Abstract
An epidemic of cat-transmitted sporotrichosis caused by Sporothrix brasiliensis has emerged as a major public health threat in Brazil in recent decades. We report the first three cases of cat-transmitted sporotrichosis caused by Sporothrix brasiliensis outside South America, and the first ever cases of cat-transmitted sporotrichosis in the United Kingdom. We outline the public health implications and outbreak response and encourage clinicians and veterinarians worldwide to be vigilant for sporotrichosis in cats and cat owners.
Keywords: Sporotrichosis, Cat-transmitted sporotrichosis, Sporothrix brasiliensis, Zoonosis: outbreak
1. Introduction
Sporotrichosis is caused by a group of saprophytic dimorphic fungi from the genus Sporothrix. Members of the genus have a worldwide distribution, particularly in tropical and subtropical regions. Sporotrichosis classically presents as cutaneous or lymphocutaneous ulcerated lesions following the traumatic inoculation of contaminated soil, plants, or organic matter, with an incubation period of 1–10 weeks. It may progress to disseminated disease, especially in the context of immunosuppression, and may manifest as osteoarticular, conjunctival or meningitic disease. Pulmonary involvement with chronic cavitating disease is rare and caused by the direct inhalation of conidia. Diagnosis is made by isolating Sporothrix spp. From lesions by fungal culture, with adjunctive histology and panfungal PCR. The treatment of choice is a prolonged course of itraconazole, and overall mortality and long-term morbidity are very low [1].
Sporothrix spp. Is a genus of thermally dimorphic ascomycetes with a saprophytic mycelial phase at 25–28 °C and a pathogenic yeast phase at 36–37 °C [2]. S. brasiliensis, alongside S. globosa, S. luriei and S. mexicana, was identified in 2007, when molecular studies revealed an additional four species within the S. schenckii complex alongside S. schenckii sensu stricto. S. brasiliensis has shown higher virulence and a preponderance for cat-transmission. Zoonotic (cat-human) and enzootic (cat-cat/cat-dog) transmission has been recorded.
Cat-transmitted sporotrichosis (CTS) has emerged as a zoonotic epidemic and public health threat in Brazil since the 1990s and is associated almost exclusively with the more virulent Sporothrix brasiliensis [3]. Over 5000 human and 5000 feline cases have been reported at a single centre in Rio de Janeiro and confirmed cases have now been reported across Brazil, as well as in Argentina [2,4]. Outside South America, cases of CTS caused by S. schenckii sensu stricto have been reported in the USA, India, Malaysia, Mexico and Panama but S. brasiliensis has not been detected [5]. Human-human transmission has never been documented.
In the United Kingdom (UK), environmentally acquired sporotrichosis in humans is rarely reported. The first case of sporotrichosis in a cat in the UK was reported in 2020 caused by Sporothrix humicola, which lies within the Sporothrix pallida complex [6] and no other cases have been identified since. There has never been a case of CTS reported in the UK prior to the cases described here.
Here we present three human cases of CTS caused by S. brasiliensis in the UK following contact with a domestic cat and outline the public health response to the outbreak. One of the cases has already been reported in isolation [7].
