Abstract
Insulin-like growth factor-1 (IGF-1) plays critical roles in the development of the central nervous system (CNS), including the retina, regulating cell proliferation, differentiation, and survival. Here, we investigated the role of IGF-1 on retinal cell proliferation using primary cultures from rat neural retina. Our data show that IGF-1 stimulates retinal cell proliferation and regulates the expression of neurotrophic factors, such as interleukin-4 and brain-derived neurotrophic factor. In addition, our results indicates that IGF-1-induced retinal cell proliferation requires activation of multiple signaling pathways, including phosphatidylinositol 3-kinase, protein kinase Src, phospholipase-C, protein kinase C delta, and mitogen-activated protein kinase pathways. We further show that activation of matrix metalloproteinases and epidermal growth factor receptor is also necessary for IGF-1 enhancing retinal cell proliferation. Overall, these results unveil potential mechanisms by which IGF-1 ensures retinal cell proliferation and support the notion that manipulation of IGF-1 signaling may be beneficial in CNS disorders associated with abnormal cell proliferation.
Keywords: Cell proliferation, Central nervous system, Epidermal growth factor, Insulin-like growth factor-1, Retina, Signaling pathway
Graphical abstract
Highlights
-
•
Insulin-like growth factor-1 (IGF-1) promotes retinal cell proliferation.
-
•
Multiple signaling pathways underlie IGF-1-induced retinal cell proliferation.
-
•
Activation of EGFR is also required for the mitogenic effect of IGF-1.
1. Introduction
Insulin-like growth factor-1 (IGF-1) and its receptor 1 (IGF-1R) play critical roles in the development of the central nervous system (CNS) (Fernandez and Torres-Alemán, 2012; O’Kusky and Ye, 2012). In humans, deficiency in Igf1 or Igf1r genes is related with severe body growth failure and abnormal CNS development such as microcephaly, mental retardation and cognitive impairments, which is consistent with findings from rodent studies using mutant mice with disrupted expression of Igf1 or Igf1r (O’Kusky and Ye, 2012). Recent studies using human retinal organoids have indicated that IGF-1 is also critical for proper retinal growth and maturation (Mellough et al., 2015; Zerti et al., 2021). Corroborating these findings, previous reports indicate that IGF-1 is essential for adequate teleost retinal growth and development by promoting retinal cell proliferation and neuronal differentiation (Becker et al., 2021; Otteson et al., 2002; Zygar et al., 2005).
In the rat retina, IGF-1 can induce retinal ganglion cell (RGC) survival and axon growth by activating phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK)/extracellular-signal regulated kinase (ERK) pathways (Dupraz et al., 2013; Granja et al., 2019; Homma et al., 2007; Liao et al., 2017; Seigel et al., 2006; Yang et al., 2013). Although extensive literature indicates that PI3K and MAPK/ERK pathways also mediate the proliferative effect of IGF-1 in different cell lines, little is known about signaling mechanisms underlying IGF-1-induced cell proliferation in the retina. In the current study, using primary cultures from rat neural retina, we showed for the first time that IGF-1 transiently stimulates retinal cell proliferation through a complex cellular mechanism that require activation of epidermal growth factor (EGF) receptor and multiple signaling cascades, including (but not limited to) PI3K and MAPK/ERK pathways.
2. Materials and methods
2.1. Materials
Fetal calf serum (FCS) and medium 199 were purchased from GIBCO (Maryland,USA). AG-1478, glutamine, penicillin, poly-L-ornithine, streptomycin, rottlerin, and Petri dishes were purchased from Sigma-Aldrich (St Louis, USA). Brefeldin A, chelerythrine chloride, LY294002, PD98059, PP1, and U73122 were purchased from Biomol International (Pennsylvania, USA). c-Jun N-terminal kinase (JNK) inhibitor (JNKi), matrix metalloproteinase (MMP) 9 inhibitor (MMP9i), and SB202190 were purchased from Calbiochem (San Diego, USA). Rabbit polyclonal anti-interleukin-4 (IL-4) antibody, rabbit polyclonal anti-brain-derived neurotrophic factor (BDNF) antibody, recombinant rat IGF-1, and recombinant rat EGF were purchased from PeproTech (New Jersey, USA). Goat anti-rabbit IgG-HRP antibody, Luminata reagent, and PVDF membrane were purchased from GE Healthcare Life Sciences (Massachusetts, USA). [metil-3H]-thymidine was purchased from Amersham (Buckinghamshire, UK) Bovine serum albumin (BSA) and rabbit polyclonal anti-β-actin antibody were purchased from Santa Cruz Biotechnology. Monodansylcadaverine (MDC) from Sigma-Aldrich (St Louis, USA) was kindly gifted by Dr. Ana L. Ventura (Fluminense Federal University, Brazil).
2.2. Animal ethics statement
This study was conducted on male and female Lister Hooded rats maintained as outbred colonies in the Animal Facility of Fluminense Federal University, under 12h light/dark cycle, at constant temperature (22–25 °C), with water and chow available ad libitum. All animal procedures were approved by Ethical Committee of Fluminense Federal University (protocol #00124/09) and performed following the National Institutes of Health's Guide for the Care and Use of Laboratory Animal.
2.3. Primary retinal cell cultures
Primary cultures from neural retinas of male and female Lister Hooded rats at postnatal day 1–2 were prepared according to established procedures (Colares et al., 2021; Mázala-de-Oliveira et al., 2022), and maintained in medium 199 supplemented with 5% FCS, 2 mM glutamine, and 100 U/mL penicillin/streptomycin. Retinal cells were seeded into poly-L-ornithine (50 μg/mL)-coated plastic Petri dishes (35 mm) at a density of 1.25 × 105 cells/cm2, incubated in 2 mL of complete medium (control cultures) or 2 mL of complete medium containing IGF-1 at different concentrations (0.1–100 ng/mL). For some experiments, retinal cultures were also exposed to the following drugs: 30 ng/mL brefeldin A, 0.75 nM MDC, 37.5 μM PD98059, 0.5 μM JNKi, 20 μM SB202190, 1 μM PP1, 25 μM LY294002 1.25 μM chelerythrine chloride, 2 μM rottlerin, 4 μM U73122, 2.5μ M AG-1478, 20 μM MMP9i, or 0.1 ng/mL EGF. All cultures were maintained in a humidified atmosphere of 5% CO2/95% air at 37 °C for the time intervals indicated in each experiment.
