Abstract
Diabetes mellitus (DM) is a metabolic disease characterized by chronic hyperglycemia. DM can lead to a number of secondary complications affecting multiple organs in the body including the eyes, kidney, heart, and brain. The most common effect of hyperglycemia on the brain is cognitive decline. It has been estimated that 20–70% of people with DM have cognitive deficits. High blood sugar affects key brain areas involved in learning, memory, and spatial navigation, and the structural complexity of the brain has made it prone to a variety of pathological disorders, including T2DM. Studies have reported that cognitive decline can occur in people with diabetes, which could go undetected for several years. Moreover, studies on brain imaging suggest extensive effects on different brain regions in patients with T2D. It remains unclear whether diabetes-associated cognitive decline is a consequence of hyperglycemia or a complication that co-occurs with T2D. The exact mechanism underlying cognitive impairment in diabetes is complex; however, impaired glucose metabolism and abnormal insulin function are thought to play important roles. In this review, we have tried to summarize the effect of hyperglycemia on the brain structure and functions, along with the potential mechanisms underlying T2DM-associated cognitive decline.
Keywords: Type 2 diabetes, Cognitive decline, Hippocampus, Neuroinflammation, Apoptosis
Introduction
Diabetes is a metabolic disorder characterized by defects in the body's ability to regulate glucose and insulin homeostasis. According to the International Diabetes Federation (IDF), type-2 diabetes mellitus (T2DM) accounts for atleast 90% of all cases of diabetes, affecting approximately 463 million people worldwide. Increased prevalence of obesity due to unhealthy diets, physical inactivity, and increased average life expectancy, has led to an increase in the prevalence of diabetes, and it has been predicted that the number of patients with T2DM will increase to 700 million by 2045 (Federation, 2019). It has been observed that the age of onset of T2DM is also reduced. Moreover, increasing evidence suggests that T2DM is a major contributor of cognitive decline in elderly as well as young individuals (Lalithambika et al., 2019, Rajamani, 2014). Due to increasing prevalence of T2DM and increased life-expectancy, diabetes-associated cognitive dysfunction has become a serious burden on the available health resources. Therefore, a deeper understanding of diabetes-associated cognitive decline will help in developing novel therapeutic options for this condition.
T2DM affects several organs of the body, including the brain. The association between cognitive decline and T2DM is poorly recognized and is sparsely addressed. The exact mechanism underlying cognitive impairment in diabetes is complex; however, impaired glucose metabolism and abnormal insulin function are often associated with cognitive impairment. Studies have shown that hyperglycemia leads to hypertension, dyslipidemia, inflammation, and abnormalities in hypothalamic-pituitary-adrenocortical axis (Rama and Sagar, 2019, Hazari et al., 2015). Moreover, chronic hyperglycemia is toxic to neurons and leads to the formation of advanced glycation end products leading to oxidative damage and neuronal injury. Inflammation and dyslipidemia are the other important factors that can cause neuronal damage leading to cognitive impairment (Naguib et al., 2020).
T2DM is closely linked to poor performance in a variety of cognitive domains as well as brain structural abnormalities (Dove et al., 2021, Mirahmadizadeh et al., 2020). T2DM and its related cognitive impairment can have a significant impact on people of all age’s quality of life (Abdellatif et al., 2020, Xia et al., 2020). Diabetic patients have a lower ability to resist against oxidative stress and have increased activation of inflammatory pathways in the cells (Sharma et al., 2020, Srikanth et al., 2020). People are more susceptible to cognitive impairment and neurodegeneration as a result of increased oxidative stress and inflammation in the body (Yang et al., 2020, Damanik and Yunir, 2021). Arpita et al. conducted a study of 1278 T2DM patients in a south Indian population to assess cognitive impairment and reported a prevalence of 35.8 % (Chakraborty et al., 2021, Subramanian et al., 2021, You et al., 2021). Individuals with diabetes are 1.5 times more likely than those without diabetes to experience cognitive decline and early stages of dementia (Lin et al., 2022).
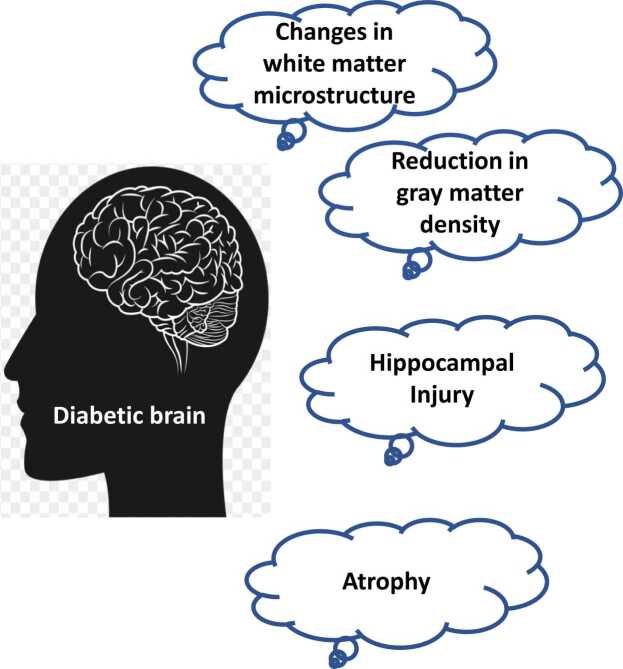
It has been reported that hyperglycemia increases the risk of damage to blood vessels within the brain. The structural changes observed in the diabetic brain are hippocampal injury, reduction in gray matter density, reduction in white matter microstructure, and atrophy (Fig. 1) (Seaquist, 2010). Furthermore, the mechanisms underlying structural brain abnormalities in T2DM may include endocrine, metabolic, and vascular pathways.
Fig. 1.
Structural changes in the diabetic brain. Diabetic patients have a number of structural alterations in the brain and these changes progress with time. These changes include atrophy, changes in white matter microstructure, hippocampal injury, and reduced gray matter density.
