Abstract
Helicobacter pylori VacA is a secreted protein toxin that forms channels in lipid bilayers and induces multiple structural and functional alterations in eukaryotic cells. A unique hydrophobic segment at the amino terminus of VacA contains three tandem repeats of a GxxxG motif that is characteristic of transmembrane dimerization sequences. To examine functional properties of this region, we expressed and analyzed ToxR-VacA-maltose binding protein fusions using the TOXCAT system, which was recently developed by W. P. Russ and D. M. Engelman (Proc. Natl. Acad. Sci. USA 96:863–868, 1999) to study transmembrane helix-helix associations in a natural membrane environment. A wild-type VacA hydrophobic region mediated insertion of the fusion protein into the inner membrane of Escherichia coli and mediated protein dimerization. A fusion protein containing a mutant VacA hydrophobic region (in which glycine 14 of VacA was replaced by alanine) also inserted into the inner membrane but dimerized significantly less efficiently than the fusion protein containing the wild-type VacA sequence. Based on these results, we speculate that the wild-type VacA amino-terminal hydrophobic region contributes to oligomerization of the toxin within membranes of eukaryotic cells.
Many Helicobacter pylori strains secrete a toxin (VacA) that is thought to play an important role in the pathogenesis of peptic ulcer disease and gastric adenocarcinoma (1, 17, 22). The most prominent effect of VacA is its capacity to induce the formation of large cytoplasmic vacuoles in eukaryotic cells. In addition, VacA interferes with the process of antigen presentation, increases the permeability of polarized epithelial cell monolayers, and forms anion-selective membrane channels (4, 9, 15, 19, 26, 28–30). Formation of channels in endosomal membranes of cells may be an important feature of the mechanism by which VacA induces cell vacuolation.
The purified VacA cytotoxin exhibits minimal activity unless it is first exposed to acidic (e.g., pH 3) or alkaline (e.g., pH 10) conditions prior to being added to eukaryotic cells (6, 13, 33). This pH activation of VacA is necessary for formation of membrane channels (4, 9, 28–30) and for efficient internalization of VacA by HeLa cells (13) and is associated with changes in VacA protein structure (2, 6, 14, 18, 33). Specifically, at neutral pH, purified VacA appears as a complex flower-shaped oligomeric structure when imaged by deep-etch electron microscopy (2, 12). Acidification to pH 3 or alkalinization to pH 10 results in the nearly complete disassembly of VacA oligomers into component monomers, which can reanneal into oligomers upon neutralization (2, 14, 33).
Transfection of HeLa cells with plasmids expressing the amino-terminal 422 amino acids of VacA is sufficient to induce vacuolation of HeLa cells (35). Small truncations, internal deletions, and several point mutations in the amino-terminal portion of VacA abrogate toxin activity when assessed in transiently transfected cells (5, 31, 34). Similarly, purified toxin from a mutant H. pylori strain, carrying a deletion of the codons for amino acids 6 through 27, fails to induce cytoplasmic vacuolation, is defective in the capacity to form membrane channels, and inhibits the activity of the wild-type toxin (31). Taken together, these data suggest that the amino-terminal region of VacA plays a very important role in toxin activity.
The amino-terminal 32 amino acids of VacA are predicted to form the only contiguous hydrophobic region in the protein that is long enough to span a membrane (Fig. 1). Analysis of this region reveals three tandem repeats of a GxxxG motif, which has been associated with transmembrane helix-helix association (23, 27). Based on these features, we hypothesized that the amino-terminal region of VacA might be capable of assuming an α-helical transmembrane conformation with the capacity to oligomerize.
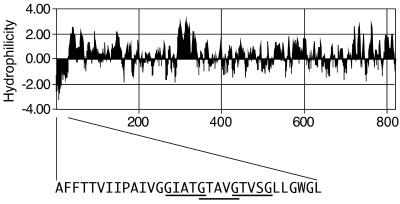
FIG. 1.
VacA hydrophilicity and localization of tandem GxxxG motifs. The predicted hydrophilicity of the mature VacA toxin from H. pylori strain 60190 (ATCC 49503, GenBank accession number U05676 [3]) was analyzed by the method of Kyte and Doolittle (10). The amino acid sequence of 32 amino-terminal residues is shown in capital letters. The three GxxxG motifs, characteristic of TM oligomerization regions, in the VacA amino-terminal region are underlined.