2.4. [3H]-thymidine incorporation assay
[3H]-thymidine incorporation assay is based on cell uptake of radiolabeled thymidine into nascent DNA during S-phase of the cell cycle (Romar et al., 2016). This assay was performed according to established procedures (dos Santos et al., 2003; Guilarducci-Ferraz et al., 2008). Briefly, after treatment with the drugs, primary retinal cultures were incubated with 0.5 μCi/mL [metil-3H]-thymidine for 1h at 37 °C. After washing with medium 199, the content of each dish was transferred to glass tubes containing 10% (v/v) trichloroacetic acid for 30min, filtered in glass microfiber filters Whatman GF/B under negative pressure, and placed to dry overnight at room temperature. The radioactivity was determined in a liquid phase scintillator (Packard, USA). Data were expressed as percentage of [3H]-thymidine uptake by control cultures.
2.5. Immunoblotting
Immunoblotting procedures were performed according to previously described (Colares et al., 2021; Mázala-de-Oliveira et al., 2022). Briefly, retinal cell lysates (60 μg total protein/lane) were resolved in 15% SDS-PAGE gels, and proteins were then blotted onto PVDF membranes using a semi-dry blotting system (Bio-Rad Laboratories, USA). Membranes were blocked with 3% (w/v) BSA in TBS-T (20 mM Tris-HCl, 160 nM NaCl, 0.1% Tween-20) for 1h at room temperature. Primary antibodies against IL-4 (1:1000), BDNF (1:1000) and β-actin (1:5000) were diluted in blocking solution and incubated with the membranes overnight at 4 °C. After incubation with anti-rabbit HRP-conjugated secondary antibody (1:15,000) for 1h at room temperature, membranes were imaged in a ChemiDoc-Pix System (Loccus Biotecnologia®, Brazil) using the Luminata reagent. Optical density determination for quantification was performed using ImageJ software, and β-actin was used as loading control.
2.6. Statistical analyses
Statistical analyses were performed using GraphPad Prism 8 software (San Diego, USA). Comparisons between multiple experimental groups were analyzed using one-way analysis of variance (ANOVA), followed by post hoc Holm-Sidak's test. Comparisons between two groups were analyzed by two-tailed paired Student's t-test. p value < 0.05 was considered statistically significant. All data are expressed as mean ± standard error of the mean (SEM).
3. Results
3.1. IGF-1 transiently stimulates retinal cell proliferation and regulates the levels of neurotrophic molecules
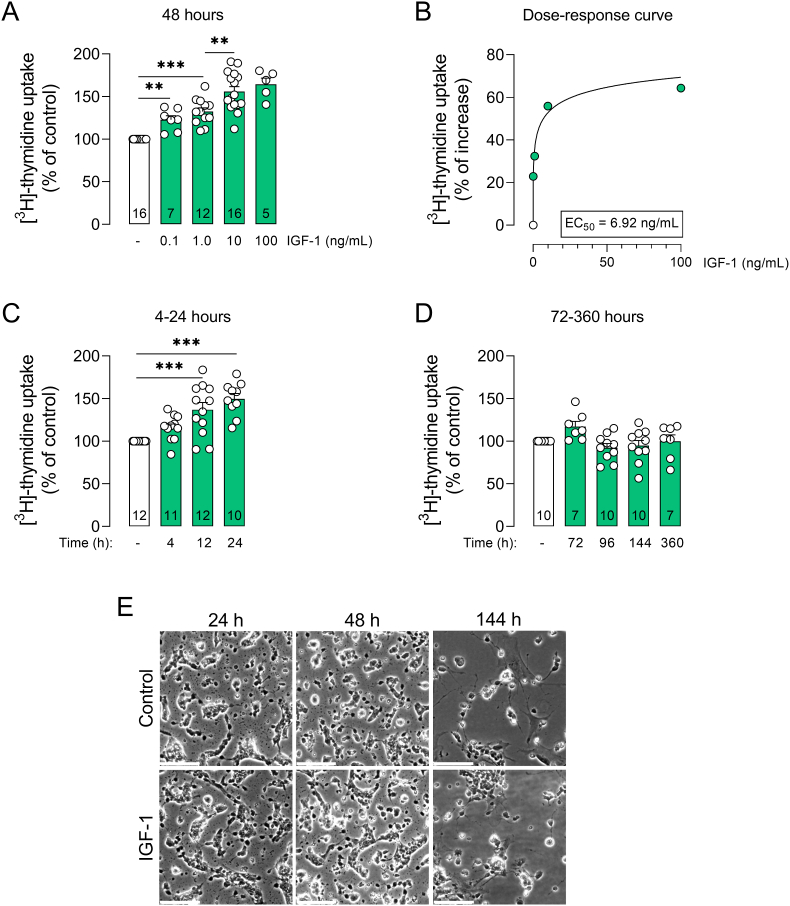
The effect of IGF-1 on retinal cell proliferation was evaluated using the [3H]-thymidine incorporation assay by directly measuring DNA synthesis during cell division, an indicative of cell proliferation. First, we found that exposure to IGF-1 for 48h increased [3H]-thymidine incorporation at all concentrations tested (0.1–100 ng/mL; Fig. 1A), with an EC50 = 6.92 ng/mL (Fig. 1B). A time course analysis indicated that treatment with IGF-1 (10 ng/mL) resulted in a transient increase in retinal cell proliferation after 12h (Fig. 1C), with no alteration after 72h (Fig. 1D). Exposure to IGF-1 (10 ng/mL) for 24h was sufficient to lead to a ∼50% increase in retinal cell proliferation similar to that observed at 48h (IGF-1 24h: 149.8 ± 6.21%; IGF-1 48h: 155.9 ± 5.65%; Fig. 1A,C,E). Hence, exposure to IGF-1 (10 ng/mL) for 24h was chosen for further study.