2. Case presentation
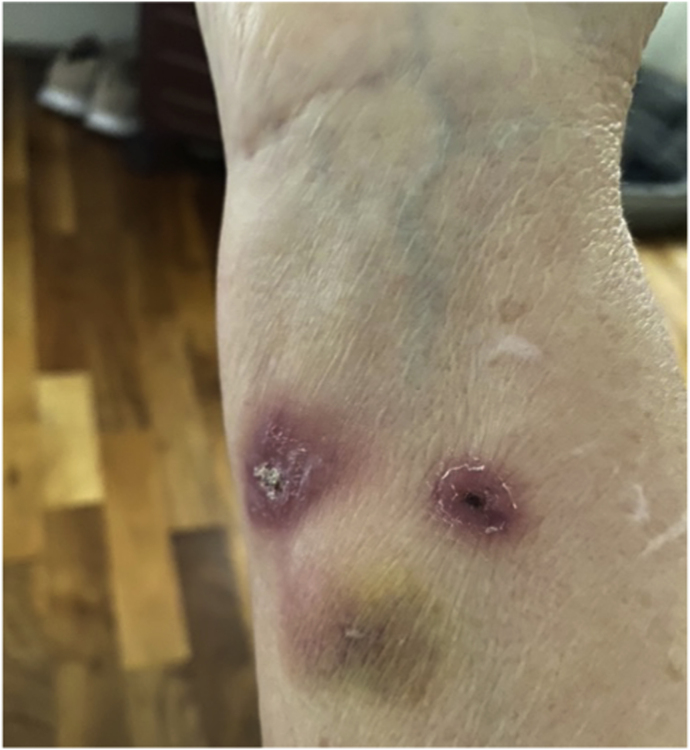
Case 1 was a 63-year-old female originally from South-Eastern Brazil who had been living in the UK for three years with no past medical history or immunosuppression. She had not travelled back to Brazil since. She presented to her local Infectious Diseases clinic having been referred by her primary care doctor with a 3-week history of right forearm lesions at the site of a scratch from a domestic cat sustained whilst feeding it medication (Fig. 1). Two small painless nodules were palpable along the lymphatics proximal to the site. She was systemically well and had a CRP of 4 mg/L. Symptoms resolved completely following six months of itraconazole 200 mg once daily.
Fig. 1.
Right forearm of Case 1 during treatment (Courtesy of patient, 2021).
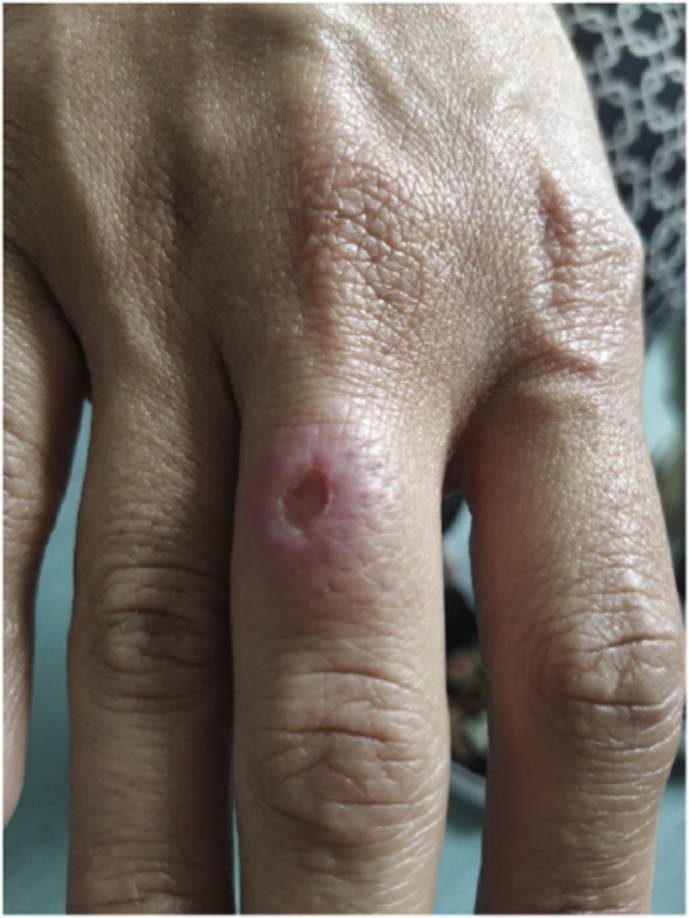
Case 2 was the daughter of Case 1 and lived with her. She was a fit and well 30-year-old. She had migrated from Brazil at the same time and had not travelled back since. She presented to the same clinic with an 8-week history of a tender non-healing ulcer on the middle finger of her dominant right hand at the site of a scratch from the same cat also following medication administration (Fig. 2). She had no lymphadenopathy, was systemically well and had a CRP of 0.8 mg/L. Symptoms resolved significantly following four weeks of itraconazole treatment, but the patient ran out of medication before her next appointment and the lesion progressed in the week off antifungals. The lesion resolved again after restarting treatment and she completed six months of itraconazole 200 mg once daily with complete resolution. Her 9-year-old son also lived in the same house but remained asymptomatic throughout and despite frequent contact with the cat, was never scratched or bitten.
