Highlights
-
•
Lattice Radiation Therapy (LRT) is a type of spatially fractionated radiation therapy that allows delivering ablative doses to large lesions without an increased toxicity, by alternating high-dose and low-dose areas as peaks and valleys.
-
•
Different LRT approaches are present in Literature and they can be defined as exclusive LRT, hybrid LRT, and metabolism guided hybrid LRT.
-
•
Available data seems to confirm LRT safety, especially for exclusive LRT.
-
•
Despite an interesting median lesions reduction approximately above 50 % 3–6 months after LRT, the very low level of evidence and the studies heterogeneity preclude drawing definitive conclusions on LRT efficacy.
Keywords: Lattice radiation therapy, Lattice radiotherapy, Spatially fractionated radiotherapy, Abscopal effect, Bulky, Radiotherapy, Radiation therapy
Abbreviations: LRT, Lattice Radiation Therapy; SFRT, Spatially Fractionated Radiation Therapy; OAR, Organ At Risk; TCP, Tumor Control Probability
Abstract
Purpose
Lattice radiation therapy (LRT) is an innovative type of spatially fractionated radiation therapy. It aims to increase large tumors control probability by administering ablative doses without an increased toxicity. Considering the rising number of positive clinical experiences, the objective of this work is to evaluate LRT safety and efficacy.
Method
Reports about LRT clinical experience were identified with a systematic review conducted on four different databases (namely, Medline, Embase, Scopus, and Cochrane Library) through the August 2022. Only LRT clinical reports published in English and with the access to the full manuscript text were considered as eligible. The 2020 update version PRISMA statement was followed.
Results
Data extraction was performed from 12 eligible records encompassing 7 case reports, 1 case series, and 4 clinical studies. 81 patients (84 lesions) with a large lesion ranging from 63.2 cc to 3713.5 cc were subjected to exclusive, hybrid, and metabolism guided LRT. Excluding two very severe toxicity with a questionable relation with LRT, available clinical experience seem to confirm LRT safety. When a complete response was not achieved 3–6 months after LRT, a median lesion reduction approximately ≥50 % was registered.
Conclusion
This systematic review appear to suggest LRT safety, especially for exclusive LRT. The very low level of evidence and the studies heterogeneity preclude drawing definitive conclusions on LRT efficacy, even though an interesting trend in terms of lesions reduction has been described.
1. Introduction
1.1. Rationale
Lattice Radiation Therapy (LRT) is an innovative radiotherapy (RT) technique that allows to concurrently administering ablative doses inside neoplastic lesions and low doses near the adjacent organs at risks (OARs) (See Fig. 1). LRT can be considered as a type of spatially fractionated radiation therapy (SFRT) and it represents the 3-dimensional (3D) configuration of the 2-dimensional (2D) GRID therapy (See Fig. 2) [1]. The heterogeneous dose distribution of a LRT plans entails the creation of a 3D array inside the planning target volume (PTV), where high-dose (vertices or hotspots) and low-dose areas (periphery) alternate like peaks and valleys. Albeit the irradiation of discrete sub volumes with ablative doses, the valleys permit to minimize the treatment related toxicity [2]. This LRT peculiarity is particularly interesting for the management of large tumors – i.e., lesions ≥5 cm -, which are traditionally not susceptible of stereotactic body radiation therapy (SBRT). Large tumors require higher doses to be controlled due to their dimensions and because they usually present many necrotic and hypoxic areas [3]. These tumors continue to grow progressively, causing disabling symptoms that are difficult to control; however, conventional RT cannot administer the required ablative doses without increasing the treatment related toxicity [4]. Other possible therapeutic options could be surgery or systemic therapy. Notwithstanding this, surgery is often excluded due to the disease stage, the patients’ performance status, and the potential high risk of complications; moreover, these large lesions are often characterized by an atypical lympho-vascular matrix that impairs systemic therapies efficacy [5]. Thus, LRT can represent an interesting strategy to fill the gap present in large lesions management, as the administration of very high doses should improve the tumor control probability (TCP), possibly achieving a durable tumor response. In addition to a promising cytoreductive action, it is hypothesized that LRT might modulate tumor microenvironment and host immune system [6]. The efficient immunogenic cell death occurring in the vertices should provoke the release of many cancers’ antigens and inflammatory cytokines (DAMPs) [7]. These molecules are thought to trigger an acute inflammatory response and to enhance the homing and the activation of the immune cells, by exploiting the more preserved vascular system in the valleys. Thereby, LRT might prime an effective immune response against cancer cells both in irradiated and not irradiated lesions, favouring their rejection.
Fig. 1.