Type-2 diabetes and cognitive decline
Glucose is the primary source of energy for every cell in the body (Howarth et al., 2012, Fioramonti and Pénicaud, 2019). Despite being only two percent of the body weight, the brain utilizes more than twenty percent of daily energy intake (Erbsloh et al., 1958). Since, neuronal cells are continually active in order to regulate important functions required for the body's survival, they require twice as much energy as cells of the body (Harris et al., 2012, Mergenthaler et al., 2013). Neurons are also active during sleep to manage the sleep cycle, in addition to other critical responsibilities. Therefore, a constant supply of glucose is required for normal brain metabolic processes, brain vitality, cerebral signal conduction, cognitive function, neurotransmission, and synaptic plasticity. Despite the fact that the brain is highly dependent on glucose, severe and long-term hyperglycemia can be harmful (Heni et al., 2015).
Diabetes has been shown in a number of studies to have a negative impact on the hippocampus and to promote neuronal death via a variety of mechanisms (Pamidi and BN, 2012). The hippocampus is a part of limbic system that is particularly vulnerable to elevated blood sugar levels and plays a role in memory, as well as emotional, reproductive and adaptive functions (Foghi and Ahmadpour, 2013, Sadeghi et al., 2016). It also helps in the formation of new memories and the association of emotions and senses like fragrance and sound with memories (Squire, 1992, Lewis, 2012). The hippocampus serves as a memory indicator, directing memories to the brain's right region for long-term storage and retrieval (Turgut and Turgut, 2011, Jarrard, 1993).
Because of its anatomical complexity, the hippocampus is vulnerable to a variety of pathological diseases, including T2DM (Pamidi and BN, 2012, Alvarez et al., 2009, Biessels et al., 1996). The hippocampus's structural complexity has made it prone to a variety of pathological disorders, including T2DM. The granular layer of the dentate gyrus (DG) continues to proliferate throughout life (Kitamura and Inokuchi, 2014, Koehl and Abrous, 2011, Kitabatake et al., 2007). Memory and learning problems can be caused by anything that disrupts the equilibrium between neuronal proliferation and death in the DG region (Van der Borght et al., 2007, Kobilo et al., 2011). Moreover, hyperglycemia suppresses granular cell growth and induces neuronal death (necrosis/apoptosis) in the CA3 region and the DG (Choi et al., 2009, Zhang et al., 2008, Li et al., 2002, Ahmadpour et al., 2010).
Effect of hyperglycemia on the brain structure
Hyperglycemia can cause nerve damage in the brain, increasing the risk of cognitive decline (Sharma et al., 2020, Srikanth et al., 2020, Vieira et al., 2018, Biessels and Whitmer, 2020). Both gray matter and white matter changes are among the T2DM-related brain structural abnormalities (Chen et al., 2021). Patients with T2DM experience cognitive decline and anatomical brain abnormalities, especially observed in the hippocampus (Li et al., 2020). Patients with T2DM exhibit brain shrinkage, as evidenced by reduced total and regional white and gray matter volumes (Moran et al., 2013). Additionally, it was found that T2DM patients had a little larger volume of white matter hyperintensities than non-T2DM patients (Moran et al., 2017). These abnormalities in the brain may serve as imaging biomarkers for T2DM alone or T2DM combined with cognitive decline (Zhang et al., 2011).
People with T2DM exhibit somewhat more global brain atrophy than those without diabetes, and this atrophy steadily worsens over time in comparison to normal ageing. Additionally, vascular lesions, especially lacunar infarcts, are more frequent. Numerous research examined the connection between brain atrophy and diabetes; some only looked at cortical or subcortical shrinkage, while others looked at both (Falvey et al., 2013, SK et al., 2003). Hippocampal atrophy has been suggested to occur in T2DM patients. Atrophy of the medial temporal lobe, in especially the hippocampus, is regarded to be a sign of neurodegeneration (Scheltens et al., 2002, Korf et al., 2007).
Global brain atrophy, which happens gradually over time compared to normal ageing, is slightly more common in adults with T2DM than in people without diabetes (Knopman et al., 2011, Knopman et al., 2005). Vascular lesions, especially lacunar infarcts, are also rising in frequency. Numerous investigations examined the relationship between brain atrophy caused by diabetes and cortical or subcortical atrophy, or both (Knopman et al., 2005). According to certain theories, those with T2DM may experience hippocampal atrophy. According to numerous studies, the hippocampus in the medial temporal lobe, in particular, is thought to atrophy as a marker of neurodegeneration (Brundel et al., 2014).
Potential mechanisms underlying hyperglycemia-induced cognitive impairment
Apoptosis
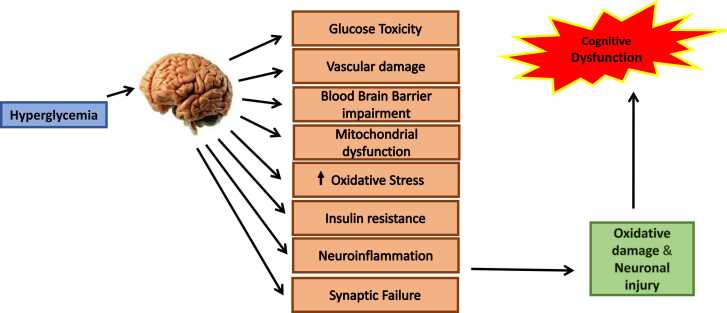
A considerable rise in apoptotic markers including Bcl-2, Bcl-xl, Bax, and caspase 3 has been observed in the hippocampus environment of diabetic mice in several preclinical studies (Li et al., 2002). In the hippocampus of STZ-induced diabetic rats, Jafari et al. reported that Caspases 3 is the most important member of the caspases family, showed a significant increase in activity. In these diabetic rats, Bax expression was dramatically raised at both the mRNA and protein levels, whereas Bcl xL and Bcl 2 expression was significantly reduced, implying that hyperglycemia-induced apoptosis in the hippocampus of diabetic rats could be mediated by mitochondria (Jafari Anarkooli et al., 2014). Several other in vitro and in vivo investigations have found that diabetic mice suffer from hippocampus cell loss, which could be a vital contributor to memory and learning problems (Fig. 3) (Li et al., 2002).
Fig. 3.
Pathway through which hyperglycemia leads to cognitive impairment. Hyperglycemia leads vascular damage, BBB impairment, mitochondrial dysfunction, increased oxidative stress, IR, neuroinflammation, synaptic failure resulting in oxidative damage and neuronal injury.