Fig. 2.
Right hand of Case 2 with ulcerated lesion on middle finger at the beginning of treatment (Courtesy of patient, 2021).
Case 3 is reported in detail elsewhere [7]. In brief, a healthy veterinarian in his late 20s was scratched by the same cat whilst examining it at his veterinary practice. Four weeks later he developed an ulcerated lesion on his middle finger at the site of the scratch, and nodular lesions tracking up his arm in a sporotrichoid pattern. Biopsies from the patient showed Periodic Acid Schiff (PAS)-positive oval yeast-like organisms with surrounding necrosis on histology, white-grey colonies after 18 days on culture on Sabouraud dextrose agar, and panfungal PCR of the recovered organism targeting the 26S rDNA gene and the partial beta tubulin gene performed exactly as described previously [8] confirmed the identity of S. brasiliensis [7]. Antifungal susceptibility testing performed by CLSI broth microdilution methodology [9] at the UKHSA National Mycology Reference Laboratory confirmed itraconazole susceptibility (MIC = 0.5 mg/L) but elevated MICs with the other azole antifungal agents (voriconazole = 16 mg/L; isavuconazole = 4 mg/L and posaconazole = 0.5 mg/L). He was treated with itraconazole, and his ulcerated lesions resolved after three months. He received a total of six months' treatment.
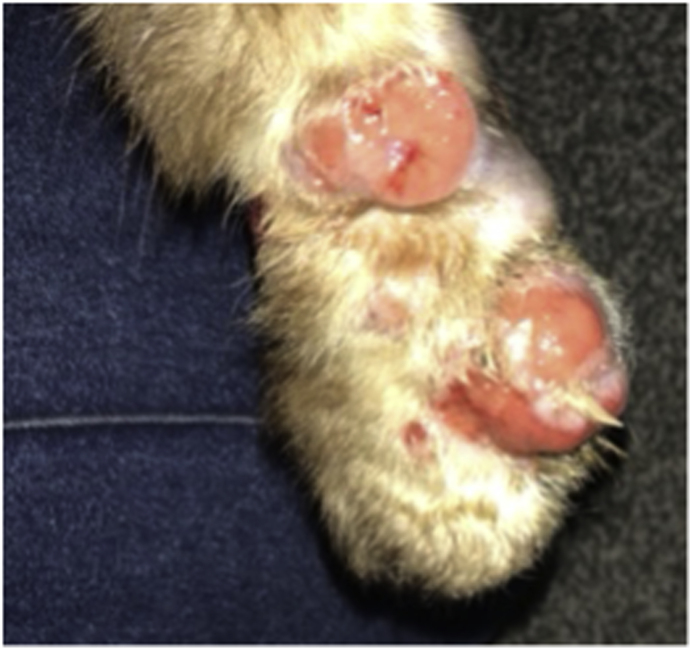
The likely source for the human cases was a 9-year-old male neutered long-haired domestic cat who had been rescued by the family in South-Eastern Brazil and brought over with them to the UK three years ago. The cat had not left the UK since. On arrival at the UK border control post, the cat had a document check for compliance with the pet travel requirements and a visual inspection to check the identity of the cat and the lack of any clinical suspicion for rabies infection. He was an indoor cat and never left the house except for a single brief escape shortly after arrival to the UK. He was never looked after elsewhere e.g., a cattery. A second indoor domestic cat also lived in the house and remained asymptomatic, but otherwise there was no interaction with other cats. The pet had developed scalp and paw lesions four months prior to presentation of the human cases (Fig. 3). The family had discussed the idea of sporotrichosis with a friend in Brazil where feline sporotrichosis is well recognised and they started the cat empirically on over the counter itraconazole. They took the cat to a vet for a more robust diagnosis who performed three biopsies of the frontal scalp and chin regions. The histology was reported as pyogranulomatous inflammation, with PAS staining confirming multifocal, monomorphous, PAS-positive spherical fungal structures measuring 4 μm with occasional areas of budding consistent with a zoonotic fungal infection such as Sporothrix spp. The cat appeared to improve on itraconazole and was seen once further by the vet before being lost to follow up. The cat died from an unknown cause six months following its initial vet appointment. The body was cremated with its bedding, collar, and toys, to reduce further potential transmission from fomites.
