Summary
Background
The delirium-sparing effect of nighttime dexmedetomidine has not been studied after surgery. We hypothesised that a nighttime dose of dexmedetomidine would reduce the incidence of postoperative delirium as compared to placebo.
Methods
This single-centre, parallel-arm, randomised, placebo-controlled superiority trial evaluated whether a short nighttime dose of intravenous dexmedetomidine (1 μg/kg over 40 min) would reduce the incidence of postoperative delirium in patients 60 years of age or older undergoing elective cardiac surgery with cardiopulmonary bypass. Patients were randomised to receive dexmedetomidine or placebo in a 1:1 ratio. The primary outcome was delirium on postoperative day one. Secondary outcomes included delirium within three days of surgery, 30-, 90-, and 180-day abbreviated Montreal Cognitive Assessment scores, Patient Reported Outcome Measures Information System quality of life scores, and all-cause mortality. The study was registered as NCT02856594 on ClinicalTrials.gov on August 5, 2016, before the enrolment of any participants.
Findings
Of 469 patients that underwent randomisation to placebo (n = 235) or dexmedetomidine (n = 234), 75 met a prespecified drop criterion before the study intervention. Thus, 394 participants (188 dexmedetomidine; 206 placebo) were analysed in the modified intention-to-treat cohort (median age 69 [IQR 64, 74] years; 73.1% male [n = 288]; 26·9% female [n = 106]). Postoperative delirium status on day one was missing for 30 (7.6%) patients. Among those in whom it could be assessed, the primary outcome occurred in 5 of 175 patients (2.9%) in the dexmedetomidine group and 16 of 189 patients (8.5%) in the placebo group (OR 0.32, 95% CI: 0.10–0.83; P = 0.029). A non-significant but higher proportion of participants experienced delirium within three days postoperatively in the placebo group (25/177; 14.1%) compared to the dexmedetomidine group (14/160; 8.8%; OR 0.58; 95% CI, 0.28–1.15). No significant differences between groups were observed in secondary outcomes or safety.
Interpretation
Our findings suggested that in elderly cardiac surgery patients with a low baseline risk of postoperative delirium and extubated within 12 h of ICU admission, a short nighttime dose of dexmedetomidine decreased the incidence of delirium on postoperative day one. Although non-statistically significant, our findings also suggested a clinical meaningful difference in the three-day incidence of postoperative delirium.
Funding
National Institute on Aging (R01AG053582).
Keywords: Delirium, Dexmedetomidine, Cardiac surgery, Neurocognition, Sleep
Research in context.
Evidence before this study
Postoperative delirium occurs in 10–30% of elderly patients recovering from cardiac surgery. Cardiac surgery and cardiopulmonary bypass induce inflammatory and metabolic changes that can contribute to postoperative delirium. Dexmedetomidine is a sedative alpha-2 adrenergic agonist associated with sleep neurophysiology, anti-inflammatory, and brain toxin clearance properties that may be delirium sparing. Before starting this trial and again upon completion, we searched the National Library of Medicine for randomised trials, systematic reviews, and meta-analyses, published in English, between January 1, 2000, and November 1, 2022, with the terms “dexmedetomidine and delirium.” We found that studies of dexmedetomidine for delirium prevention have focused on mechanically ventilated and critically ill intensive care unit patients. We identified one study of dexmedetomidine for delirium prevention in non-mechanically ventilated general surgical patients, and one study in cardiac surgical patients. There was no study on the effect of a short-term nighttime administration of intravenous dexmedetomidine on delirium prevention in any patient population.
Added value of this study
We conducted a blinded randomised-controlled trial in patients having surgery with cardiopulmonary bypass. We tested the hypothesis that a short nighttime infusion of dexmedetomidine in non-mechanically ventilated patients reduces the incidence of next-day postoperative delirium.
Implications of all the available evidence
Our results suggested that a short nighttime infusion of dexmedetomidine decreased the incidence of delirium on postoperative day one. Although non-statistically significant, our findings also suggested a clinically meaningful decrease in the 3-day incidence of delirium in patients recovering from cardiac surgery. This finding suggests that prolonged infusions of dexmedetomidine, which are more burdensome to administer, may not be necessary. Thus, non-intravenous formulations may generalise the delirium-sparing benefits of dexmedetomidine to patients in general surgical/medical units and non-hospital care settings.
Introduction
Postoperative delirium, a behavioural state characterised by an acute change in cognition and attention, occurs in 10–30% of patients older than 60 years recovering from cardiac surgery.1, 2, 3, 4, 5, 6, 7, 8 It is a cause of distress to patients, families, and caregivers.9 Further, it is associated with long-term cognitive deficits,10, 11, 12 prolonged hospitalisation and institutionalisation,12,13 and increased mortality,14,15 resulting in total attributable healthcare costs of approximately $32.9 billion annually.16 Multicomponent non-pharmacological postoperative delirium prevention strategies are resource intensive, highlighting the need for easy to implement prophylactic pharmacological strategies.17 At present, definitive guidelines17,18 or large clinical trials do not support the use of any medication for postoperative delirium prevention.
Sleep is an evolutionarily conserved altered arousal state associated with physiology that may confer cardiovascular, immune, and cognitive benefits.19,20 Various types of sleep disturbance have been associated with postoperative delirium, suggesting sleep as a modifiable risk factor.21 Dexmedetomidine is a sedative alpha-2 adrenergic agonist that approximates sleep. Patients who are sedated with dexmedetomidine are arousable and responsive in a manner that is very similar to that seen in people who are sleeping.22 A continuous infusion of dexmedetomidine has been associated with a neurophysiological state that closely approximates non-rapid eye movement (non-REM) stage II sleep with delta (1–4 Hz) and transient time-domain spindle oscillations (13–16 Hz).23,24 Further, a short nighttime dose of intravenous dexmedetomidine has been associated with non-REM stages II and III sleep neurophysiology in non-mechanically ventilated healthy volunteers.25 In addition to promoting sleep neurophysiology, other beneficial properties of dexmedetomidine, such glymphatic clearance26 and immune modulation27 may persist long after drug administration. However, randomised controlled trials of dexmedetomidine for delirium have primarily focused on mechanically ventilated and critically ill intensive care unit (ICU) patients.28, 29, 30
The Minimizing ICU Neurological Dysfunction with Dexmedetomidine induced Sleep (MINDDS) trial was designed to evaluate whether a nighttime dose of dexmedetomidine reduces the incidence of postoperative delirium in non-mechanically ventilated patients older than 60 years after major cardiac surgery. It was hypothesised that a dexmedetomidine intervention beginning on postoperative day zero would reduce the incidence of postoperative day one delirium, and consequently, the overall incidence of postoperative delirium.
Methods
Study design and participants
This single-centre, parallel-arm, randomised, placebo-controlled superiority trial was performed at Massachusetts General Hospital, an urban tertiary care teaching hospital in Boston, MA, with a cardiac surgical volume of approximately 1300 patients annually. Patients were recruited between March 2, 2017 and July 29, 2021 at a single centre in Boston, MA, in the United States. The final follow-up of participants was completed on February 16, 2022. The Mass General Brigham Institutional Review Board approved this study before the implementation of any study procedures (Protocol 2016P000742). Verbal informed consent was obtained from all participants before administering the baseline functional and cognitive assessments, followed by written informed consent before surgery.