Oxidative stress
Oxidative stress has been related to the onset and progression of diabetes, as well as the problems that come with it. Hyperglycemia leads to the formation of reactive oxygen species (ROS), other oxidative stress markers and reactive nitrogen species (RNS) (Cheong et al., 2020). Furthermore, hyperglycemia is linked to a reduction in antioxidant levels in the brain (Valko et al., 2007). In diabetic rats, increased oxidative stress has been linked to the development of cognitive deficits (Fukui et al., 2002, Comin et al., 2010).
Circulating microRNAs (miRNAs) can be used as biomarkers of T2D (Zampetaki et al., 2010, Prattichizzo et al., 2016, Kato et al., 2013). miRNAs are a class of non-coding RNAs which interact with the 3′ untranslated region (3′ UTR) of target mRNAs to induce mRNA degradation and translational repression (Bartel, 2009). The two candidate miRNAs (miR-192 and miR-193b) were reported as markers of pre-diabetes by Parrizas et al. in a cohort study (Párrizas et al., 2015). Furthermore, de Candia et al. reported a unique miRNA signature link to prediabetics with respect to disease progression (De Candia et al., 2017).
La Sala et al. demonstrated the association between circulating miR-21 and glycaemic dysfunctions and provided novel and valuable insights into the molecular characterization of impaired glucose tolerance (IGT) status (La Sala et al., 2019). An increase in levels of circulating miR-21 was observed in IGT subjects. In addition, results showed a positive correlation of miR-21 and postprandial glucose levels with ROS and insulin resistance index. It has also been reported that miR-21 could be an important modulator of ROS homeostasis and antioxidant pathways, and defective antioxidant response is one of the major causes of cellular damage (La Sala et al., 2016).
Hyperglycemia is one of the major risk factors of AD (Shieh et al., 2020, Li et al., 2017, An et al., 2018, Jash et al., 2020). High blood glucose levels increase oxidative stress leading to the production of lipid peroxidation byproducts, such as 4-hydroxynonenal (HNE), which lowers the antioxidant defense mechanism in AD patients. AD patients may have increased levels of HNE in the brain and blood, thereby, enhancing the production of Aβ (Arimon et al., 2015, Di Domenico et al., 2017, Liou et al., 2019). Sanotra et al. showed that this could be a result of both HNE adducts and Aβ being neutralized by associated autoantibodies. When HNE adducts and levels of Aβ continue to increase, it may deplete these crucial neutralizing antibodies and promote a cellular environment for neurodegeneration, leading to pathologic states such as AD (Sanotra et al., 2022). Further research on HNE immune responses is needed to get more insight of the pathogenesis from hyperglycemia to AD.
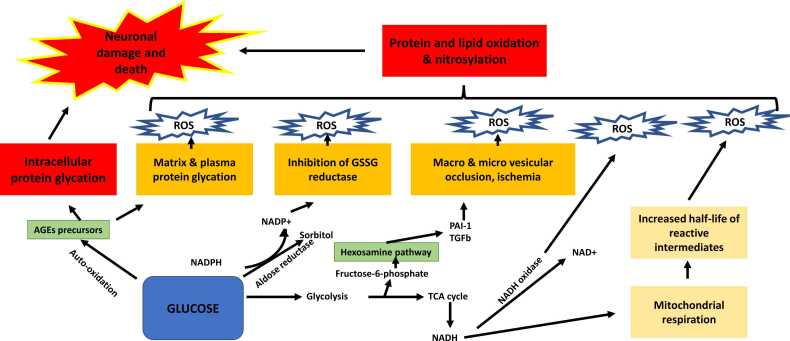
It is well known that hyperglycemia-induced neurotoxicity is mainly due to increased production of advanced glycation end-products (AGEs), increased polyol pathway flux, activation of protein kinase C (PKC) isoforms, and increased hexosamine pathway flux, (Brownlee, 2001) all of which leads to an increase in oxidative damage and vascular complications shown in Fig. 2. Few studies suggest that blood-brain barrier (BBB) permeability is reduced in diabetic animal models due to degeneration of the cerebral vasculature (Prasad et al., 2014, Ueno et al., 2016). However, there is conflicting information about cerebral microcirculation and BBB disruption in diabetic rodent models with chronic hyperglycemia (Weiss et al., 2009, Huber et al., 2006, Rom et al., 2019, Xu et al., 2013).
Fig. 2.
Mechanisms underlying diabetes-associated cognitive decline. Abnormally high levels of blood glucose can lead to the activation of numerousmetabolic pathways like polyol pathway, advanced glycation end products (AGE) pathway, protein kinase C (PKC) pathway, and hexosamine pathway which in turn leads to neuronal damage.
Nerve cells are particularly susceptible to hyperglycemia because neuronal glucose uptake is highly dependent on external glucose concentration, which is 4–5 times greater in diabetics. It has been shown that the levels of neurotrophic support factors, such as nerve growth factor and insulin-like growth factor, are decreased in diabetic patients, leading to nerve malnourishment. As shown in Fig. 2, all of these pathways function together to provide a platform that leads to neuronal dysfunction and nerve injury in diabetic animals (Sims-Robinson et al., 2010).
Impaired neuronal insulin signaling
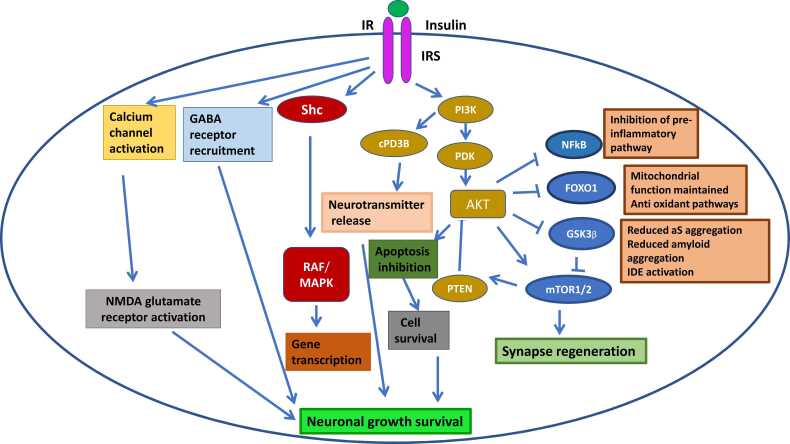
Insulin plays an important role in neuroprotection. Insulin activates insulin receptor substrates 1 and 2 by binding to the insulin receptor (IR). IRS1 is generally located in the cerebral cortex, while IRS2 is mostly found in the hypothalamus (Mullins et al., 2017, Arnold et al., 2018). Insulin attaches to IR/IRS and activates a variety of secondary messengers through three main mechanisms:
a. The IR-Shc-MAP kinase (MAPK) pathway is activated, which promotes synaptic plasticity, protein expression for cell growth and maintenance (Yao et al., 2004). Insulin also regulates protein transcription, translation, and post-translational modification via the MAPK pathway (Kelly et al., 2003, Dou et al., 2005).