Fig. 1.
IGF-1 stimulates transient cell proliferation in retinal cell cultures. (A,B) Primary cultures from rat neural retinas were treated with IGF-1 (0.1–100 ng/mL) for 48h, or (C,D) IGF-1 (10 ng/mL) for the indicated time intervals. Plots represent mean (±SEM) [3H]-thymidine uptake expressed as percentage of control group (set on 100%; white bars), with sample size provided within each bar. Data were analyzed by one-way ANOVA followed by Holm-Sidak's test (**p < 0.01, ***p < 0.001). EC50 value was calculated in GraphPad Prism 8 using a nonlinear curve fit ([IGF-1] vs. normalized response - method of least squares with variable slope). (E) Representative images (bright-field) of retinal cells treated with IGF-1 (10 ng/mL) for 24h, 48h or 144h (scale bar = 20 μm).
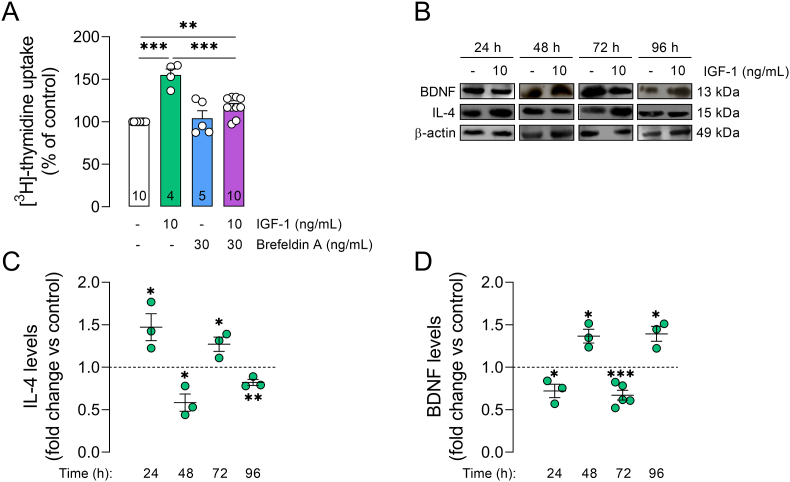
We also found that inhibition of vesicular protein transport, using brefeldin A (30 ng/mL), prevented the mitogenic effect induced by IGF-1 on retinal cells (Fig. 2A), suggesting that IGF-1 can also induce the secretion of some neurotrophic molecule important for cell proliferation. Previously, we have shown that exposure to IGF-1 (10 ng/mL) for 24h stimulates secretion of IL-4 by retinal cells, while IL-4 increases IGF-1R levels/activation for ensuring RGC survival (Granja et al., 2019). Here, we showed that exposure to IGF-1 cyclically regulates protein levels of IL-4 over time, increasing IL-4 levels in retinal cells after 24h (Fig. 2B and C). Taken together, these findings suggest that IL-4 may play an important role on retinal cell proliferation induced by IGF-1. In addition, IGF-1 regulates protein levels of BDNF over time, decreasing BDNF levels after 24h (Fig. 2B,D), a neurotrophin that inhibits retinal cell proliferation (dos Santos et al., 2003).
Fig. 2.
IGF-1 regulates expression of neurotrophic molecules in retinal cell cultures. (A) Primary retinal cultures were treated with an inhibitor of vesicular protein transport (brefeldin A; 30 ng/mL) or IGF-1 (10 ng/mL) for 24h, and cell proliferation was assessed by [3H]-thymidine incorporation assay. Plots represent mean (±SEM) [3H]-thymidine uptake expressed as percentage of control group (set on 100%; white bars), with sample size provided within each bar. Additionally, (B-D) cultured retinal cells were exposed to IGF-1 (10 ng/mL) for the indicated time periods for assessing IL-4 and BDNF levels by immunoblotting. Plots represent mean (±SEM) protein optical density (normalized to β-actin) expressed as fold change relative to control cultures (represented as a dashed line), with N = 3–5 experiments using independent retinal cultures. Data were analyzed by (A) one-way ANOVA followed by Holm-Sidak's test or (C,D) two-tailed paired Student's t-test (*p < 0.05, **p < 0.01, ***p < 0.001).
3.2. Multiple intracellular signaling pathways underlie IGF-1-induced retinal cell proliferation
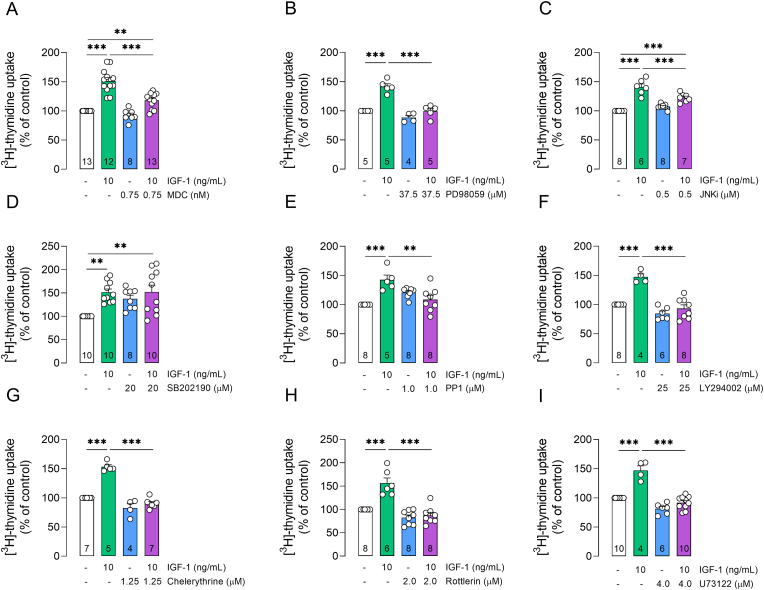
Previous studies have indicated that IGF-1R endocytosis is required for stimulation of MAPK/ERK pathway and cell proliferation induced by IGF-1 (Chow et al., 1998; Girnita et al., 2007; Solomon-Zemler et al., 2017). MDC, an endocytosis inhibitor, was proven to inhibit clathrin-mediated IGF-1R endocytosis and consequent MAPK/ERK activation and cell proliferation triggered by IGF-1 (Chow et al., 1998; Solomon-Zemler et al., 2017). To assess the impact of receptor internalization on IGF-1-induced proliferative effect, we treated retinal cells with MDC and IGF-1. Accordingly, treatment with MDC inhibited the IGF-1-induced retinal cell proliferation (Fig. 3A), suggesting that receptor endocytosis is a critical step for retinal cell proliferation triggered by IGF-1.