The study protocol has been previously published31 and is included in Supplementary Appendix 2, including a history of substantive changes throughout the study (Supplementary Appendix 3). The study was registered as NCT02856594 on ClinicalTrials.gov on August 5, 2016, before the enrolment of any participants. This study is being reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
All patients scheduled for cardiac surgery were screened for participation, and eligible patients were approached during the preoperative surgical visit. Patients were eligible for inclusion if they were 60 years or older, scheduled to undergo a cardiac surgical procedure with planned postoperative admission to the cardiac surgical intensive care unit (CSICU) for 24 h or more, and were scheduled for a same-day surgical admission. Patients were excluded from participation if they were allergic to dexmedetomidine, had renal (requiring dialysis) or liver failure (Child-Pugh score > 5), were on chronic benzodiazepine or antipsychotic therapy, had severe deficit(s) due to structural or anoxic brain damage, were admitted to the ICU for more than 2 days in the month before surgery, previously underwent cardiac surgery within 1 year of surgery, were undergoing a surgical procedure requiring total circulatory arrest, or were SARS-CoV-2 positive or symptomatic (e.g., fever, cough, loss of taste/smell). Participants who were blind, deaf, or unable to communicate in English were excluded due to their inability to complete the cognitive assessments, as were patients experiencing circumstances for which long-term follow-up might be difficult (e.g., homelessness, active psychotic disorder, or substance abuse).
Following enrolment, participants were dropped from the study if they met any of the prespecified dropout criteria before drug administration, including participants who were (i) scheduled to undergo a second surgical procedure during their hospital stay, (ii) intubated for more than 12 h postoperatively, or (iii) became SARS-CoV-2 positive or symptomatic.
Randomisation and masking
Participants were randomised to receive either intravenous dexmedetomidine or placebo in a 1:1 ratio using permuted block randomisation. The randomisation sequence was generated by a trial statistician not otherwise involved in the study using R statistical software, stratifying by planned surgical procedure (isolated cardiac bypass graft surgery vs. another cardiac surgical procedure requiring bypass [with or without CABG]) with random block sizes of two, four, and six. After the research team obtained written informed consent, independent research pharmacists dispensed either dexmedetomidine or placebo centrally according to the computer-generated randomisation list. To achieve blinding among care teams, participants, and research staff assessing outcomes, intravenous dexmedetomidine and placebo (0.9% saline or, if in short supply, 5% dextrose in water) were distributed in bags of equal volume that were identical in appearance.
Interventions
A nighttime dose of placebo or dexmedetomidine (1 μg/kg over 40 min, maximal dose of 80 μg) was administered intravenously following extubation on the night of surgery and subsequently every night throughout the CSICU stay for up 3 days postoperatively. For participants extubated by 8:30 PM, the study medication was administered at a targeted time of 9:00 PM. In the study participants admitted to the CSICU and extubated after 8:30 PM, but before 2:00 AM the following day, the study medication was administered within 30 min of extubation. If the participant was extubated after 2:00 AM, no study medication was administered on the night of surgery. Instead, the study medication was administered the following evening. Regardless of the timing of study drug administration on the night of surgery, participants who remained admitted to the ICU received nightly doses of dexmedetomidine or placebo to target a time of 9:00 PM on subsequent nights. The nightly study drug was discontinued upon transfer out of the CSICU. Clinicians were asked to refrain from administering dexmedetomidine to enrolled participants in the operating room or CSICU.
Participants in both groups received the institutional standards of care for their perioperative surgical and anaesthetic management. All other care decisions were at the discretion of the primary clinical care team.
Outcomes
The primary outcome was delirium occurring on postoperative day one. Delirium was assessed by trained members of the study team using the Confusion Assessment Method (CAM), a standardised tool that evaluates the four features of delirium, namely acute onset and fluctuating course, inattention, disorganised thinking, and an altered level of consciousness.32 Delirium was assessed twice daily (morning and afternoon, with at least 6 h separating assessments) for the first 3 days postoperatively or until hospital discharge, whichever came first. Participants who remained delirious past day three were assessed until postoperative day five or hospital discharge. For those participants who remained delirious after day five, assessments continued until day seven or hospital discharge, whichever came first. Each study day, delirium was defined as present if it occurred at either the morning or afternoon assessment. Delirium severity was assessed as a secondary outcome using the long CAM-Severity (CAM-S) score that can be derived from components of the CAM.33 The CAM-S ranges from 0 (no delirium features) to 19, with higher scores indicating worsening delirium severity.
Postoperative cognitive and health-related quality of life were assessed as secondary outcomes. Postoperative cognitive function was assessed using the Abbreviated Montreal Cognitive Assessment (a-MoCA), a validated screening tool adapted from the MoCA that has demonstrated sensitivity in assessing mild cognitive impairment.34,35 The a-MoCA ranges from 0 (worst) to 22 (best) points and does not require visual cues or writing and can be administered over the phone. Health-related quality of life was assessed in five domains (global physical and mental health, physical function, pain, applied cognition, and sleep quality) using the validated Patient-Reported Outcomes Measurement Information System® Short Forms (PROMIS SF). This included the PROMIS Global Health SF V.1.1, PROMIS Physical Function SF 8b V.1.2, PROMIS Pain Interference SF 8a V.1.0, PROMIS Applied Cognition Abilities SF 8a V.1.0, and PROMIS Sleep Disturbance SF 4A V.1.0, respectively. Scores from each assessment were converted to a T-score for analysis with a mean of 50 and a standard deviation of 10, with lower scores on the PROMIS Pain Interference SF and PROMIS Sleep Disturbance SF considered better, whereas higher scores on all other assessments were considered better. Participants were contacted via telephone by trained study staff blinded to group assignment at baseline and at 30, 90, and 180 days postoperatively to complete these assessments. Up to five attempts were made to contact participants. Additional secondary outcomes included delirium/coma-free days in the ICU and hospital, length of hospital stay, and all-cause mortality in-hospital and at 30, 90, and 180 days postoperatively.
Adverse events
An independent Data Safety Monitoring Board performed an annual review of safety data. However, no formal stopping rules were specified for efficacy, futility, or safety. Expected adverse outcomes following cardiac surgery were collected from the Society of Thoracic Surgeons database after study completion, including readmission, surgical site infection, reoperation, stroke, renal failure, and sternal wound infection. The incidence of heart rate less than 40 beats per minute (bpm), systolic blood pressure less than 90 mmHg, and vasoactive medications given from the start of study drug infusion until 6 h afterward were abstracted from the medical record of enrolled participants.
Power analysis
Assuming a 15% incidence of delirium in the placebo group and a 5% in the dexmedetomidine group, it was determined that 185 participants per group would be required to detect an absolute difference of 10% between groups with an alpha of 0.05 and 80% power. This study, therefore, aimed to enrol 370 patients who received the study intervention on postoperative day zero to ensure the per-protocol primary outcome sensitivity analysis was adequately powered.
Given the ongoing COVID-19 pandemic and changes in the scheduling of elective surgical procedures, an unplanned, blinded interim analysis was performed using the marginal event rate across groups in July 2021. Given the lower than anticipated event rate and concerns that enrolling additional participants would be unlikely to remediate the statistical power given the original effect size assumptions, it was recommended by the study team and approved by the Data Safety Monitoring Board that the trial close to enrolment. At the time, 394 participants comprised the modified intention-to-treat cohort, and 334 participants were included in the per-protocol cohort. Enrolment was thus terminated on July 29, 2021, and active follow-up continued only for previously enrolled participants.
