Abstract
Expression of type III proteins of Pseudomonas aeruginosa in patients with cystic fibrosis (CF) was investigated by measuring the immune response against components of the type III pathway. Twenty-three of the 33 sera contained antibodies against PcrV, a protein involved in translocation of type III cytotoxins into eukaryotic cells, and 11 of 33 had antibodies against ExoS, while most CF sera contained antibodies against PopB and PopD, components of the type III apparatus. These data indicate that P. aeruginosa commonly expresses components of the type III translocation apparatus in adult CF patients.
Cystic fibrosis (CF) is the most common lethal autosomal genetic disorder in Caucasians, affecting approximately 1 in 2,500 live births. The CF lung is frequently colonized by one or more bacterial pathogens, especially Staphylococcus aureus and Pseudomonas aeruginosa (1). P. aeruginosa produces a number of virulence determinants, which are either cell surface associated or secreted, which contribute to its pathogenicity. Secreted virulence factors, including proteases (20, 24), phospholipases (28), siderophores (21, 29), and exotoxins (12), provide nutrients for growth, enhance invasive potential, or directly damage host tissue.
The identification of a type III pathway in P. aeruginosa (8) implicates several new classes of cytotoxins as virulence factors of P. aeruginosa, which may act at the site of infection and contribute to subversion of the innate and immune responses of the host. P. aeruginosa produces several type III secreted cytotoxins, including ExoU, ExoY, ExoS, and ExoT. The role of these toxins in P. aeruginosa pathogenicity has been studied in models of acute lung infection and in tissue culture (6, 7, 25–27). The presence of antibodies against P. aeruginosa antigens has been used to implicate the expression of these antigens during chronic infections of CF patients (2, 3, 11, 13, 15, 18, 23). Using this approach, we present evidence that P. aeruginosa expresses components of the type III pathway in chronic lung infections of adult patients with CF.
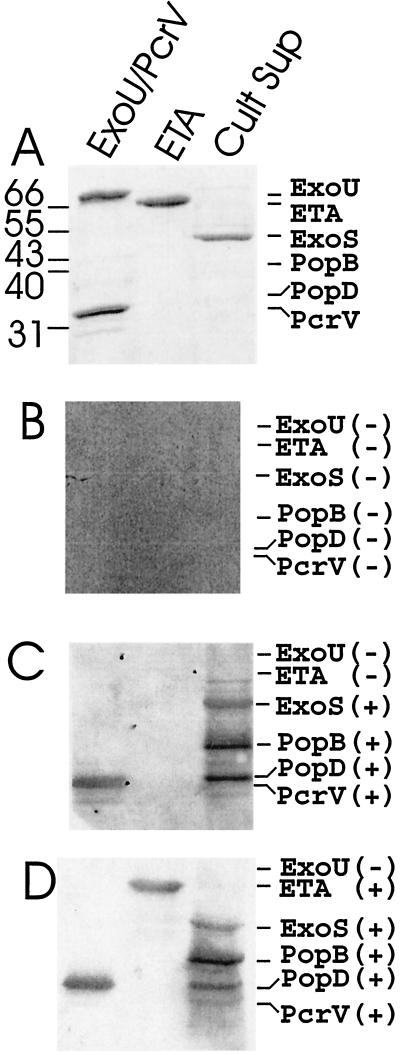
Patients were genotyped to identify their mutation in the CF transmembrane regulator, and sera were collected under National Heart, Lung, and Blood Institute Institutional Review Board protocol 98-H-0062. The presence of antibodies against components of the type III pathway was determined by an enhanced chemiluminesence (ECL) Western blot procedure, using a 1/2,000 dilution of sera, unless noted otherwise. Each analysis included an evaluation of the immune reactivity of serum from patient 4, which served as an internal control and provided a mechanism to evaluate the relative reactivity of each serum (10). In the initial analysis, purified components of the type III system (PcrV and ExoU, recombinant proteins purified from Escherichia coli) and a P. aeruginosa culture extract, which was enriched for ExoS but also contained PopB and PopD, were used as antigens. As a positive control, sera were also assayed for antibodies