Fig. 3.
Cat paw with lesions at the beginning of treatment (Courtesy of patient, 2021).
The cluster of cases were referred to the UK Health Security Agency (UKHSA) for public health input and management given that this organism had never been isolated in the UK before and probable zoonotic transmission had occurred. An Incident Management Team (IMT) was set up with representation from the local Health Protection Team, National Emerging Infections and Zoonoses team and the National Mycology Reference Laboratory of UKHSA, the Animal and Plant Health Agency (APHA), and the local clinical teams. Two meetings took place two weeks apart; the first was when the cat was believed to still be alive. A dynamic risk assessment was conducted and the ongoing risk to the both the human and feline community in the neighbourhood was assessed to be low given the cat was on itraconazole treatment, remained permanently indoors and the co-habiting cat had never developed symptoms despite living together for over three years. There were also no other notifications of cases to UKHSA in the last year. There has never been documented human-human transmission of sporotrichosis and all three cases were improving on treatment. A letter raising occupational awareness of CTS and its treatment is planned for submission to ‘Veterinary Record’, the journal of the British Veterinary Association, but given the low ongoing risk to the wider population outside of the household, the local medical community (primary care facilities and other acute trusts) were not alerted. The second meeting after further contact with Cases 1 and 2 revealed that the cat had died. There were no ongoing public health concerns or exposures and the IMT advised active monitoring of the three cases, the co-habiting cat and a second vet who had been bitten by the index cat but had not developed symptoms.
3. Discussion
Here we present the first confirmed outbreak of cat-transmitted S. brasiliensis outside Brazil and Argentina, the first cases of cat-transmitted sporotrichosis in the UK, and the first record of S. brasiliensis in a cat outside South America. These cases have implications for public health measures globally.
Cat-transmitted sporotrichosis due to Sporothrix brasiliensis is a major public health problem in Brazil, having spread over the past 30 years from the South-East with Rio de Janeiro as an epicentre, to across Brazil, highlighted by a recent case series of 121 patients treated at an Infectious Diseases unit in Natal, North-Eastern Brazil. In addition, 21 cases have been reported in Argentina [2], with retrospective molecular studies tracing circulation of S. brasiliensis back to the 1980s [10]. More recently, a father and son were diagnosed with CTS in 2017 in Paraguay following contact with their sick cat. The family had moved from South-Eastern Brazil nine months previously along with the cat, who had been symptomatic for four months, implying a potential five-month incubation period if acquired in Brazil. The cat had died before the cases sought medical advice [11]. S. brasiliensis was not confirmed but was the most likely species given the Brazilian travel history.
Clinically, a case series of 178 patients with CTS in Rio de Janeiro from 1998 to 2001 found over 90% of patients admitted contact with cats, and 65% had received a traumatic injury from a cat. 55% presented with lymphocutaneous disease, 25% localised cutaneous, and 16% widespread cutaneous, with a median of seven lesions. 30% had concurrent arthritis, and of those not lost to follow-up, all either resolved spontaneously or with itraconazole [3].