Fig. 3.
IGF-1 requires receptor endocytosis and activity of multiple kinases to enhance retinal cell proliferation. Primary retinal cultures were treated with IGF-1 (10 ng/mL) or following drugs for 24h: (A) MDC (endocytosis inhibitor; 0.75 nM), (B) PD98059 (MAPK/ERK inhibitor; 37.5 μM), (C) JNKi (JNK inhibitor; 0.5 μM), (D) SB202190 (p38-MAPK inhibitor; 20 μM), (E) PP1 (Src inhibitor; 1 μM), (F) LY294002 (PI3K inhibitor; 25 μM), (G) chelerythrine chloride (pan-PKC inhibitor; 1.25 μM), (H) rottlerin (PKCδ inhibitor; 2 μM), or (I) U73122 (phospholipase-C inhibitor; 4 μM). Plots represent mean (±SEM) [3H]-thymidine uptake expressed as percentage of control group (set on 100%; white bars), with sample size provided within each bar. Data were analyzed by one-way ANOVA followed by Holm-Sidak's test (**p < 0.01, ***p < 0.001).
MAPK signaling cascades are key modulators of cell proliferation (Zhang and Liu, 2002). Here, we assessed the involvement of three MAPK cascades on IGF-1-induced retinal cell proliferation by treating retinal cells with inhibitors of MEK/ERK (PD98059), JNK (JNKi), or p38-MAPK (SB202190) pathways. Our results indicated that activation of MAPK/ERK and JNK pathways (but not p38-MAPK) underlie the proliferative effect of IGF-1 on retinal cells (Fig. 3B–D). In addition, treatment with pharmacological inhibitors of Src (PP1, Fig. 3E), PI3K (LY294002, Fig. 3F), PKC (chelerythrine chloride and rottlerin, Fig. 3G and H), or phospholipase-C (U73122, Fig. 3I) prevented increased [3H]-thymidine uptake by retinal cells exposed to IGF-1, suggesting that IGF-1 stimulates retinal cell proliferation also by activation of Src, PI3K, PKCδ and phospholipase-C (PLC).
3.3. IGF-1-induced retinal cell proliferation requires activation of EGFR
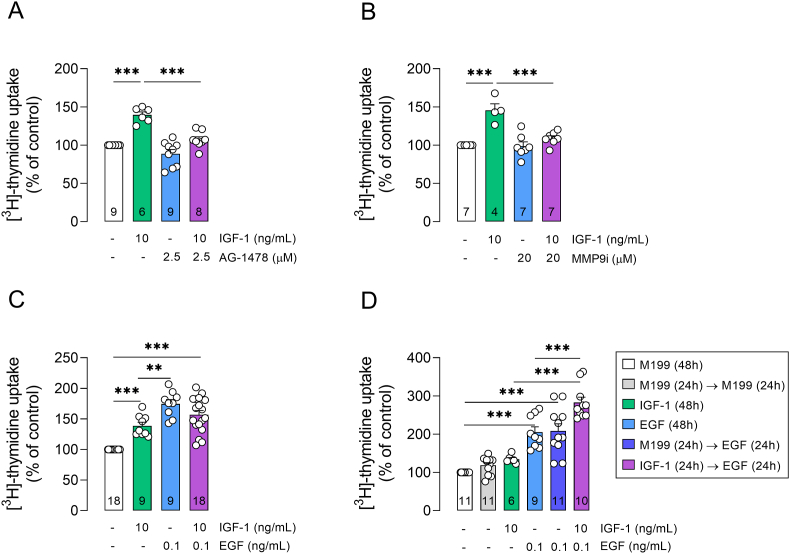
IGF-1 has been reported to transactivate EGFR to activate the MAPK/ERK pathway and promote cell cycle progression in different cell lines (Ahmad et al., 2004; Meng et al., 2007; Roudabush et al., 2000; Saxena et al., 2008; Zhou et al., 2006). In these cells, IGF-1 can transactivate EGFR through MMP-mediated shedding of heparin-binding EGF, while inhibition of MMP can prevent IGF-1-induced ERK activation and cell proliferation (Roudabush et al., 2000; Saxena et al., 2008; Zhou et al., 2006). So, we next assessed the involvement of EGFR and MMP on IGF-1-induced retinal cell proliferation. Retinal cells were treated with an EGFR inhibitor (AG-1478) or MMP9 inhibitor (MMPi). Both AG-1478 and MMP9i inhibited IGF-1-induced cell proliferation (Fig. 4A and B). Although co-exposure to EGF (0.1 ng/mL) and IGF-1 for 24h did not increase mitogenic responsiveness to IGF-1 (Fig. 4C), treatment with EGF for 24h after initial exposure to IGF-1 potentiated IGF-1-induced retinal cell proliferation (Fig. 4D). Taken together, these results suggest that activation of EGFR plays a critical role on IGF-1-induced retinal cell proliferation.
Fig. 4.
Activation of EGFR mediates IGF-1-stimulated retinal cell proliferation. Primary retinal cultures were treated with IGF-1 (10 ng/mL), (A) AG-1478 (EGFR inhibitor; 2.5 μM), (B) MMP9i (MMP9 inhibitor; 20 μM), or (C) EGF (0.1 ng/mL) for 24h. Additionally, (D) cultured retinal cells were treated with IGF-1 for 24h, after which media were removed and novel medium containing EGF was applied for additional 24h, in parallel with primary retinal cultures exposed only to IGF-1 or EGF for 48h. Control cultures had their medium replaced by a novel medium after 24h (M199 → M199), which did not impact [3H]-thymidine uptake compared to control cultures without medium change (M199 48h). Plots represent mean (±SEM) [3H]-thymidine uptake expressed as percentage of control group (set on 100%; white bars), with sample size provided within each bar. Data were analyzed by one-way ANOVA followed by Holm-Sidak's test (**p < 0.01, ***p < 0.001).
