing, which includes a variety of receptors and integration pathways (Altner and Loftus, 1985; Frank et al., 2017; Marin et al., 2020). Such a system appears to be conserved across invertebrates (Russell et al., 2014; Enjin, 2017). Moreover, at high temperatures more cuticular hydrocarbons (CHCs) melt and those that are liquid show a viscosity decline (Baumgart et al., 2022). Therefore, a theoretical expectation exists and some empirical data support the idea that temperature change alone should elicit a desiccation resistance response. In an initial experiment, we first further test this theoretical expectation (complementing Baumgart et al., 2022) by examining traits of the cuticle associated with water management. These are contact angle between water and the cuticle (Helbig et al., 2011; Gunderson et al., 2015; Hensel et al., 2016) (a functional trait) and the general elemental composition of the cuticle (a structural trait, as a proxy for hydrocarbon types, which we lacked the instrumentation to measure) (Menzel et al., 2007, 2022; Nickerl et al., 2014; Schmüser et al., 2020), recalling that in the Collembola we examine here, tracheae are absent, precluding water loss via such a gas exchange system (Hopkin, 1997).
We also examine the extent to which desiccation resistance might respond to laboratory selection under high temperatures. We do so to inform perspectives on what the relative roles of environmental filtering and post-introduction evolutionary change might be given the focus on such matters in forecasting and understanding invasions (Bertelsmeier and Keller, 2018; Liu et al., 2020).
2. Material and methods
2.1. Collection, identification, and alien species assignment
For this study, sixteen springtail species (nine alien, seven indigenous) were collected mostly from across Australia (two species were collected on the sub-Antarctic island of South Georgia to broaden the latitudinal scope of the study) (Table 1), between 2013 and 2015 as part of a broader study investigating differences in traits among indigenous and alien springtail species across a broad latitudinal gradient (Janion-Scheepers et al., 2018). Hemiedaphic (litter-dwelling) species (Christiansen, 1964) were the main focus of this study. At each site, leaf litter samples were collected and extracted using Tullgren funnels. Individuals were initially assigned to species in the field based on morphological characteristics with between 50 and 100 individuals collected per species. Adults were maintained in 70 mL plastic vials lined with moist, mixed Plaster-of-Paris:Charcoal powder (9:1) base substrates until their return to the laboratory (Janion-Scheepers et al., 2018), typically within one to two weeks of collection.
Table 1.
Species investigated in this study, including their status (alien or indigenous), mean survival time (in minutes ± s.d., at 76% relative humidity) at the low temperature treatment acclimation values and trial conditions for each species, and the latitude from which they were collected. Note that all species were collected in Australia, except for H. viatica and M. caeca, which were collected on the sub-Antarctic Island of South Georgia.
| Order/Species | Family | Status | Survival time | Test/ acclimation temperatures | Latitude (decimal) |
|---|---|---|---|---|---|
| Poduramorpha | |||||
| Hypogastrura purpurescens | Hypogastruridae | Alien | 846 ± 278 | 15 °C, 25 °C | 37.9°S |
| Hypogastrura viatica | Hypogastruridae | Alien | 941 ± 252 | 7 °C, 17 °C | 54.2°S |
| Triacanthella sp. | Hypogastruridae | Indigenous | 151 ± 32 | 15 °C, 25 °C | 31.2°S |
| Neanura muscorum | Neanuridae | Alien | 580 ± 134 | 15 °C, 25 °C | 37.9°S |
| Deuteraphorura sp. 2 | Onychiuridae | Alien | 102 ± 32 | 15 °C, 25 °C | 37.9°S |
| Orthonychiurus sp. | Onychiuridae | Alien | 85 ± 31 | 15 °C, 25 °C | 37.9°S |
| Entomobryomorpha | |||||
| Hemisotoma thermophila | Isotomidae | Alien | 24 ± 5 | 20 °C, 30 °C | 20.3°S |
| Mucrosomia caeca | Isotomidae | Indigenous | 168 ± 46 | 7 °C, 17 °C | 54.2°S |
| Isotomurus sp. | Isotomidae | Alien | 56 ± 14 | 20 °C, 30 °C | 20.3°S |
| Lepidocyrtus (Ascocyrtus) sp. 2 | Entomobryidae | Indigenous | 86 ± 18 | 20 °C, 30 °C | 17.8°S |
| Lepidocyrtus sp. 1 | Entomobryidae | Indigenous | 88 ± 21 | 20 °C, 30 °C | 16.0°S |
| Lepidocyrtus sp. 2 | Entomobryidae | Alien | 185 ± 51 | 20 °C, 30 °C | 16.1°S |
| Lepidocyrtus sp. 5 | Entomobryidae | Alien | 82 ± 29 | 20 °C, 30 °C | 20.3°S |
| Lepidocyrtus sp. 6 | Entomobryidae | Indigenous | 99 ± 33 | 15 °C, 25 °C | 21.2°S |
| Lepidocyrtus sp. 13 | Entomobryidae | Indigenous | 128 ± 30 | 15 °C, 25 °C | 37.9°S |
| Lepidocyrtus sp. 14 | Entomobryidae | Indigenous | 94 ± 34 | 20 °C, 30 °C | 20.3°S |
Species were identified to genus- and, where species had been described, to species-level using available keys (e.g., Fjellberg, 1998; Greenslade et al., 2014). DNA barcoding was used to confirm species identifications and/or to establish a reliable means for ongoing association of unnamed species with this study. Mitochondrial DNA extraction and sequencing of the cytochrome c oxidase subunit I gene was undertaken by the Biodiversity Institute of Ontario, University of Guelph, Canada, following standard protocols developed for Collembola (Hogg and Hebert, 2004; Janion-Scheepers et al., 2018). Sequences of 47 specimens from 16 species were compared with the > 75 000 springtail sequences available through the Barcode of Life Data Systems (BOLD) (www.barcodinglife.org; SI Appendix: Supplementary Information Table S1). Individuals that could not be identified using available keys and which were not represented in BOLD were examined by one of the authors (CJ-S), in discussion with other systematic experts, and assigned to uniquely identifiable species based on morphological characteristics and/or a barcoding gap of at least 2.5% (Meyer and Paulay, 2005). Sequences are available on BOLD (www.boldsystems.org) as part of Project COLMU (Collembola of Monash University). Species clearly identified as indigenous to Australia in faunal treatments and those similarly identified as alien were considered as such in the classification of species as alien or indigenous. Undescribed species not represented in BOLD previously, or represented only from individuals already collected across Australia, New Zealand or south of the Wallace line were considered indigenous. Following previous authors (Cicconardi et al., 2017), undescribed species that had sequences present in BOLD from other distant tropical regions (such as the Neotropics) or from the Holarctic (typically Europe) were considered alien species (Supplementary Information Table S1). Given the extensive nature of the BOLD information on springtails (> 75 000 sequences, representing several thousand species), and the systematic expertise we consulted, we are confident in the species assignments to alien or indigenous species. Species numbers conform to the codes used for operational taxonomic units in our Australian barcoding efforts (Janion-Scheepers et al., 2018; Phillips et al., 2020).
2.2. Colony maintenance
Species were reared at constant rearing temperatures that typically reflect the average soil temperatures of the sites at which they were collected (following Janion-Scheepers et al., 2018) in controlled-temperature incubators (MIR-154-PE, SANYO Electric Co. Ltd., Osaka, Japan) (Table 1). Thus, the sub-Antarctic species were reared at 7 °C, whereas the tropical species were reared at 20 °C and the temperate species at 15 °C. A 12 light:12 dark light cycle was used. iButton Hygrochron® data loggers (Maxim Integrated, USA) deployed in the cabinets verified temperatures were typically within ± 0.7 °C of the intended temperature (data not shown). Adults of the F2 generation were used for experiments to minimize any carry-over effects from the environment of origin, including parental effects, and to reduce the possibility of adaptation to laboratory conditions (Hoffmann and Sgrò, 2017). Between 50 and 100 adults from the collected (F0) individuals were randomly assigned to two to four 70 mL plastic vials. The vials were lined with moistened Plaster-of-Paris:Charcoal powder (9:1) substrate. De-ionised water was added once to twice a week to maintain high humidity (∼ 100%) and, depending on the species, individuals were fed two to three times a week with algae from the bark of Platanus sp. or on slime mold ad libitum (Janion-Scheepers et al., 2018; Hoskins et al., 2015), enabling individuals to select nutrients optimally.
2.3. Temperature effects on water balance characteristics of the cuticle
Previous work has used temperature to induce acclimation effects in water balance characteristics (rate of water loss and survival time) (Chown et al., 2007; Phillips et al., 2020). Although the approach has a clear theoretical foundation (see Introduction), few tests have been conducted to determine whether traits associated with the cuticle, through which most water is lost in the springtails examined here (Hopkin, 1997), respond to thermal acclimation (see Baumgart et al., 2022 for an exception using ants). Here we first provide such a test.
Individuals of Orthonychirus sp. were collected from 1 L leaf litter samples from the Jock Marshall Reserve on the Monash University Clayton Campus, in the early spring of 2020 using the Tullgren funnel method. Collected individuals were maintained in groups of approximately 100 individuals in 200 mL plastic vials with a 1 cm plaster of Paris and charcoal (9:1) base to maintain humidity. They were housed in controlled-temperature incubators (MIR-154-PE, SANYO) set at 15 °C on a 12 light:12 dark light cycle as above. Animals were fed as described above for the main acclimation trials.
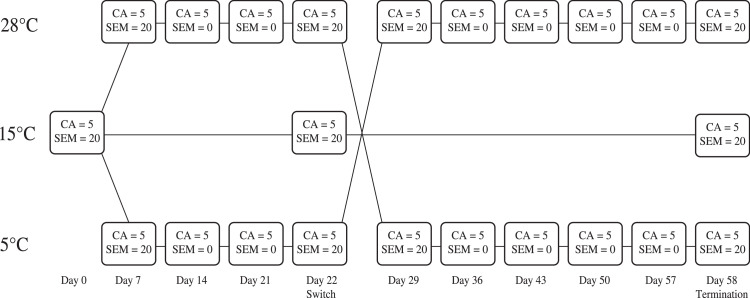
Two experimental and one control group of individuals was then established by dividing the total colony into thirds. The control population was maintained at 15 °C as above. One experimental population was then placed at 5 °C and the other at 28 °C (both maintained in controlled-temperature incubators [MIR-154-PE, SANYO] on a 12 light:12 dark light cycle). Populations were sampled weekly (Fig. 1) and their contact angle measured to determine if the hydrophobicity of the cuticle had changed. Once the contact angle measurements showed no further change (Day 21, checked on Day 22), the 5 °C and 28 °C populations were switched to the alternative temperature, and the measurements continued until stability of the traits and subsequent termination of the experiment in Day 58.
Fig. 1.
Experimental approach for exploring the effects of temperature on cuticle traits. CA = contact angle, SEM = scanning electron microscopy with EDS. Numbers after the equals signs refer to sample sizes in each case. Zero indicates no individuals measured. Large numbers to the left indicate treatment temperatures: 15 °C = control; 5 °C and 28 °C are experimental treatments, with the switch of populations to the opposite temperature indicated after Day 22.
Two traits were measured. The first, as noted, is the contact angle between a water droplet and the individual cuticle, which indicates changes to the surface properties of the cuticle that determine whether the surface is wettable or hydrophobic (Hensel et al., 2016). Generally, contact angles of 90° and above are considered hydrophobic (Gunderson et al., 2015), and those of 150° and above, superhydrophobic (Gunderson et al., 2014). A contact angle of 180° implies an unwettable surface. Measurements of contact angle were made weekly, following previous approaches (Gunderson et al., 2014, 2015), using an Attension Theta Flex tensiometer (Biolin Scientific, Gothenburg, Sweden) with picolitre dispenser (Microdrop Technologies GmbH, Norderstedt, Germany) attachment. Here, for each individual a single 1500 pl droplet of water purified by reverse osmosis was dispensed onto the dorsal side of the abdomen. The contact angle was measured by the OneAttension (Biolin Scientific) software for a period of 10 s and the average recorded as the final measurement for an individual.
