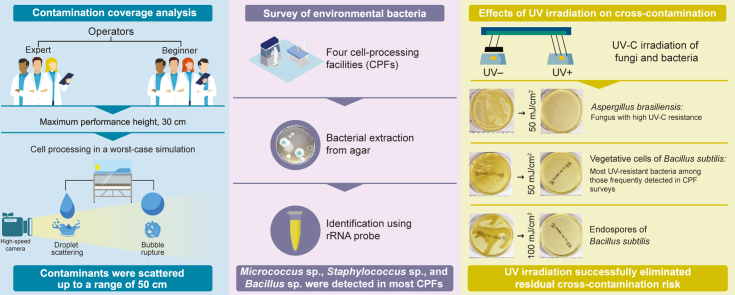
Abstract
Introduction
During changeover in cell-product processing, it is essential to minimize cross-contamination risks. These risks differ depending on the patient from whom the cells were derived. Human error during manual cell-product processing increases the contamination risk in biosafety cabinets. Here, we evaluate the risk of cross-contamination during manual cell-processing to develop an evidence-based changeover method for biosafety cabinets.
Methods
Contaminant coverage was analyzed during simulated medium preparation, cell seeding, and waste liquid decanting by seven operators, classified by skill. Environmental bacteria were surveyed at four participating facilities. Finally, we assessed the effect of conventional UV irradiation in biosafety cabinets on bacteria and fungi that pose a cross-contamination risk.
Results
Under simulated conditions, scattered contamination occurred via droplets falling onto the surface from heights of 30 cm, and from bubbles rupturing at this height. Visible traces of contaminants were distributed up to 50 cm from the point of droplet impact, or from the location of the pipette tip when the bubble ruptured. In several facilities, we detected Bacillus subtilis, of which the associated endospores are highly resistant to disinfection. Irradiation at 50 mJ/cm2 effectively eliminated Bacillus subtilis vegetative cells and Aspergillus brasiliensis, which is highly resistant to UV. Bacillus subtilis endospores were eliminated at 100 mJ/cm2.
Conclusions
Under these simulated optimal conditions, UV irradiation successfully prevents cross-contamination. Therefore, following cell-product processing, monitoring the UV dose in the biosafety cabinet during cell changeover represents a promising method for reducing cross-contamination.
Keywords: Changeover, Cross-contamination, UV irradiation, Biosafety cabinet, Cell-product processing
Graphical abstract
Highlights
-
•
Cross-contamination during changeover poses risks in cell processing.
-
•
We evaluated simulated contamination risk in tasks performed by seven operators.
-
•
Contamination from falling droplets and ruptured bubbles was widely scattered.
-
•
Several environmental bacteria were detected at the four participating facilities.
-
•
UV irradiation decontaminates Aspergills brasiliensis and Bacillus subtilis.
1. Introduction
Cell-processing facilities are known to harbor risks, including environmental bacteria [1], which may contaminate biosafety cabinets during processing [2]. During autologous cell-product processing, cross-contamination can occur during changeover. In particular, the use of high-viscosity fetal bovine or human serum as culture media is often associated with droplet scattering and bubble rupture. The droplets generated by such processes may remain on the walls and floor of the aseptic operation area and contaminate the products in subsequent processes. However, no analysis has been conducted to determine the risk of contamination due to these human manual operations, nor the extent to which contamination from droplets and bursting bubbles becomes scattered. During changeover, it is necessary to determine whether the risk is acceptable or unacceptable. For example, human tissues, such as digestive epithelial tissues or blood, may be contaminated with bacteria or fungi [3,4], which may remain as microparticles after cell-product processing, posing an ongoing contamination risk. Thus, the risks posed by cross-contamination differ depending on the patient from whom the cells are derived, and on the type of processing task. Given that human tissue-derived materials and cell products cannot be sterilized, the risk of cross-contamination during cell-processing must be minimized. Biosafety cabinet decontamination can minimize these risks.
Currently, each cell-processing facility must establish its own changeover methods, as no international guidelines exist for changeover. The most promising changeover decontamination methods, as alternatives to formaldehyde, include fogging with hydrogen peroxide or peracetic acid. However, fogging after each processing task is labor-intensive and requires residual gas measurement after each application. Furthermore, residual hydrogen peroxide adversely affects cell proliferation [5]. Hence, decontamination by disinfectant fogging is not ideal. Biosafety cabinet decontamination is generally achieved using UV irradiation, the most common technique for surface sterilization [6], and is confirmed by sampling environmental bacteria after processing each product. UV irradiation eliminates microorganisms by causing the formation of dimers in nucleic acids [7,8]. However, the effectiveness of UV during cell-processing changeover has not been previously reported, and no standard protocols are available. Furthermore, although the times required for cell-processing tasks in human-cell cultures have been reported [9], the contamination risks have not been adequately examined.
To evaluate the cross-contamination risk, we first analyzed contamination scatter by task type, under worst-case simulated conditions. Second, we surveyed environmental bacteria in several participating cell-processing facilities to identify frequently detected bacteria and to select the target bacteria for decontamination experiments. Finally, we evaluated the effects of UV irradiation on the frequently detected and UV-resistant bacteria. Based on these results, we propose an evidence-based changeover method for biosafety cabinets.