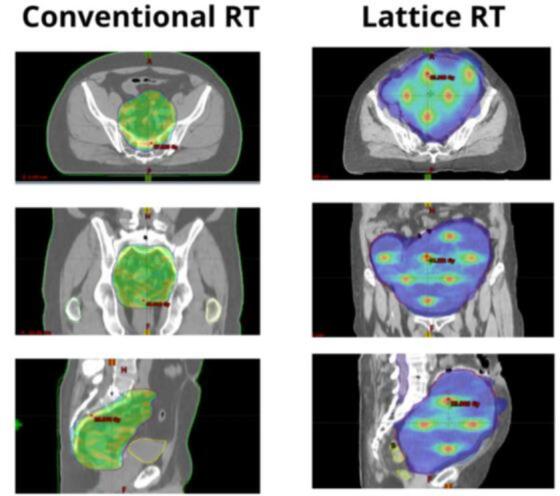
Fig. 1 shows the difference between the dose distribution of a conventional RT (on the left) and a LRT (on the right). In this example the conventional RT is a neoadjuvant irradiation of a rectal cancer, where the green area displays the isodose 25 Gy of the treatment plan. In is worth noting how the target irradiation is homogeneous. By contrast on the right it is illustrated a LRT treatment where the blue area represents the low dose regions (namely, the periphery or the valleys), while the red hotspots are the dose peaks (namely, the vertices). The right column shows how in LRT the irradiation is heterogeneous allowing delivering lower doses in the lesion periphery –i.e., near OARs-, while ablative doses are administered to the inner subvolumes. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
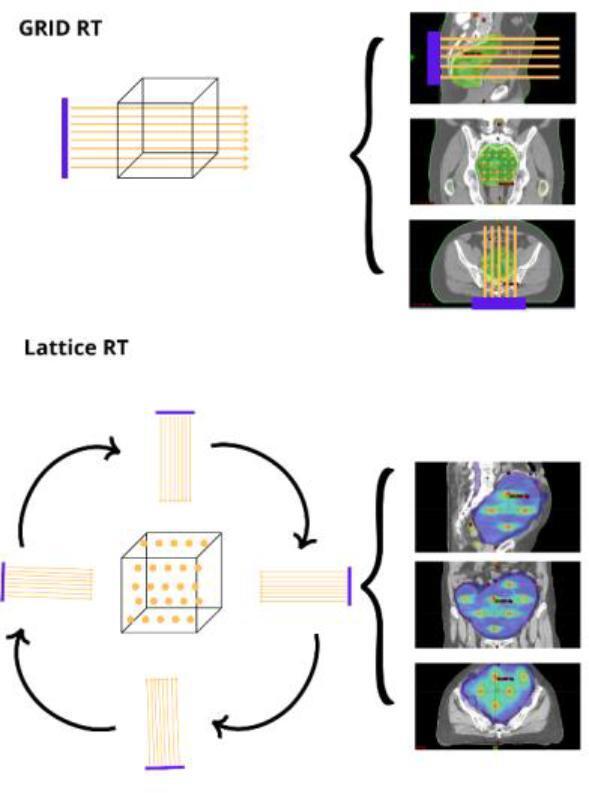
The figure shows the difference between the GRID RT (above) and the LRT (below). GRID RT is a 2D-dimensional approach where a group of parallel photon beams go through the target with a space disposition similar to a group of pens in a closed hand. By contrast, in LRT the photon beams are delivered with different directions. Hence, the LRT allows creating a three-dimensional array or matrix where the area of high and low doses alternates in all spatial directions.
1.2. Purpose
Considering the rising interest, different LRT experiences can be found in Literature. In light of this, the aim of our systematic review is to assess LRT safety and efficacy by analysing available data on LRT in clinical practice (i.e., case reports, case series, and clinical trials). This systematic review was registered as open-ended registration in OSF registries on the September 19, 2022 (osf.io/htjpg).
2. Method
2.1. Research question
To address the purpose of this review we decided to frame the following research question: “Which are the available clinical results in terms of toxicity and response, in the oncological patients subjected to LRT?” The population (P) consisted in the oncological patients, the intervention (I) was LRT, and the outcomes (O) were the reported data about toxicity and response. Both palliative and curative settings (S) were encompassed, without any time limit (T) on follow up and publication data. The definition of a comparator (C) was judged unfeasible and not considered.
2.2. Data sources
We conducted our systematic review on 4 different databases, namely Medline, Embase, Scopus, and Cochrane Library. The 2020 update version of the Preferred Reporting Items for Systematic reviews and meta-Analyses (PRISMA) statement was followed in this systematic review [8].
2.3. Search strategy
As LRT can be considered as a kind of SFRT, we decided to encompass papers about SFRT to minimize the risk of evidence selection bias, with the attempt to consider all available records dealing with LRT. The last search was performed on the 16th of August 2022 and all backward records were collected, without any data limitation. To individuate and assess all available informative papers, we adopted the following research strategy: Lattice radiation therapy[Title/Abstract] OR lattice radiotherapy[Title/Abstract] OR spatially fractionated radiation therapy[Title/Abstract] OR spatially fractionated radiotherapy[Title/Abstract].
2.4. Eligibility criteria
As we wanted to investigate the current clinical data on LRT use in clinical practice, inclusion criteria were as follows: case reports; case series; retrospective studies; clinical prospective studies; reports published in English language; and access to the full text of the manuscript. We excluded reports about preclinical experience, opinion articles, old version of the same reports, off-topic articles, reviews, purposes of study or feasibility articles, planning-dosimetry studies, and articles dealing with types of SFRT different from LRT. The retrieved reports that matched these criteria were considered as eligible.
2.5. Data extraction
Three unblinded reviewers (FI, AC, SFG) on structured collection forms independently performed the data collection (i.e., record exclusion, reports examination, and data extraction). In case of any disagreements between the reviewers, the disagreements were resolved by consensus and by involving two externals third reviewers (EA, SC). A further control was performed by others reviewers (PC, CI) to evaluate reports excluded and data extraction. The extracted data were about author, publication year, report type (i.e., case report, case series, retrospective study or clinical prospective trial), number of patients, number of lesions, patients’ sex, range of Eastern Cooperative Oncology Group scale Performance Status (ECOG PS), median age, tumor type, tumor stage, median dimension, LRT dosages in the vertices and in the periphery. In addition, we decided to focus on LRT schedule (dose in vertices, periphery, and number of fractions), presence of a sequential RT (conventional or hypofractionated), LRT reticule design, RT technique adopted, and the reported acute toxicity (according to Common Terminology Criteria for Adverse Events (CTCAEv5 criteria). To detect the efficacy of LRT we considered the last follow up computed tomography (CT) clearly reported in the eligible articles, to investigate tumor response (according to Response Evaluation Criteria in Solid Tumors (RECIST criteria), and tumor reduction in case of partial response (PR). The presence of a prior, concurrent, sequential systemic therapy administration was encompassed as it could have acted as a confounder.