4. Discussion
We have previously reported that IGF-1 can activate PI3K/AKT pathway in the rat retina and that expression of IGF-1 and p-IGF-1R declines during postnatal retinal development (Maturana-Teixeira et al., 2015) in parallel with a remarkable decrease in retinal cell proliferation (Braga et al., 2013). Here, we showed that IGF-1 induces retinal cell proliferation through activation of the PI3K pathway. In addition, inhibition of vesicular protein transport blocked the mitogenic effect of IGF-1, suggesting that IGF-1 can induce the secretion of some neurotrophic molecule important for cell proliferation. A possible neurotrophic molecule is IL-4, since IGF-1 stimulates IL-4 secretion by retinal cells, and IL-4 promotes activation of IGF-1R/PI3K/AKT pathway in primary retinal cultures (Granja et al., 2019). Consistent with this, our present findings indicated that IGF-1 transiently upregulated levels of IL-4 in parallel with increased retinal cell proliferation. It is also possible that IGF-1 regulates the secretion of other molecules important for cell proliferation, such as vascular endothelial growth factor (VEGF), since IGF-1 can enhance VEGF expression and signaling in the retina (Ruberte et al., 2004; Smith et al., 1999), and VEGF also stimulates retinal progenitor cell proliferation (Hashimoto et al., 2006; Nishiguchi et al., 2007).
We also showed that IGF-1 requires activation of MAPK/ERK and JNK pathways for enhancing retinal cell proliferation. Furthermore, blocking activation of EGFR prevented retinal cell proliferation triggered by IGF-1, suggesting that activation of EGFR mediates IGF-1-induced retinal cell proliferation. Accordingly, previous studies have indicated that transactivation of EGFR is required for IGF-1 to activate MAPK/ERK pathway and induce cell proliferation in different cell lines (Ahmad et al., 2004; Meng et al., 2007; Roudabush et al., 2000; Saxena et al., 2008; Zhou et al., 2006). In these cells, IGF-1 can transactivate EGFR via MMP-mediated proteolytic release of EGF-like ligands from the cell surface (Roudabush et al., 2000; Saxena et al., 2008; Zhou et al., 2006) or via Src activation (Meng et al., 2007). It has been reported that IGF-1 can activate MMPs in Müller glial cells (MGCs) (Lorenc et al., 2015, 2018), the main type of glia in the vertebrate retina. In MGCs, activation of different receptors has been reported to activate ERK through a mechanism that requires both MMP- and Src-mediated transactivation of EGFR (Harun-Or-Rashid et al., 2014, 2016). In the current study, we indicated that activity of both MMP9 and Src is necessary for IGF-1-induced retinal cell proliferation. However, whether IGF-1 induces transactivation of EGFR in retinal cells by MMP9 or Src remains to be investigated.
Intracellular activation of PKCδ exerts a central role for IGF-1 ensuring cell proliferation in different cell types (Czifra et al., 2006; Li et al., 1998; Takahashi et al., 2015). Interestingly, a recent study identified that PKCδ can also be secreted from liver cancer cells and behave as a growth factor, inducing cell proliferation through IGF-1R and subsequently ERK activation (Yamada et al., 2021). Previously, we have shown that activation of PKCδ stimulates releasing of neurotrophic factors by retinal cells and promotes RGC survival (Braga et al., 2018; de Rezende Corrêa et al., 2010). Here, we identified that PKCδ is also required for IGF-1-induced retinal cell proliferation. Furthermore, we showed that PLC is another central protein for IGF-1-stimulated retinal cell proliferation, similarly to that reported in other cell types (Faenza et al., 2005; Xu et al., 2001).
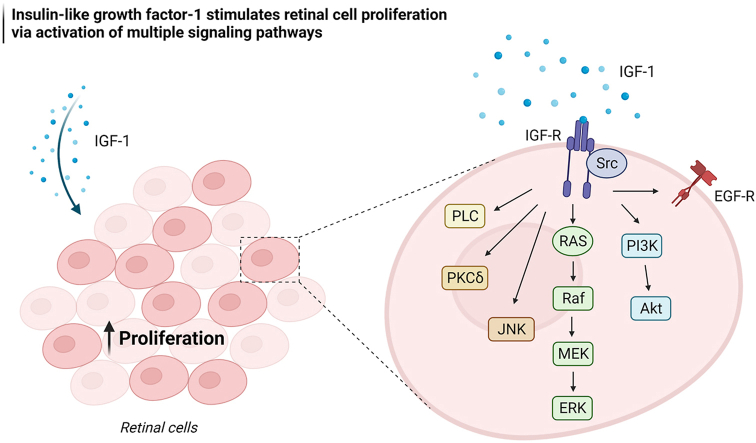
In summary, the current study indicates that IGF-1 stimulates cell proliferation in rat retinal cells, which is according to previous findings in fish (Becker et al., 2021; Otteson et al., 2002; Zygar et al., 2005), mice (Wang et al., 2018) and human (Mellough et al., 2015; Zerti et al., 2021) models. Furthermore, we unveil potential mechanisms by which IGF-1 ensures retinal cell proliferation (Fig. 5), including secretion of neurotrophic factors, activation of EGFR, and activation of multiple kinases associated with mitogenesis. A better understanding of these mechanisms could help establish new therapeutic strategies, since homeostatic proliferation is crucial for proper retinal development, and its disruption is associated with retinal abnormalities such as dysplasia, retinal degeneration and retinal tumors.
Fig. 5.