b. By regulating neuronal transmission via cyclic nucleotide phosphodiesterase 3B, the phosphatidylinositol 3-kinase (PI3K) pathway influences cognitive function, and memory (cPD3B) and information processing. Inhibition of neuronal apoptosis is also linked to the PI3K-Akt pathway (Zhao et al., 2004). Glycogen synthase kinase 3 (GSK3), forkhead box O1 (FOXO1), mammalian target of rapamycin (mTOR) and nuclear factor B (NFkB) are some downstream effectors of PI3K-Akt. Memory and learning are directly influenced by these effectors.
c. Direct activation of NMDA glutamate receptors to increase the opening of calcium channels at synapses to mediate neurotransmission (Wan et al., 1997). Increased calcium uptake promotes NMDA-mediated neurotransmission, which increases the recruitment of functional GABA receptors to postsynaptic sites and enhances GABA transmission, regulating synaptic inhibition for learning and memory tasks (Fig. 4) (Cheong et al., 2020).
Fig. 4.
Insulin signaling pathway. Insulin activates IRS1 and 2 by binding to the IR. Insulin attaches to IR/IRS and activates a variety of secondary messengers through three main mechanisms:PI3K/AKT pathway, MAPK pathway, NMDA glutamate receptor activation pathway.
Suppressed insulin activity
Despite the fact that the brain is not an insulin-dependent organ, insulin crosses the BBB and binds to receptors on glial cells and neurons. Although it is unknown whether insulin resistance exists in the CNS, emerging research revealed that insulin insensitivity may play a role in the development of obesity and T2DM. Insulin has multiple functions in the brain, and causes increased glucose uptake in the neurons of the hippocampus and frontal lobes, two of the most important regions involved in memory regulation. Insulin also aids in the formation of new memories by increasing synaptic connections between brain cells. Insulin also controls the metabolism and release of acetylcholine, a neurotransmitter that is important for cognition. Finally, insulin plays a role in blood vessel formation (Arnold et al., 2018).
Insulin increases the production of insulin degrading enzymes (IDE) and generates the extracellular release of the β-amyloid peptide (Aβ) (Young et al., 2006). Insulin deficiency leads to the buildup of Aβ. There is a reduction in insulin receptors and insulin in the brain when there is hyperinsulinemia or insulin resistance (Kawamura et al., 2012). Because IDE degrades insulin, high insulin levels causes IDE consumption, which leads to an increase in Aβ deposition. As a result of the increased Aβ buildup, cognitive impairment occurs (Biessels et al., 2006, Kodl and Seaquist, 2008, Craft, 2005).
Brain insulin resistance
Insulin resistance is characterized as a decrease in the body's sensitivity to insulin (Goldstein, 2002). Insulin resistance is the inability of brain cells to respond to insulin. Insulin receptor downregulation, insulin receptor inability to bind insulin, or improper insulin signaling cascade activation could all contribute to this lack of responsiveness. At the cellular level, this dysfunction impacts neuroplasticity, receptor modulation, and neurotransmitter synthesis, as well as processes directly engaged in insulin metabolism, such as neuronal glucose absorption in GLUT4-expressing neurons, and insulin homeostatic and inflammatory responses (Mielke et al., 2005).
In the brain, insulin and related proteins are necessary for cell survival. A range of brain processes, including learning and memory, appear to be governed by glucose and insulin. Chronically high or low blood glucose levels can cause brain damage and cognitive impairment by disrupting insulin activity. Insulin insensitivity in our liver, fat, and muscle cells/tissues may also corresponds to insulin sensitivity in our central nervous system (insulin resistance at the level of brain), according to new findings. The regions of the brain involved in cognition, memory, and learning are affected. Insulin plays a role in the cell-level memory formation process known as long-term potentiation. Insulin also regulates acetylcholine, a chemical messenger that plays a role in memory (Arnold et al., 2018, Cholerton et al., 2016).
Neuroinflammation
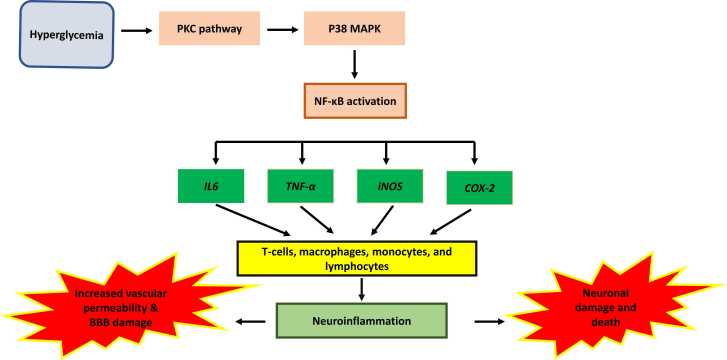
The expression of pro-inflammatory cytokines in the brain rises in diabetic individuals, resulting in neuronal damage (Gaspar et al., 2016). It's thought that the transcription factor NF-kB is involved in cognitive function. BAY 11–7082 (BAY) is a pharmacological inhibitor of IkB (inhibitor of kappa B alpha) phosphorylation that reduces IL-6 and TNF levels while inhibiting NF-kB activation. BAY improves learning and memory in T2DM rats without compromising glycemic control (Kumar Datusalia and Sunder Sharma, 2016). Furthermore, microglial activity is enhanced in diabetic human postmortem hippocampus, indicating increased inflammation (Valente et al., 2010).