To test this hypothesis, we used the TOXCAT system, which was developed by Russ and Engelman to study transmembrane helix-helix association in a natural membrane environment (23, 24). The TOXCAT system has been used to study the well-characterized transmembrane dimerization sequence of glycophorin A (24) and to select transmembrane dimerization sequences from a randomized library (23). A similar system has been used to study dimerization of synaptobrevin II and syntaxin 1A (11) and the self-assembly of membrane-spanning leucine zipper motifs (8). In TOXCAT, a putative transmembrane sequence (TM) is cloned between the transcription-activator domain of Vibrio cholerae ToxR and the periplasmic domain of the Escherichia coli maltose binding protein (MBP) to produce a ToxR-TM-MBP fusion protein. Expression of the ToxR-TM-MBP fusion protein in E. coli allows the detection of transmembrane oligomerization in two steps. As a first step, membrane localization of the fusion protein is determined based on complementation of a nonpolar malE mutant E. coli strain. If a ToxR-TM-MBP fusion protein inserts into the inner membrane such that the MBP domain localizes to the periplasmic space, cells are able to transport maltose and thus can grow in maltose-minimal medium. In contrast, cells expressing fusion proteins that remain cytoplasmic fail to grow in maltose-minimal medium (24). As a second step, dimerization of the fusion protein is determined based on expression of the cat gene, which is under the control of the dimerization-dependent transcription activator ToxR (reviewed in reference (7)). E. coli strains expressing ToxR-TM-MBP fusion proteins that dimerize are chloramphenicol resistant, whereas strains expressing fusion proteins that lack a dimerization sequence remain chloramphenicol sensitive (24).
A DNA fragment encoding the amino-terminal 32 amino acids of VacA was PCR amplified from H. pylori strain 60190 (ATCC 49503) using primers OP1582(5′-CCCCGCTAGCGCCTTTTTTACAACCGTG; underlined nucleotides indicate an NheI site) and OP1583 (5′-CCCCAGATCTTGAGCCCCCAGCCAAGAAGCCC; underlined nucleotides indicate a BglII site), digested with NheI and BglII, and ligated into NheI-BamHI-digested pccKAN (a plasmid containing the cat gene under the control of the V. cholerae ctx promoter, and the transcription-activator domain of ToxR and the periplasmic domain of MBP separated by a kanamycin resistance cassette [24]), generating plasmid pccVacA-wt. DNA sequence analysis confirmed the proper construction of pccVacA-wt.
Additionally, we constructed (using the method of Perrin and Gilliland [20]) a related plasmid, pccVacA-G14A, that was identical to pccVacA-wt except that it contained a mutation within the vacA sequence such that the glycine at position 14 was replaced with alanine. DNA sequence analysis confirmed the proper construction of pccVacA-G14A. We chose to express this mutation because glycine 14 constitutes part of a GxxxG motif, and because the G14A substitution has been associated previously with decreased VacA-induced vacuolation in a transient-transfection assay of toxin activity (34).
Plasmid pccVacA-wt, plasmid pccVacA-G14A, and plasmids encoding fusion proteins with the wild-type TM region from glycophorin A (pccGpA-wt) or a nondimerizing mutant glycophorin A sequence (pccGpA-G83I) (24) were introduced into E. coli strain MM39 (araD lacIΔU1269 malE444 Strr [24]), and transformants were selected on ampicillin-containing medium. The expression levels of the fusion proteins produced by plasmids pccGpA-wt, pccGpA-G83I, pccVacA-wt, and pccVacA-G14A then were analyzed by immunoblotting with anti-MBP antiserum (New England Biolabs, Beverly, Mass.). Each of the plasmid-containing strains produced similar levels of the ToxR-TM-MBP fusion proteins (Fig. 2).
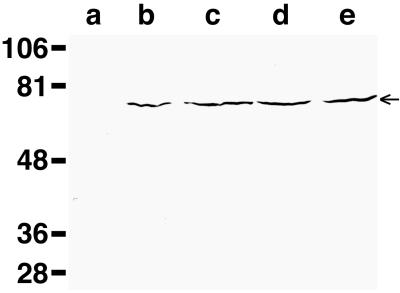
FIG. 2.