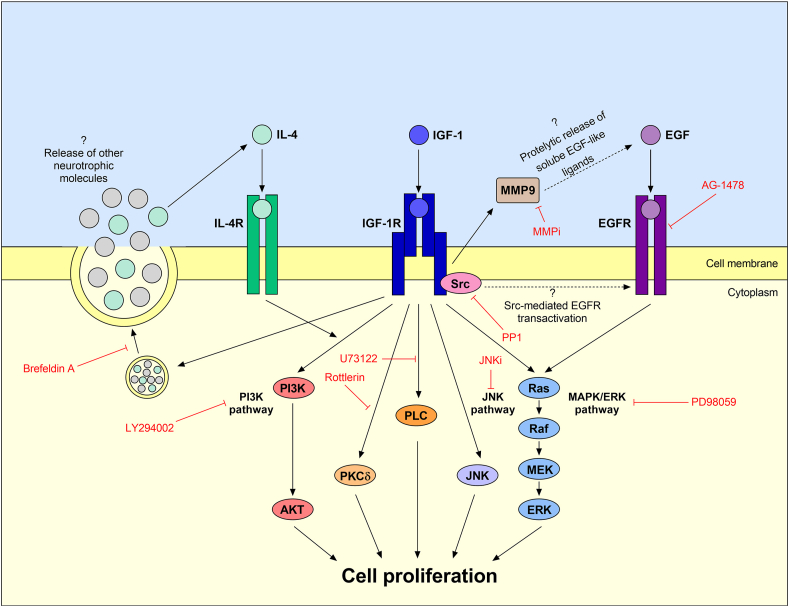
A simplified model of cellular signaling pathways underlying IGF-1-induced cell proliferation in the retina. IGF-1 activates PI3K/AKT pathway in retinal cells and increases protein levels and release of IL-4, which stimulates IGF-1R/PI3K/AKT pathway. Inhibition of protein vesicular secretion or PI3K activity prevents the mitogenic effect of IGF-1. IGF-1-induced retinal cell proliferation also requires activation of PKCδ, PLC, JNK, and MAPK/ERK (Ras-Raf-MEK-ERK) pathways. EGF is another neurotrophic factor that induces retinal cell proliferation by activating MAPK/ERK pathway. EGFR activation is also necessary for IGF-1 ensuring retinal cell proliferation. Furthermore, IGF-1-induced retinal cell proliferation requires activation of both MMP9 and Src. Although it has been reported that IGF-1 can transactivate EGFR in different cell types via MMP-mediated proteolytic release of EGF-like ligands or via Src activation, further studies are needed to investigate whether these mechanisms of EGFR transactivation also happen in retinal cells.
CRediT authorship contribution statement
Camila Saggioro de Figueiredo: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Ícaro Raony: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Simone Vidal Medina: Conceptualization, Methodology, Investigation. Eliezer de Mello Silva: Conceptualization, Methodology, Investigation. Aline Araujo dos Santos: Resources, Funding acquisition, Writing – review & editing. Elizabeth Giestal-de-Araujo: Conceptualization, Supervision, Resources, Funding acquisition, Writing – review & editing, All authors contributed to and have approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro, and Instituto Nacional de Ciência e Tecnologia em Neuroimunomodulação. We thank Dr. Ana L. Ventura (Fluminense Federal University) for sharing MDC and Arnaldo P. Andrade for suggestions in writing this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crneur.2022.100068.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Ahmad T., Farnie G., Bundred N.J., Anderson N.G. The mitogenic action of insulin-like growth factor I in normal human mammary epithelial cells requires the epidermal growth factor receptor tyrosine kinase. J. Biol. Chem. 2004;279(3):1713–1719. doi: 10.1074/jbc.M306156200. [DOI] [PubMed] [Google Scholar]
- Becker C., Lust K., Wittbrodt J. Igf signaling couples retina growth with body growth by modulating progenitor cell division. Development. 2021;148(7):dev199133. doi: 10.1242/dev.199133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga L.E.G., Granja M.G., da Silva G.M., Giestal-de-Araujo E., dos Santos A.A. PMA increases M3 muscarinic receptor levels and decreases retinal cells proliferation through a change in the levels of cell-cycle regulatory proteins. Neurosci. Lett. 2013;550:29–34. doi: 10.1016/j.neulet.2013.06.045. [DOI] [PubMed] [Google Scholar]
- Braga L.E.G., Miranda R.L., Granja M.G., Giestal-de-Araujo E., Dos Santos A.A. PKC delta activation increases neonatal rat retinal cells survival in vitro: involvement of neurotrophins and M1 muscarinic receptors. Biochem. Biophys. Res. Commun. 2018;500(4):917–923. doi: 10.1016/j.bbrc.2018.04.193. [DOI] [PubMed] [Google Scholar]
- Chow J.C., Condorelli G., Smith R.J. Insulin-like growth factor-I receptor internalization regulates signaling via the Shc/mitogen-activated protein kinase pathway, but not the insulin receptor substrate-1 pathway. J. Biol. Chem. 1998;273(8):4672–4680. doi: 10.1074/jbc.273.8.4672. [DOI] [PubMed] [Google Scholar]
- Colares T.G., de Figueiredo C.S., de Oliveira Jesus Souza L., Dos Santos A.A., Giestal-de-Araujo E. Increased retinal ganglion cell survival by exogenous IL-2 depends on IL-10, dopamine D1 receptors, and classical IL-2/IL-2R signaling pathways. Neurochem. Res. 2021;46(7):1701–1716. doi: 10.1007/s11064-021-03313-1. [DOI] [PubMed] [Google Scholar]
- Czifra G., Tóth I.B., Marincsák R., Juhász I., Kovács I., Ács P., Kovács L., Blumberg P.M., Bíró T. Insulin-like growth factor-I-coupled mitogenic signaling in primary cultured human skeletal muscle cells and in C2C12 myoblasts. A central role of protein kinase Cδ. Cell. Signal. 2006;18(9):1461–1472. doi: 10.1016/j.cellsig.2005.11.007. [DOI] [PubMed] [Google Scholar]
- de Rezende Corrêa G., da Silva Cunha K.C., Dos Santos A.A., de Araujo E.G. The trophic effect of ouabain on retinal ganglion cell is mediated by EGF receptor and PKC δ activation. Neurochem. Res. 2010;35(9):1343–1352. doi: 10.1007/s11064-010-0190-7. [DOI] [PubMed] [Google Scholar]
- dos Santos A.A., Medina S.V., Sholl-Franco A., de Araujo E.G. PMA decreases the proliferation of retinal cells in vitro: the involvement of acetylcholine and BDNF. Neurochem. Int. 2003;42(1):73–80. doi: 10.1016/S0197-0186(02)00059-1. [DOI] [PubMed] [Google Scholar]
- Dupraz S., Grassi D., Karnas D., Nieto Guil A.F., Hicks D., Quiroga S. The insulin-like growth factor 1 receptor is essential for axonal regeneration in adult central nervous system neurons. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0054462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faenza I., Billi A.M., Follo M.Y., Fiume R., Martelli A.M., Cocco L., Manzoli L. Nuclear phospholipase C signaling through type 1 IGF receptor and its involvement in cell growth and differentiation. Anticancer Res. 2005;25(3B):2039–2041. [PubMed] [Google Scholar]
