Abstract
Objective
Though multicomponent exercise training was found beneficial in improving the physical functionality, the effects of multicomponent exercise training on muscle oxygenation are still unclear. The purpose of this study was to investigate the effects of multicomponent exercise training on muscle oxygenation in young and older participants.
Methods
In this study, 17 young adults (Y) and 18 healthy older adults (E) were recruited to receive a multicomponent exercise training for 12 weeks, 2–3 sessions per week. Muscle oxygenation, muscle strength, and electromyography data were collected and compared pre- and post-training. Muscle oxygen saturation (SpO2) during isometric knee extension tests involving voluntary contraction (VOL) and electrical stimulation (ES) was measured by near-infrared spectroscopy. The SpO2 kinetics in the contraction and recovery phases were calculated using a tangential model to extract ΔSpO2 and inflection time (IF).
Results
Muscle strength significantly increased in the post-training (234.31 ± 83.2 N·m, p < 0.05). The post-training ΔSpO2 of the ES in the Y (8.43 ± 5.35%) significantly increased and was higher than that in the E (2.78 ± 3.03%, p < 0.05). In the recovery phase, the post-training IF of VOL (7.07 ± 3.31s) was significantly shorter than that of the pre-training period (8.73 ± 4.46s, p < 0.05). Additionally, the median frequency of electromyography significantly decreased in the post-training period (103.84 ± 21.75 Hz, p < 0.05).
Conclusion
The multicomponent exercise training improved the muscle strength, neuromuscular performance, and muscle aerobic function irrespective of age. The primary adaptation of the muscles to the multicomponent exercise training between the two groups varied.
Keywords: Near infrared spectroscopy, Oxygen demand, Exercise training, Electrical stimulation
1. Introduction
A multicomponent exercise training is an effective method for improving and maintaining the physical function and performance in older adults, specifically when the aerobic and resistance exercises are combined.1,2 Evidence suggests that multicomponent training has positive effects on muscle mass, muscle strength, and cardiorespiratory fitness in older adults.3 The exercise training type is the key factor that influences the adaptation of the intramuscular oxygenation performance.4,5 Aerobic exercise training improves the muscle oxidative capacity,1 whereas resistance training increases the oxygen demand of the contracting muscles.6 However, the influence of multicomponent exercise training on the muscle metabolism is still not completely understood.
A decline in the muscle oxidative capacity during exercise is directly associated with age-related changes in muscle composition.7,8 Muscle performance, measured on the basis of the oxidative metabolism, is influenced by the preferential atrophy of type II fibres usually associated with aging.9 Furthermore, changes in the muscle composition affect the microvascular O2 pressure, thereby decreases or increases O2 extraction in the contracting muscles.8,10 Compared with young adults, older adults require considerably more recovery time for muscle reoxygenation during the recovery phase, whereas their muscle deoxygenation is faster during the exercise phase11,12; moreover, age influences the oxygenation performance.11,13
The recruitment pattern of the human skeletal muscles during an electrical stimulation (ES) is nonselective.14 Therefore, fast motor units can be activated at relatively low force levels.14 The composition and recruitment of the fast muscle fibers is indirectly reflected through the neuromuscular ES.15 During such stimulation, the recruitment of the fast motor units increases the oxygen demand of the contracting muscles and enhances the oxygen saturation (SpO2) level in the recovery phase.16,17 Evaluating the ES-induced muscle metabolic change can provide a comprehensive understanding of the muscle function.18 Moreover, a study on neuromuscular changes reported a significant reduction in the threshold value of the motor unit recruitment following a short-term muscle strengthening training.19 Therefore, an increase could be observed in the oxygen demand during the ES-induced muscle contraction following a short-term exercise training because of the decreased threshold of the motor unit recruitment.
Various approaches, including the transcutaneous oxygen measurement, pulse oximetry, and functional magnetic resonance imaging,20,21 have been used for examining tissue oxygenation caused by oxygen utilization in the skeletal muscles. Near infrared spectroscopy (NIRS) is a recently developed, noninvasive optical technique that has been used for evaluating the hemodynamics of the muscle tissues.22, 23, 24 The different wavelengths of near infrared light were used for estimating the light absorption of oxygenated and deoxygenated hemoglobin that can reflect oxygenation performance in contracting muscle.25 Furthermore, one advantage of NIRS compared to other techniques is its ability to measure the instantaneous oxygenation response in a less restricted environment.26 Thus, NIRS is extensively used for monitoring the muscle oxygenation performance during dynamic exercises22 and is a valid and reliable technique reflecting the adaptation of exercise training.20 Furthermore, it was suggested that the application of NIRS is suitable for aging related issues. Research are needed to further investigate the influence of different exercise training modalities on muscle oxygenation for older adults.5
Most studies have investigated the influence of single component exercise training by including one or several age-based groups at a time4,6; however, few studies have examined the metabolic adaptation of muscle oxygenation after multicomponent exercise training.5 Notably, no study has yet compared the adaptation of muscle oxygenation after multicomponent exercise training between young and older adult groups. To bridge this gap, this study examined the effects of a 12-week multicomponent exercise training regimen on the adaptation of muscle oxygenation through NIRS and compared them between young and older participants. The influences of multicomponent exercise training regimen on the muscle oxygenation kinetics was investigated in the muscle contraction and recovery phases, which included voluntary contraction (VOL) and ES-induced contraction. Furthermore, the effects of training on neuromuscular changes were determined on the basis of the surface electromyographic data, which was quantified using the median frequency (MF) of the frequency spectrum.
2. Methods
2.1. Participants
Seventeen young adults (10 females and 7 males, 19.70 ± 1.59 years) and 18 healthy, community-dwelling older adults (11 females and 7 males, 61.57 ± 5.02 years) participated in the study. No participants had any cardiac and musculoskeletal diseases. After being fully informed of the procedures, the participants gave their written consent. The experiments were approved by the Human Experiment and Ethics Committee of National Cheng Kung University Hospital, Taiwan (B-ER-100-404).