Expression of ToxR-TM-MBP fusion proteins. E. coli strains MM39 (lane a), MM39 pccGpA-wt (lane b), MM39 pccGpA-G83I (lane c), MM39 pccVacA-wt (lane d), and MM39 pccVacA-G14A (lane e) were cultured in Luria-Bertani medium to an optical density at 600 nm of approximately 0.35. Equal culture volumes were pelleted, lysed in sodium dodecyl sulfate-sample buffer, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblotted using anti-MBP antisera (New England Biolabs). The 65-kDa ToxR-TM-MBP fusion proteins are indicated by the arrow. Sizes are indicated in kilodaltons on the left.
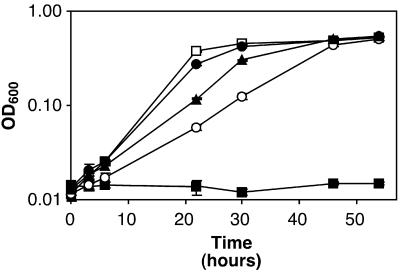
To determine whether these putative transmembrane sequences promoted membrane localization of the ToxR-TM-MBP fusion proteins, bacteria were inoculated into M9-maltose medium (Fig. 3) (25). As reported previously by Russ and Engelman, E. coli strains containing plasmids encoding TM regions from glycophorin A (either pccGpA-wt or pccGpA-G83I) were able to grow in this medium, whereas MM39 with no plasmid was unable to grow in maltose-minimal medium (24). Similar to strains carrying the glycophorin A control plasmids, E. coli MM39 containing either pccVacA-wt or pccVacA-G14A grew in maltose-minimal medium (Fig. 3). These results indicate that the VacA amino-terminal hydrophobic region is able to insert into and span a lipid bilayer.
FIG. 3.
Membrane localization mediated by the VacA N-terminal region. E. coli strains MM39 (■), MM39 pccGpA-wt (□), MM39 pccGpA-G83I (●), MM39 pccVacA-wt (○), and MM39 pccVacA-G14A (▴) were inoculated at an initial optical density at 600 nm (OD600) of 0.014 to 0.024 into M9 broth containing 0.4% maltose, and cultures were incubated at 37°C. Results represent the mean (± standard deviation) optical density at 600 nm from triplicate cultures. All strains grew in glucose-minimal medium (data not shown).
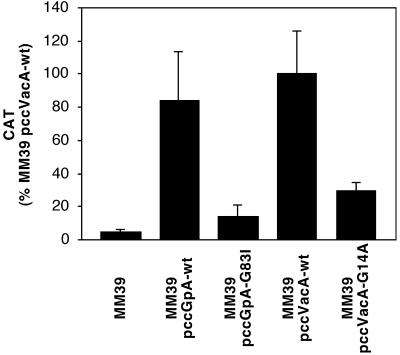
To determine whether the TM regions mediated protein dimerization, E. coli MM39 strains were tested for chloramphenicol acetyltransferase (CAT) activity (Fig. 4). As reported previously by Russ and Engelman, E. coli strain MM39 containing a plasmid expressing the wild-type glycophorin A TM domain (pccGpA-wt) produced high levels of CAT activity, whereas MM39 containing a plasmid expressing the mutant glycophorin A TM domain (pccGpA-G83I) or MM39 containing no plasmid produced significantly less CAT activity (24). Similar to the strain carrying pccGpA-wt, E. coli MM39 containing pccVacA-wt produced high levels of CAT activity, whereas MM39 containing pccVacA-G14A produced significantly less CAT activity (Fig. 4). These results indicate that the wild-type VacA amino-terminal region is able to mediate protein dimerization. The transmembrane segments containing the wild-type and the G14A-mutant VacA sequences are not predicted to differ substantially in hydrophobicity, and the previous experiment (Fig. 3) indicated that both segments efficiently mediated insertion of the fusion proteins into the inner membrane of E. coli. The finding that a single amino acid substitution alters the capacity of the VacA hydrophobic region to promote protein dimerization is consistent with the hypothesis that one or more GxxxG motifs are important for this interaction.
FIG. 4.
Protein dimerization mediated by the VacA N-terminal region. E. coli strains MM39, MM39 pccGpA-wt, MM39 pccGpA-G83I, MM39 pccVacA-wt, and MM39 pccVacA-G14A were cultured in Luria-Bertani medium to an optical density of approximately 0.35 at 600 nm. CAT activity from each strain was quantified using the Quan-T-CAT assay system (Amersham-Pharmacia Biotech). Results represent the mean and standard deviation from triplicate cultures. MM39 pccVacA-G14A produced significantly less CAT activity than MM39 pccVacA-wt (Student's t test, P < 0.003), and MM39 pccGpA-G83I produced significantly less CAT activity than MM39 pccGpA-wt (Student's t test; P < 0.0002).