An outbreak of S. brasiliensis CTS in the UK raised two relevant public health questions which were discussed during the IMT meetings. The first is where the cat acquired the pathogen. One possibility is autochthonous acquisition in the UK, which would imply circulating S. brasiliensis in the environment, other cats or elsewhere, and would be the most concerning. This is less likely given that the cat always remained indoors, with no exposure to other animals beyond the other domestic cat which remained asymptomatic. Also, S. brasiliensis has never been identified outside South America, making unrecognised environmental contamination in the UK particularly unlikely. The more likely alternative is that the cat was colonised with S. brasiliensis for several years following initial acquisition in Brazil, where it had lived outdoors in a very high prevalence part of the country, and an additional more recent event such as acquired immunodeficiency e.g., feline immunodeficiency virus (FIV), or traumatic inoculation, or both, triggered a more invasive disease. Although the cat was visually inspected as per protocol on first arrival to the UK, the reason for inspection is to assess any clinical signs associated with infection with rabies or trauma/welfare issues during travel. Small skin lesions or asymptomatic carriage would not be detected during border checks. However, incubation periods of this length have not been reported previously and the role of healthy cats in transmitting S. brasiliensis appears to be minor, with only one case of colonisation in a study of 175 healthy cats in Rio de Janeiro [12], suggesting asymptomatic colonisation is uncommon.
The second question was that if the pathogen had been imported, what could be done to stop this happening again in the future. In this scenario, the cat remained indoors, but if it had been accustomed to outdoor living then S. brasiliensis could have been spread to other cats and the environment, introducing a novel and virulent species into the UK. Between 2017 and 2021, an average of 90 cats/year were imported to the UK from Brazil according to APHA data. Although numbers are low, this does represent a potential ongoing risk. Numbers may be higher in other countries with closer ties to Brazil such as Portugal. Countries importing cats from endemic areas may wish to consider voluntary pre-import screening for sporotrichosis. An enzyme-linked immunosorbent assay (ELISA) has been developed in Brazil for diagnosing cats with good sensitivity and specificity for Sporothrix spp., but it requires a blood test and does not distinguish S. brasiliensis [13]. Given the expense and limited availability of testing in non-endemic countries, raising awareness of the clinical signs, symptoms, and risk factors of sporotrichosis in imported cats amongst veterinarians may be a more pragmatic step to facilitate the early recognition and containment of feline sporotrichosis if missed prior to importation.
Given the potential risk of CTS linked to imported cats in the UK and globally, clinicians should be aware of the clinical features of sporotrichosis and consider it early in cat owners with non-healing ulcers with or without lymph node involvement, especially if there is a history of feline bites or scratches. Skin biopsies of the lesion should be sent for fungal culture, histology with fungal staining, and panfungal PCR when suspected.
In conclusion, we have presented the first three reported cases of cat-transmitted S. brasiliensis infection to humans outside Brazil and Argentina, likely acquired from an indoor domestic cat which had previously lived in South-Eastern Brazil three years previously. This suggests that S. brasiliensis can lay dormant for many years and has implications for global public health. Veterinarians must be vigilant in taking a travel history when seeing cats with unexplained lesions. Clinicians must suspect CTS as part of the differential in cat owners with lymphocutaneous manifestations. Animal health authorities worldwide should re-examine their border control policies to consider pre-import screening of cats from endemic areas and working with public health agencies to improve awareness amongst those owning or treating cats from endemic areas of the signs and symptoms of feline sporotrichosis to prevent zoonotic transmission.
Conflicts of interest
There are none.
Acknowledgements
D.A.J. is funded by the Wellcome Trust (no. 219551/Z/19/Z), the Medical Research Council (grant no. MR/V037315/1) and Cystic Fibrosis Trust (grant no. SRC015). D.A.J. is funded by the Department of Health and Social Care (DHSC) Centre for Antimicrobial Optimisation (CAMO), Imperial College London. The views expressed in this publication are those of the authors and not necessarily those of the DHSC, National Health Service or National Institute for Health Research (NIHR).