2.2. Experimental design
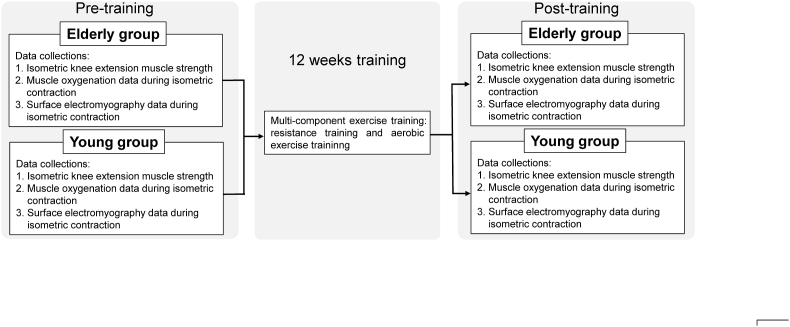
Fig. 1. shows the experimental design of this study. The young (Y) and older adult (E) groups received a supervised multicomponent exercise training regimen in 2–3 sessions per week for 12 weeks, prior to which they had no exercise training experience. Data on the maximal muscle strength during isometric knee extension, muscle oxygenation, and electromyography collected from pre- and post-assessments were compared.
Fig. 1.
Schematic of the experimental design. The maximal muscle strength during isometric knee extension, muscle oxygenation, and electromyography data of the vastus lateralis muscle for both groups were collected and compared at the pre- and post-training periods.
2.3. Multicomponent exercise training regimen
Table 1 shows the multicomponent exercise training regimen of this study that is comprised of aerobic exercise and resistance training. All participants performed a 45-min aerobic exercise after 30 min of resistance training. The resistance training consisted of lower and upper body exercises was performed on weight machines. The lower limb exercises included seated leg extensions, double-leg presses, and leg curls. The upper limb exercises included vertical bench presses, seated latissimus pull downs, tricep pushdowns, and bicep curls. The training intensity was 15 repetitions maximum (RM) with two sets per exercise throughout the 12-week training period and the repetition tempo was required for 2 s eccentric phase and 1 s concentric phase, respectively. According to the overload principle, when 15 repetitions of the subscribed exercise were accomplished for three consecutive training days, the load was increased by 1.5–10 kg based on the upper or lower body as well as the participant's physical condition.
Table 1.
Multicomponent exercise training regimen.
| Duration | Training content | Intensity | Time | Frequency |
|---|---|---|---|---|
|
12 Weeks |
Resistance training |
15 repetitions maximum |
30 min |
2–3 days per week |
| ||||
| Aerobic exercise |
Weeks 1–4: RPE 13 Weeks 4–8: RPE 13-14 Weeks 9–12: RPE 13-15 |
45 min | ||
|
RPE, rating of perceived exertion.
The aerobic exercise included 5–10 min of warm-up, a 30-min aerobic dance training session, and a 10 to 15-min cool-down period. The aerobic dance exercise included walk in place, run in place, heel steep, jumping jacks, knee jumps, grapevine, V step and other combined exercises. A rating of perceived exertion (RPE) scale was used for determining the intensity of the aerobic dance exercise. The Borg scale is the most frequently used RPE scale utilizing a rating range of 6–20.27 In this study, the exercise intensity of the aerobic dance training was set to and controlled at the RPE scales of 13–15 for each participant. In addition, all exercise sessions were supervised for ensuring correct lifting and landing techniques and for monitoring the appropriate durations of the exercise and rest intervals.
2.4. Evaluation of muscle oxygenation through NIRS
Instantaneous muscle oxygenation measurements were estimated noninvasively by using the frequency domain NIRS (Imagent, ISS Inc., Champaign, IL, USA). NIR light of two wavelengths (690 and 830 nm) were used for estimating the optical parameters of hemoglobin; they were delivered to the muscle tissue by an emitter.28,29 Because of the light scattering effect, NIR light travels through the muscle tissue and is collected by the detector. The changes in the absorption and scattering coefficients at two wavelengths can be used to estimate the oxygenated hemoglobin (HbO2) and deoxygenated hemoglobin (Hb) concentrations,29 following which the blood volume (total hemoglobin concentration, [tHb] = [HbO2]+[Hb], μM) and the SpO2 (100 × [HbO2]/[tHb], %) were derived.28 The SpO2 reflects the dynamic balance between the oxygen delivery and oxygen consumption in the investigated skeletal muscle tissue. A study reported that the SpO2 can be an indirect indicator of the muscle oxygen demand, and a considerable decrease in the SpO2 indicates a higher oxygen demand in the contracting muscle.30 In this study, the SpO2 data were collected from the vastus lateralis (VL) muscle by using a multidistance probe at a 5-Hz sampling rate.
The skin was first cleaned and abraded with alcohol to remove contamination before taking measurements. To reduce motion artefacts, the NIRS probe was firmly placed on the skin overlying the middle part of the VL and was secured using an adhesive tape and elastic wrap. The NIRS multidistance probe has four emitter positions with emitter–detector distances ranging from 1.95 to 3.53 cm. The thicknesses of the adipose tissue at the site of the probe, measured using a skinfold calliper (Slim Guide, Creative Health Products, Plymouth, MI, USA), were 7.89 ± 3.48 and 6.77 ± 2.16 mm in the Y and E groups, respectively. Because the adipose tissue thickness was less than half the source–detector distance,31 the penetration depth of the NIRS signal thus reasonably reflected the hemodynamic change in the VL muscle. Moreover, the multiple-distance probe was applied to minimize the effect of individual adipose tissue thickness on effective optical signals.32 For the pre-training measurements, the optimal light intensity with effective optical coefficients was estimated using an NIRS device for each participant. The recorded optimal light intensity was used for the post-training measurements for reducing variations between the two oxygenation measurements. The measurements of muscle oxygenation were recorded throughout the examination comprising 30-s rest and isometric contraction phases and a 120-s recovery phase.