Statistical analysis
Descriptive statistics of the data are presented as mean (standard deviation, SD), median (interquartile range [IQR]), or frequency (proportion) depending on variable type and distribution. The normality of continuous variables was confirmed with a visual inspection of the data. The primary analysis was performed on a modified intention-to-treat cohort, which included randomised participants that did not meet a prespecified dropout criterion. A sensitivity analysis was conducted in the per-protocol cohort, which included randomised participants who received the study drug on the night after surgery. All models were adjusted for the randomisation strata. In the primary analysis, differences in the incidence of delirium on postoperative day one between the dexmedetomidine and placebo groups were assessed using logistic regression, conditional on the randomisation strata (planned isolated CABG or not). In a sensitivity analysis, the primary model was re-specified, adjusting for additional prognostic variables that have previously been associated with the incidence of delirium, namely age, preoperative a-MoCA score, cardiopulmonary bypass time, and sex.10,36 A second sensitivity analysis was undertaken using multiple imputation using chained equations which was derived from the prognostic variables and the chart review for delirium keywords. This model was conducted using multiple imputations by chained equations, with 100 iterations, using a predictive mean matching algorithm. The pooled estimates were combined using Rubin's rules. Chart review for delirium keywords used a natural language processing algorithm to identify probable cases of postoperative delirium.37 Probable cases were assessed (using delirium, agitated/agitation, disorientation/disoriented as keywords) independently by two clinicians who were unaware of group assignment, and discordant assessments were adjudicated by a third independent clinician. Results of these models are presented as adjusted odds ratios (OR) with 95% confidence intervals (CI).
A similar analysis was performed using logistic regression to evaluate whether the incidence of binary secondary outcomes such as mortality varied by randomisation group. Ordered logistic regression was employed to assess delirium free days. Continuous secondary outcomes, including delirium severity and length of hospital and ICU stay, were assessed using a generalised linear model with linear or logit link function. Effect estimates are presented as the mean difference (MD) between randomisation groups with an associated 95% CI.
The MINDDS statistical analyses plan was finalised prior to accessing the data and maintained the pre-specified approach to data analyses for consistency and transparency in reporting. Based on recommendations received during the review process, Fisher's exact test for the primary outcome was reported, in which the exact one-tailed probability was doubled for reporting. In addition, the following post hoc analyses were performed to assess the impact of missing data on the primary outcome: (1) coded missing data on postoperative day one as positive for delirium if delirium was present on postoperative day two, (2) coded missing data on postoperative day one as positive for delirium if delirium was present on postoperative day two, and negative for delirium if delirium was absent on day two, (3) coded missing data on postoperative day one as positive for delirium if delirium was present on postoperative day two or if data were missing on day two, and (4) coded missing data on postoperative day one as positive for delirium if delirium was present on postoperative day two or if data were missing on day two, and negative for delirium if delirium was absent on day two. An additional post hoc analysis was performed utilising exact logistic regression for outcomes with low event rates. Finally, a time to event analysis was performed for postoperative delirium through day three, with results reported in a Kaplan Meier curve and assessed for differences between randomisation strata using a Log–Rank test.
For all analyses, two-sided p-values <0.05 were considered statistically significant. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC), R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria), and RStudio (RStudio PBC, Boston, MA). A copy of the complete statistical analysis plan, including details of the sensitivity models, is outlined in Supplementary Appendix 4.
Role of the funding source
This study was funded by grant R01AG053582 from the National Institutes of Health National Institute on Aging (NIH NIA). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Study authors (O.A., A.M. and T.T.H.) had full access to verify the study data and take responsibility for the integrity of the data and the accuracy of the analyses. All authors accept responsibility for the decision to submit for publication.
Results
Patients
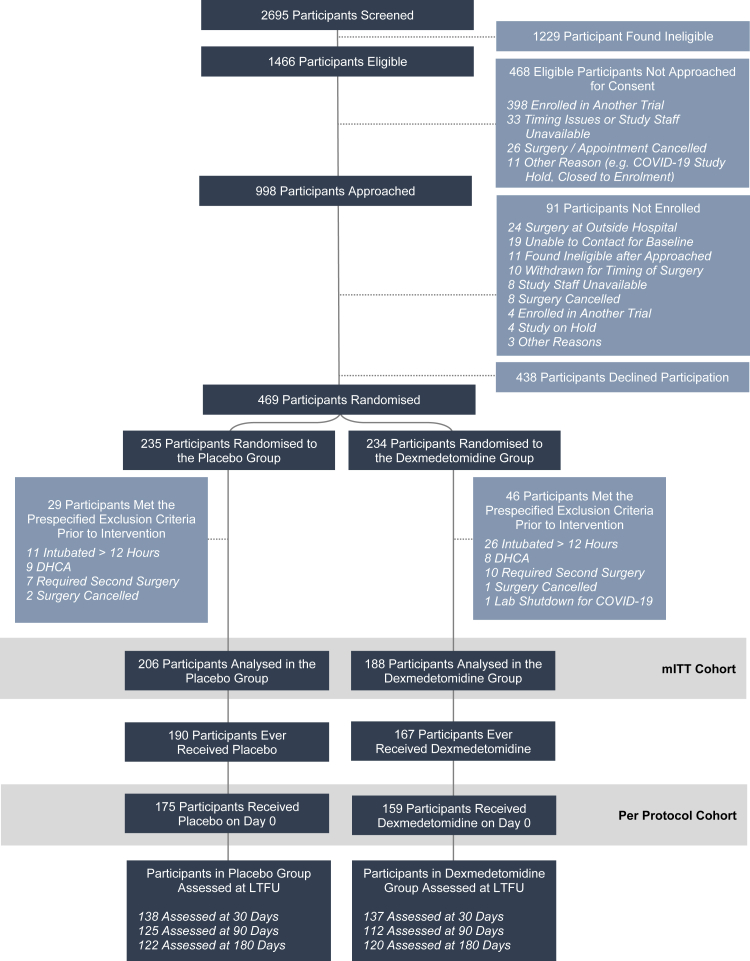
A total of 2695 patients were screened, 1229 of whom met at least one exclusion criterion (Fig. 1). Of the remaining 1466 eligible patients, 469 patients underwent randomisation to placebo (n = 235) or dexmedetomidine (n = 234). A total of 75 patients met at least one prespecified drop criterion before the study intervention. Ultimately 394 patients were included in the modified intention-to-treat cohort for analysis, including 206 randomised to placebo and 188 to dexmedetomidine.
Fig. 1.
Eligibility, randomisation, and follow-up. Participant flow in the study is described at each step, including information about the number of participants analysed in each group. Of the 1453 eligible patients, a total of 469 were randomised, and ultimately 394 were analysed in the modified intention to treat cohort. This included 188 participants in the dexmedetomidine group and 206 in the placebo group. Abbreviations: DHCA (deep hypothermic circulatory arrest), LTFU (long term follow up), mITT (modified intention-to-treat).
The demographic and in-hospital characteristics of the participants are shown in Table 1. Overall, participants were 69 [IQR 64, 74] years old and predominantly male. The median a-MoCA scores were 19.0 [IQR 18.0, 20.0] in the placebo group and 19.0 [17.0, 20.0] in the dexmedetomidine group at enrolment. A total of 59 (31.4%) participants in the dexmedetomidine group and 47 (22.8%) patients in the placebo group met the a-MOCA criterion for cognitive impairment (a-MOCA ≤ 17) at baseline.
Table 1.