2. Materials and methods
2.1. Cell-processing analytical procedures
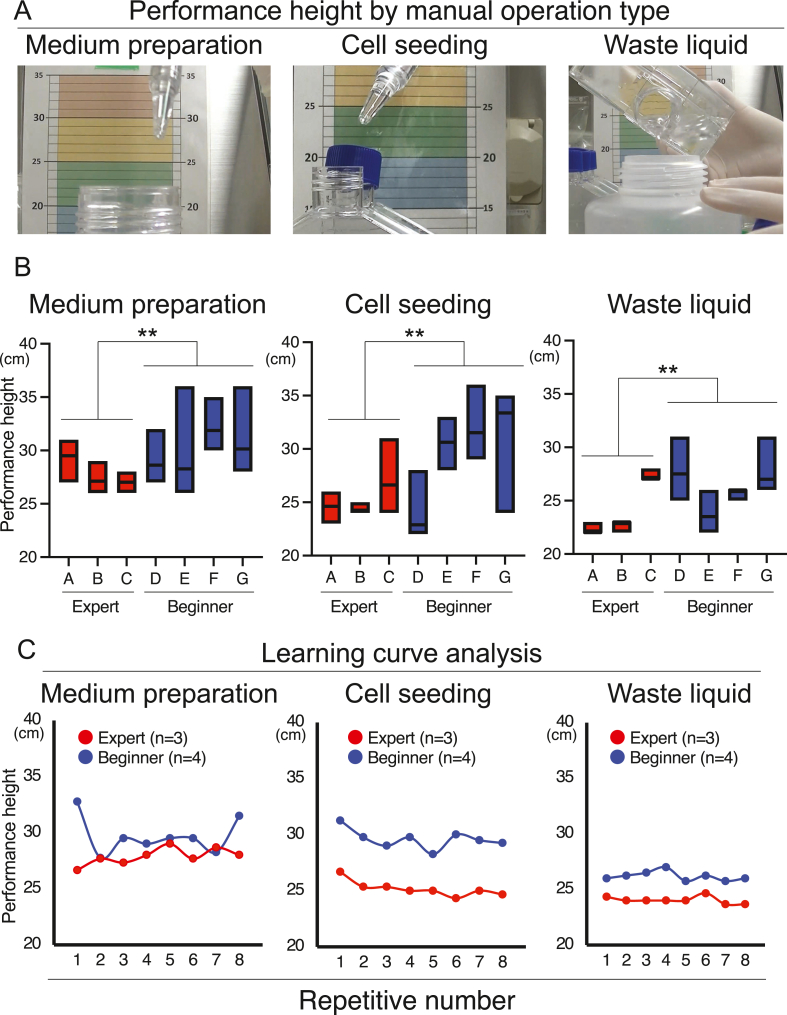
Seven operators, classified as expert or beginner based on their in-house qualifications (Table 1) participated in the study. Biosafety cabinets with downflow air (MHE-130AJ and MHE-131AJ: PHC Corporation, Tokyo, Japan), fitted with a camera, were used. We simulated the actual operations performed in mass culture (medium preparation, cell seeding, and decanting of waste liquid using flasks; Fig. 1A), repeated eight times by each operator. For medium preparation, the operators transferred the maximum volume of basic medium into a 1000 mL bottle (height: 220 mm, Corning Inc., NY, USA), using a 50 mL pipette (Sumitomo Bakelite Co., Ltd., Tokyo, Japan). For cell seeding, all of the medium in the 1000 mL bottle was then extracted using a 50 mL pipette and seeded into eight culture flasks (height: 190 mm, Thermo Fisher Scientific Inc., MA, USA). For this step, the pipettes were used repeatedly, to imitate actual mass culture production, under the assumption that the same patient's cells were being handled. The culture medium was then decanted from the eight culture flasks into a waste liquid bottle (height: 195 mm, Sanplatec Co., Osaka, Japan). The height above the floor of the biosafety cabinet at which each task was performed (“performance height”) was measured; the maximum performance height reached during each operation was recorded as the height for that task.
Table 1.
Adherent-cell culture experience within each group.
| Category | Years of experience | In-house qualification |
|---|---|---|
| Expert | 6.5 | + |
| 4 | + | |
| 5 | + | |
| Beginner | 3 | – |
| 0 | – | |
| 0 | – | |
| 0 | – |
Fig. 1.
Contamination during cell processing. (A) Performance height by processing task. (B) Data are presented as mean and range. ∗∗p < 0.01 (Welch's t-test). (C) Learning-curve analysis. The data are the means for three experts (in red) and four beginners (in blue), based on eight repetitions of the tasks.
2.2. High-speed camera
Droplet movement and bubble rupture inside the biosafety cabinets were monitored using a Fastcam Mini AX camera (Photron Limited, Tokyo, Japan), at 2000 frames per second. For image highlighting we used Photron FASTCAM Viewer 4 (Photron).