2.5. Muscle oxygenation measurement during the voluntary and ES-induced isometric contractions
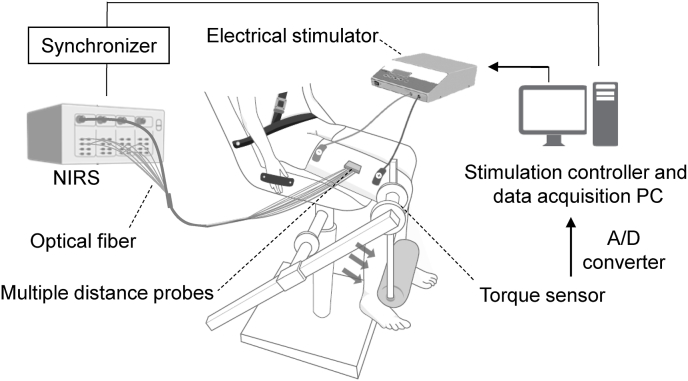
To collect the NIRS data during the voluntary isometric contraction, all participants performed an isometric knee extension test by using a custom-made isometric strength device, as described elsewhere.6 The torque value (N·m) of the knee extension was displayed on a monitor placed in front of the participant performing the test for real-time visual feedback. An external trigger was used for synchronizing the NIRS and torque acquisitions during the isometric knee extension test. In addition to the voluntary contraction, participants from both groups performed an ES-induced isometric knee extension test for acquiring muscle oxygenation data (Fig. 2). A commercial biphasic electrical stimulator (ZMI Inc., Taiwan) was used with a 20 Hz stimulus frequency and 300 pulse width for recording the neuromuscular ES. The electrodes (10 4.5 cm2) were positioned such that the cathode was over the motor point of the quadriceps and the anode was positioned center-to-center at 20 cm distal to the cathode. The start and end of the neuromuscular ES were controlled using a stimulation controller.
Fig. 2.
Experimental setup. The participant sat on a custom-made isometric torque measurement device with an electrical stimulator. An electrical stimulator is used to generate an electrical current into the right quadriceps for stimulating muscle. A multidistance optical probe was placed on the vastus lateralis muscle for acquiring the muscle oxygenation data during the isometric contraction. A/D: analogue to digital converter, NIRS: near infrared spectroscope, PC: personal computer.
At pre-training, the maximal voluntary contraction (MVC) of isometric knee extension was evaluated using a Biodex System 3 PRO dynamometer (Biodex Medical Systems Inc., Shirley, NY, USA), and the torque value of 20% MVC was set as the target level for the voluntary isometric knee extension test. After the MVC test, the participants were asked to perform isometric knee extension test by using a custom-made isometric strength device under two conditions: (1) an ES at 50 mA for 30 s and (2) a VOL at a relative exercise intensity (20% MVC) for 30 s. To avoid muscle fatigue, the participants were given sufficient time to rest between the tests. During the two types of isometric knee extension tests, the start and end of the exercise were signaled to the participants by visual and audio cues. Moreover, some instructed trials were provided for all participants for familiarizing with isometric knee extension at the target torque level. After completing the 12 weeks of training, both groups repeated the ES-induced and voluntary isometric contraction tests at the same exercise intensities as pre-training for additional muscle oxygenation measurements.
2.6. Surface electromyography measurement during the voluntary isometric contraction
The surface electromyographic (sEMG) activity was recorded continuously during the voluntary isometric contraction by using a Biopac MP100 data acquisition system with EMG 100C amplifier (Biopac Systems Inc., Santa Barbara, CA, USA). The VL muscle sEMG activity was monitored using two surface Ag–AgCl circular electrodes positioned 2 cm apart over the distal half of the muscle belly and aligned longitudinally along the muscle fibers. The ground electrode was placed over the bony medial epicondyle area of the femur. The sEMG signals were amplified at a gain of 500 and then sampled at 2 k·Hz for further processing. Before positioning the electrodes for measurements, the skin was abraded for reducing the skin–electrode impedance values to less than 5 kΩ.
2.7. Data analysis
A hyperbolic tangent equation was used to fit the muscle tissue oxygenation curves (Y) measured during the isometric contraction and recovery phases as a function of time (t) for determining the SpO2 kinetics:
In the contraction phase, the ΔSpO2 was estimated using the maximum and minimum SpO2 values by using the parameters d−a and d+a, respectively, and the ΔSpO2 was used as an indirect indicator of the muscle oxygen demand.6 The coefficients derived in the recovery phase, the IF and c/b, represent the time required for yielding a range of 50% of the change. The IF reflects the half-reoxygenation time of the SpO2 in the recovery phase and was used for evaluating the effect of exercise training on the muscle aerobic function.28
In addition to the SpO2 kinetics data, frequency spectrum analysis was conducted for evaluating the sEMG data. The raw sEMG signals were processed using a band-pass filter (bandwidth: 10–400 Hz) and detrended prior to performing the fast Fourier transform. The MF was then calculated for determining the distribution of the sEMG frequency content. All data analyses were performed using Matlab (Version 7.6, The MathWorks Inc., Natick, MA, USA).
2.8. Statistical analyses
The data on the physical characteristics of the participants, namely the age, height, body weight, body mass index, adipose tissue thickness at the site of the NIRS probe, and maximal muscle strength during isometric knee extension, the SpO2 kinetic parameters, namely ΔSpO2 and IF, and the MF of the sEMG are presented as the mean ± standard deviation (SD). The adipose tissue thickness, muscle strength, SpO2 kinetics, and MF data were analyzed using the two-way analysis of variance (ANOVA) for the mixed design, and a post hoc test was conducted for assessing the effects of age (as an inter-participant factor) and training (as an intra-participant factor) on the measured variables. When an interaction was observed, the differences for each measured variable between the pre- and post-training periods were compared using the paired t-test. The differences between the groups for each measured variable were compared using the independent t-test. The statistical analyses were performed using SPSS software (Version 17.0, SPSS Inc., Chicago, IL, USA). The results were considered statistically significant at p < 0.05.
3. Results
3.1. Participant characteristics
Baselines for the physical characteristics of the participants are given in Table 2. No significant difference in the adipose tissue thickness at the site of the NIRS probe between the two groups was observed. The two-by-two factorial ANOVA revealed significant effects of training and age on the muscle strength. The post-training muscle strength in both Y and E groups was significantly higher (234.31 ± 83.2 N·m) than was the pre-training muscle strength (210.07 ± 87.11 N m) (F = 5.470, p = 0.026, η2 = 0.154). However, the muscle strength of the E group at both pre- and post-training periods was significantly lower (185.85 ± 50.23 N m) (F = 6.875, p = 0.014, η2 = 0.186) than that of the Y group at both training periods (258.15 ± 88.05 N·m).