Participant characteristics at baseline in the intention-to-treat cohort.
| Placebo N = 206 |
Dexmedetomidine N = 188 |
|
|---|---|---|
| Demographics | ||
| Age, years | 70.0 [65.0, 75.0] | 67.5 [63.0, 73.0] |
| Sex | ||
| Male | 144 (69.9) | 144 (76.6) |
| Female | 62 (30.1) | 44 (23.4) |
| Height, centimetres | 175.3 [166.4, 180.3] | 175.3 [167.6, 180.3] |
| Weight, kilograms | 85.3 [72.4, 95.3] | 85.3 [76.2, 97.2] |
| Body mass index, kg/m2 | 27.66 [24.78, 30.96] | 28.11 [25.03, 31.97] |
| Self-reported race | ||
| White | 202 (98.1) | 182 (96.8) |
| Asian | 2 (1.0) | 5 (2.7) |
| American Indian or Alaskan Native | 0 (0.0) | 1 (0.5) |
| Other | 1 (0.5) | 0 (0.0) |
| Unknown | 1 (0.5) | 0 (0.0) |
| Ethnicity | ||
| Hispanic or Latino | 2 (1.0) | 1 (0.5) |
| Not Hispanic of Latino | 194 (94.2) | 179 (95.2) |
| Not documented | 10 (4.9) | 8 (4.3) |
| Marital status at enrolment | ||
| Married | 159 (77.2) | 135 (71.8) |
| Divorced | 16 (7.8) | 20 (10.6) |
| Single | 13 (6.3) | 14 (7.4) |
| Widowed | 17 (8.3) | 16 (8.5) |
| Other | 0 (0.0) | 3 (1.6) |
| Unknown | 1 (0.5) | 0 (0.0) |
| Highest level of education | ||
| 8th grade but less than high school graduate | 0 (0.0) | 4 (2.1) |
| High school graduate, GED | 30 (14.6) | 29 (15.4) |
| Some college, associate's degree | 43 (20.9) | 41 (21.8) |
| Bachelor's degree | 61 (29.6) | 57 (30.3) |
| Master's degree | 42 (20.4) | 26 (13.8) |
| Doctoral degree | 30 (14.6) | 30 (16.0) |
| Unknown | 0 (0.0) | 1 (0.5) |
| Comorbidities and past medical history | ||
| Diabetes | 40 (19.4) | 44 (23.4) |
| Hypertension | 156 (75.7) | 151 (80.3) |
| Heart failurea | 54/200 (27.0) | 63/183 (34.4) |
| Prior myocardial infarction | 18 (8.7) | 23 (12.2) |
| Previous cardiac intervention | 65 (31.6) | 63 (33.5) |
| Peripheral arterial disease | 16 (7.8) | 17 (9.0) |
| Cerebrovascular disease | 25 (12.1) | 20 (10.6) |
| Liver disease | 7 (3.4) | 8 (4.3) |
| Syncope | 9 (4.4) | 4 (2.1) |
| Sleep apnoea | 46 (22.3) | 40 (21.3) |
| Chronic lung disease | 31 (15.0) | 25 (13.3) |
| Baseline neurocognitive and PROMIS scores | ||
| Delirium at baseline | 0 (0.0) | 0 (0.0) |
| Abbreviated MoCA | 19.0 [18.0, 20.0] | 19.0 [17.0, 20.0] |
| PROMIS scoresb | ||
| Global health—physical | 50.8 [44.9, 57.7] | 50.8 [42.3, 57.7] |
| Global health—mental | 56.0 [48.3, 62.5] | 56.0 [50.8, 62.5] |
| Physical function | 46.4 [40.1, 52.5] | 44.6 [38.8, 52.5] |
| Pain interference | 40.7 [40.7, 51.2] | 40.7 [40.7, 55.4] |
| Applied cognition | 51.2 [45.9, 62.7] | 52.4 [45.9, 62.7] |
| Sleep disturbancec | 50.5 [43.8, 54.3] | 50.5 [43.8, 56.1] |
Data is presented as mean (standard deviation), median [quartile 1, quartile 3], or n (%) depending on variable type and distribution.
Abbreviations: GED (General Educational Development), MoCA (Montreal Cognitive Assessment), PROMIS (Patient-Reported Outcomes Measurement Information System).
History of heart failure was missing for 11 participants, including 5 in the dexmedetomidine group and six participants in the placebo group.
All PROMIS scores are translated to T-scores for reporting.
Baseline PROMIS sleep disturbance scores were introduced after enrolment began, therefore sleep disturbance scores are missing in the first 14 enrolled participants (7 in each group). No other data was missing unless specified.
Study drug administration
Surgical characteristics and study drug administration details are shown in Table 2. The study drug was given to 334 participants on the night of postoperative day zero, including 175 (85.0%) in the placebo group and 159 (84.6%) in the dexmedetomidine group. Seven patients who were supposed to receive the study drug on day zero did not, including 1.6% of (3/188) participants in the dexmedetomidine group and 1.9% (4/206) of participants in the placebo group. The remaining patients did not receive the study medication on day zero in accordance with the study protocol because they were extubated after 2 AM or discharged from the ICU. Participants in both groups received the study drug a median of 1.0 day [IQR 1.0, 1.0], consistent with protocol given their length of stay in the ICU (median 25.4 [23.0, 42.0] hours vs. 26.0 [23.0, 47.0] hours in the placebo and dexmedetomidine groups, respectively). Of those randomised to receive the study medication, 92.2% of participants in the placebo group (190/206) and 88.8% of participants in the dexmedetomidine group (167/188) ultimately received at least one dose of the study drug.
Table 2.
Surgical characteristics and study drug administration in the intention-to-treat cohort.
| Placebo N = 206 |
Dexmedetomidine N = 188 |
|
|---|---|---|
| Surgical characteristics | ||
| Cardiopulmonary bypass time, minutes | 129.0 [96.0, 163.0] | 125.0 [92.0, 162.0] |
| Cross clamp timea, minutes | 92.0 [71.0, 116.0] | 89.0 [70.0, 117.0] |
| Strata at randomisation: Isolated CABG surgery | 39 (18.9) | 39 (20.7) |
| Procedure type performed | ||
| Isolated CABG | 38 (18.4) | 37 (19.7) |
| AV replacement + CABG | 25 (12.1) | 18 (9.6) |
| AV replacement + MV replacement | 4 (1.9) | 3 (1.6) |
| AV replacement | 48 (23.3) | 40 (21.3) |
| MV repair | 34 (16.5) | 41 (21.8) |
| MV repair + CABG | 5 (2.4) | 6 (3.2) |
| MV replacement + CABG | 1 (0.5) | 3 (1.6) |
| MV replacement | 5 (2.4) | 3 (1.6) |
| Other | 46 (22.3) | 37 (19.7) |
| Afternoon surgery | 48 (23.3) | 33 (17.6) |
| Study drug administration | ||
| Received the study drug (Ever) | 190 (92.2) | 167 (88.8) |
| Received the study drug on POD 0 | 175 (85.0) | 159 (84.6) |
| Number of days receiving the study drug | ||
| 0 | 16 (7.8) | 21 (11.2) |
| 1 | 153 (74.3) | 136 (72.3) |
| 2 | 25 (12.1) | 21 (11.2) |
| 3 | 11 (5.3) | 10 (5.3) |
| 4 | 1 (0.5) | 0 (0.0) |
Data is presented as mean (standard deviation), median [quartile 1, quartile 3], or n (%) depending on variable type and distribution.
Abbreviations: AV (aortic valve), CABG (coronary artery bypass graft), MV (mitral valve), POD (postoperative day).
Cross clamp time was missing for one participant in the dexmedetomidine group who did not have their aorta clamped.