The second trait is cuticular elemental composition because cuticular hydrocarbons respond profoundly to changes in desiccation stress in a range of arthropods including springtails (Helbig et al., 2011; Nickerl et al., 2014; Schmüser et al., 2020; Menzel et al., 2007, 2022). Elemental composition was measured on Day 0, 7, 22, 29 and 58, using scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDS) (Sugiura et al., 2008; Yudha et al., 2019). We were unable to determine the specific structure of the compound mixture (such as alkenes, alkanes), but consider the elemental composition assessment indicative, given previous work (Schmüser et al., 2020; Baumgart et al., 2022; Menzel et al., 2022). Specimens for SEM-EDS were immediately freeze-dried to preserve cuticle structure and stored at −20 °C until analysis could be conducted. They were then mounted dorsal side up to aluminum sample stages with the use of silver glue, and sputter coated with approximately a 1 nm layer of iridium. The silver glue and iridium improve conductance of the samples and prevent deleterious overcharging effects during analysis. Once mounted and coated, SEM-EDS analysis was conducted using a FEI Nova Nano SEM 450 FEGSEM (FEI Company, Hillsboro, USA) with a Bruker Quantax 400 X-ray attachment (Bruker Corporation, Billerica, USA). For each individual the same protocol was followed: an accelerating voltage of 5 kVe was used, with the sample positioned 5.1 mm from the electron gun, and the image zoomed and focused on a 120 × 90 µm area on the abdomen of the individual. The Bruker ESPRIT software was then used to perform the SEM-EDS analysis. Using the “Line Scan” function an EDS analysis was conducted along a 100 µm line with a scan depth of 0.2–0.3 µm. This depth corresponds to the epicuticle in Collembola which comprises significant hydrocarbon-based waxes (Noble-Nesbitt, 1963; Nickerl et al., 2014), though a part of the exocuticle may also have been measured.
From these scans, the major elements comprising the cuticle were determined automatically by the ESPRIT software, and netto counts of each element were recorded, as well as percentage contribution of each element for each specimen investigated. A netto count is indicative of the concentration of that element along the course of the line scan. In SEM-EDS analysis, certain elements are deliberately not detected/recorded by the sensor. Crucially for this study, this includes hydrogen, which is a key component of hydrocarbons. Changes in hydrocarbons can still reliably be informed by measures of carbon change especially, given the way in which components of the hydrocarbons respond to changes in water availability (Menzel et al., 2007, 2022; Nickerl et al., 2014 Schmüser et al., 2020; Baumgart et al., 2022).
2.4. Acclimation to assess phenotypic plasticity
Following previous work using temperature to induce acclimation effects (Chown et al., 2007; Phillips et al., 2020), prior to the experimental trials, all species were subject to two temperature treatments (referred to as ‘acclimation’ hereafter), in controlled-temperature incubators (MIR-154-PE, SANYO). Experimentally, to ensure that acclimation treatments are not confounded with starvation, individuals have to be fed, which necessarily requires the provision of moist food (Hoskins et al., 2015). In turn, for small animals such as Collembola, this might mean providing a confounding effect in the form of altering the exposure humidity away from, for example, some alternative humidities to elicit a desiccation response. Hence, an exposure to temperature also avoids such a confounding effect. Acclimation treatments lasted seven days, given that complete responses usually occur within seven days in terrestrial arthropods, including Collembola (Weldon et al., 2011; Janion-Scheepers et al., 2018; Kuyucu and Chown, 2021). Acclimation treatments were as follows: sub-Antarctic (7 °C or 17 °C), temperate (15 °C or 25 °C) and tropical (20 °C or 30 °C) (Table 1). The experiments were undertaken after these one-week exposures to the acclimation temperature treatments.
2.5. Desiccation resistance experiments
An experimental protocol for desiccation resistance, measured as survival time at 76% relative humidity, was established based on previous work (Kærsgaard et al., 2004; Chown et al., 2007; Leinaas et al., 2009; Phillips et al., 2020). Choice of such a relative humidity might seem, at first, too low for Collembola living in the litter layer. Individual springtails living in such a layer, in a variety of environments are, however, routinely likely to encounter conditions as dry or drier than this. For example, at a temperature of 20 °C and a relative humidity of 76%, vapor pressure deficit (VPD, the drying power of the air relevant to a springtail) is 0.56 kPa (Campbell and Norman, 1998). Yet such a VPD is less than those which might be found in litter in a variety of environments (Liu et al., 2021). Even in the relatively cold, moist, sub-Antarctic, relative humidities within the vegetation layer (often below closed canopies) can routinely encompass 76% (Lee et al., 2009) (Supplementary Information Figure S1). Moreover, use of such a relative humidity enables comparisons across a wide range of species and settings, especially when capabilities of springtails which live in a variety of habitats and soil depths are being investigated (Liu et al., 2021). Lower humidities are typically not considered suitable for use with hemiedaphic springtails because they are rarely encountered (though not impossible) (Liu et al., 2021), and because they preclude reasonable experimental assessment.
Experiments were undertaken at two test temperatures for each species matching the acclimation temperatures they had been exposed to for a week, in a full factorial design (Supplementary Information Figure S2). An intermediate temperature, or set of temperatures, could have been used for both the acclimation and test temperatures across all species. Such intermediate temperatures would, however, have likely brought sublethal effects during acclimation to the species from the sub-Antarctic and may have done so for others (Janion et al., 2010; Janion-Scheepers et al., 2018; Phillips et al., 2020). Moreover, our interest is in the responses of animals at temperatures typical of their habitats, rather than those that might be considered unusual. Similarly, had we used intermediate test temperatures, we would not have had a full factorial design required for testing the predictions of the hypotheses being examined.
Springtails were contained within glass vials covered with fine mesh, which were then housed in sealed, 70 mL plastic pots containing 15 mL of saturated NaCl solution as a desiccant. Saturated NaCl was used because it provides a consistent relative humidity of 76% from 0 °C to 30 °C. Each pot contained two glass vials with ∼ 5 springtails per vial, and an iButton Hygrochron® data logger (Maxim Integrated, USA) to verify temperature and relative humidity. Throughout the experiments, conducted in controlled-temperature rooms, springtails were examined every 10 min under a Leica M80 microscope (Leica Microsystems Pty Ltd, Wetzlar, Germany), and time to death (minutes) was recorded for each individual. Four to five replicates of 10 individuals were used per experiment. Typical times to death (average for all species of 232 min, maximum for any individual of 1490 min) were well within the range of starvation resistance of even small soil-dwelling species, such as Folsomia candida, which can be up to six weeks (Hilligsøe and Holmstrup, 2003). Following the experiment, springtails were dried at 40 °C for 24 h and then weighed in groups using a high-resolution (0.1 μg) microbalance (Mettler-Toledo XP2U, Switzerland) to obtain an estimate of individual dry body mass per species per test temperature and acclimation temperature. Thus, the experimental unit of analysis is the individual, but masses were considered to be identical for individuals in each species x test temperature x acclimation temperature treatment. Despite the resolution of the microbalance, individual dry weights could not be estimated within the acceptable resolution of the balance.
2.6. Selection experiment
Laboratory natural selection (Gibbs, 1999) was used to determine whether desiccation resistance (measured as survival time of a 76% relative humidity treatment, as above) responds to rearing at elevated temperatures, following the previous rationale concerning the influence of temperature on desiccation resistance capabilities. The animals used were surplus individuals from an experiment examining laboratory natural selection on critical thermal limits, described in full elsewhere (Janion-Scheepers et al., 2018). Four species, used in the previous trials (as described), were selected for this experiment. That is, a tropical alien species (Desoria trispinata), a tropical indigenous species (Lepidocyrtus (Ascocyrtus) sp. 2), a temperate alien species (Orthonychiurus sp.), and a temperate indigenous species (Lepidocyrtus sp. 10).
For each species, mass-reared colonies were established from approximately 100 individuals of field-caught individuals. For two generations, these were maintained on a 12:12 h light:dark cycle at constant temperatures representing mean temperatures from their environment of collection (temperate = 15 °C [mean ± s.d.: 14.88 ± 0.56 °C], tropical = 20 °C [mean ± s.d.: 20.16 ± 0.29 °C], verified with iButton Hygrochron® data loggers (Maxim Integrated, USA)). Springtails were maintained at an intermediate density (70 - 100 individuals) in 70 mL plastic vials with a saturated Plaster-of-Paris:Charcoal powder (9:1) substrate, and were supplied with bark from Platanus sp. trees ad libitum for shelter and food (Hoskins et al., 2015).
At the F2 generation, laboratory natural selection was initiated, with springtails of each species being assigned to one of two treatment conditions. Individuals were exposed to either a high (selection group) or low (control group) constant temperature for successive generations. Collembola in the control group were maintained under the original rearing temperatures (temperate = 15 °C [mean ± s.d.: 14.88 ± 0.56 °C], tropical = 20 °C [mean ± s.d.: 20.16 ± 0.29 °C], temperatures measured using iButton Hygrochron® data loggers, (Maxim Integrated, USA)), and those in the selection group were maintained under higher temperatures (temperate = 25 °C [mean ± s.d.: 24.89 ± 0.54 °C], tropical = 27 °C [mean ± s.d.: 27.08 ± 0.61 °C]). These warmer temperatures were selected as 25 °C and 27 °C were the highest temperatures at which the temperate and tropical species could still reproduce, respectively (Janion-Scheepers et al., 2018). Within the treatment and control group for each species were two, independent, replicate lines with a starting population of 150 individuals which were divided between two separate vials. Springtails were maintained as previously described under each of the temperature conditions, and eggs were collected and transferred to new vials 2 - 3 times a week to ensure that generations remained discrete. Eggs from multiple vials within replicate lines for each species/treatment condition were combined within generations to maintain genetic diversity.
Desiccation resistance (assessed as above) was determined for temperate species after eight generations of laboratory natural selection and for tropical species after twelve generations. To assess desiccation resistance in terms of both plasticity and evolution in a cross-tolerance framework, a crossed factorial design was used (see Supplementary Information Figure S3). For each species, within each treatment and replicate line (from now on called an ‘experimental unit’), this involved transferring individuals from the temperature that they had been selected under to either the opposite temperature treatment, or, leaving them at the same temperature, for seven days prior to the desiccation experiment. This week-long acclimation was implemented to disentangle the effects of short-term plasticity versus a longer-term evolutionary response to the specific laboratory selection temperature treatments. Following acclimation, the desiccation experiment was conducted under each of the two temperatures. Thus, for each experimental unit there were three variables: the selection temperature, the acclimation temperature, and the test temperature. New individuals from each experimental unit were used to assess desiccation resistance for each combination of variables.
2.7. Statistical analyses
All analyses were conducted in R version 4.0.2 (R Core Team, 2020) implemented in R Studio v. 1.3.1056, with plots made using the ggplot2 package. All code and data files are archived in the Monash Bridges (Figshare) repository: https://doi.org/10.26180/21670898.v1.
For the assessment of cuticular trait variation, both contact angles and cuticular carbon content were compared using linear models. Controls were examined for absence of differences over time. The experimental treatments were expected to show variation between both the temperatures and days, with an interaction effect demonstrating that the switch between temperatures resulted in significant change. Given the clear nature of the acclimation effects, orthogonal polynomial contrasts were not considered necessary for the analyses.
Predictions of the phenotypic plasticity hypothesis were examined in two ways. First, within each species, the effects of test temperature and acclimation temperature were examined with the expectation of distinct differences between the indigenous and alien species such that the latter would consistently show greater desiccation resistance than the former following high temperature acclimation (Chown et al., 2007). To do so, generalized linear models assuming a quasipoisson distribution and a log link function (given the strong right skew to survival time data (Phillips et al., 2020), see Supplementary Information Figure S4) were implemented for each species examining the effects of acclimation and test temperatures (fixed factors) and their interactions on survival time. Mass was typically not significant in these models and was excluded. Second, mean survival time values (minutes) per species per test temperature and per acclimation temperature were calculated. Then all values were included in a single generalized linear model (assuming a quasipoisson distribution and a log link function), with test temperature, acclimation temperature and dry mass treated as continuous predictors and species status (indigenous or alien) as a fixed factor. All interactions were investigated and those that were not significant were removed from models to result in a minimum adequate model (Crawley, 2013). Because acclimation temperature was not significant in these models, the analyses were repeated now treating acclimation as a fixed factor recorded as either low (for low temperature acclimations irrespective of their absolute value) or high. Acclimation was again not significant. These analyses were repeated transforming test temperature to vapor pressure deficit (Campbell and Norman, 1998) to account for differences in drying power of the air between different temperatures at a constant relative humidity of 76%. Because of the straightforward nature of the transformation, the expectation is, however, for little difference in the outcome of the analyses.