Table 2.
Physical characteristics of the participants (mean ± SD).
| E group | Y group | |
|---|---|---|
| Age (years) | 61.50 ± 5.15 | 19.82 ± 1.59 |
| Height (cm) | 159.61 ± 8.09 | 166.11 ± 7.80 |
| Body weight (kg) | 63.76 ± 9.56 | 62.54 ± 10.51 |
| BMI (Body weight/height2) | 24.89 ± 2.69 | 22.61 ± 3.10 |
| pre-training | post-training | pre-training | post-training | Main effects | |
|---|---|---|---|---|---|
| Adipose tissue thickness (mm) | 6.94 ± 2.59 | 6.70 ± 2.48 | 7.81 ± 3.65 | 7.81 ± 3.59 | – |
| Maximal muscle strength (N·m) | 181.60 ± 44.98 | 190.10 ± 53.1 | 238.54 ± 86.88 | 278.52 ± 86.18 | §※p < 0.05 |
BMI, body mass index; E, older group; NIRS, near infrared spectroscopy; Y, young group.
Two-by-two factorial ANOVA results (p < 0.05): §main effect for age; ※main effect for training; #interaction effect; − no main effect or interaction effect.
3.2. Oxygen saturation kinetics
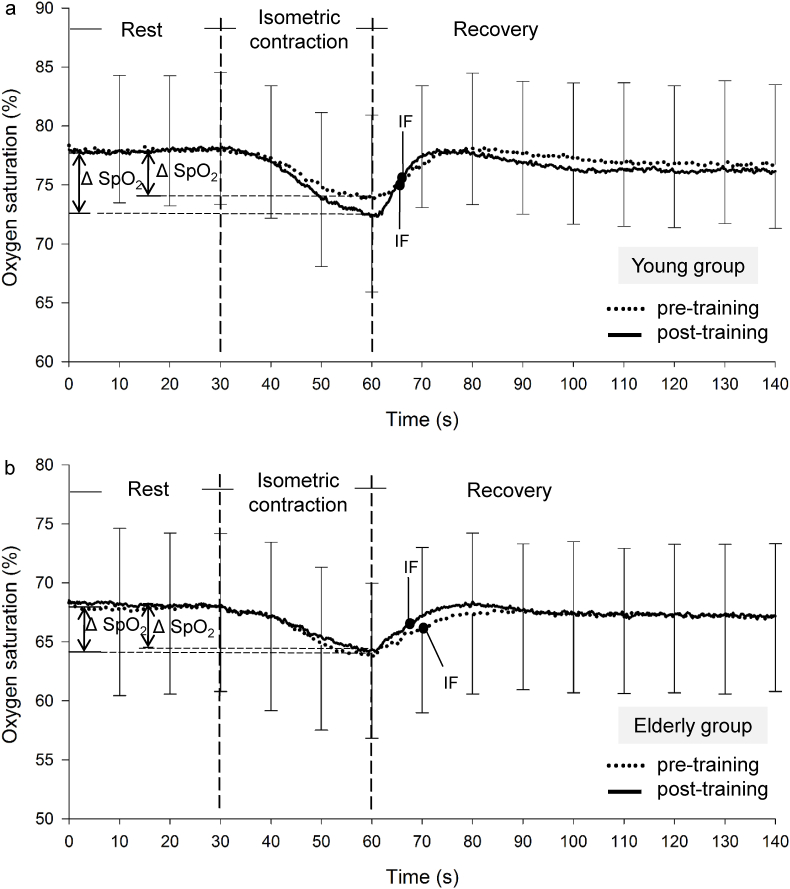
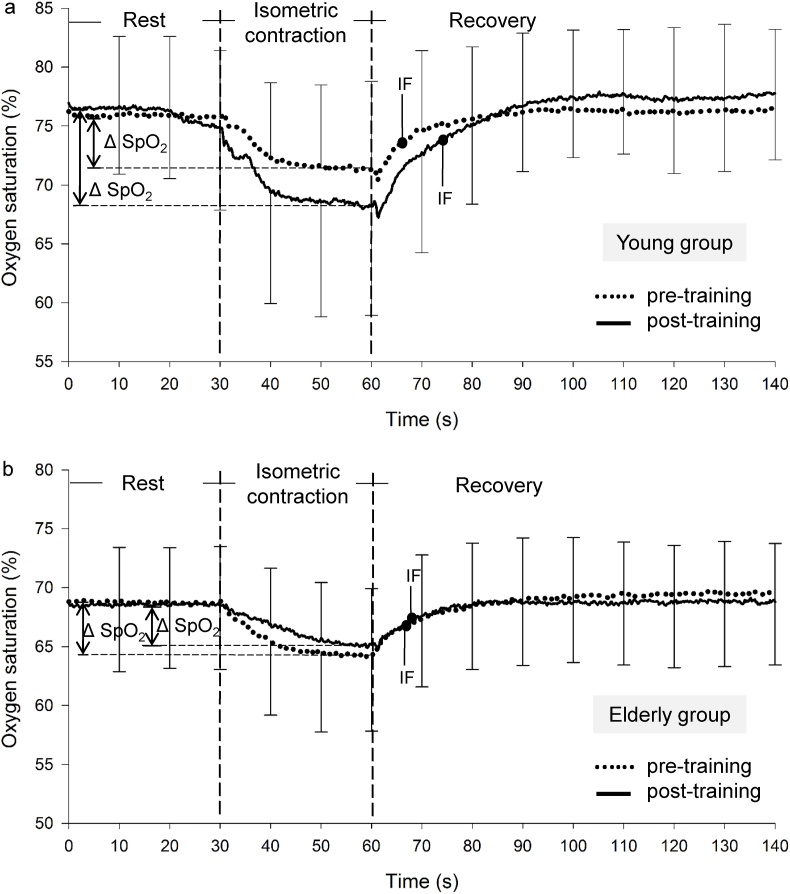
Fig. 3 shows the representative SpO2 kinetics at the pre- and post-training periods recorded during the VOL test performed by the participants from both groups. The SpO2 decreased in all participants from the beginning of the isometric contraction. After the 12 weeks of training, the ΔSpO2 did not significantly increase or decrease in either group. However, the decreased IF in the recovery phase at the post-training period was more apparent in the E group than in the Y group. Fig. 4 shows the representative SpO2 kinetics at the pre- and post-training periods recorded during the 50 mA ES test for both groups; the ΔSpO2 of the Y group but not the E group increased in the post-training tests.