2.3. Contamination coverage analysis
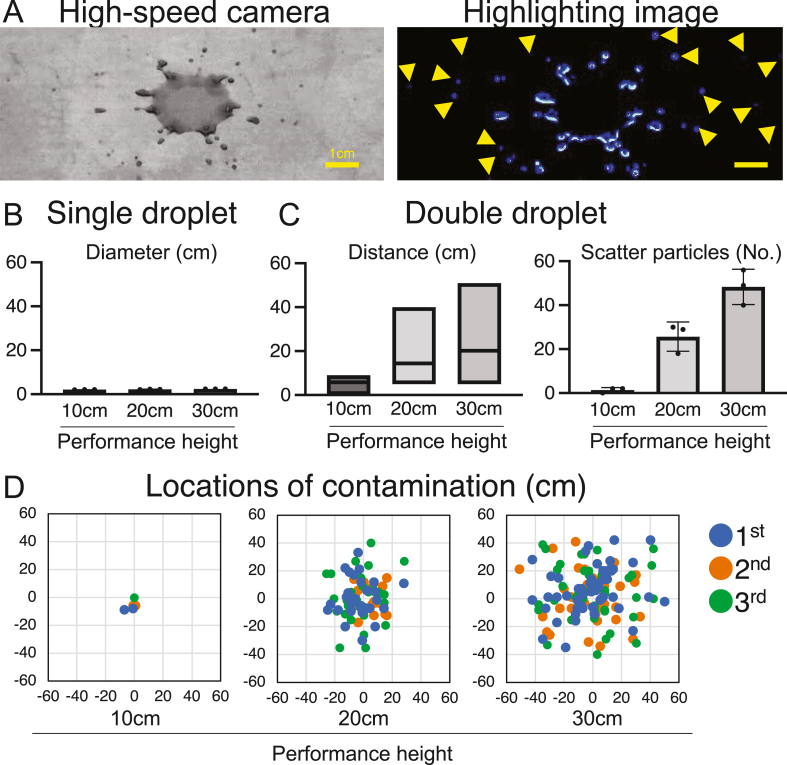
To measure contamination coverage arising from droplet scattering or bubble-rupture, we used DMEM containing 5 × phenol red (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) and 20% fetal bovine serum (Thermo Fisher Scientific). Using a 50 mL pipette, this culture medium was dropped onto a SUS304 special-use stainless steel plate (5 cm × 5 cm; AS ONE Co., Osaka, Japan), from heights of 10, 20, or 30 cm, over a sheet of graph paper placed on the floor of the biosafety cabinet. The biosafety cabinet floor was manufactured from SUS304 stainless steel. The contamination-scatter on the graph paper was then photographed, and the distances of particle scatter from the landing point were quantified. The SUS304 plate, which we used to prevent inhibition of scattering, was placed at the center of the graph paper. Droplet volume was measured separately from the experiment using a Practum612-1S balance (Sartorius AG, Göttingen, Germany). The size of the bubble before rupturing was quantified using ImageJ (National Institutes of Health, MD, USA). The scatter patterns were independently analyzed three times.
2.4. Environmental bacteria survey
At four anonymized and cooperating cell-processing facilities, we conducted environmental monitoring using agar to sample bacteria. Bacteria were present in each area of the facilities. The definition of a clean area was similar to that used in our previous studies [1]. “General environment” refers to the uncontrolled area. The controlled areas (grades C and D) were defined according to the cleanliness definitions in the Consideration on Aseptic Operation in Cell Culture Processing Facilities protocol, based on the Japanese Society for Regenerative Medicine Safety Act. Considering that environmental monitoring is not typically conducted in uncontrolled areas, we requested that testing be performed in a small number of uncontrolled locations. Within the controlled areas, environmental monitoring was conducted in accordance with each facility's standard practices. The bacteria extracted from the agar were identified using a ribosomal RNA-specific primer and probe set, as previously reported [1]. Species suggested by BLAST analysis, with an identification rate >99%, were considered candidate species.
2.5. UV irradiation
Each biosafety cabinet was equipped with a 15 W UV-C germicidal lamp (Sankyo Denki Co., Kanagawa, Japan). The irradiation dose was measured using a UV intensity meter (UVC-254SD; SATOTECH, Kanagawa, Japan) at 254 nm (range: 220–280 nm).
2.6. Bacteria and fungi
Using a micropipette, 100 μL drops of the bacterial and fungal dilutions were added to SUS304 plates (5 cm × 5 cm), which were subsequently irradiated with UV. Bacteria that are highly resistant to UV radiation, as well as those frequently detected in the cell-processing facilities, were used. Vegetative cells of Bacillus subtilis (NBRC 3134; National Institute of Technology and Evaluation [NBRC], Chiba, Japan) were diluted to 1 × 106 CFU in physiological saline (Otsuka Pharmaceutical Co. Ltd., Tokushima, Japan). Bacillus subtilis endospores (1 × 108 CFU, NBRC 13722, Bioball Multishot 10E8; bioMérieux, Marcy l’Etoile, France) dissolved in rehydration fluid (bioMérieux) were diluted to 1 × 106 CFU with physiological saline. After irradiation, bacteria were seeded in soybean-casein digest agar medium (Nissui-seiyaku Ltd., Tokyo, Japan) and incubated at 37 °C. Bacterial seeding with or without UV irradiation was triplicated independently.
The fungus Aspergillus brasiliensis (NCPF 2275) was obtained from Bioball Multishot 10E8 (1 × 108 CFU; bioMérieux) dissolved in rehydration fluid, and subsequently diluted to 1 × 106 CFU with physiological saline. After irradiation, the fungi were seeded on Sabouraud agar medium (Nissui-seiyaku) and incubated at 30 °C. Fungus seeding with or without UV irradiation was triplicated independently.
2.7. Statistical analysis
GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA) was employed for all statistical analyses. Results are presented as the mean ± SD, or with a range. Two-group analysis was performed using Welch's t-test, and correlation analysis was performed using Pearson's correlation. P < 0.05 was considered significant (two-tailed tests).
3. Results
3.1. Contamination from manual processing
To simulate worst-case scenarios for manual operation, we focused on performance height. Following eight replicates of the three simulated mass-culture operations (medium preparation, cell seeding, and waste liquid decanting), the maximum performance heights were evaluated for the seven operators (three experts and four beginners).
The maximum performance heights for each task were significantly lower for experts (27.9 ± 1.5 cm) than beginners (29.7 ± 2.8 cm) for medium preparation (CoVs: 5.5% and 9.5%), as well as for cell seeding (25.2 ± 1.7 cm and 29.6 ± 4.8 cm; CoVs: 6.8% and 16.2%, respectively), and waste liquid decanting (24.0 ± 2.4 cm and 26.2 ± 2.2 cm; CoVs: 9.8% and 8.4%, respectively; Fig. 1B). Learning-curve analysis revealed that variability was particularly high among beginners (Fig. 1C). Based on these results, we defined 30 cm as the maximum worst-case scenario performance height.