3. Results
3.1. Review flowchart and eligible article selection
At the end of the bibliographic research, we found 79 records, 118 records, 94 records, and 38 records, in Medline, Embase, Scopus, and Cochrane Library, respectively. After removing duplicates, 239 records remained for the screening. During the screening 112 records were unanimously excluded by the authors, as they were completely off-topic and did not match with the theme of LRT. It is of note that no reports about LRT use in clinical practice should have been lost, as by evaluating unretrieved records titles, the authors unanimously agreed that they did not have the eligibility criteria. Of the 127 reports sought for retrieval, 5 were not retrieved and, as a result, 122 reports were assessed for eligibility. Only full records about case reports, case series, and clinical trials with LRT were considered eligible. Of the 122 reports assessed 110 papers were excluded because they were: abstracts (n = 10: Reason A), planning-dosimetry studies (n = 23: Reason B), studies about 2D-GRID therapy (n = 27: Reason C), purposes of study or feasibility articles (n = 6: Reason D), opinion articles (n = 5: Reason E), preclinical studies (n = 9: Reason F), studies which finally resulted off-topic (n = 11: Reason G), reviews (n = 18: Reason H), old version of the same reports (n = 1; Reason I). Finally, 12 articles were considered as eligible. Table 1 displayed the flow of information through the different phases of our systematic review (see Table 1). Considering that Dicer et al. [9] describe two completely different cases in their case report, we decided to consider each case separately. Results are discussed qualitatively; no quantitative analysis was performed due to data heterogeneity.
Table 1.
The table illustrates the flow of information through the different phases of our review.
 |
3.2. Summary of patient characteristics
Considering the LRT novelty, it was not surprising that 7 out of 12 eligible articles were case reports, there was 1 case series [10] and 4 clinical studies with 1 retrospective study [11], and 3 prospective trials (See Table 2) [12], [13], [14]. It is worth to underline that 6 papers were published in 2022. Globally the sum of the patients’ number amount to 81, with an age range between 33 and 91 years, whereas the number of irradiated lesions is 84. Since Borzov et al. [12] did not report patients’ sex, of the remaining 78 patients, the females and the males were 55 % and 45 %, respectively, with an initial ECOG PS ranging between 0 and 2. Noteworthy, the paper of Schiff JP et al. does not report the ECOG PS, however considering the reported anamnestic information it should amount to 3–4 [15].
Table 2.
Summary of patients’ main characteristic. Abbreviations: M (Metastasis) N/A (not available); adk (adenocarcinoma); scc (squamocellular carcinoma). References in chronological order of appearance: Blanco Suarez et al.2018 [17]; Amendola et al. 2018 [18]; Amendola et al. 2019 [10]; Amendola et al. 2020 [11]; Duriseti et al. 2021 [11]; Jiang et al. 2021 [6]; Iori et al. [16]; Dicer et al 2022 [15]; Borzov et al. 2022 [12]; Ferini et al 2022 [19]; Schiff JP et al. 2022 [15]; Ferini et al. [14].
| Report type | N of patients | N of lesions | Median Age (Range) |
Sex Females Males |
ECOG PS | Tumor | Stage | Median dimension (Range) |
Systemic Therapy (conurrent or sequential) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Blanco Suarez et al. 2015 | Case report | 1 | 1 | 62 | 1F |
N/A | M. ovarian carcinoma | IV | 1495 cc | Concurrent chemotherapy |
| Amendola et al. 2018 | Case report | 1 | 1 | 72 | 1 M | N/A | NSCLC | IIIA (T3N1M0) | 218.5 cc | Concurrent chemotherapy |
| Amendola et al. 2019 | Case serie | 1 | 1 | 71 (48–87) |
1F 9 M |
0–1 | NSCLC | IIIA -IV | 195 CC (46 – 487 cc) |
No |
| Amendola et al. 2020 | Clinical Study |
10 | 10 | 64 (44–90) |
10F | N/A | Cervix Cancer | IIIA-IIIB | 200.35 cc (74.1 – 414.4 cc) |
Concurrent Chemotherapy (weekly cisplatin) |
| Duriseti et al. 2021 | Clinical Study | 20 | 22 | 67 (31–86) |
11F 9 M |
0–2 | 9 Soft tissue sarcoma 7 NSCLC 1 Thymic carcinoma 1 mesothelioma 1 endometrial adk 1 colonic adk |
N/A | 579.2 cc (54.2–3714.5 cc) |
No |
| Jiang et al. 2021 | Case Report | 1 | 1 | 33 | 1F | N/A | M. of NSCLC | IV | 63.2 cc | Concurrent immunotherapy (Pembrolizumab) |
| Iori et al. 2022 | Case Report | 1 | 1 | 69 | 1 M | 2 | Sarcomatoid lung cancer | IV (cT4bN3M1c) | 946.8 cc | Sequential chemotherapy (Vinorelbine) |
| Dicer et al. 2022 | Case Report | 1 | 1 | 59 | 1F | N/A | M. of anal cancer | IV |
N/A | No |
| Dicer et al. 2022 | Case Report | 1 | 1 | 70 | 1 M | N/A | M. of rectosigmoid cancer | IV (T3N1M1) |
494 cc | No |
| Borzov et al. 2022 | Clinical Study | 3 | 3 | N/A | N/A | N/A | Soft tissue sarcoma | N/A | 172 cc | No |
| Ferini et al. 2022 | Case Report | 1 | 1 | 75 | 1 M | N/A | Cutaneous squamous cell carcinoma | IV | 334.88 cc | Sequential target therapy (cemipilumab) |
| Schiff JP et al. 2022 | Case Report | 1 | 1 | 85 | 1F | N/A | Endometrial cancer | IV (IVB) |
1539 cc | No |
| Ferini et al. 2022 | Clinical Study | 30 | 31 | 74.9 (42–91) |
10F 20 M |
0–2 | 8 adk 7 scc 5 sarcoma 2 ductal carcinoma 2 urotelial carcinoma 1 melanoma |
N/A | 146.8 cc (50.9 – 2039.7 cc) |
No |
In all studies, LRT was used to manage very large lesion with volumes ranging from 63.2 cc to 3713.5 cc. The prevalent tumor types were lung cancer and soft tissue sarcoma. Nearly all patients had a stage IV disease and in most of the cases, the treatment had a palliative-cytoreductive intent, however, Borzov et al. reported the case of a preoperative LRT [12]. Both metastases and primary lesions were treated with LRT. Although the extent of accuracy is variable, it is reported that nearly all patients of the eligible articles experimented a disabling burden of symptoms due to the bulky lesions before LRT.