Fig. 3.
Typical oxygen saturation (SpO2) data during the voluntary contraction (VOL). The SpO2 kinetics data (mean ± SD) measured during the voluntary contraction at pre- (…) and post- (—) trainings in the young (a) and older (b) groups. Vertical dashed lines represent the beginning (at 30 s) and the end (at 60 s) of the isometric contraction.
Fig. 4.
Typical oxygen saturation (SpO2) data during the electrical stimulation (ES). The SpO2 kinetics data (mean ± SD) measured during a 50 mA electrical stimulation at the pre- (…) and post- (—) trainings in the young (a) and older (b) groups. Vertical dashed lines represent the beginning (at 30 s) and the end (at 60 s) of the isometric contraction.
Table 3 shows the SpO2 kinetics data of the voluntary and ES-induced contractions recorded during the muscle contraction and recovery phases. The two-by-two factorial ANOVA revealed a significant age and training and the ΔSpO2 of the ES (F = 5.009, p = 0.032, η2 = 0.132). The results of the paired t tests showed that the ΔSpO2 of the ES increased significantly in the Y group (p = 0.034) after the 12 weeks of training. The independent t tests showed that the post-training ΔSpO2 of the ES was significantly higher in the Y group than in the E group (p = 0.000).
Table 3.
SpO2 kinetics and median frequency of sEMG (mean ± SD).
| E (pre-training) | E (post-training) | Y (pre-training) | Y (post-training) | ||
|---|---|---|---|---|---|
| Contraction phase | Interaction | ||||
| ΔSpO2 (VOL) | 4.03 ± 2.61 | 3.72 ± 2.13 | 4.04 ± 1.43 | 5.21 ± 5.28 | – |
| ΔSpO2 (ES) | 3.11 ± 3.42 | 2.78 ± 3.03 | 4. 91 ± 3.63 | d&8.43 ± 5.35 | cp < 0.05 |
| Recovery phase | Main effects | ||||
| IF (s) (VOL) | 10.66 ± 4.82 | 7.84 ± 3.76 | 6.69 ± 2.99 | 6.25 ± 2.63 | a,bp < 0.05 |
| IF (s) (ES) | 8.27 ± 5.93 | 7.96 ± 4.85 | 7.49 ± 5.17 | 12.21 ± 8.11 | – |
| Contraction phase | Main effects | ||||
| MF (Hz) | 111.41 ± 25.02 | 103.90 ± 26.20 | 119.06 ± 18.83 | 103.79 ± 17.01 | bp < 0.05 |
E, older group; ES, electrical stimulation; IF, inflection time; MF, median frequency; sEMG, surface electromyography; SpO2, oxygen saturation; VOL, voluntary contraction; Y, young group.
Two-by-two factorial ANOVA results (p < 0.05):
main effect for age;
main effect for training;
interaction effect; − no main effect or interaction effect; &significant difference compared with the pre-training period;
significant difference compared with the E group at the post-training period.
The IF was analyzed in the recovery phase. The two-by-two factorial ANOVA revealed significant effects for training and age. The post-training IF of the VOL in both Y and E groups was significantly shorter (7.07 ± 3.31s) than the pre-training IF (8.73 ± 4.46s) (F = 4.398, p = 0.044, η2 = 0.118). The IF of the VOL of the E group in the pre- and post-training periods was significantly longer (9.25 ± 4.49s) (F = 8.240, p = 0.007, η2 = 0.200) than that of the Y group at both training periods (6.47 ± 2.78s).
3.3. MF of the surface electromyographic frequency spectrum
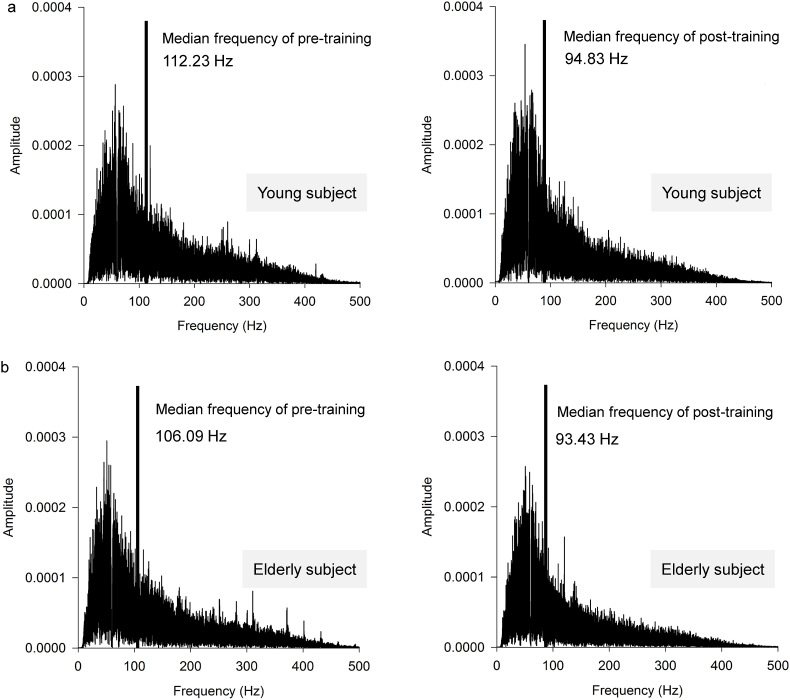
Fig. 5 shows a representative frequency spectrum of the sEMG. The MF decreased in the post-training period in both the older and young participants; however, the decrease was higher among the young participants. The results of the two-by-two factorial ANOVA revealed significant effects for training. The post-training MF value in both Y and E groups was significantly lower (103.84 ± 21.75 Hz) than the pre-training MF value (115.23 ± 22.15 Hz) (F = 5.809, p = 0.022, η2 = 0.154).