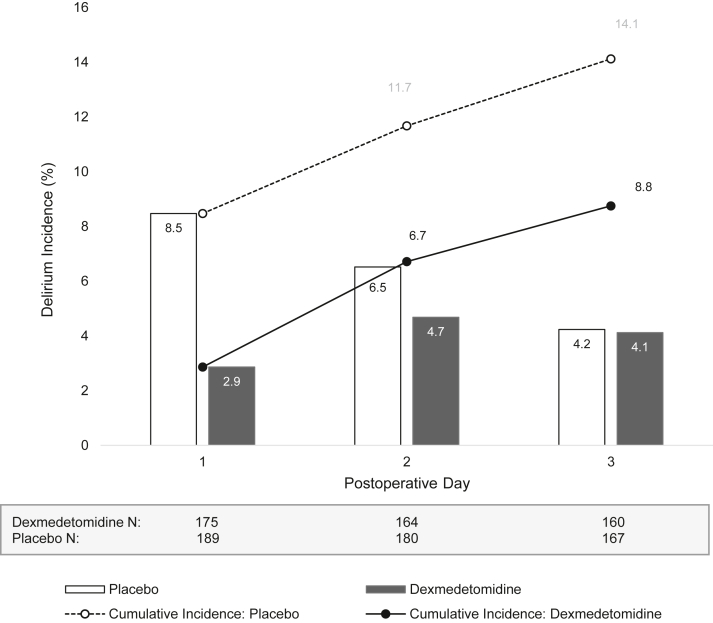
Postoperative delirium
Overall, 16 (8.5%) participants in the placebo group and 5 (2.9%) participants in the dexmedetomidine group experienced delirium on postoperative day one (Table 3; Fig. 2). After controlling for randomisation strata, dexmedetomidine reduced the incidence of delirium on postoperative day one as compared to placebo (OR 0.32; 95% CI, 0.10–0.83; P = 0.029). Results of sensitivity analyses that included prognostic adjustment (OR 0.31; 95% CI, 0.09–0.88) or imputation for missing data based on chart review and prognostic variables (OR 0.33; 95% CI, 0.12–0.91) were consistent with the primary model (Supplementary Appendix 5). Results from additional analyses requested during the review process were also consistent with the primary model (Supplementary Appendices 6 and 7). Similar interpretations were observed in the per-protocol cohort.
Table 3.
Outcomes and clinical characteristics of the intention-to-treat cohort conditional on the randomisation strata.
| Placebo N = 206 |
Dexmedetomidine N = 188 |
Effect estimateg (95% CI) | P value | |
|---|---|---|---|---|
| Delirium outcomes | ||||
| Postoperative day 1 | ||||
| Delirium (primary outcome)a | 16/189 (8.5) | 5/175 (2.9) | 0.32 (0.10, 0.83) | 0.029 |
| Delirium severityb | 3.0 [2.0, 4.0] | 3.0 [2.0, 4.0] | 0.95 (0.87, 1.04) | 0.24 |
| Postoperative days 1–3c | ||||
| Delirium | 25/177 (14.1) | 14/160 (8.8) | 0.58 (0.28, 1.15) | 0.12 |
| Delirium severityb | 3.0 [2.0, 5.0] | 3.0 [2.0, 4.0] | 0.96 (0.89, 1.03) | 0.24 |
| Delirium free days to day 3c | 1.74 (0.89, 3.55) | 0.11 | ||
| 0 | 2/177 (1.1) | 0/160 (0.0) | ||
| 1 | 7/177 (4.0) | 6/160 (3.8) | ||
| 2 | 16/177 (19.0) | 8/160 (5.0) | ||
| 3 | 152/177 (85.9) | 146/160 (91.3) | ||
| Hospital clinical characteristics | ||||
| Length of hospital stay, daysb | 6.0 [5.0, 7.0] | 6.0 [5.0, 8.0] | 1.02 (0.95, 1.09) | 0.67 |
| Length of ICU stay, hoursb | 25.4 [23.0, 42.0] | 26.0 [23.0, 47.0] | 1.06 (0.94, 1.19) | 0.34 |
| Readmitted to the ICU | 5 (2.4) | 6 (3.2) | 1.33 (0.39, 4.68) | 0.64 |
| Total postoperative ventilation time, hoursb | 5.02 [3.98, 7.85] | 5.02 [3.97, 6.75] | 0.95 (0.85, 1.06) | 0.34 |
| Discharged to homed | 167/205 (81.5) | 161/185 (87.0) | 1.52 (0.88, 2.68) | 0.14 |
| Clinical characteristics within 30 days postoperatively | ||||
| Readmittede | 19/205 (9.3) | 14/183 (7.7) | 0.81 (0.39, 1.66) | 0.56 |
| Surgical site infection | 3 (1.5) | 2 (1.1) | 0.69 (0.09, 4.26) | 0.69 |
| Reoperation for bleeding | 2 (1.0) | 1 (0.5) | 0.54 (0.02, 5.65) | 0.61 |
| Stroke | 1 (0.5) | 0 (0.0) | NA | 1.00 |
| Renal failure | 1 (0.5) | 3 (1.6) | 3.31 (0.42, 67.19) | 0.30 |
| Atrial fibrillation | 83 (40.3) | 65 (34.6) | 0.79 (0.52, 1.18) | 0.25 |
| Mortality | ||||
| In hospital mortality | 1 (0.5) | 3 (1.6) | 3.31 (0.42, 67.19) | 0.30 |
| 30 days | 1 (0.5) | 3 (1.6) | 3.31 (0.42, 67.19) | 0.30 |
| 90 days | 1 (0.5) | 4 (2.1) | 4.46 (0.65, 87.67) | 0.19 |
| 180 days | 4 (1.9) | 4 (2.1) | 1.11 (0.26, 4.75) | 0.89 |
| Neurocognitive and PROMIS scores at follow up | ||||
| Number assessed at follow up | ||||
| 30 days | 138 | 137 | – | – |
| 90 days | 125 | 112 | – | – |
| 180 days | 122 | 120 | – | – |
| Abbreviated MoCAb | ||||
| 30 days | 20.0 [19.0, 21.0] | 20.0 [18.0, 21.0] | 0.97 (0.95, 1.00) | 0.063 |
| 90 days | 20.0 [19.0, 21.0] | 20.0 [19.0, 21.0] | 1.00 (0.97, 1.03) | 0.97 |
| 180 days | 21.0 [19.0, 22.0] | 20.0 [19.0, 21.0] | 0.98 (0.94, 1.01) | 0.13 |
| PROMIS global health – physicalf | ||||
| 30 days | 47.7 [42.3, 54.1] | 50.8 [44.9, 54.1] | 1.1 (−1.0, 3.1) | 0.30 |
| 90 days | 54.1 [47.7, 57.7] | 54.1 [47.7, 59.8] | 0.1 (−2.2, 2.4) | 0.91 |
| 180 days | 54.1 [50.8, 61.9] | 57.7 [50.8, 61.9] | −0.8 (−3.0, 1.4) | 0.48 |
| PROMIS global health—mentalf | ||||
| 30 days | 56.0 [50.8, 62.5] | 59.0 [50.8, 67.6] | 1.3 (−0.8, 3.4) | 0.22 |
| 90 days | 62.5 [56.0, 67.6] | 59.0 [53.3, 67.6] | −0.0 (−2.2, 2.1) | 0.97 |
| 180 days | 62.5 [53.3, 67.6] | 62.5 [53.3, 67.6] | −0.5 (−2.7, 1.7) | 0.64 |
| PROMIS physical functionf | ||||
| 30 days | 39.1 [35.5, 44.6] | 40.8 [36.8, 46.4] | 1.2 (−0.6, 3.1) | 0.19 |
| 90 days | 48.2 [42.2, 52.5] | 48.8 [43.4, 56.1] | 0.7 (−1.5, 2.9) | 0.53 |
| 180 days | 50.4 [46.4, 59.7] | 50.4 [45.5, 59.7] | −0.9 (−2.9, 1.2) | 0.42 |
| PROMIS pain interference f | ||||
| 30 days | 52.3 [40.7, 58.1] | 49.9 [40.7, 55.8] | −1.6 (−3.7, 0.4) | 0.12 |
| 90 days | 40.7 [40.7, 49.9] | 40.7 [40.7, 51.2] | 0.9 (−1.1, 2.9) | 0.36 |
| 180 days | 40.7 [40.7, 40.7] | 40.7 [40.7, 47.9] | −0.3 (−2.0, 1.3) | 0.69 |
| PROMIS applied cognitionf | ||||
| 30 days | 53.0 [47.7, 62.7] | 54.6 [48.6, 62.7] | 1·2 (−0.8, 3.1) | 0.24 |
| 90 days | 54.6 [47.7, 62.7] | 53.0 [49.5, 62.7] | 0.3 (−1.9, 2.5) | 0.78 |
| 180 days | 54.6 [48.6, 62.7] | 54.6 [47.7, 62.7] | −0.3 (−2.3, 1.7) | 0.76 |
| PROMIS sleep disturbancef | ||||
| 30 days | 50.5 [43.8, 56.1] | 50.5 [43.8, 56.1] | −1.0 (−3.3, 1.2) | 0.37 |
| 90 days | 48.4 [41.1, 52.4] | 48.4 [43.8, 52.4] | 0.4 (−1.7, 2.6) | 0.71 |
| 180 days | 46.2 [41.1, 50.5] | 46.2 [41.1, 51.5] | −0.2 (−2.5, 2.0) | 0.84 |
Data is presented as mean (standard deviation), median [quartile 1, quartile 3], or n (%) depending on variable type and distribution.