3.3. Treatment characteristics
The most widely used RT technique is Image Guided Radiotherapy-Volumetric Modulated Arc Therapy (IGRT-VMAT) with daily cone beam (CB) CT, although Dicer et al. used a step and shoot IMRT with a magnetic resonance imaging (MRI)-Linac, and Jiang et al used a CyberKnife. Different LRT schedules have been adopted. Iori et al., Duriseti et al, Schiff JP et al., and Dicer et al. delivered an exclusive LRT in 5 fractions reaching a vertices daily dose of 11 Gy, 13.34 Gy, and 10 Gy, respectively. With reference to the periphery, they compressively administered 20 Gy, 20 Gy, and 30 Gy, respectively [16], [13], [15], [9]. By contrast Blanco Suarez et al., Ferini et al., Amendola et al., and Borzov et al. decided to add a sequential conventional or hypofractionated RT after 1–3 upfront LRT fractions with a lower dose-escalation between the vertices and the valleys [17], [14], [11], [12]. It is interesting to underline that there is a median dose escalation between the periphery and the vertices around 275 %, with a range between 167 and 600 %. Most of the treatments were performed with 6 MV photon x or flattening filter free photon x, using a VMAT. A little different is the report of Jiang et al., as they delivered a single fraction with 20 Gy in the vertices with CyberKnife, and of Dicer et al. since they delivered the treatment using a step and shoot IMRT with a MRI Linac [6], [9]. More lacking are the data about the lattice array design, i.e., vertices number, dimension, and their spatial location. Although there is a general agreement about centre-to-centre distance (range 2 – 6 cm) and vertices diameter (range 0.8 – 1.5 cm), more lacking is the information about lattice array design. Some papers do not report this information in detail while in others the lattice array design was arbitrary decided according to the judgment of the Radiation Oncologists and the Medical Physicists, taking into account the size and the location of the tumor with respect to the organs at risk (OARs) [12], [10], [11], [14]. In others, an ad hoc created script decided the vertices optimal number and their location [16]. Notwithstanding this, it is observable a consensus about removing vertices closer than 1.5 cm to the OARs to minimize LRT toxicity (See Table 3).
Table 3.
The table illustrates the different LRT schedule adopted and the retrievable information about Veritces and Pheriphery dose, Vertices diameter, center to center distance, and RT technique used to administer the treatment with the energy of the photon beam. In the last column there are the information reported by the authors about lattice array creation. Abbreviations: V (Vertices), P (Periphery), Fx (fractions), FFF (Flattening Filter Free); pz (patients); N/A (not available).
| V dose |
P dose |
Fx | Sequential RT | V Diameter |
Center to center distance | Tecnique | Photon Energy | Reported information on lattice array design | |
|---|---|---|---|---|---|---|---|---|---|
| Blanco Suarez et al. 2015 | 9 Gy | 3 Gy | 3 |
1.8 Gy: 5 fractions. + 2 Gy (peripheral dose) + 5 Gy as IB: 5 fractions + 1.8 Gy 5 fractions + 2 Gy (peripheral dose) + 5 Gy (strips): 5 fractions. |
1.5–2 cm | N/A | VMAT | N/A | N/A |
| Amendola et al. 2018 | 18 Gy | 3 Gy | 1 | 58 Gy (2 Gy/die) |
1.5 cm | 2 cm | VMAT | N/A | N/A |
| Amendola et al. 2019 | 18 Gy | 9 Gy | 3 | 45–58 Gy (1.8–2 Gy/die) |
0.8–1.2 cm | Average 3.6 cm | VMAT | 6 MV | It was decided arbitrary according to the size and the location of the tumor with respet to the OARs |
| Amendola et al. 2020 | 24 Gy | 9 Gy | 3 | 39.60–45.00 Gy (1.8–2 Gy/die) |
1.0–1.5 cm | N/A | VMAT | N/A | It was decided arbitrary according to the size and the location of the tumor with respet to the OARs, in a range between 2 and 11. |
| Jiang et al. 2021 | 20 Gy | 5 Gy | 1 | No | 1.0 cm | 2.0 cm | CyberKnife | 6 MV | N/A |
| Duriseti et al. 2021 | 66.7 Gy | 20 Gy | 5 | No | 1.5 cm | 6 cm | VMAT | N/A | It was based on a 3 × 3 × 3 cm grid guide. In axial planes where vertices are placed are separated by 3 cm in plane. Within a plan, vertices are separated by 6 cm center to center (4.5 cm edge to edge) in orthogonal axes, and 3√2 cm along the diagonal |
| Iori et al. 2022 | 55 Gy | 20 Gy | 5 | No | 1.5 cm | 6 cm | VMAT | 6 MV FFF |
The vertices were separated by 3 cm in the axial plane. The center to center vertex distance was of 6 cm (4.5 cm edge to edge) in orthogonal axes, and of 3√2 cm along the diagonal axes (spatial distance). The best disposition was decided by a homemade script that calculated the best number of positionable vertices and they spatial geometry according to target dimension and OARs proximity |
| Dicer et al. 2022 |
50 Gy | 30 Gy | 5 | No | N/A | N/A | MRI Linac | 6 MV FFF | It was decided arbitrary according to the size and the location of the tumor with respet to the OARs |