We speculate that the 32-amino-acid VacA hydrophobic region functions similarly when present at the amino terminus of H. pylori VacA, inserting into the plasma membrane and mediating transmembrane helix-helix associations. In one possible model, acidic or alkaline pH-induced activation of VacA would result in exposure of the previously buried N-terminal hydrophobic sequence, and once exposed, this region would insert into the plasma membrane of eukaryotic cells and promote toxin oligomerization. This model is consistent with the capacity of acidic pH to increase exposure of hydrophobic VacA sequences (14) and to promote insertion of VacA into lipid bilayers or the plasma membrane of eukaryotic cells to form anion-selective channels (4, 9, 29).
Although the present results indicate that the 32-amino-acid VacA hydrophobic region promotes dimerization of TOXCAT fusion proteins, it seems clear that other regions of VacA also probably contribute to toxin oligomerization. For example, a 58-kDa carboxy-terminal fragment of VacA (in which the 32-amino-acid hydrophobic region is absent) is capable of dimerization (21). Moreover, VacA-(Δ6-27) (which lacks a large portion of the hydrophobic region) is capable of assembling into oligomeric structures that are indistinguishable from wild-type VacA (31). Similarly, other regions of VacA in addition to the 32-amino-acid VacA segment studied here may promote toxin insertion into membranes (14, 16, 32). For example, both the amino-terminal 34-kDa portion of VacA (which contains the hydrophobic region) and a carboxy-terminal 58-kDa portion of VacA (which lacks the hydrophobic region) insert into liposomal membranes (14, 16).
Thus, although the results of this study indicate that the N-terminal hydrophobic region of VacA promotes transmembrane dimerization of fusion proteins, it must be acknowledged that the conformation and function of this segment might be considerably different in the context of the native VacA protein interacting with the plasma membrane of a eukaryotic cell, rather than the TOXCAT fusion protein interacting with the inner membrane of E. coli. Further experiments will be required to elucidate the true function of this VacA segment. Nevertheless, the capacity of a G14A mutation to abrogate both VacA cytotoxicity (34) and dimerization of TOXCAT fusion proteins suggests that the TOXCAT model will be a useful approach for studying the function of this important region of VacA.
Acknowledgments
We thank Arlene Vinion-Dubiel for helpful discussions and William Russ and Donald Engelman (who developed TOXCAT with support from the NIH) for providing reagents and helpful comments. DNA oligonucleotides were synthesized by the Vanderbilt University DNA Chemistry Core Facility, and DNA sequencing was performed by the Vanderbilt University DNA Sequencing Laboratory.
This work was supported by NIH grants AI39657 and DK53623 and by the Medical Research Department of the Department of Veterans Affairs.
REFERENCES
- 1.Cover T L. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1996;20:241–246. doi: 10.1111/j.1365-2958.1996.tb02612.x. [DOI] [PubMed] [Google Scholar]
- 2.Cover T L, Hanson P I, Heuser J E. Acid-induced dissociation of VacA, the Helicobacter pylori vacuolating cytotoxin, reveals its pattern of assembly. J Cell Biol. 1997;138:759–769. doi: 10.1083/jcb.138.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cover T L, Tummuru M K R, Cao P, Thompson S A, Blaser M J. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994;269:10566–10573. [PubMed] [Google Scholar]