Abbreviations: ICU (intensive care unit), MoCA (Montreal Cognitive Assessment), PROMIS (Patient-Reported Outcomes Measurement Information System).
A total of 30 participants (13 in dexmedetomidine group and 17 in the placebo group) were missing the primary outcome, incidence of delirium on postoperative day 1.
Values were log transformed for analysis, with resulting effect estimates presented as a ratio of geometric means. Effect estimates can be interpreted as the percent increase (or decrease) in for every one-unit increase (e.g. 1.02 corresponds to a 2% increase in the dexmedetomidine group).
A total of 57 participants (28 in dexmedetomidine group and 29 in the placebo group) had missing delirium assessments through postoperative day three. One additional participant was assessed as delirious through day three, but the delirium severity score was missing.
Discharge status was missing for 4 participants (3 in dexmedetomidine group and 1 in the placebo group).
Readmission status was missing for 6 participants (5 in dexmedetomidine group and 1 in the placebo group). No other data was missing unless specified.
All PROMIS scores are translated to T-scores for reporting.
Effect estimates are reported as odds ratios (binary outcomes) or mean difference (continuous outcomes) comparing the dexmedetomidine group to the placebo group (reference), conditional on the randomisation strata.
Fig. 2.
Delirium incidence. The incidence of delirium is reported for each postoperative day, stratified by the dexmedetomidine or placebo group status. In addition, the cumulative incidence of delirium occurring at any time up until (or including) that postoperative day is reported with a line for each randomisation group.
A higher proportion of participants experienced delirium within the first 3 days postoperatively in the placebo group (25/177; 14.1%) compared to the dexmedetomidine group (14/160; 8.8%). However, this result was not statistically significant (OR 0.58; 95% CI, 0.28–1.15). No statistically significant difference was observed in delirium severity on postoperative day one or in the first 3 days postoperatively between study groups, nor were there differences in the delirium-free days to day three.
Secondary outcomes
The median hospital length of stay was 6·0 days in both groups, with no statistically significant difference between the dexmedetomidine and placebo (Table 3). Data on length of ICU stay, readmission to the ICU, total postoperative ventilation time, and discharge to home were not statistically different between groups.
Cognitive function (a-MOCA) and quality of life (PROMIS applied cognition, global health, pain interference, physical function, and sleep disturbance scores) were assessed in 69.8% (n = 275), 60.2% (n = 237), and 61.4% (n = 242) of participants at 30, 90, and 180–days after surgery, respectively. At 180–days, 14.2% (17/120) of participants in the dexmedetomidine group and 10.7% (13/122) of participants contacted in the placebo group met the a-MOCA criterion for cognitive impairment (≤17 is positive for cognitive impairment). Overall median scores for these assessments improved over time for all measures. However, no difference was observed between randomisation groups for these secondary outcomes.
All patients received isoflurane to maintain general anesthesia. Hypnotics, analgesics, and other relevant medications administered intraoperatively or in the immediate postoperative period were similar (Supplementary Appendix 8). Patient demographics, surgical characteristics, and outcomes for the per protocol cohort were similar to the modified intention-to-treat cohort (Supplementary Appendices 9–11). A Kaplan Meir curve was constructed to show the clinically relevant decrease in the cumulative incidence of postoperative delirium through postoperative day three, though this was not statistically significant (Supplementary Appendix 12).
Safety endpoints
Clinical characteristics within 30 days, including readmission to the hospital, surgical site infection, reoperation for bleeding, stroke, renal failure, and atrial fibrillation, were not statistically different between groups (Table 3). Two patients in the dexmedetomidine group experienced an isolated bradycardic event (heart rate < 40 bpm), one on postoperative day zero (heart rate 34 bpm) and another on postoperative day one (heart rate 35 bpm). The incidence of hypotension, defined as any systolic blood pressure less than 90 mmHg, was not significantly different (10.6% [20/188] vs. 6.3% [13/206] on the day of surgery; 13.3% [25/188] vs. 18.0% [37/206] on day one), nor were the actual systolic blood pressures values during these events (median 84 [81, 87] vs. 85 [82, 87] mmHg) between the dexmedetomidine and placebo groups, respectively. The cumulative norepinephrine equivalent from the start of study drug infusion until 6 h afterward was higher in the dexmedetomidine group compared to the placebo group (median 0.075 [IQR 0.000, 0.246] vs. 0.000 [0.000, 0.123] mcg/kg/min; p < 0.0001).
Discussion
In this randomised controlled clinical trial that compared a nighttime dose of dexmedetomidine to placebo in extubated patients recovering from cardiac surgery, dexmedetomidine reduced the incidence of delirium on postoperative day one. A clinically meaningful, albeit not statistically significant, difference persisted through postoperative day three. Other in-hospital secondary outcomes, such as ICU and hospital delirium/coma-free days, the severity of delirium, length of hospital stay, major inpatient morbidity, and inpatient mortality, did not differ between groups. Furthermore, no differences in secondary outcomes were observed for variables including a-MOCA, PROMIS (applied cognition, physical function, global health physical, global health mental, pain interference, sleep disturbance) scores, or all-cause mortality after hospital discharge up to 180 days postoperatively. Safety endpoints were similar between groups.
Although the incidence of postoperative delirium after cardiac surgery has reduced markedly over the past decade, the 14% incidence of postoperative delirium within 3 days after cardiac surgery in the MINDDS cohort is consistent with recent studies.2,4,8 Despite this lower incidence of delirium, the risk reduction of our intervention was prominent. Thus, the data presented support nighttime dexmedetomidine as a prophylactic treatment to reduce the public health burden of postoperative delirium and as a possible strategy to improve the ICU patient experience. Compared to a prolonged drug infusion, nighttime dexmedetomidine is likely to improve drug administration utilisation and compliance, given that it is less burdensome to administer and is unlikely to prolong ICU stay. Further, non-parenteral formulations of dexmedetomidine currently being developed for various indications38, 39, 40 may extend the delirium-sparing benefits of nighttime dexmedetomidine to patients in less-monitored care settings.
Two previous randomised controlled trials have studied the effect of prolonged dexmedetomidine infusions on postoperative delirium in a mixed population of mechanically and non-mechanically ventilated post-surgical patients.2,41 The trials differed with respect to the dose and duration of dexmedetomidine, delirium outcome measure, and findings. Indeed, the cumulative drug dose and timing regimen alongside different outcome choices has hampered the ability for consensus evidence in this area. In a study of non-cardiac surgical patients, a low dose continuous infusion of dexmedetomidine (0.1 μg/kg/h from ICU admission until 8 AM on postoperative day one, mean duration of approximately 15 h) reduced the incidence of delirium in the first 7 days after surgery.41 This dosing strategy was associated with a reduced length of ICU stay.42 Conversely, in a trial conducted in cardiac surgical patients, a continuous sedative infusion of dexmedetomidine (started at the surgical incision and increased to 0.4 μg/kg/h postoperatively, mean duration of approximately 24 h) did not decrease the incidence of postoperative delirium in the first 5 days after surgery.2 Rather, a clinically significant increase in the incidence of delirium and a longer length of ICU stay were reported in the dexmedetomidine group.