| Borzov et al. 2022 | 20 Gy | 8 Gy | 1 | 50 Gy (2 Gy/die) |
1.0 cm | N/A | VMAT | N/A |
It is reported that the V location was determined by a senior radiation oncologist and a senior physicist in collaboration with a senior radiologist and an orthopedic surgeon considering target dimension and OARs proximity |
| Ferini et al. 2022 | 15 Gy | * | 1 | 30 Gy (3 Gy × 10 fx) |
1.0 cm | 2 cm | VMAT | N/A | Placed arbitrary in the target (GTV) by RO with the maintaining of 2 cm center to center separation.) |
| Schiff JP et al. 2022 | 66.7 Gy | 20 Gy | 5 | No | 1.5 cm | 4 cm | VMAT | N/A |
Based on hotspots with diameter of r 1.5 cm and a 4 cm distance from center to center inside the target. The hotspots position allowed the creation of the higher number possible of hotspots in the target lesion. There was a longitudinal distance of 3 cm between located vertices. |
| Ferini et al. 2022 | 15 Gy* | * | 1–3 | 18 Gy/3 fx: 1 pz 20 Gy/4 fx: 17 pz 22.4 Gy/4 fx: 1 pz 30 Gy/3 fx: 10 pz 30 Gy/5 fx: 1 pz 40.5 Gy/15 fx: 1 pz |
N/A | 2 cm | VMAT | N/A | Chose arbitrary by RO according to tumor mass and near OAR with a minimum distance of 2 cm center to center |
Note: * The dose prescription was made on the vertices; *It is a median value with a range between 10 and 27 Gy in 1/3 fractions). References in chronological order of appearance: Blanco Suarez et al.2018 [17]; Amendola et al. 2018 [18]; Amendola et al. 2019 [10]; Amendola et al. 2020 [11]; Duriseti et al. 2021 [11]; Jiang et al. 2021 [6]; Iori et al. [16]; Dicer et al 2022 [15]; Borzov et al. 2022 [12]; Ferini et al 2022 [19]; Schiff JP et al. 2022 [15]; Ferini et al. [14].
3.4. Clinical outcomes and acute toxicity
Eligible article reports different radiological or metabolic follow up schedule (See Table 4). In the majority of reports the follow up is made with clinical visits and radiological imaging to assess tumor response according to RECIST criteria. For few patients it is also reported the metabolic response evaluated with positron emission tomography (PET)/CT according to PERCIST criteria. Apart from Borzov’s paper, where 2 pathological complete responses (CR) and 1 partial response (PR) were highlighted by the pathologist after the surgery, it is interesting to underline how LRT contributed to reach 13 CR with no residual tumor and 45 PR with a tumor reduction ranging between 41 % and 96 %. Albeit these interesting results, in several studies a concurrent or sequential systemic therapy or immunotherapy was administered [6], [17], [18], [11], [16], [19]. Thus, there is a risk of intervention bias that affects the data on lesions regressions. In addition, it is important to underline that the follow up schedules to assess target lesion response, vary widely across the eligible reports. Noteworthy that all symptomatic patients experiment a significant symptoms relief after LRT. With reference to toxicity, we decided to adopt the CTCAEv5 scale as it is widely used across the eligible articles. LRT toxicity depends on the lesions location and on its proximity to the OARs. As different follow up time are reported, we decided to focus on acute toxicity in this review. The study of Duriseti et al. is the most accurate and precise relating to treatment toxicity [13]. According to its data, one patient experimented G4 toxicity, however, its relation with LRT was questionable as it was mainly provoked by a sepsis condition despite the LRT on an abdominal lesion [13]. It is of interest the fatal case reported by Schiff JP et al. In their paper it is illustrated the death of an old and frail patient affected by a large endometrial cancer due to a tumor lysis syndrome probably caused by the dramatic lattice induced tumor necrosis [15]. With reference to the other papers, no other acute toxicity ≥G3 was reported. LRT was associated with a transient G1 asthenia, while the treatments of thorax lesion were associated with G1 esophagitis and pneumonia. Conversely, LRT on abdominopelvic lesions was associated with G1 diarrhoea [10], [16]. With reference to large lesions of the head and neck district, LRT was associated with G2 mucositis and G1 dysphagia [14]. Mainly, it worth highlighting that several patients were reported to have experimented no toxicity despite the elevate dose administered (See Table 4).
Table 4.
On left, the table illustrates the data on tumor response to LRT according to the different follow up schedule adopted in the eligible articles. We reported when the follow up CT was performed and how many patients were reassessed. In addition, we reported the median tumor regression of PR. Conversely, on the right it is shown the acute toxicity associated with LRT, the number of patients that experimented it, and the type of toxicity. Abbreviations: N/A (not available); PR (partial response); CR (complete response); PD (progressive disease); pz (patients).