against exotoxin A (ETA; Berna Products or List Biochemicals), a type II secreted virulence factor, since others (2) have reported the presence of antibodies against ETA in sera of CF patients infected with P. aeruginosa. Reactivity was scored as positive or negative, as shown in Fig. 1 and listed in Table 1. Figure 1A shows the mobility of ExoU (74 kDa), PcrV (33 kDa), ETA (66 kDa), ExoS (49 kDa), PopB (40 kDa), and PopD (34 kDa) during sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Figure 1B shows a Western blot using serum from a healthy non-CF volunteer which did not possess antibodies against these P. aeruginosa antigens. Sera from all of 10 healthy non-CF volunteers lacked antibodies against the six P. aeruginosa antigens (data not shown). Figure 1C and D also shows a Western blot using sera from two patients with CF, which contained antibodies against several components of the type III system of P. aeruginosa. Thirty of the 33 CF sera contained antibodies that recognized PopB, a component of the type III apparatus, which is used to deliver type III cytotoxins into eukaryotic cells. Of the 30 sera that possessed antibodies to PopB, 24 possessed antibodies to PopD, 19 possessed antibodies to PcrV and ETA, 3 possessed antibodies to PcrV but not ETA, and 5 possessed antibodies to ETA but not PcrV. Three sera with antibodies to PopB did not express antibodies to either PcrV or ETA.
FIG. 1.
Immunoreactivity of CF sera to components of the type III secretion apparatus, type III secreted cytotoxins, and the type II secreted ETA. Samples of purified proteins (1 μg) or culture supernatant of P. aeruginosa PA103 (pUCPExoS) were subjected to SDS-PAGE (12% gels) and either stained with Coomassie blue (A) or transferred to nitrocellulose for reaction with serum diluted 1/2,000 from a healthy volunteer (B) or two CF patients (C and D). ECL was used to detect immunoreactivity, with goat anti-human immunoglobulin G-horseradish peroxidase as the secondary antibody. Scans of X-ray film are shown. Panel B was overexposed to enhance potential detection of small amounts of antibodies to the type III proteins in the healthy volunteer serum. At the left of panel A are the positions of (in kilodaltons) molecular weight marker proteins; to the right of panels B to D are the positions of migration of the antigens. Signs in parentheses indicate positive (+) or negative (−) reactivity of the sera.
TABLE 1.
Immunoreactivity patterns of sera from 33 adult patients with CF
| No. of CF patients with indicated immunoreactivity | Immunoreactivitya
|
|||
|---|---|---|---|---|
| PopB | PcrV | ETA | ExoS | |
| 19 | + | + | + | 10b |
| 3 | + | + | − | 1b |
| 5 | + | − | + | − |
| 3 | + | − | − | − |
| 3 | − | − | − | − |
Detection (+) or absence (−) of an immune response to the indicated antigen in sera, diluted 1/2,000, of CF patients when sera from MCW4 elicited an immune response to PcrV (the internal positive control), as determined by ECL detection.
Number of CF patients showing an immune response to the indicated antigens and also ExoS.