Fig. 5.
Typical surface electromyographic frequency spectrum (sEMG) data. The sEMG data measured during the voluntary contraction (VOL) at the pre- and post-trainings in the young (a) and older (b) participants. Vertical solid lines represent the median frequency at the pre- and post-trainings.
4. Discussion
To further investigate the influence of a multicomponent exercise training on the skeletal muscles, the oxygen demand of the working muscles and the muscle aerobic function were evaluated through NIRS, and neuromuscular changes were estimated through the sEMG. The major findings of this study are as follows: (1) The multicomponent exercise training improved the muscle performance irrespective of age. (2) A considerable increase in the muscle strength observed in the young participants was possibly because of the improvements in the neuromuscular performance, whereas the older participants showed an evident decrease of reoxygenation time in the recovery phase.
4.1. ΔSpO2 in the VOL and ES-induced contraction phases
To avoid a severe blood flow occlusion,6 muscle oxygenation was evaluated at a low isometric contraction intensity. The ΔSpO2 during the VOL isometric contraction showed no significant difference at the pre-training period between the two groups, and the ΔSpO2 for both groups did not significantly increase after the 12 weeks of training regimen (Table 3). Exercise training increases the capillary density, oxidative capacity, and mitochondrial density, thus increasing oxygen extraction from arterioles and capillaries in the skeletal muscles.4 Multicomponent exercise training has beneficial effects on the aerobic capacity.1 A study on resistance training indicated that a long-term training regimen increases the oxygen demand of the working muscles in both young and older participants.6 However, in this study, the multicomponent exercise training regimen did not increase the ΔSpO2 during muscle contraction. Although the muscle strength significantly increased in the post-training period (Y group, + 16.76%; E group, + 4.68%), the metabolism requirement of the working muscle based on the oxygen demand did not change. By contrast, an earlier study indicated that exercise training increases the motor unit recruitment, firing rate, and synchronization.33 The related neural alterations can be observed as a decrease in the frequency of the sEMG signal or an increase in the sEMG amplitude.33 In this study, the post-training MF in both Y and E groups was significantly lower than at the pre-training MF, and the Y group showed a larger decrease (−12.82%) than the E group (−6.74%) did. Therefore, the sEMG and ΔSpO2 data of the contraction phase suggested that the increased muscle strength following the multicomponent exercise training regimen was accompanied by the neuromuscular changes but did not show a significant increase in the metabolic requirements during the VOL.
In contrast to the E group, the ΔSpO2 of ES in the Y group significantly increased at the post-training period. The influence of the adipose tissue thickness on the ES was limited because no significant difference was observed in the thickness between the two groups. The recruitment patterns of the human skeletal muscles during the ES are different to those during the VOL. The ES can be used to preferentially activate the fast motor units at relatively low force levels.14 Studies on the ES evaluated the level of muscle oxygenation and indicated that preferentially activating the fast motor units increases the oxygen demand.17,30 Moreover, studies have indicated that the force development and neural drive of the skeletal muscles can be enhanced through resistance training.34 Specifically in young adults, an enhanced muscle strength following a training regimen is accompanied by a reduction in the motor unit recruitment thresholds.19 According to the results of this study, the 12-week multicomponent training regimen significantly increased the muscle strength. Thus, the resistance training of the multicomponent training regimen might have decreased the motor unit recruitment threshold for the Y group, enhancing the recruitment of the fast motor units during the ES and resulting with a high oxygen demand. However, the ΔSpO2 of the ES did not increase in the E group, suggesting a significant difference in the training adaptation between the older and young participants. Specifically, the primary muscle adaptation to the multicomponent exercise training regimen in the older participants may not reflect the improvement in the motor unit recruitment.
4.2. IF in the recovery phase
The IF of the VOL in the recovery phase was longer in the E group (9.25 ± 4.49s) than in the Y group (6.47 ± 2.78s). Ichimura et al. suggested that the half-recovery time of muscle oxygenation in older adults is longer because of the impaired muscle aerobic function.12 The results of this study are consistent with the aforementioned result, showing that the decline in the muscle aerobic function in the older participants delayed the recovery time of muscle oxygenation. Moreover, the IF of the VOL in both groups decreased following the 12 weeks of training regimen, and the decrease was higher in the E group (−26.45%) than in the Y group (−6.57%). Aerobic exercise training can enhance the muscle aerobic function in both young and older people.35 Coggan et al. reported that older participants who underwent an exercise training regimen showed a higher muscle oxidative capacity and muscle blood flow than did untrained participants.36 Exercise training can reduce the reoxygenation time because of the improved oxygen delivery to the working muscles.12 However, Lin et al. reported that the muscle aerobic function (i.e., reoxygenation time) in older participants did not significantly improve even after a long-term resistance training regimen.6 This result suggests that the improved muscle aerobic function of the E group observed in this study could be attributed to the aerobic exercise training of the multicomponent exercise training regimen. Although the older participants did not exhibit a higher enhancement in the muscle strength than the young participants did, a considerable improvement in the muscle aerobic function in the older participants was observed following the 12 weeks of training.
Although the IF of the VOL in the E group reduced considerably in the post-training period, the IF of the ES did not vary significantly considering the effects of training and age. In addition, the IF of the ES in the Y group did not increase or decrease significantly after the 12 weeks of training regimen. The half-recovery time of muscle oxygenation after the voluntary contraction was used for evaluating the muscle aerobic function.12 Hamaoka et al. indicated that muscle reoxygenation is considerably correlated to phosphocreatine resynthesis.37 Phosphocreatine is resynthesized through the creatine kinase reaction.38 However, McNeil et al. showed that the ES-induced muscle contraction enhances the skeletal muscle metabolism through the glycolytic pathway, leading to a considerable production of anaerobic metabolites (i.e., lactate).16 During recovery after an ES, the accumulated blood lactate may decrease phosphocreatine resynthesis.39 However, a study suggested that the increased blood flow (i.e., rapid reoxygenation) in the skeletal muscles during recovery after an ES was for the removal of anaerobic metabolites,16 and the evaluation of the muscle oxidative capacity could be affected by the abundant amount of lactate. Therefore, further investigation is required for a comprehensive understanding of the influence of the ES on muscle oxygenation when using an ES for assessing the effects of training regimens.