| Tumor response (Recists Criteria) |
Median PR tumor reduction (Range) |
Acute Toxicity (CTCAE) |
|||||
|---|---|---|---|---|---|---|---|
| Time after LRT | Response | N pz | Grade | N pz | Type | ||
| Blanco Suarez et al. 2015 | 13 months | PR | 1 | 71 % | G1 G2 G3 G4 |
0 0 0 0 |
/ |
| Amendola et al. 2018 | 6 years | CR | 1 | / | G1 G2 G3 G4 |
0 0 0 0 |
/ |
| Amendola et al. 2019 | 6 month | PR PD |
9 1 |
48 % (15–83 %) |
G1 G2 G3 G4 |
1 0 0 0 |
G1: pneumonitis |
| Amendola et al. 2020 | 4 months | CR PR Death |
5 4 1 |
55 % (41 – 76 %) |
G1 G2 G3 G4 |
1 1 0 0 |
G1: diarrhea (1 pz)G2: cystitis (1 pz) |
| Jiang et al. 2021 | 5 month | CR | 1 | / | G1 G2 G3 G4 |
0 0 0 0 |
/ |
| Duriseti et al. 2021 | 4.5 months | PR PD Death |
10 1 9 |
47.4 % (5.3–96.1 %) |
G1 G2 G3 G4 |
N/A 1 0 1 |
G2: radiation pneumonitis (1 pz)G4: genitourinary and gastointestina (1 pz) (only 8 patients completed 90 days follow up) |
| Iori et al. 2022 | 6 month | PR | 1 | 75 % | G1 G2 G3 G4 |
1 0 0 0 |
G1: asthenia, esophagitis |
| Dicer et al. 2022 | 3 month | CR | 1 | / | G1 G2 G3 G4 |
0 0 0 0 |
/ |
| Dicer et al. 2022 | 1 month | PR | 1 | 54 % | G1 G2 G3 G4 |
0 0 0 0 |
/ |
| Borzov et al. 2022 | / | CR PR |
2 1 |
* | G1 G2 G3 G4 |
0 0 0 0 |
/ |
| Ferini et al. 2022 | 6 months | PR | 1 | 55 % | G1 G2 G3 G4 |
0 0 0 0 |
/ |
| Schiff JP et al. 2022 | ~ 3 weeks | ** | 1 | 28 % | G5 | 1 | The patient died |
| Ferini et al. 2022 | 10 months |
CR PR PD |
5 24 1 |
N/A | G1 G2 G3 G4 |
8 1 0 0 |
G1: dysphagia (2 pz); skin (5 pz); diarrhea (1 pz) G2: mucosisits (1 pz) ; |
Note:* Patients underwent surgery after LRT. It is reported that the pathologist highlighted two pathological CR and a PR. No data on lesions dimension immediately before surgery is available as well as the intercourse time; ** Despite a PR at approximately 3 weeks, the patient died due to a tumor lysis syndrome. References in chronological order of appearance: Blanco Suarez et al.2018 [17]; Amendola et al. 2018 [18]; Amendola et al. 2019 [10]; Amendola et al. 2020 [11]; Duriseti et al. 2021 [11]; Jiang et al. 2021 [6]; Iori et al. [16]; Dicer et al 2022 [15]; Borzov et al. 2022 [12]; Ferini et al 2022 [19]; Schiff JP et al. 2022 [15]; Ferini et al. [14].
4. Discussion
LRT is an innovative RT technique that allows delivering ablative doses to large lesions to achieve a significant and durable local response, without an increased toxicity [1]. Its peculiarity is the heterogeneous dose gradient generated in the target, which raises the total dose inside the tumor, keeping low the OARs parasitic dose. Two main LRT approaches are detectable in Literature. The first is an exclusive LRT treatment [6], [13], [16], [9], where five LRT fractions are administered every other day or consecutively. The second is a non-exclusive LRT that could be defined as a hybrid LRT, where a conventional or hypofractionated RT follows one to three upfront LRT fractions [9], [10], [11], [14], [17], [18], [19]. The idea of exclusive LRT is closer to SBRT, where ablative doses are delivered in few fractions to improve the TCP, while the concept of hybrid LRT is more similar to GRID RT [20]. In GRID RT, a sequential course of conventional RT usually starts on the day following the GRID RT administration, with the pro of an increase total dose delivered and the con of a longer treatment. It is worth underlying that no available data can support the superiority of one approach over the other, therefore, both strategies should warrant further investigations. Considering available reports, another interesting difference is the LRT array design. In some cases, the authors followed a geometrical architecture for vertices location whereas in others, the hotspots position was completely arbitrary. Again, no data are available about which strategy should be preferred; however, we believe that a geometrical array as the one used by Duriseti et al. may represent a better option to allow the array reproducibility, thereby increasing the study external validity [13]. In fact, a geometrical organization could entail a higher trials homogeneity; moreover, it may allow locating the optimal vertices-valleys alternation as well as the optimal number of vertices, using opportunely designed script. Another interesting strategy could be to guide vertices position according to a metabolic data. This idea of a metabolism guided LRT was investigated by Ferini et al. and it entails the hotspot location on the high 18F-FDG uptake areas, to administer higher doses to the more active neoplastic nice [14]. Their paper reports interesting results in terms of toxicity and local control with a hybrid metabolism guided LRT; however, the completely different sequential RT schedules administered may act as confounders, as they expose the results to the risk of measurements bias, and they increase the study population heterogeneity [14]. In addition, there might be a selection bias because the sequential RT schedule as well as the vertices’ location was decided according to the radiation oncologists’ preference case by case.
Notwithstanding these differences, a consensus is present around a vertex diameter between 1.0 cm and 1.5 cm, since it should avoid as much as possible a dose drop in the hotspots, despite organ physiological motion. By contrast, the median center-to-center distance was around 2 cm and 6 cm, for hybrid and exclusive LRT, respectively. Of note that the higher center-to-center distance in exclusive LRT entails a more pronounced dose gradient between vertices and valleys in comparison with a hybrid LRT. In all reports, the dose escalation allows to reach ablative dose in the hotspots, however, the gradient is more pronounced in exclusive LRT.
With reference to efficacy, LRT appears a valid option to increase large lesion response considering the impressive results reported by the different experiences in Literature. When a CR is not achieved, all papers show a very positive trend in terms of PR, with median reduction approximately ≥50 % after 3–6 months. However, the studies heterogeneity and their short follow up preclude drawing a clear conclusion or performing any quantitative analysis. In fact, the studies presents significant differences in terms of LRT schedules, follow up times of reassessment CT, and associated systemic therapies.