TNF levels and microglia/macrophage activation were found to be higher in the brains of mice on a high-fat diet, indicating pro-inflammatory changes in the brain (Puig et al., 2012). The spatial-recognition memory of diabetic and obese db/db mice was reduced, which was connected to higher levels of pro-inflammatory cytokines (IL-1, TNF, and IL-6), implying a relationship between inflammation and memory impairment (Dinel et al., 2011). The relationship between oxidative stress and neuroinflammation is widely understood. NF-kB is a regulator of TNF and interleukins and a modulator of reactive oxygen species (ROS). It is involved in the commencement of the inflammatory cascade. Increased ROS production and cognitive impairment occur from TNF upregulation, which inhibits insulin signaling (Fig. 5) (Kuhad et al., 2009).
Fig. 5.
Hyperglycemia leads to neuroinflammation. Hyperglycemia activates PKC pathway which in turn activates NF-kB pathway leading to neuroinflammation, increased vascular permeability, and BBB damage.
Synaptic dysfunction
Synaptic damage is the most common cause of brain malfunction (Morrison and Baxter, 2012), and the severity of synaptic alterations is related to the severity of cognitive loss (Hawkins and Byrne, 2015). Amyloid plaques are the primary cause of synaptic dysfunction, but neuroinflammation and microglial activation also plays an important role (Moore et al., 2019). Mitochondria plays a role in synaptic degeneration because of a lack of ATP synthesis (energy failure), impaired production of neurotransmitter precursors and metabolites, increased production of reactive oxygen species (ROS), decreased Ca+ + handling, dysregulation of mitochondrial dynamics, and mitochondrial dependent cell signaling transduction (Tait and Green, 2012, Guo et al., 2017, Belenguer et al., 2019). The hippocampus, a part of the brain known to play a role in memory formation in animals, is severely compromised by diabetes, and electrophysiological studies show that diabetes decreases synaptic plasticity in hippocampal slices (Biessels et al., 2002, Trudeau et al., 2004, Duarte et al., 2019, Garcia-Serrano and Duarte, 2020).
Conclusion
Brain function is intimately linked to glucose metabolism. T2DM is now widely understood to be linked to poor cognitive performance. Free radicals and reactive oxygen species (ROS) have been identified as the primary drivers of neuronal death in diabetic mice. Studies have reported that ROS and the resulting oxidative stress play a pivotal role in apoptosis. The information provided in this review demonstrates the overlap between the biological pathways driving diabetes and cognitive impairment. Hyperglycemia causes dysregulation of several extracellular and intracellular signaling cascades in the CNS, resulting in decreased neuronal and synaptic function and, as a result, an increase in neuronal death. An understanding of how each molecular pathway intersects and affects the other is critical for the development of future drug intervention strategies for diabetes-associated cognitive dysfunction.
Funding resource
This review did not receive any specific grant from any of the funding agencies.
References
- Abdellatif G.A., et al. Mild cognitive impairment among type ii diabetes mellitus patients attending university teaching hospital. Open Access Maced. J. Med. Sci. 2020;8(E):105–111. [Google Scholar]
- Ahmadpour S., et al. Neuronal death in dentate gyrus and ca3 in diabetic rats: effects of insulin and ascorbic acid. Hormozan. J. Med. Sci. 2010;13:13–16. [Google Scholar]
- Alvarez E.O., et al. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav. Brain Res. 2009;198(1):224–230. doi: 10.1016/j.bbr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- An Y., et al. Evidence for brain glucose dysregulation in Alzheimer's disease. Alzheimer's Dement. 2018;14(3):318–329. doi: 10.1016/j.jalz.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimon M., et al. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol. Dis. 2015;84:109–119. doi: 10.1016/j.nbd.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S.E., et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat. Rev. Neurol. 2018;14(3):168–181. doi: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenguer P., et al. Mitochondria and the brain: bioenergetics and beyond. Neurotox. Res. 2019;36(2):219–238. doi: 10.1007/s12640-019-00061-7. [DOI] [PubMed] [Google Scholar]
- Biessels G.J., et al. Ageing and diabetes: implications for brain function. Eur. J. Pharmacol. 2002;441(1–2):1–14. doi: 10.1016/s0014-2999(02)01486-3. [DOI] [PubMed] [Google Scholar]
- Biessels G.J., et al. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- Biessels G.J., Whitmer R.A. Cognitive dysfunction in diabetes: how to implement emerging guidelines. Diabetologia. 2020;63(1):3–9. doi: 10.1007/s00125-019-04977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels G.-J., et al. Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes. 1996;45(9):1259–1266. doi: 10.2337/diab.45.9.1259. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Brundel M., Kappelle L.J., Biessels G.J. Brain imaging in type 2 diabetes. Eur. Neuropsychopharmacol. 2014;24(12):1967–1981. doi: 10.1016/j.euroneuro.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Chakraborty A., et al. Age related prevalence of mild cognitive impairment in type 2 diabetes mellitus patients in the Indian population and association of serum lipids with cognitive dysfunction. Front. Endocrinol. 2021:12. doi: 10.3389/fendo.2021.798652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., et al. Identifying type 2 diabetic brains by investigating disease-related structural changes in MRI. Front. Neurosci. 2021:1418. doi: 10.3389/fnins.2021.728874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong J.L., et al. The association between type 2 diabetes mellitus and Parkinson’s disease. J. Parkinson's Dis. 2020;10(3):775–789. doi: 10.3233/JPD-191900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.-H., et al. Effects of streptozotocin-induced type 1 diabetes on cell proliferation and neuronal differentiation in the dentate gyrus; correlation with memory impairment. Korean J. Anat. 2009;42(1):41–48. [Google Scholar]