5. Conclusion
In the current study, the muscle oxygenation data acquired through NIRS was used for investigating the muscle metabolism. The increase ES-induced ΔSpO2 and lower MF in the young participants suggest neuromuscular adaptations following the 12 weeks of training regimen, which may have caused an increase in the muscle strength rather showing any improvements in the muscle metabolism. Although the older participants did not show a significant enhancement in the VOL- or ES-induced ΔSpO2 during muscle contraction, they showed a more evident improvement in the muscle aerobic function because of the considerable decrease in the reoxygenation time than the young participants did. The current study considered age and training effect but was delimited to gender differences. The gender differences can be investigated in future research. The results suggest that the 12-week multicomponent exercise training regimen used in this study enhanced the muscle strength, neuromuscular performance, and muscle aerobic function with variations in the young and older participants.
6. Practical applications
According to this study, it was shown that NIRS was suitable for investigating the muscle metabolism not only during exercise but also in the recovery phase. Additionally, the multicomponent exercise training has a benefit to improve muscle function with different aspects.
CRediT authorship contribution statement
Tai-You Lin conceived, designed and wrote the manuscript.
Jia-Jin J. Chen made critical suggestions and revisions on the study.
Linda L. Lin made critical suggestions and revisions on the study.
Wei-Tsun Ou Yang recorded the surface electromyographic data.
Meng-Yu Chen recruited participants for this study.
Yueh-Chang Tsai supervised the all exercise sessions in this study.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to thank the Institute of Physical Education, Health and Leisure Studies, for assisting with subject recruitment and exercise training. This work was supported by the National Science Council of Taiwan under grant NSC 102-2410-H-006-120-MY2.
References
- 1.Villareal D.T., Smith G.I., Sinacore D.R., Shah K., Mittendorfer B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity. 2011;19:312–318. doi: 10.1038/oby.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouaziz W., Lang P.-O., Schmitt E., Kaltenbach G., Geny B., Vogel T. Health benefits of multicomponent training programmes in seniors: a systematic review. Int J Clin Pract. 2016;70:520–536. doi: 10.1111/ijcp.12822. [DOI] [PubMed] [Google Scholar]
- 3.Labata-Lezaun N., González-Rueda V., Llurda-Almuzara L., et al. Effectiveness of multicomponent training on physical performance in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2022 doi: 10.1016/j.archger.2022.104838. [DOI] [PubMed] [Google Scholar]
- 4.Uchiyama K., Miaki H., Terada S., Hoso M. Effect of muscle strength training and muscle endurance training on muscle deoxygenation level and endurance performance. J Phys Ther Sci. 2011;23:349–355. [Google Scholar]
- 5.Fiogbé E., de Vassimon-Barroso V., de Medeiros Takahashi A.C. Exercise training in older adults, what effects on muscle oxygenation? A systematic review. Arch Gerontol Geriatr. 2017;71:89–98. doi: 10.1016/j.archger.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Lin T.-Y., Lin L.L., Ho T.-C., Chen J.-J.J. Investigating the adaptation of muscle oxygenation to resistance training for elders and young men using near-infrared spectroscopy. Eur J Appl Physiol. 2014;114:187–196. doi: 10.1007/s00421-013-2763-z. [DOI] [PubMed] [Google Scholar]
- 7.Conley K.E., Jubrias S.A., Esselman P.C. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(Pt 1):203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkendall D.T., Garrett W.E. The effects of aging and training on skeletal muscle. Am J Sports Med. 1998;26:598–602. doi: 10.1177/03635465980260042401. [DOI] [PubMed] [Google Scholar]
- 9.Tieland M., Trouwborst I., Clark B.C. Skeletal muscle performance and ageing. J. cachexia sarcopenia muscle. 2018;9:3–19. doi: 10.1002/jcsm.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira L.F., McDonough P., Behnke B.J., Musch T.I., Poole D.C. Blood flow and O 2 extraction as a function of O 2 uptake in muscles composed of different fiber types. Respir Physiol Neurobiol. 2006;153:237–249. doi: 10.1016/j.resp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 11.DeLorey D.S., Paterson D.H., Kowalchuk J.M. Effects of ageing on muscle O2 utilization and muscle oxygenation during the transition to moderate-intensity exercise. Appl Physiol Nutr Metabol. 2007;32:1251–1262. doi: 10.1139/H07-121. [DOI] [PubMed] [Google Scholar]
- 12.Ichimura S., Murase N., Osada T., et al. Age and activity status affect muscle reoxygenation time after maximal cycling exercise. Med Sci Sports Exerc. 2006;38:1277–1281. doi: 10.1249/01.mss.0000227312.08599.f1. [DOI] [PubMed] [Google Scholar]
- 13.Gepner Y., Wells A.J., Gordon J.A., et al. Differences in muscle oxygenation between young and middle-aged recreationally active men during high-volume resistance exercise. Kinesiology. 2019;51:3–11. [Google Scholar]
- 14.Gregory C.M., Bickel C.S. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther. 2005;85:358–364. [PubMed] [Google Scholar]
- 15.Heyters M., Carpentier A., Duchateau J., Hainaut K. Twitch analysis as an approach to motor unit activation during electrical stimulation. Can J Appl Physiol. 1994;19:451–461. doi: 10.1139/h94-037. [DOI] [PubMed] [Google Scholar]