- 4.Czajkowsky D M, Iwamoto H, Cover T L, Shao Z. The vacuolating toxin from Helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc Natl Acad Sci USA. 1999;96:2001–2006. doi: 10.1073/pnas.96.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Bernard M, Burroni D, Papini E, Rappuoli R, Telford J, Montecucco C. Identification of the Helicobacter pylori VacA toxin domain active in the cell cytosol. Infect Immun. 1998;66:6014–6016. doi: 10.1128/iai.66.12.6014-6016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bernard M, Papini E, de Filippis V, Gottardi E, Telford J, Manetti R, Fontana A, Rappuoli R, Montecucco C. Low pH activates the vacuolating toxin of Helicobacter pylori, which becomes acid and pepsin resistant. J Biol Chem. 1995;270:23937–23940. doi: 10.1074/jbc.270.41.23937. [DOI] [PubMed] [Google Scholar]
- 7.DiRita V J. Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae. Mol Microbiol. 1992;6:451–458. doi: 10.1111/j.1365-2958.1992.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 8.Gurezka R, Laage R, Brosig B, Langosch D. A heptad motif of leucine residues found in membrane proteins can drive self-assembly of artificial transmembrane segments. J Biol Chem. 1999;274:9265–9270. doi: 10.1074/jbc.274.14.9265. [DOI] [PubMed] [Google Scholar]
- 9.Iwamoto H, Czajkowsky D M, Cover T L, Szabo G, Shao Z. VacA from Helicobacter pylori: a hexameric chloride channel. FEBS Lett. 1999;450:101–104. doi: 10.1016/s0014-5793(99)00474-3. [DOI] [PubMed] [Google Scholar]
- 10.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 11.Laage R, Rohde J, Brosig B, Langosch D. A conserved membrane-spanning amino acid motif drives homomeric and supports heteromeric assembly of presynaptic SNARE proteins. J Biol Chem. 2000;275:17481–17487. doi: 10.1074/jbc.M910092199. [DOI] [PubMed] [Google Scholar]
- 12.Lupetti P, Heuser J E, Manetti R, Massari P, Lanzavecchia S, Bellon P L, Dallai R, Rappuoli R, Telford J L. Oligomeric and subunit structure of the Helicobacter pylori vacuolating cytotoxin. J Cell Biol. 1996;133:801–807. doi: 10.1083/jcb.133.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClain M S, Schraw W, Ricci V, Boquet P, Cover T L. Acid-activation of Helicobacter pylori vacuolating cytotoxin (VacA) results in toxin internalization by eukaryotic cells. Mol Microbiol. 2000;37:433–442. doi: 10.1046/j.1365-2958.2000.02013.x. [DOI] [PubMed] [Google Scholar]
- 14.Molinari M, Galli C, de Bernard M, Norais N, Ruysschaert J M, Rappuoli R, Montecucco C. The acid activation of Helicobacter pylori toxin VacA: structural and membrane binding studies. Biochem Biophys Res Commun. 1998;248:334–340. doi: 10.1006/bbrc.1998.8808. [DOI] [PubMed] [Google Scholar]
- 15.Molinari M, Salio M, Galli C, Norais N, Rappuoli R, Lanzavecchia A, Montecucco C. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J Exp Med. 1998;187:135–140. doi: 10.1084/jem.187.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moll G, Papini E, Colonna R, Burroni D, Telford J, Rappuoli R, Montecucco C. Lipid interaction of the 37-kDa and 58-kDa fragments of the Helicobacter pylori cytotoxin. Eur J Biochem. 1995;234:947–952. doi: 10.1111/j.1432-1033.1995.947_a.x. [DOI] [PubMed] [Google Scholar]
- 17.Montecucco C, Papini E, de Bernard M, Telford J L, Rappuoli R. Helicobacter pylori vacuolating cytotoxin and associated pathogenic factors. In: Alouf J E, Freer J H, editors. The comprehensive sourcebook of bacterial protein toxins. San Diego, Calif: Academic Press; 1999. pp. 264–286. [Google Scholar]
- 18.Pagliaccia C, Wang X M, Tardy F, Telford J L, Ruysschaert J M, Cabiaux V. Structure and interaction of VacA of Helicobacter pylori with a lipid membrane. Eur J Biochem. 2000;267:104–109. doi: 10.1046/j.1432-1327.2000.00970.x. [DOI] [PubMed] [Google Scholar]
- 19.Papini E, Satin B, Norais N, de Bernard M, Telford J L, Rappuoli R, Montecucco C. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J Clin Investig. 1998;102:813–820. doi: 10.1172/JCI2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrin S, Gilliland G. Site-specific mutagenesis using asymmetric polymerase chain reaction and a single mutant primer. Nucleic Acids Res. 1990;18:7433–7438. doi: 10.1093/nar/18.24.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reyrat J M, Lanzavecchia S, Lupetti P, de Bernard M, Pagliaccia C, Pelicic V, Charrel M, Ulivieri C, Norais N, Ji X, Cabiaux V, Papini E, Rappuoli R, Telford J L. 3D imaging of the 58 kDa cell binding subunit of the Helicobacter pylori cytotoxin. J Mol Biol. 1999;290:459–470. doi: 10.1006/jmbi.1999.2877. [DOI] [PubMed] [Google Scholar]