- Cholerton B., et al. Type 2 diabetes, cognition, and dementia in older adults: toward a precision health approach. Diabetes Spectr. 2016;29(4):210–219. doi: 10.2337/ds16-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comin D., et al. Vitamin E improves learning performance and changes the expression of nitric oxide-producing neurons in the brains of diabetic rats. Behav. Brain Res. 2010;210(1):38–45. doi: 10.1016/j.bbr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance syndrome and Alzheimer's disease: age-and obesity-related effects on memory, amyloid, and inflammation. Neurobiol. Aging. 2005;26(1):65–69. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Damanik J., Yunir E. Type 2 diabetes mellitus and cognitive impairment. Acta Med. Indones. 2021;53(2):213–220. [PubMed] [Google Scholar]
- De Candia P., et al. A unique plasma microRNA profile defines type 2 diabetes progression. PloS One. 2017;12(12) doi: 10.1371/journal.pone.0188980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico F., Tramutola A., Butterfield D.A. Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic. Biol. Med. 2017;111:253–261. doi: 10.1016/j.freeradbiomed.2016.10.490. [DOI] [PubMed] [Google Scholar]
- Dinel A.-L., et al. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PloS One. 2011;6(9) doi: 10.1371/journal.pone.0024325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou J.-T., et al. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn. Mem. 2005;12(6):646–655. doi: 10.1101/lm.88005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A., et al. The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimer's Dement. 2021;17(11):1769–1778. doi: 10.1002/alz.12482. [DOI] [PubMed] [Google Scholar]
- Duarte J., et al. Impact of caffeine consumption on type 2 diabetes-induced spatial memory impairment and neurochemical alterations in the hippocampus. Front. Neurosci. 2019;12:1015. doi: 10.3389/fnins.2018.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbsloh F., Bernsmeier A., Hillesheim H. The glucose consumption of the brain & its dependence on the liver. Archiv fur Psychiatrie und Nervenkrankheiten. Ver. Mit. Z. fur die Gesamt Neurol. und Psychiatr. 1958;196(6):611–626. doi: 10.1007/BF00344388. [DOI] [PubMed] [Google Scholar]
- Falvey C.M., et al. Macro-and microstructural magnetic resonance imaging indices associated with diabetes among community-dwelling older adults. Diabetes Care. 2013;36(3):677–682. doi: 10.2337/dc12-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federation, I.D., IDF diabetes atlas ninth. Dunia: IDF, 2019.
- Fioramonti, X. and L. Pénicaud, Carbohydrates and the brain: roles and impact, in Feed your mind-How does nutrition modulate brain function throughout life? 2019, IntechOpen.
- Foghi K., Ahmadpour S. Diabetes mellitus type 1 and neuronal degeneration in ventral and dorsal hippocampus. Iran. J. Pathol. 2013;9(1):33–37. [Google Scholar]
- Fukui K., et al. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Ann. N. Y. Acad. Sci. 2002;959(1):275–284. doi: 10.1111/j.1749-6632.2002.tb02099.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Serrano A.M., Duarte J. Brain metabolism alterations in type 2 diabetes: what did we learn from diet-induced diabetes models? Front. Neurosci. 2020;14:229. doi: 10.3389/fnins.2020.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar J.M., et al. Inside the diabetic brain: role of different players involved in cognitive decline. ACS Chem. Neurosci. 2016;7(2):131–142. doi: 10.1021/acschemneuro.5b00240. [DOI] [PubMed] [Google Scholar]
- Goldstein B.J. Insulin resistance as the core defect in type 2 diabetes mellitus. Am. J. Cardiol. 2002;90(5):3–10. doi: 10.1016/s0002-9149(02)02553-5. [DOI] [PubMed] [Google Scholar]
- Guo L., Tian J., Du H. Mitochondrial dysfunction and synaptic transmission failure in Alzheimer’s disease. J. Alzheimer's Dis. 2017;57(4):1071–1086. doi: 10.3233/JAD-160702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J.J., Jolivet R., Attwell D. Synaptic energy use and supply. Neuron. 2012;75(5):762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Hawkins R.D., Byrne J.H. Associative learning in invertebrates. Cold Spring Harb. Perspect. Biol. 2015;7(5) doi: 10.1101/cshperspect.a021709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazari M.A.H., et al. Cognitive impairment in type 2 diabetes mellitus. Int. J. Diabetes Mellit. 2015;3(1):19–24. [Google Scholar]
- Heni M., et al. Impaired insulin action in the human brain: causes and metabolic consequences. Nat. Rev. Endocrinol. 2015;11(12):701–711. doi: 10.1038/nrendo.2015.173. [DOI] [PubMed] [Google Scholar]
- Howarth C., Gleeson P., Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 2012;32(7):1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J.D., VanGilder R.L., Houser K.A. Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. Am. J. Physiol. -Heart Circ. Physiol. 2006;291(6):H2660–H2668. doi: 10.1152/ajpheart.00489.2006. [DOI] [PubMed] [Google Scholar]
- Jafari Anarkooli, I., H. Barzegar Ganji, and M. Pourheidar, The protective effects of insulin and natural honey against hippocampal cell death in streptozotocin-induced diabetic rats. Journal of diabetes research, 2014. 2014. [DOI] [PMC free article] [PubMed]
- Jarrard L.E. On the role of the hippocampus in learning and memory in the rat. Behav. Neural Biol. 1993;60(1):9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Jash K., et al. Cognitive dysfunction: a growing link between diabetes and Alzheimer's disease. Drug Dev. Res. 2020;81(2):144–164. doi: 10.1002/ddr.21579. [DOI] [PubMed] [Google Scholar]
- Kato M., Castro N.E., Natarajan R. MicroRNAs: potential mediators and biomarkers of diabetic complications. Free Radic. Biol. Med. 2013;64:85–94. doi: 10.1016/j.freeradbiomed.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T., Umemura T., Hotta N. Cognitive impairment in diabetic patients: can diabetic control prevent cognitive decline? J. Diabetes Investig. 2012;3(5):413–423. doi: 10.1111/j.2040-1124.2012.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly Á., Laroche S., Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J. Neurosci. 2003;23(12):5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabatake Y., et al. Adult neurogenesis and hippocampal memory function: new cells, more plasticity, new memories? Neurosurg. Clin. 2007;18(1):105–113. doi: 10.1016/j.nec.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Inokuchi K. Role of adult neurogenesis in hippocampal-cortical memory consolidation. Mol. Brain. 2014;7(1):1–8. doi: 10.1186/1756-6606-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D., et al. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology. 2011;76(22):1879–1885. doi: 10.1212/WNL.0b013e31821d753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D.S., et al. Cardiovascular risk factors and cerebral atrophy in a middle-aged cohort. Neurology. 2005;65(6):876–881. doi: 10.1212/01.wnl.0000176074.09733.a8. [DOI] [PubMed] [Google Scholar]