- Fernandez A.M., Torres-Alemán I. The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 2012;13(4):225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- Girnita L., Shenoy S.K., Sehat B., Vasilcanu R., Vasilcanu D., Girnita A., Lefkowitz R.J., Larsson O. β-Arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. J. Biol. Chem. 2007;282(15):11329–11338. doi: 10.1074/jbc.M611526200. [DOI] [PubMed] [Google Scholar]
- Granja M.G., Braga L.E.G., de Oliveira R.M., de Mello Silva E., Gonçalves-de-Albuquerque C.F., Silva A.R., de Castro-Faria-Neto H.C., Dos Santos A.A., Giestal-de-Araujo E. IGF-1 and IGF-1R modulate the effects of IL-4 on retinal ganglion cells survival: the involvement of M1 muscarinic receptor. Biochem. Biophys. Res. Commun. 2019;519(1):53–60. doi: 10.1016/j.bbrc.2019.08.124. [DOI] [PubMed] [Google Scholar]
- Guilarducci-Ferraz C.V.V., da Silva G.M., Torres P.M.M., Dos Santos A.A., de Araújo E.G. The increase in retinal cells proliferation induced by FGF2 is mediated by tyrosine and PI3 kinases. Neurochem. Res. 2008;33(5):754–764. doi: 10.1007/s11064-007-9491-x. [DOI] [PubMed] [Google Scholar]
- Harun-Or-Rashid M., Konjusha D., Galindo-Romero C., Hallböök F. Endothelin B receptors on primary chicken müller cells and the human MIO-M1 müller cell line activate ERK signaling via transactivation of epidermal growth factor receptors. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0167778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harun-Or-Rashid M., Lindqvist N., Hallböök F. Transactivation of EGF receptors in chicken Müller cells by α2A-adrenergic receptors stimulated by brimonidine. Investig. Ophthalmol. Vis. Sci. 2014;55(6):3385–3394. doi: 10.1167/iovs.13-13823. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Zhang X.M., Chen B.Y.K., Yang X.J. VEGF activates divergent intracellular signaling components to regulate retinal progenitor cell proliferation and neuronal differentiation. Development. 2006;113:2201–2210. doi: 10.1242/dev.02385. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma K., Koriyama Y., Mawatari K., Higuchi Y., Kosaka J., Kato S. Early downregulation of IGF-I decides the fate of rat retinal ganglion cells after optic nerve injury. Neurochem. Int. 2007;50(5):741–748. doi: 10.1016/j.neuint.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Li W., Jiang Y.X., Zhang J., Soon L., Flechner L., Kapoor V., Pierce J.H., Wang L.H. Protein kinase C-δ is an important signaling molecule in insulin-like growth factor I receptor-mediated cell transformation. Mol. Cell Biol. 1998;18(10):5888–5898. doi: 10.1128/MCB.18.10.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao R., Yan F., Zeng Z., Farhan M., Little P., Quirion R., Srivastava L.K., Zheng W. Amiodarone-induced retinal neuronal cell apoptosis attenuated by IGF-1 via counter regulation of the PI3k/Akt/FoxO3a pathway. Mol. Neurobiol. 2017;54(9):6931–6943. doi: 10.1007/s12035-016-0211-x. [DOI] [PubMed] [Google Scholar]
- Lorenc V.E., Jaldín-Fincati J.R., Luna J.D., Chiabrando G.A., Sánchez M.C. IGF-1 regulates the extracellular level of active MMP-2 and promotes müller glial cell motility. Investig. Ophthalmol. Vis. Sci. 2015;56(11):6948–6960. doi: 10.1167/iovs.15-17496. [DOI] [PubMed] [Google Scholar]
- Lorenc V.E., Subirada Caldarone P.V., Paz M.C., Ferrer D.G., Luna J.D., Chiabrando G.A., Sánchez M.C. IGF-1R regulates the extracellular level of active MMP-2, pathological neovascularization, and functionality in retinas of OIR mouse model. Mol. Neurobiol. 2018;55(2):1123–1135. doi: 10.1007/s12035-017-0386-9. [DOI] [PubMed] [Google Scholar]
- Maturana-Teixeira S., Braga L.E.G., Carpi Santos R., Calaza K.D.C., Giestal-de-Araujo E., Leao-Ferreira L.R. The (Na+/K+)-ATPase activity in the developing rat retina: the role of insulin-like growth factor-I (IGF-I) Cell. Mol. Neurobiol. 2015;35(2):243–254. doi: 10.1007/s10571-014-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mázala-de-Oliveira T., de Figueiredo C.S., de Rezende Corrêa G., da Silva M.S., Miranda R.L., de Azevedo M.A., Cossenza M., Dos Santos A.A., Giestal-de-Araujo E. Ouabain-Na+/K+-ATPase signaling regulates retinal neuroinflammation and ROS production preventing neuronal death by an autophagy-dependent mechanism following optic nerve axotomy in vitro. Neurochem. Res. 2022;47(3):723–738. doi: 10.1007/s11064-021-03481-0. [DOI] [PubMed] [Google Scholar]
- Mellough C.B., Collin J., Khazim M., White K., Sernagor E., Steel D.H., Lako M. IGF-1 signaling plays an important role in the formation of three-dimensional laminated neural retina and other ocular structures from human embryonic stem cells. Stem Cell. 2015;33(8):2416–2430. doi: 10.1002/stem.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng D., Shi X., Jiang B.H., Fang J. Insulin-like growth factor-I (IGF-I) induces epidermal growth factor receptor transactivation and cell proliferation through reactive oxygen species. Free Radic. Biol. Med. 2007;42(11):1651–1660. doi: 10.1016/j.freeradbiomed.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Nishiguchi K.M., Nakamura M., Kaneko H., Kachi S., Terasaki H. The role of VEGF and VEGFR2/Flk1 in proliferation of retinal progenitor cells in murine retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2007;48(9):4315–4320. doi: 10.1167/iovs.07-0354. [DOI] [PubMed] [Google Scholar]