- 22.Reyrat J M, Pelicic V, Papini E, Montecucco C, Rappuoli R, Telford J L. Towards deciphering the Helicobacter pylori cytotoxin. Mol Microbiol. 1999;34:197–204. doi: 10.1046/j.1365-2958.1999.01592.x. [DOI] [PubMed] [Google Scholar]
- 23.Russ W P, Engelman D M. The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol. 2000;296:911–919. doi: 10.1006/jmbi.1999.3489. [DOI] [PubMed] [Google Scholar]
- 24.Russ W P, Engelman D M. TOXCAT: a measure of transmembrane helix association in a biological membrane. Proc Natl Acad Sci USA. 1999;96:863–868. doi: 10.1073/pnas.96.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratories; 1989. [Google Scholar]
- 26.Satin B, Norais N, Telford J, Rappuoli R, Murgia M, Montecucco C, Papini E. Effect of Helicobacter pylori vacuolating toxin on maturation and extracellular release of procathepsin D and on epidermal growth factor degradation. J Biol Chem. 1997;272:25022–25028. doi: 10.1074/jbc.272.40.25022. [DOI] [PubMed] [Google Scholar]
- 27.Senes A, Gerstein M, Engelman D M. Statistical analysis of amino acid patterns in transmembrane helices: the GxxxG motif occurs frequently and in association with beta-branched residues at neighboring positions. J Mol Biol. 2000;296:921–936. doi: 10.1006/jmbi.1999.3488. [DOI] [PubMed] [Google Scholar]
- 28.Szabo I, Brutsche S, Tombola F, Moschioni M, Satin B, Telford J L, Rappuoli R, Montecucco C, Papini E, Zoratti M. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J. 1999;18:5517–5527. doi: 10.1093/emboj/18.20.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tombola F, Carlesso C, Szabo I, de Bernard M, Reyrat J M, Telford J L, Rappuoli R, Montecucco C, Papini E, Zoratti M. Helicobacter pylori vacuolating toxin forms anion-selective channels in planar lipid bilayers: possible implications for the mechanism of cellular vacuolation. Biophys J. 1999;76:1401–1409. doi: 10.1016/S0006-3495(99)77301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tombola F, Oregna F, Brutsche S, Szabo I, Del Giudice G, Rappuoli R, Montecucco C, Papini E, Zoratti M. Inhibition of the vacuolating and anion channel activities of the VacA toxin of Helicobacter pylori. FEBS Lett. 1999;460:221–225. doi: 10.1016/s0014-5793(99)01348-4. [DOI] [PubMed] [Google Scholar]
- 31.Vinion-Dubiel A D, McClain M S, Czajkowsky D M, Iwamoto H, Ye D, Cao P, Schraw W, Szabo G, Blanke S R, Shao Z, Cover T L. A dominant negative mutant of Helicobacter pylori vacuolating toxin (VacA) inhibits VacA-induced cell vacuolation. J Biol Chem. 1999;274:37736–37742. doi: 10.1074/jbc.274.53.37736. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Wattiez R, Paggliacia C, Telford J L, Ruysschaert J, Cabiaux V. Membrane topology of VacA cytotoxin from H. pylori. FEBS Lett. 2000;481:96–100. doi: 10.1016/s0014-5793(00)01978-5. [DOI] [PubMed] [Google Scholar]
- 33.Yahiro K, Niidome T, Kimura M, Hatakeyama T, Aoyagi H, Kurazono H, Imagawa K, Wada A, Moss J, Hirayama T. Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase beta. J Biol Chem. 1999;274:36693–36699. doi: 10.1074/jbc.274.51.36693. [DOI] [PubMed] [Google Scholar]
- 34.Ye D, Blanke S R. Mutational analysis of the Helicobacter pylori vacuolating toxin amino terminus: identification of amino acids essential for cellular vacuolation. Infect Immun. 2000;68:4354–4357. doi: 10.1128/iai.68.7.4354-4357.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye D, Willhite D C, Blanke S R. Identification of the minimal intracellular vacuolating domain of the Helicobacter pylori vacuolating toxin. J Biol Chem. 1999;274:9277–9282. doi: 10.1074/jbc.274.14.9277. [DOI] [PubMed] [Google Scholar]