These latter analyses also provided a test of the prediction of the ideal weed hypotheses that invasive species have, on average, a longer survival time than indigenous species. In such an approach, however, outcomes may be influenced by the phylogenetic relatedness of species. Taking phylogenetic effects into consideration where multiple independent data points representing different treatment regimes are available for single species remains complicated, with various approaches still being investigated (Uyeda et al., 2018). Therefore, we excluded acclimation given its minimal influence, as documented in the previous analyses, and used survival time data measured at a test temperature as close to 20 °C as possible for each species, while still including test temperature (and in a second analysis, VPD) as a covariate.
To take phylogenetic effects into consideration we adopted a phylogenetic generalised least squares (PGLS) approach (Garland and Ives, 2000). Here, the covariance matrix for the species trait values was constructed adopting a Brownian Motion (BM) model of evolution, assuming that evolutionary change along a branch is proportional to the branch length. Type I error rates of the alternative Ornstein-Uhlenbeck model are high when the phylogeny is small (< 200 taxa), and its model parameter is often too low to be biologically distinguishable from the BM model (Cooper et al., 2016). Thus, this model was not investigated. Pagel's lambda (λ) (Pagel, 1999) was estimated by maximum likelihood to indicate the degree of phylogenetic correlation in the data. This index is more robust to incompletely resolved phylogenies and sub-optimal branch length information than some others (Molina-Venegas and Rodríguez, 2017). The PGLS analyses were implemented in the ‘caper’ (v. 1.0.1, Orme, 2018) and ‘APE’ (v. 5.3, Paradis et al., 2004) packages in R.
For the PGLS, a phylogeny for the species was constructed following previous work on some of these species (Janion-Scheepers et al., 2018), with species relative positions based on the cytochrome c oxidase subunit I gene phylogeny, or in a few cases on morphological similarity adjudicated by one of us (CJ-S). The barcoding placements were obtained from a neighbor-joining tree using standard methods. For the final tree, branch lengths were assigned using Grafen's method (Grafen, 1989), and the tree (Supplementary Information Figure S5) is available as a Newick file.
For the selection experiment, nested mixed effects models were used to examine the main and interactive effects of the factors: acclimation temperature (high or low), test temperature (high or low), and treatment (selection [high temperature] or control [low temperature] lines) on the response variable of survival time (minutes) for each of the four species. ‘Replicate line’ was nested within ‘treatment’ as a random effect. Given the right skew of the response variable, data were log transformed (log10) prior to analysis. Mixed effects models were performed using the lme4 package (version 1.1–23), with p-values provided via Satterthwaite's degrees of freedom method via lmerTest.
3. Results
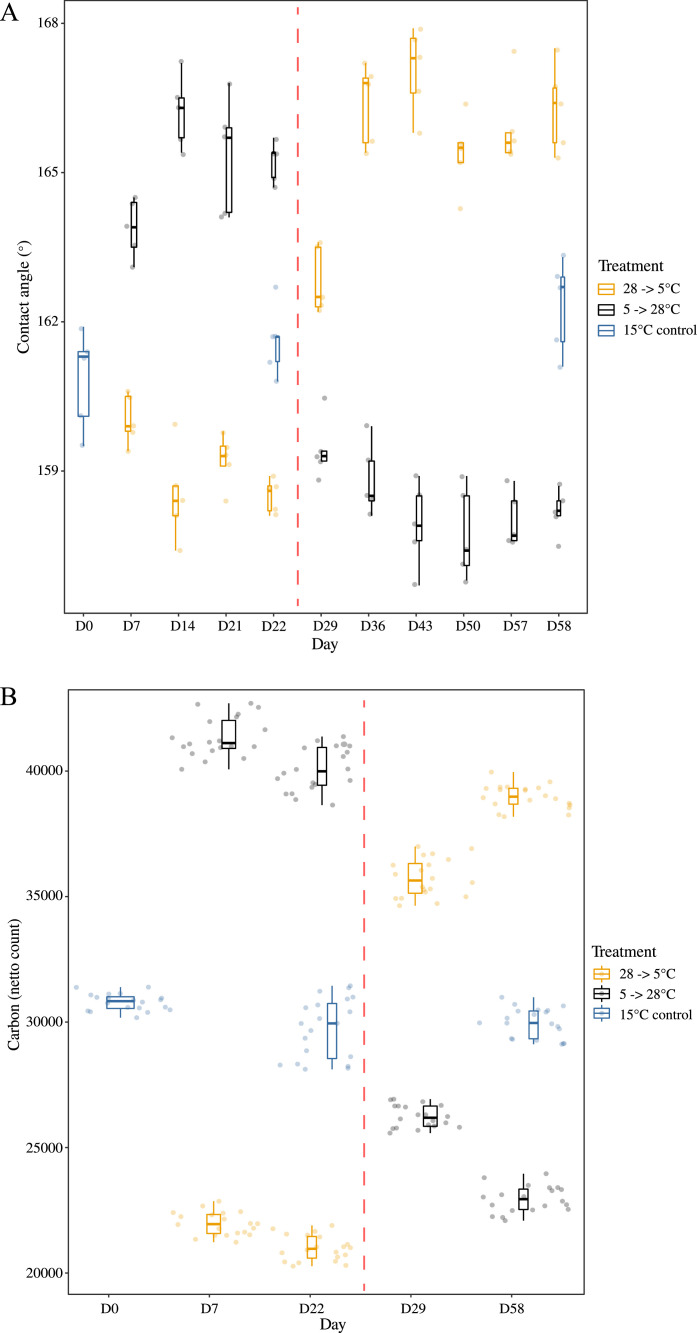
Both contact angle and carbon netto count of the cuticle responded strongly to the experimental conditions, differing from the control within a week, and responding to the reciprocal switch in temperature within the same period (Fig. 2A). Contact angle values did not vary significantly between the controls over time (F(2,12) = 3.474, p = 0.645), but varied over time and between the two treatments, with a significant interaction substantiating the large response to the temperature switch (Table 2A). By contrast, carbon netto count declined significantly over time in the controls (F(2,57) = 9.664, p < 0.001), but the effect size was small compared with the overall variation found in the experiment (Fig. 2B). The two treatments varied significantly through time with a clear switch associated with the change in acclimation conditions (Table 2B, Fig. 2B).
Fig. 2.
Boxplots with point data illustrating the differences in A. contact angle and B. carbon netto count among the treatments and controls and the rapid reciprocal change in the experimental group values when switched from one treatment to the other after Day 22. Termination on Day 58.
Table 2.
Outcome (ANOVA table) of the linear models examining: A. differences in contact angle among the two treatments (5 °C and 28 °C) and their change through time (as illustrated in Fig. 2A); B. differences in netto carbon counts among the two treatments (5 °C and 28 °C) and their change through time (as illustrated in Fig. 2B).
| Variable | df | Sums of squares | Mean square | F | p |
|---|---|---|---|---|---|
| A. Contact angle | |||||
| Acclimation | 1 | 93.7 | 93.7 | 173.89 | <0.00001 |
| Week | 9 | 18.1 | 2.01 | 3.73 | 0.0006 |
| Accl:Week | 9 | 1146.5 | 127.39 | 236.42 | <0.00001 |
| Residuals | 80 | 43.1 | 0.54 | ||
| B. Netto carbon counts | |||||
| Acclimation | 1 | 4.17 × 108 | 4.17 × 108 | 1078.59 | <0.00001 |
| Week | 3 | 2.59 × 107 | 8.64 × 106 | 22.32 | <0.00001 |
| Accl:Week | 3 | 1.05 × 1010 | 3.49 × 109 | 9008.71 | <0.00001 |
| Residuals | 152 | 5.88 × 107 | 3.87 × 105 | ||
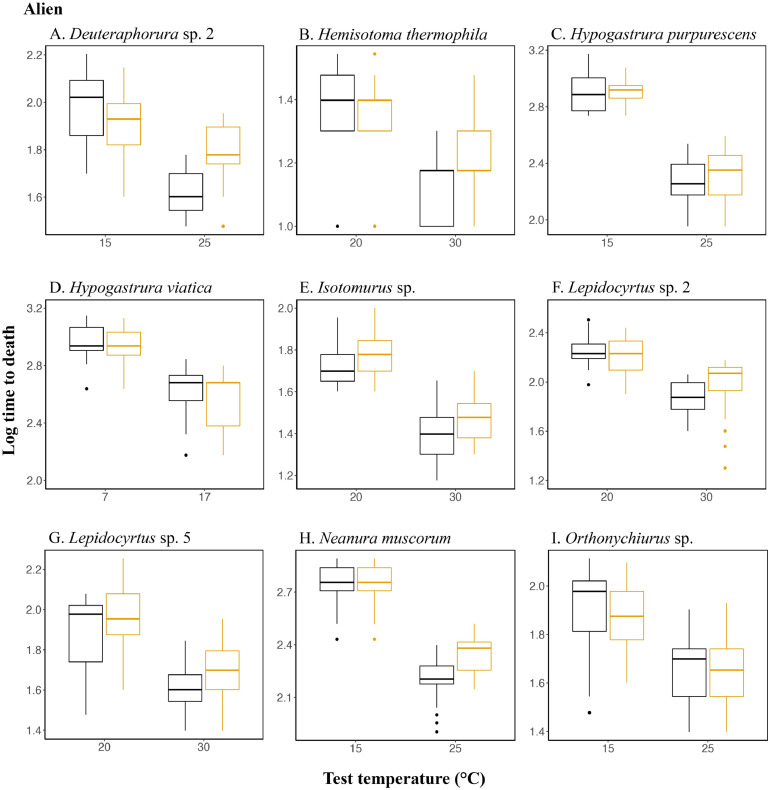
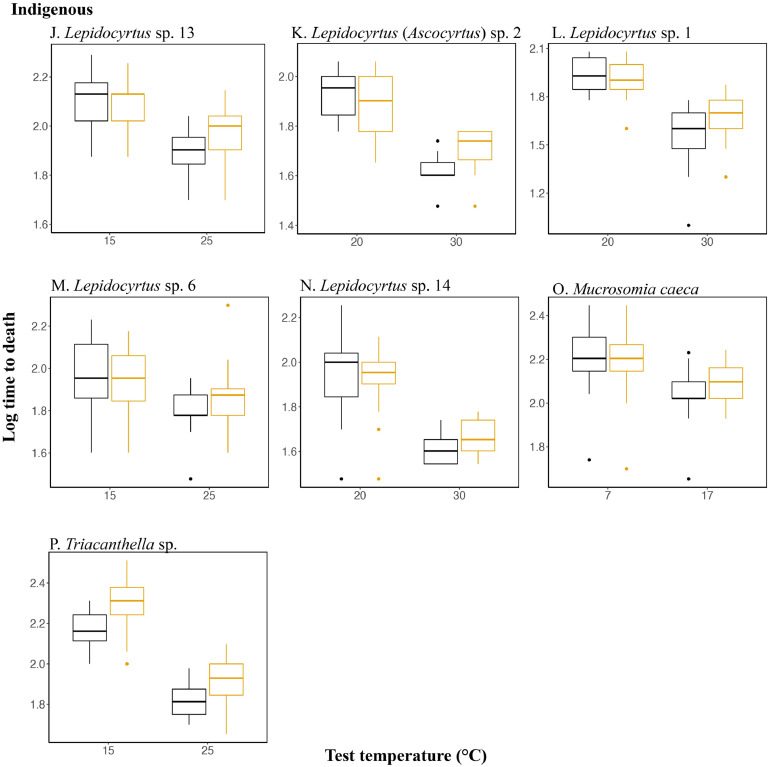
As might be expected, given its effect on saturation vapor pressure (Campbell and Norman, 1998), test temperature had a significant effect on survival time in all 16 species investigated (Table 3). Acclimation effects were less pronounced, however. The most prominent effect was an interaction between acclimation and test temperature such that in nine of the species, survival time was significantly longer in the high temperature treatment following high temperature acclimation than after low temperature acclimation (Table 3, Fig. 3). A beneficial acclimation effect was only observed in the alien temperate Deuteraphorura sp. 2 (Fig. 3A), while only the indigenous temperate Triacanthella sp. showed longer survival times following higher temperature acclimation at both high and low test temperatures (Fig. 1P). No consistent differences were found among the indigenous and alien species.