This MINDDS trial builds on these two trials2,41 with some methodological differences. First, the MINDDS trial study cohort was comprised entirely of non-mechanically ventilated patients. Thus, postoperative delirium was assessed in all patients using the long version of the CAM, increasing the rigor of the delirium diagnosis. This approach, coupled with nighttime dexmedetomidine, was structured to improve the reliability of delirium outcome assessments and to reduce confounds inherent to sedative drug-induced altered arousal. Also, the delirium prevention benefit of a single nighttime dose of dexmedetomidine may not extend to mechanically ventilated patients, especially those with sepsis or sedated with medications to maintain unconsciousness. Second, given various endotypes of postoperative delirium may exist, objective drop criteria were implemented to enable inferences on treatment effects that may otherwise be undecipherable in a heterogeneous population. Finally, a multi-day delirium outcome measure may obscure temporal relationships between treatment and response. Therefore, postoperative day one delirium was prespecified as the primary outcome, given that the intervention was expected to be administered in the ICU on postoperative day zero.
This trial has some notable limitations. The study consisted of predominantly white non-Hispanic male patients enrolled from a single geographic region who may have been at low risk for postoperative delirium. However, the trial demographics are consistent with recent studies of postoperative delirium after cardiac surgery.2,6,43 In addition, the trial was initially powered (n = 370) to enable inferences in the per-protocol sensitivity analyses and stopped early because of COVID-19-related enrolment challenges. Thus, the per protocol sensitivity analyses may be underpowered. This trial is also limited by restricting delirium assessments to the first 3 days postoperatively, with the primary outcome only evaluating the first postoperative day, thereby potentially missing delirium occurring later in their hospital stay. Of relevance, the primary outcome also occurred in a lower percentage of patients than anticipated when the trial was planned, was not differentiated by delirium subtype (hypoactive versus hyperactive), and most patients were discharged from the ICU within 1 day. However, given the observed effect size and reduction in delirium in the dexmedetomidine group, this trial was powered to make inferences on the prespecified modified intention-to-treat cohort. Although we hypothesised a sleep associated mechanism for the dexmedetomidine effect, we did not assess for in-hospital sleep quality using validated questionnaires. However, subjective and objective sleep assessments, which are often discordant,44 may not inform on biological processes associated with sleep such as immune modulation. While unanticipated, more participants in the dexmedetomidine group met a dropout criterion for the study after randomisation (e.g., prolonged intubation) but prior to any study intervention or assessment. However, given that strict blinding was adhered to, this was likely a chance finding. Finally, 7.6% of delirium assessments were missing on postoperative day one. However, the results of prespecified and additional sensitivity analyses requested during the review process that accounted for missing data were consistent with the primary analysis.
In patients older than 60 years with low baseline risk of postoperative delirium admitted to the ICU after cardiac surgery and extubated within 12 h of ICU admission, a post-extubation nighttime dose of dexmedetomidine may reduce the incidence of delirium on postoperative day one.
Contributors
J.Z.Q., M.B.W., K.T.S., S.S., D.A.D., L.B., E.N.B., T.T.H., and O.A. were responsible for study conception and design. J.Z.Q., A.M., T.B.M., M.B.W., K.T.S., S.S., D.A.D., L.B., E.N.B., T.T.H., and O.A. contributed to the acquisition, analysis and interpretation of the data. O.A., A.M. and T.T.H. had full access to verify the study data and take responsibility for the integrity of the data and the accuracy of the analyses. Authors J.Z.Q., A.M., and O.A. drafted the manuscript and J.Z.Q., A.M., T.B.M., M.B.W., K.T.S., S.S., D.A.D., L.B., E.N.B., T.T.H., and O.A. revised the article for important intellectual content. All authors (J.Z.Q., A.M., T.B.M., M.B.W., K.T.S., S.S., D.A.D., L.B., E.N.B., T.T.H., O.A.) confirm they access to the study data, approve of the final version of the manuscript for publication, and accept responsibility for the decision to submit for publication.
Data sharing statement
De-identified data and a data dictionary will be made available upon reasonable request to researchers who provide a methodologically sound proposal and whose use of the data has been approved the study authors. Data will be made available immediately following publication, with no anticipated end date, to achieve the aims specified in the approved proposal. Proposals should be directed to oluwaseun.akeju@mgh.harvard.edu.
Declaration of interests
A.M. reports receiving funding from Roche Diagnostics and the University of Chicago for statistical consulting projects related to biomarkers in preeclampsia. T.T.H. reports receiving personal fees from Anesthesiology and Headache. L.B. receives salary support from K23 HL128882/NHLBI NIH. L.B. receives technologies and devices from iNO Therapeutics LLC, Praxair Inc., Masimo Corp., and grants from “Fast Grants for COVID-19 Research” at Mercatus Center of George Mason University and from iNO Therapeutics LLC. E.N.B. is a cofounder of PASCALL, a company developing closed-loop physiological control systems, and a cofounder of Neuradia, a company promoting the recovery of consciousness. E.N.B. and O.A. are listed as inventors on brain monitoring patents assigned to Massachusetts General Hospital. No other disclosures were reported.
Acknowledgments
This study was supported by the National Institutes of Health National Institute on Aging (NIA) R01AG053582.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2022.101796.