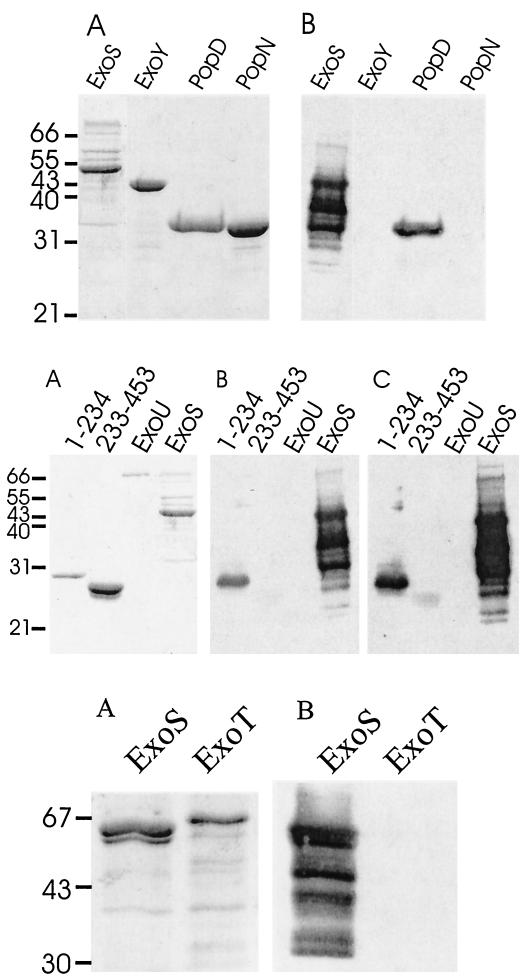
Both native and recombinant forms of ExoS purify as high-molecular-weight aggregates which contain several additional P. aeruginosa proteins (16, 17). This caused concern that the immunoreactivity ascribed to ExoS could be due to reaction with a contaminating protein of similar molecular weight. An experiment was performed to determine if sera that contained antibodies to ExoS also reacted with two recombinant fragments of ExoS that had been produced in E. coli. Figure 2 (middle, A) shows the electrophoretic migration of two deletion peptides of ExoS: ExoS(1–234), which comprises the N-terminal 234 amino acids, and ExoS(233–453), which comprises the C-terminal 222 amino acids (14, 22). Serum MCW27, which reacted strongly with ExoS produced in P. aeruginosa, reacted also with ExoS(1–234) and ExoS(233–453) (Fig. 2, middle, B), indicating that this serum contained antibodies to ExoS. Serum from patient MCW4 also exhibited weak but detectable immunoreactivity with ExoS(1–234) (data not shown). Both sera contained greater reactivity with the N terminus than the C terminus of ExoS, indicating that the N terminus possessed an immunodominant epitope(s). The presence of antibodies against ExoS in these two sera is consistent with the conclusion that P. aeruginosa had produced this cytotoxin during the course of infection. Analysis of immune specificity showed that the responses of serum from patient MCW27 (Fig. 2, bottom, B) or MCW4 (data not shown) were specific to ExoS, with little observed reactivity to ExoT.
FIG. 2.
Detection of anti-PopD and anti-ExoS antibodies in CF serum. Samples of the indicated purified proteins (1 μg) were subjected to SDS-PAGE and stained with Coomassie blue (A) or subjected to Western blotting, using serum at a 1/2,000 dilution from CF patient MCW27. Scans of X-ray film exposed for 10 (B) or 30 (C)s to the ECL-developed Western blot are shown.
PopB and PopD are components of the type III apparatus which are required for translocation of type III secreted cytotoxins into eukaryotic cells (9, 26). To test if the reactivity of CF sera to PopD was specific, the reactivity of serum MCW27 to purified recombinant PopD was determined (Fig. 2, top, B). This serum reacted with recombinant PopD but not other purified P. aeruginosa proteins, confirming that CF sera contained antibodies to PopD. Expression of PopB as a recombinant protein in E. coli has not been completed, which precluded analysis of the reactivity of this serum directly with this antigen.
P. aeruginosa is an opportunistic pathogen which causes both acute and chronic infections in humans. Type III cytotoxins have been associated with epithelial cell pathology (19) and lung injury (5). Specifically, ExoS has been reported to accelerate injury in cultured epithelial cells (19). In addition, PcrV, a component of the type III pathway, can elicit a protective immune response against lung injury mediated by P. aeruginosa (25). These findings demonstrate that the type III cytotoxins contribute to the pathogenicity of P. aeruginosa in acute lung infections. Recent studies have shown that some CF clinical isolates of P. aeruginosa, utilize the type III system to elicit toxicity towards human polymorphonuclear neutrophils, which is independent of the cytotoxin ExoU (4). The presence of antibodies against type III cytotoxins and components of the type III apparatus indicates that P. aeruginosa expresses the type III pathway during chronic infection of the CF lung.
Acknowledgments
This study was supported by grants AI30162 to J.T.B. and AI31665 and AI01289 to D.W.F.