Table 3.
Outcomes of the generalized linear models (quasipoisson distribution, log link) estimating the effects of acclimation and treatment temperature and their interaction on survival time (as a measure of desiccation resistance) in each of the species examined in this study.
| Species | Estimate ± S.E. | t | p |
|---|---|---|---|
| Alien | |||
| Deuteraphorura sp. 2 | |||
| Intercept | 4.622 ± 0.045 | 103.48 | < 0.0001 |
| Acclimation (25) | −0.193 ± 0.066 | −2.903 | 0.0044 |
| Test temperature (25) | −0.853 ± 0.082 | −10.438 | < 0.0001 |
| Acclimation:Test | 0.572 ± 0.111 | 5.162 | <0.0001 |
| Residual deviance 719.2; df = 116; quasipoisson dispersion parameter = 6.084 | |||
| Hemisotoma thermophila | |||
| Intercept | 3.184 ± 0.033 | 95.521 | < 0.0001 |
| Acclimation (30) | −0.022 ± 0.048 | −0.458 | 0.649 |
| Test temperature (30) | −0.579 ± 0.056 | −10.406 | < 0.0001 |
| Acclimation:Test | 0.261 ± 0.076 | 3.423 | 0.0008 |
| Residual deviance 181.73; df = 159; quasipoisson dispersion parameter = 1.100 | |||
| Hypogastrura purpurescens | |||
| Intercept | 6.740 ± 0.039 | 174.19 | < 0.0001 |
| Acclimation (25) | −0.032 ± 0.057 | −0.561 | 0.576 |
| Test temperature (25) | −1.434 ± 0.077 | −18.654 | < 0.0001 |
| Acclimation:Test | 0.151 ± 0.108 | 1.403 | 0.163 |
| Residual deviance 6242.7; df = 152; quasipoisson dispersion parameter = 41.800 | |||
| Hypogastrura viatica | |||
| Intercept | 6.847 ± 0.035 | 193.38 | < 0.0001 |
| Acclimation (17) | −0.033 ± 0.053 | −0.627 | 0.532 |
| Test temperature (17) | −0.735 ± 0.070 | −10.438 | <0.0001 |
| Acclimation:Test | −0.105 ± 0.111 | −0.940 | 0.349 |
| Residual deviance 9498.4; df = 152; quasipoisson dispersion parameter = 60.137 | |||
| Isotomurus sp. | |||
| Intercept | 4.022 ± 0.038 | 105.61 | < 0.0001 |
| Acclimation (30) | 0.083 ± 0.051 | 1.614 | 0.108 |
| Test temperature (30) | −0.732 ± 0.065 | −11.201 | < 0.0001 |
| Acclimation:Test | 0.026 ± 0.088 | 0.295 | 0.769 |
| Residual deviance 623.16; df = 184; quasipoisson dispersion parameter = 3.481 | |||
| Lepidocyrtus sp. 2 | |||
| Intercept | 5.219 ± 0.039 | 132.86 | < 0.0001 |
| Acclimation (30) | −0.129 ± 0.059 | −2.203 | 0.029 |
| Test temperature (30) | −0.841 ± 0.075 | −11.158 | < 0.0001 |
| Acclimation:Test | 0.404 ± 0.100 | 4.026 | < 0.0001 |
| Residual deviance 2185.2; df = 167; quasipoisson dispersion parameter = 12.535 | |||
| Lepidocyrtus sp. 5 | |||
| Intercept | 4.409 ± 0.047 | 93.311 | < 0.0001 |
| Acclimation (30) | 0.157 ± 0.060 | 2.619 | 0.010 |
| Test temperature (30) | −0.695 ± 0.081 | −8.632 | < 0.0001 |
| Acclimation:Test | 0.086 ± 0.104 | 0.823 | 0.412 |
| Residual deviance 1172.3; df = 166; quasipoisson dispersion parameter = 6.786 | |||
| Neanura muscorum | |||
| Intercept | 6.363 ± 0.029 | 222.85 | < 0.0001 |
| Acclimation (25) | 0.008 ± 0.041 | 0.200 | 0.842 |
| Test temperature (25) | −1.262 ± 0.074 | −16.972 | < 0.0001 |
| Acclimation:Test | 0.300 ± 0.100 | 2.997 | 0.003 |
| Residual deviance 4481.8; df = 167; quasipoisson dispersion parameter = 25.534 | |||
| Orthonychiurus sp. | |||
| Intercept | 4.433 ± 0.038 | 116.63 | < 0.0001 |
| Acclimation (25) | −0.073 ± 0.058 | −1.255 | 0.211 |
| Test temperature (25) | −0.601 ± 0.058 | −10.307 | < 0.0001 |
| Acclimation:Test | 0.085 ± 0.088 | 0.970 | 0.333 |
| Residual deviance 1360.5; df = 209; quasipoisson dispersion parameter = 6.167 | |||
| Indigenous | |||
| Lepidocyrtus (Ascocyrtus) sp. 2 | |||
| Intercept | 4.455 ± 0.032 | 141.04 | < 0.0001 |
| Acclimation (30) | −0.088 ± 0.045 | −1.974 | 0.0501 |
| Test temperature (30) | −0.704 ± 0.054 | −13.148 | < 0.0001 |
| Acclimation:Test | 0.288 ± 0.074 | 3.878 | 0.0002 |
| Residual deviance 562.8; df = 155; quasipoisson dispersion parameter = 3.263 | |||
| Lepidocyrtus sp. 1 | |||
| Intercept | 4.473 ± 0.355 | 126.04 | < 0.0001 |
| Acclimation (30) | −0.033 ± 0.048 | −0.684 | 0.495 |
| Test temperature (30) | −0.744 ± 0.062 | −12.111 | < 0.0001 |
| Acclimation:Test | 0.168 ± 0.079 | 2.113 | 0.036 |
| Residual deviance 789.44; df = 181; quasipoisson dispersion parameter = 4.194 | |||
| Lepidocyrtus sp. 6 | |||
| Intercept | 4.600 ± 0.037 | 124.69 | < 0.0001 |
| Acclimation (25) | −0.065 ± 0.055 | −1.180 | 0.239 |
| Test temperature (25) | −0.432 ± 0.059 | −7.392 | < 0.0001 |
| Acclimation:Test | 0.210 ± 0.082 | 2.567 | 0.011 |
| Residual deviance 1512.9; df = 212; quasipoisson dispersion parameter = 7.308 | |||
| Lepidocyrtus sp. 13 | |||
| Intercept | 4.855 ± 0.031 | 155.85 | < 0.0001 |
| Acclimation (25) | −0.037 ± 0.044 | −0.847 | 0.398 |
| Test temperature (25) | −0.497 ± 0.055 | −9.051 | < 0.0001 |
| Acclimation:Test | 0.248 ± 0.072 | 3.458 | 0.0007 |
| Residual deviance 830.0; df = 158; quasipoisson dispersion parameter = 5.110 | |||
| Lepidocyrtus sp. 14 | |||
| Intercept | 4.543 ± 0.034 | 133.45 | < 0.0001 |
| Acclimation (30) | −0.043 ± 0.047 | −0.911 | 0.364 |
| Test temperature (30) | −0.828 ± 0.067 | −12.33 | < 0.0001 |
| Acclimation:Test | 0.154 ± 0.092 | 1.678 | <0.0951 |
| Residual deviance 968.5; df = 176; quasipoisson dispersion parameter = 5.227 | |||
| Mucrosomia caeca | |||
| Intercept | 5.122 ± 0.028 | 181.54 | < 0.0001 |
| Acclimation (17) | −0.027 ± 0.038 | −0.714 | 0.476 |
| Test temperature (17) | −0.423 ± 0.045 | −9.341 | < 0.0001 |
| Acclimation:Test | 0.182 ± 0.060 | 3.009 | 0.003 |
| Residual deviance 2509.0; df = 280; quasipoisson dispersion parameter = 8.807 | |||
| Triacanthella sp. | |||
| Intercept | 5.022 ± 0.037 | 136.11 | < 0.0001 |
| Acclimation (25) | 0.298 ± 0.046 | 6.508 | < 0.0001 |
| Test temperature (25) | −0.807 ± 0.062 | −12.948 | < 0.0001 |
| Acclimation:Test | −0.092 ± 0.082 | −1.122 | 0.264 |
| Residual deviance 1443.3; df = 178; quasipoisson dispersion parameter = 7.848 | |||
Fig. 3.
Boxplots illustrating the effects of different acclimation treatments [low temperature (black) or high temperature (orange) for 1 week] on desiccation resistance (provided here as log10 time to death in minutes) for alien and indigenous Collembola species measured under 76% relative humidity at test temperatures equivalent to acclimation temperatures in a full factorial design (see Table 1 for temperature details).
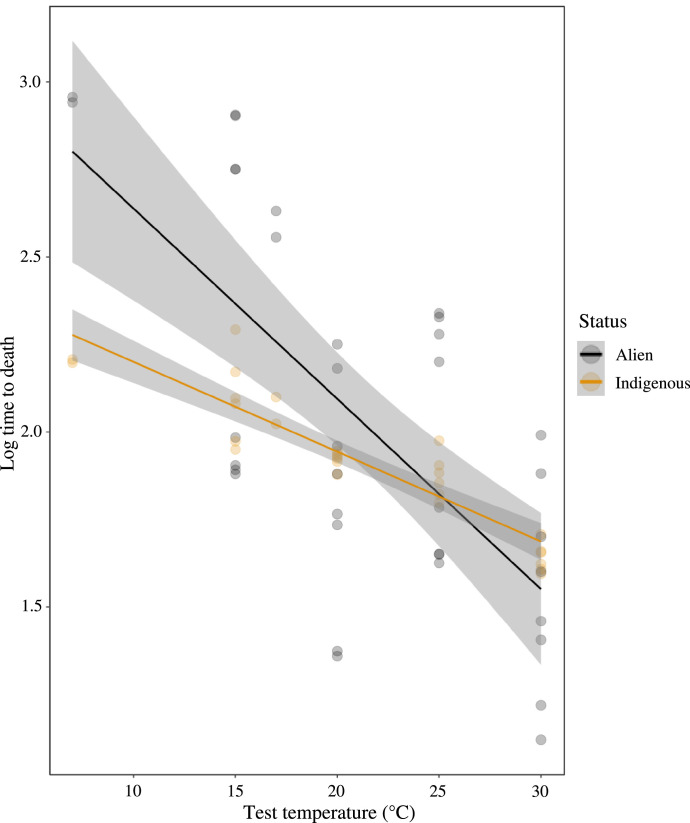
When the species mean data were investigated, similar outcomes were found irrespective of whether acclimation was included as a continuous predictor, using all acclimation temperature values, or categorical predictor, allocating all low temperature values to a low temperature categorical predictor, and all high temperature values to a high temperature predictor (Table 4). In each case, the only interaction effect that was positive, was between status (indigenous or alien) and test temperature (or VPD, see Supplementary Information Table S2). Thus, the difference between the two groups was more pronounced at the low than high temperatures (Fig. 4). In the PGLS analysis, survival time showed strong phylogenetic signal (λ = 0.975), with neither test temperature (t = −1.223, p = 0.243) nor status (t = 0.687, p = 0.504) being significant. The outcomes were virtually identical using VPD (phylogenetic signal λ = 0.975), again with neither test temperature (t = −1.223, p = 0.243) nor status (t = 0.687, p = 0.504) being significant).
Table 4.