- Kobilo T., Yuan C., van Praag H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn. Mem. 2011;18(2):103–107. doi: 10.1101/lm.2001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodl C.T., Seaquist E.R. Cognitive dysfunction and diabetes mellitus. Endocr. Rev. 2008;29(4):494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl M., Abrous D.N. A new chapter in the field of memory: adult hippocampal neurogenesis. Eur. J. Neurosci. 2011;33(6):1101–1114. doi: 10.1111/j.1460-9568.2011.07609.x. [DOI] [PubMed] [Google Scholar]
- Korf E., et al. Diabetes mellitus, hypertension and medial temporal lobe atrophy: the LADIS study. Diabet. Med. 2007;24(2):166–171. doi: 10.1111/j.1464-5491.2007.02049.x. [DOI] [PubMed] [Google Scholar]
- Kuhad A., et al. Suppression of NF-κβ signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol. Biochem. Behav. 2009;92(2):251–259. doi: 10.1016/j.pbb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Kumar Datusalia A., Sunder Sharma S. NF-κB inhibition resolves cognitive deficits in experimental type 2 diabetes mellitus through CREB and glutamate/GABA neurotransmitters pathway. Curr. Neurovasc. Res. 2016;13(1):22–32. doi: 10.2174/1567202612666151030104810. [DOI] [PubMed] [Google Scholar]
- La Sala L., et al. Oscillating glucose induces microRNA-185 and impairs an efficient antioxidant response in human endothelial cells. Cardiovasc. Diabetol. 2016;15(1):1–9. doi: 10.1186/s12933-016-0390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Sala L., et al. Circulating microRNA-21 is an early predictor of ROS-mediated damage in subjects with high risk of developing diabetes and in drug-naïve T2D. Cardiovasc. Diabetol. 2019;18(1):1–12. doi: 10.1186/s12933-019-0824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalithambika C.V., et al. Cognitive impairment and its association with glycemic control in type 2 diabetes mellitus patients. Indian J. Endocrinol. Metab. 2019;23(3):353. doi: 10.4103/ijem.IJEM_24_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. Hippocampus plays multiple choice. Nat. Rev. Neurosci. 2012;13(9) doi: 10.1038/nrn3318. 600-600. [DOI] [PubMed] [Google Scholar]
- Li M., et al. Atrophy patterns of hippocampal subfields in T2DM patients with cognitive impairment. Endocrine. 2020;68(3):536–548. doi: 10.1007/s12020-020-02249-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.-C., et al. Visit-to-visit variations in fasting plasma glucose and HbA1c associated with an increased risk of Alzheimer disease: Taiwan diabetes study. Diabetes Care. 2017;40(9):1210–1217. doi: 10.2337/dc16-2238. [DOI] [PubMed] [Google Scholar]
- Li Z.-G., et al. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 2002;946(2):221–231. doi: 10.1016/s0006-8993(02)02887-1. [DOI] [PubMed] [Google Scholar]
- Lin C.-F., Liu H.-C., Lin S.-Y. Kidney function and risk of physical and cognitive impairment in older persons with type 2 diabetes at an outpatient clinic with geriatric assessment implementation. Diabetes Metab. Syndr. Obes.: Targets Ther. 2022;15:79. doi: 10.2147/DMSO.S341935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou C.J., et al. Altered brain expression of insulin and insulin-like growth factors in frontotemporal lobar degeneration: another degenerative disease linked to dysregulation of insulin metabolic pathways. ASN Neuro. 2019;11 doi: 10.1177/1759091419839515. 1759091419839515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergenthaler P., et al. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36(10):587–597. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke J.G., et al. A biochemical and functional characterization of diet‐induced brain insulin resistance. J. Neurochem. 2005;93(6):1568–1578. doi: 10.1111/j.1471-4159.2005.03155.x. [DOI] [PubMed] [Google Scholar]
- Mirahmadizadeh A., et al. The prevalence of undiagnosed type 2 diabetes and prediabetes in Eastern Mediterranean region (EMRO): a systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2020;160 doi: 10.1016/j.diabres.2019.107931. [DOI] [PubMed] [Google Scholar]
- Moore Z., Taylor J.M., Crack P.J. The involvement of microglia in Alzheimer's disease: a new dog in the fight. Br. J. Pharmacol. 2019;176(18):3533–3543. doi: 10.1111/bph.14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C., et al. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care. 2013:4036–4042. doi: 10.2337/dc13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C., et al. Neuroimaging and its relevance to understanding pathways linking diabetes and cognitive dysfunction. J. Alzheimer's Dis. 2017;59(2):405–419. doi: 10.3233/JAD-161166. [DOI] [PubMed] [Google Scholar]
- Morrison J.H., Baxter M.G. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 2012;13(4):240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins R.J., et al. Insulin resistance as a link between amyloid-beta and tau pathologies in Alzheimer’s disease. Front. Aging Neurosci. 2017;9:118. doi: 10.3389/fnagi.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib R., et al. Cognitive impairment among patients with diabetes in Saudi Arabia: a cross-sectional study. Middle East Curr. Psychiatry. 2020;27(1):1–11. [Google Scholar]
- Pamidi N., BN S.N. Effect of streptozotocin induced diabetes on rat hippocampus. Bratisl. Lek. Listy. 2012;113(10):583–588. doi: 10.4149/bll_2012_130. [DOI] [PubMed] [Google Scholar]
- Párrizas M., et al. Circulating miR-192 and miR-193b are markers of prediabetes and are modulated by an exercise intervention. J. Clin. Endocrinol. Metab. 2015;100(3):E407–E415. doi: 10.1210/jc.2014-2574. [DOI] [PubMed] [Google Scholar]
- Prasad S., et al. Diabetes mellitus and blood-brain barrier dysfunction: an overview. J. Pharmacovigil. 2014;2(2):125. doi: 10.4172/2329-6887.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prattichizzo F., et al. Extracellular microRNAs and endothelial hyperglycaemic memory: a therapeutic opportunity? Diabetes Obes. Metab. 2016;18(9):855–867. doi: 10.1111/dom.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig K.L., et al. Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamani U. Causes of neurodegeneration in diabetes: possible culprits and therapeutic targets. Brain Disord. Ther. 2014;3(4):1–6. [Google Scholar]
- Rama, M.R. and G.R. Sagar, Association of Cognitive Impairment and Type 2 Diabetes Mellitus: A Case-Control Study. 2019.