REFERENCES
- 1.Abman S H, Ogle J W, Harbeck R J, Butler-Simon N, Hammond K B, Accurso F J. Early bacteriologic, immunologic, and clinical courses of young infants with cystic fibrosis identified by neonatal screening. J Pediatr. 1991;119:211–217. doi: 10.1016/s0022-3476(05)80729-2. [DOI] [PubMed] [Google Scholar]
- 2.Brauner A, Cryz S J, Granstrom M, Hanson H S, Lofstrand L, Strandvik B, Wretlind B. Immunoglobulin G antibodies to Pseudomonas aeruginosa lipopolysaccharides and exotoxin A in patients with cystic fibrosis or bacteremia. Eur J Clin Microbiol Infect Dis. 1993;12:430–436. doi: 10.1007/BF01967437. [DOI] [PubMed] [Google Scholar]
- 3.Cordon S M, Elborn J S, Rayner R J, Hiller E J, Shale D J. IgG antibodies in early Pseudomonas aeruginosa infection in cystic fibrosis. Arch Dis Child. 1992;67:737–740. doi: 10.1136/adc.67.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dacheux D, Toussaint B, Richard M, Brochier G, Croize J, Attree I. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils. Infect Immun. 2000;68:2916–2624. doi: 10.1128/iai.68.5.2916-2924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish J P, Fleiszig S M, Wu C, Mende-Mueller L, Frank D W. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 6.Fleiszig S M, Lee E J, Wu C, Andika R C, Vallas V, Portoles M, Frank D W. Cytotoxic strains of Pseudomonas aeruginosa can damage the intact corneal surface in vitro. CLAO J. 1998;24:41–47. [PubMed] [Google Scholar]
- 7.Fleiszig S M, Wiener-Kronish J P, Miyazaki H, Vallas V, Mostov K E, Kanada D, Sawa T, Yen T S, Frank D W. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank D W. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol. 1997;26:621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- 9.Frithz-Lindsten E, Holmstrom A, Jacobsson L, Soltani M, Olsson J, Rosqvist R, Forsberg A. Functional conservation of the effector protein translocators PopBYopB and PopDYopD of Pseudomonas aeruginosa and Yersinia pseudotuberculosis. Mol Microbiol. 1998;29:1155–1165. doi: 10.1046/j.1365-2958.1998.00994.x. [DOI] [PubMed] [Google Scholar]
- 10.Ganesan A K, Vincent T S, Olson J C, Barbieri J T. Pseudomonas aeruginosa exoenzyme S disrupts Ras-mediated signal transduction by inhibiting guanine nucleotide exchange factor-catalyzed nucleotide exchange. J Biol Chem. 1999;274:21823–21829. doi: 10.1074/jbc.274.31.21823. [DOI] [PubMed] [Google Scholar]
- 11.Giordano A, Magni A, Filadoro F, Graziani C, Quattrucci S, Cipriani P. Study of IgG antibodies to Pseudomonas aeruginosa in early cystic fibrosis infection. New Microbiol. 1998;21:375–378. [PubMed] [Google Scholar]
- 12.Iglewski B H, Liu P V, Kabat D. Mechanism of action of Pseudomonas aeruginosa exotoxin A adenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect Immun. 1977;15:138–144. doi: 10.1128/iai.15.1.138-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klinger J D, Straus D C, Hilton C B, Bass J A. Antibodies to proteases and exotoxin A of Pseudomonas aeruginosa in patients with cystic fibrosis: demonstration by radioimmunoassay. J Infect Dis. 1978;138:49–58. doi: 10.1093/infdis/138.1.49. [DOI] [PubMed] [Google Scholar]