Outcomes of the generalized linear models (quasipoisson distribution, log link) estimating the effects of acclimation, treatment temperature, status and mean mass, and their significant interactions on survival time (as a measure of desiccation resistance). Acclimation treatment was either treated as a continuous variable with all values included, or as a categorical variable with treatments for each species assigned either to a low (L) or high (H) category. Supplementary Information Table S2 provides the same information, but for analyses with test temperature replaced by vapor pressure deficit.
| Analysis | Estimate ± S.E. | t | p |
|---|---|---|---|
| Acclimation continuous | |||
| Intercept | 7.807 ± 0.279 | 28.02 | < 0.0001 |
| Test temperature | −0.127 ± 0.016 | −7.739 | < 0.0001 |
| Status (Indigenous) | −2.216 ± 0.514 | −4.313 | <0.0001 |
| Acclimation temperature | −0.014 ± 0.013 | −1.041 | 0.3022 |
| Mean mass | 4.341 ± 1.482 | 2.929 | 0.0049 |
| Test:Status | 0.083 ± 0.027 | 3.042 | 0.0035 |
| Residual deviance 3493; df = 58; quasipoisson dispersion parameter = 65.273 | |||
| Acclimation categorical | |||
| Intercept | 7.689 ± 0.265 | 29.003 | < 0.0001 |
| Test temperature | −0.134 ± 0.015 | −9.116 | < 0.0001 |
| Status (Indigenous) | −2.227 ± 0.515 | −4.293 | <0.0001 |
| Acclimation temperature (L) | −0.025 ± 0.157 | −0.156 | 0.876 |
| Mean mass | 4.307 ± 1.484 | 2.903 | 0.0052 |
| Test:Status | 0.084 ± 0.028 | 3.045 | 0.0035 |
| Residual deviance 3562; df = 58; quasipoisson dispersion parameter = 65.531 | |||
Fig. 4.
The relationship between test temperature and desiccation resistance (log10 of survival time in minutes) for the alien (black circles) and indigenous (orange circles) Collembola species investigated here, including data from both acclimation treatments. Fitted lines are taken from the generalised linear model of the relationships (Table 3).
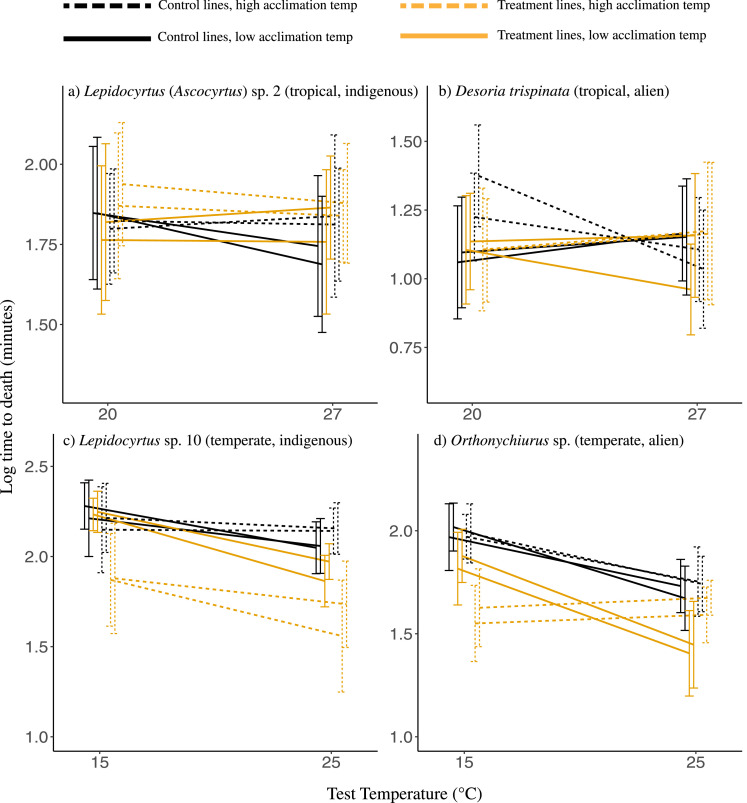
For the selection experiment, test temperature had a significant effect on survival for all four species (Table 5, Fig. 5). Unlike the other three species, the alien Desoria trispinata survived for longer at the higher temperature (Table 5). However, this result is likely due to their overall very low desiccation resistance and thus short survival time generally (Supplementary Information Figure S6). Desoria trispinata was also the only species for which acclimation temperature had a significant effect (Table 5). Treatment (control or selection lines) only had a significant effect for Orthonychiurus sp., with individuals from selected lines having shorter survival times than those in control lines (Table 5, Fig. 5). For the other three species, though the main effect of treatment was not significant, interactions between treatment and acclimation temperature, and between treatment and test temperature were significant, though the differences between groups were relatively small (Table 5, Fig. 5). Perhaps the most notable of these is for Lepidocyrtus sp. 10., where individuals from selected lines performed worse than those in all other groups when acclimated to high temperatures.
Table 5.
Outcomes of nested mixed effects models estimating the effects of acclimation temperature, test temperature and treatment (control or selection lines) and their interaction on survival time (as a measure of desiccation resistance) for four springtail species that were selected under high (selection) or low (control) temperatures for multiple generations. In each model, replicate line is nested within treatment as a random effect. P-values calculated using Satterthwaite's degrees of freedom approach.
| Species | Estimate ± S.E. | t | p |
|---|---|---|---|
| Indigenous Lepidocyrtus (Ascocyrtus) sp. 2 | |||
| Intercept | 1.848 ± 0.034 | 55.044 | < 0.0001 |
| Acclimation temperature (high) | −0.036 ± 0.034 | −1.048 | 0.2952 |
| Test temperature (high) | −0.13 ± 0.036 | −3.555 | 0.0004 |
| Treatment (treatment) | −0.056 ± 0.047 | −1.198 | 0.2691 |
| Acclimation temp x Test temp | 0.142 ± 0.05 | 2.859 | 0.0044 |
| Acclimation temp x Treatment | 0.148 ± 0.048 | 3.063 | 0.0023 |
| Test temp x Treatment | 0.149 ± 0.05 | 2.99 | 0.0029 |
| Acclimation temp x Test temp x Treatment | −0.207 ± 0.068 | −3.043 | 0.0025 |
| Random Effects | Percentage of variation explained | ||
| Replicate line | 2.15% | ||
| Residual | 97.85% | ||
| Alien Desoria trispinata | Estimate ± S.E. | t | p |
| Intercept | 1.078 ± 0.028 | 38.443 | < 0.0001 |
| Acclimation temperature (high) | 0.221 ± 0.033 | 6.666 | < 0.0001 |
| Test temperature (high) | 0.081 ± 0.032 | 2.551 | 0.012 |
| Treatment (treatment) | 0.043 ± 0.04 | 1.076 | 0.3158 |
| Acclimation temp x Test temp | −0.309 ± 0.047 | −6.514 | < 0.0001 |
| Acclimation temp x Treatment | −0.238 ± 0.046 | −5.141 | < 0.0001 |
| Test temp x Treatment | −0.144 ± 0.045 | −3.183 | 0.0015 |
| Acclimation temp x Test temp x Treatment | 0.437 ± 0.065 | 6.677 | < 0.0001 |
| Random Effects | Percentage of variation explained | ||
| Replicate line | 1.27% | ||
| Residual | 98.73% | ||
| Indigenous Lepidocyrtus sp. 10 | Estimate ± S.E. | t | p |
| Intercept | 2.242 ± 0.034 | 65.51 | < 0.0001 |
| Acclimation temperature (high) | −0.061 ± 0.034 | −1.791 | 0.0738 |
| Test temperature (high) | −0.189 ± 0.033 | −5.649 | < 0.0001 |
| Treatment (treatment) | −0.002 ± 0.048 | −0.047 | 0.9645 |
| Acclimation temp x Test temp | 0.157 ± 0.047 | 3.296 | 0.0010 |
| Acclimation temp x Treatment | −0.305 ± 0.047 | −6.556 | < 0.0001 |
| Test temp x Treatment | −0.133 ± 0.047 | −2.86 | 0.0044 |
| Acclimation temp x Test temp x Treatment | −0.062 ± 0.066 | −0.941 | 0.3471 |
| Random Effects | Percentage of variation explained | ||
| Replicate line | 3.03% | ||
| Residual | 96.97% | ||
| Alien Orthonychiurus sp. | Estimate ± S.E. | t | p |
| Intercept | 1.99368 ± 0.02716 | 73.41 | < 0.0001 |
| Acclimation temperature (high) | −0.01449 ± 0.02373 | −0.611 | 0.5417 |
| Test temperature (high) | −0.29485 ± 0.02524 | −11.682 | < 0.0001 |
| Treatment (treatment) | −0.14669 ± 0.03891 | −3.77 | 0.0204 |
| Acclimation temp x Test temp | 0.06305 ± 0.0355 | 1.776 | 0.0762 |
| Acclimation temp x Treatment | −0.24488 ± 0.03445 | −7.108 | < 0.0001 |
| Test temp x Treatment | −0.12619 ± 0.0354 | −3.565 | 0.0004 |
| Acclimation temp x Test temp x Treatment | 0.40308 ± 0.04985 | 8.086 | < 0.0001 |
| Random Effects | Percentage of variation explained | ||
| Replicate line | 3.68% | ||
| Residual | 96.32% | ||
Fig. 5.
Line graph illustrating desiccation resistance under 76% relative humidity (as the mean ± s.d. of log10 time to death in minutes) at two test temperatures for four species of springtails that had evolved at a high (orange) or low (black) temperatures for successive generations, which were then exposed to a high (dashed lines) or low (solid lines) acclimation temperature for one week prior to experimentation. Repeated lines represent replicates of each of the four treatments for each species.
4. Discussion
Here we set out to test predictions from the two major traits-based hypothesis of invasion ecology (Enders et al., 2020). The ideal weed hypothesis makes the prediction that invasive alien species success depends on a specific basal form of a trait, while the plasticity hypothesis makes the prediction that invasive alien species are more phenotypically plastic than their indigenous counterparts. Evidence supporting these predictions, in the form of reliable differences in traits on average between indigenous and alien species, is emerging for a range of taxa (van Kleunen et al., 2010b, 2015; Davidson et al., 2011; Kelley, 2014; Capellini et al., 2015; Allen et al., 2017; Gallien et al., 2019; Redding et al., 2019; Milanović et al., 2020), including for thermal traits of arthropods (Janion et al., 2010; Weiner et al., 2010; Yu et al., 2012; Jarošík et al., 2015; Janion-Scheepers et al., 2018; da Silva et al., 2021). The situation for desiccation resistance is not well understood, however, despite the importance of this trait for the survival, distribution and abundance of terrestrial species (Addo-Bediako et al., 2001; Chown et al., 2007, 2011; Peguero et al., 2019; Staubus et al., 2019; Ajayi et al., 2020; Kellermann et al., 2020; Phillips et al., 2020; da Silva et al., 2021). In the Collembola investigated here, from a range of climatic areas, no support for the plasticity hypothesis was found. Support for the ideal weed hypothesis was identified, although strong phylogenetic signal indicates that it may be mediated through particular taxa.
In the case of the predictions of the plasticity hypothesis, no consistent differences in plasticity between the indigenous and alien species were found. The only consistent pattern (in 9 of the 16 species investigated) was an increase in survival time at high test temperatures following acclimation to such temperatures, relative to acclimation to a lower temperature – supporting a form of the hotter is better hypothesis (Huey et al., 1999; Chown et al., 2007). Only two other studies have sought to investigate differences between indigenous and alien Collembola species in phenotypic plasticity of survival time, both of which considered species from sub-Antarctic island assemblages (Chown et al., 2007; Phillips et al., 2020). Our results are consistent with those of the more comprehensive study of 12 species from sub-Antarctic Macquarie Island (Phillips et al., 2020). This study failed to find consistent differences between indigenous and alien species in phenotypic plasticity just as we have here. By contrast, a study of six species from sub-Antarctic Marion Island (Chown et al., 2007) revealed that alien species tended to have longer survival times following high acclimation temperatures than following acclimation at low temperatures, whereas the converse was true in the indigenous species. In our current study, only the indigenous Triacanthella sp. showed the effect found for alien species on Marion Island. Therefore, the trend emerging thus far is one where the predictions of the plasticity hypothesis (Enders et al., 2020) are not generally upheld for hemiedaphic Collembola, though exceptions may exist in some assemblages. Thus, it seems reasonable to conjecture that phenotypic plasticity of desiccation resistance might not be a major contributor to the success of alien Collembola.