- Rom S., et al. Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol. Neurobiol. 2019;56(3):1883–1896. doi: 10.1007/s12035-018-1195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi A., et al. The effect of diabetes mellitus on apoptosis in hippocampus: cellular and molecular aspects. Int. J. Prev. Med. 2016:7. doi: 10.4103/2008-7802.178531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanotra M.R., et al. Serum levels of 4-hydroxynonenal adducts and responding autoantibodies correlate with the pathogenesis from hyperglycemia to Alzheimer’s disease. Clin. Biochem. 2022;101:26–34. doi: 10.1016/j.clinbiochem.2021.12.005. [DOI] [PubMed] [Google Scholar]
- Scheltens P., et al. Structural magnetic resonance imaging in the practical assessment of dementia: beyond exclusion. Lancet Neurol. 2002;1(1):13–21. doi: 10.1016/s1474-4422(02)00002-9. [DOI] [PubMed] [Google Scholar]
- Seaquist E.R. The final frontier: how does diabetes affect the brain? Diabetes. 2010;59(1):4–5. doi: 10.2337/db09-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G., et al. Cognitive impairments in type 2 diabetes, risk factors and preventive strategies. J. Basic Clin. Physiol. Pharmacol. 2020;31(2) doi: 10.1515/jbcpp-2019-0105. [DOI] [PubMed] [Google Scholar]
- Shieh J.C.-C., Huang P.-T., Lin Y.-F. Alzheimer’s disease and diabetes: insulin signaling as the bridge linking two pathologies. Mol. Neurobiol. 2020;57(4):1966–1977. doi: 10.1007/s12035-019-01858-5. [DOI] [PubMed] [Google Scholar]
- Sims-Robinson C., et al. How does diabetes accelerate Alzheimer disease pathology? Nat. Rev. Neurol. 2010;6(10):551–559. doi: 10.1038/nrneurol.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SK S., Sun S.W., Ju W.K., Lin S.J., Cross A.H., Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Squire L.R. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992;99(2):195. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Srikanth V., et al. Type 2 diabetes and cognitive dysfunction—towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 2020;8(6):535–545. doi: 10.1016/S2213-8587(20)30118-2. [DOI] [PubMed] [Google Scholar]
- Subramanian M., Vasudevan K., Rajagopal A. Cognitive impairment among older adults with diabetes mellitus in Puducherry: a community-based cross-sectional study. Cureus. 2021;13(1) doi: 10.7759/cureus.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait S.W., Green D.R. Mitochondria and cell signalling. J. Cell Sci. 2012;125(4):807–815. doi: 10.1242/jcs.099234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau F., Gagnon S., Massicotte G. Hippocampal synaptic plasticity and glutamate receptor regulation: influences of diabetes mellitus. Eur. J. Pharmacol. 2004;490(1–3):177–186. doi: 10.1016/j.ejphar.2004.02.055. [DOI] [PubMed] [Google Scholar]
- Turgut Y.B., Turgut M. A mysterious term hippocampus involved in learning and memory. Child'S. Nerv. Syst. 2011;27(12):2023–2025. doi: 10.1007/s00381-011-1513-y. [DOI] [PubMed] [Google Scholar]
- Ueno M., et al. Blood‐brain barrier damage in vascular dementia. Neuropathology. 2016;36(2):115–124. doi: 10.1111/neup.12262. [DOI] [PubMed] [Google Scholar]
- Valente T., et al. Immunohistochemical analysis of human brain suggests pathological synergism of Alzheimer's disease and diabetes mellitus. Neurobiol. Dis. 2010;37(1):67–76. doi: 10.1016/j.nbd.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Valko M., et al. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Van der Borght K., et al. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behav. Neurosci. 2007;121(2):324. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- Vieira M.N., Lima-Filho R.A., De Felice F.G. Connecting Alzheimer's disease to diabetes: underlying mechanisms and potential therapeutic targets. Neuropharmacology. 2018;136:160–171. doi: 10.1016/j.neuropharm.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Wan Q., et al. Recruitment of functional GABA A receptors to postsynaptic domains by insulin. Nature. 1997;388(6643):686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- Weiss N., et al. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2009;1788(4):842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Xia S.-S., et al. The factors contributing to cognitive dysfunction in type 2 diabetic patients. Ann. Transl. Med. 2020;8(4) doi: 10.21037/atm.2019.12.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Guo L., Tian G. Diabetes cognitive impairments and the effect of traditional Chinese herbs. Evid. -Based Complement. Altern. Med. 2013:2013. doi: 10.1155/2013/649396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., et al. Relationship between self-care behavior and cognitive function in hospitalized adult patients with type 2 diabetes: a cross-sectional study. Diabetes Metab. Syndr. Obes.: Targets Ther. 2020;13:207. doi: 10.2147/DMSO.S236966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W.-D., et al. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41(4):625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- You Y., et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta Diabetol. 2021;58(6):671–685. doi: 10.1007/s00592-020-01648-9. [DOI] [PubMed] [Google Scholar]
- Young S.E., Mainous A.G., Carnemolla M. Hyperinsulinemia and cognitive decline in a middle-aged cohort. Diabetes Care. 2006;29(12):2688–2693. doi: 10.2337/dc06-0915. [DOI] [PubMed] [Google Scholar]
- Zampetaki A., et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010;107(6):810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- Zhang J., et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol. Psychiatry. 2011;70(4):334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Zhang W.J., et al. Impairment of hippocampal neurogenesis in streptozotocin‐treated diabetic rats. Acta Neurol. Scand. 2008;117(3):205–210. doi: 10.1111/j.1600-0404.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- Zhao W.-Q., et al. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 2004;490(1–3):71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]