- 14.Knight D A, Finck-Barbancon V, Kulich S M, Barbieri J T. Functional domains of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1995;63:3182–3186. doi: 10.1128/iai.63.8.3182-3186.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kronborg G, Fomsgaard A, Galanos C, Freudenberg M A, Hoiby N. Antibody responses to lipid A, core, and O sugars of the Pseudomonas aeruginosa, lipopolysaccharide in chronically infected cystic fibrosis patients. J Clin Microbiol. 1992;30:1848–1855. doi: 10.1128/jcm.30.7.1848-1855.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulich S M, Frank D W, Barbieri J T. Expression of recombinant exoenzyme S of Pseudomonas aeruginosa. Infect Immun. 1995;63:1–8. doi: 10.1128/iai.63.1.1-8.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulich S M, Frank D W, Barbieri J T. Purification and characterization of exoenzyme S from Pseudomonas aeruginosa 388. Infect Immun. 1993;61:307–313. doi: 10.1128/iai.61.1.307-313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagace J, Peloquin L, Kermani P, Montie T C. IgG subclass responses to Pseudomonas aeruginosa a- and b-type flagellins in patients with cystic fibrosis: a prospective study. J Med Microbiol. 1995;43:270–276. doi: 10.1099/00222615-43-4-270. [DOI] [PubMed] [Google Scholar]
- 19.McGuffie E M, Frank D W, Vincent T S, Olson J C. Modification of Ras in eukaryotic cells by Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1998;66:2607–2613. doi: 10.1128/iai.66.6.2607-2613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIver K S, Kessler E, Olson J C, Ohman D E. The elastase propeptide functions as an intramolecular chaperone required for elastase activity and secretion in Pseudomonas aeruginosa. Mol Microbiol. 1995;18:877–889. doi: 10.1111/j.1365-2958.1995.18050877.x. [DOI] [PubMed] [Google Scholar]
- 21.Meyer J M, Stintzi A, Poole K. The ferripyoverdine receptor FpvA of Pseudomonas aeruginosa PAO1 recognizes the ferripyoverdines of P. aeruginosa PAO1 and P. fluorescens ATCC 13525. FEMS Microbiol Lett. 1999;170:145–150. doi: 10.1111/j.1574-6968.1999.tb13367.x. [DOI] [PubMed] [Google Scholar]
- 22.Pederson K J, Vallis A J, Aktories K, Frank D W, Barbieri J T. The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol Microbiol. 1999;32:393–401. doi: 10.1046/j.1365-2958.1999.01359.x. [DOI] [PubMed] [Google Scholar]
- 23.Pressler T, Pedersen S S, Espersen F, Hoiby N, Koch C. IgG subclass antibody responses to alginate from Pseudomonas aeruginosa in patients with cystic fibrosis and chronic P. aeruginosa infection. Pediatr Pulmonol. 1992;14:44–51. doi: 10.1002/ppul.1950140109. [DOI] [PubMed] [Google Scholar]
- 24.Rust L, Pesci E C, Iglewski B H. Analysis of the Pseudomonas aeruginosa elastase (lasB) regulatory region. J Bacteriol. 1996;178:1134–1140. doi: 10.1128/jb.178.4.1134-1140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawa T, Yahr T L, Ohara M, Kurahashi K, Gropper M A, Wiener-Kronish J P, Frank D W. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med. 1999;5:392–398. doi: 10.1038/7391. [DOI] [PubMed] [Google Scholar]
- 26.Vallis A J, Finck-Barbancon V, Yahr T L, Frank D W. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect Immun. 1999;67:2040–2044. doi: 10.1128/iai.67.4.2040-2044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallis A J, Yahr T L, Barbieri J T, Frank D W. Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect Immun. 1999;67:914–920. doi: 10.1128/iai.67.2.914-920.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasil M L, Graham L M, Ostroff R M, Shortridge V D, Vasil A I. Phospholipase C: molecular biology and contribution to the pathogenesis of Pseudomonas aeruginosa. Antibiot Chemother. 1991;44:34–47. doi: 10.1159/000420295. [DOI] [PubMed] [Google Scholar]
- 29.Vasil M L, Ochsner U A, Johnson Z, Colmer J A, Hamood A N. The Fur-regulated gene encoding the alternative sigma factor PvdS is required for iron-dependent expression of the LysR-type regulator PtxR in Pseudomonas aeruginosa. J Bacteriol. 1998;180:6784–6788. doi: 10.1128/jb.180.24.6784-6788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]