References
- 1.Barros MB. de L., de Almeida Paes R., Schubach A.O. Sporothrix schenckii and sporotrichosis. Clin. Microbiol. Rev. 2011 Oct;24(4):633–654. doi: 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etchecopaz A., Toscanini M.A., Gisbert A., Mas J., Scarpa M., Iovannitti C.A., et al. Sporothrix brasiliensis: a review of an emerging South American fungal pathogen, its related disease, presentation and spread in Argentina. J Fungi. 2021 Feb 26;7(3):170. doi: 10.3390/jof7030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastos de Lima Barros M., Oliveira Schubach A de, Francesconi do Valle A.C., Gutierrez Galhardo M.C., Conceicao‐Silva F., Pacheco Schubach T.M., et al. Cat‐transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin. Infect. Dis. 2004 Feb 15;38(4):529–535. doi: 10.1086/381200. [DOI] [PubMed] [Google Scholar]
- 4.de Oliveira Bento A., de Sena Costa A.S., Lima S.L., do Monte Alves M., de Azevedo Melo A.S., Rodrigues A.M., et al. The spread of cat-transmitted sporotrichosis due to Sporothrix brasiliensis in Brazil towards the Northeast region. Pappas G, editor. PLoS Neglected Trop. Dis. 2021 Aug 30;15(8) doi: 10.1371/journal.pntd.0009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gremião I.D.F., Miranda L.H.M., Reis E.G., Rodrigues A.M., Pereira S.A. Zoonotic epidemic of sporotrichosis: cat to human transmission. Sheppard DC. PLoS Pathog. 2017 Jan 19;13(1) doi: 10.1371/journal.ppat.1006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makri N., Paterson G.K., Gregge F., Urquhart C., Nuttall T. First case report of cutaneous sporotrichosis (Sporothrix species) in a cat in the UK. J Feline Med Surg Open Rep. 2020 Jan;6(1) doi: 10.1177/2055116920906001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rachman R., Ligaj M., Chinthapalli S., Serafino Wani R. Zoonotic acquisition of cutaneous Sporothrix braziliensis infection in the UK. BMJ Case Rep. 2022 May;15(5) doi: 10.1136/bcr-2021-248418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borman A.M., Desnos-Ollivier M., Campbell C.K., Bridge P.D., Dannaoui E., Johnson E.M. Novel taxa associated with human fungal black-grain mycetomas: emarellia grisea gen. Nov., sp. nov., and emarellia paragrisea sp. nov. Warnock DW, editor. J. Clin. Microbiol. 2016 Jul;54(7):1738–1745. doi: 10.1128/JCM.00477-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander Barbara, Gary Procop, Dufresne Philippe, Espinel-Ingroff Ana, Fuller Jeff, Ghannoum Mahmoud, et al. M38: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. third ed. Clinical and Laboratory Standards Institute; 2017. https://clsi.org/media/1894/m38ed3_sample.pdf [Internet] Available from: [Google Scholar]
- 10.Córdoba S., Isla G., Szusz W., Vivot W., Hevia A., Davel G., et al. Molecular identification and susceptibility profile of Sporothrix schenckii sensu lato isolated in Argentina. Mycoses. 2018 Jul;61(7):441–448. doi: 10.1111/myc.12760. [DOI] [PubMed] [Google Scholar]
- 11.García Duarte J.M., Wattiez Acosta V.R., Fornerón Viera P.M.L., Aldama Caballero A., Gorostiaga Matiauda G.A., Rivelli de Oddone V.B., et al. Sporotrichosis transmitted by domestic cat. A family case report. Nachrichtentechnik. 2017 Dec 30;9(2):67–76. [Google Scholar]
- 12.Macêdo-Sales P.A., Souto S.R.L.S., Destefani C.A., Lucena R.P., Machado R.L.D., Pinto M.R., et al. Domestic feline contribution in the transmission of Sporothrix in Rio de Janeiro State, Brazil: a comparison between infected and non-infected populations. BMC Vet. Res. 2018 Dec;14(1):19. doi: 10.1186/s12917-018-1340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes G.F., Lopes-Bezerra L.M., Bernardes-Engemann A.R., Schubach T.M.P., Dias M.A.G., Pereira S.A., et al. Serodiagnosis of sporotrichosis infection in cats by enzyme-linked immunosorbent assay using a specific antigen, SsCBF, and crude exoantigens. Vet. Microbiol. 2011 Jan;147(3–4):445–449. doi: 10.1016/j.vetmic.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]