3.2. Contamination area from single droplets and ruptured bubbles
We next examined the droplet-scattering contamination risk, focusing on droplets and bubble rupture.
Based on high-speed photography and image-highlighting, minute droplets were scattered when second droplets landed from a height of 20 cm (Fig. 2A; Supplemental Videos 1 and 2). Droplet volume, measured separately from the experiment, was 83.3 ± 15.1 μL. Single droplets contaminated a limited area, and did not scatter on landing (Fig. 2B). Double droplets falling from performance heights of 10, 20, and 30 cm scattered particles to distances of 5.0 ± 4.6 cm, 30 ± 11.8 cm, and 47.7 ± 4.9 cm (Fig. 2C), and generated 1.3 ± 1.2, 25.7 ± 6.7, and 48 ± 8.0 particles, respectively. These findings indicate that the scattering area and number of scattered particles increased with performance height (Fig. 2C and D).
Fig. 2.
Contamination from falling droplets. (A) Particle scattering by double droplets: high-speed photography (left) and highlighted image (right). Scale bar, 1 cm. Yellow arrowheads indicate the scattered particles. (B) Contamination from a single droplet. (C) Contamination from a double droplet. Distance from the point of impact is shown as the mean and range (left). The number of scattered particles is shown by mean ± SD (right). (D) Locations of contamination from double droplets. The scatter plots were evaluated independently three times.
Supplementary video related to this article can be found at https://doi:10.1016/j.reth.2022.12.003.
The following is/are the supplementary data related to this article:
High-speed camera capture of falling droplets.1
Video of falling droplets.
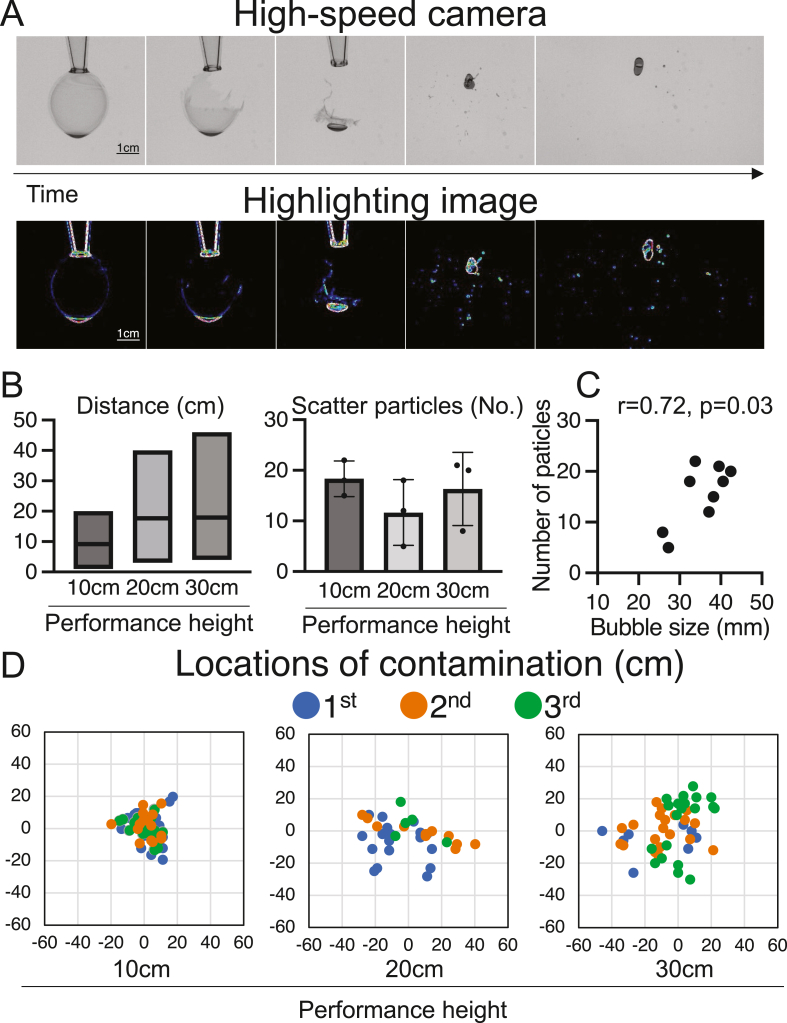
Bubble rupture generated multiple scattered water particles (Fig. 3A, Supplemental Videos 3 and 4), with scatter distances of 9.1 ± 4.7 cm, 17.7 ± 9.0 cm, and 17.9 ± 9.1 cm for performance heights of 10, 20, and 30 cm (Fig. 3B), generating 18.3 ± 3.5, 11.7 ± 6.5, and 16.3 ± 7.2 particles, respectively. The mean size ±SD of the bubble before rupturing was 35.3 ± 5.8 mm. The mean number of scattered particles and the mean bubble size prior to rupture were correlated (Fig. 3C). Substantial scattering of particles from higher performance heights was observed, although the number of particles generated did not vary with performance height (Fig. 3D). These results indicate that scattering can cause contamination at distances up to 50 cm. Scattered contamination may also involve environmental bacteria.
Fig. 3.
Contamination following bubble rupture. (A) Bubble rupture: high-speed photography (top), and highlighted image (bottom). Scale bar, 1 cm. (B) Contamination following bubble rupture. Distance from the bubble rupture location is indicated as the mean and range (left). The number of scattered water particles is shown as mean ± SD (right). (C) Pearson's correlation between bubble size and the number of scattered particles. (D) Contamination scatter following bubble rupture. The scatter plots were evaluated independently three times.