An interesting point it the combination of LRT with systemic therapies as chemotherapies, target therapies and immunotherapies [17], [18], [11]. Many systemic drugs act as radiosensitizers and they concurrent administration with LRT treatment could enhance tumor response. In these cases, it is difficult to evaluate whether tumor response was determined mainly by LRT properties or by the chemo-radio combination. This element increases works heterogeneity and influences the reported responses and toxicities. An analogous analysis is assessable around the combination with IT, since LRT might also have an important immunomodulatory activity, superior to conventional RT [6]. Vertices ablative doses can provoke a more efficient immunogenic cells death, boosting damage-associated molecular patterns (DAMPs) release. Concurrently, valleys low doses could preserve the residual blood flow and allow a more efficient DAMPs circulation as well as immune cells homing and activation. Thereby, LRT may increase tumor immunogenicity and strengthen the immune rejection of cancer cells both in irradiated and non-irradiated sites (abscopal effect). This hypothesis seems to find confirm in Jiang et al. report, where only the combination of LRT with an immune checkpoint blockade was able to achieve a CR [6]. However, it is unclear to what extent cytoreduction and immunomodulation affect lesion response respectively.
An interesting point is the data about tolerability. Only Schiff JP and colleagues reported a fatal case, however, it should not be ignored that the treated patients was probably not eligible to LRT due to the comorbidities and a poor ECOG PS [15]. In addition, the patient had a severe kidney failure that impaired the ion balance; consequently, it is reasonable to assume how the marked tumor necrosis caused by the LRT determined a lethal tumor lysis syndrome. Nonetheless, they reported how the patient well tolerated the treatment itself, as a result, it seems logical to hypothesize that the renal failure played a key role – the creatinine was 2.07 mg/dL before treatment administration-. Another relevant case is the one of the Patient 11 of Duriseti et colleagues [13]. This patient experimented a G4 urosepsis after the second LRT fraction on an abdominal leyomiosarcoma (3719.5 cc), however, the relation with LRT treatment was questionable and the authors defined the toxicity as “possibly associated with LRT” [13]. Excluding these two compromised patients, the other clinical experience appear to suggest that LRT is safe despite hotspots dose escalation, as no other no toxicity ≥G3 was registered. It is of note that most of the authors report a mild and limited toxicity, that is quite similar to the one associated with SBRT. This data is particularly interesting considering that most of the treatments had a palliative intent, many patients were compromised or symptomatic due to the advanced disease stage, and lesions dimension. Notwithstanding this, the fatal case cannot be overlooked as it suggests how a renal function assessment should probably precede LRT administration.
Albeit these impressive preliminary data in terms of efficacy and safety, the evidence reported by this systematic review present different limitations. With reference to efficacy, the quality of evidence about LRT efficacy is low as 7 eligible reports were case reports, the case series associated several completely different irradiation schedules – that act as confounders - after three upfront LRT fractions, and the available clinical studies present a high risk of bias or have a different primary endpoint. For instance, the work of Borzov et al. has a consistent risk of reporting bias since much information on patients follow up are lacking, while the study of Duriseti et al. has as primary endpoint the LRT short-term safety [12], [13]. The ongoing phase II clinical trial (NCT04553471) will provide more reliable data as it specifically design to evaluate the efficacy of exclusive LRT. With reference to the LRT safety data are more solid as the LITE SABR M1 provide positive evidence in terms of acute toxicity, that are consistent with the other clinical experiences we could find in Literature. A similar encouraging data is also reported in the Lattice_01 trial, where no G3 toxicity was reported despite the different treatment schedule [13], [14].
5. Conclusion
This systematic review represents a synthesis of the available data and a basis for further analysis. Considering the rising interest around LRT potentialities, different strategies to deliver a LRT treatment can be adopted. These LRT approach could be defined as exclusive LRT, hybrid LRT, and metabolism guided hybrid LRT. Current data do not allow preferring one approach to the others, and all deserve further investigation. Despite the promising preliminary results in term of efficacy, the current level of evidence is low, and further studies are mandatory to have robust evidence of LRT efficacy. Conversely, available data seems to confirm LRT safety, especially for exclusive LRT, providing that a correct patient selection is performed. Considering the LRT potentialities, it is possible to forecast important repercussions in the clinical practice, with a more widespread use of LRT in the management of large lesion, moreover, LRT immunomodulatory properties might be integrated in the future as a key component in multimodal treatments with immunotherapy to managed both the localized and the widespread disease.
Statements & declarations
Funding: This study was partially supported by Italian Ministry of Health – Ricerca Corrente Annual Program 2023.
Ethics approval: This is a systematic review, and no ethical approval is required.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to express our gratitude to Maria Chiara Bassi and Simone Cocchi (Biblioteca Medica Pietro Giuseppe Corradini), who helped us with the bibliographic research of this project.