In part this outcome may be the consequence of selecting temperature as the acclimation treatment for desiccation stress. Doing so is reasonable, however, on both theoretical and empirical grounds. In the former case, temperature has a pronounced effect on vapor pressure deficit (Campbell and Norman, 1998), and thermo- and hygro-sensing in arthropods and other invertebrates are closely integrated (Altner and Loftus, 1985; Russell et al., 2014; Enjin, 2017; Frank et al., 2017; Marin et al., 2020). In the latter because such responses of desiccation resistance to changes in environmental temperature only have been documented previous for springtails and for insects (Terblanche and Chown, 2006; Chown et al., 2007; Leinaas et al., 2009; Fischer and Kirtse, 2018; Phillips et al., 2020) and because both a previous investigation of ants (Baumgart et al., 2022) and the experiment here on springtail cuticle responses to temperature with no change in humidity reveal pronounced effects. In the experiment on ants, warm acclimation resulted in cuticular hydrocarbon changes and an improvement of desiccation tolerance, though species-specific difference in response between the two Lasius species investigated were found (Baumgart et al., 2022). Here we verified such responses for springtails, finding strong responses to temperature, in ways that were similar in reciprocal acclimations (i.e. a switch between the 5 °C and 28 °C temperature conditions), in both contact angle and in the netto carbon count of the cuticle. Both of these traits are strongly related to water relations in springtails and in insects (Menzel et al., 2007, 2022; Helbig et al., 2011; Nickerl et al., 2014; Gunderson et al., 2015; Hensel et al., 2016; Schmüser et al., 2020; Baumgart et al., 2022). What precise changes in hydrocarbons were taking place in the species we investigated here would be useful to understand, but were beyond the scope of this verification of temperature response despite constant ∼100% humidity of the acclimation treatments.
By contrast with these findings, basal resistance differed significantly and substantially between the indigenous and alien species. An effect of test temperature demonstrated, however, that this difference became weaker with an increase in test temperature and therefore vapor pressure deficit. Thus, differences are marked under the conditions that hemiedaphic springtails, dwelling in litter (Christeiansen 1964; Liu et al., 2021), are most likely to encounter. Such a finding of differences in basal resistance, in this case survival time, accords with the differences found between indigenous and alien species in the sub-Antarctic Macquarie Island assemblage (Phillips et al., 2020). Nonetheless, a major difference between our results and those from that study, is the marked effect of phylogenetic correlation found here (λ = 0.975) relative to the situation for the Macquarie Island Collembola fauna (λ = 0). In fact, a difference so large here that it rendered the effects of test temperature, status, and their interactions insignificant.
Clearly this outcome has to do, in part, with the exceptionally long survival times of the species in the family Hypogastruridae and Neanuridae (Table 1), which in this study were dominated by alien species. Some species in these families are among the most successful invaders, particularly those investigated here (Convey et al., 1999; Greenslade, 2002, 2018), with ecological investigations demonstrating that invasive hypogastrurids can displace other Collembola species at local scales (Terauds et al., 2011; Chown et al., 2022). Moreover, they are among the most desiccation tolerant species known, at least as far as comparisons, even with atmobiotic species, have revealed (Liu et al., 2021). Nonetheless, it is clear that members of the family Entomobryidae and Isotomidae can be successful invaders too (Greenslade, 2018), but differences among them in desiccation resistance are less clear. In consequence, it appears that other characteristics, which may be phylogenetically conserved, likely also play a role in the outcome of invasions, though extent of habitat disturbance and other local factors probably also influence outcomes (Richardson and Pyšek, 2006; Moles et al., 2007; Enders et al., 2020). Disentangling the factors which lead to invasion success in the Collembola can clearly benefit from a combination of approaches applied across a range of scales which combine both ecological and trait-based studies. So far, this approach has yet to be fully applied in this group (see discussion in Phillips et al., 2020), though it is now widely used for plants, providing clear insights into the factors enabling success along the invasion pathway (van Kleunen et al., 2010a; Powell et al., 2011; Hulme and Bernard-Verdier, 2018).
One of the factors which may be important is, of course, the extent to which introduced species are likely to adapt to local conditions (Oduor et al., 2016; Enders et al., 2020), relative to simply being filtered out along the invasion pathway owing to unsuitable local conditions (Kolar and Lodge, 2001; Renault et al., 2018). Here, we used laboratory natural selection (Gibbs, 1999) to determine if differences in adaptation might exist between indigenous and invasive species in response to varying environmental conditions. By contrast with what we expected, especially given evidence for heritability of desiccation resistance at least in some arthropods (Hoffmann and Parsons, 1993; Gibbs et al., 1997; Kellermann et al., 2009), laboratory natural selection had little effect on the indigenous or alien species after 8–12 generations. Recent work has revealed, however, that heritability for desiccation-related traits can be highly variable among species from different regions (e.g., tropical versus temperate areas) and experimental conditions (Kellermann and van Heerwaarden, 2019). Here, we found no differences among species from tropical or temperate regions, and little response to laboratory natural selection either. Obviously, selection must have led to differences among major groups and species of Collembola at some point, but what circumstances may have given rise to such differences is not clear. As is the case with thermal traits, perhaps short-term extreme conditions are most important (Kingsolver and Buckley, 2017; Janion-Scheepers et al., 2018). Irrespective, the outcomes here suggest that environmental filtering may have an important role to play, as is the case with invasions involving other taxa (Renault et al., 2018).
In interpreting these results, consideration of other responses to desiccation stress are also important. Arthropods, including springtails, may alter survival of dry conditions by either limiting water loss rate through various adaptive and/or short-term plastic responses, or they may alter initial body water content, or simply tolerate greater water loss (Hadley, 1994; Chown and Nicholson, 2004). The approach taken here does not reveal which of these strategies are used by the species in question. An investigation of the relative contributions in other springtail species has revealed, however, that alterations to change rates of water loss are most common, with changes in the tolerance of water loss being less important (Kærsgaard et al., 2004; Leinaas et al., 2009). Although body water content change in responses to laboratory selection has been found in insects, it is thought to be less common in the wild (Gibbs et al., 2003). These questions deserve greater consideration in the examination of variation in responses to dry conditions by indigenous and introduced springtails.
In conclusion, our work has indicated that basal desiccation resistance may be a factor influencing the success of invasive Collembola. The extent to which this is as a consequence of the identity of the invasive species in a particular assemblage, or the role of environmental filtering or adaptation cannot, however, yet be resolved. Importantly, it does appear that phenotypic plasticity of desiccation tolerance plays less of a role in differentiating indigenous from alien Collembola species than might be the case for plasticity of other traits in other taxa. Ultimately, the most revealing outcome is how much still needs to be done to understand the factors underlying Collembola invasions, that are significant in many parts of the world (Cicconardi et al., 2017; Greenslade, 2018; Phillips et al., 2020), with indications that they may displace local species and alter ecosystem structure and potentially functioning (Convey et al., 1999; Terauds et al., 2011; Leinaas et al., 2015; Treasure et al., 2019; Potapov et al., 2020; Chown et al., 2022).
CRediT authorship contribution statement
Steven L Chown: Conceptualization, Formal analysis, Data curation, Writing – original draft. Charlene Janion-Scheepers: Conceptualization, Investigation, Writing – review & editing. Angus Marshall: Investigation, Data curation. Ian J Aitkenhead: Investigation, Data curation, Formal analysis, Writing – review & editing. Rebecca Hallas: Investigation, Data curation. WP Amy Liu: Formal analysis, Data curation, Writing – review & editing. Laura M Phillips: Conceptualization, Formal analysis, Investigation, Data curation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Two anonymous reviewers provided insightful comments on previous versions. We thank the editor and editorial team for their patience with the time taken to complete the cuticle response experiments because of Melbourne's extended pandemic lockdowns. This work was supported by Australian Research Council Discovery Project DP190100341 and by Australian Research Council SRIEAS Grant SR200100005 Securing Antarctica's Environmental Future.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cris.2022.100051.
Appendix. Supplementary materials
Data Availability
Data and code are open access via Monash Bridges (Figshare) at: https://doi.org/10.26180/21,670,898.v1
References
- Addo-Bediako A., Chown S.L., Gaston K.J. Revisiting water loss in insects: a large scale view. J. Insect Physiol. 2001;47:1377–1388. doi: 10.1016/s0022-1910(01)00128-7. [DOI] [PubMed] [Google Scholar]
- Ajayi O.S., Appel A.G., Chen L., Fadamiro H.Y. Comparative cutaneous water loss and desiccation tolerance of four Solenopsis spp. (Hymenoptera: formicidae) in the southeastern United States. Insects. 2020;11:418. doi: 10.3390/insects11070418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen W.L., Street S.E., Capellini I. Fast life history traits promote invasion success in amphibians and reptiles. Ecol. Lett. 2017;20:222–230. doi: 10.1111/ele.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altner H., Loftus R. Ultrastructure and function of insect thermo- and hygroreceptors. Annu. Rev. Entomol. 1985;30:273–295. [Google Scholar]
- Baumgart L., Wittke M., Morsbach S., Abou B., Menzel F. Why do ants differ in acclimatory ability? Biophysical mechanisms behind cuticular hydrocarbon acclimation across species. J. exp. Biol. 2022;225 doi: 10.1242/jeb.243847. [DOI] [PubMed] [Google Scholar]
- Bertelsmeier C., Keller L. Bridgehead effects and role of adaptive evolution in invasive populations. Trends Ecol. Evol. 2018;33:527–534. doi: 10.1016/j.tree.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Blackburn T.M., Pyšek P., Bacher S., Carlton J.T., Duncan R.P., Jarosík V., Wilson J.R.U., Richardson D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011;26:333–339. doi: 10.1016/j.tree.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Bradie J., Leung B. A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeog. 2017;44:1344–1361. [Google Scholar]
- Bradshaw C.J.A., Leroy B., Bellard C., Roiz D., Albert C., Fournier A., Barbet-Massin M., Salles J.-.M., Simard F., Courchamp F. Massive yet grossly underestimated global costs of invasive insects. Nature Commun. 2016;7:12986. doi: 10.1038/ncomms12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G.S., Norman J.M. Second ed. Springer; New York: 1998. An Introduction to Environmental Biophysics. [Google Scholar]