Supplementary video related to this article can be found at https://doi:10.1016/j.reth.2022.12.003
The following are the supplementary data related to this article:
High-speed camera capture of bubble rupture.
Video of bubble rupture.
3.3. Environmental bacteria survey
To identify bacteria that are highly resistant to decontamination, we surveyed the environmental bacteria in the cell-processing facilities: Micrococcus sp., Staphylococcus sp., and Bacillus sp. were detected in the controlled areas at most of the facilities (Table 2). The endospore-forming Bacillus sp., which is highly resistant to decontamination, was selected as the candidate species.
Table 2.
Cell-processing facility environmental bacteria survey.
| Facility | General environmental bacteria | Grade D | Grade C |
|---|---|---|---|
| A | Micrococcus sp. |
Cutibacterium sp. Staphylococcus sp. Micrococcus sp. |
Kocuria sp. Bacillus sp. Micrococcus sp. |
| B | Staphylococcus sp. |
Kyotococcus sp. Cutibacterium sp. Staphylococcus sp. |
Micrococcus sp. |
| C | No examinations |
Bacillus sp. Corynebacterium sp. |
Micrococcus sp. Staphylococcus sp. |
| D |
Bacillus sp. Micrococcus sp. |
Staphylococcus sp. Bacillus sp. |
Kocuria sp. Staphylococcus sp. Lysinibacillus sp. Roseomonas sp. |
Grades were defined according to cleanliness definitions in the Consideration on Aseptic Operation in Cell Culture Processing Facilities protocol based on the Safety Act of the Japanese Society for Regenerative Medicine.
3.4. Decontamination effects of UV irradiation
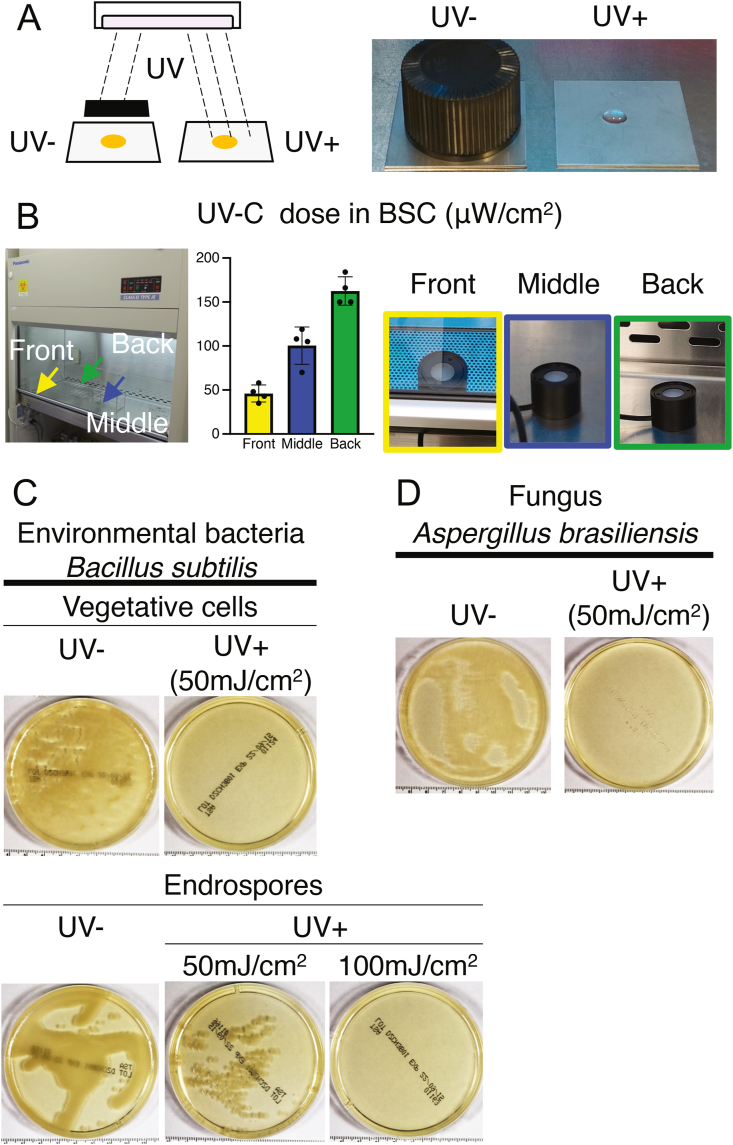
Assuming that environmental bacteria are present when there is the residual scattered contamination in biosafety cabinets contains environmental bacteria, we analyzed the effects of UV irradiation on contamination (Fig. 4A). We evaluated UV decontamination of the vegetative cells and endospores of Bacillus subtilis and of Aspergillus brasiliensis, which are highly UV-resistant.
Fig. 4.
Effects of UV irradiation on contamination risk. (A) Schema illustrating UV irradiation within biosafety cabinets. (B) UV-C dose in biosafety cabinets (μW/cm2). Means ± SD for four different biosafety cabinets, with UV lamps replaced within the preceding two years. (C) Effects of UV irradiation on vegetative cells and endospores of Bacillus subtilis, a frequently detected environmental bacterium. (D) Effects of UV irradiation on Aspergillus brasiliensis, a fungus that is highly resistant to UV irradiation. In both cases, samples were adjusted to 1 × 106 CFU per 100 μL of physiological saline.
The UV irradiation levels were 46 ± 9.6, 100.5 ± 21.2, and 162.5 ± 16.2 μW/cm2, at the front, middle, and back of the cabinets, respectively (Fig. 4B). The effects of this UV irradiation on Bacillus subtilis were examined: colony formation was not observed for vegetative cells at 50 mJ/cm2 or endospores at 100 mJ/cm2, whereas endospore colony formation was observed at 50 mJ/cm2 (Fig. 4C). For Aspergillus brasiliensis, no colony formation was observed at 50 mJ/cm2 (Fig. 4D). Therefore, under proper operations, UV irradiation successfully eliminated the risk of residual cross-contamination.