- O’Kusky J., Ye P. Neurodevelopmental effects of insulin-like growth factor signaling. Front. Neuroendocrinol. 2012;33(3):230–251. doi: 10.1016/j.yfrne.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otteson D.C., Cirenza P.F., Hitchcock P.F. Persistent neurogenesis in the teleost retina: evidence for regulation by the growth-hormone/insulin-like growth factor-I axis. Mech. Dev. 2002;117(1-2):137–149. doi: 10.1016/S0925-4773(02)00188-0. [DOI] [PubMed] [Google Scholar]
- Romar G.A., Kupper T.S., Divito S.J. Research techniques made simple: techniques to assess cell proliferation. J. Invest. Dermatol. 2016;136(1):e1–e7. doi: 10.1016/j.jid.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Roudabush F.L., Pierce K.L., Maudsley S., Khan K.D., Luttrell L.M. Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. J. Biol. Chem. 2000;275(29):22583–22589. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- Ruberte J., Ayuso E., Navarro M., Carretero A., Nacher V., Haurigot V., George M., Llombart C., Casellas A., Costa C., Bosch A. Increased ocular levels of IGF-1 in transgenic mice lead to diabetes-like eye disease. J. Clin. Investig. 2004;113(8):1149–1157. doi: 10.1172/JCI19478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena N.K., Taliaferro-Smith L., Knight B.B., Merlin D., Anania F.A., O'Regan R.M., Sharma D. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008;68(23):9712–9722. doi: 10.1158/0008-5472.CAN-08-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigel G.M., Lupien S.B., Campbell L.M., Ishii D.N. Systemic IGF-I treatment inhibits cell death in diabetic rat retina. J. Diabetes Complicat. 2006;20(3):196–204. doi: 10.1016/j.jdiacomp.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Smith L.E., Shen W., Perruzzi C., Soker S., Kinose F., Xu X., Robinson G., Driver S., Bischoff J., Zhang B., Schaeffer J.M. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat. Med. 1999;5(12):1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- Solomon-Zemler R., Sarfstein R., Werner H. Nuclear insulin-like growth factor-1 receptor (IGF1R) displays proliferative and regulatory activities in non-malignant cells. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0185164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Uehara H., Ogawa H., Umemoto H., Bando Y., Izumi K. Inhibition of EP2/EP4 signaling abrogates IGF-1R-mediated cancer cell growth: involvement of protein kinase C-θ activation. Oncotarget. 2015;6(7):4829. doi: 10.18632/oncotarget.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Zhang Y., Ni N., Tang Z., Bai Z., Shen B., Sun H., Gu P. Insulin-like growth factor-1 regulation of retinal progenitor cell proliferation and differentiation. Cell Cycle. 2018;17(4):515–526. doi: 10.1080/15384101.2018.1431594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A., Suh P.G., Marmy-Conus N., Pearson R.B., Seok O.Y., Cocco L., Gilmour R.S. Phosphorylation of nuclear phospholipase C β1 by extracellular signal-regulated kinase mediates the mitogenic action of insulin-like growth factor I. Mol. Cell Biol. 2001;21(9):2981–2990. doi: 10.1128/MCB.21.9.2981-2990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Oikawa T., Kizawa R., Motohashi S., Yoshida S., Kumamoto T., Saeki C., Nakagawa C., Shimoyama Y., Aoki K., Tachibana T. Unconventional secretion of PKCδ exerts tumorigenic function via stimulation of ERK1/2 signaling in liver CancerUnconventional secretion of PKCδ in liver cancer. Cancer Res. 2021;81(2):414–425. doi: 10.1158/0008-5472.CAN-20-2009. [DOI] [PubMed] [Google Scholar]
- Yang X., Wei A., Liu Y., He G., Zhou Z., Yu Z. IGF-1 protects retinal ganglion cells from hypoxia-induced apoptosis by activating the Erk-1/2 and Akt pathways. Mol. Vis. 2013;19:1901. 2013. [PMC free article] [PubMed] [Google Scholar]
- Zerti D., Molina M.M., Dorgau B., Mearns S., Bauer R., Al-Aama J., Lako M. IGFBPs mediate IGF-1's functions in retinal lamination and photoreceptor development during pluripotent stem cell differentiation to retinal organoids. Stem Cell. 2021;39(4):458–466. doi: 10.1002/stem.3331. [DOI] [PubMed] [Google Scholar]
- Zhang W., Liu H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12(1):9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Meng D., Yan B., Jiang B.H., Fang J. Transactivation of epidermal growth factor receptor by insulin-like growth factor 1 requires basal hydrogen peroxide. FEBS Lett. 2006;580(22):5161–5166. doi: 10.1016/j.febslet.2006.08.068. [DOI] [PubMed] [Google Scholar]
- Zygar C.A., Colbert S., Yang D., Fernald R.D. IGF-1 produced by cone photoreceptors regulates rod progenitor proliferation in the teleost retina. Dev. Brain Res. 2005;154(1):91–100. doi: 10.1016/j.devbrainres.2004.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.