- Capellini I., Baker J., Allen W.L., Street S.E., Venditti C. The role of life history traits in mammalian invasion success. Ecol. Lett. 2015;18:1099–1107. doi: 10.1111/ele.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown S.L., Bergstrom D.M., Houghton M., Kiefer K., Terauds A., Leihy R.I. Invasive species impacts on sub-Antarctic Collembola support the Antarctic climate-diversity-invasion hypothesis. Soil Biol. Biochem. 2022;166 [Google Scholar]
- Chown S.L., Nicolson S.W. First ed. Oxford University Press; Oxford: 2004. Insect Physiological ecology. Mechanisms and Patterns. [Google Scholar]
- Chown S.L., Slabber S., McGeoch M.A., Janion C., Leinaas H.P. Phenotypic plasticity mediates climate change responses among invasive and indigenous arthropods. Proc. R. Soc. B. 2007;274:2661–2667. doi: 10.1098/rspb.2007.0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown S.L., Sørensen J.G., Terblanche J.S. Water loss in insects: an environmental change perspective. J. Insect Physiol. 2011;57:1070–1084. doi: 10.1016/j.jinsphys.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Christiansen K. Bionomics of Collembola. Annu. Rev. Entomol. 1964;9:147–178. [Google Scholar]
- Cicconardi F., Borges P.A.V., Strasberg D., Oromi P., Lopez H., Perez-Delgado A.J., Casquet J., Caujape-Castells J., Fernandez-Palacios J.M., Thebaud C., Emerson B.C. MtDNA metagenomics reveals large-scale invasion of belowground arthropod communities by introduced species. Molec. Ecol. 2017;26:3104–3115. doi: 10.1111/mec.14037. [DOI] [PubMed] [Google Scholar]
- Convey P., Greenslade P., Arnold R.J., Block W. Collembola of sub-Antarctic South Georgia. Polar Biol. 1999;22:1–6. [Google Scholar]
- Cooper N., Thomas G.N., Venditti C., Meade A., Freckleton R.P. A cautionary note on the use of Ornstein-Uhlenbeck models in macroevolutionary studies. Biol. J. Linn. Soc. 2016;118:64–77. doi: 10.1111/bij.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley M.J. John Wiley & Sons; Chichester: 2013. The R Book. [Google Scholar]
- da Silva C.R.B., Beaman J.E., Dorey J.B., Barker S.J., Congedi N.C., Elmer M.C., Galvin S., Tuiwawa M., Stevens M.I., Alton L.A., Schwarz M.P., Kellermann V. Climate change and invasive species: a physiological performance comparison of invasive and endemic bees in Fiji. J. Exp. Biol. 2021;224 doi: 10.1242/jeb.230326. [DOI] [PubMed] [Google Scholar]
- Davidson A.M., Jennions M., Nicotra A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011;14:419–431. doi: 10.1111/j.1461-0248.2011.01596.x. [DOI] [PubMed] [Google Scholar]
- Diagne C., Leroy B., Vaissiere A.C., Gozlan R.E., Roiz D., Jaric I., Salles J.M., Bradshaw C.J.A., Courchamp F. High and rising economic costs of biological invasions worldwide. Nature. 2021;592:571–576. doi: 10.1038/s41586-021-03405-6. [DOI] [PubMed] [Google Scholar]
- Enders M., Havemann F., Ruland F., Bernard-Verdier M., Catford J.A., Gómez-Aparicio L., Haider S., Heger T., Kueffer C., Kühn I., Meyerson L.A., Musseau C., Novoa A., Ricciardi A., Sagouis A., Schittko C., Strayer D.L., Vilà M., F Essl F., Hulme P.E., van Kleunen M., Kumschick S., Lockwood J.L., Mabey A.L., McGeoch M.A., Palma E., Pyšek P., Saul W.-.C., Yannelli F.A., Jeschke J.M., Belmaker J. A conceptual map of invasion biology: integrating hypotheses into a consensus network. Global Ecol. Biogeogr. 2020;29:978–991. doi: 10.1111/geb.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjin A. Humidity sensing in insects-from ecology to neural processing. Curr. Opin. Insect Sci. 2017;24:1–6. doi: 10.1016/j.cois.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Fischer K., Kirste M. Temperature and humidity acclimation increase desiccation resistance in the butterfly Bicyclus anynana. Entomol. Exp. Appl. 2018;166:289–297. [Google Scholar]
- Fjellberg A. Brill; Leiden: 1998. Fauna Entomologica Scandinavica Volume 42. The Collembola of Fennoscandia and Denmark. Part I: Poduromorpha. [Google Scholar]
- Frank D.D., Enjin A., Jouandet G.C., Zaharieva E.E., Para A., Stensmyr M.C., Gallio M. Early integration of temperature and humidity stimuli in the Drosophila brain. Curr. Biol. 2017;27:2381–2388. doi: 10.1016/j.cub.2017.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R.V., Falster D.S., Maitner B.S., Salguero-Gomez R., Vandvik V., Pearse W.D., Schneider F.D., Kattge J., Poelen J.H., Madin J.S., Ankenbrand M.J., Penone C., Feng X., Adams V.M., Alroy J., Andrew S.C., Balk M.A., Bland L.M., Boyle B.L., Bravo-Avila C.H., Brennan I., Carthey A.J.R., Catullo R., Cavazos B.R., Conde D.A., Chown S.L., Fadrique B., Gibb H., Halbritter A.H., Hammock J., Hogan J.A., Holewa H., Hope M., Iversen C.M., Jochum M., Kearney M., Keller A., Mabee P., Manning P., McCormack L., Michaletz S.T., Park D.S., Perez T.M., Pineda-Munoz S., Ray C.A., Rossetto M., Sauquet H., Sparrow B., Spasojevic M.J., Telford R.J., Tobias J.A., Violle C., Walls R., Weiss K.C.B., Westoby M., Wright I.J., Enquist B.J. Open Science principles for accelerating trait-based science across the Tree of Life. Nature Ecol. Evol. 2020;4:294–303. doi: 10.1038/s41559-020-1109-6. [DOI] [PubMed] [Google Scholar]
- Gallien L., Thornhill A.H., Zurell D., Miller J.T., Richardson D.M. Global predictors of alien plant establishment success: combining niche and trait proxies. Proc. R. Soc. B. 2019;286 doi: 10.1098/rspb.2018.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T., Ives A.R. Using the past to predict the present: confidence intervals for regression equations in phylogenetic comparative methods. Am. Nat. 2000;155:346–364. doi: 10.1086/303327. [DOI] [PubMed] [Google Scholar]
- Gibbs A.G. Laboratory selection for the comparative physiologist. J. exp. Biol. 1999;202:2709–2718. doi: 10.1242/jeb.202.20.2709. [DOI] [PubMed] [Google Scholar]
- Gibbs A.G., Chippindale A.K., Rose M.R. Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J. exp. Biol. 1997;200:1821–1832. doi: 10.1242/jeb.200.12.1821. [DOI] [PubMed] [Google Scholar]
- Gibbs A.G., Fukuzato F., Matzkin L.M. Evolution of water conservation mechanisms in Drosophila. J. exp. Biol. 2003;206:1183–1192. doi: 10.1242/jeb.00233. [DOI] [PubMed] [Google Scholar]
- Grafen A. The phylogenetic regression. Phil. Trans. R. Soc. B. 1989;326:119–157. doi: 10.1098/rstb.1989.0106. [DOI] [PubMed] [Google Scholar]
- Greenslade P. Assessing the risk of exotic Collembola invading subantarctic islands: prioritising quarantine management. Pedobiologia (Jena) 2002;46:338–344. [Google Scholar]
- Greenslade P. Why are there so many exotic springtails in Australia? A review. Soil Org. 2018;90:141–156. [Google Scholar]
- Greenslade P., Ireson J., Skarzynski D. Biology and key to the Australian species of Hypogastrura and Ceratophysella (Collembola: hypogastruridae) Austral Entomol. 2014;53:53–74. [Google Scholar]
- Gundersen H., Leinaas H.P., Thaulow C. Surface structure and wetting characteristics of Collembola cuticles. PLoS ONE. 2014;9:e86783. doi: 10.1371/journal.pone.0086783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H., Thaulow C., Leinaas H.P. Seasonal change in the wetting characteristics of the cuticle of the Collembola Cryptopygus clavatus (Schott, 1893) Zoomorphology. 2015;134:211–218. doi: 10.1007/s00435-015-0254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley N.F. Academic Press; San Diego: 1994. Water Relations of Terrestrial Arthropods. [Google Scholar]
- Helbig R., Nickerl J., Neinhuis C., Werner C. Smart skin patterns protect springtails. PLoS ONE. 2011;6:e25105. doi: 10.1371/journal.pone.0025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel R., Neinhuis C., Werner C. The springtail cuticle as a blueprint for omniphobic surfaces. Chem. Soc. Rev. 2016;45:323–341. doi: 10.1039/c5cs00438a. [DOI] [PubMed] [Google Scholar]
- Hill M.P., Clusella-Trullas S., Terblanche J.S., Richardson D.M. Drivers, impacts, mechanisms and adaptation in insect invasions. Biol. Invas. 2016;18:883–891. [Google Scholar]
- Hilligsøe H., Holmstrup M. Effects of starvation and body mass on drought tolerance in the soil collembolan Folsomia candida. J. Insect Physiol. 2003;49:99–104. doi: 10.1016/s0022-1910(02)00253-6. [DOI] [PubMed] [Google Scholar]
- Hoffmann A.A., Parsons P.A. Direct and correlated responses to selection for desiccation resistance: a comparison of Drosophila melanogaster and D. simulans. J. Evol. Biol. 1993;6:643–657. [Google Scholar]
- Hoffmann A.A., Sgrò C.M. Comparative studies of critical physiological limits and vulnerability to environmental extremes in small ectotherms: how much environmental control is needed? Integr. Zool. 2017;13:355–371. doi: 10.1111/1749-4877.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg I.D., Hebert P.D.N. Biological identification of springtails (Hexapoda: collembola) from the Canadian Arctic, using mitochondrial DNA barcodes. Can. J. Zool. 2004;82:749–754. [Google Scholar]
- Holway D.A., Suarez A.V., Case T.J. Role of abiotic factors in governing susceptibility to invasion: a test with Argentine ants. Ecology. 2002;83:1610–1619. [Google Scholar]
- Hopkin S. Oxford University Press; Oxford: 1997. Biology of the Springtails (Insecta: Collembola) [Google Scholar]
- Hoskins J.L., Janion-Scheepers C., Chown S.L., Duffy G.A. Growth and reproduction of laboratory-reared neanurid Collembola using a novel slime mould diet. Sci. Rep. 2015;5:11957. doi: 10.1038/srep11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey R.B., Berrigan D., Gilchrist G.W., Herron J.C. Testing the adaptive significance of acclimation: a strong inference approach. Am. Zool. 1999;39:323–336. [Google Scholar]
- Hulme P.E., Bernard-Verdier M. Comparing traits of native and alien plants: can we do better? Funct. Ecol. 2018;32:117–125. [Google Scholar]
- Janion C., Leinaas H.P., Terblanche J.S., Chown S.L. Trait means and reaction norms: the consequences of climate change/invasion interactions at the organism level. Evol. Ecol. 2010;24:1365–1380. [Google Scholar]
- Janion-Scheepers C., Phillips L., Sgrò C.M., Duffy G.A., Hallas R., Chown S.L. Basal resistance enhances warming tolerance of alien over indigenous species across latitude. Proc. Natnl. Acad. Sci. U.S.A. 2018;115:145–150. doi: 10.1073/pnas.1715598115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarošík V., Kenis M., Honěk A., Skuhrovec J., Pyšek P. Invasive insects differ from non-invasive in their thermal requirements. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0131072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke J.M., Strayer D.L. Are threat status and invasion success two sides of the same coin? Ecography. 2008;31:124–130. [Google Scholar]
- Kærsgaard C.W., Holmstrup M., Malte H., Bayley M. The importance of cuticular permeability, osmolyte production and body size for the desiccation resistance of nine species of Collembola. J. Insect Physiol. 2004;50:5–15. doi: 10.1016/j.jinsphys.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Kearney M., Porter W.P. Ecologists have already started rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006;21:481–482. doi: 10.1016/j.tree.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Kellermann V., McEvey S.F., Sgrò C.M., Hoffmann A.A. Phenotypic plasticity for desiccation resistance, climate change, and future species distributions: will plasticity have much impact? Am. Nat. 2020;196:306–315. doi: 10.1086/710006. [DOI] [PubMed] [Google Scholar]
- Kellermann V., van Heerwaarden B. Terrestrial insects and climate change: adaptive responses in key traits. Physiol. Entomol. 2019;44:99–115. [Google Scholar]
- Kellermann V., van Heerwaarden B., Sgrò C.M., Hoffmann A.A. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science. 2009;325:1244–1246. doi: 10.1126/science.1175443. [DOI] [PubMed] [Google Scholar]