4. Discussion
Manual cell-product processing using culture media in biosafety cabinets carries the risk of contamination from droplet scattering and bubble rupture [10,11]. We estimated contamination scatter and evaluated the effects of UV irradiation on visible contamination, focusing on Bacillus subtilis, which was detected in several cell-processing facilities, and Aspergillus Brasiliensis, both highly resistant to UV. UV irradiation prevented colony formation, even by endospores, which are highly resistant to disinfection, suggesting that appropriate UV irradiation can avoid cross-contamination during changeover. Furthermore, our findings indicate that appropriate education and training in cell-processing can reduce both the height at which tasks are performed and the amount of variation in how they are performed, thereby reducing contamination.
Experiments evaluating human manual operations revealed that education and training improve performance height stability during cell culturing. Mean performance height was lower for the experts than beginners, although this does not necessarily mean that lower performance heights significantly reduce contamination risk. In fact, a low performance height may increase the contamination risk, for example, by increasing the incidence of bumping into the culture bottles. In cell processing, regardless of experience, the operator's focus should be on ensuring traceability for each action. For example, if droplets or bubble ruptures occur, it is important to note each one. To establish a safe processing environment for cell products, future studies should analyze the effects of movement speed, stability, and sway on manual operation cell contamination risks.
However, even with optimized education and training, human error is inevitable. To evaluate this risk, we analyzed contamination under simulated conditions. Double droplets falling from 30 cm scattered particles up to 50 cm from the point of impact. Although wiping could remove such contamination, it would be difficult during cell-processing to trace droplets scattered up to 50 cm. Therefore, a larger area should be wiped, assuming that particles are scattered up to 50 cm. When scattered contamination is visible, it can be readily cleaned. Nonetheless, it is difficult to guarantee decontamination by wiping, which is also not a reproducible method [[12], [13], [14], [15]]. Studies on wiping to remove anticancer-agent residues from biosafety cabinets have focused on the removal agents, such as isopropyl alcohol or sodium dodecyl sulfate [16,17]. Here, we assumed that wiping is ineffective and, therefore, evaluated the effectiveness of UV lamps, which are commonly fitted in biosafety cabinets.
In the participating cell-processing facilities, we detected environmental bacteria, including Bacillus, that are indigenous to the human epidermis [18], and that are commonly found in cleanrooms [1,19]. These may have been introduced from adjacent environments, possibly by operators. The endospores of Bacillus, a soil bacterium, are resistant to disinfection [20]. Bacillus contamination within a biosafety cabinet must therefore be appropriately and effectively decontaminated. We applied UV irradiation to eliminate Bacillus subtilis and Aspergillus brasiliensis, both of which are resistant to UV irradiation [21]. With 50–100 mJ/cm2 irradiation (equivalent to 10–20 min of irradiation in the middle of the biosafety cabinet), no bacterial or fungal colonies were formed, regardless of their high initial concentration of 1.0 × 106 CFU. In prior studies, UV irradiation after seeding on agar medium has not achieved significant decontamination [22,23]. Our findings indicate that cross-contamination during changeover can be avoided without the need to introduce additional equipment into the biosafety cabinet.
In Japan, new clinical trials of cell products are underway [24,25], cell products are being approved, and there are increasing expectations for new medical technology [26,27]. Of the 16 cell products approved in Japan up to November 2022, 14 are autologous tissue- or cell-derived products. However, a maximum of 200 units of autologous tissue- or cell-derived products are sold each year in Japan, based on the market size; hence, mechanization-based automation is not yet feasible from a cost perspective.
UV irradiation must reach the target to have a decontaminating effect. Although UV light has a strong decontamination effect, it has low effective depth and does not effectively pass through opaque materials: for instance, little UV-C light penetrates through the filter-tip cases left in biosafety cabinets (data not shown). Taking advantage of these characteristics, UV irradiation is used to inactivate viruses in plasma [28,29]. In biosafety cabinets, the presence of high protein content on surfaces or layers of dry residue left for long periods may reduce the irradiation dose reaching the target, thereby diminishing its decontamination effect. Furthermore, the effectiveness of UV sterilization of biofilms is expected to be limited [[30], [31], [32]]. However, under optimal operating conditions, i.e., with proper wipe-cleaning whenever visible contamination occurs, UV radiation will retain its optimal levels of effectiveness. The development of equipment that facilitates proper wipe-cleaning of biosafety cabinets will further reduce cross-contamination during changeover.
5. Limitations
This study has two main limitations. First, we did not address the effectiveness of UV irradiation on disinfection-resistant nonenveloped viruses, like the B19 parvovirus, in raw human materials. Parvovirus B19 nucleic acid was detected in >15% of human synovial tissue and bone marrow samples from over 100 patients [33]. Therefore, its residual contamination risk after cell production should be assessed. Although parvovirus B19 reportedly remains active after irradiation at 30 mJ/cm2 [22], it is inactivated at 100 mJ/cm2 [34], suggesting that it can be inactivated by longer periods of irradiation.
Second, precise measurement of UV irradiation requires chemical dosimetry based on the potassium iodide reaction [35,36], which we did not use. Relative to precise chemical dosimetry UV readings, our UV measurements have an error of ±10%. We expect excess UV irradiation to achieve sufficient inactivation. In addition, since there is no international standard for UVC dosimetry equipment, the results presented in this study cannot be directly appropriated at other facilities. As such, the findings of this study should be verified at each facility.