Appendix A. Supplementary data
References
- 1.Greaves D., Psaltis P.J., Ross T.J., et al. Cognitive outcomes following coronary artery bypass grafting: a systematic review and meta-analysis of 91,829 patients. Int J Cardiol. 2019;289:43–49. doi: 10.1016/j.ijcard.2019.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turan A., Duncan A., Leung S., et al. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomised placebo-controlled trial. Lancet. 2020;396(10245):177–185. doi: 10.1016/S0140-6736(20)30631-0. [DOI] [PubMed] [Google Scholar]
- 3.Eertmans W., De Deyne C., Genbrugge C., et al. Association between postoperative delirium and postoperative cerebral oxygen desaturation in older patients after cardiac surgery. Br J Anaesth. 2020;124(2):146–153. doi: 10.1016/j.bja.2019.09.042. [DOI] [PubMed] [Google Scholar]
- 4.Sauer A.C., Veldhuijzen D.S., Ottens T.H., Slooter A.J.C., Kalkman C.J., van Dijk D. Association between delirium and cognitive change after cardiac surgery. Br J Anaesth. 2017;119(2):308–315. doi: 10.1093/bja/aex053. [DOI] [PubMed] [Google Scholar]
- 5.Lopez M.G., Hughes C.G., DeMatteo A., et al. Intraoperative oxidative damage and delirium after cardiac surgery. Anesthesiology. 2020;132(3):551–561. doi: 10.1097/ALN.0000000000003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramaniam B., Shankar P., Shaefi S., et al. Effect of intravenous acetaminophen vs. placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery: the DEXACET randomized clinical trial. JAMA. 2019;321(7):686–696. doi: 10.1001/jama.2019.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djaiani G., Silverton N., Fedorko L., et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology. 2016;124(2):362–368. doi: 10.1097/ALN.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 8.Royse C.F., Saager L., Whitlock R., et al. Impact of methylprednisolone on postoperative quality of recovery and delirium in the steroids in cardiac surgery trial: a randomized, double-blind, placebo-controlled substudy. Anesthesiology. 2017;126(2):223–233. doi: 10.1097/ALN.0000000000001433. [DOI] [PubMed] [Google Scholar]
- 9.Williams S.T., Dhesi J.K., Partridge J.S.L. Distress in delirium: causes, assessment and management. Eur Geriatr Med. 2020;11(1):63–70. doi: 10.1007/s41999-019-00276-z. [DOI] [PubMed] [Google Scholar]
- 10.Saczynski J.S., Marcantonio E.R., Quach L., et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bickel H., Gradinger R., Kochs E., Forstl H. High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dement Geriatr Cogn Disord. 2008;26(1):26–31. doi: 10.1159/000140804. [DOI] [PubMed] [Google Scholar]
- 12.Kat M.G., Vreeswijk R., de Jonghe J.F., et al. Long-term cognitive outcome of delirium in elderly hip surgery patients. A prospective matched controlled study over two and a half years. Dement Geriatr Cogn Disord. 2008;26(1):1–8. doi: 10.1159/000140611. [DOI] [PubMed] [Google Scholar]
- 13.Marcantonio E.R., Flacker J.M., Michaels M., Resnick N.M. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618–624. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 14.Moskowitz E.E., Overbey D.M., Jones T.S., et al. Post-operative delirium is associated with increased 5-year mortality. Am J Surg. 2017;214(6):1036–1038. doi: 10.1016/j.amjsurg.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 15.Pedemonte J.C., Sun H., Franco-Garcia E., et al. Postoperative delirium mediates 180-day mortality in orthopaedic trauma patients. Br J Anaesth. 2021;127(1):102–109. doi: 10.1016/j.bja.2021.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gou R.Y., Hshieh T.T., Marcantonio E.R., et al. One-year medicare costs associated with delirium in older patients undergoing major elective surgery. JAMA Surg. 2021;156(5):430–442. doi: 10.1001/jamasurg.2020.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes C.G., Boncyk C.S., Culley D.J., et al. American Society for Enhanced Recovery and Perioperative Quality Initiative joint consensus statement on postoperative delirium prevention. Anesth Analg. 2020;130(6):1572–1590. doi: 10.1213/ANE.0000000000004641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldecoa C., Bettelli G., Bilotta F., et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34(4):192–214. doi: 10.1097/EJA.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 19.Nedergaard M., Goldman S.A. Glymphatic failure as a final common pathway to dementia. Science. 2020;370(6512):50–56. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winkelman J.W. CLINICAL PRACTICE. Insomnia disorder. N Engl J Med. 2015;373(15):1437–1444. doi: 10.1056/NEJMcp1412740. [DOI] [PubMed] [Google Scholar]
- 21.Fadayomi A.B., Ibala R., Bilotta F., Westover M.B., Akeju O. A systematic review and meta-analysis examining the impact of sleep disturbance on postoperative delirium. Crit Care Med. 2018;46(12):e1204–e1212. doi: 10.1097/CCM.0000000000003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebert T.J., Hall J.E., Barney J.A., Uhrich T.D., Colinco M.D. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93(2):382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Akeju O., Pavone K.J., Westover M.B., et al. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology. 2014;121(5):978–989. doi: 10.1097/ALN.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akeju O., Kim S.E., Vazquez R., et al. Spatiotemporal dynamics of dexmedetomidine-induced electroencephalogram oscillations. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0163431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akeju O., Hobbs L.E., Gao L., et al. Dexmedetomidine promotes biomimetic non-rapid eye movement stage 3 sleep in humans: a pilot study. Clin Neurophysiol. 2018;129(1):69–78. doi: 10.1016/j.clinph.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benveniste H., Lee H., Ding F., et al. Anesthesia with dexmedetomidine and low-dose isoflurane increases solute transport via the glymphatic pathway in rat brain when compared with high-dose isoflurane. Anesthesiology. 2017;127(6):976–988. doi: 10.1097/ALN.0000000000001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li R., Lai I.K., Pan J.Z., Zhang P., Maze M. Dexmedetomidine exerts an anti-inflammatory effect via alpha 2 adrenoceptors to prevent lipopolysaccharide-induced cognitive decline in mice. Anesthesiology. 2020;133(2):393–407. doi: 10.1097/ALN.0000000000003390. [DOI] [PubMed] [Google Scholar]
- 28.Hughes C.G., Mailloux P.T., Devlin J.W., et al. Dexmedetomidine or propofol for sedation in mechanically ventilated adults with sepsis. N Engl J Med. 2021;384(15):1424–1436. doi: 10.1056/NEJMoa2024922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shehabi Y., Howe B.D., Bellomo R., et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. 2019;380(26):2506–2517. doi: 10.1056/NEJMoa1904710. [DOI] [PubMed] [Google Scholar]
- 30.Skrobik Y., Duprey M.S., Hill N.S., Devlin J.W. Low-dose nocturnal dexmedetomidine prevents ICU delirium. A randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2018;197(9):1147–1156. doi: 10.1164/rccm.201710-1995OC. [DOI] [PubMed] [Google Scholar]
- 31.Shelton K.T., Qu J., Bilotta F., et al. Minimizing ICU neurological dysfunction with dexmedetomidine-induced sleep (MINDDS): protocol for a randomised, double-blind, parallel-arm, placebo-controlled trial. BMJ Open. 2018;8(4) doi: 10.1136/bmjopen-2017-020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inouye S.K., van Dyck C.H., Alessi C.A., Balkin S., Siegal A.P., Horwitz R.I. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 33.Inouye S.K., Kosar C.M., Tommet D., et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160(8):526–533. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horton D.K., Hynan L.S., Lacritz L.H., Rossetti H.C., Weiner M.F., Cullum C.M. An abbreviated montreal cognitive assessment (MoCA) for dementia screening. Clin Neuropsychol. 2015;29(4):413–425. doi: 10.1080/13854046.2015.1043349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong A., Nyenhuis D., Black S.E., et al. Montreal cognitive assessment 5-minute protocol is a brief, valid, reliable, and feasible cognitive screen for telephone administration. Stroke. 2015;46(4):1059–1064. doi: 10.1161/STROKEAHA.114.007253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Neal J.B., Billings F.T., 4th, Liu X., et al. Risk factors for delirium after cardiac surgery: a historical cohort study outlining the influence of cardiopulmonary bypass. Can J Anaesth. 2017;64(11):1129–1137. doi: 10.1007/s12630-017-0938-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge W., Alabsi H., Jain A., et al. Identifying patients with delirium based on unstructured clinical notes: observational study. JMIR Form Res. 2022;6(6) doi: 10.2196/33834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chamadia S., Pedemonte J.C., Hobbs L.E., et al. A pharmacokinetic and pharmacodynamic study of oral dexmedetomidine. Anesthesiology. 2020;133(6):1223–1233. doi: 10.1097/ALN.0000000000003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chamadia S., Hobbs L., Marota S., et al. Oral dexmedetomidine promotes non-rapid eye movement stage 2 sleep in humans. Anesthesiology. 2020;133(6):1234–1243. doi: 10.1097/ALN.0000000000003567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preskorn S.H., Zeller S., Citrome L., et al. Effect of sublingual dexmedetomidine vs. placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727–736. doi: 10.1001/jama.2022.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su X., Meng Z.T., Wu X.H., et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–1902. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]
- 42.Wu X.H., Cui F., Zhang C., et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: a pilot randomized controlled trial. Anesthesiology. 2016;125(5):979–991. doi: 10.1097/ALN.0000000000001325. [DOI] [PubMed] [Google Scholar]
- 43.Shaefi S., Shankar P., Mueller A.L., et al. Intraoperative oxygen concentration and neurocognition after cardiac surgery. Anesthesiology. 2021;134(2):189–201. doi: 10.1097/ALN.0000000000003650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landry G.J., Best J.R., Liu-Ambrose T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci. 2015;7:166. doi: 10.3389/fnagi.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.