References
- 1.Wu X., Perez N.C., Zheng Y., Li X., Jiang L., Amendola B.E., et al. The technical and clinical implementation of LATTICE radiation therapy (LRT) Radiat Res. 2020;194(6):737–746. doi: 10.1667/RADE-20-00066.1. PMID: 33064814. [DOI] [PubMed] [Google Scholar]
- 2.Duriseti S., Kavanaugh J., Goddu S., Price A., Knutson N., Reynoso F., et al. Spatially fractionated stereotactic body radiation therapy (Lattice) for large tumors. Adv Radiat Oncol. 2021;6(3) doi: 10.1016/j.adro.2020.100639. PMID: 34195486; PMCID: PMC8233471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferini G., Valenti V., Tripoli A., Illari S.I., Molino L., Parisi S., et al. Lattice or oxygen-guided radiotherapy: what if they converge? possible future directions in the era of immunotherapy. Cancers (Basel) 2021;13(13):3290. doi: 10.3390/cancers13133290. PMID: 34209192; PMCID: PMC8268715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allibhai Z., Taremi M., Bezjak A., Brade A., Hope A.J., Sun A., et al. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;87(5):1064–1070. doi: 10.1016/j.ijrobp.2013.08.020. Epub 2013 Oct 24 PMID: 24210082. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Zhao L, Li XF. Hypoxia and the tumor microenvironment. Technol Cancer Res Treat. 2021;20:15330338211036304. doi: 10.1177/15330338211036304. PMID: 34350796; PMCID: PMC8358492. [DOI] [PMC free article] [PubMed]
- 6.Jiang L., Li X., Zhang J., Li W., Dong F., Chen C., et al. Combined high-dose LATTICE radiation therapy and immune checkpoint blockade for advanced bulky tumors: the concept and a case report. Front Oncol. 2021;12(10) doi: 10.3389/fonc.2020.548132. PMID: 33643893; PMCID: PMC7907519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cytlak U.M., Dyer D.P., Honeychurch J., Williams K.J., Travis M.A., Illidge T.M. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat Rev Immunol. 2022;22(2):124–138. doi: 10.1038/s41577-021-00568-1. Epub 2021 Jul 1 PMID: 34211187. [DOI] [PubMed] [Google Scholar]
- 8.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29(372) doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dincer N., Ugurluer G., Korkmaz L., Serkizyan A., Atalar B., Gungor G., et al. Magnetic resonance imaging-guided online adaptive lattice stereotactic body radiotherapy in voluminous liver metastasis: two case reports. Cureus. 2022;14(4):e23980. doi: 10.7759/cureus.23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amendola B.E., Perez N.C., Wu X., Amendola M.A., Qureshi I.Z. Safety and efficacy of lattice radiotherapy in voluminous non-small cell lung cancer. Cureus. 2019;11(3):e4263. doi: 10.7759/cureus.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amendola B.E., Perez N.C., Mayr N.A., Wu X., Amendola M. Spatially fractionated radiation therapy using lattice radiation in far-advanced bulky cervical cancer: a clinical and molecular imaging and outcome study. Radiat Res. 2020;194(6):724–736. doi: 10.1667/RADE-20-00038.1. PMID: 32853384. [DOI] [PubMed] [Google Scholar]
- 12.Borzov E., Bar-Deroma R., Lutsyk M. Physical aspects of a spatially fractionated radiotherapy technique for large soft tissue sarcomas. Phys Imaging Radiat Oncol. 2022;4(22):63–66. doi: 10.1016/j.phro.2022.04.010. PMID: 35572042; PMCID: PMC9092247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duriseti S., Kavanaugh J.A., Szymanski J., Huang Y., Basarabescu F., Chaudhuri A., et al. LITE SABR M1: a phase I trial of Lattice stereotactic body radiotherapy for large tumors. Radiother Oncol. 2022;167:317–322. doi: 10.1016/j.radonc.2021.11.023. Epub 2021 Dec 4 PMID: 34875286. [DOI] [PubMed] [Google Scholar]
- 14.Ferini G., Parisi S., Lillo S., Viola A., Minutoli F., Critelli P., et al. Impressive results after “metabolism-guided” lattice irradiation in patients submitted to palliative radiation therapy: preliminary results of LATTICE_01 Multicenter Study. Cancers. 2022;14:3909. doi: 10.3390/cancers14163909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiff J.P., Spraker M.B., Duriseti S., Shaikh S., Murad H.F., Mutch D.G., et al. Tumor lysis syndrome in a patient with metastatic endometrial cancer treated with lattice stereotactic body radiation therapy. Adv Radiat Oncol. 2021;7(1) doi: 10.1016/j.adro.2021.100797. PMID: 34761139; PMCID: PMC8567179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iori F., Botti A., Ciammella P., Cozzi S., Orlandi M., Iori M., et al. How a very large sarcomatoid lung cancer was efficiently managed with lattice radiation therapy: a case report. Ann. Palliat Med. 2022;11(11):3555–3561. doi: 10.21037/apm-22-246. [DOI] [PubMed] [Google Scholar]
- 17.Blanco Suarez J.M., Amendola B.E., Perez N., Amendola M., Wu X. The use of lattice radiation therapy (LRT) in the treatment of bulky tumors: a case report of a large metastatic mixed mullerian ovarian tumor. Cureus. 2015;7(11):e389. doi: 10.7759/cureus.389. PMID: 26719832; PMCID: PMC4689595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amendola B.E., Perez N.C., Wu X., Blanco Suarez J.M., Lu J.J., Amendola M. Improved outcome of treating locally advanced lung cancer with the use of Lattice Radiotherapy (LRT): A case report. Clin Transl Radiat Oncol. 2018;12(9):68–71. doi: 10.1016/j.ctro.2018.01.003. PMID: 29594253; PMCID: PMC5862683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferini G., Castorina P., Valenti V., Illari S.I., Sachpazidis I., Castorina L., et al. A novel radiotherapeutic approach to treat bulky metastases even from cutaneous squamous cell carcinoma: its rationale and a look at the reliability of the linear-quadratic model to explain its radiobiological effects. Front Oncol. 2022;23(12) doi: 10.3389/fonc.2022.809279. PMID: 35280772; PMCID: PMC8904747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer J., Eley J., Schmid T.E., Combs S.E., Dendale R., Prezado Y. Spatially fractionated proton minibeams. Br J Radiol. 2019;92(1095) doi: 10.1259/bjr.20180466. 20180466, Epub 2018 Nov 7. PMID: 30359081; PMCID: PMC6541186. [DOI] [PMC free article] [PubMed] [Google Scholar]