6. Conclusions
We evaluated the risks of cross-contamination in the processing of human-cell products, considering medium preparation, cell seeding, and waste liquid decanting. Contamination occurred from droplets and ruptured bubbles when tasks were performed at heights of ca. 30 cm. Particles scattered by droplets falling from 30 cm travelled up to 50 cm from the point of impact, making them difficult to accurately trace. To improve decontamination, we evaluated the effectiveness of UV irradiation, which effectively decontaminated Bacillus subtilis, which was detected in several cell-processing facilities, and Aspergillus brasiliensis, both of which are highly resistant to UV irradiation. These findings will be useful for improving biosafety cabinet decontamination during changeover of products from different patients.
Authors contributions
Conception and design: MM, KY, TT, and TY; data acquisition: MM, KY, TT, TY, and YT; data analysis and interpretation: MM, KY, TT, TY, and YT; drafting of the manuscript: MM; and revision of the manuscript for important intellectual content: MM, KY, TT, TY, KW, NS, and IS. All authors have read and approved the final manuscript.
Declarations of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Kimiko Takanashi and Mayumi Tsukamoto for managing our laboratory, as well as the cell-processing facilities for sampling of environmental bacteria. This research was funded by a joint research grant from the Terumo Corporation, Japan.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Mizuno M., Endo K., Katano H., Tsuji A., Kojima N., Watanabe K., et al. The environmental risk assessment of cell-processing facilities for cell therapy in a Japanese academic institution. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogawa Y., Mizutani M., Okamoto R., Kitajima H., Ezoe S., Kino-oka M. Understanding the formation and behaviors of droplets toward consideration of changeover during cell manufacturing. Regen Ther. 2019;12:36–42. doi: 10.1016/j.reth.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizutani M., Samejima H., Terunuma H., Kino-oka M. Experience of contamination during autologous cell manufacturing in cell processing facility under the Japanese Medical Practitioners Act and the Medical Care Act. Regen Ther. 2016;5:25–30. doi: 10.1016/j.reth.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takagi R., Kobayashi S., Yamato M., Owaki T., Kasai Y., Hosoi T., et al. How to prevent contamination with Candida albicans during the fabrication of transplantable oral mucosal epithelial cell sheets. Regen Ther. 2015;1:1–4. doi: 10.1016/j.reth.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chihara R., Kitajima H., Ogawa Y., Nakamura H., Tsutsui S., Mizutani M., et al. Effects of residual H2O2 on the growth of MSCs after decontamination. Regen Ther. 2018;9:111–115. doi: 10.1016/j.reth.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutler T.D., Zimmerman J.J. Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Anim Health Res Rev. 2011;12:15–23. doi: 10.1017/S1466252311000016. [DOI] [PubMed] [Google Scholar]
- 7.Setlow R.B. Cyclobutane-type pyrimidine dimers in polynucleotides. Science. 1966;153:379–386. doi: 10.1126/science.153.3734.379. [DOI] [PubMed] [Google Scholar]
- 8.Beukers R., Berends W. The effects of u.v.-irradiation on nucleic acids and their components. Biochim Biophys Acta. 1961;49:181–189. [Google Scholar]
- 9.Kanie K., Sasaki H., Ikeda Y., Tamada M., Togawa F., Kato R. Quantitative analysis of operators' flow line in the cell culture for controlled manual operation. Regen Ther. 2019;12:43–54. doi: 10.1016/j.reth.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moutsatsou P., Ochs J., Schmitt R.H., Hewitt C.J., Hanga M.P. Automation in cell and gene therapy manufacturing: from past to future. Biotechnol Lett. 2019;41:1245–1253. doi: 10.1007/s10529-019-02732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doulgkeroglou M.-N., Di Nubila A., Niessing B., König N., Schmitt R.H., Damen J., et al. Automation, monitoring, and standardization of cell product manufacturing. Front Bioeng Biotechnol. 2020;8:811. doi: 10.3389/fbioe.2020.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyan K., Zuckerman J.M., Cutler C., Modupe K., Ray D., Marmolejo L., et al. A multiphase intervention of novel color additive for bleach disinfectant wipes improves thoroughness of cleaning in an academic medical center. Am J Infect Control. 2022;50:469–472. doi: 10.1016/j.ajic.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Rutala W.A., Weber D.J. Best practices for disinfection of noncritical environmental surfaces and equipment in health care facilities: a bundle approach. Am J Infect Control. 2019;47:A96–A105. doi: 10.1016/j.ajic.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald T., Sholtz L.A., Marion N., Turner P., Carling P.C., Rupp M.E. Maintenance of environmental services cleaning and disinfection in the ICU after a performance improvement project. Am J Infect Control. 2012;40:E159. [Google Scholar]
- 15.Frota O.P., Ferreira A.M., Koch R., de Andrade D., Rigotti M.A., Borges N.M.A., et al. Surface cleaning effectiveness in a walk-in emergency care unit: influence of a multifaceted intervention. Am J Infect Control. 2016;44:1572–1577. doi: 10.1016/j.ajic.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 16.Anastasi M., Rudaz S., Queruau Lamerie T., Odou P., Bonnabry P., Fleury-Souverain S. Efficacy of two cleaning solutions for the decontamination of 10 antineoplastic agents in the biosafety cabinets of a hospital pharmacy. Ann Occup Hyg. 2015;59:895–908. doi: 10.1093/annhyg/mev031. [DOI] [PubMed] [Google Scholar]