- Kelley A.L. The role thermal physiology plays in species invasion. Conserv. Physiol. 2014;2:cou045. doi: 10.1093/conphys/cou045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsolver J.G., Buckley L.B. Quantifying thermal extremes and biological variation to predict evolutionary responses to changing climate. Phil. Trans. R. Soc. B. 2017;372 doi: 10.1098/rstb.2016.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar C.S., Lodge D.M. Progress in invasion biology: predicting invaders. Trends Ecol. Evol. 2001;16:199–204. doi: 10.1016/s0169-5347(01)02101-2. [DOI] [PubMed] [Google Scholar]
- Kuyucu A.C., Chown S.L. Time course of acclimation of critical thermal limits in two springtail species (Collembola) J. Insect Physiol. 2021;130 doi: 10.1016/j.jinsphys.2021.104209. [DOI] [PubMed] [Google Scholar]
- Lee J.E., Janion C., Marais E., Van Vuuren B.J., Chown S.L. Physiological tolerances account for range limits and abundance structure in an invasive slug. Proc. R. Soc. B. 2009;276:1459–1468. doi: 10.1098/rspb.2008.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinaas H.P., Bengtsson J., Janion-Scheepers C., Chown S.L. Indirect effects of habitat disturbance on invasion: nutritious litter from a grazing resistant plant favours alien over native Collembola. Ecol. Evol. 2015;5:3462–3471. doi: 10.1002/ece3.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinaas H.P., Slabber S., Chown S.L. Effects of thermal acclimation on water loss rate and tolerance in the collembolan Pogonognathellus flavescens. Physiol. Entomol. 2009;34:325–332. [Google Scholar]
- Liu C., Wolter C., Xian W., Jeschke J.M. Most invasive species largely conserve their climatic niche. Proc. Natnl. Acad. Sci. U.S.A. 2020;117:23643–23651. doi: 10.1073/pnas.2004289117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.P.A., Phillips L.M., Terblanche J.S., Janion-Scheepers C., Chown S.L. An unusually diverse genus of Collembola in the Cape Floristic Region characterised by substantial desiccation tolerance. Oecologia. 2021;195:873–885. doi: 10.1007/s00442-021-04896-w. [DOI] [PubMed] [Google Scholar]
- Marin E.C., Buld L., Theiss M., Sarkissian T., Roberts R.J.V., Turnbull R., Tamimi I.F.M., Pleijzier M.W., Laursen W.J., Drummond N., Schlegel P., Bates A.S., Li F., Landgraf M., Costa M., Bock D.D., Garrity P.A., Jefferis G. Connectomics analysis reveals first-, second-, and third-order thermosensory and hygrosensory neurons in the adult Drosophila brain. Curr. Biol. 2020;30:3167–3182. doi: 10.1016/j.cub.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel F., Blaimer B.B., Schmitt T. How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait. Proc. R. Soc. B. 2007;284 doi: 10.1098/rspb.2016.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel F., Morsbach S., Martens J.H., Räder P., Hadjaje S., Poizat M., Abou B. Communication versus waterproofing: the physics of insect cuticular hydrocarbons. J. exp. Biol. 2022;222 doi: 10.1242/jeb.210807. [DOI] [PubMed] [Google Scholar]
- Meyer C.P., Paulay G. DNA barcoding: error rates based on comprehensive sampling. PLoS Biol. 2005;3:2229–2238. doi: 10.1371/journal.pbio.0030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanović M., Knapp S., Pyšek P., Kühn I. Trait–environment relationships of plant species at different stages of the introduction process. NeoBiota. 2020;58:55–74. [Google Scholar]
- Moles A.T., Gruber M.A.M., Bonser S.P. A new framework for predicting invasive plant species. J. Ecol. 2007;96:13–17. [Google Scholar]
- Molina-Venegas R., Rodríguez M.A. Revisiting phylogenetic signal; strong or negligible impacts of polytomies and branch length information? BMC Evol. Biol. 2017;17:53. doi: 10.1186/s12862-017-0898-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerl J., Tsurkan M., Hensel R., Neinhuis C., Werner C. The multilayered protective cuticle of Collembola: a chemical analysis. J. R. Soc. Interface. 2014;11 doi: 10.1098/rsif.2014.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble-Nesbitt J. The fully formed intermoult cuticle and associated structures of Podura aquatica (Collembola) Quart. J. Micr. Sci. 1963;104:253–270. [Google Scholar]
- Oduor A.M.O., Leimu R., van Kleunen M., Mack R. Invasive plant species are locally adapted just as frequently and at least as strongly as native plant species. J. Ecol. 2016;104:957–968. [Google Scholar]
- Orme D., 2018. The caper package: comparative analysis of phylogenetics and evolution in R, https://cran.r-project.org/web/packages/caper/vignettes/caper.pdf.
- Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- Paradis E., Claude J., Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Parkash R., Rajpurohit S., Ramniwas S. Changes in body melanisation and desiccation resistance in highland vs. lowland populations of D. melanogaster. J. Insect Physiol. 2008;54:1050–1056. doi: 10.1016/j.jinsphys.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Peguero G., Sol D., Arnedo M., Petersen H., Salmon S., Ponge J.F., Maspons J., Emmett B., Beier C., Schmidt I.K., Tietema A., De Angelis P., Kovacs-Lang E., Kroel-Dulay G., Estiarte M., Bartrons M., Holmstrup M., Janssens I.A., Penuelas J. Fast attrition of springtail communities by experimental drought and richness-decomposition relationships across Europe. Global Change Biol. 2019;25:2727–2738. doi: 10.1111/gcb.14685. [DOI] [PubMed] [Google Scholar]
- Phillips L.M., Aitkenhead I., Janion-Scheepers C., King C.K., McGeoch M.A., Nielsen U.N., Terauds A., Liu W.P.A., Chown S.L. Basal tolerance but not plasticity gives invasive springtails the advantage in an assemblage setting. Conserv. Physiol. 2020;8:coaa049. doi: 10.1093/conphys/coaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapov A., Bellini B.C., Chown S.L., Deharveng L., Janssens F., Kováč L., Kuznetsova N., Ponge J.-.F., Potapov M., Querner P., Russell D., Sin X., Zhang F., Berg M.P. Towards a global synthesis of Collembola knowledge – challenges and potential solutions. Soil Org. 2020;92:161–188. [Google Scholar]
- Powell K.I., Chase J.M., Knight T.M. A synthesis of plant invasion effects on biodiversity across spatial scales. Am. J. Bot. 2011;98:539–548. doi: 10.3732/ajb.1000402. [DOI] [PubMed] [Google Scholar]
- Core Team R. R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A language and Environment For Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Redding D.W., Pigot A.L., Dyer E.E., Sekercioglu C.H., Kark S., Blackburn T.M. Location-level processes drive the establishment of alien bird populations worldwide. Nature. 2019;571:103–106. doi: 10.1038/s41586-019-1292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault D., Laparie M., McCauley S.J., Bonte D. Environmental adaptations, ecological filtering, and dispersal central to insect invasions. Annu. Rev. Entomol. 2018;63:345–368. doi: 10.1146/annurev-ento-020117-043315. [DOI] [PubMed] [Google Scholar]
- Reznick D.N., Losos J., Travis J. From low to high gear: there has been a paradigm shift in our understanding of evolution. Ecol. Lett. 2019;22:233–244. doi: 10.1111/ele.13189. [DOI] [PubMed] [Google Scholar]
- Richardson D.M., Pyšek P. Plant invasions: merging the concepts of species invasiveness and community invasibility. Prog. Phys. Geog. 2006;30:409–431. [Google Scholar]
- Russell J., Vidal-Gadea A.G., Makay A., Lanam C., Pierce-Shimomura J.T. Humidity sensation requires both mechanosensory and thermosensory pathways in Caenorhabditis elegans. Proc. Natnl. Acad. Sci. U.S.A. 2014;111:8269–8274. doi: 10.1073/pnas.1322512111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmüser L., Zhang W., Marx M.T., Encinas N., Vollmer D., Gorb S., Baio J.E., Räder H.J., Weidner T. Role of surface chemistry in the superhydrophobicity of the springtail Orchesella cincta (Insecta: collembola) ACS Appl. Mater. Interf. 2020;12:12294–12304. doi: 10.1021/acsami.9b21615. [DOI] [PubMed] [Google Scholar]
- Seebens H., Blackburn T.M., Dyer E.E., Genovesi P., Hulme P.E., Jeschke J.M., Pagad S., Pysek P., Winter M., Arianoutsou M., Bacher S., Blasius B., Brundu G., Capinha C., Celesti-Grapow L., Dawson W., Dullinger S., Fuentes N., Jager H., Kartesz J., Kenis M., Kreft H., Kuhn I., Lenzner B., Liebhold A., Mosena A., Moser D., Nishino M., Pearman D., Pergl J., Rabitsch W., Rojas-Sandoval J., Roques A., Rorke S., Rossinelli S., Roy H.E., Scalera R., Schindler S., Stajerova K., Tokarska-Guzik B., van Kleunen M., Walker K., Weigelt P., Yamanaka T., Essl F. No saturation in the accumulation of alien species worldwide. Nature Commun. 2017;8:14435. doi: 10.1038/ncomms14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberloff D., Martin J.L., Genovesi P., Maris V., Wardle D.A., Aronson J., Courchamp F., Galil B., Garcia-Berthou E., Pascal M., Pyšek P., Sousa R., Tabacchi E., Vila M. Impacts of biological invasions: what's what and the way forward. Trends Ecol. Evol. 2013;28:58–66. doi: 10.1016/j.tree.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Staubus W.J., Bird S., Meadors S., Meyer W.M., 3rd Distributions of invasive arthropods across heterogeneous urban landscapes in southern California: aridity as a key component of ecological resistance. Insects. 2019;10:29. doi: 10.3390/insects10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M., Horibe Y., Kawada H., Takagi M. Insect spiracle as the main penetration route of pyrethroids. Pest. Biochem. Physiol. 2008;91:135–140. [Google Scholar]
- Szűcs M., Vercken E., Bitume E.V., Hufbauer R.A. The implications of rapid eco-evolutionary processes for biological control - a review. Entomol. Exp. Appl. 2019;167 598-165. [Google Scholar]
- Terauds A., Chown S.L., Bergstrom D.M. Spatial scale and species identity influence the indigenous–alien diversity relationship in springtails. Ecology. 2011;92:1436–1447. doi: 10.1890/10-2216.1. [DOI] [PubMed] [Google Scholar]
- Terblanche J.S., Chown S.L. The relative contributions of developmental plasticity and adult acclimation to physiological variation in the tsetse fly, Glossina pallidipes (Diptera, Glossinidae) J. exp. Biol. 2006;209:1064–1073. doi: 10.1242/jeb.02129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treasure A.M., le Roux P.C., Mashau M.H., Chown S.L. Species-energy relationships of indigenous and invasive species may arise in different ways - a demonstration using springtails. Sci. Rep. 2019;9:13799. doi: 10.1038/s41598-019-48871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda J.C., Zenil-Ferguson R., Pennell M.W. Rethinking phylogenetic comparative methods. Syst. Biol. 2018;67:1091–1109. doi: 10.1093/sysbio/syy031. [DOI] [PubMed] [Google Scholar]
- Van Kleunen M., Bossdorf O., Dawson W. The ecology and evolution of alien plants. Annu. Rev. Ecol. Evol. Syst. 2018;49:25–47. [Google Scholar]
- Van Kleunen M., Dawson W., Maurel N. Characteristics of successful alien plants. Mol. Ecol. 2015;24:1954–1968. doi: 10.1111/mec.13013. [DOI] [PubMed] [Google Scholar]
- Van Kleunen M., Dawson W., Schlaepfer D., Jeschke J.M., Fischer M. Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecol. Lett. 2010;13:947–958. doi: 10.1111/j.1461-0248.2010.01503.x. [DOI] [PubMed] [Google Scholar]
- Van Kleunen M., Weber E., Fischer M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010;13:235–245. doi: 10.1111/j.1461-0248.2009.01418.x. [DOI] [PubMed] [Google Scholar]
- Weiner S.A., Noble K., Upton C.T., Woods W.A., Starks P.T. A role for thermoregulation in the Polistes dominulus invasion: a comparison of the thermoregulatory abilities of the invasive wasp P. dominulus and the native wasp P. fuscatus. Insect. Soc. 2010;58:185–190. [Google Scholar]
- Weldon C.W., Terblanche J.S., Chown S.L. Time-course for attainment and reversal of acclimation to constant temperature in two Ceratitis species. J. Thermal Biol. 2011;36:479–485. [Google Scholar]
- Yu H., Wan F.H., Guo J.Y. Different thermal tolerance and hsp gene expression in invasive and indigenous sibling species of Bemisia tabaci. Biol. Invas. 2012;14:1587–1595. [Google Scholar]
- Yudha S.S., Falahudin A., Triawan D.A., Adfa M. A SEM study of unexplored wax microtubes produced by caterpillar living on common banana leaves. Micron. 2019;125 doi: 10.1016/j.micron.2019.102729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code are open access via Monash Bridges (Figshare) at: https://doi.org/10.26180/21,670,898.v1