- 16.McNeil C.J., Murray B.J., Rice C.L. Differential changes in muscle oxygenation between voluntary and stimulated isometric fatigue of human dorsiflexors. J Appl Physiol. 2006;100:890–895. doi: 10.1152/japplphysiol.00921.2005. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe K., Yoshida T., Ishikawa T., Kawade S., Moritani T. Effect of the combination of whole-body neuromuscular electrical stimulation and voluntary exercise on metabolic responses in human. Front Physiol. 2019;10:291. doi: 10.3389/fphys.2019.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muthalib M., Kerr G., Nosaka K., Perrey S. Local muscle metabolic demand induced by neuromuscular electrical stimulation and voluntary contractions at different force levels: a NIRS study. Eur J trans myology. 2016;26 doi: 10.4081/ejtm.2016.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patten C., Kamen G. Adaptations in motor unit discharge activity with force control training in young and older human adults. Eur J Appl Physiol. 2000;83:128–143. doi: 10.1007/s004210000271. [DOI] [PubMed] [Google Scholar]
- 20.Pereira M.I., Gomes P.S., Bhambhani Y.N. A brief review of the use of near infrared spectroscopy with particular interest in resistance exercise. Sports Med. 2007;37:615–624. doi: 10.2165/00007256-200737070-00005. [DOI] [PubMed] [Google Scholar]
- 21.Robertson P., Hart B. Assessment of tissue oxygenation. Respir Care Clin N Am. 1999;5:221–263. [PubMed] [Google Scholar]
- 22.Angleri V., de Oliveira R., Biazon T.M., et al. Effects of drop-set and pyramidal resistance training systems on microvascular oxygenation: a near-infrared spectroscopy approach. Int J Exerc Sci. 2020;13:1549. [PMC free article] [PubMed] [Google Scholar]
- 23.Lin T.-Y., Wu J.-S., Lin L.L., Ho T.-C., Lin P.-Y., Chen J.-J.J. Assessments of muscle oxygenation and cortical activity using functional near-infrared spectroscopy in healthy adults during hybrid activation. IEEE Trans Neural Syst Rehabil Eng. 2015;24:1–9. doi: 10.1109/TNSRE.2015.2429655. [DOI] [PubMed] [Google Scholar]
- 24.Barstow T.J. Understanding near infrared spectroscopy and its application to skeletal muscle research. J Appl Physiol. 2019;126:1360–1376. doi: 10.1152/japplphysiol.00166.2018. [DOI] [PubMed] [Google Scholar]
- 25.Tuesta M., Yáñez-Sepúlveda R., Verdugo-Marchese H., Mateluna C., Alvear-Ordenes I. Near-infrared spectroscopy used to assess physiological muscle adaptations in exercise clinical trials: a systematic review. Biology. 2022;11:1073. doi: 10.3390/biology11071073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagerwaard B., Nieuwenhuizen A.G., De Boer V.C., Keijer J. In vivo assessment of mitochondrial capacity using NIRS in locomotor muscles of young and elderly males with similar physical activity levels. GeroScience. 2020;42:299–310. doi: 10.1007/s11357-019-00145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borg G.A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 28.Hamaoka T., McCully K.K., Quaresima V., Yamamoto K., Chance B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt. 2007;12 doi: 10.1117/1.2805437. 062105-062105-062116. [DOI] [PubMed] [Google Scholar]
- 29.Lin P.-Y., Lin S.-I., Penney T., Chen J.-J.J. Review: applications of near infrared spectroscopy and imaging for motor rehabilitation in stroke patients. J Med Biol Eng. 2009;29:210–211. [Google Scholar]
- 30.Muthalib M., Jubeau M., Millet G.Y., Maffiuletti N.A., Nosaka K. Comparison between electrically evoked and voluntary isometric contractions for biceps brachii muscle oxidative metabolism using near-infrared spectroscopy. Eur J Appl Physiol. 2009;107:235–241. doi: 10.1007/s00421-009-1118-2. [DOI] [PubMed] [Google Scholar]
- 31.Ferrari M., Mottola L., Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29:463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 32.Franceschini M.A., Fantini S., Paunescu L.A., Maier J.S., Gratton E. Influence of a superficial layer in the quantitative spectroscopic study of strongly scattering media. Appl Opt. 1998;37:7447–7458. doi: 10.1364/ao.37.007447. [DOI] [PubMed] [Google Scholar]
- 33.Creer A.R., Ricard M.D., Conlee R.K., Hoyt G.L., Parcell A.C. Neural, metabolic, and performance adaptations to four weeks of high intensity sprint-interval training in trained cyclists. Int J Sports Med. 2004;25:92–98. doi: 10.1055/s-2004-819945. [DOI] [PubMed] [Google Scholar]
- 34.Duchateau J., Semmler J.G., Enoka R.M. Training adaptations in the behavior of human motor units. J Appl Physiol. 2006;101:1766–1775. doi: 10.1152/japplphysiol.00543.2006. [DOI] [PubMed] [Google Scholar]
- 35.Thompson L.V. Effects of age and training on skeletal muscle physiology and performance. Phys Ther. 1994;74:71–81. doi: 10.1093/ptj/74.1.71. [DOI] [PubMed] [Google Scholar]
- 36.Coggan A.R., Abduljalil A.M., Swanson S.C., et al. Muscle metabolism during exercise in young and older untrained and endurance-trained men. J Appl Physiol. 1993;75 doi: 10.1152/jappl.1993.75.5.2125. 2125-2125. [DOI] [PubMed] [Google Scholar]
- 37.Hamaoka T., Iwane H., Shimomitsu T., et al. Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J Appl Physiol. 1996;81:1410–1417. doi: 10.1152/jappl.1996.81.3.1410. [DOI] [PubMed] [Google Scholar]
- 38.Thomas C., Sirvent P., Perrey S., Raynaud E., Mercier J. Relationships between maximal muscle oxidative capacity and blood lactate removal after supramaximal exercise and fatigue indexes in humans. J Appl Physiol. 2004;97:2132–2138. doi: 10.1152/japplphysiol.00387.2004. [DOI] [PubMed] [Google Scholar]
- 39.Arthur P.G., West T.G., Brill R.W., Schulte P.M., Hochachka P.W. Recovery metabolism of skipjack tuna (Katsuwonus pelamis) white muscle: rapid and parallel changes in lactate and phosphocreatine after exercise. Can J Zool. 1992;70:1230–1239. [Google Scholar]