- 17.Adé A., Chauchat L., Ouellette Frève J.-F., Gagné S., Caron N., Bussières J.-F. Comparison of decontamination efficacy of cleaning solutions on a biological safety cabinet workbench contaminated by cyclophosphamide. Can J Hosp Pharm. 2017;70:407–414. doi: 10.4212/cjhp.v70i6.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeeuwen P.L.J.M., Boekhorst J., van den Bogaard E.H., de Koning H.D., van de Kerkhof P.M.C., Saulnier D.M., et al. Microbiome dynamics of human epidermis following skin barrier disruption. Genome Biol. 2012;13:R101. doi: 10.1186/gb-2012-13-11-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandle T. A review of cleanroom microflora: types, trends, and patterns. PDA J Pharm Sci Technol. 2011;65:392–403. doi: 10.5731/pdajpst.2011.00765. [DOI] [PubMed] [Google Scholar]
- 20.Setlow P. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol. 2006;101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 21.Racchi I., Scaramuzza N., Hidalgo A., Cigarini M., Berni E. Sterilization of food packaging by UV-C irradiation: is Aspergillus brasiliensis ATCC 16404 the best target microorganism for industrial bio-validations? Int J Food Microbiol. 2021;357 doi: 10.1016/j.ijfoodmicro.2021.109383. [DOI] [PubMed] [Google Scholar]
- 22.Narita K., Asano K., Naito K., Ohashi H., Sasaki M., Morimoto Y., et al. Ultraviolet C light with wavelength of 222 nm inactivates a wide spectrum of microbial pathogens. J Hosp Infect. 2020;105:459–467. doi: 10.1016/j.jhin.2020.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Harrington B., Valigosky M. Monitoring ultraviolet lamps in biological safety cabinets with cultures of standard bacterial strains on TSA blood agar. Lab Med. 2007;38:165–168. [Google Scholar]
- 24.Sekiya I., Koga H., Otabe K., Nakagawa Y., Katano H., Ozeki N., et al. Additional use of synovial mesenchymal stem cell transplantation following surgical repair of a complex degenerative tear of the medial meniscus of the knee: a case report. Cell Transplant. 2019;28:1445–1454. doi: 10.1177/0963689719863793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizuno M., Endo K., Katano H., Amano N., Nomura M., Hasegawa Y., et al. Transplantation of human autologous synovial mesenchymal stem cells with trisomy 7 into the knee joint and 5 years of follow-up. Stem Cells Transl Med. 2021;10:1530–1543. doi: 10.1002/sctm.20-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shineha R., Inoue Y., Yashiro Y. A comparative analysis of attitudes toward stem cell research and regenerative medicine between six countries – a pilot study. Regen Ther. 2022;20:187–193. doi: 10.1016/j.reth.2022.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shineha R., Inoue Y., Ikka T., Kishimoto A., Yashiro Y. A comparative analysis of attitudes on communication toward stem cell research and regenerative medicine between the public and the scientific community. Stem Cells Transl Med. 2018;7:251–257. doi: 10.1002/sctm.17-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J., Mauser A., Chao S.F., Remington K., Treckmann R., Kaiser K., et al. Virus inactivation and protein recovery in a novel ultraviolet-C reactor. Vox Sang. 2004;86:230–238. doi: 10.1111/j.0042-9007.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 29.Chan H.-L., Gaffney P.R., Waterfield M.D., Anderle H., Peter Matthiessen H., Schwarz H.-P., et al. Proteomic analysis of UVC irradiation-induced damage of plasma proteins: serum amyloid P component as a major target of photolysis. FEBS Lett. 2006;580:3229–3236. doi: 10.1016/j.febslet.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Cortesão M., de Haas A., Unterbusch R., Fujimori A., Schütze T., Meyer V., et al. Aspergillus niger spores are highly resistant to space radiation. Front Microbiol. 2020;11:560. doi: 10.3389/fmicb.2020.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo X., Zhang B., Lu Y., Mei Y., Shen L. Advances in application of ultraviolet irradiation for biofilm control in water and wastewater infrastructure. J Hazard Mater. 2022;421 doi: 10.1016/j.jhazmat.2021.126682. [DOI] [PubMed] [Google Scholar]
- 32.Otter J.A., Vickery K., Walker J.T., deLancey Pulcini E., Stoodley P., Goldenberg S.D., et al. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. J Hosp Infect. 2015;89:16–27. doi: 10.1016/j.jhin.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe K., Otabe K., Shimizu N., Komori K., Mizuno M., Katano H., et al. High-sensitivity virus and mycoplasma screening test reveals high prevalence of parvovirus B19 infection in human synovial tissues and bone marrow. Stem Cell Res Ther. 2018;9:80. doi: 10.1186/s13287-018-0811-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugawara H., Motokawa R., Abe H., Yamaguchi M., Yamada-Ohnishi Y., Hirayama J., et al. Inactivation of parvovirus B19 in coagulation factor concentrates by UVC radiation: assessment by an in vitro infectivity assay using CFU-E derived from peripheral blood CD34+ cells. Transfusion. 2001;41:456–461. doi: 10.1046/j.1537-2995.2001.41040456.x. [DOI] [PubMed] [Google Scholar]
- 35.Rahn R.O., Stefan M.I., Bolton J.R., Goren E., Shaw P.-S., Lykke K.R. Quantum yield of the iodide-iodate chemical actinometer: dependence on wavelength and concentration. Photochem Photobiol. 2007;78:146–152. doi: 10.1562/0031-8655(2003)078<0146:qyotic>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Qiang Z., Li W., Li M., Bolton J.R., Qu J. Inspection of feasible calibration conditions for UV radiometer detectors with the KI/KIO3 actinometer. Photochem Photobiol. 2015;91:68–73. doi: 10.1111/php.12356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High-speed camera capture of falling droplets.1
Video of falling droplets.
High-speed camera capture of bubble rupture.
Video of bubble rupture.